Summary
Adipose-derived stem/stromal cells (ASCs), together with adipocytes, vascular endothelial cells, and vascular smooth muscle cells, are contained in fat tissue. ASCs, like the human bone marrow stromal/stem cells (BMSCs), can differentiate into several lineages (adipose cells, fibroblast, chondrocytes, osteoblasts, neuronal cells, endothelial cells, myocytes, and cardiomyocytes). They have also been shown to be immunoprivileged, and genetically stable in long-term cultures. Nevertheless, unlike the BMSCs, ASCs can be easily harvested in large amounts with minimal invasive procedures. The combination of these properties suggests that these cells may be a useful tool in tissue engineering and regenerative medicine.
Keywords: mesenchymal stem cells, adipose tissue, ASCs, osteogenic differentiation, bone tissue engineering
Introduction
Stem cells are characterized by their ability to produce self-renewing progenitor cells that can generate one or more specialized cell types. They are divided into embryonic stem cells (ESCs) and adult stem cells (1). The use of adult stem cells has awakened huge interest. Even if they have a lower capacity of differentiating (2, 3) than ESCs (4, 5), they circumvent ethical issues and show less tumorigenicity. The first multipotent mesenchymal stem cells (MSCs) identified (6) were the bone marrow stromal/stem cells (BMSCs). Currently, these cells are most frequently used in regenerative medicine for their high differentiation potential and low morbidity during harvesting (2, 7, 8). Nevertheless, harvesting of BMSCs by bone marrow aspiration is a painful procedure, and the number of cells acquired is usually low. An alternative source of multipotent stem cells, with similar properties to those of BMSCs, is the adipose tissue (9–12). In fact, adipose stem cells (ASCs) can be easily harvested, obtaining large amount with minimal risk, and moreover they show the ability to be readily expanded, and also the capacity to undergo adipogenic, osteogenic, chondrogenic, neurogenic, and myogenic differentiation in vitro (3, 10–17). For these reasons ASCs are currently used for tissue regeneration or reconstruction in clinical trials. In fact, during recent years, the number of trials investigating the efficacy in treating conditions such as type I and II diabetes, liver and cardiovascular disease, limb ischemia, amyotrophic lateral sclerosis, and lipodystrophy, has increased. Moreover, ASCs appear to be more genetically stable in long-term cultures (18) compared to BMSCs (19), and show immunosuppressive properties (20, 21). Indeed, these cells are subject to study in case of immunosuppression (20–22), graft-versus-host disease (23–26), soft tissue augmentation (27, 28), multiple sclerosis (29), and bone tissue repair (30, 31). In view of all these properties, the studies of clinical and basic research are focused on ASCs, but more knowledge of the features of this cell population is needed in order to standardize the clinical strategies (32, 33). The aim of this review is to examine the differentiative capacity of ASCs and their potential role in tissue engineering.
Adipose tissue: an alternative source of stem cells
Adipose tissue, derived from the mesenchyme, is found in bone marrow, around internal organs, under the skin, and in breast tissue. In humans, it is one of the most abundant tissue types, a metabolic reservoir for packaging, storing, and releasing high-energy substrates (34), and it also has many endocrinologic properties, given that it secretes numerous polypeptides, hormones, growth factors, and cytokines (35, 36). Adipose tissue is composed of a heterogeneous set of cell populations that are defined as stromal vascular fraction (SVF) (3, 37). The SVF comprises the stromal cells, ASCs (10, 11, 38), vascular endothelial cells and their progenitors, vascular smooth muscle cells, and also cells with hematopoietic progenitor activity (39, 40). Currently, this tissue is probably considered to be one of the richest sources of adult stem cells in the human body, and therefore it holds great promise for utilization in tissue repair and regeneration.
Adipose stem cells
Recently, ASCs have been isolated from adipose tissue (2, 11). These cells, like other MSCs (i.e. BMSCs), show a spindle or stellate shape, the capacity to adhere to plastic to form fibroblast-like colonies (Figure 1), an extensive proliferative capacity, and the ability to differentiate into several multilineage cells such as osteoblasts, adipocytes, chondroblasts, myocytes, tendocytes, and ligament cells (3, 10–17, 41). In addition to their potential to differentiate, ASCs have also been shown to possess some level of plasticity, transdifferentiating in vitro into epithelial cells, hepatocytes, and neural cells (42–44). In culture, these cells express stem cell-surface markers such as: CD13, CD29, CD44, CD73, CD90, CD105, CD133 and CD166 (45–51). Therefore, ASCs match the four criteria for the identification of human mesenchymal stem cells proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (52): they have to be plastic-adherent when maintained under standard culture conditions; they must have the ability to osteogenic, adipogenic, and chondrogenic differentiation; they must express CD73, CD90, CD105; they must lack the expression of hematopoietic linage markers (CD14, CD11b, CD34, CD45, CD19, CD79). Moreover, ASCs can be easily harvested in great amounts from fat tissue; i.e., after cosmetic liposuctions, once adipose tissue is digested with collagenase (63), approximately ~5000 cell/ml fat can be obtained (64). For the reasons mentioned above, ASCs may represent a valuable and alternative tool in tissue engineering. Some studies have also reported that ASCs possess the ability to suppress a mixed lymphocyte reaction in a dose-dependent and time-dependent manner (53, 54), that they are immunoprivileged (20, 21), due to lack of major histocompatibility complex class II expression (45, 55), and they have the capacity to suppress the proliferation of activated allogeneic lymphocytes (54–56). This suggests that ASCs can have potential as immunoprivileged universal donor cells with the capacity to be used in the allogeneic setting and to reduce graft-versus-host disease (20, 54). Nevertheless, recent studies have shown that the immunosuppressive effects of ASCs may induce tumorigenesis (57, 58). Conversely, other studies show that these cells possess tumorsuppressive capacity (59–61). The debate is still open at this time.
