Abstract
Obesity and obstructive sleep apnea (OSA) tend to co-exist. Little is known about the effects of OSA, obesity, or their treatment on central aortic pressures and large artery stiffness. We randomized 139 adults with obesity (BMI>30 kg/m2) and moderate-to-severe-OSA to: 1) CPAP therapy (n=45); 2) weight loss (WL) therapy (n=48); or 3) combined CPAP and WL (n=46) for 24 weeks. We assessed the effect of these interventions on central pressures and carotid-femoral pulse wave velocity (CF-PWV; a measure of large artery stiffness), measured with arterial tonometry. Central systolic pressure was reduced significantly only in the combination arm (−7.4 mmHg, 95%CI: −12.5 to −2.4 mmHg; P=0.004), without significant reductions detected in either the weight loss-only (−2.3 mmHg; 95%CI: −7.5 to 3.0, P=0.39) or the CPAP-only (−3.1 mmHg; 95%CI:−8.3 to 2.0, P=0.23) arms. However, none of these interventions significantly changed central pulse pressure, pulse pressure amplification or the central augmentation index. The change in mean arterial pressure (P=0.008) and heart rate (P=0.027) induced by the interventions were significant predictors of the change in CF-PWV. However, after adjustment for mean arterial pressure and heart rate, no significant changes in CF-PWV were observed in any group. In obese subjects with OSA, combination therapy with WL and CPAP is effective in reducing central systolic pressure. However, this effect is largely mediated by changes in mean, rather than central pulse pressure. WL and CPAP, alone or in combination, did not reduce large artery stiffness in this population.
Keywords: obstructive sleep apnea, obesity, blood pressure, central hemodynamics, arterial stiffness
Introduction
Obstructive sleep apnea (OSA) is associated with an elevated risk of cardiovascular disease.1,2 Blood pressure is an important determinant of cardiovascular risk. Prior studies have examined the effects of continuous positive airway pressure (CPAP) therapy or weight loss on blood pressure independently in both observational and randomized controlled settings. However, they were not designed to account for the comparative or simultaneous effect of these interventions.3–11 Accounting for the effect of OSA versus obesity is important, given that OSA and obesity are strongly associated and often have overlapping associations with cardiovascular risk factors. We recently reported the primary results of the Cardiovascular effects of Obstructive Sleep Apnea (COSA) trial, which aimed to assess the independent effect of obesity and OSA on various cardiovascular risk factors in an experimental design.12 In this, cardiovascular effects of sleep apnea (COSA) trial, subjects with moderate-severe OSA were randomized to weight loss (WL) alone, CPAP alone, or combined therapy with CPAP and WL for 24 weeks, to assess the incremental effects of combination therapy over each individual therapy.12 This trial demonstrated an incremental benefit of combination therapy over CPAP alone (but not compared to weight loss alone) in reducing serum C-reactive protein levels, insulin resistance and dyslipidemia. These results indicated that obesity (but not OSA) was the primary causal factor in these abnormalities. However, a secondary analysis of blood pressure changes demonstrated that systolic blood pressure (SBP) was reduced in all study arms. Although no significant between-group differences were present in intent-to-treat analyses, a greater reduction in the combined-intervention group than either of the monotherapy groups was observed in per-protocol analyses that included subjects compliant with therapy, suggesting that both OSA and obesity independently contribute to hypertension.
In general, blood pressure is determined by a steady component (mean arterial pressure, MAP), which is strongly dependent on microvascular resistance, and a pulsatile component, which is strongly dependent on conduit artery properties. The pulsatile component, often represented by pulse pressure, is increasingly recognized as an important determinant of cardiovascular risk. However, pulse pressure (and systolic pressure) is different in the arm compared to the central aorta.13 Central aortic pulse pressure, in particular, is impacted by wave reflections from the peripheral arterial tree returning to the heart.14–17 Central pulsatile hemodynamics and arterial stiffness are independent predictors of cardiovascular risk.13,18–20 Whereas arterial stiffness has been linked to OSA in multiple observational studies,21–25 little experimental data are available regarding the effect of CPAP, weight loss, or both, on either central hemodynamics or arterial stiffness.
In this study, we report on the results of an ancillary study of the COSA trial, in which changes in central blood pressure and carotid-femoral pulse wave velocity (CF-PWV) were assessed, in response to randomized CPAP therapy, WL therapy, or both.
