Abstract
The human vagina constitutes a complex ecosystem created through relationships established between host mucosa and bacterial communities. In this ecosystem, classically defined strict bacterial aerobes and anaerobes thrive as communities in the microaerophilic environment. Levels of CO2 and O2 present in the vaginal lumen are impacted by both the ecosystem’s physiology and the behavior and health of the human host. Study of such complex relationships requires controlled and reproducible causational approaches that are not possible in the human host that, until recently, was the only place these intact bacterial communities thrived. To address this need we have utilized our ex vivo human vaginal mucosa culture system to support controlled, reproducible colonization by vaginal microbiomes (VMB) collected from healthy and symptomatic donors. Parallel vaginal epithelial cells (VEC)-VMB co-cultures were exposed to increasingly microaerophilic conditions to study the impact of CO2 concentrations upon the anaerobic bacteria associated with dysbiosis and inflammation. Our data suggest that in the context of intact VMBs, increased CO2 concentrations favored specific lactobacilli species defined as aerobes or microaerophiles when grown as monocultures. The observed community changes also led to shifts in host VEC phenotypes with significant changes in the host transcriptome, including altered expression of select molecular transporter genes. These findings support the need for additional study of the environmental changes associated with behavior and health upon the symbiotic and adversarial relationships that are formed in microbial communities present in the human vaginal ecosystem.
Keywords: vaginal microbiome, Lactobacillus jensenii, Lactobacillus crispatus, microaerophiles, anaerobes, aerobes
1. Introduction
The vaginal microbiome (VMB) plays critical roles in maintaining women’s reproductive and general health [1]. VMB communities are part of a complex ecosystem, the “eco-ome”, that contribute to profound changes of the host mucosa created by the stratified squamous vaginal epithelial cell (VEC) multilayer as well as individual bacterial phenotypes. The bacterial phenotypes are altered by their ongoing survival efforts that can be favored or limited by host changes including the alteration of CO2/O2 levels in the vaginal lumen. During periods of eubiosis, the symbiotic relationship of the VMB and VEC creates optimal carbon supplies for the bacteria and protective chemical and physical barriers for the host to exclude pathogens including bacterial and viral sexually-transmitted infections (STIs) [1]. Conversely, dysbiotic VMB reported to be dominated by expanded populations of anaerobic bacteria, cause inflammation and increased susceptibility to many pathogenic outcomes [2].
VMB dysbiosis can lead to the symptomatic syndrome bacterial vaginosis (BV), the most common bacterial infection of reproductive age women (14–49 years), afflicting 29.2% women overall with a higher prevalence in non-Caucasian women [2–4]. It is becoming increasingly clear that VMB differ among women of different age and racial background but the contributing factors are still being elucidated [5–7]. Categorical schemes have been described to aid identification of associations between specific VMBs with clinical outcomes. Ravel and co-workers used next generation sequencing (NGS) to classify 6 VMB community state types (CSTs) based on the most abundant Lactobacillus species present in the community. Specifically CST I communities are dominated by L. crispatus, CST II by L. gasseri, CST III by L. iners, and CST V by L. jensenii [7]. CST IV communities carry a mixed population of lactobacilli (IVA) or are dominated by anaerobes associated with BV generally lacking a substantial number of lactobacilli (IVB). A more recent schema linked VMB profile with host health state leading to 4 categorical “cervicotypes” (CTs) [8]. For this schema, CT1 showed L. crispatus domination, CT2 was dominated by L. iners, CT3 by Gardnerella spp, and CT4 containing mixed VMB communities that included Prevotella spp [8].
Additional NGS and other ‘omic approaches correlated to clinical datasets have provided associative outcomes that implicate growing lists of environmental, behavioral and genetic confounders influencing the VMB profile [9–13]. Such associations have linked inflammatory profiles and VMBs associated with symptomatic BV dominated by traditionally defined anaerobes including G. vaginalis and Atopobium vaginae suggesting that conditions related to increased CO2 levels in the vaginal lumen may support VMB shifts to inflammatory states and increased susceptibility to STI pathogens. The vaginal lumen is considered a microaerophilic to slightly hypoxic environment that can be impacted by introduction of ambient air (AA) by sexual activity as well as vaginal hygiene methods (e.g. tampon usage, douching, etc.) [14, 15]. The limited published clinical data suggest that typical vaginal lumen CO2 levels are related to the CO2 present in venous blood and are approximately AA supplemented with 5% CO2 [14, 16]. It is likely that CO2 levels are impacted also by both the carbon metabolism of the VEC multilayer as well as the composition of VMB community.
To test the hypothesis that increased CO2 may be a predisposing factor for creating dysbiotic VMBs dominated by anaerobic bacteria, we utilized our ex vivo human vaginal mucosal culture system [17, 18]. The air-interfaced, apical surface of the VEC cultures support colonization with intact VMBs transplanted from samples collected from woman during routine gynecological exams. The system allows controlled manipulation of environmental, genetic and behavioral confounders not easily controlled in clinical research to study traditionally defined aerobic and anaerobic bacteria in the context of a stabilized community exposed to controlled gas mixtures in the created “vaginal lumen”. For our studies, VEC cultures were created with cells from a Caucasian (V19I) or an African American (BVEC02I) donor and colonized with representative VMB under typical (AA+5% CO2 and ~20% O2) and increased CO2 (AA+10%CO2 and 18.9% O2) environments.
VMB shifts created by the increasing CO2 would be predicted to produce compensatory changes in VEC contributions to the eco-ome including differently regulated gene expression. As an initial study of the molecular signals associated with alterations of the vaginal environment, we quantified changes in immune response and molecular transporter gene expression with custom RT-PCR arrays as an extension of our previous work [19]. Collectively, the results from two genetically distinct VEC donors suggest that increasing CO2 in the vaginal lumen favors selected lactobacilli that are defined as microaerophiles or aerobes and are considered probiotic organisms. Interestingly, in dysbiotic VMBs that lacked detectable lactobacilli, G. vaginalis was enhanced by higher CO2 levels. The data suggest that current bacterial species categorization based solely upon single species lab cultivation incubated under aerobic or anaerobic conditions may prove insufficient for predicting behavior in complex communities cultivated on human mucosae.
