Abstract
In targeted drug delivery systems, it is desirable that the delivery of hydrophobic drugs to a cell or tissue is achieved with little to no side effects. To ensure that the drugs do not leak during circulation, encapsulation stability of the drug carrier in serum is critical. In this paper, we report on a modified FRET-based method to evaluate encapsulation stability of amphiphilic assemblies and cross-linked polymer assemblies in serum. Our results show that serum components can act as reservoirs for hydrophobic molecules. We also show that serum albumin is likely to be the primary determinant of this property. This work highlights the importance of assessing encapsulation stability in terms of dynamics of guest molecules, as it provides the critical distinction between hydrophobic molecules bound inside amphiphilic assemblies and the molecules that are bound to the hydrophobic pockets of serum albumin.
Graphical abstract
Introduction
Development of new drug molecules is expensive and time-consuming, as the discovery, development, and launch of a new drug has been estimated to be nearly $2 billion and 20 years.1,2 Because of this, there has been a recent focus on approaches to developing new drug delivery vehicles as an alternative to introducing new drugs in the market. The promise here is often based on the development of an excipient that delivers drug molecules to specific tissues (e.g., at a tumor site).3-6 Therefore, administration of toxic and insoluble drugs to a specific target site has spurred tremendous interest in the field of drug delivery.7-9
Polymer nanoparticles have attracted much attention as carrier for drug delivery.10-15 If endowed with amphiphilic character, polymer-based vehicles can increase the solubility of the insoluble hydrophobic drugs in aqueous solution.3, 10-15 The protected drug can dramatically reduce side effects, associated with nonspecific homing in healthy tissues.16-18 Through careful molecular design, the encapsulated drug can be programmed for release under specific stimulus relevant to the target site and thus increase the therapeutic efficiency of the drugs.19 The surface of these nanoparticles are often endowed with stealth property (mostly consisting of poly(ethylene glycol) (PEG)) for long circulation due to reduced biofouling.20 Moreover, the targeting effect are also achieved through the decoration of targeting ligands on the surface.21 In addition to therapeutics, such systems are useful for diagnostics and theranostics as well.22-24 To fully realize the potential of such systems, many factors should be considered in their design. Among these characteristics, encapsulation stability is crucial, especially when carrying drug molecules with potentially high off-target toxicities. Stable encapsulation of the hydrophobic drug molecule inside the carrier under serum conditions is the prerequisite for drug delivery to reduce side effects. Unstable encapsulation will result in premature release of encapsulated guests during circulation, increasing the probability of adverse side effects and reducing the therapeutic efficacy of the system due to the low dose that eventually reaches the target site.25-26 Although numerous amphiphilic delivery vehicles have been designed for drug delivery, very few interrogate their dynamic interaction and stability in serum or in the presence of serum proteins, a feature that is critical to their performance in vivo. 27
In previous literature, most of the opinions about the encapsulation stability of the carriers concern the release of the guest molecules from carrier into an aqueous solution.28-30 Encapsulation itself is often defined by the loading capacity of the host, that is, the amount of guest molecule that a host assembly can hold. This capacity is dictated by the thermodynamic distribution coefficient of the guest molecule between the host and the solvent. It does not provide indications on the stability of encapsulation in terms of guest exchange dynamics with the bulk media. Understanding encapsulation stability in these terms is crucial, as this carries clear implications to the potential leakage of encapsulated guest molecules from the vehicles as they pass through a biological system.31, 32 In this paper, we analyze the encapsulation stability of assemblies (non-cross-linked or cross-linked) in more complex and biologically relevant serum conditions, where we also identify albumin as the protein that plays a significant role in affecting the encapsulation stability of these assemblies.
Experimental Section
The monomers and polymers used in this study were obtained from commercial sources or synthesized using previously reported procedures.33,34 The details were shown in the Supporting Information.
Preparation of the Nanogel
Dye-loaded nanoassembly and the cross-linked nanogel with different cross-link degrees were prepared through the encapsulation of two FRET pair dyes (DiI and DiO) into one host container. First, 10 mg of the PEG-co-PDS methacrylate polymer was dissolved in 1 mL of deionized water. Then, 20 μL of the DiI stock solution (5 mg/mL in acetone) and 40 μL DiO stock solution (2.5 mg/mL in acetone) were added and the solution was stirred without cap overnight to evaporate the organic solvent. Then, different amounts of DTT were added to cross-link the amphiphile to form nanogels with different cross-link degrees at room temperature for 24 h. The dye loaded nanocontainers were purified through filtration on 220 μm filter and then dialysis under water with a dialysis bag (cutoff = 7 k) for 24 h. The nanogel stock solution was stored at 2 mg/mL for further FRET experiments. The blank nanogel was prepared without the addition of dyes through the same procedure and the concentration of the stock solution was also 2 mg/mL.
