Abstract
Background
Although HIV infection is associated with well-known oral pathologies, there remains a dearth of comparative studies aimed at determining the association between HIV infection/exposure and early childhood caries.
Methods
This is a cross-sectional study using a convenience sample of 3 groups of children receiving care at a tertiary care hospital in Nigeria. The groups include HIV infected (HI); HIV exposed but uninfected (HEU); and HIV unexposed and uninfected (HUU) children aged 6 through 72 months. Medical records were reviewed and caregivers were interviewed for socio-demographic, maternal and birth factors as well as early feeding and dietary information. Oral examinations were performed by trained dentist-examiners.
Results
Of 335 children enrolled, 33 (9.9%) presented with caries. In an adjusted analysis, compared to HUU children, HI children had significantly greater odds of having caries (OR=2.58; 95%CI=1.04–6.40; p=0.04); but there was no statistically significant difference in HEU children (OR=2.01; 95%CI=0.56–7.23; p=0.28). Factors significantly associated with higher caries prevalence include low CD4 counts and percentage, older age, longer duration of breastfeeding and spontaneous membrane rupture during delivery.
Conclusions
Caries was more prevalent in HIV infected children. These findings support the need to target HIV infected children for oral health prevention and treatment services particularly in Nigeria and other developing countries.
Introduction
HIV infection is characterized by progressive impairment of the immune system and the development of opportunistic infections particularly in the oral cavity. About 30–80% of HIV infected (HI) individuals present with one or more oral diseases (often termed “oral manifestations of HIV”). (1–3)
Despite the overall reduction in incidence of oral manifestations of HIV in the last decade, largely attributed to the introduction of highly active antiretroviral therapy (HAART), there have been reports of increased caries experience in HI populations.(4) Dental caries is associated with clinical, social, psychological and economic consequences particularly in children. Early childhood caries (ECC), which could interrupt balanced nutrition, development and consequently lead to failure to thrive, is a significant problem particularly in developing nations such as Nigeria (which has the second highest burden of HIV infection in the world after South Africa).(5) Although there is still no clear consensus, Obileye et al reported caries prevalence of 39.5% among Nigerian HI children while other studies have reported low prevalence rates in HI children or no association between HIV infection and caries.(6–8)
In Nigeria, where 3.4 million people are living with HIV, about 200,000 children are born to HIV infected women every year.(9) With improved antiretroviral (ART) scale-up for the prevention of mother-to-child HIV transmission (PMTCT), there is evidence suggesting that the growing population of HIV exposed but uninfected (HEU) children have an increased susceptibility to early life infections and greater immunological impairment compared to HIV-unexposed/uninfected (HUU) children born to HIV uninfected mothers. (10–13)
Given the above, there is a need to further examine the relationship between caries and HIV infection particularly in regions where the burden of HIV infection is highest i.e. sub-Saharan Africa. Furthermore, there is no current report evaluating the effect of HIV perinatal exposure without infection (HEU) on caries prevalence, compared to HUU children. Notably, comparative studies are limited in the literature worldwide and non-existent in Nigeria. The objectives of this study were to compare the prevalence of early childhood caries in HI and HEU children with HUU children (as controls) and to identify other factors associated with early childhood caries in Nigerian children. We hypothesized that the prevalence of caries will be higher in HI and HEU children, compared to HUU children.
Methods
Study Design and Population
This was a cross sectional study of children aged 6–72 months receiving care and treatment from February through October 2014, at the University of Benin Teaching Hospital (UBTH) in Benin City, Edo State, Nigeria. We enrolled a convenience sample of three groups of children, HI children, HEU children and HUU children as they attended routine well-child/monitoring clinics. Only children aged 6–72 months, who had at least one tooth erupted and whose parents or guardians provided written informed consent, were eligible to participate. This age range was chosen to reflect the risk of ECC. Children whose HIV status could not be determined were excluded from the study. This study was approved by the institutional review boards at UBTH and the University of Maryland, Baltimore (UMB).
Determination of HIV perinatal exposure and HIV infection
Prenatal exposure to HIV was determined based on review of maternal and child medical records and current HIV status of child. Pregnant women who attend the antenatal clinic at UBTH are routinely tested for HIV-1 antibodies by enzyme-linked immunosorbent assay (ELISA; Vironostica, BioMerieux) and confirmed by Western blot (Immunetics, Boston). Pediatric HIV infection was ascertained by the laboratory detection of HIV DNA using PCR in children < 18 months of age and by detection of antibodies with HIV serology in children > 18 months of age. Blood was collected from all participants (regardless of HIV exposure or infection status) for CD4+ measurements (counts and percentages).
Oral Examination
ECC is defined as the presence of one or more decayed (noncavitated or cavitated lesions), missing (due to caries), or filled tooth surfaces in any primary tooth in a child 71 months of age or younger.(14) To determine the presence of decayed lesions, standardized oral clinical examination was conducted blindly (without knowledge of their HIV infection or exposure status) by 2 similarly trained and calibrated dentist-examiners according to the National Institute of Dental and Craniofacial Research (NIDCR) criteria (15). All teeth present in the oral cavity were assessed with the aid of artificial light, a dental mirror and a dental probe to detect white spots and cavitated lesions, and children were classified as either “caries free” or “caries affected” based on the presence of at least one cavity or white-spot lesion on any tooth surface in the mouth. Radiography was not used in this study. The 2 examiners were calibrated to ensure inter-examiner and intra-examiner reliability for the presence or absence of caries per tooth in about 31 children with 342 teeth (8 carious). The inter-examiner Cohen’s Kappa value was 0.84 and intra-examiner Cohen’s Kappa values for the two examiners were 0.91 and 0.89. Caries severity was measured using the dft index (number of decayed or filled primary teeth) and DMFT index (number of decayed, missing or filled permanent teeth). The presence of other mucosal and oro-dental conditions such as candidiasis, salivary gland swellings, gingivitis, ulcers and lymph node swellings, were observed and recorded by the dentist-examiners.