2. Materials and Methods
2.1 Ethics statement
Collection of vaginal microbiome (VMB) samples from healthy volunteers was approved by the University of Texas Medical Branch’s institutional review board. Adult subjects provided written informed consent during enrollment prior to collection of vaginal swabs. Each sample was provided a unique study number by the clinical team and provided as de-identified material to the research team. VEC cultures were either purchased commercially (V19I) as previously described [18, 20] or established from de-identified, discarded surgical material (BVEC02I) collected during surgical prolapse repair.
2.2 Clinical VMB
Vaginal swabs, collected by clinical staff, were obtained from healthy women during routine gynecological examinations using a sterile calcium alginate swab (Fisherbrand, Pittsburgh, PA) passed over the mid-vaginal wall avoiding external sites via the placement of a standard speculum. The swabs were immediately placed into 2ml of sterile, Ca++/Mg++-free Dulbecco’s Phosphate Buffered Saline (DPBS; Cellgro, Herndon, VA), and transported at 4°C to the lab. The subsequent procedures were all completed in a sterile class II biosafety cabinet. Each swab was thoroughly vortexed to release the collected bacteria and human cells and then aliquoted for DNA and RNA separately into MagNA Pure 96 External lysis buffer (Roche, Basel, Switzerland) for molecular evaluations or mixed with sterile glycerol (10% w/v) as a cryo-protectant for viable VMB community aliquots. All aliquots were stored at −80°C until utilized. DNA or RNA was extracted on an automated MagNA Pure 96 (Roche). VMBs were characterized by customized qPCR array as described previously [17]. Any VMB samples molecularly positive for STI, yeast or Y chromosome were excluded from the study.
2.3 Ex vivo VEC multilayer production and VMB colonization
Immortalized human VEC (V19I and BVEC02I) were cultured as described [20] and used to produce stratified squamous vaginal multilayers [17, 18]. Briefly, VEC from one of 2 donors were plated in 24 (106 cells/transwell) or 96 (105 cells/transwell) well transwell format and cultured at 37°C with 5% CO2. After monolayer formation, each culture was subjected to air-interfacing by removal of the apical growth medium. The multilayers were refed basally every other day with antibiotic- and serum-free medium as described [17] leading to stratification and differentiation of multilayers.
At maturation (7–9 days) VMB were diluted to 103-104 total genomes and applied to the apical surface of the multilayers. VMB were selected from our cryo-repository based on community profiles established from initial swab evaluations. In supplementation studies we used 1,000 cfu of our previous L. jensenii clinical isolate [18]. Parallel triplicate cultures were created and immediately placed in prepared 37°C, humidified incubators containing AA supplemented with either 5% or 10% CO2. The cultures were maintained for 48h before harvesting in MagNA Pure 96 External lysis buffer (Roche). Each study was repeated at least once.
2.4 VMB profiling and VEC molecular transporter gene expression evaluation by custom qPCR array analyses
Half of each lysate was subjected to automated DNA extraction using MagNA Pure 96 DNA and Viral NA Small Volume kit (Roche). Molecular evaluation of VMBs was completed by qPCR array as previously described [17]. RNA extraction was completed using the second half of each cell lysate using the MagNA Pure 96 Cellular RNA large volume kit (Roche) and then converted immediately to cDNA (iScript; Bio-Rad, Hercules, CA). Custom qPCR arrays were built for selected immune response-related genes and molecular transporters (Supplemental Tables 1 and 2) [21–23] and subjected to qPCR with SYBR green detection in CFX amplification systems (Bio-Rad) as described previously [24]. Gene targets were selected following previously identified gene expression in human vaginal mucosa tissues [19]. Melt temperatures for each amplified target were matched to historic controls and genes with melt curves outside ± 0.6 C were eliminated from further analysis. Ct values for triplicate independent replicates were tabulated and genes present 2 out of 3 times were determined as authentic for detection and further analysis. Expression levels were normalized to 3 housekeeping genes (C1orf4, CHMP2A and PSMB2) [23]. After normalization, the standardized delta delta Ct algorithm was used to compare gene expression levels. Statistical significance was determined using t-tests and P values of ≤ 0.05 were concluded as significant. Heat map clustering analyses were performed using QCanvas [25]. Input data were scaled to a maximum of ±10-fold change for visualization.
2.5 Statistics
All experiments were repeated at least once. A minimum of 2 representative VMB for each dominant lactobacilli or dysbiotic VMBs were cultivated in triplicate for each environmental condition. Most data are presented as averaged proportions of targeted bacterial species within the community. Replicate data was used to calculate average absolute genomic copies in parallel 5% and 10% CO2 groups and statistical significance was determined using Student’s t test with P<0.05 considered significant. Statistical evaluations and proportional graphing were completed with Prism v7.0a (GraphPad, San Diego, CA).
3. Results
3.1 Increased CO2 shifts VMB community profiles
The impact of a variety of activities like intercourse, douching, diet, and menstruation alter the vaginal ecosystem producing potential changes in the physiology of both bacteria and VEC that in turn further alter health status [26–28]. To begin the study of these complex interactions we have assessed the impact of the fluctuation of the gaseous composition in the vaginal lumen. The impact of changing O2/CO2 ratios on VMB profiles was measured using our ex vivo culture system with V19I VEC multilayers colonized with selected VMBs. Initial evaluations of 25 distinct VMB were completed to uncover potential trends in community alteration. These studies included at least 2 representatives for each lactobacilli-dominated and dysbiotic community that were cultivated in the presence of either 5 or 10% CO2 supplemented AA in triplicate cultures. Surprisingly, these initial studies illustrated that increased luminal CO2 favored selected lactobacilli and suggested a hierarchy in this genus that was contrary to the aerobic categorization of their cultivation as individual species in the laboratory. Further, this enhancement was associated with reductions in bacteria categorized as strict anaerobes in selected communities (e.g. G. vaginalis).
