SUMMARY
Necrotizing fasciitis is a life-threatening infection requiring urgent surgical and medical therapy. Our objective was to estimate the mortality burden of necrotizing fasciitis in the United States, and to identify time trends in the incidence rate of necrotizing fasciitis-related mortality. We obtained data from the National Center for Health Statistics, which receives information from death certificates from all states, including demographic information and cause of death. The U.S. Multiple Cause of Death Files were searched from 2003 through 2013 for a listing of NF (ICD10 code M72.6) as either the underlying or contributing cause of death. We identified a total of 9,871 necrotizing fasciitis-related deaths in the U.S. between 2003 and 2013 (Figure 1), corresponding to a crude mortality rate of 4.8 deaths per 1,000,000 person-years, without a significant time trend. Compared to white individuals, the incidence rate of necrotizing fasciitis-associated death was greater among black, Hispanic, and American Indian individuals, and lower among Asian individuals. Streptococcal infection was most commonly identified in cases where a pathogen was reported. Diabetes mellitus and obesity were more commonly observed among necrotizing fasciitis-related deaths compared with deaths due to other causes. Racial differences in the incidence of necrotizing fasciitis-related deaths merits further investigation.
Keywords: Necrotizing fasciitis, epidemiology, mortality, Streptococcus, Staphylococcus
INTRODUCTION
Necrotizing fasciitis is a life-threatening infection requiring urgent surgical and medical therapy, and the suspected diagnosis must be quickly established by surgical exploration [1]. Development of necrotizing fasciitis may follow traumatic injury, surgical intervention, or may be spontaneous with a clear antecedent injury [2]. The rapid progression of infection hinges on the expression of bacterial toxins that permits the infection to spread rapidly along fascial planes, and the appearance of the overlying skin often does not reflect the degree of destruction in deeper tissue layers [3]. Disruption of vascular flow rapidly leads to tissue necrosis, and the patient quickly develops an overwhelming sepsis syndrome which is fatal without tissue debridement and supportive care that includes effective antimicrobial therapy [4]. Although a clinical prediction rule was developed to guide decision-making [5], in subsequent work it was found to have limited sensitivity [6].
Cases of necrotizing fasciitis can been classified based on anatomic location and the microbial etiology. Bacterial pathogens that have been implicated in necrotizing fasciitis include group A Streptococcus (GAS), Staphylococcus aureus, Clostridium species, and mixed gram-negative and anaerobic organisms [7]. Mixed infections with aerobic and anaerobic bacteria are classified as Type I, and mono-microbial infections with GAS or S. aureus are classified as Type II necrotizing fasciitis [8]. In a population-based study in the United States between 1996 and 2004, the incidence rate of invasive GAS infection was 3.5 cases 100,000 person years, with 7.2% of these cases presenting with necrotizing fasciitis [9].
There are little data available regarding time trends in the epidemiology of necrotizing fasciitis in the United States, despite concerns for an increasing burden of skin and soft tissue infections due to pathogens such as methicillin-resistant S. aureus (MRSA) [10]. Our objective was to estimate the mortality burden of necrotizing fasciitis in the United States over a 10-year period, and to identify temporal trends in the incidence of necrotizing fasciitis-related mortality during this period. In secondary analyses, we sought to characterize demographic factors and co-morbid conditions associated with necrotizing fasciitis-related death during this period.
METHODS
Setting
Data were obtained from the National Center for Health Statistics, which receives information from death certificates from all 50 states, including demographic information and cause of death. Years 2003 through 2013 were included, given the change in cause of death codes from the International Classification of Disease 9th Revision (ICD-9) to the 10th revision (ICD-10) that occurred prior to 2003 [11]. The multiple cause of death (MCOD) data file includes both underlying and contributing causes (both diseases and injuries) that ultimately led to the individual’s death.