Methods
Study design
This was a randomized, parallel-group, three-armed trial comparing the effects of CPAP, weight loss or both (CPAP plus weight loss) among subjects with 1) obesity, defined as body mass index ≥ 30 kg/m2; 2) moderate-to-severe OSA, defined as the presence of an apnea-hypopnea index (AHI) ≥ 15 events/hour, and; 3) serum C-reactive protein (CRP) level ≥1 mg/dl. The study design, along with the detailed inclusion and exclusion criteria has been described previously.12 Briefly, study participants were initially screened with a home-based sleep monitor. For those with AHI ≥10 events/hour, this was followed by diagnostic polysomnography (PSG). Subjects with AHI ≥15 on PSG were randomized using a permuted-block design with stratification according to enrollment site (Hospital of University of Pennsylvania and VA Medical Center), sex and statin use.
This ancillary study was initiated with funding awarded by the American Heart Association several months after the primary National Heart, Lung and Blood Institute-funded trial was initiated. Once the ancillary trial was implemented, however, all subjects in the main trial were also enrolled in this ancillary study.
Interventions
The primary interventions for this trial were CPAP therapy and weight loss, either alone or in combination. For subjects in the CPAP-only and the combined-intervention arms, CPAP was initially calibrated in the laboratory followed by continued therapy with either a fixed pressure or an auto-adjusting CPAP. Adherence was monitored weekly with the help of a router attached to the device. Subjects in the weight loss and the combined-intervention arm received individual weekly counseling sessions targeted towards a goal caloric intake and progressively increasing durations of unsupervised exercise. Cognitive behavioral strategies including self-monitoring, goal-setting and problem-solving were used to promote compliance to weight loss recommendations.
Outcome assessments
Assessments were performed at baseline, 8 weeks, and 24 weeks after the initiation of therapy and are detailed further in online supplement. Central pressure waveforms were obtained with applanation tonometry of the carotid artery, using a high-fidelity Millar tonometer (Millar Instruments, Houston, TX) and a Sphygmocor PWV Vx System (Atcor Medical; Sydney, Australia). We recorded radial artery waveforms from the wrist of the dominant arm. Radial waveforms were calibrated according to sphygmomanometric systolic and diastolic pressures measured in the brachial artery. Mean arterial pressure (MAP) was obtained via integration of the radial pressure waveform. Carotid pressure waveforms were calibrated using radial MAP and diastolic blood pressure (DBP), which varies minimally along the arterial tree. Because some amplification of the pulse pressure occurs between the aorta and the carotid artery, we performed a second set of sensitivity analyses, in which we obtained central (aortic) pressures using a generalized transfer function applied to the radial pressure waveform, as previously described.26,27
Carotid femoral pulse wave velocity (CF-PWV), considered the non-invasive “gold-standard” index of large artery stiffness19,28, was measured using the SphygmoCor system. Briefly, carotid-to-femoral transit time (ΔT) was computed from the foot-to-foot time difference between sequentially acquired carotid and femoral waveforms, using the intersecting tangents method, and the QRS complex of the ECG as a fiducial point. The distance between the sternal notch and the carotid artery was subtracted from the distance between the sternal notch and the femoral artery, in order to estimate the path length (L), and PWV was computed as L/ΔT. Distance measurements were performed using a rigid caliper, to avoid an artificial effect of obesity in distance measurements that can occur with flexible tape measures.
Statistical analysis
Continuous data are described as mean ± standard deviation, or counts (percentages) as appropriate. Statistical analyses were carried out: (a) On a primary modified intention-to-treat (ITT) population, defined as all participants who were randomized to a study group and had at least one outcome assessment observation after randomization, and; (b) In a per-protocol analysis restricted to participants who met pre-specified minimum requirements for weight loss (at least 5% of baseline weight) and adherence to CPAP therapy (use for an average of at least 4 hours per night on at least 70% of the total number of nights). The intent-to-treat analysis best represents the expected therapeutic effects of our interventions as implemented in the trial. The per-protocol analysis is most informative about causal relationships between OSA or obesity on the endpoints; this is based on the fact that any incremental benefit of effective combination therapy (weight loss combined with effective CPAP treatment), relative to effective CPAP alone, must be due to the effects of obesity that are independent of the effects of OSA. Conversely, any incremental benefit of effective combination therapy, as compared with effective weight loss alone, must be due to the effects of OSA that are independent of the effects of obesity.12 The effects of the interventions on end points were analyzed with the use of general linear mixed models, with all measurements available used to estimate intervention effects at 24 weeks. Estimates are presented as mean [95% CI]. Analyses were performed with SAS software, version 9.2 (SAS Institute).