FRET Experiment
FRET dye-loaded nanogel (or nanoassembly) (0.02 mL), 0.08 mL of blank nanogel (or nanoassemly), and 0.9 mL of H2O were mixed and the evolution of fluorescent spectrum was analyzed over time.
Serum Stability Study
Nanocontainer solution (0.05 mL) (different cross-link degree) was mixed with 0.95 mL of serum (100%) and the evolution of fluorescent spectrum was analyzed over time. In control experiments, 0.05 mL of the nanocontainer solution was mixed with 0.95 mL of water.
The FRET experiment was also performed with the albumin solution, for which the procedure is the same except that the 0.95 mL serum is changed to albumin solution. In this case, to obtain the desired final concentration of 40 mg/mL, 0.02 mL of the nanocontainer (different cross-link degree, 2 mg/mL) was mixed with 0.4 mL of albumin (100 mg/mL) and 0.58 mL of H2O.
Characterization
1H NMR and 13C NMR spectra were recorded on a 400 MHz Bruker NMR spectrometer. Molecular weight of the polymers was measured by gel permeation chromatography (GPC, Waters) using a PMMA standard with a refractive index detector. THF was used as eluent with a flow rate of 1 mL/min. Dynamic light scattering (DLS) measurements were performed using a Malvern Nanozetasizer. TEM images were recorded in JEOL 2000FX transmission electron microscope. UV-visible absorption spectra were recorded on a Varian (model EL 01125047) spectrophotometer. The fluorescence spectra were obtained from a JASCO FP-6500 spectrofluorimeter.
Results and Discussion
We evaluate the encapsulation stability of nanoassemblies in serum, using the recently developed self-cross-linking nanogels as the initial test case.34-36 Here, we define the non-cross-linked amphiphilic assemblies as “nanoassemblies” and the cross-linked assemblies as “nanogel”. Accordingly, we prepared a random copolymer, comprised of PEG-methacrylate and PDS-methacrylate using RAFT polymerization. At a 3:7 ratio of these two monomers, the molecular weight of the polymer was found to be about 14k by gel permeation chromatography (GPC). Synthesis and characterization details are provided in the Supporting Information. In water, this amphiphilic random copolymer afforded a self-assembled aggregate of about 12 nm, according to dynamic light scattering (DLS) measurements, which was stabilized by chemical cross-linking using dithiothreitol (DTT). The amount of DTT used in the reaction had a direct correlation with the cross-link density, which was experimentally assessed using the percentage of the pyridothione byproduct (Figure S3-S5). The amount of this byproduct could be conveniently assessed using its distinct absorption spectrum. The size of the nanogel remained at ∼12 nm after cross-linking as well (Figure S6A), which suggested that there is no crosstalk among the nanoaggregates in solution during the cross-linking process. TEM image of the nanogel loaded with two different dyes are shown in Figure S6B. The nanogel showed very uniform size of ∼10 nm, which is consistent with the DLS results.
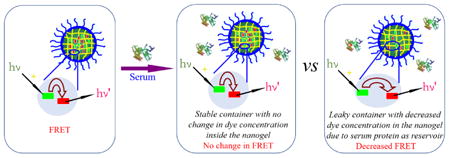
We use fluorescence resonance energy transfer (FRET) to monitor the encapsulation stability of the nanogels. We have previously reported on a FRET-based method to assess this process.32 However, note that the current method is intently different, as there is a critical difference in our objective in this study. Our prior study was focused on understanding the exchange dynamics of hydrophobic guest molecules among the nanoaggregates. Our goal here is to understand the encapsulation in serum and our premise for looking into this process is that certain serum proteins are capable of binding to hydrophobic molecules and therefore affect the encapsulation stability of nanocarriers. To realize the current goal, it is important that we modify our FRET strategy and the modified strategy is represented in Scheme 1. Briefly here, the FRET donor and the FRET acceptor molecules are coencapsulated in the nanoassembly first. Therefore, the proximity of these two molecules within the assembly then ensures that there is a high degree of FRET at the outset. We hypothesized then that when these assemblies are introduced into the serum, if serum proteins act as reservoirs for hydrophobic molecules, these dye molecules would diffuse into the isolated binding pockets of these proteins where these molecules are spatially apart. This process will cause the dye molecules to lose it FRET properties. Note however that this process will only happen if the encapsulation stability of the nanoassembly is poor. If the encapsulation stability is high, then there should be no change in FRET over time. In order to distinguish the difference between our previous FRET method and the method used here, we simply call it as the modified FRET-base method.