Case Definition
The U.S. Multiple Cause of Death Files were searched from 2003 through 2013 for a listing of necrotizing fasciitis (ICD10 code M72.6) as either the underlying or contributing cause of death. Analysis was not restricted to cases where necrotizing fasciitis was reported as the underlying cause of death, as the instructions for completing the death certificate refer to the underlying cause of death as “the disease or injury which initiated the train of morbid events leading directly or indirectly to death or the circumstances of the accident or violence which produced the fatal injury” [12]. Thus, necrotizing fasciitis arising from an injury or surgical procedure may be recorded as a contributing cause of death, rather than the underlying cause of death. To explore the underlying microbiological diagnosis, each death record was examined for ICD-10 codes corresponding to streptococcal infection. (ICD-10 codes A40.0, A40.1, A40.2, A40.3, A40.8, A40.9, A49.1, M00.0), staphylococcal infection. (ICD-10 codes A41.0, A41.1, A41.2, A49.0, M00.2), gas gangrene (A48.0), gram negative organisms (A41.5), and anaerobes (A41.4).
Necrotizing Fasciitis Mortality Rates Adjusted for Age, Race/ethnicity, and Sex
Characteristics of individuals with necrotizing fasciitis-related deaths in the U.S. were summarized according to demographic characteristics. Age at death was defined based on the following age groups: < 1 year, 1–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, >=85 years. Age-adjusted mortality rates and rate ratios (RRs) were calculated with 95% confidence intervals (CIs) using bridged-race population estimates of U.S. census population data, which allows for comparison of different race categories across various collection systems [15]. Goodness-of-fit was examined by testing the statistical significance of the model deviance with the chi-squared test, along with visual inspection of the plot of variance-versus-mean.
Time Trend Analysis
Count data were modeled using maximum likelihood analysis in a negative binomial regression model [13]. A negative binomial regression model was employed, rather than a Poisson model, because a violation of the assumption of equal mean and variance (overdispersion) was observed that reached statistical significance [14]. In order to test for a temporal trend in the rate of necrotizing fasciitis-associated deaths in the population, year was included as a dummy variable in the negative binomial regression model, with the population as the offset.
Matched Case-Control Analysis for Associated Diagnoses
To identify co-morbid conditions associated with necrotizing fasciitis-related death, a previously validated approach developed by Redelings and colleagues [16] was employed. For each record of necrotizing fasciitis-related death during the study period, a random sample of deaths from the multiple cause of death dataset during the same year was selected, and matched at a 10:1 ratio (controls:case) by age category, sex, and race/ethnicity. Groups of co-morbidities were defined based on the leading ICD-10 diagnoses among all necrotizing fasciitis-related deaths. For each co-morbidity, we calculated a matched odds ratio (MOR) and the associated 95% CI. All analyses were performed in Stata v13.0 (Statacorp, TX).
RESULTS
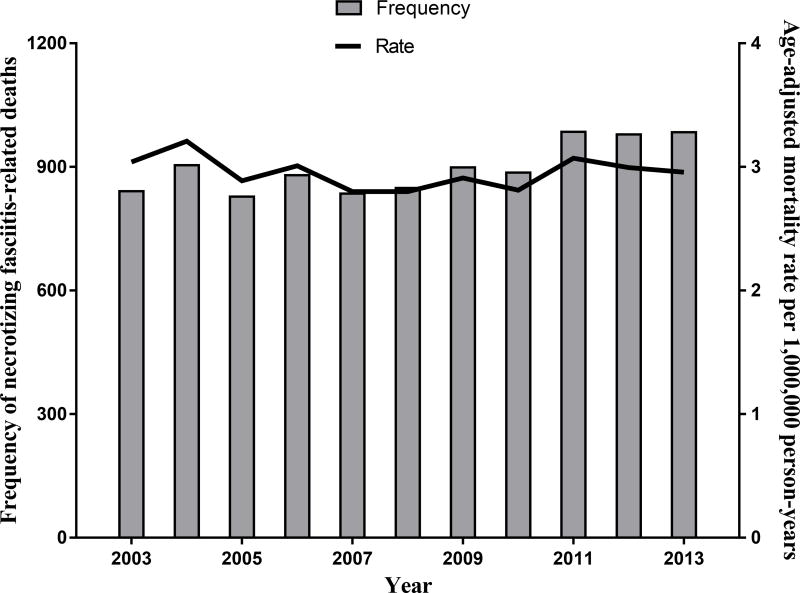
A total of 9,871 necrotizing fasciitis-related deaths in the U.S. between 2003 and 2013 (Figure 1) were identified, corresponding to a summary mortality rate of 4.8 deaths per 1,000,000 person-years. Necrotizing fasciitis was reported as the underlying cause in 4,185 deaths (42%), and a contributing cause of death in 5,686 deaths (58%). An autopsy was reported in 885 of 9,871 deaths (9%), and autopsy status was unknown in an additional 1,034 deaths (11%). In a negative binomial regression model, there was not a significant time trend in the incidence rate of necrotizing fasciitis-associated deaths during the study period (p=0.47).