Measurement of Covariates
Information regarding each child’s sociodemographic profile (age and sex), clinical status, birth factors and oral hygiene practices were collected via medical chart reviews and structured questionnaires. Maternal age, education and employment status were also collected and recorded from mother or caregiver (in cases where mother was dead or unavailable). A previously validated food and drink frequency questionnaire was adapted and administered to examine the child’s current and early infant feeding practices.(16) Regular intake of sugary food or drinks was defined as consuming foods or drinks containing refined sugar 2 or more times a week.
Statistical Analysis
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). We calculated the proportion of children who had caries or one or more oral diseases among the 3 groups. We also determined the dft/DMFT indices and compared them across the groups. Unadjusted odds ratios for the associations between caries and HIV infection/exposure were calculated. To determine group differences (HI vs. HUU, and HEU vs HUU), associations between categorical variables were assessed using Pearson’s Chi-square or Fisher’s exact test where appropriate. For continuous variables, a t-test and/or an ANOVA F test were performed. Covariates found to be associated with caries and HIV infection or exposure were examined for confounding and effect modification in stratified analyses. A multivariable logistic regression model evaluated the association between HIV infection or exposure on the odds of having one or more carious lesions while controlling for confounders and allowing for effect modification. Because we hypothesized that immunosuppression mediates the relationship between HIV infection/exposure and caries, we did not include CD4 lymphocyte counts or percentages in the final multivariable logistic regression model. Thereafter, we evaluated the relationship between CD4 counts/percentages on caries without including HIV infection and exposure status.
Results
Study Population
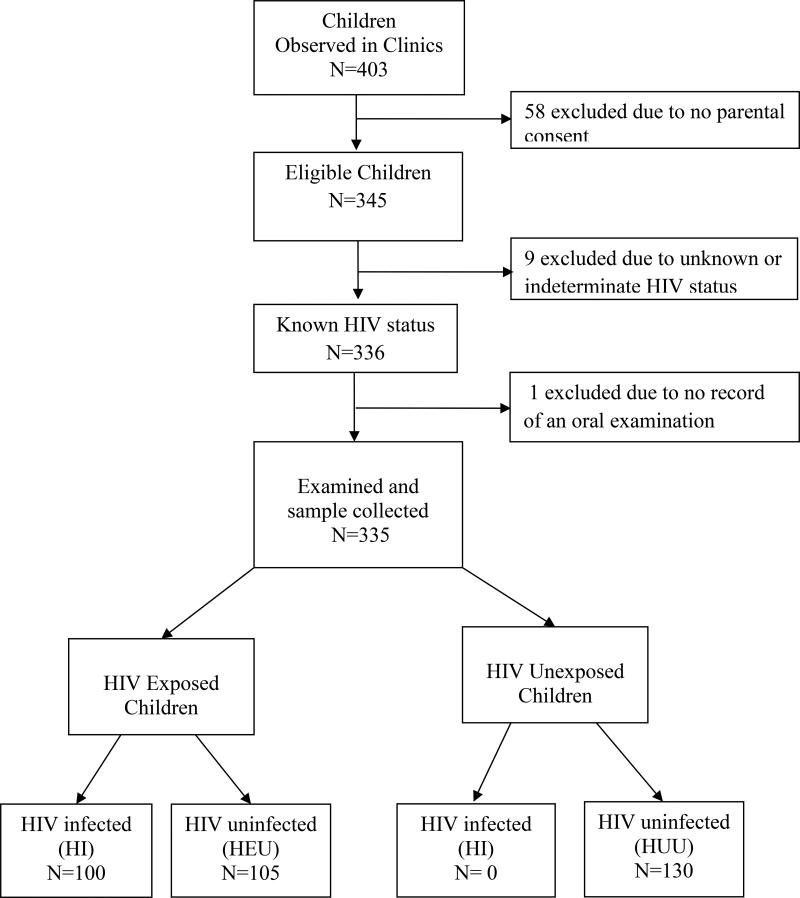
A total of 335 children participated in the study. Of these, 100 were HI, 105 were HEU and 130 were HUU children (Figure 1). The characteristics of the children are described in Table 1 – where 167 (49.9%) were female and the mean age at enrollment was 40.6 months (standard deviation [SD] = 18.1; range, 9–72 months).
Figure 1. Identification of Study Population.
Figure shows a flow chart describing recruitment of study participants. The study groups are as follows: HI= HIV infected; HEU = HIV exposed but uninfected; HUU= HIV unexposed and uninfected
Table 1.