Scheme 1.
Encapsulation Stability of the Container under Serum Condition.
To evaluate this method first, we used blank nanoassemblies (i.e. nanoassemblies without any loaded hydrophobic guest molecules) as the model system. The un-cross-linked nanoassembly, formed from the polymer P1 loaded with the FRET pair, was mixed with the blank nanoassembly from the same polymer. Evolution of FRET in such a solution is shown in Figure 1A. The fluorescence peak at ∼509 nm for donor gradually increased over time, compared to the acceptor fluorescence. The FRET ratio (Ia/Ia+Id) decreased gradually from 0.68 to 0.49 after 4 h, as shown in Figure 1B. These results indicate that the distance between the dye molecules increased over time. To confirm that this is really due to the blank nanoassemblies acting as a reservoir for the leaking dye molecules, we performed a control experiment where the FRET change of the same dye-loaded nanoassembly was monitored in the absence of any blank assemblies. Indeed, the FRET ratio was unchanged over the same time period (Figure S7). These results suggest that the uncross-linked nanoassemblies are leaky but have the thermodynamic propensity to host the hydrophobic molecules. In the presence of a reservoir that can bind to these leaking hydrophobic molecules, the concentration and therefore the proximity of the FRET pairs would decrease with time.
Fig. 1.
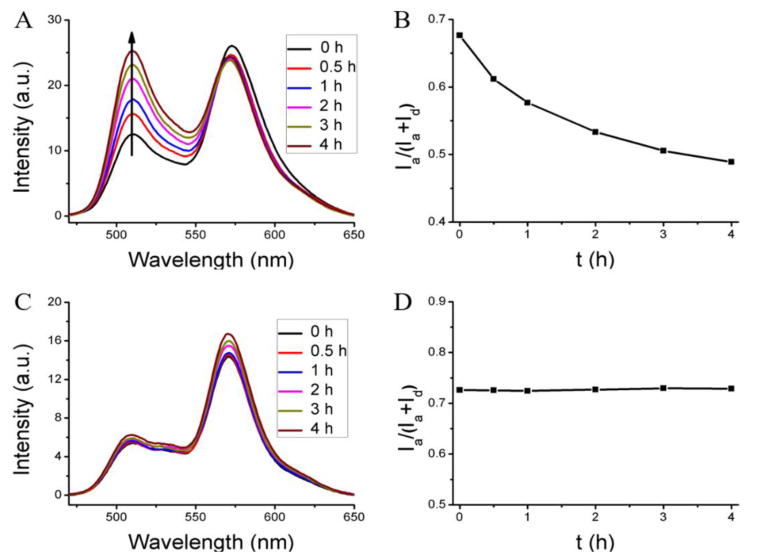
FRET evolution of the dye-loaded host with different blank containers. (A, B) The interaction of the dye (DiI+DiO) loaded nanoassembly with the corresponding blank nanoassembly (leaky assembly and the presence of a reservoir that can accept guests). (C, D) The interaction of the dye (DiI+DiO) loaded nanogel with the blank nanogel (stably encapsulating nanogel or the lack of a reservoir to accept the guest molecule).
Similarly, we also studied the encapsulation stability of the corresponding cross-linked nanoassembly, that is, the nanogel. The experiments here were carried out with nanogels in which all the available PDS units were used up for cross-linking, that is, 100% cross-linked with respect to the PDS units. Interestingly, there is no change in FRET in the nanogels when a dye-loaded nanogel is mixed with an empty nanogel (Figure1C, D). This lack of dye distribution could be due to either (i) the absence of an effective reservoir in the empty nanogel, that is, the empty nanogel does not allow hydrophobic molecules to be encapsulated when cross-linked or (ii) the crosslinked nanogel effectively encapsulates the hydrophobic guest molecules such that it does not allow any leakage. To test these possibilities, two control experiments were performed and the results are shown in Figure S8. In one experiment, the FRET dyes-loaded uncrosslinked assembly was mixed with the blank nanogel. Here, too, no temporal evolution of FRET was observed (Figure S8A,C). Since our prior experiments suggest that the un-cross-linked nanoassembly is leaky, the fact that there is no fluorescence change here suggests that the cross-linked nanogel is not capable of acting as a reservoir for the dye molecules. In the second control experiment, we mixed dye-loaded cross-linked nanogels with the empty un-cross-linked nanoassembly. In this case again, there was no evolution of FRET over time as shown in Figure S8B, D. Since we have established that the un-cross-linked nanoassembly can be a good reservoir for hydrophobic dyes in solution, the fact that there is no FRET change over time here suggests that the encapsulation of the molecules inside the nanogels is stable. Overall, the experiments here suggest that the cross-linked nanogels do not allow any hydrophobic small molecules to enter or exit them, once cross-linked.