Figure 1.
Annual frequency and rate of necrotizing fasciitis related deaths in the U.S., 2003–2013.
An underlying microbiologic diagnosis was provided in 546 of 9,871 deaths (6%). Among these 546 deaths, streptococcal infection was identified in 260 deaths (48%), staphylococcal infection in 119 deaths (22%), gram-negative infection in 114 deaths (21%), gas gangrene in 28 deaths (5%), anaerobic infection in 15 deaths (3%), and a mixed infection was identified in 10 deaths (2%). Thus, type II (mono-microbial) necrotizing fasciitis due to either Staphylococcus or Streptococcus was identified in 379 of 546 deaths (69%) where a microbiologic diagnosis was included in the cause of death information.
Characteristics of individuals with necrotizing fasciitis-related death during this period are shown in Table 1. Advancing age was associated with an increasing rate of necrotizing fasciitis-related mortality, as compared with a reference group of 35 to 44-year old individuals, but no difference in mortality rates was observed among younger age categories. After adjusting for age, the rate of necrotizing fasciitis-related mortality among men was 1.25 times greater than the rate among women (95% CI 1.20 to 1.30). The age-adjusted mortality rate among black individuals was nearly two times the rate among white individuals (1.97, 95% CI 1.87 to 2.06), and the rate among Native Americans was nearly three times the rate among white individuals (2.86, 95% CI 2.50 to 3.21). In contrast, necrotizing fasciitis-related mortality among Asian individuals was 28% lower than white individuals (age-adjusted mortality rate 0.72, 95% CI 0.65, 0.80).
Table 1.
Necrotizing fasciitis-related mortality rates per 1,000,000 person-years
| Characteristic | Number of deaths (%) |
Age-adjusted mortality rate per 1,000,000 person-years (95% CI) |
Age-adjusted mortality rate ratios (95% CI) |
|---|---|---|---|
| Age categories* (N=9,870) | |||
| < 1 year old | 34 (0.3) | 0.77 (0.51, 1.03) | 0.45 (0.30, 0.61) |
| 1–4 years old | 5 (0.1) | 0.03 (0.00, 0.05) | 0.02 (0.00, 0.03) |
| 5–14 years old | 34 (0.3) | 0.08 (0.05, 0.10) | 0.04 (0.03, 0.06) |
| 15–24 years old | 73 (0.7) | 0.15 (0.12, 0.19) | 0.09 (0.07, 0.11) |
| 25–34 years old | 297 (3.0) | 0.67 (0.59, 0.74) | 0.39 (0.34, 0.44) |
| 35–44 years old | 794 (8.0) | 1.71 (1.59, 1.83) | Reference |
| 45–54 years old | 1,876 (19.0) | 3.91 (3.74, 4.09) | 2.29 (2.10, 2.48) |
| 55–64 years old | 2,461 (24.9) | 6.56 (6.30, 6.81) | 3.83 (3.52, 4.17) |
| 65–74 years old | 1,893 (19.2) | 8.22 (7.85, 8.59) | 4.80 (4.41, 5.20) |
| 75–84 years old | 1,585 (16.1) | 10.99 (10.45, 11.53) | 6.42 (5.87, 6.97) |
| >84 years old | 818 (8.3) | 14.27 (13.29, 15.25) | 8.34 (7.52, 9.15) |
| Sex | |||
| Female | 4,807 (48.7) | 2.65 (2.57, 2.73) | Reference |
| Male | 5,063 (51.3) | 3.31 (3.22, 3.39) | 1.25 (1.20, 1.30) |
| Race/ethnicity | |||
| White, non-Hispanic | 6,548 (66.4) | 2.58 (2.51, 2.65) | Reference |
| Black, non-Hispanic | 1,694 (17.2) | 5.07 (4.88, 5.26) | 1.97 (1.87, 2.06) |
| Hispanic | 1,209 (12.3) | 3.67 (3.54, 3.80) | 1.42 (1.36, 1.49) |
| Asian, non-Hispanic | 255 (2.6) | 1.87 (1.68, 2.05) | 0.72 (0.65, 0.80) |
| American Indian or Alaskan Native, non-Hispanic | 164 (1.7) | 7.37 (6.46, 8.27) | 2.86 (2.50, 3.21) |
Age-specific mortality rates are reported
We aggregated ICD10 codes by block to describe the most common conditions included in the death certificates for necrotizing fasciitis-related deaths (Table 2). Diabetes mellitus was the most common co-morbid condition, identified in 21% of all deaths. Renal disease, ischemic heart disease, and substance use were also frequently reported as co-morbid conditions. In a matched case-control study of co-morbid conditions, diabetes mellitus, renal failure, and obesity were significantly associated with necrotizing fasciitis-related death, compared with death due to other causes (Table 3). In contrast, heart disease and substance use were less commonly observed among necrotizing fasciitis-related deaths, compared to other causes.