Characteristics of Study Population (N=335)
| Study Group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Total (N=335) | HI (N=100) | HEU (N=105) | HUU (N=130) | |||||
|
|
|||||||||
| n | (%) | (%) | (%) | (%) | p-value* | ||||
| Demographics | |||||||||
| Sex | 0.41 | ||||||||
| Male | 168 | (50.2) | 55 | (55.0) | 53 | (50.5) | 60 | (46.2) | |
| Female | 167 | (49.9) | 45 | (45.0) | 52 | (49.5) | 70 | (53.9) | |
| Age in months, mean (standard deviation [SD]) | 40.6 | (18.1) | 48.23 | (17.7) | 34.63 | (18.2) | 39.52 | (21.2) | <0.0001 |
| Age Categories | <0.0001 | ||||||||
| < 24 months | 85 | (25.4) | 9 | (9.0) | 36 | (34.23) | 40 | (30.8) | |
| 24 to <36 months | 71 | (21.2) | 24 | (24.0) | 25 | (23.8) | 22 | (16.9) | |
| 36 to <48 months | 55 | (16.4) | 16 | (16.0) | 16 | (15.2) | 23 | (17.7) | |
| 48 to < 60 months | 43 | (12.8) | 16 | (16.0) | 17 | (16.2) | 10 | (7.7) | |
| 60 to <72 months | 81 | (24.2) | 35 | (35.0) | 11 | (10.5) | 35 | (26.9) | |
| Immunologic Status | |||||||||
| CD4 count, cells/mm3 mean (SD) | 1131 | (553) | 1018 | (647) | 1147 | (492) | 1206 | (512) | 0.03 |
| CD4 count, cells/mm3 | <0.0001 | ||||||||
| < 750 | 76 | (28.4) | 38 | (39.0) | 18 | (17.1) | 20 | (15.9) | |
| ≥ 750 | 259 | (71.6) | 62 | (60.0) | 87 | (82.9) | 110 | (84.6) | |
| CD4 percent, % mean (SD) | 28.69 | (13.3) | 25.45 | (12.3) | 27.37 | (12.7) | 32.25 | (13.8) | 0.0003 |
| CD4 percent, % | 0.003 | ||||||||
| CD4 % < 22 | 95 | (28.4) | 39 | (39.0) | 35 | (33.3) | 21 | (16.1) | |
| CD4 % ≥ 22 | 240 | (71.6) | 61 | (60.0) | 70 | (66.7) | 109 | (83.8) | |
| ART Exposure | |||||||||
| Currently on ART | 94 | (28.1) | 94 | (94.0) | 0 | (0) | 0 | <0.0001 | |
| ART Prophylaxis only | 86 | (25.7) | 1 | (1.0) | 85 | (80.9) | 0 | ||
| No ART exposure | 155 | (46.3) | 5 | (5.0) | 20 | (19.0) | 130 | (100.0) | |
| Early feeding Practices | |||||||||
| Duration of Breastfeeding, months; mean (SD) | 8.59 | (6.5) | 8.99 | (6.6) | 3.77 | (4.7) | 12.15 | (5.1) | <0.0001 |
| Breast feeding categories | <0.0001 | ||||||||
| Never breast fed | 68 | (20.3) | 15 | (15.0) | 50 | (47.6) | 3 | (2.3) | |
| ≤ 12 months | 157 | (46.9) | 50 | (50.0) | 45 | (42.9) | 62 | (47.7) | |
| > 12 months | 110 | (32.8) | 35 | (35.0) | 10 | (9.5) | 65 | (50.0) | |
| Diet | |||||||||
| Regular Intake of Sugary drinks and foods | 0.61 | ||||||||
| Yes | 111 | (34.9) | 30 | (30.9) | 36 | (37.1) | 45 | (36.9) | |
| No | 207 | (65.1) | 67 | (69.1) | 63 | (63.6) | 77 | (63.1) | |
| Sleeping off while bottle/breast feeding | 0.33 | ||||||||
| Regularly | 44 | (13.5) | 13 | (13.0) | 18 | (17.3) | 13 | (10.6) | |
| None/ Rarely | 283 | (86.5) | 87 | (87.0) | 86 | (82.7) | 110 | (89.4) | |
| Oral Hygiene and Health | |||||||||
| Number of erupted primary teeth, mean (SD) | 17.21 | (4.8) | 18.63 | (3.5) | 16.60 | (5.0) | 16.61 | (5.2) | 0.002 |
| Frequency of teeth cleaning | 0.35 | ||||||||
| Less than once daily | 32 | (9.5) | 6 | (6.0) | 12 | (11.4) | 14 | (10.8) | |
| At least once daily | 303 | (90.4) | 94 | (94.0) | 93 | (88.6) | 116 | (89.2) | |
| Birth Factors | |||||||||
| Gestational age at Birth in weeks, mean (SD) | 36.97 | (2.8) | 37.65 | (2.8) | 36.82 | (3.7) | 36.60 | (1.6) | 0.02 |
| Mode of Delivery | 0.007 | ||||||||
| Caesarean | 26 | (7.8) | 3 | (3.0) | 15 | (14.3) | 8 | (6.1) | |
| Vaginal | 309 | (92.2) | 97 | (97.0) | 90 | (85.7) | 122 | (93.8) | |
| Membrane Rupture | 0.04 | ||||||||
| Spontaneous | 222 | (67.5) | 73 | (73.0) | 66 | (62.9) | 83 | (63.8) | |
| Induced/Artificial | 107 | (32.5) | 21 | (21.0) | 39 | (37.1) | 47 | (36.1) | |
| Maternal Factors | |||||||||
| Mother's age at Enrollment | 0.08 | ||||||||
| Mean, (SD) | 33.36 | (6.1) | 34.57 | (5.5) | 33.30 | (6.4) | 32.61 | (6.3) | |
| Highest education completed | 0.0004 | ||||||||
| None | 8 | (2.5) | 5 | (5.6) | 3 | (2.9) | 0 | 0.0 | |
| Started Primary | 62 | (19.4) | 26 | (28.9) | 22 | (21.4) | 14 | (11.0) | |
| Started Secondary | 156 | (48.7) | 41 | (45.6) | 57 | (55.3) | 58 | (45.7) | |