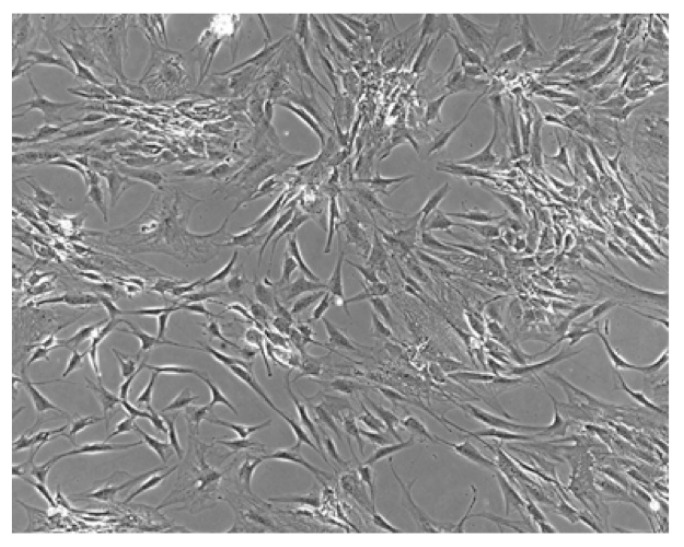
Figure 1.
ASCs undifferentiated. Observation with phase contrast microscope: ASCs with shaped form.
Many names have been utilized to describe these cells: processed lipoaspirate cells (PLA), preadipocytes, adipose-derived adult stem (ADAS) cells, adipose-derived stromal cells (ADSCs), adipose stromal cells (ASCs), multipotent adipose-derived stem cells (MADS), and adipose mesenchymal stem cells (AdMSCs) (37). To solve the problem, the International Fat Applied Technology Society has proposed a standardized nomenclature by adopting the term adipose derived stem/stromal cells (ASCs) to identify the isolated, plastic-adherent, multipotent cell population (39, 62).
ASCs: from ward to bench
ASCs can be isolated from adipose tissue coming from plastic surgery or biopsies. When the starting material is obtained from liposuction procedures, the isolation is faster as a result of finely minced tissue fragments (65). Using whole tissue pieces as starting material, the tissue is minced manually (35). In our lab, after having obtained informed consent in accordance with the Institutional Review Board protocol, we have utilized the following practice: the adipose tissue biopsies are immediately placed in McCOY’S 5A medium supplemented with 22mM HEPES and 100 IU/ml penicillin, 100 μg/ml streptomycin, pH 7.4, and transported to the laboratory, with processing within 30 min of excision. In the lab the samples were minced into small pieces (0.2–0.5mm) and the fragments were washed with McCOY’S 5A medium, centrifuged at 200 g for 10 min, resuspended in Ham’s F12 Coon’s modification medium supplemented with 20% FBS and 3 mg/ml collagenase type I, digested for 3 h at 37°C, mechanically dispersed and passed through a sterile 230-mm stainless steel tissue sieve. The undigested tissue trapped in the sieve was discarded, while the infranatant containing the preadipocyte fraction was collected and the cells were sedimented by centrifugation at 300 g for 5 min. The pellet was incubated with an erythrocyte lysis buffer for 2 min at room temperature, and the remaining cells were cultured in 100 mm culture plates in growth medium: Ham’s F12 Coon’s modification medium supplemented with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 1 ng/ml basic fibroblast growth factor, and incubated at 37°C in humid atmosphere with 5% CO2. The medium was refreshed twice a week (66).
ASCs: differentiation potential
ASCs can differentiate into several different cell types (Table 1) (10, 11, 15, 67–74) and the lineage-specific differentiation is directly related to the expression of specific phenotypic markers and mature tissue genes. However, the mechanisms that drive these cells into the specialized lineage are not yet clear.
Table 1.
Differentiation potential of ASCs.
Adipogenic differentiation
ASCs have a notable capacity to differentiate into adipocytes (75–77), which is very important in developing techniques to repair soft tissue defects, especially after oncological surgery (78). The adipogenic induction medium contains insulin or IGF-1, triiodothyronine, transferrin, isobutylmethylxanthine, hydrocortisone or dexamethasone, indomethacin or thiazolidinedione, pantothenate, and serum (10, 11, 79, 80). A week after induction, lipid containing vacuoles accumulate in ASCs, which can be detected by Oil Red O or Nile red staining (Figure 2) (11, 81), and express several genes and proteins involved in lipid biosynthesis, metabolism and accumulation, including peroxisome-proliferating activated receptor γ, adipocyte fatty-acid binding protein, and leptin can be detected (10, 11, 82–84).
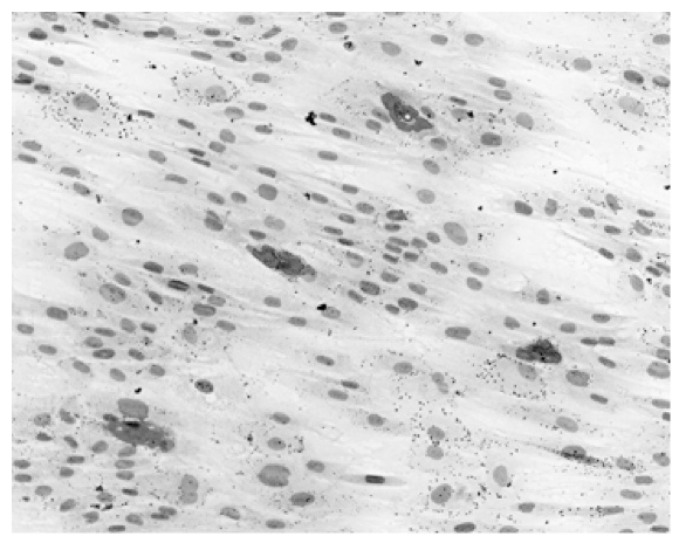
Figure 2.
Adipogenic differentiation of ASCs. Oil Red O staining: adipocytes in red, nuclei in blue-violet.