Table 2.
Most common co-morbid diagnoses among individuals with necrotizing fasciitis-related deaths in the United States, 2003–2013 (N=9,817)
| Co-morbid Condition | ICD10 Block Codes | Number of deaths (%) |
|---|---|---|
| Diabetes mellitus | E10–E14 | 2,109 (21) |
| Other forms of heart disease | I30–I52 | 1,972 (20) |
| Renal failure | N17–N19 | 1,592 (16) |
| Other diseases of the respiratory system | J95–J99 | 1,002 (10) |
| Mental and behavioral disorders due to psychoactive substance use | F10–F19 | 934 (10) |
| Ischemic heart diseases | I20–I25 | 753 (8) |
| Hypertensive diseases | I10–I15 | 717 (7) |
| Diseases of liver | K70–K77 | 617 (6) |
| Obesity and other hyperalimentation | E65–E68 | 519 (5) |
| Chronic lower respiratory diseases | J40–J47 | 338 (3) |
| Influenza and pneumonia | J09–J18 | 323 (3) |
| Metabolic disorders | E70–E90 | 304 (3) |
| Malignant neoplasms of digestive organs | C15–C26 | 294 (3) |
| Malignant neoplasms, stated or presumed to be primary, of lymphoid, hematopoietic and related tissue | C81–C96 | 282 (3) |
Table 3.
Medical conditions associated with necrotizing fasciitis deaths in the United States, 2003–2013.
| Condition | ICD-10 Codes |
Necrotizing fasciitis- related deaths (%) N=9,871 |
Matched controls* (%) N=98,710 |
Matched OR (95% CI) |
|---|---|---|---|---|
| Diabetes mellitus | E10–E14 | 2,109 (21%) | 10.316 (10%) | 2.37 (2.25, 2.50) |
| Heart disease | I00–I99 | 3,301 (33%) | 49,007 (50%) | 0.49 (0.47, 0.51) |
| Renal failure | N17–N19 | 1,695 (17%) | 8,223 (8%) | 2.31 (2.18, 2.45) |
| Substance use | F10–F19 | 934 (9%) | 12,076 (12%) | 0.74 (0.69, 0.80) |
| Liver disease | K70–K77 | 617 (6%) | 5,872 (6%) | 1.06 (0.97, 1.15) |
| Obesity | E65–E68 | 519 (5%) | 1,928 (2%) | 2.83 (2.56, 3.13) |
Each case of necrotizing fasciitis-related death was matched to 10 controls by age, sex, and race/ethnicity
DISCUSSION
In the United States from 2003 to 2013, the overall mortality rate for necrotizing fasciitis-related death was 4.8 per 1,000,000 person-years. Contrary to our hypothesis, there was not a significant time trend in the number of cases of fatal necrotizing fasciitis during the study period. Streptococcal infection was most commonly identified in cases where a pathogen was reported, although most cases did not have a microbiologic diagnosis established in the death certificate.