Note that all the experiments so far were carried out in water. Next, we were interested in assessing whether materials present in the biological system, especially in the serum, can act as a reservoir for the hydrophobic guest molecules encapsulated within these assemblies. Thus, the encapsulation stability of nanogels in serum conditions was studied. Here, we also varied the cross-link density of the nanogels to assess the effect of this variation upon the encapsulation stability. Since the components in the serum are being evaluated as the potential reservoir for the dye molecules, the dyes-encapsulated nanogels were simply incubated in serum and the temporal evolution of FRET was evaluated.
This experiment was first carried out with the un-cross-linked nanoassembly. Since we know that these nanoassemblies are leaky from our experiments in water, if there is FRET evolution in this system then it would confirm the premise that components in serum can act as reservoir for hydrophobic molecules. If this evolution is not present, then we could safely conclude that serum components do not have viable reservoirs for these hydrophobic molecules. In serum, the un-cross-linked nanoassemblies indeed exhibited a rather fast decrease in the FRET ratio from 0.60 to 0.45 in 7 h with an average leakage coefficient (Λ) of 0.021 h-1 as shown Figure 2A,B. The magnitude of decrease is similar to that found with the empty nanoassembly, confirming that serum components can quite rapidly accept hydrophobic guest molecules present in solution.
Fig. 2.
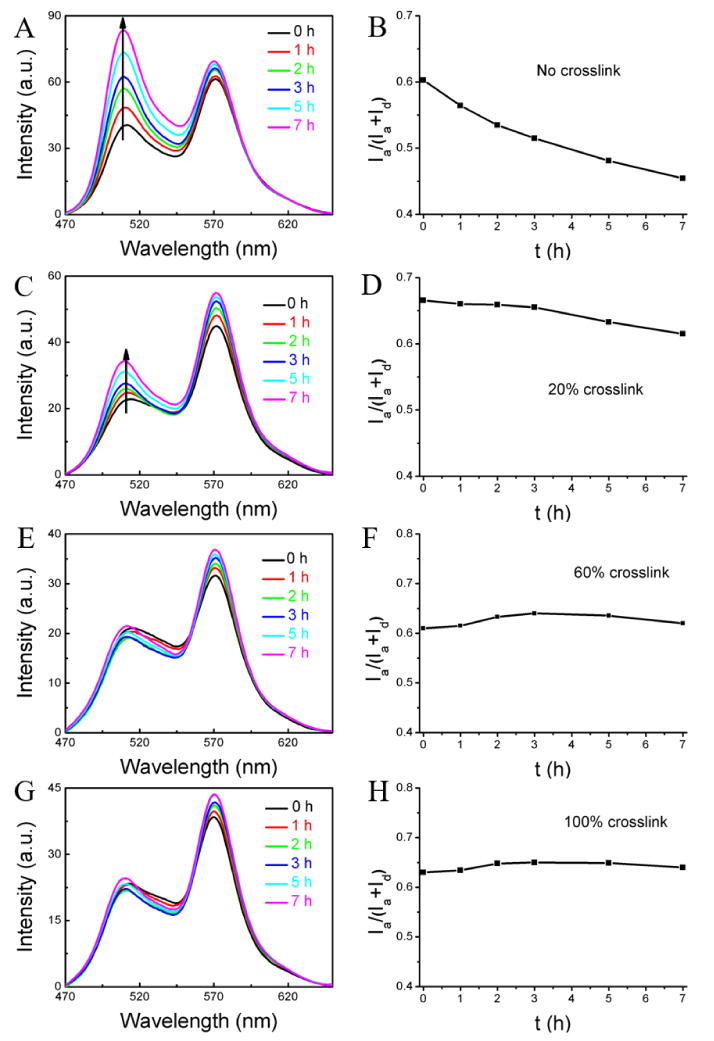
Encapsulation stability of the nanogel with different cross-link degrees in serum: (A, B) non-crosslinked nanoassembly, (C, D) nanogel with 20% cross-link density, (E, F) nanogel with 60% cross-link density, (G, H) nanogel with 100% cross-link density. Condition: mixture of 0.05 mL of the nanogel (2 mg/mL) and 0.95 mL 100% serum.