Osteogenic differentiation
In vitro osteogenic differentiation of ASCs can be obtained using medium supplemented with ascorbic acid, b-glycerophosphate, dexamethasone and/or 1,25 vitamin D3 (3, 85, 86). Another factor that can strongly induce the osteogenic differentiation is bone morphogenic protein 2 (BMP2), belonging to the transforming growth factor-β (TGF-β) superfamily. Indeed, some studies show in vitro and in vivo differentiation of ASCs in osteoblast-like cells when transfected with BMP2 gene (87, 88). ASCs cultured in the presence of these factors express genes and proteins associated with the osteoblast phenotype, including alkaline phosphatase (Figure 3A), type I collagen, osteopontin, osteonectin, Runx2, BMP-2, BMP-4, and BMP receptors I and II (11, 35, 76, 77, 85, 89, 90). Moreover, when cultured between 14 and 28 days in vitro in osteogenic medium, ASCs start to produce calcium phosphate mineral within their extracellular matrix, which can be detected by Alizarin Red or von Kossa staining (Figure 3B) (10, 11). Importantly, ASCs undergoing osteogenic induction are able to adhere to scaffolds, migrate, proliferate, and differentiate when transplanted in bone tissue in vivo (91–93). This type of construct can be effective to regenerate damaged bone tissue (92, 94–97). Finally, since it is demonstrated that while the adipogenic potential is unchanged compared to age, the osteogenic potential decreases with an increase of the same (98). All these conditions should be evaluated for designing clinical treatment for patients.
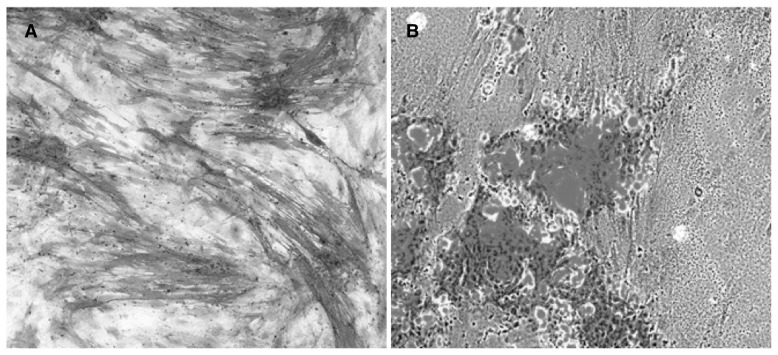
Figure 3.
Osteogenic differentiation of ASCs. A. ALP activity (in red) in osteogenic differenziate ASCs; B. Hydroxyapatite deposits: calcium deposits in red, ASCs in gray-blue.
Chondrogenic differentiation
Many studies have demonstrated the ability of ASCs to undergo chondrocytic differentiation (10, 17, 68, 99) using medium supplemented with insulin growth factor, ascorbate-2-phosphate, dexamethasone, L-proline, BMP6 and 7, and TGF-β (100–106). Routinely, for chondrogenic differentiation, ASCs are cultured in micro mass culture or pellet culture systems (8, 107). These practices mimic the embryonic development conditions of cartilage tissue, increase the cell-to-cell interaction, and lead to the production of a cartilage-like matrix (108). Alternatively, ASCs can be seeded into polyglycolic acid scaffolds that mimic the composition of the native cartilage (74, 109). Moreover, it is demonstrated that ASCs on elastin-like polypeptide material can grow and express the chondrogenic phenotype without the chondrogenic medium (110). So, when ASCs are maintained in vitro in an appropriate 3D environment, with or without induction medium, they will start to synthesize the cartilage extracellular matrix proteins, including COL II, COL VI, and aggrecan (68, 109, 111). The potential of ASCs in cartilage tissue engineering is also demonstrated in in vivo studies in animal models (112).
Myogenic and cardiomyogenic differentiation
Using specific medium that contains induction factors such as dexamethasone and hydrocortisone, it is possible to obtain ASCs myogenic differentiation (10, 113). In vitro, after undergoing these culture conditions, ASCs express muscle-specific marker including a heavy chain of myosin, myogenic determination factor 1 and myogenin (10, 11, 69), and they adopt elongated, multinucleated structures that closely resemble myotubes (10, 11, 69). In vivo studies showed that the implantation of ASCs in muscular dystrophy mice restores the dystrophin expression in the muscles (114). These results are particularly promising for genetic diseases characterized by progressive muscle degeneration.
ASCs also have the capacity to differentiate in cardiomyocytes, when seeded in medium supplemented with interleukin 3 and 6 (70, 115–117). Nevertheless, few studies are reported, and in most of them a low percentage of differentiation has been reported (118–120). In vitro expanded ASCs have been shown to improve cardiac function when administered in animal model with myocardial infarction (120).
Endothelial differentiation
It has been reported that ASCs have the potential for endothelial differentiation (33, 121–125) and can participate in blood vessel formation, as they are able to secrete a number of proangiogenic factors, such as vascular endothelial growth factor and platelet-derived growth factor (13, 126–130). These characteristics are very important, because the regenerated tissues need to contain vascular systems to allow both the tissue and the differentiated cells to survive. Studies performed on animal models have allowed the development of an osteogenic and vasculogenic construct using human adipose stromal-vascular cell fractions (125, 131). These studies prove that human ASCs under perfusion flow in a three-dimensional environment are able to form bone tissue and blood vessels after implantation in nude mice. What is more, the blood vessels formed by human ASCs were functionally connected to the mouse vascular network and contained mouse erythrocytes (125, 131).
ASCs: transdifferentiation potential
It has long been believed that tissue-specific progenitor cells can only differentiate into native tissue cell types. Recent studies have challenged this view. Many experiments have revealed that adult stem cells may retain the potential to transdifferentiate from one phenotype to another, either in vitro or after transplantation in vivo (132, 133). ASCs can differentiate, upon specific stimulus, into cells of ectodermal and endodermal origin such as pancreatic cells (16, 134), hepatocytes (135), epithelial cells (113, 136) and neuronal cells (11, 137, 138), although the mechanisms are not yet clear.