There are limited prior data that estimate the disease burden of necrotizing fasciitis in the U.S.. In a study of medical claims records for cellulitis diagnoses, the incidence of necrotizing fasciitis in adults was estimated to be 40 cases per 1,000,000 person-years [17]. The incidence of necrotizing fasciitis among inpatient admissions in Texas varied between 59 cases per 1,000,000 person-years from 2001 to 2002 and 76 cases per 1,000,000 person-years from 2009 to 2010, with an overall in-hospital mortality rate of 9.3% [18]. A larger study of necrotizing soft tissue infections using the Nationwide Inpatient Sample found that the number of hospital discharges for necrotizing soft tissue infections peaked in 2004, with an overall decline between 1998 and 2010 [19], although this approach may underestimate deaths that occur following discharge from the hospital [20]. Our estimate of 4.8 cases of fatal necrotizing fasciitis per 1,000,000 person-years is similar in scale to the population incidence rate that has recently been reported from New Zealand [21].
During the study period, we observed that fatal cases of necrotizing fasciitis were more common among older individuals, and the greatest number of cases observed among individuals age 55 to 64. The association of advancing age with necrotizing fasciitis mortality increased for each age category beyond the reference group of 35 to 44 year olds. In contrast with a previous report from Cook County, Illinois, [22], we did not observe an increasing necrotizing fasciitis-associated mortality rate among individuals in the youngest age groups, and in fact observed declining mortality rates for every age category below the reference group.
In an adjusted analysis using population census data, we observed a greater incidence rate among black, Hispanic, and American Indian individuals, when compared to white individuals, and a lower incidence rate among Asian individuals. The observed relationship between race/ethnicity and necrotizing fasciitis-related mortality is likely multifactorial in etiology. In a population-level study of sepsis syndrome in the U.S., racial differences in outcomes were partly explained by differences in organ impairment [23]. The mortality rate was not adjusted for the presence of specific co-morbidities, income level, or insurance status, which may further contribute to the observed mortality differences. Finally, disparities in access to health care, leading to delays in the recognition and appropriate management of necrotizing fasciitis, provide an additional explanation for the finding of increased mortality rates among Hispanic, black, and American Indian individuals, as compared with white individuals [24].
In a matched case-control study, co-morbid conditions associated with necrotizing fasciitis-related deaths in the United States were identified, based on sampling of deaths certificate data from the entire population. Diabetes mellitus, obesity, and renal failure were significantly associated with necrotizing fasciitis-related death. In a hospital-based retrospective study of 299 patients with necrotizing fasciitis in New Zealand, diabetes and obesity were also found to be frequent co-morbid conditions, identified in 32% and 23% of patients, respectively [25]. It is notable that a protective effect of diabetes mellitus among patients with necrotizing fasciitis has been reported previously in analyses of hospital discharge data [18, 26], which our approach was unable to address. Although outbreaks of necrotizing fasciitis have been reported in association with black-tar heroin use [27], there was no association between necrotizing fasciitis-related death and ICD10 codes for substance use.
This study has several important limitations. Errors in the population estimates from the census data will be reflected in the calculated mortality rates. The completeness of death certificate depends on the information available to the clinician at the time of death, and the appropriate recognition of all relevant contributing causes of death [28]. Additionally, relevant factors such as income level and health insurance status are not included on the death certificate, and there is limited information regarding the severity of co-morbid factors such as diabetes mellitus, or the degree of organ failure. Strengths of the study include the completeness of death certificate reporting in the United States, and prior work with ICD coding to capture incident cases of necrotizing fasciitis in settings such as the National Surgical Quality Improvement Program [29].
In summary, we estimate the U.S. mortality burden of necrotizing fasciitis to be 4.8 deaths per 1,000,000 person-years, with a stable annual incidence rate during the period 2003–2013. Age, sex, and race were independently associated with the rate of necrotizing fasciitis-related deaths during the study period. The observed racial differences in the mortality rate of necrotizing fasciitis merits further investigation.
Acknowledgments
Financial Support: Dr. Vinnard is supported by NIAID (K23AI102639-02).