Representative VMB were selected to complete focused confirmatory analyses designed to identify the Lactobacillus spp that were most or least favored by increased CO2. Absolute abundance values for selected organisms within communities cultivated in parallel at 5% or 10% supplemented CO2 were used to establish average abundance and compared statistically to identify significantly increased titers of lactobacilli in general and L. crispatus and L. jensenii specifically (Table 1; P<0.05). There were general trends across multiple VMB that did not achieve significance indicating increases in L. gasseri were common to VMB lacking L. jensenii or L. crispatus. L. iners and G. vaginalis were suppressed by cultivation at AA+10% CO2 in VMB that had L. jensenii or L. crispatus (Table 1).
Table 1.
Absolute abundance comparisons of selected bacteria cultivated in VMB communities at AA+5 or 10% CO2
| AA+5% CO2 | AA+10% CO2 | p value* | |||
|---|---|---|---|---|---|
| V19I VEC | |||||
| Ava. abundance** | SDEV | Avg. abundance | SDEV | ||
| Lactobacillus spp | 4.88 | 5.22 | 5.45 | 5.53 | 0.04 |
| L. crispatus | 3.45 | 3.41 | 4.19 | 4.31 | 0.003 |
| L. jensenii | 3.98 | 4.18 | 4.91 | 5.04 | 0.022 |
| L. gasseri | 4.16 | 4.56 | 4.65 | 4.83 | 0.26 |
| L. iners | 4.35 | 4.97 | 4.10 | 4.24 | 0.6 |
| G. vaginalis | 4.13 | 4.05 | 4.24 | 4.3 | 0.51 |
| BVEC02I VEC | |||||
| Ava. abundance | SDEV | Avg. abundance | SDEV | ||
| Lactobacillus spp | 4.87 | 4.67 | 5.16 | 5.06 | 0.3 |
| L. crispatus | 4.23 | 3.72 | 4.51 | 4.49 | 0.14 |
| L. jensenii | 4.58 | 4.47 | 5.13 | 4.8 | 0.0001 |
| L. gasseri | 3.84 | 3.79 | 4.38 | 4.6 | 0.3 |
| L. iners | 3.3 | 3.26 | 3.68 | 3.6 | 0.09 |
| G. vaginalis | 3.96 | 3.42 | 4.12 | 3.65 | 0.01 |
Absolute abundance values for genomic titers of each indicated bacteria were established by qPCR and averaged from the 25 tested VMB. Only positive values were considered.
p values were established by Student’s T test. P<0.05 are indicated in bold
Absolute abundance averages and standard deviations are provided in log10
To illustrate the impact of elevated CO2 on representative VMB, proportional bar charts are presented in Figure 1. The first VMB that was dominated by L. crispatus but also contained a substantial proportion of G. vaginalis illustrates the impact of 10% CO2 that led to L. crispatus enhancement while G. vaginalis loads were reduced (Fig. 1A VMB1 bright blue v bright red color blocks). A second VMB dominated by L. gasseri also with a large portion of G. vaginalis, again showed reduction of G. vaginalis and an expansion of L. gasseri dominance in high CO2 relative to 5% supplementation (Fig. 1A; VMB2 turquoise v bright red). VMB3 was selected to illustrate a community dominated by L. iners at 5% supplemented CO2 (Fig. 1A; orchid) that lacked other lactobacilli. Remarkably, L. iners, categorized as an anaerobic vaginal lactobacillus when grown as a single species [29, 30], was not enhanced by increasing the CO2 levels showing a marked reduction in proportion (Fig. 1A VMB3). In this community, G. vaginalis became the dominant organism suggesting this VMB transitioned towards dysbiosis in the higher CO2 environment. A second VMB dominated by L. iners but containing small proportions of both L. crispatus and L. jensenii was tested (VMB4). At 10% CO2, this community also showed marked reduction in L. iners and a coincident expansion of both L. crispatus and L. jensenii (Fig. 1A VMB4). In fact, VMBs in our panel of 25 that were dominated by or contained measurable L. iners (n=7) consistently experienced a reduction in this species during cultivation in V19I cultures at 10% CO2.
Figure 1. VMB community profiles changed in response to increased luminal CO2 in the VEC19I vaginal mucosa model.

To determine the effects higher CO2 conditions have on the VMB profile, selected clinical VMBs were transplanted to matured V19I multilayer cultures and cultivated in either 5% or 10% CO2 supplemented AA. (A) Paired proportional bar charts, established by qPCR array, revealed the impact of increased CO2 (5, left bar or 10%, right bar). Native Lactobacillus spp dominated over anaerobic BV-associated bacterial species growth in all VMB (VMB1, VMB2, VMB5, VMB6) with the exception of L. iners (VMB3, VMB4). (B) Eubiotic (VMB7) or dysbiotic (VMB1) bacterial communities that lacked L. jensenii were supplemented with 103 L. jensenii CFU prior to application to sterile V19I multilayers. The resulting community profiles confirmed L. jensenii was favored over native Lactobacillus spp in AA+10% CO2.
To confirm the trends observed with the microaerophilic lactobacilli and begin to develop a hierarchy for CO2 enhancement, VMB5 was selected as a representative dysbiotic community that was dominated by G. vaginalis but contained small proportions of L. crispatus (2%), L. iners (10%) and L. jensenii (14%) at 5% CO2. When cultivated with 10% CO2 supplementation, VMB5 showed increases in the proportion of L. jensenii and a non-significant increase in L. crispatus. Coincident contractions of G. vaginalis and L. iners were observed as well (Fig. 1A). Expansion of L. jensenii was also illustrated in the VMB2 community where a significant 13-fold increase was detected (P=0.029) indicating this species was the most affected by increased CO2 regardless of community profile. VMB6, dominated by L. jensenii, showed proportions of 97.8% at 5% CO2 and 99.2% in the higher CO2 illustrating the consistent dominance of this species in multiple community types (Fig. 1A). Across all tested VMB including those that were already dominated by L. jensenii, an average 8-fold increase in absolute abundance was observed (Table 1, P=0.022).