Next, we were interested in assessing the effect of cross-link density upon the encapsulation stability. At a cross-link density of 20%, the container was found to be still leaky, but the kinetics of the leakage seems to be significantly slower. In this case, the FRET ratio changed from 0.67 to 0.62 with average Λ of 0.007 h-1 (Figure 2C and 2D). Further increase in the cross-link density to 60% and 100% resulted in very stable encapsulation, where there is no change in FRET ratio over the entire observation time (Figure 2E-2H). This dependence of encapsulation stability on the cross-link density offers the opportunity to tune the molecular release kinetics in these systems.
The results above suggest that the cross-linked polymer nanogels exhibit increased encapsulation stability inside the serum condition and the leakage dynamics can be tuned by varying the cross-linking density. Next, we were interested in the effect of decross-linking these nanogels in the serum conditions, that is, whether reversing the cross-linking process will result in rendering the nanogels leaky and release the contents to the serum proteins. In order to test the possibility, the cross-linked nanogel (100% cross-link degree), which showed very high stability under serum conditions, was subjected to the presence of 10 mM glutathione (GSH), a disulfide reducing agent. Indeed, while the encapsulation is stable in the absence of GSH under identical conditions, the FRET ratio decreased significantly from 0.72 to 0.49 after 8 h with the average Λ of 0.029 h-1 in the presence of GSH (Figure 3A,B). To further confirm that the observed decrease in FRET ratio is indeed due to the ability of serum components to act as reservoirs for hydrophobic molecules, FRET evolution of the dyes-loaded nanogel was investigated in water in the presence of same concentration of GSH, but in the absence of any reservoir for hydrophobic molecules. As shown in Figure 3C,D, there is no FRET change, meaning no dye release from the nanogel. These results further confirm that the observed FRET change in serum is indeed due to the ability of serum proteins to act as reservoirs for hydrophobic guest molecules.
Fig. 3.
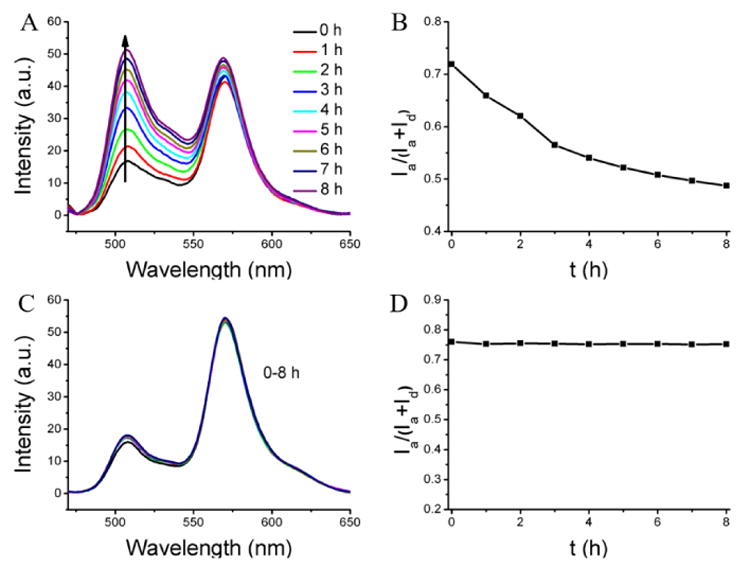
Destabilization of the nanogel through the de-cross-linking. (A, B) FRET evolution of dyes-loaded nanogel in serum in the presence of the GSH. (C, D) FRET evolution of dyes-loaded in water in the presence of the GSH.
As this is potentially a general methodology for assessing encapsulation stability of supramolecular assemblies in serum, we were also interested in evaluating other nanoassemblies that are capable of encapsulating hydrophobic guest molecules. Thus, small molecule surfactants (cetyltrimethylammonium bromide (CTAB) and tween 80), amphiphilic block copolymer assemblies (P85 and F127) and amphiphilic random copolymer assemblies were all studied to quantify their encapsulation stability in serum (Figure 4). While most of these molecules were obtained from commercial sources, the random copolymer was synthesized in our laboratories, the synthetic details of which are outlined in the Supporting Information.
Fig. 4.
