Abstract
We report the first case of infective endocarditis following urinary tract infection (UTI) caused by Globicatella sanguinis in an 87-year-old Japanese woman with recurrent episodes of UTI. We identified the pathogen using the Rapid ID32 Strep system. Accurate identification of this infection is important and essential for the effective antimicrobial coverage to this pathogen.
Keywords: Globicatella sanguinis, Infective endocarditis, Urinary tract infection, Rapid ID 32 strep, 16S rRNA sequencing
Introduction
G. sanguinis was described in 1992 as a new genus and species of catalase-negative, facultatively anaerobic, non-motile, non-hemolytic, Gram-positive cocci (GPC) forming short chains or pairs by Collins, et al. [1]. Only 42 isolates from clinical specimens and 13 case reports about UTI, bacteremia, or meningitis have been reported (Table 1). These reports suggest that G. sanguinis can colonize inguinal skin [2] and aged female patients with a history of cerebrovascular disease are susceptible to G. sanguinis. However, the epidemiology and the clinical significance of this pathogen remain largely unknown. G. sanguinis is an unusual pathogen that it could be misidentified or misdiagnosed with viridans streptococci (or may be overlooked) due to its colonial morphology [3]. We successfully identified the organism using the Rapid ID32 Strep system. We present the first case of an infective endocarditis by G. sanguinis following a UTI.
Table 1.
Clinical reported 42 cases of G. sanguinis infections.
| Reference | Country | Age | Gender | Underlying conditions | Presenting signs and symptoms | Site of isolation | Infection | Identification | Antibiotic treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| [2] | France | 56 | F | N/A | meningitis | CSF | meningitis | 16S rRNA sequencing | CTX, FOM | alive |
| [3] | USA | N/A | F | N/A | N/A | blood | bacteremia | Rapid ID 32 Strep + BBL Crystal Rapid Gram-Positive ID kit + BBL Crystal Gram-Positive ID kit + RapID STR | N/A | N/A |
| [3] | USA | N/A | F | N/A | N/A | blood | bacteremia | same as above | N/A | N/A |
| [3] | USA | N/A | M | N/A | N/A | urine | urinary | same as above | N/A | N/A |
| [3] | USA | N/A | N/A | N/A | N/A | blood | bacteremia | same as above | N/A | N/A |
| [3] | USA | N/A | N/A | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 69 | M | N/A | N/A | urine | urinary | same as above | N/A | N/A |
| [3] | USA | 85 | F | N/A | N/A | urine | urinary | same as above | N/A | N/A |
| [3] | USA | N/A | N/A | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 1 | M | N/A | N/A | CSF | meningitis | same as above | N/A | N/A |
| [3] | USA | 84 | F | N/A | N/A | blood | sepsis | same as above | N/A | N/A |
| [3] | canada | N/A | F | N/A | N/A | urine | N/A | same as above | N/A | N/A |
| [3] | USA | 90 | F | N/A | N/A | blood | urosepsis | same as above | N/A | N/A |
| [3] | USA | 68 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | canada | 82 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | canada | 79 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | N/A | N/A | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 1 | M | N/A | N/A | blood | septocemia | same as above | N/A | N/A |
| [3] | USA | N/A | N/A | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 58 | M | N/A | N/A | blood | septocemia | same as above | N/A | N/A |
| [3] | USA | 82 | F | N/A | N/A | blood | septocemia | same as above | N/A | N/A |
| [3] | canada | 2 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | canada | 92 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | canada | N/A | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 70 | F | N/A | N/A | blood | endocarditis | same as above | N/A | N/A |
| [3] | canada | 43 | F | N/A | N/A | CSF | N/A | same as above | N/A | N/A |
| [3] | canada | 85 | M | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | canada | 1 | F | N/A | N/A | blood | N/A | same as above | N/A | N/A |