Endodermal tissue-derived differentiation
Pancreatogenic differentiation
Some studies report that ASCs can be differentiated into cells with a pancreatic endocrine phenotype expressing insulin, glucagon, and somatostatin (16). Indeed, if transplanted into diabetic mice, they can regulate blood glucose levels by releasing human insulin (139), or if transplanted in pancreatectomized rats, to induce C-peptide-positive cells from ASCs by observing expression of genes related to early pancreatic differentiation (140).
Hepatogenic differentiation
The differentiation of ASCs into hepatocyte-like cells has also been investigated (15, 141, 142). When hepatogenically induced, ASCs express albumin and α-fetoprotein, and show LDL uptake and production of urea. When these cells are predifferentiated in vitro, and after a transplantation into a SCID mouse model, they express albumin and promote the hepatic integration in vivo (135).
Epithelial differentiation
Currently, there are studies which show that, under optimized conditions, ASCs express epithelial marker protein and complete their epithelialization after implantation (113, 136), i.e. a tissue-engineered airway construct with a 3D structure of fibrin and ASCs was marked as a prototype vocal fold replacement.
Ectodermal tissue-derived differentiation
Neurogenic differentiation
Many studies have demonstrated the neurogenic potential of ASCs (11, 137, 138). When seeded in medium containing factors such as valproic acid, insuline, hydroxyanisole, epidermal growth factor, fibroblast growth factor, and indomethacin (11), ASCs express neuronal and/or oligodendrocytic markers (14, 143, 144) such as neuron specific enolase, neuron specific nuclear protein, nestin, intermediate filament M, and glial fibrillary acidic protein. Moreover, they adopt a neuronal morphology with a small cell body and multiple neurite-like projections (11). However, chemical signal transduction properties of ASCs in neurogenesis need to be characterized further to use these cells in the regeneration of the central or peripheral nervous system following traumatic injury.
Possible bio-applications of the ASCs
ASCs in immunologic disorders
Graft-versus-host disease (GvHD)
GvHD is a disorder that can be manifested after tissue or organ allogenic transplant. These cases are characterized by massive activity of the donor’s immunologic system cells versus host. Some in vivo studies have reported good results in humans, using ASCs for treating severe and acute GvHD (23–26).
Diabetes
One study, effected in insulinopenic diabetic patients treated using insulin-producing ASCs transfused, reported 30–50% decreased insulin requirements in all patients with a 4- to 26-fold increase in serum c-peptide levels during a follow-up period of 2.9 months on average (145).
Immunosuppression by ASCs
The use of ASCs for immunologic diseases, such as autoimmune-induced rheumatoid arthritis (RA) or inflammatory bowel disease, is currently under investigation (20–22, 146). Results show that the systemic infusion of ASCs in human patients is an important regulator of immune tolerance, with the ability to suppress T-cell and inflammatory responses, and inducing the activation of antigen-specific regulatory T-cells.
ASCs in tissue engineering
Tissue engineering is a field of research that uses biomaterials, growth factors, and stem cells to repair, replace or regenerate tissues and organs damaged by diseases or injuries (147, 148). ASCs are ideal candidates for utilization in tissue engineering practices, especially if the receiver is at the same time the donor (autologous transplant), since, as demonstrated above, they are able to self-renew, to commit to multiply cell lineages (2, 12), and easily harvested in large amounts with minimal invasive procedures (40).
Soft-tissue augmentation
The extensive in vitro proliferative capacity of ASCs is very important for the volume return of tissue in oncological patients after surgery, especially in breast cancer cases. Also, soft-tissue augmentation is a practice often used in plastic surgery. These techniques consist in the utilization of hyaluronic acid-based spongy scaffolds seeded with ASCs and then implanted subcutaneously (149).
Bone tissue repair
Many studies have been carried out to evaluate the ASCs or scaffold-ASCs construct capacity in bone tissue repair. At this time, three of these studies report success. In the first case, in the animal model, ASCs are plated on an apatite-coated resorbable scaffold. This construct has been used for the treatment of a murine calvarial defect. The results demonstrated a range of 70–90% closure of the defect within 12 weeks (96). In the second case, with a patient affected by keratocyst, maxillary reconstruction, has been done utilizing synthetic bone combined with autologous ASCs, previously expanded ex vivo. The results show mature bone structures developed within the construct (31). In the third case, the patient affected by severe calvarial fracture was treated with autologous ASCs applied in combination with autologous bone. The ASCs were supported in place using autologous fibrin glue, and mechanical fixation was achieved with resorbable macroporous sheets. The postoperative course was uneventful and new bone formation was observed three months after the reconstruction (30). However, further studies are required to assess and verify the safe outcome of the clinical procedure using in vitro expanded stem cells. In our laboratory, we have studied the growing and osteogenic differentiation ability of ASCs on hard biocompatible biomaterials, usually used in orthopedic-prothesic replacement (66). ASCs were seeded on titanium alloy (Ti6Al4V) and, at different times, were evaluated for cell growth and adhesion, and osteogenic differentiation. The results show good cell expansion and adhesion on the Ti6Al4V alloy surface. Moreover, when seeded in the presence of osteogenic medium, ASCs express the osteoblastic phenotype, which is characterized by an increase, time-dependent, of alkaline phosphatase activity, by deposits of osteopontin, osteonectin, and hydroxyapatite (Figure 4), and by the expression of a pattern of genes characteristic of the same phenotype as ALP, RUNX2, SMAD1, OCN and OPN.