Results
Study participants
Of the 181 total participants who underwent randomization in the parent trial, 139 participated were included in this sub-study (CPAP: n=45, WL: n=48, CPAP+WL: n=46). Seventy-one participants were found to meet adherence criteria and were included in the per-protocol analysis. A flowchart of study participants at each stage is shown in figure S1.
Baseline characteristics of the tonometry subsample of study participants as compared with those without tonometry measurements are shown in Table 1. The mean age of the participants was 49.4±11.2 years and 42.3% of the subjects were female. The age and gender distribution of the sub-study population was not significantly different from those not included in the sub-study. However, there was a significantly higher proportion of Caucasians and lower proportion of African-Americans among subjects enrolled in this sub-study. Diastolic blood pressure and heart rate were significantly but slightly lower among subjects enrolled in this sub-study. There were no other significant differences between the two populations with respect to tobacco use, alcohol consumption, or use of antihypertensive agents or statins.
Table 1.
Comparison of baseline characteristics of subjects with and without tonometry measurements.
| Characteristic | No Tonometry (n = 42) | Tonometry (n =139) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age - years Mean ± SD | 47.7±11.0 | 49.2±11.2 | 0.367 |
| % Female | 43.2% | 42.3% | 0.921 |
| Race | 0.007 | ||
| Black | 60.5% | 34.3% | |
| White | 39.5% | 61.9% | |
| Other | - | 3.7% | |
| Comorbidities and medication use | |||
| Tobacco use | 0.272 | ||
| Never smoker | 77.3% | 68.6% | |
| Current/Past | 22.7% | 31.4% | |
| Alcohol use | 0.120 | ||
| None | 43.2% | 29.2% | |
| < 3 drinks | 50.0% | 51.1% | |
| 3–7 drinks | 6.8% | 14.6% | |
| 7–14 drinks | - | 5.1% | |
| Statin use | 20.5% | 20.6% | 0.985 |
| Antihypertensive use | 43.2% | 37.2% | 0.480 |
| Days/week exercise Mean±SD | 1.7±2.3 | 1.3±1.6 | 0.184 |
| BMI Mean±SD | 39.4±6.7 | 38.6±6.4 | 0.475 |
| Hemodynamic Parameters | |||
| SBP Mean±SD | 129.4±12.7 | 127.4±10.4 | 0.322 |
| DBP Mean±SD | 81.3±8.29 | 78.6±7.19 | 0.044 |
| MAP Mean±SD | 100.5±9.3 | 98.1±7.6 | 0.095 |
| Pulse Pressure Mean±SD | 48.1±9.2 | 48.8±8.8 | 0.632 |
| Heart rate Mean±SD | 76.0±10.7 | 69.8±9.9 | 0.001 |
General baseline characteristics of the tonometry subsample of study participants randomized to weight loss, CPAP, or both, are shown in Table 2. No significant differences were observed in various characteristics, except for a slightly greater BMI at baseline among subjects randomized to CPAP. Changes in body weight, BMI and peripheral blood pressure are summarized in supplemental table S1.
Table 2.