As a final confirmation of the dominance of L. jensenii under higher CO2 conditions, healthy (VMB7) and dysbiotic (VMB1) communities that lacked detectable L. jensenii were selected for exogenous supplementation with a clinical L. jensenii isolate (1,000 cfu) [18]. In both VMB, the exogenous L. jensenii became the dominant organism after 48 hours in 5% CO2 conditions (Fig. 1B). At 10% CO2, the non-native, exogenous L. jensenii expanded from 74.1% to 86.5 % in the healthy and from 70% to 92% in the dysbiotic VMBs tested (Fig. 1B). Both native VMB contained proportions of L. crispatus that also expanded after cultivation in higher CO2 levels as seen in previous studies but failed to surpass the growth of the exogenous L. jensenii (Fig. 1B).
3.2 Increased CO2 enhanced L. jensenii in African American VEC but other vaginal bacteria were differently impacted
Current clinical data associate African lineages with VMBs that have reduced Lactobacillus content and higher proportions of anaerobes [7, 31]. Utilizing VEC from genetically distinct donors, the multilayer culture system offers the opportunity to establish distinct eco-omes to directly compare VMB community formation. For these studies, we compared the data from VMB created in either V19I VEC (Caucasian; haplogroup T2b) or BVEC02I cells from a distinct donor (African American; haplogroup L1b1). The same clinical VMBs were used to colonize BVEC02I VEC with the exception of one community (VMB1) that was no longer available and was substituted with a similar community (VMB8).
In contrast to the consistent contraction of L. iners proportions observed in V19I VEC, BVEC02I VEC produced VMB communities with enhanced L. iners proportions at higher CO2 levels (Fig. 2A VMB8, VMB3 and VMB4). The trend was consistent but did not achieve statistical significance across the tested VMB (Table 1, P=0.09). The marked increase in L. iners in these three VMB included both eubiotic (VMB8 and 4) and dysbiotic (VMB 3) communities. Consistent with the results in V19I, BVEC02I VEC also supported a dominant expansion of L. jensenii at the 10% CO2 supplementation. Across the tested VMB, an average 3.5-fold increase in L. jensenii was observed (Table 1; P=0.0001). VMB2, 5 and 6 communities cultivated in BVEC02I cultures produced similar L. jensenii proportions to those observed in V19I at AA+10% CO2 (Fig. 1A vs. 2B). In BVEC02I cultures, VMB5 also illustrated an increase in L. crispatus consistent with the outcomes in V19I during cultivation in 10% CO2. However, the average increase of 2-fold across the tested VMB was not significant (Table 1, P=0.14).
Figure 2. VMB community profiles established in BVEC02I cultures.
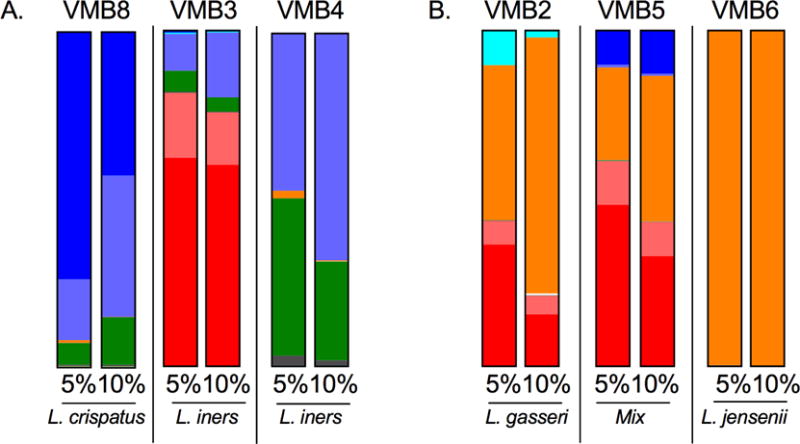
VMB communities were cultivated in BVEC02I ecosystems with AA+5 or 10% luminal CO2 as shown in Figure 1. The same VMB were tested with the exception of VMB8 that was substituted for VMB1 because of its similar profile. (A) VMB with native L. iners with or without G. vaginalis illustrated the observed expansion of L. iners in 10% CO2. (B) VMB with native L. jensenii confirmed that results in V19I cultures supporting the conclusion that this lactobacilli was most enhanced by elevated luminal CO2. The color legend is depicted in Fig. 1.
3.3 Luminal CO2 VMB shifts produced VEC transcription changes in immune response genes and molecular transporters
VMB shifts are known to cause changes in the inflammatory state and overall health of the vaginal mucosa [8]. An inflammatory state can cause tissue damage and create disruptions in the vaginal mucosa leaving women more susceptible to STIs like HIV [32–34]. These same conditions also appear to impact the efficacy of topically applied antimicrobials [17, 34, 35]. To begin to elucidate the molecular mechanisms associated with compensatory changes in the ecosystem, we exploited the shifted VMB outcomes to study associated differences in expression of selected genes related to immune responses and molecular transport. RNA from triplicate cultures was harvested from sterile V19I VEC or multilayers colonized with VMB1 or VMB6 with or without L. jensenii supplementation that were cultivated in 5% or 10% CO2. The RNA was converted to cDNA and subjected to custom RT-PCR arrays to quantify changes in expression of selected immune response genes and molecular transporters (Supplemental Tables 1 and 2). After normalization the fold change expression patterns were statistically compared and selected significant changes are described below and in Figures 3 and 4. Genes that were impacted by CO2 in sterile cultures were first identified and then eliminated from further consideration. Remaining targets were used to create baseline gene expression values for sterile cultures at 5% and 10% supplemented CO2 and used as comparators for parallel cultures created with VMB with or without L. jensenii supplementation as shown in Fig. 1B. Up-regulated expression is depicted on the heat map in red, down regulation by green and average fold change is shown in the table.