| Post-secondary | 84 | (26.2) | 14 | (15.6) | 18 | (17.5) | 52 | (40.9) | |
| Other | 10 | (3.1) | 4 | (4.4) | 3 | (2.9) | 3 | (2.4) | |
| Employment Status | <0.0001 | ||||||||
| Professional | 46 | (14.5) | 3 | (3.4) | 7 | (6.9) | 36 | (27.7) | |
| Clerical /Skilled | 43 | (13.5) | 13 | (14.6) | 14 | (13.7) | 15 | (11.5) | |
| Trading/Unskilled | 190 | (59.7) | 61 | (68.5) | 67 | (65.7) | 62 | (47.7) | |
| Unemployed | 40 | (12.6) | 12 | (13.5) | 14 | (13.7) | 14 | (10.8) | |
| Marital status | 0.02 | ||||||||
| Married | 301 | (94.4) | 82 | (90.1) | 97 | (96.0) | 122 | (96.1) | |
| Single | 6 | (1.9) | 4 | (4.4) | 2 | (2.0) | 0 | 0.0 | |
| Separated/Divorced | 5 | (1.6) | 0 | (0.0) | 1 | (1.0) | 4 | (3.1) | |
| Widowed | 7 | (2.2) | 5 | (5.5) | 1 | (1.0) | 1 | (0.8) | |
|
| |||||||||
| Oral Pathologies | |||||||||
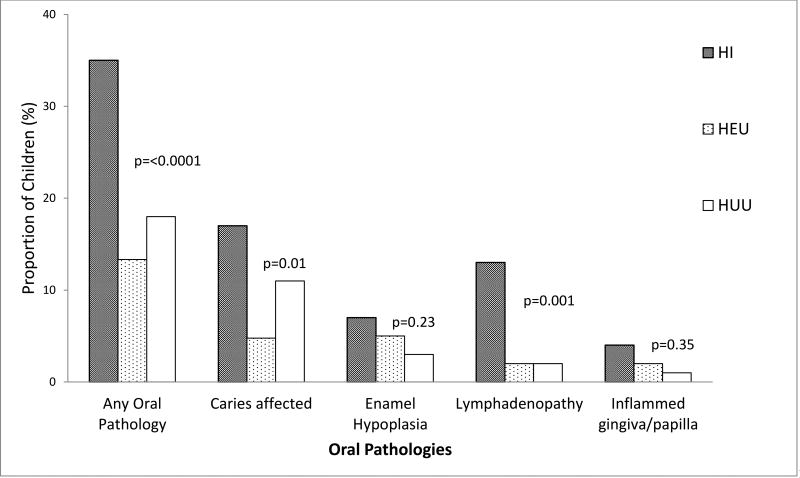
| Presence of any oral pathology | 67 | (20.0) | 35 | (35.0) | 14 | (13.3) | 18 | (18.0) | <0.0001 |
| Caries affected | 33 | (9.9) | 17 | (17.0) | 5 | (4.8) | 11 | (11.0) | 0.01 |
| Hypoplastic teeth | 15 | (4.5) | 7 | (7.0) | 5 | (5.0) | 3 | (3.0) | 0.23 |
| Lymphadenopathy | 17 | (5.1) | 13 | (13.0) | 2 | (2.0) | 2 | (2.0) | <0.0001 |
| Inflamed gingiva/papilla | 7 | (2.1) | 4 | (4.0) | 2 | (2.0) | 1 | (1.0) | 0.33 |
| Caries Indices | |||||||||
| dft score (mean + SD) | 0.33 | (1.1) | 0.62 | (1.6) | 0.13 | (0.8) | 0.27 | (1.0) | 0.01 |
| DMFT score (mean + SD) | 0.003 | (0.1) | 0 | 0 | 0.01 | (0.1) | 0.45 | ||
p value for overall comparison across the 3 groups (ANOVA)
HEU - HIV exposed but uninfected; HI- HIV infected; HUU - HIV Unexposed Uninfected;
ART- Antiretroviral Treatment; SD- Standard Deviation
Dft –decayed, filled primary teeth
DMFT – decayed, missing filled permanent teeth
Socio-Demographic, Clinical, Dietary and Maternal Characteristics by Study Group
Differences in demographic, immunologic, dietary, birth and maternal characteristics of the children were examined by study group as shown in Table 1. Overall, the 3 study groups significantly differed from each other in terms of age, mean CD4 lymphocyte count, mean CD4 percent values, ART exposure, duration of breastfeeding, number of primary teeth erupted, gestational age at birth, mode of delivery, membrane rupture method (spontaneous or induced/artificial), maternal education, mother’s employment and marital status (based on comparisons across all groups).
HI vs. HUU children
Compared to HUU children, HI children were significantly different with respect to certain characteristics (Table 1). HI children were more likely to be older, be born at an older gestational age, and with a larger number of erupted primary teeth. HI children weighed less at the time of this study, had lower CD4 lymphocyte counts and percentages, and were less likely to be delivered via artificial membrane rupture or be breastfed when compared to HUU children. Among the HI group, six children were not receiving ART at the time of enrollment. Among those on ART, the median duration of time on ART was 15 months [interquartile range (IQR): 2–34 months] and 78% of them were on the zidovudine (ZDV)-containing first-line regimen (ZDV/3TC/NVP). Furthermore, mothers of HI children were more likely to be dead or aged over 40 than mothers of HUU children. They were also less likely to have a post-secondary education, professional employment or a married partner at the time of the study when compared to HUU children.
HEU vs. HUU children
In general, HEU children were more like HUU children than HI children (Table 1). Compared to HUU children, HEU children were younger and were less likely to be breastfed when compared to HUU children. Although HEU children did not differ significantly from HUU children in terms of CD4 counts and percentages, birth factors such as birth weight, gestational age and type of membrane rupture for delivery, their mothers were less likely to have a post-secondary education or professional employment at the time of the study.