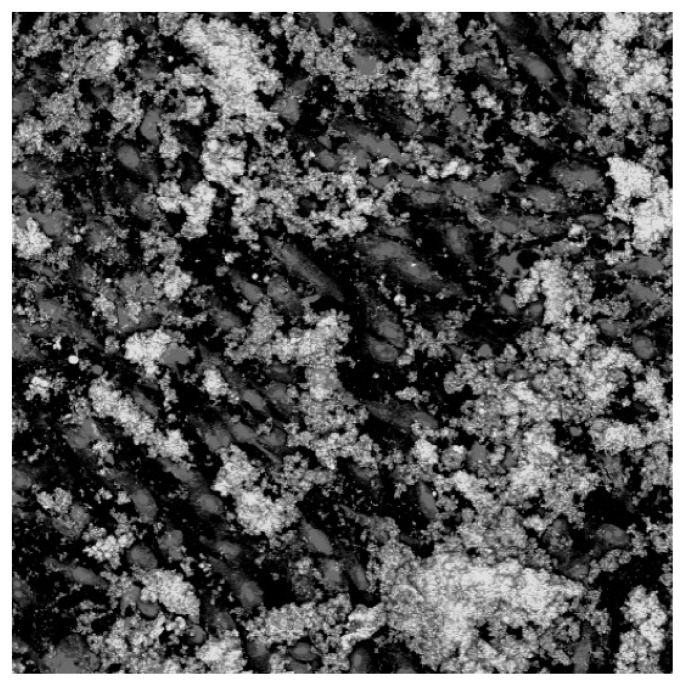
Figure 4.
ASCs seeded on titanium alloy (Ti6Al4V). LSM confocal microscopy: nucleuses in red, calcium deposits in green.
Clinical trials
In the view the results described above, and to improve the safety and efficacy of ASCs for tissue regeneration or reconstruction, 18 clinical trials have been initiated (Table 2), and only 2 have been completed so far. The clinical trials using ASCs can be consulted on www.clinicaltrials.gov.
Table 2.
Clinical trials utilizing ACSc.
| Trial | Condition | Design |
|---|---|---|
| Study of Autologous Fat Enhanced w/Regenerative Cells Transplanted to Reconstruct Breast Deformities After Lumpectomy | Breast Neoplasms Carcinoma, Ductal, Breast Mammoplasty Mastectomy, Segmental, Lumpectomy, Breast Reconstruction |
Phase IV |
| Safety and Efficacy of Autologous Cultured Adipocytes in Patient with Depressed Scar | Depressed Scar | Phase II/III, Completed |
| Efficacy and Safety of Adipose Stem Cells to Treat Complex Perianal Fistulas Not Associated to Crohn’s Disease (FATT1) | Complex Perianal Fistulas | Phase III, Completed |
| Safety and Efficacy of Autologous Adipose-Derived Stem Cell Transplantation in Type 2 Diabetics | Type 2 Diabetes Mellitus | Phase I/II |
| Intraarterial Infusion of Autologous Mesenchymal Stem Cells From Adipose Tissue in Diabetic Patients With Chronic Critical Limb Ischemia | Chronic Critical Limb Ischemia | Phase I/II |
| Safety and Efficacy of Autologous Adipose-Derived Stem Cell Transplantation in Patients With Type 1 Diabetes Mellitus | Type 1 Diabetes | Phase I/II |
| Safety and Efficacy Study of Autologous Cultured Adipose-Derived Stem Cells for the Crohn’s Fistula | Crohn’s Fistula | Phase I |
| Safety Study of Autologous Cultured Adipose-Derived Stem Cells for the Fecal Incontinence | Fecal Incontinence | Phase I |
| Autologous Mesenchymal Stem Cells From Adipose Tissue in Patients With Secondary Progressive Multiple Sclerosis | Secondary Progressive Multiple Sclerosis | Phase I/II |
| Allogenic Stem Cells Derived From Lipoaspirates for the Treatment of Recto-Vaginal Fistulas Associated to Crohn’s Disease (ALOREVA) | Rectovaginal Fistula Crohn Disease | Phase I/II |
| Randomized Clinical Trial of Adipose Derived Stem Cells in the Treatment of Pts With STElevation Myocardial Infarction | Myocardial Infarction Coronary Arteriosclerosis Cardiovascular Disease Coronary Disease |
Phase I |
| A Randomized Clinical Trial of Adipose-Derived Stem Cells in Treatment of Non Revascularizable Ischemic Myocardium | Ischemic Heart Disease Coronary Arteriosclerosis Cardiovascular Disease Coronary Disease Coronary Artery Disease |
Phase I |
| Autologous Adipose-Derived Stem Cell Transplantation in Patients With Lipodystrophy | Lipodystrophy | Phase I |
| Liver Regeneration Therapy by Intrahepatic Arterial Administration of Autologous Adipose Tissue Derived Stromal Cells | Liver Cirrhosis | Phase I |
| Autologous Stem Cells Derived From Lipoaspirates for the Non-Surgical Treatment of Complex Perianal Fistula | Anal Fistula | Phase II |
| Long-term Safety and Efficacy of Adipose-derived Stem Cells to Treat Complex Perianal Fistulas in Phase II Patients Participating in the FATT-1 Randomized Controlled Trial | Complex Perianal Fistula | Phase II |
| Liver Regeneration Therapy Using Autologous Adipose Tissue Derived Stromal Cells | Liver Cirrhosis | Recruiting |
| Tissue Partitioning in Early Childhood | Changes in Bone Mineral Content Changes in Bone Marrow Adipose Tissue |
No yet recruiting |
Future perspectives
It is well know that adipose tissue can be harvested in large quantities with minimal invasive procedures, obtaining large amounts of ASCs with high plasticity (134, 150–153). Moreover these ASCs exhibit immunomodulatory and angiogenetic properties (20, 21). These properties can have opposite effects. Reports have shown that the immunosuppressive capacity of the ASCs may in some cases favour the growth of tumour cells (57, 58), even if other studies report contrary results (59–61). In addition, although ASCs appear to be fairly stable genetically in long-term cultures, some chromosomal aberrations also arise transiently, at least when cells are cultured for approximately one month (18, 154). However, it is demonstrated that ASCs can be cultured safely during a standard ex vivo expansion period of approximately 6–8 weeks (155). Consequently, it is very important to improve methods to assess the safety of ASCs, not only in vitro, but also in vivo and clinically, to prevent anaphylactic reactions and tumorigenicity in the recipient.