Figure 3. Clustering and fold change evaluations of selected immune response genes related to the shifted VMB community profiles produced by increased luminal CO2.
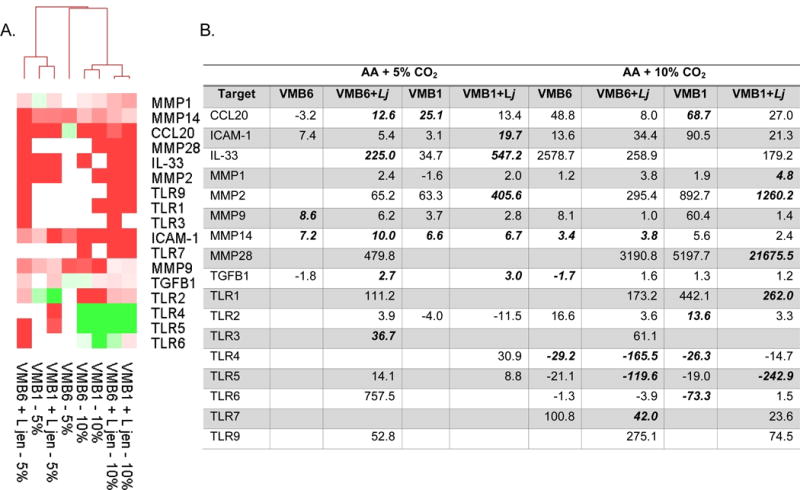
Custom RT-PCR arrays were used to analyze gene expression using delta delta Ct comparisons of sterile multilayers and parallel cultures colonized by the indicated VMB with or without L. jensenii (L jen) supplementation. (A) Heat map relational clustering of genes that had significantly altered average expression (triplicate cultures) for at least one condition are indicated as up-regulated (red) or down-regulated (green) relative to the sterile control baseline expression levels. (B) Average fold change values for the clustered data are provided for those conditions that led to altered gene expression. Values that were significantly different (p<0.05) are bolded. At least 3 values are considered for each condition.
Figure 4. Clustering and fold change evaluations of selected molecular transporter genes related to the shifted VMB community profiles produced by increased luminal CO2.
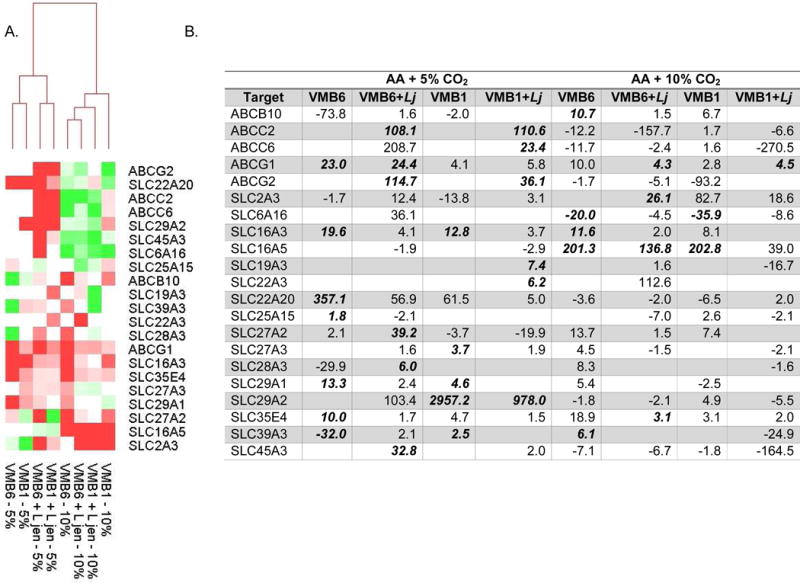
Data for selected molecular transporter genes are presented as described in Fig. 3. (A) Heat map relational clustering illustrated grouping of 5% CO2 conditions regardless of VMB or L. jensenii supplementation. (B) Average fold change values for the clustered data again are provided for those conditions that led to altered gene expression. Values that were significantly different (p<0.05) are bolded. At least 3 values are considered for each condition.
Expression patterns for the immune response and signaling genes impacted by shifted VMB (Fig. 3A) clustered the samples based on CO2 supplementation (5% CO2 outcomes clustered on the left). Genes that were generally up-regulated by any VMB community, regardless of CO2 levels, included MMPs 1, 9, 14 and 28 as well as ICAM-1 (Fig. 3). Interestingly, these genes are associated with epithelial barrier integrity. Immune modifiers, including CCL20, IL33 and TGFB1 also were generally up-regulated when bacterial communities were added to the V19I VEC (Fig. 3). With regard to shifted VMB impacts, Toll-like receptors (TLR) 4, 5 and 6, responsible for recognition of bacterial products, were significantly down-regulated (4- to 242-fold reduced, p<0.05; Fig. 3) following cultivation in 10% CO2 while no change or slight increases were observed in 5% CO2 equivalents. These changes may be attributed to the significant expansion of L. jensenii in these communities and a more probiotic community profile. In contrast, TLR1, 2, 3, 7 and 9 expression levels were either unchanged or significantly increased (24- to 275-fold; p<0.05) in AA+10% CO2 conditions. TLR1 and 3 were most impacted by the VMB6 L. jensenii-dominated community that showed up-regulation at both CO2 concentrations. TLR3, 7 and 9 recognize modified nucleotides and CpG motifs generally related to viral challenge.
Expression of molecular transporter genes, divided into ATP-binding cassette (ABC) and solute carrier (SLC) superfamilies [36, 37], were evaluated using a similar paradigm. In previous work, we employed microarray, qRT-PCR and immunolabeling to evaluate human vaginal tissues and in VEC cultures to identify the subset of ABC and SLC transporters that were expressed [1, 19]. Selected vaginally-expressed transporter genes were evaluated in cultures with shifted VMB profiles created by CO2 supplementation in the VEC model. Genes impacted by elevated CO2 in sterile cultures were eliminated from additional evaluation and then clustered creating a clean separation of samples based on CO2 levels (Fig. 4).