Caries Prevalence
Overall, 9.9% of children presented with one or more carious lesions. Compared to 11% caries prevalence in HUU children, 17% of HI children (p=0.05) and 5% of HEU children (p=0.26) had caries (Table 1). Most of the carious lesions were found in the primary dentition; only one child (in the HUU group) had a carious lesion in his permanent dentition. Figure 2 shows the distribution of all oral findings by study group.
Figure 2. Distribution of Oral Pathologies among HIV infected (HI), HIV exposed but uninfected (HEU) and HIV unexposed and uninfected (HUU) children.
This figure shows the distribution of oral pathologies by study group. P value was derived from an ANOVA test of comparison across all study groups. HI= HIV infected; HEU = HIV exposed but uninfected; HUU= HIV unexposed and uninfected
Table 2 shows the risk factors associated with caries in the unadjusted and adjusted analyses. In the unadjusted analysis, HIV infection, older age, immunosuppression (CD4 lymphocyte count), longer duration of breast feeding, spontaneous (vs. artificial) membrane rupture were associated with a higher caries prevalence. In a multivariable model excluding CD4 measures (Table 2), HIV infection remained significantly associated with caries (OR =2.58; 95% CI=1.04–6.40, P=0.04). HEU children did not significantly differ from HUU children in terms of caries prevalence. HIV exposure and lack of infection (HEU vs HUU) was not independently associated with caries (OR= 2.01; 95% CI =0.56–7.23, p=0.28).
Table 2.
Risk Factors associated with Caries
| Caries Affected | Caries Unaffected | Unadjusted | Adjusted* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Risk factors | N | n | (%) | n | (%) | OR | (95%CI) | p value φ | OR | (95%CI) | p value |
| Study Group | |||||||||||
| HI | 100 | 17 | (17.0) | 83 | (83.0) | 2.22 | 0.99–4.97 | 0.05 | 2.58 | 1.04 – 6.40 | 0.04 |
| HEU | 105 | 5 | (4.8) | 100 | (95.2) | 0.54 | 0.18–1.61 | 0.27 | 2.01 | 0.56 – 7.23 | 0.28 |
| HUU | 130 | 11 | (11.0) | 119 | (91.5) | ref | ref | ||||
| Sex | |||||||||||
| Female | 167 | 17 | (10.2) | 150 | (89.8) | 1.62 | 0.78–3.38 | 0.19 | 1.68 | 0.74–3.81 | 0.21 |
| Male | 168 | 11 | (6.5) | 157 | (93.4) | ref | ref | ||||
| Age (in months), mean (SD) | 56.09 (12.0) | 38.89 (19.93) | 1.05 | 1.03–1.07 | <0.0001 | 1.04 | 1.02–1.07 | 0.0007 | |||
| ref | ref | ||||||||||
| CD4 lymphocyte count, mean (SD) | 894 (437) | 1201 (501) | 0.99 | 0.99– 1.00 | 0.006 | ||||||
| CD4 lymphocyte percent, mean (SD) | 25.48 (11.3) | 29.04 (13.5) | 0.97 | 0.95– 1.01 | 0.14 | ||||||
| ART Exposure | |||||||||||
| Currently on ART | 94 | 16 | (17.0) | 78 | (83.0) | 2.07 | 0.96 – 4.46 | 0.06 | |||
| ART Prophylaxis only | 86 | 3 | (3.5) | 83 | (96.5) | 0.36 | 0.10 – 1.30 | 0.12 | |||
| No ART exposure | 155 | 14 | (9.0) | 141 | (91.0) | ref | |||||
| Duration of Breastfeeding in months, mean (SD) | 13.30 (7.1) | 8.09 (8.8) | 1.14 | 1.07–1.21 | <0.0001 | 1.13 | 1.05– 1.21 | 0.0006 | |||
| Breast feeding categories | |||||||||||
| Never breast fed | 68 | 4 | (5.9) | 64 | (94.1) | 1.03 | 0.30–3.46 | 0.96 | |||
| > 12 months | 110 | 20 | (18.2) | 90 | (81.8) | 3.65 | 1.59–8.37 | 0.002 | |||
| ≤ 12 months | 157 | 9 | (5.7) | 148 | (94.3) | ref | |||||
| Regular Intake of Sugary drinks/foods | |||||||||||
| Yes | 111 | 12 | (10.8) | 99 | (89.2) | 1.27 | 0.61–2.67 | 0.52 | |||
| No | 207 | 20 | (9.7) | 187 | (90.3) | ref | |||||
| Sleeping off while bottle/breast feeding | |||||||||||
| Regularly | 44 | 6 | (13.6) | 38 | (86.4) | 1.54 | 0.60– 3.98 | 0.37 | |||
| None/ Rarely | 283 | 27 | (9.5) | 256 | (90.5) | ref | |||||
| Number of Erupted 1° Teeth | 19.18 (3.1) | 16.98 (4.9) | 1.19 | 1.02–1.39 | 0.02 | ||||||
| Frequency of Teeth cleaning | |||||||||||
| Less than once daily | 32 | 1 | (3.1) | 31 | (96.9) | 0.27 | 0.04– 2.07 | 0.21 | |||
| At least once daily | 303 | 32 | (10.6) | 271 | (89.4) | ref | |||||
| Membrane Rupture | |||||||||||
| Induced/Artificial | 109 | 4 | (3.7) | 105 | (96.3) | 0.26 | 0.09–0.77 | 0.01 | 0.31 | 0.10–1.00 | 0.05 |
| Spontaneous | 226 | 29 | (12.8) | 197 | (87.2) | ref | |||||
HEU; HIV exposed but uninfected; HI- HIV infected; HUU - HIV Unexposed Uninfected; ART – Antiretroviral Treatment; SD- Standard Deviation, OR- Odds Ratio; CI- Confidence Interval
P value from Chi/Fisher exact, T test , ANOVA or logistic regression model where appropriate;
Multivariable logistic regression model includes study group, age (as a continuous variable), sex, duration of breast feeding (as a continuous variable) and membrane rupture
Table 3 shows the association between caries and CD4 measure within models that did not include the study group (HIV infection or exposure). Specifically, having CD4 counts <750 cells/mm3 was significantly associated with caries prevalence in an unadjusted (OR=3.29; 95%CI =1.57–6.50, P=0.002) and adjusted analyses (OR=2.70; 95%CI =1.17–6.20, P=0.02). Low CD4 percentages (<22%) were not significantly associated with caries in a univariate unadjusted analysis (OR=1.60; 95%CI =0.75–3.43, P=0.23). However, in an adjusted analysis, there was trend towards an association between low CD4 percentage values and caries prevalence (OR=2.30; 95%CI =0.96–5.53, P=0.06).