References
- 1.Dazzi F, Ramasamy R, Glennie S, et al. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 4.Bongso A, Fong CY, Ng SC, et al. Isolation and culture of inner cell mass cells from human blastocysts. Human Reproduction. 1994;9:2110–2117. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 7.Jaiswal RK, Jaiswal N, Bruder SP, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. The Journal of Biological Chemistry. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA. Tissue engineering craniofacial defects with adult stem cells? Are we ready yet? Pediatric Research. 2008;63:478–486. doi: 10.1203/PDR.0b013e31816bdf36. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation Research. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 14.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochemical and Biophysical Research Communications. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 15.Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochemical and Biophysical Research Communications. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 16.Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochemical and Biophysical Research Communication. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 17.Winter A, Breit S, Parsch D, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis and Rheumatism. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 18.Meza-Zepeda LA, Noer A, Dahl JA, et al. Highresolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. Journal of Cellular and Molecular Medicine. 2008;12:553–563. doi: 10.1111/j.1582-4934.2007.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl JA, Duggal S, Coulston N, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. The International Journal of Developmental Biology. 2008;52:1033–1042. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn’s disease. Expert Opinion on Biological Therapy. 2008;8:1417–1423. doi: 10.1517/14712598.8.9.1417. [DOI] [PubMed] [Google Scholar]
- 23.Fang B, Song Y, Liao L, et al. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplantation Proceedings. 2007;39:3358–3362. doi: 10.1016/j.transproceed.2007.08.103. [DOI] [PubMed] [Google Scholar]
- 24.Fang B, Song Y, Lin Q, et al. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatric Transplantation. 2007;11:814–817. doi: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 25.Fang B, Song Y, Zhao RC, et al. Using human adipose tissue-derived mesenchymal stem cells as salvage therapy for hepatic graft-versus-host disease resembling acute hepatitis. Transplantation Proceedings. 2007;39:1710–1713. doi: 10.1016/j.transproceed.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 26.Fang B, Song YP, Liao LM, et al. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplantation. 2006;38:389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plastic Surgery. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatologic Surgery. 2008;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 29.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. Journal of Translational Medicine. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lendeckel S, Jodicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. Journal of Craniomaxillofacial Surgery. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Mesimaki K, Lindroos B, Tornwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stemcells. International Journal of Oral and Maxillofacial Surgery. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010;42:211–216. doi: 10.1016/j.tice.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Froehlich H, Gulati R, Boilson B, Witt T, et al. Carotid repair using autologous adipose-derived endothelial cells. Stroke. 2009;40:1886–1891. doi: 10.1161/STROKEAHA.108.539932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Rada T, Reis RL, Gomes ME. Adipose tissuederived stem cells and their application in bone and cartilage tissue engineering. Tissue Engineering Part B: Reviews. 2009;15:113–125. doi: 10.1089/ten.teb.2008.0423. [DOI] [PubMed] [Google Scholar]
- 36.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 37.Schaffler A, Buchler C. Adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 38.Katz AJ, Llull R, Hedrick MH, et al. Emerging approaches to the tissue engineering of fat. Clinics in Plastic Surgery. 1999;26:587–603. [PubMed] [Google Scholar]
- 39.Daher SR, Johnstone BH, Phinney DG, et al. Adipose stromal/stem cells: basic and translational advances: the IFATS collection. Stem Cells. 2008;26:2664–2665. doi: 10.1634/stemcells.2008-0927. [DOI] [PubMed] [Google Scholar]
- 40.Fraser JK, Wulur I, Alfonso Z, et al. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Huang JI, Zuk PA, Jones NF, et al. Chondrogenic potential of multipotential cells from human adipose tissue. Plastic and Reconstructive Surgery. 2004;113:585–594. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 42.Dawn B, Bolli R. Adult bone marrow-derived cells: Regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 45.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 46.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 47.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 49.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 50.Rebelatto CK, Aguiar AM, Moretao MP, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Experimental Biology and Medicine. 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 51.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. Journal of Cellular Physiology. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 52.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 53.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effects of human adipose tissue-derived stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 54.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 55.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 56.Lazarus H, Curtin P, Devine S, et al. Role of mesenchymal stem cells (MSC) in allogeneic transplantation: Early phase I clinical results. Blood. 2009;96:1691. [Google Scholar]
- 57.Yu JM, Jun ES, Bae YC, et al. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells and Development. 2008;17:463–473. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 58.Muehlberg FL, Song YH, Krohn A, et al. Tissue resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 59.Kucerova L, Altanerova V, Matuskova M, et al. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Research. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 60.Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS ONE. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grisendi G, Bussolari R, Cafarelli L, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Research. 2010;70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 62.Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 63.Williams SK, McKenney S, Jarrell BE. Collagenase lot selection and purification for adipose tissue digestion. Cell Transplant. 1995;4:281–289. doi: 10.1177/096368979500400306. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y, Liu T, Song K, et al. Adipose-derived stem cell: A better stem cell than bmsc. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 65.Tremolada C, Palmieri G, Ricordi C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplantation. 2010;19:1217–1223. doi: 10.3727/096368910X507187. [DOI] [PubMed] [Google Scholar]