Relative to sterile cultures, three transporters, ABCG1, SLC16A3 and SLC35E4 were consistently upregulated by the presence of VMB bacteria regardless of community or CO2 supplementation (Fig. 4). Eight transporters were consistently down-regulated in the presence of VMB communities at 10% CO2 in contrast to 5% CO2 where they were up-regulated or unchanged relative to sterile baselines (top half of the heat map, Fig. 4). These included ABCC2, C6 and G2 that encode proteins (MRP2, MRP6 and a “White family xenobiotic transporter”, respectively) involved in multidrug resistance and movement of antimicrobials and xenobiotics [37–40]. SLC6A16, 22A20, 25A15, 29A2 and 45A3 were down regulated in AA+10% CO2 and generally up regulated in 5% CO2 relative to sterile baselines (Fig. 4). These genes encode proteins associated with transport of organic anions. Of interest to the vaginal mucosa, SLC22A20 encodes a protein (OAT6) that transports estrone sulfate [41]. SLC29A2 encodes HNP36 involved in nucleotide transport including nucleoside analogs employed as antivirals [42, 43]. VMB shifts to L. jensenii dominated communities also led to the increased expression on SLC16A5, a gene that encodes a transporter of monocarboxylates including lactate and pyruvate [44]. Collectively these data illustrate the potential of the system to elucidate the molecular mechanisms in the changing eco-ome associated with VMB communities shifted by controlled luminal CO2.
4. Discussion
Human vaginal bacterial communities function as part of a complex ecosystem important to maintaining reproductive health and serving as a first line defense against STI pathogens [31]. Several studies have defined the bacterial profile of common VMB in women [6, 7, 45] producing data identifying factors that influence typical community composition including age, race and health status [7, 46]. Additional clinical data illustrate VMB communities are disrupted through sexual intercourse, menstruation, douching, diet, infection, antibiotic therapy, hormonal changes and other yet to be defined factors [26–28] emphasizing the complex interplay of the bacterial community, the mucosa status and vaginal lumen changes. In this regard, clinical data from 1984 suggested the vaginal environment is disrupted by tampon insertion that may introduce AA into the vaginal cavity enriching O2 levels [15]. However, a more recent report described increased CO2 levels with coincident decreased O2 as the tampon resides in the vaginal cavity [14]. These data led to a hypothetical association between altered CO2/O2 levels and pathogenic bacterial growth [15] but causal studies were hindered by limited modeling capabilities and clinical research approaches confounded by human genetics and behavior. Our ex vivo vaginal model system allowed the controlled study of increasing CO2 in the simulated vaginal lumen measuring impacts on both VMB community profiles and the phenotype of cultured vaginal mucosa.
Parallel cultivation of transplanted VMB representing eubiotic and dysbiotic communities in 2 genetic backgrounds provided unexpected results that were contrary to predictions based on the categorization of individual species of bacteria. Specifically, in the context of the vaginal eco-ome, elevated CO2 conditions favored aerobes and microaerophiles considered to be probiotic rather than strict anaerobes associated with BV and inflammation [1]. Such definitions are challenged generally when species are considered in the context of complex communities that thrive in microaerophilic environments such as the vaginal vault. Recent work from Srinivasan and colleagues has begun to enhance lab based cultivation systems without human cells that will help to clarify the nature of the bacterial community members as individual species [30].
The data generated in our ex vivo system also support the conclusion that community-based relationships alter the behavior of individual species relative to lab cultivation. Such physiologic changes allow strict anaerobes and aerobes to thrive in microaerophilic environments including the vagina. Some in vitro studies support this finding given that culture conditions affect the production of metabolites and antimicrobials associated with vaginal health such as H2O2 and lactic acid [47–49]. Understanding the impact of species relationships in the VMB will be a crucial step forward in the development of optimized probiotic approaches that have had mixed results to date [47].
Our data show the VMB can be shifted in response to potential vaginal environmental changes such as fluctuations in O2 and CO2 and that these responses vary between our two VEC genetic backgrounds. In both donor cultures L. jensenii was expanded consistently and significantly during cultivation with increased luminal CO2 in diverse eubiotic and dysbiotic VMB communities. This organism, defined as a microaerophile, has been studied extensively for probiotic contributions to vaginal health [50] and was tested as a probiotic supplement in a number of published studies [50–53]. L. crispatus also showed enhancement in elevated CO2 conditions but was surpassed by native or exogenous L. jensenii. We previously established that these organisms, as single additives, tempered inflammatory responses in the VEC culture system [18]. L. gasseri trended towards enhanced growth in selected VMBs but was not increased significantly if either of the other lactobacilli were present in the community.
Although both VEC types favored lactobacilli in general, there were distinct outcomes consistent with clinical data suggesting African genetic lineages support VMB that are dominated by L. iners and bacterial anaerobes [6, 7, 26, 31, 45]. Our limited data support this clinical finding and add validity to the ex vivo model’s predictions and utility. In the VEC-VMB culture system, while there were similarities in the responses of the two tested haplogroups there also were differences in the favored microbiota. Specifically, BVEC02I eco-omes favored L. iners and G. vaginalis in general and at higher luminal CO2; these same bacteria were generally inhibited in the V19I cultures. The trends in these initial experiments support the need for additional studies of VMBs representing distinct communities to better predict optimal probiotic approaches that may need to be tailored to genetic differences. The data suggest increased luminal CO2 and the associated molecular changes might enhance supplementation by probiotic lactobacilli that often are excluded by established communities [47, 49, 54].