| [3] | USA | 3 | F | N/A | N/A | blood | septicemia | same as above | N/A | N/A |
| [4] | Japan | 80 | F | colon cancer, brain stroke,dementia, HTN | fever, pyuria | urine | urinary | 16S rRNA sequencing | ABPC | alive |
| [5] | Korea | 85 | F | parkinson's disease, asthma, hypertwnsion, staying at nursing home | pain and swelling of left arm, fever | blood | bacteremia | partial 16S rRNA sequencing | VCM + CTRX | alive |
| [7] | USA | 72 | F | obesity, gastrip lap banding, tabacco | Hip pain | Hip synovium | prosthetic joint infection | API 20TREP + Vitek 2 g-Positive ID card + MALDI-TOF MS | VCM | alive |
| [7] | USA | 54 | F | obesity, DM, gastric bypass, tabacco | fatigue and fever | blood | bacteremia | MALDI-TOF MS | LZD | alive |
| [12] | Taiwan | 80 | F | chronic diarrhea, DM | cardiac arrest | blood | bacteremia | 16S rRNA sequencing | N/A | died |
| [12] | Taiwan | 92 | F | dementia, CHF | fever, cough | blood | urosepsis | 16S rRNA sequencing | CXM = > CAZ | alive |
| [13] | Denmark | 23 | F | intravenous drug use. Right-sided endocarditis, hepatitis C | pneumonia | blood | bacteremia | Rapid ID32 Strep, partial 16S rRNA sequencing | CXM = > PC | alive |
| [13] | Denmark | 82 | F | alzheimer's disease, hypertension | dehydration | blood | urosepsis | Rapid ID32 Strep, partial 16S rRNA sequencing | PC | alive |
| [13] | Denmark | 56 | M | crohn's disease, atrial fibrillation | erysipelas, dyspnoea, fever | blood | bacteremia | Rapid ID32 Strep, partial 16S rRNA sequencing | CXM | alive |
| [14] | Japan | 94 | M | dementia, CHF, nephrolithiasis | back pain, fever | blood | bacteremia | 16S rRNA sequencing | ABPC/SBT = > VCM | alive |
| [15] | Germany | 69 | F | VPS | meningitis | CSF | meningitis | Rapid ID32 Strep, phoenix PMIC/ID-56 | CTRX | alive |
| [16] | India | 70 | M | Craniectomy | meningitis | CSF | meningitis | Vitek 2 ID | LVFX, CPZ, SBT, AMK = > VCM, LVFX | alive |
DM, diabetes mellitus; CHF, chronic heart failure; VPS, ventriculoperitoneal shunt; CSF, cerebrospinal fluid; VCM, vancomycin; CTRX, ceftriaxone; LZD, linezolid; CXM, cefuroxime; CAZ, ceftazidime; PC, penicillin; ABPC/SBT, ampicillin/sulbactam; ABPC,levofloxacin ampicillin; CTX, cefotaxime; FOM, fosfomycin; LVFX, levofloxacin; CPZ, cefoperazone; SBT, sulbactam; AMK,amikacin; MEPM, meropenem
Case report
An 87 year-old Japanese woman was admitted to our hospital with recurrent episode of UTI, with a fever higher than 38.5 °C for 5 days, and with hematuria despite taking oral levofloxacin 500 mg and acetaminophen 1200 mg daily. She had been bedridden at nursing home since a subarachnoid hemorrhage and surgical construction of ventriculo-peritoneal shunt, and she had gastrostomy 10 years ago. On examination, she had body temperature of 36.5 °C, blood pressure 92/35 mmHg, pulse 84 bpm, respiratory rate 16 breaths/min, and pulse oxygen saturation 98% on room air. Physical examination showed Glasgow Coma Scale (GCS) of 7 (E2, V2, M3), inner lip bleeding, and systolic murmur at cardiac apex. Laboratory data at the admission was leucocyte count 35,600/mL, hemoglobin level 10.7 g/dL, platelet count 4.3000/mL, C-reactive protein 21.1 mg/dL, procalcitonin level 20.5 ng/mL, albumin 1.9 mg/dL, blood urea nitrogen 212 mg/dL, creatinine 3.9 ng/mL, uric acid 11.1 mg/dL, and lactate dehydrogenase 299 IU/L. Urine microscopy showed the presence of leukocytosis and numerous bacteria. Abdominal CT scans revealed bilateral hydronephrosis and hydroureters besides distended urinary bladder. After inserting urinary bladder catheter, pyuria and hematuria were obtained. Urinary gram stain showed GPC (1 + ) in pairs and short chains and Gram negative rods (GNR) (2+). She was diagnosed with infected hydronephrosis due to neurogenic bladder and started to receive ceftriaxone (CTRX) 2 g every 24 h empirically.