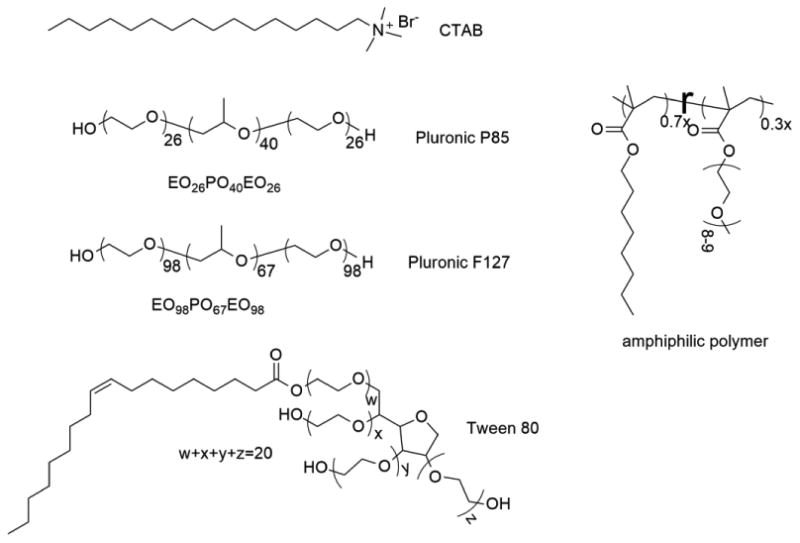
Structure of different amphiphilic molecules used in this study.
The FRET pair dye molecules (DiO and DiI) were encapsulated together in the CTAB micelle and the possible evolution of FRET was evaluated in serum. The results were shown in Figure 5A. After the CTAB micelle was injected into the serum, the FRET ratio decreased dramatically. In fact, there is strong donor emission with almost no accompanying acceptor emission. Interestingly, the CTAB micelle exhibited an inherent lack of encapsulation stability even in water (Figure S11A). Tween 80 micelle showed gradual decrease of the FRET ratio from 0.69 to 0.43 after 5 h with average Λ of 0.051 h-1, while there is no FRET change in water (Figure S11B).
Fig. 5.
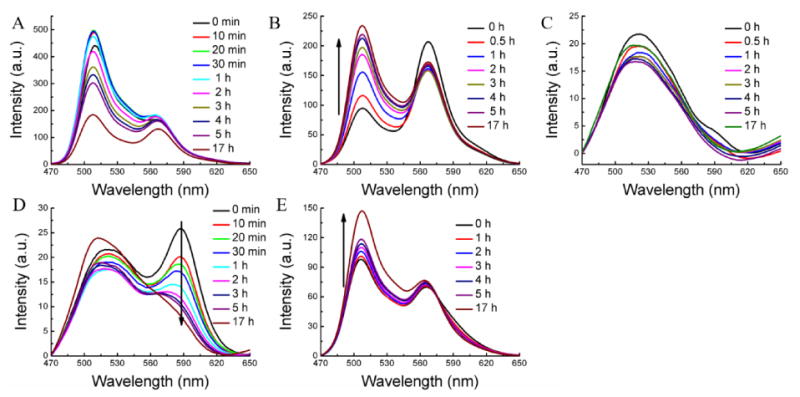
Encapsulation stability of the other amphiphilic assemblies in serum. (A) CTAB, (B) Tween 80, (C) Pluronic P85, (D) Pluronic F127, and (E) amphiphilic polymer.
The encapsulation stability of pluronic block copolymer (P85, EO26PO40EO26) micellar assemblies, which are widely pursued in biomedical applications,37, 38 exhibited dramatic FRET ratio change immediately after injection to the serum. As shown in Figure 5C, there is no emission peak at ∼569 nm for the acceptor even just after the injection of the micelle into serum condition. The encapsulation stability of another pluronic block copolymer F127, structurally related but higher molecular weight, was also studied. The FRET ratio in this case gradually changed from 0.50 to 0.41 after 5 h with an average Λ of 0.018 h-1 (Figure 5D). Although the encapsulation stability of F127 is quite poor in serum, it should be noted that its encapsulation stability is substantially higher than its lower molecular weight counterpart P85.
We then measured the guest exchange dynamics of an architecturally different amphiphilic assembly, formed from a random copolymer POMA-PEG. This random copolymer also did show decrease of the FRET with time, although the rate of decrease was found to be quite slow. In this case, the FRET ratio was changed from 0.42 to 0.38 after 5 h and further to 0.33 after 17 h. Interestingly, this random copolymer showed higher encapsulation stability than the un-cross-linked PDS-co-PEG methacrylate random copolymer, which is attributed to the possible higher hydrophobicity of the interior of the assembly.39 The inherent stability of all these assemblies were also assessed in water in the absence of any reservoir. Other than CTAB-based micelles, all these amphiphilic assemblies did exhibit inherent encapsulation stability, that is, high dynamic encapsulation stabilities in water.