Bacterial products and metabolites have been the focus of research efforts to identify biomarkers and molecular mechanisms associated with health and inflammation [50]. Metabolomic and proteomic approaches have identified a number of odorants and enzymatic activities that support clinical diagnostics of BV (e.g. BV Blue® sialidase detection and the Whiff test in Amsel’s criteria). These molecules also serve to alter the ecosystem in undefined ways associated with VMB shifts and vaginal mucosal changes. Utilizing the ex vivo model system, we completed gene expression studies to establish if transcription changes in the VEC could be specifically attributed to shifted VMB profiles caused by the elevated luminal CO2. Using customized PCR arrays developed during our previous transcriptomic studies of vaginal mucosa [19] we identified a set of immune response genes including targets related to epithelial barrier integrity and pathogen recognition. Consistent with our findings that L. jensenii tempered inflammation [18], VMB shifts that led to expansion of this probiotic organism significantly decreased expression of TLRs 4, 5 and 6 that recognize pathogenic bacterial products [20].
The underlying molecular changes that affect efficacy of vaginally applied drugs remain unclear but likely involve altered movement of drug from the lumen to deeper cell layers and intracellular locations targeted by pathogens. Molecular transporters collectively mobilize metabolites, signaling molecules and carbon sources to and from the mucosa and, until recently, had not been characterized in the vaginal tract [19, 36, 37, 55]. In fact, molecular transporter expression patterns produced from vaginal ecosystems may help to explain differences in clinical results for vaginally-delivered antiretroviral therapies [1, 19, 36, 37, 56–58]. Consistent with previous data suggesting VMB impacted the efficacy of tenofovir against HIV-1 [17] we found ABC and SLC transporters were impacted by the VMB shifts associated with elevated CO2. Collectively the results for the selected molecular transporters suggested biomarkers for physiological changes will be identified through such studies and can be used to predict the physiological and metabolic changes that would favor probiotic therapies. Such approaches also could enhance vaginally-applied drugs and vaccines.
Highlights.
The human vagina is a complex ecosystem easily influenced by external stimuli.
Environmental and behavioral factors shift vaginal bacterial communities.
Increased luminal CO2 in the vagina favors lactobacilli over anaerobic bacteria.
Microbiome shifts result in differential transporter and immune gene expression.
Acknowledgments
The authors thank Jeanne Marrazzo, M.D. of Harborview Medical Center, Seattle, WA for creative discussions and suggestions. The authors also thank the UTMB clinical research group who supported collection of the VMB samples.
Funding statement
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U19AI113048 and R01AI100744. The authors also acknowledge support from the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pyles RB, Moss JA, Baum MM. Vaginal Mucosal HIV Prep: Fundamental Insights and Practical Considerations. In: ur Rahman Atta., editor. Frontiers in Clinical Drug Research: HIV. 2. Oak Park, IL: Bentham Science Publishers; 2015. pp. 33–165. [Google Scholar]
- 2.Marrazzo JM. Interpreting the epidemiology and natural history of bacterial vaginosis: are we still confused? Anaerobe. 2011;17(4):186–90. doi: 10.1016/j.anaerobe.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 4.Sobel JD. What’s new in bacterial vaginosis and trichomoniasis? Infect Dis Clin North Am. 2005;19(2):387–406. doi: 10.1016/j.idc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Forney LJ, Gajer P, Williams CJ, Schneider GM, Koenig SS, McCulle SL, et al. Comparison of self-collected and physician-collected vaginal swabs for microbiome analysis. J Clin Microbiol. 2010;48(5):1741–8. doi: 10.1128/JCM.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1(2):121–33. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 7.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–76. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris MJ, Norori J, Zozaya-Hinchliffe M, Martin DH. Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J Clin Microbiol. 2007;45(3):1016–8. doi: 10.1128/JCM.02085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 11.Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Delanghe J, Van Simaey L, et al. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstraelen H, Verhelst R, Claeys G, Temmerman M, Vaneechoutte M. Culture-independent analysis of vaginal microflora: the unrecognized association of Atopobium vaginae with bacterial vaginosis. Am J Obstet Gynecol. 2004;191(4):1130–2. doi: 10.1016/j.ajog.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Yeoman CJ, Thomas SM, Miller ME, Ulanov AV, Torralba M, Lucas S, et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One. 2013;8(2):e56111. doi: 10.1371/journal.pone.0056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill DR, Brunner ME, Schmitz DC, Davis CC, Flood JA, Schlievert PM, et al. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J Appl Physiol (1985) 2005;99(4):1582–91. doi: 10.1152/japplphysiol.01422.2004. [DOI] [PubMed] [Google Scholar]
- 15.Wagner G, Bohr L, Wagner P, Petersen LN. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am J Obstet Gynecol. 1984;148(2):147–50. doi: 10.1016/s0002-9378(84)80165-9. [DOI] [PubMed] [Google Scholar]
- 16.Wagner G, Ottesen B. Vaginal physiology during menstruation. Ann Intern Med. 1982;96(6 Pt 2):921–3. doi: 10.7326/0003-4819-96-6-921. [DOI] [PubMed] [Google Scholar]