Footnotes
Conflict of Interest: None
References
- 1.Stevens DL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2014;59:e10–52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 2.Hasham S, et al. Necrotising fasciitis. British Medical Journal. 2005;330:830. doi: 10.1136/bmj.330.7495.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clinical Infectious Diseases. 2007;44:705–710. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 4.Anaya DA, et al. Predictors of mortality and limb loss in necrotizing soft tissue infections. Archives of Surgery. 2005;140:151–157. doi: 10.1001/archsurg.140.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Wong CH, et al. The LRINEC (laboratory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Critical Care Medicine. 2004;32:1535–1541. doi: 10.1097/01.ccm.0000129486.35458.7d. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: clinical suspicion should trump scoring systems. Journal of Emergency Medicine. 2013;44:928–931. doi: 10.1016/j.jemermed.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Brook I, Frazier EH. Clinical and microbiological features of necrotizing fasciitis. Journal of Clinical Microbiology. 1995;33:2382. doi: 10.1128/jcm.33.9.2382-2387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarani B, et al. Necrotizing fasciitis: current concepts and review of the literature. Journal of the American College of Surgery. 2009;208:279–288. doi: 10.1016/j.jamcollsurg.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 9.O'Loughlin RE, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clinical Infectious Disease. 2007;45:853–862. doi: 10.1086/521264. [DOI] [PubMed] [Google Scholar]
- 10.Miller LG, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. New England Journal of Medicine. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. International Classification of Diseases. 1992 10th Revision. Available a: www.who.int/classifications/icd/en/
- 12.Hyattsville: National Center for Health Statistics; 2013. Instruction for Classifying the Underlying Cause of Death, 2013. Available at: www.cdc.gov/nchs/data/dvs/2a_2013.pdf. [Google Scholar]
- 13.Lloyd-Smith JO. Maximum Likelihood Estimation of the Negative Binomial Dispersion Parameter for Highly Overdispersed Data, with Applications to Infectious Diseases. PLoS ONE. 2007;2:e180. doi: 10.1371/journal.pone.0000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver CG, et al. Analyzing hospitalization data: potential limitations of Poisson regression. Nephrology Dialysis Transplantation. 2015;30:1244–1249. doi: 10.1093/ndt/gfv071. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics; Centers for Disease Control and Prevention (CDC); US Census Bureau. United States Bridged-Race Population Estimates 2010 - NCHS. 2011 Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 16.Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. American Journal of Epidemiology. 2007;166:104–108. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]
- 17.Simonsen SME, et al. Cellulitis incidence in a defined population. Epidemiology and Infection. 2006;134:293–299. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oud L, Watkins P. Contemporary trends of the epidemiology, clinical characteristics, and resource utilization of necrotizing fasciitis in Texas: a population-based cohort study. Critical Care Research and Practice. 2015 doi: 10.1155/2015/618067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psoinos CM, et al. Contemporary trends in necrotizing soft tissue infections in the United States. Surgery. 2013;153:819–827. doi: 10.1016/j.surg.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall WB, et al. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. American Journal of Respiratory and Critical Care Medicine. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das DK, Baker MG, Venugopal K. Increasing incidence of necrotizing fasciitis in New Zealand: a nationwide study over the period 1990 to 2006. Journal of Infection. 2011;63:429–433. doi: 10.1016/j.jinf.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin MS, et al. The epidemiology of necrotizing fasciitis including factors associated with death and amputation. Epidemiology and Infection. 2009;137:1609–1614. doi: 10.1017/S0950268809002532. [DOI] [PubMed] [Google Scholar]
- 23.Mayr FB, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. Journal of the American Medical Association. 2010;303:2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Critical Care Medicine. 2013;41:2784–2793. doi: 10.1097/CCM.0b013e3182a84a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das DK, Baker MG, Venugopal K. Risk factors, microbiological findings and outcomes of necrotizing fasciitis in New Zealand: a retrospective chart review. BMC Infectious Diseases. 2012;12:348. doi: 10.1186/1471-2334-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulla ZD, Gibbs SG, Aaronoff DM. Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis. Epidemiology and Infection. 2007;135:868–76. doi: 10.1017/S0950268806007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura AC, et al. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clinical Infectious Diseases. 2004;38:e87. doi: 10.1086/383471. [DOI] [PubMed] [Google Scholar]
- 28.Messite J, Stellman SD. Accuracy of death certificate completion: the need for formalized physician training. Journal of the American Medical Association. 1996;275:794–796. [PubMed] [Google Scholar]
- 29.Faraklas I, et al. Development and validation of a necrotizing soft-tissue infection mortality risk calculator using NSQIP. Journal of the American College of Surgery. 2013;217:153–160. doi: 10.1016/j.jamcollsurg.2013.02.029. [DOI] [PubMed] [Google Scholar]