Finally, we were interested in identifying the critical component of the serum that is responsible for acting as the reservoir for hydrophobic guest molecules. Since the major component of serum is albumin and since this protein does have the ability to bind hydrophobic molecules and transport them, it is reasonable to hypothesize that this could be the primary determinant of the observed processes. The concentration of the albumin was chosen to be 40 mg/mL, as close to its plasma concentration (35-50 mg/mL).40 First, the un-cross-linked PEG-co-PDS methacrylate polymer assembly was indeed found to be leaky in the presence of serum albumin, where the FRET ratio decreased from 0.56 to 0.44 after 3 h with an average Λ of 0.038 h-1 (Figure 6A,B). On the other hand, the cross-linked (100%) nanogel exhibits high encapsulation stability with no FRET change (Figure 6C,D). These results were very similar to those found in the serum. Further, we also studied the effect of crosslink degree on the encapsulation stability of the nanogel inside the albumin solution (Figure S12). The nanogel with 20% cross-link degree showed slight leakage, while the nanogel with 60% cross-link degree exhibited no leakage at all. Once again, these results were found to be similar to those found in the serum. Similarly, in the presence of 10 mM GSH, the 100% cross-linked nanogel showed leakage with the FRET ratio decrease from 0.51 to 0.38 after 8 h (average Λ = 0.0156 h-1) in the albumin solution (Figure S13). All these results suggest that albumin is likely to be the primary reservoir for hydrophobic guest molecules from leaky amphiphilic assemblies in serum.
Fig. 6.
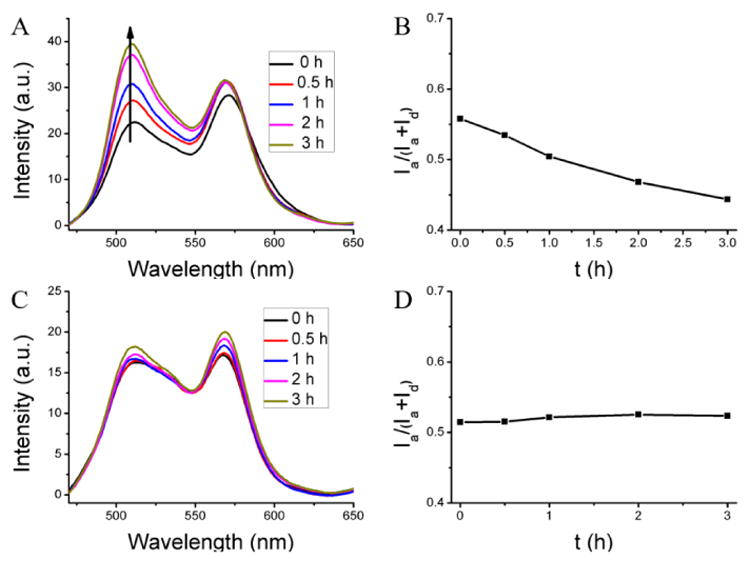
Encapsulation stability of the nanogel in albumin solution. (A, C) The fluorescent spectra, (B, D) the FRET ratio evolution in albumin solution. (A, B) From un-cross-linked nanoassembly, (B, D) from 100% cross-linked nanogel.
Conclusions
In this manuscript, we report on a FRET-based method that is used to evaluate the encapsulation stability of hydrophobic molecules within amphiphilic nanoscopic assemblies in serum. The primary premise here is that we hypothesized that serum proteins could act as reservoirs for hydrophobic guest molecules, if molecular assemblies exhibit poor encapsulation stability. This encapsulation stability is not apparent, when one evaluates these supramolecular assemblies in aqueous phase or even in buffer solutions. With a simple assessment in aqueous phase, only CTAB micelles exhibit poor inherent encapsulation stability. However, when rigorously evaluated in the presence of reservoirs that these assemblies are likely to encounter in biological systems, many of these assemblies were found to be quite leaky. Finally, our results also suggest that serum albumin, which is the major constituent of serum protein mixtures, could be the primary reservoir for the hydrophobic molecules. From a big picture perspective, the current studies highlight the possibility that when leaky assemblies are evaluated in vivo, the pharmacokinetic properties evaluated in the process could really be reporting on the albumin-bound drug molecules. Thus, this study highlights the importance of evaluating supramolecular assemblies for dynamic leakage over time in the presence of a reservoir to fully understand their potential as delivery vehicles.