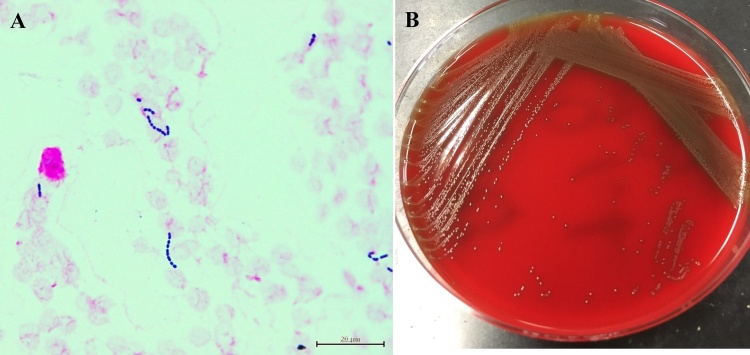
On day 3, her blood cultures from admission were growing aerobic, a-hemolytic GPC in short chains in 2 of 4 bottles (Fig. 1A, B). On day 4, the isolates were identified as G. sanguinis with a high certainty (98.8%) by Rapid ID32 Strep (bioMerieux, Lyon, France). In order to double-check the results, we performed 16S rRNA sequencing because the species is rare and for confirmation of the diagnosis. Extended-spectrum b-lactamase producing Escherichia coli (ESBL-producing E. coli) simultaneously was observed in the two sets of blood culture bottles. G. sanguinis and ESBL-producing E.coli in her urine culture were also identified. She was diagnosed with bacteremia due to UTI caused by both of the organisms. The antimicrobial treatment was altered to meropenem 0.5 g every 12 h, for a 14-day course, considering antimicrobial susceptibility testing results (Table 2) by WalkAway 40 SI system (Beckman Coulter, California, USA) and her renal function test results. Urine culture obtained before treating with meropenem showed the growth of G. sanguinis again, but her repeated blood and urine cultures after treatment were negative.
Fig. 1.
(A) Microscopic appearance of the isolates from her blood after Gram staining (×1000). (B) morphology of colonies on sheep blood agar.
Table 2.
Susceptibility Testing Results of G. sanguinis.
| MIC mg/mL | Interpretation | |
|---|---|---|
| AMPC/CVA | < = 0.25 | * |
| ABPC | < = 0.06 | S |
| AZM | < = 0.25 | S |
| CDTR-PI | 1 | * |
| CFPM | 1 | S |
| CTX | 2 | I |
| CTM | >4 | * |
| CTRX | 2 | I |
| CZOP | 1 | S |
| CP | < = 4 | S |
| CLDM | < = 0.12 | S |
| EM | < = 0.12 | S |
| LVFX | 8 | R |
| MEPM | 0.25 | S |
| MINO | < = 0.5 | S |
| PCG | 0.06 | S |
| ST | >4 | * |
| VCM | 0.25 | S |
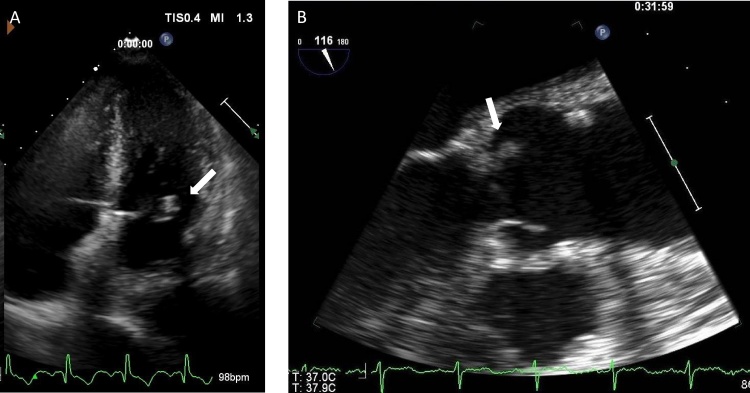
On day 6 of the treatment with meropenem, transthoracic echocardiography revealed a 3 mm (in diameter) vegetation (Fig. 2A) on the mitral valve besides mild aortic, mitral and tricuspid regurgitation. Meeting with Duke’s criteria (1 major and 3 minor criteria), she was eventually diagnosed with IE due to UTI-associated bacteremia. Her CT scans showed there was no abscess formation on the entire body. On day 10 of meropenem, a 2 mm vegetation on left coronary cusp of aortic valve was discovered by transesophageal echocardiography (TEE) (Fig. 2B). We presumed that G. sanguinis caused the endocarditis and administered her a 2 week of ampicillin 2 g every 8 h intravenously after completion of the meropenem treatment. After the 2 weeks of ampicillin, no vegetation was detected on TEE. She showed improvement and was discharged from the hospital to a nursing facility. She was in the hospital for 40 days.