- 17.Pyles RB, Vincent KL, Baum MM, Elsom B, Miller AL, Maxwell C, et al. Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One. 2014;9(3):e93419. doi: 10.1371/journal.pone.0093419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose WA, 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One. 2012;7(3):e32728. doi: 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunawardana M, Mullen M, Moss JA, Pyles RB, Nusbaum RJ, Patel J, et al. Global expression of molecular transporters in the human vaginal tract: implications for HIV chemoprophylaxis. PLoS One. 2013;8(10):e77340. doi: 10.1371/journal.pone.0077340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59(3):212–24. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 21.Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15(44):5549–57. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santiago GL, Deschaght P, El Aila N, Kiama TN, Verstraelen H, Jefferson KK, et al. Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am J Obstet Gynecol. 2011;204(5):450 e1–7. doi: 10.1016/j.ajog.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg E, Levanon EY. Human housekeeping genes, revisited. Trends Genet. 2013;29(10):569–74. doi: 10.1016/j.tig.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 24.McGowin CL, Whitlock GC, Pyles RB. High-throughput multistrain polymerase chain reaction quantification of Chlamydia trachomatis from clinical and preclinical urogenital specimens. Diagn Microbiol Infect Dis. 2009;64(2):117–23. doi: 10.1016/j.diagmicrobio.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim N, Park H, He N, Lee HY, Yoon S. QCanvas: An Advanced Tool for Data Clustering and Visualization of Genomics Data. Genomics Inform. 2012;10(4):263–5. doi: 10.5808/GI.2012.10.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002;40(6):2147–52. doi: 10.1128/JCM.40.6.2147-2152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Culhane JF, Rauh V, McCollum KF, Elo IT, Hogan V. Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am J Obstet Gynecol. 2002;187(5):1272–6. doi: 10.1067/mob.2002.127311. [DOI] [PubMed] [Google Scholar]
- 28.Smith CB, Noble V, Bensch R, Ahlin PA, Jacobson JA, Latham RH. Bacterial flora of the vagina during the menstrual cycle: findings in users of tampons, napkins, and sea sponges. Ann Intern Med. 1982;96(6 Pt 2):948–51. doi: 10.7326/0003-4819-96-6-948. [DOI] [PubMed] [Google Scholar]
- 29.Falsen E, Pascual C, Sjoden B, Ohlen M, Collins MD. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol. 1999;49(Pt 1):217–21. doi: 10.1099/00207713-49-1-217. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan S, Munch MM, Sizova MV, Fiedler TL, Kohler CM, Hoffman NG, et al. More Easily Cultivated Than Identified: Classical Isolation With Molecular Identification of Vaginal Bacteria. J Infect Dis. 2016;214(Suppl 1):S21–8. doi: 10.1093/infdis/jiw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. 2012;160(4):267–82. doi: 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev. 2013;37(5):762–92. doi: 10.1111/1574-6976.12029. [DOI] [PubMed] [Google Scholar]
- 33.Spear GT, St John E, Zariffard MR. Bacterial vaginosis and human immunodeficiency virus infection. AIDS Res Ther. 2007;4:25. doi: 10.1186/1742-6405-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, et al. Microbiome Composition and Function Drives Wound-Healing Impairment in the Female Genital Tract. PLoS Pathog. 2016;12(9):e1005889. doi: 10.1371/journal.ppat.1005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravel J, Gajer P, Fu L, Mauck CK, Koenig SS, Sakamoto J, et al. Twice-daily application of HIV microbicides alter the vaginal microbiota. MBio. 2012;3(6) doi: 10.1128/mBio.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim RB. Drug transporters in HIV Therapy. Top HIV Med. 2003;11(4):136–9. [PubMed] [Google Scholar]
- 37.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Prochazkova J, Lanova M, Pachernik J. Multidrug resistance-associated ABC transporters - too much of one thing, good for nothing. Biomol Concepts. 2012;3(4):319–31. doi: 10.1515/bmc-2012-0006. [DOI] [PubMed] [Google Scholar]
- 39.Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, et al. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279(1):74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20) Am J Physiol Renal Physiol. 2006;291(2):F314–21. doi: 10.1152/ajprenal.00497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuchi Y, Furihata T, Hashizume M, Iikura M, Chiba K. Characterization of ribavirin uptake systems in human hepatocytes. J Hepatol. 2010;52(4):486–92. doi: 10.1016/j.jhep.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Guallar JP, Cano-Soldado P, Aymerich I, Domingo JC, Alegre M, Domingo P, et al. Altered expression of nucleoside transporter genes (SLC28 and SLC29) in adipose tissue from HIV-1-infected patients. Antivir Ther. 2007;12(6):853–63. [PubMed] [Google Scholar]
- 44.Murakami Y, Kohyama N, Kobayashi Y, Ohbayashi M, Ohtani H, Sawada Y, et al. Functional characterization of human monocarboxylate transporter 6 (SLC16A5) Drug Metab Dispos. 2005;33(12):1845–51. doi: 10.1124/dmd.105.005264. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(Pt 8):2565–73. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 46.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1(1):29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet. 2014;289(3):479–89. doi: 10.1007/s00404-013-3064-9. [DOI] [PubMed] [Google Scholar]
- 48.Heczko PB, Tomusiak A, Adamski P, Jakimiuk AJ, Stefanski G, Mikolajczyk-Cichonska A, et al. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double-blind, placebo-controlled trial. BMC Womens Health. 2015;15:115. doi: 10.1186/s12905-015-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79–86. doi: 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed] [Google Scholar]
- 50.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutt P, Lapp E, Stsepetova J, Smidt I, Taelma H, Borovkova N, et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb Ecol Health Dis. 2016;27:30484. doi: 10.3402/mehd.v27.30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin R, Sanchez B, Urdaci MC, Langella P, Suarez JE, Bermudez-Humaran LG. Effect of iron on the probiotic properties of the vaginal isolate Lactobacillus jensenii CECT 4306. Microbiology. 2015;161(Pt 4):708–18. doi: 10.1099/mic.0.000044. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto HS, Xu Q, Fichorova RN. Homeostatic properties of Lactobacillus jensenii engineered as a live vaginal anti-HIV microbicide. BMC Microbiol. 2013;13:4. doi: 10.1186/1471-2180-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mastromarino P, Hemalatha R, Barbonetti A, Cinque B, Cifone MG, Tammaro F, et al. Biological control of vaginosis to improve reproductive health. Indian J Med Res. 2014;140(Suppl):S91–7. [PMC free article] [PubMed] [Google Scholar]
- 55.Brown KC, Paul S, Kashuba AD. Drug interactions with new and investigational antiretrovirals. Clin Pharmacokinet. 2009;48(4):211–41. doi: 10.2165/00003088-200948040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou T, Hu M, Cost M, Poloyac S, Rohan L. Short communication: expression of transporters and metabolizing enzymes in the female lower genital tract: implications for microbicide research. AIDS Res Hum Retroviruses. 2013;29(11):1496–503. doi: 10.1089/AID.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51(5):546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21(14):1899–907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]