Table 3.
Effect of Immunosuppression on Caries Prevalence (N=335)
| Caries Affected | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4 categories* | N | n | % | Unadjusted OR |
(95% CI) | P value | Adjusted** OR |
(95% CI) | P value |
| CD4 lymphocyte count (cells/mm3) | |||||||||
| <750 | 76 | 15 | (19.7) | 3.29 | 1.57–6.91 | 0.002 | 2.70 | 1.17–6.21 | 0.02 |
| ≥750 | 259 | 18 | (6.9) | ref | ref | ||||
| CD4 percentage values in % | |||||||||
| <22 | 95 | 12 | (12.6) | 1.60 | 0.75–3.43 | 0.23 | 2.30 | 0.96–5.53 | 0.06 |
| ≥22 | 240 | 21 | (8.7) | ref | ref | ||||
OR- Odds Ratio; CI- Confidence Interval
Multivariable CD4 categories based on CDC’s 2014 and 2008 case definitions for stages of HIV infection (Centers for Disease Control and Prevention (CDC), 2014; Schneider et al., 2008)
Multivariable logistic regression model included the CD4 category of interest (for CD4 lymphocyte counts or CD4 percent values), age (as a continuous variable), sex, duration of breast feeding (as a continuous variable) and method of membrane rupture (spontaneous or induced/artificial)
Severity of Caries
Overall the mean dft score was 0.33 +1.15 (mean ±SD) (Table 1) and among the 33 children who had caries, the mean dft score was 3.27 (SD =1.91) (not shown in Table). About 82% of children who presented with dental caries had a dft score ≥2. Compared to HUU children, HI children had more severe caries as indicated by a higher dft score (0.62 vs 0.27; P=0.04); however there were no significant differences in DFMT scores as the carious lesions were found mostly in the primary/deciduous dentition (Table 1). In an adjusted analysis (not shown in Table), the mean dft score for HI children was significantly higher by 0.38 when compared to HUU children (P=0.02). HEU children did not have a significantly higher dft score compared to HUU children (P=0.38).
Discussion
In this first comparative study of the association between HIV exposure or infection and dental caries in young Nigerian children, we observed a higher prevalence of dental caries in the HI group when compared to the HUU group. However, HIV exposure alone, without a diagnosis of infection, did not appear to significantly increase the odds of having caries or the presentation of any oral pathology compared to HUU children.
These study findings must be considered in the context of the age distribution of this study population. PMTCT has been successfully practiced in UBTH (evidenced by perinatal HIV transmission rates of about 2% in the last 5 years); hence 50% of the HI children eligible and available for recruitment in this study were beyond the age of 4. (17) Consequently, HI children were considerably older than HEU and HUU children enrolled in this study. However, even after adjustment for age and other key covariates, there is moderate evidence to suggest that HIV infection is an independent risk factor for caries. The possible reason for this finding could be that an immunocompromised condition could increase the risk of microbial colonization, particularly cariogenic bacteria, leading to tooth decay. Several studies have reported that the high prevalence of caries in HI children is associated with reduced CD4+ T-lymphocyte values.(18–21) Our results also support the fact that, even in a population with well controlled HIV infection, immunosuppression remained significantly associated with caries. In addition to compromised immunity, several authors have attributed the higher caries prevalence in HI children to reduced salivary flow, high sucrose levels in pediatric syrups and/or changes in bacterial profiles.(22–24)
We observed a non-significant increase in odds in HEU children compared to HUU children. We had hypothesized that HEU children, reported to have an impaired immune system due to perinatal exposure to HIV and an increased susceptibility to infection early in life, are more likely to acquire cariogenic bacteria and subsequently have caries, but results from this study do not support this. (25–30). It is possible that HIV infection has a more disruptive effect on the oral microbiome and subsequently oral health compared to perinatal HIV exposure.