- 66.Tognarini I, Sorace S, Zonefrati R, et al. In vitro differentiation of human mesenchymal stem cells on Ti6Al4V surfaces. Biomaterials. 2008;29:809–824. doi: 10.1016/j.biomaterials.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez AM, Pisani D, Dechesne CA, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erickson GR, Gimble JM, Franklin DM, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 69.Mizuno H, Zuk PA, Zhu M, et al. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 70.Rangappa S, Fen C, Lee EH, et al. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 71.Ashjian PH, Elbarbary AS, Edmonds B, et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg. 2003;111:1922–1931. doi: 10.1097/01.PRS.0000055043.62589.05. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez AM, Elabd C, Delteil F, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315:255–263. doi: 10.1016/j.bbrc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 73.Huang JI, Beanes SR, Zhu M, et al. Rat extramedullary adipose tissue as a source of osteochondrogenic progenitor cells. Plast Reconstr Surg. 2002;109:1033–1041. doi: 10.1097/00006534-200203000-00037. [DOI] [PubMed] [Google Scholar]
- 74.Awad HA, Wickham MQ, Leddy HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 75.Mauney JR, Nguyen T, Gillen K, et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3d scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y, Lin H, Zhang J, et al. Crosslinked three-dimensional demineralized bone matrix for the adipose-derived stromal cell proliferation and differentiation. Tissue Eng Part A. 2009;15:13–21. doi: 10.1089/ten.tea.2008.0039. [DOI] [PubMed] [Google Scholar]
- 77.Hong L, Colpan A, Peptan IA, et al. 17-beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007;13:1197–1203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- 78.Brayfield C, Marra K, Rubin JP. Adipose stem cells for soft tissue regeneration. Handchir Mikrochir Plast Chir. 2010;42:124–128. doi: 10.1055/s-0030-1248269. [DOI] [PubMed] [Google Scholar]
- 79.Hauner H, Entenmann G, Wabitsch M, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. Journal of Clinical Investigation. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sen A, Lea-Currie YR, Sujkowska D, et al. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. Journal of Cellular Biochemistry. 2001;81:312–319. doi: 10.1002/1097-4644(20010501)81:2<312::aid-jcb1046>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 81.Gimble JM, Morgan C, Kelly K, et al. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. Journal of Cellular Biochemistry. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- 82.Moldes M, Zuo Y, Morrison RF, et al. Peroxisomeproliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. The Biochemical Journal. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Won Park K, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metabolism. 2008;8:454–457. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiological Reviews. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 85.Halvorsen YD, Franklin D, Bond AL, et al. Extracellular matrix mineralization and osteoblast gene expres-sion by human adipose tissue-derived stromal cells. Tissue Engineering. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 86.Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells–their utility and potential in bone formation. International Journal of Obesity and Related Metabolic Disorders. 2000;24(Suppl 4):S41–S44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 87.Lee SJ, Kang SW, Do HJ, Han I, Shin DA, Kim JH, Lee SH. Enhancement of bone regeneration by gene delivery of bmp2/runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials. 2010;31:5652–5659. doi: 10.1016/j.biomaterials.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 88.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2-transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 89.Lee JH, Rhie JW, Oh DY, Ahn ST. Osteogenic differentiation of human adipose tissue-derived stromal cells (hascs) in a porous three dimensional scaffold. Biochem Biophys Res Commun. 2008;370:456–460. doi: 10.1016/j.bbrc.2008.03.123. [DOI] [PubMed] [Google Scholar]
- 90.Strem BM, Hicok KC, Zhu M, et al. Multipotential differentiation of adipose tissue-derived stem cells. The Keio Journal of Medicine. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 91.Jeon O, Rhie JW, Kwon IK, et al. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008;14:1285–1294. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y, Wang T, Wu L, et al. Ectopic and in situ bone formation of adipose tissue-derived stromal cells in biphasic calcium phosphate nanocomposite. J Biomed Mater Res A. 2007;81:900–910. doi: 10.1002/jbm.a.31149. [DOI] [PubMed] [Google Scholar]
- 93.Li X, Yao J, Wu L, et al. Osteogenic induction of adipose-derived stromal cells: Not a requirement for bone formation in vivo. Artif Organs. 2009;34:46–54. doi: 10.1111/j.1525-1594.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 94.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gastaldi G, Asti A, Scaffino MF, et al. Human adipose-derived stem cells (hASCs) proliferate and differentiate in osteoblast-like cells on trabecular titanium scaffolds. J Biomed Mater Res. 2010;94A:790–799. doi: 10.1002/jbm.a.32721. [DOI] [PubMed] [Google Scholar]
- 96.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 97.Shen FH, Zeng Q, Lv Q, et al. Osteogenic differentiation of adipose-derived stromal cells treated with GDF-5 cultured on a novel three-dimensional sintered microsphere matrix. Spine J. 2006;6:615–623. doi: 10.1016/j.spinee.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 98.Zhu M, Kohan E, Bradley J, et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. Journal of Tissue Engineering and Regenerative Medicine. 2009;3:290–301. doi: 10.1002/term.165. [DOI] [PubMed] [Google Scholar]
- 99.Dragoo JL, Samimi B, Zhu M, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740–747. [PubMed] [Google Scholar]
- 100.Hennig T, Lorenz H, Thiel A, et al. Reduced chondrogenic potential of adipose tissuederived stromal cells correlates with an altered TGFbeta receptor and bmp profile and is overcome by bmp-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 101.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissuederived mesenchymal stem cells: Greater doses of growth factor are necessary. J Orthop Res. 2009;27:612–619. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 102.Kim BS, Kang KS, Kang SK. Soluble factors from ascs effectively direct control of chondrogenic fate. Cell Prolif. 2010;43:249–261. doi: 10.1111/j.1365-2184.2010.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Awad HA, Halvorsen YD, Gimble JM, Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 104.Knippenberg M, Helder MN, Zandieh Doulabi B, et al. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochemical and Biophysical Research Communications. 2006;342:902–908. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 105.Diekman BO, Estes BT, Guilak F. The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. Journal of Biomedical Materials Research Part A. 2010;93A:994–1003. doi: 10.1002/jbm.a.32589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis and Rheumatism. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 107.Denker AE, Nicoll SB, Tuan RS. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-beta 1. Differentiation. 1995;59:25–34. doi: 10.1046/j.1432-0436.1995.5910025.x. [DOI] [PubMed] [Google Scholar]
- 108.Wei Y, Sun X, Wang W, et al. Adipose-derived stem cells and chondrogenesis. Cytotherapy. 2007;9:712–716. doi: 10.1080/14653240701620596. [DOI] [PubMed] [Google Scholar]
- 109.Mahmoudifar N, Doran PM. Chondrogenic differentiation of human adipose-derived stem cells in polyglycolic acid mesh scaffolds under dynamic culture conditions. Biomaterials. 2010;31:3858–3867. doi: 10.1016/j.biomaterials.2010.01.090. [DOI] [PubMed] [Google Scholar]