Fig. 2.
(A) Parasternal long axis view on transthoracic echocardiogram shows a vegetation on posterior leaflet of mitral valve (arrow). (B) Parasternal long axis view on transesophageal echocardiogram shows a vegetation on left coronary cusp of aortic valve (arrow).
Discussion
We believe that this is the first case of having IE followed with UTI caused G. sanguinis complicated with ESBL-producing E.coli infection. G. sanguinis looks like viridans streptococci in Gram-stain morphology and colonial morphology including hemolysis pattern on sheep blood agar but has difference in not producing leucine aminopeptidase (LAP) and growth in the presence of 6.5% NaCl. Susceptibility to the third-generation cephalosporins is also different: G. sanguinis is resistant while a-streptococcus is susceptible [3]. Although we successfully identified G. sanguinis by rapid ID32 Strep with a high certainty (98.8%), there are some past cases that failed G. sanguinis identification by the same methods [4]. There may be two reasons; one is that G. sanguinis shows various biochemical reaction depending on strain [5] and the other is that the data of G. sanguinis had not been collected on the database of the rapid ID32 Strep system until 2006. If rapid ID32 Strep fails to identify G. sanguinis, 16S rRNA sequencing is required. We additionally attempted Bruker matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany) with MBT Compass software using MALDI Biotyper library version 5.0. The identification process suggested not G. sanguinis but G. sulfidifaciens with cutoff score of 1.97 (Table 3). G. sulfidifaciens, first described in 2001 isolated only from animals, has 99.2% similarity in 16S rRNA gene sequencing to G. sanguinis [6] but is different in biochemical reaction. Although, Miller et al. succeeded to identify G. sanguinis with Bruker MALDI-TOF MS with MBT 6903 MSP library database [7], MALDI-TOF MS is not so useful to identify the organism because there are only 3 G. sanguinis strains appeared in the database. An update of the database is needed.
Table 3.
Results of Bruker MALDI-TOF MS analysis.
| Rank (Quality) | Matched Pattern | Score value |
|---|---|---|
| 1 (+) | Globicatella sulfidifaciens 11_ 0100356_001_01 LGL | 1.97 |
| 2 (-) | Stenotrophomonas maltophilia 10942 CHB | 1.44 |
| 3 (-) | Pseudomonas boreopolis LMG 979T HAM | 1.39 |
| 4 (-) | Stenotrophomonas sp 109_Neb28 NFI | 1.37 |
| 5 (-) | Lactobacillus pentosus DSM 16366 DSM | 1.33 |
In our case, both G. sanguinis and E. coli were found in blood culture when the patient was admitted. IE caused by E. coli is rare (<1%) and G. sanguinis has been reported to be an opportunistic pathogen [8], [9], [10]. Patients diagnosed with E.colli IE are reported to be often diabetic with underlying heart disease or have prosthetic valves. Surgery is often necessary and the mortality rate is high (17%) [11]. Therefore, we speculated that IE caused by G. sanguinis followed a subacute clinical course, similar to viridans streptococcus, and her IE was caused by G. sanguinis. In order to verify our speculation, identifying G. sanguinis IE correctly with Rapid ID 32 Strep, collecting more cases of G. sanguinis IE, and then revealing clinical feature of G. sanguinis IE are required.
Ethics statement
Written informed consent to publish clinical details was obtained from the patient. A copy of the consent form is available for review by the Editor of this journal.
Conflict of interest
We have no conflict of interest to disclose.
Acknowledgements
We thank for the diligent and thorough critical reading of our manuscript by Masami Yoshihara, the medical interpreter and the medical coordinator, Tokyo Saiseikai Central Hospital.