Other factors that were associated with caries, such as age, CD4 count and duration of breastfeeding, have been previously reported. (20, 31) An increased odds of caries among those who were born after a spontaneous membrane rupture (vs. an artificial membrane rupture) has not been reported elsewhere. Our study provides marginal evidence to suggest that children born after an induced /artificial membrane rupture in this study population are less likely to develop caries compared to those born after a spontaneous membrane rupture; however, these findings must be taken with caution. Although other studies have reported that mode of delivery is associated with ECC, it remains unclear whether type of membrane rupture or mode of delivery can determine the composition of oral microbiota and subsequently influence cariogenesis. (32, 33)
Despite the well-known role sugary substrates play in cariogenesis, our study did not show a significant association between regular sugar intake (in terms of consumption of foods with high cariogenic potential) and caries.(34, 35) Reported consumption of refined sugar was low in this population. It is possible that we were unable to accurately measure or assign true cariogenic potential of dietary fermentable carbohydrates recorded within the food and drink questionnaire. Parents/Wards might have differentially under-reported consumption of refined sugar; however, other studies have suggested no correlation between caries experience and the intake/frequency of sugar intake in a population with carbohydrate-rich diet.(36, 37)
Our study has some limitations. Within a cross sectional design, we were not able to assess baseline factors and fully examine the changes in oral health and associated risk factors over time. Prospective studies are required to examine the relationship between HIV, immune status and caries. With our population of children aged 6 to 72 months, there is a high degree of heterogeneity in terms of age. We admit to the possibility of residual confounding by other factors associated with age and although the convenience sample may not be completely representative of the entire population of young, HI, HEU and HUU children in Nigeria; we are confident that our findings are generalizable to children at risk for ECC. Improved access to ART within this setting made it difficult to evaluate the prevalence of caries in HIV infected children who are not receiving ART or in children with progressive HIV infection. Again, in the future, a multi-center prospective study design will address this issue. For this study, we relied on clinical detection while radiographic assessments are often required for a definitive caries diagnosis. Although this limitation has the potential of underestimating the prevalence of caries, we do not expect this to significantly impact our results.
Despite these limitations, to our knowledge, this is the largest study comparing caries prevalence in HI and HEU children to HUU children, as well as documenting other risk factors for ECC in Nigerian children. In conclusion, we observed that HI children experience more caries and non-HIV related oral diseases than their uninfected counterparts even when receiving ART; however, CD4+ cell counts and percentages appear to be stronger determinants. A compromised immune status might play a more important role (than HIV infection or perinatal exposure) in the colonization of cariogenic bacteria such as S. mutans which subsequently increases the risk of developing caries.
The results of this study suggest that HI children on ART are in need of an integrated oral health management-based strategy focusing on clinical and preventive treatment. We observed higher, but not significant, odds of presenting with caries in HEU children compared to HUU children so there is a need to evaluate whether perinatal HIV exposure alone significantly increases the risk of acquiring cariogenic bacteria. Large longitudinal studies will allow us examine the responses of the oral microbiota to immunologic and dietary changes over time.
Acknowledgments
This study was supported by Fogarty AIDS International Training and Research Program (D43TW01041) and the National Institutes of Health (R01DE025174). The authors would like to thank the medical and dental staff at the University of Benin Teaching Hospital (UBTH) and the laboratory staff at the Institute of Human Virology Nigeria (IHVN) for their tireless efforts throughout all the phases of this study’s implementation. Finally, special thanks go to all the participating families, for their time and invaluable contributions.
References
- 1.Arendorf TM, Bredekamp B, Cloete CA, Sauer G. Oral manifestations of HIV infection in 600 South African patients. J Oral Pathol Med. 1998 Apr;27(4):176–9. doi: 10.1111/j.1600-0714.1998.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 2.Dios PD, Ocampo A, Miralles C, Limeres J, Tomas I. Changing prevalence of human immunodeficiency virus-associated oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Oct;90(4):403–4. doi: 10.1067/moe.2000.110030. [DOI] [PubMed] [Google Scholar]
- 3.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Mar;89(3):299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Westhausen AM, Priepke F, Bergmann FJ, Reichart PA. Decline in the rate of oral opportunistic infections following introduction of highly active antiretroviral therapy. J Oral Pathol Med. 2000 Aug;29(7):336–41. doi: 10.1034/j.1600-0714.2000.290708.x. [DOI] [PubMed] [Google Scholar]
- 5.Niederman R, Feres M, Ogunbodede E. Dentistry. In: Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN, editors. Essential Surgery: Disease Control Priorities. Edition. Vol. 1. Washington (DC): International Bank for Reconstruction and Development / The World Bank; 2015. [PubMed] [Google Scholar]
- 6.Okunseri C, Badner V, Wiznia A, Rosenberg M. Prevalence of oral lesions and percent CD4+ T-lymphocytes in HIV-infected children on antiretroviral therapy. AIDS Patient Care STDS. 2003 Jan;17(1):5–11. doi: 10.1089/108729103321042863. [DOI] [PubMed] [Google Scholar]
- 7.Obileye MF, Agbelusi GA, Orenuga OO, Temiye EO. Dental caries status of HIV infected children in Nigeria. Nig Q J Hosp Med. 2009 Sep-Dec;19(4):210–3. doi: 10.4314/nqjhm.v19i4.54530. [DOI] [PubMed] [Google Scholar]
- 8.Sahana S, Krishnappa SS, Krishnappa VS. Low prevalence of dental caries in children with perinatal HIV infection. J Oral Maxillofac Pathol. 2013 May;17(2):212–6. doi: 10.4103/0973-029X.119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi BH, Stringer JS, Moodley D. Antiretroviral drug regimens to prevent mother-to-child transmission of HIV: a review of scientific, program, and policy advances for sub-Saharan Africa. Current HIV/AIDS Reports. 2013:1–10. doi: 10.1007/s11904-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filteau S. The HIV-exposed, uninfected African child. Tropical Medicine & International Health. 2009;14(3):276–87. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 11.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. The Lancet. 2007;369(9571):1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007 Aug 15;196(4):562–9. doi: 10.1086/519847. [DOI] [PubMed] [Google Scholar]
- 13.Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, Waindi EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20(2):275–80. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 14. http://www.aapd.org/assets/1/7/D_ECC.pdf [Internet] Available from: http://www.aapd.org/assets/1/7/D_ECC.pdf.