- 110.Betre H, Ong SR, Guilak F, et al. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 111.Koga H, Engebretsen L, Brinchmann JE, et al. Mesenchymal stem cellbased therapy for cartilage repair: a review. Knee Surgery, Sports Traumatology, Arthroscopy. 2009;17:128912–97. doi: 10.1007/s00167-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 112.Jin X, Sun Y, Zhang K, et al. Ectopic neocartilage formation from predifferentiated human adipose derived stem cells induced by adenoviral-mediated transfer of hTGF beta2. Biomaterials. 2007;28:2994–3003. doi: 10.1016/j.biomaterials.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142–150. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- 114.Rodriguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:2167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaustad KG, Boquest AC, Anderson BE, et al. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 116.Planat-Benard V, Menard C, Andre M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 117.van Dijk A, Niessen HW, Zandieh Doulabi B, et al. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell and Tissue Research. 2008;334:457–467. doi: 10.1007/s00441-008-0713-6. [DOI] [PubMed] [Google Scholar]
- 118.Bai X, Pinkernell K, Song YH, et al. Genetically selected stem cells from human adipose tissue express cardiac markers. Biochemical and Biophysical Research Communications. 2007;353:665–671. doi: 10.1016/j.bbrc.2006.12.103. [DOI] [PubMed] [Google Scholar]
- 119.Song YH, Gehmert S, Sadat S, et al. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochemical and Biophysical Research Communications. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 120.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart Journal. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 121.Kang Y, Park C, Kim D, et al. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc Res. 2010;80:310–316. doi: 10.1016/j.mvr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 122.Madonna R, De Caterina R. In vitro neovasculogenic potential of resident adipose tissue precursors. Am J Physiol Cell Physiol. 2008;295C:1271–1280. doi: 10.1152/ajpcell.00186.2008. [DOI] [PubMed] [Google Scholar]
- 123.Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, et al. Human adipose stem cells: A potential cell source for cardiovascular tissue engineering. Cells Tissues Organs. 2008;187:263–274. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- 124.Verseijden F, Posthumus-van Sluijs SJ, Pavljasevic P, et al. Adult human bone marrow- and adipose tissue-derived stromal cells support the formation of prevascular-like structures from endothelial cells in vitro. Tissue Eng Part A. 2010;16:101–114. doi: 10.1089/ten.TEA.2009.0106. [DOI] [PubMed] [Google Scholar]
- 125.Scherberich A, Galli R, Jaquiery C, et al. Threedimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells. 2007;25:1823–1829. doi: 10.1634/stemcells.2007-0124. [DOI] [PubMed] [Google Scholar]
- 126.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 127.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 128.Madonna R, Geng Y-J, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1723–1729. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 129.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 130.De Francesco F, Tirino V, Desiderio V, et al. Human CD34+/CD90+ ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE. 2009;4:e6537. doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muller AM, Mehrkens A, Schafer DJ, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–135. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 132.Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 133.Ng AM, Saim AB, Tan KK, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci. 2005;10:192–199. doi: 10.1007/s00776-004-0884-2. [DOI] [PubMed] [Google Scholar]
- 134.Okura H, Komoda H, Fumimoto Y, et al. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs. 2009;12:123–130. doi: 10.1007/s10047-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 135.Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58:570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 136.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010;120:125–131. doi: 10.1002/lary.20719. [DOI] [PubMed] [Google Scholar]
- 137.Nakada A, Fukuda S, Ichihara S, et al. Regeneration of central nervous tissue using a collagen scaffold and adipose-derived stromal cells. Cells Tissues Organs. 2009;190:326–335. doi: 10.1159/000223233. [DOI] [PubMed] [Google Scholar]
- 138.Ryu HH, Lim JH, Byeon YE, et al. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang HM, Kim J, Park S, et al. Insulin-secreting cells from human eyelid-derived stem cells alleviate type I diabetes in immunocompetent mice. Stem Cells. 2009;27:1999–2008. doi: 10.1002/stem.127. [DOI] [PubMed] [Google Scholar]
- 140.Lee J, Han DJ, Kim SC. In vitro differentiation of human adipose tissue-derived stem cells into cells with pancreatic phenotype by regenerating pancreas extract. Biochemical and Biophysical Research Communications. 2008;375:547–551. doi: 10.1016/j.bbrc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 141.Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 142.Talens-Visconti R, Bonora A, Jover R, et al. Hepatogenic differentiation of human mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells. World Journal of Gastroenterology. 2006;12:5834–5845. doi: 10.3748/wjg.v12.i36.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dhar S, Yoon ES, Kachgal S, et al. Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Engineering. 2007;13:2625–2632. doi: 10.1089/ten.2007.0017. [DOI] [PubMed] [Google Scholar]
- 144.Aluigi MG, Coradeghini R, Guida C, et al. Preadipocytes commitment to neurogenesis 1: preliminary localisation of cholinergic molecules. Cell Biology International. 2009;33:594–601. doi: 10.1016/j.cellbi.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 145.Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplantation Proceedings. 2008;40:1135–1139. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 146.Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Diseases of the Colon and Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 147.Guilak F. Functional tissue engineering: the role of biomechanics in reparative medicine. Annals of the New York Academy of Sciences. 2002;961:193–195. doi: 10.1111/j.1749-6632.2002.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 148.Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Seminars in Cell & Developmental Biology. 2009;20:646–655. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stillaert FB, Di Bartolo C, Hunt JA, et al. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29:3953–3959. doi: 10.1016/j.biomaterials.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 150.Goudenege S, Pisani DF, Wdziekonski B, et al. Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of myod. Mol Ther. 2009;17:1064–1072. doi: 10.1038/mt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kang SK, Putnam LA, Ylostalo J, et al. Neurogenesis of rhesus adipose stromal cells. J Cell Sci. 2004;117:4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- 152.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 153.Safford KM, Safford SD, Gimble JM, et al. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 154.Grimes BR, Steiner CM, Merfeld-Clauss S, et al. Interphase FISH demonstrates that human adipose stromal cells maintain a high level of genomic stability in long-term culture. Stem Cells and Development. 2009;18:717–724. doi: 10.1089/scd.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Research. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]