Supplementary Material
Acknowledgments
We thank U.S. Army Research Office (W911NF-15-1-0568) and National Institutes of Health (CA169140) for support.
References
- 1.Avorn J. N Engl J Med. 2015;372:1877–1879. doi: 10.1056/NEJMp1500848. [DOI] [PubMed] [Google Scholar]
- 2.Dickson M, Gagnon JP. Discov Med. 2004;4:172–179. [PubMed] [Google Scholar]
- 3.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 4.Medina SH, El-Sayed MEH. Chem Rev. 2009;109:3141–3157. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RA. Nature. 1984;394:427–428. doi: 10.1038/28756. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka K, Harada A, Nagasaki Y. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Feijen JJ. Controlled Release. 2012;161:473–483. doi: 10.1016/j.jconrel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Blanco E, Shen H, Ferrari M. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Annu Rev Chem Biomol Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamaly N, Yameen B, Wu J, Farokhzad OC. Chem Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masood F. Mater Sci Eng C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Mora-Huertasa CE, Fessia H, Elaissari A. Int J Pharm. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. J Controlled Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 15.Kurniasih IN, Keilitz J, Haag R. Chem Soc Rev. 2015;44:4145–4164. doi: 10.1039/c4cs00333k. [DOI] [PubMed] [Google Scholar]
- 16.De Jong WH, Borm PJ. Int J Nanomedicine. 2008;3:133–49. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver CL, LaRosa JM, Luo X, Cui X. ACS Nano. 2014;8:1834–1843. doi: 10.1021/nn406223e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambikanandan M, Ganesh S, Aliasgar S. J Pharm Pharmaceut Sci. 2003;6:252–273. [Google Scholar]
- 19.Mura S, Nicolas J, Couvreur P. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 20.Waku T, Matsusaki M, Kaneko T, Akashi M. Macromolecules. 2007;40:6385–6392. [Google Scholar]
- 21.Ulbrich K, Holá K, Šubr V, Bakandritsos A, Tuček J, Zbořil R. Chem Rev. 2016;116:5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Piaoa Y, Hyeon T. Chem Soc Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Chen W, Wang W, Shen J, Guo R, Gong F, Lin S, Cheng D, Chen G, Shuai X. ACS Nano. 2012;6:10646–10657. doi: 10.1021/nn3037573. [DOI] [PubMed] [Google Scholar]
- 24.Omidi Y. Bioimpacts. 2011;1:145–147. doi: 10.5681/bi.2011.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller T, Breyer S, van Colen G, Mier W, Haberkorn U, Geissler S, Voss S, Weigandt M, Goepferich A. Int J Pharm. 2013;445:117–124. doi: 10.1016/j.ijpharm.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Cheng X, Soeriyadi AH, Sagnella SM, Lu X, Scott JA, Lowe SB, Kavallaris M, Gooding JJ. Biomater Sci. 2014;2:121–130. doi: 10.1039/c3bm60148j. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh A, Buettner CJ, Manos AA, Wallace AJ, Tweedle MF, Goldberger JE. Biomacromolecules. 2014;15:4488–4494. doi: 10.1021/bm501311g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen SC, Chan DPY, Shoichet MS. Nano today. 2012:7, 53–65. [Google Scholar]
- 29.Kim S, Shi Y, Kim JY, Park K, Chen JX. Expert Opin Drug Deliv. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 30.Zou P, Chem H, Paholak HJ, Sun DX. J Tumor. 2013;18:7–15. [Google Scholar]
- 31.Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Proc Natl Acad Sci U S A. 2008;105:6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiwpanich S, Ryu J, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:10683–10685. doi: 10.1021/ja105059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Basu S, Thayumanavan S. Macromolecules. 2006;39:5595–5597. [Google Scholar]
- 34.Ryu JH, Chacko R, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. J Am Chem Soc. 2010;132:17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Thayumanavan S. J Am Chem Soc. 2017;139:2306–2317. doi: 10.1021/jacs.6b11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu J, Bickerton S, Zhuang J, Thayumanavan S. Biomacromolecules. 2012;13:1515–1522. doi: 10.1021/bm300201x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabanov AV, Batrakova EV, Alakhov VY. J Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 38.Kabanov AV, Batrakova EV, Alakhov VY. Adv Drug Delivery Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 39.Dube N, Seo JW, Dong H, Shu JY, Lund R, Mahakian LM, Ferrara KW, Xu T. Biomacromolecules. 2014;15:2963–2970. doi: 10.1021/bm5005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busher JT. In: Serum Albumin and Globulin, Chapter 101 in Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd. Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