- 15.Kaste LM, Selwitz RH, Oldakowski RJ, Brunelle JA, Winn DM, Brown LJ. Coronal caries in the primary and permanent dentition of children and adolescents 1–17 years of age: United States, 1988–1991. J Dent Res. 1996 Feb;75(Spec No):631–41. doi: 10.1177/002203459607502S03. [DOI] [PubMed] [Google Scholar]
- 16.Omoni AO, Christian PS, Sadoh WE, Okechukwu A, Olateju E, Omoigberale A, et al. Immunologic outcomes of antiretroviral therapy among HIV-infected Nigerian children and its association with early infant feeding and nutritional status at treatment initiation. Pediatr Infect Dis J. 2013 Jul;32(7):e291–7. doi: 10.1097/INF.0b013e31828b2a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esene H, Omoigberale AI. Prevalence of HIV among Exposed Infants in University of Benin Teaching Hospital, Benin City, Edo State, Nigeria. JMBR. 2012;11:105, 106–115. [Google Scholar]
- 18.Beena JP. Prevalence of dental caries and its correlation with the immunologic profile in HIV-Infected children on antiretroviral therapy. Eur J Paediatr Dent. 2011 Jun;12(2):87–90. [PubMed] [Google Scholar]
- 19.Phelan JA, Mulligan R, Nelson E, Brunelle J, Alves ME, Navazesh M, et al. Dental caries in HIV-seropositive women. J Dent Res. 2004 Nov;83(11):869–73. doi: 10.1177/154405910408301109. [DOI] [PubMed] [Google Scholar]
- 20.Hicks MJ, Flaitz CM, Carter AB, Cron SG, Rossmann SN, Simon CL, et al. Dental caries in HIV-infected children: a longitudinal study. Pediatr Dent. 2000 Sep-Oct;22(5):359–64. [PubMed] [Google Scholar]
- 21.Baqui A, Meiller T, Jabra-Rizk M, Zhang M, Kelley J, Falkler W. Association of HIV viral load with oral diseases. Oral Dis. 1999 Oct;5(4):294–8. doi: 10.1111/j.1601-0825.1999.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Saxena D, Chen Z, Norman RG, Phelan JA, Laverty M, et al. HIV infection affects Streptococcus mutans levels, but not genotypes. J Dent Res. 2012 Sep;91(9):834–40. doi: 10.1177/0022034512454298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nittayananta W, Talungchit S, Jaruratanasirikul S, Silpapojakul K, Chayakul P, Nilmanat A, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010 May;39(5):397–406. doi: 10.1111/j.1600-0714.2009.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomarico L, Czauski G, Portela M, de Souza I, Kneipp L, de Araújo Soares R, et al. Cariogenic and erosive potential of the medication used by HIV-infected children: pH and sugar concentration. Community Dent Health. 2008;25(3):170–2. [PubMed] [Google Scholar]
- 25.Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr. 2012 Dec;58(6):505–8. doi: 10.1093/tropej/fms019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One. 2012;7(10):e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012 Jun 9;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 28.Filteau S, Baisley K, Chisenga M, Kasonka L, Gibson RS. CIGNIS Study Team. Provision of micronutrient-fortified food from 6 months of age does not permit HIV-exposed uninfected Zambian children to catch up in growth to HIV-unexposed children: a randomized controlled trial. J Acquir Immune Defic Syndr. 2011 Feb 1;56(2):166–75. doi: 10.1097/QAI.0b013e318201f6c9. [DOI] [PubMed] [Google Scholar]
- 29.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011 Jan;30(1):45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 30.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007 Jun;26(6):519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 31.Colak H, Dulgergil CT, Dalli M, Hamidi MM. Early childhood caries update: A review of causes, diagnoses, and treatments. J Nat Sci Biol Med. 2013 Jan;4(1):29–38. doi: 10.4103/0976-9668.107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattanaporn K, Saraithong P, Khongkhunthian S, Aleksejuniene J, Laohapensang P, Chhun N, et al. Mode of delivery, mutans streptococci colonization, and early childhood caries in three-to five-year-old Thai children. Community Dent Oral Epidemiol. 2013 Jun;41(3):212–23. doi: 10.1111/cdoe.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Caufield PW, Dasanayake AP, Wiener HW, Vermund SH. Mode of delivery and other maternal factors influence the acquisition of Streptococcus mutans in infants. J Dent Res. 2005 Sep;84(9):806–11. doi: 10.1177/154405910508400905. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson BE, Quensel CE, Lanke LS, Lundqvist C, Grahnen H, Bonow BE, et al. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep 11;(3–4):232–64. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- 35.Krasse B. The Vipeholm Dental Caries Study: recollections and reflections 50 years later. J Dent Res. 2001 Sep;80(9):1785–8. doi: 10.1177/00220345010800090201. [DOI] [PubMed] [Google Scholar]
- 36.Giacaman R, Fernandez C, Diaz S. Fermentable carbohydrate dietary consumption measured by a cariogenicity scoring system and caries experience in youth and adults<br />. Rev chil nutr. 2012;39:116, 117–122. [Google Scholar]
- 37.Ohlund I, Holgerson PL, Backman B, Lind T, Hernell O, Johansson I. Diet intake and caries prevalence in four-year-old children living in a low-prevalence country. Caries Res. 2007;41(1):26–33. doi: 10.1159/000096102. [DOI] [PubMed] [Google Scholar]