References
- 1.Collons M.D., Aguirre M., Facklam R.R. Globicatella sanguis gen-nov, a new Gram-positive catalase bacterium from human sources. J Appl Bacteriol. 1992;73:433–437. doi: 10.1111/j.1365-2672.1992.tb05000.x. [DOI] [PubMed] [Google Scholar]
- 2.Héry-Arnaud G., Doloy A., Ansart S. Globicatella sanguinis meningitis associated with human carriage. J Clin Microbiol. 2010;48:1491–1493. doi: 10.1128/JCM.01299-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shewmaker P.L., Steigerwalt A.G., Shealey L. DNA relatedness, phenotypic characteristics, and antimicrobial susceptibilities of Globicatella sanguinis strains. J Clin Microbiol. 2001;39:4052–4057. doi: 10.1128/JCM.39.11.4052-4057.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurogi S. A case of urinary tract infection caused by Globicatella sanguinis. J Jpn Soc Clin Microbiol. 2016;26:27–31. [Google Scholar]
- 5.Yang H.S., Kim Y.J., Lee M.S. Globicatella sanguinis bacteremia in a non-Immunocompromised patient identified by 16S rRNA gene sequencing: first case in korea. Clin Lab. 2016;62:1825–1827. doi: 10.7754/Clin.Lab.2016.151215. [DOI] [PubMed] [Google Scholar]
- 6.Vandamme P., Hommez J., Snauwaert C. Globicatella sulfidifaciens sp. nov., isolated from purulent infections in domestic animals. Int J Syst Evol Microbiol. 2001;51:1745–1749. doi: 10.1099/00207713-51-5-1745. [DOI] [PubMed] [Google Scholar]
- 7.Miller A.O., Buckwalter S.P., Henry M.W. Globicatella sanguinis osteomyelitis and bacteremia:review of an emerging human pathogen with an expanding spectrum of disease. Open Forum Infect Dis. 2017;19:277. doi: 10.1093/ofid/ofw277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morpeth S., Murdoch D., Cabell C.H. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 9.Micol R., Lortholary O., Jaureguy F. Escherichia coli native valve endocarditis. Clin Microbiol Infect. 2006;12:401–403. doi: 10.1111/j.1469-0691.2006.01375.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruoff K.L. Miscellaneous catalae negative Gram-positive cocci emerging opportunists. J Clin Microbiol. 2002;40:1129–1133. doi: 10.1128/JCM.40.4.1129-1133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branger S. Escherichia coli endocarditis: seven new cases in adults and review of the literature. Eur J Clin Microbiol Infect Dis. 2005;24:537–541. doi: 10.1007/s10096-005-1379-6. [DOI] [PubMed] [Google Scholar]
- 12.Lau Woo S.K.P.C., Li N.K. Globicatella bacteraemia identified by 16S ribosomal RNA gene sequencing. J Clin Pathol. 2006;59:303–307. doi: 10.1136/jcp.2005.028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul-Redha R.J. Globicatella sanguinis bacteremia idenfitied by partial 16S rRNA gene sequencing. Scand J Infect Dis. 2007;39:745–748. doi: 10.1080/00365540701203527. [DOI] [PubMed] [Google Scholar]
- 14.Matsunami M. Urosepsis caused by Globicatella sanguinis and Corynebacterium riegelii in an adult: case report and literature review. J Infect Chemother. 2012;18:552–554. doi: 10.1007/s10156-011-0335-x. [DOI] [PubMed] [Google Scholar]
- 15.Seegmüller I. Globicatella sanguinis is an etiological agent of ventriculoperitoneal shunt-associated meningitis. J Clin Microbiol. 2007;45:666–667. doi: 10.1128/JCM.01774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Jain N. Globicatella sanguinis meningitis in a post head trauma patient: first case report from Asia. J Infect Dev Ctries. 2012;23(6):592–594. doi: 10.3855/jidc.1947. [DOI] [PubMed] [Google Scholar]; (b) Branger S. Escherichia coli endocarditis: seven new cases in adults and review of the literature. Eur J Clin Microbiol Infect Dis. 2005;24:537–541. doi: 10.1007/s10096-005-1379-6. [DOI] [PubMed] [Google Scholar]