Baseline characteristics of study participants with tonometry measurements. Plus minus values are mean ± SD. SpO2 denotes oxygen saturation level as measured by oximetry. MAP = mean arterial pressure, SBP = systolic blood pressure, CF-PWV = Carotid-femoral pulse wave velocity, PP = pulse pressure
| Characteristic | Weight loss (n=48) | CPAP (n=45) | Weight loss + CPAP (n=46) | P value |
|---|---|---|---|---|
| Age - years mean±SD | 49.0±10.7 | 48.9±11.3 | 49.8±12.1 | 0.921 |
| Male sex – no. (%) | 30 (62.5) | 27 (60) | 23 (50) | 0.416 |
| Race – no. (%) | 0.657 | |||
| White | 29 (60.4) | 26 (57.8) | 31 (67.4) | |
| Black | 16 (33.3) | 18 (40) | 14 (30.4) | |
| Mixed or other | 3 (6.2) | 1 (2.2) | 1 (2.2) | |
| Height – cm | 172.6±10.5 | 171.7±9.1 | 171.4±9.4 | 0.826 |
| Weight – kg | 111.4±21.1 | 119.5±21.5 | 112.5±25.2 | 0.180 |
| Waist size - cms | 117.0±14.2 | 124.4±14.3 | 120.5±15.3 | 0.055 |
| Body – mass index | 37.2±4.9 | 40.7±7.5 | 38.1±6.3 | 0.026 |
| Hypertension – no. (%) | 18 (37.5) | 18 (40) | 16 (34.8) | 0.948 |
| Percentage of time SpO2 <90% | 4.2±6.2 | 8.2±15.3 | 6.4±11.6 | 0.262 |
| AHI-events/hr | 38.3±18.5 | 43.1±21.3 | 45.3±26.0 | 0.304 |
| Oxygen desaturation index – no. of events/hour | ||||
| >3% drop from baseline | 22.8±18.2 | 29.1±23.5 | 27.9±26.5 | 0.381 |
| >4% drop from baseline | 18.5±16.3 | 23.9±21.9 | 23.3±25.6 | 0.418 |
| Medication use – no. (%) | ||||
| Statin | 10 (21.3) | 10 (22.2) | 9 (24.3) | 0.951 |
| Antihypertensive | 19 (39.6) | 17 (37.8) | 16 (34.8) | 0.889 |
| Hemodynamics (Mean±SD) | ||||
| Peripheral SBP | 124.8±10.2 | 129.1±10.6 | 128.4±10.2 | 0.099 |
| Peripheral MAP | 96.6±7.6 | 99.3±7.3 | 98.6±7.7 | 0.204 |
| Carotid SBP | 110.6±17.0 | 113.3±14.6 | 113.2±13.8 | 0.624 |
| Carotid MAP | 89.9±9.8 | 92.9±9.6 | 92.2±9.3 | 0.287 |
| Peripheral Augmentation Index | 78.5±16.2 | 74.1±13.5 | 75.5±17.5 | 0.409 |
| Central Augmentation Index | 117.5±32.2 | 116.3±22.5 | 116.2±26.6 | 0.964 |
| CF-PWV | 7.8±1.4 | 8.1±1.7 | 8.2±1.4 | 0.633 |
| Peripheral PP | 46.9±7.6 | 49.7±9.9 | 49.6±7.6 | 0.201 |
| Central PP | 34.8 (14.4) | 34.9 (12.4) | 36.0 (10.6) | 0.892 |
Systolic blood pressure
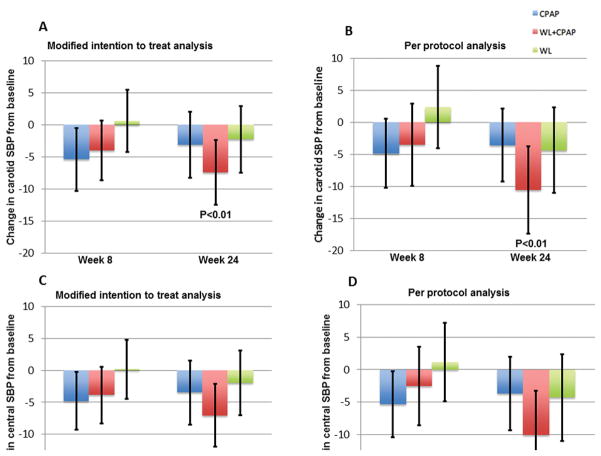
In intent-to-treat analyses, central systolic blood pressure assessed with carotid arterial tonometry was reduced significantly only in the combination arm (−7.4 mmHg, 95%CI: −12.5 to −2.4 mmHg; P=0.004), without significant reductions detected in either the weight loss-only (−2.3 mmHg; 95%CI: −7.5 to 3.0, P=0.39) or the CPAP-only (−3.1 mmHg; 95%CI: −8.3 to 2.0, P=0.23) arms (Figure 1A). Similarly, in the per-protocol compliant sub-sample, central systolic blood pressure decreased significantly in the combination arm (−10.6 mmHg, 95%CI: −17.4 to −2.8, P=0.003), without significant reductions detected in either the weight loss-only (−4.3 mmHg, 95%CI: −11.0 to 2.4, P=0.20) or the CPAP-only arm (−3.6 mmHg, 95%CI: −9.3 to 2.1, P=0.21) (Figure 1B). These differences were not seen at 8 weeks (Figure 1).
Figure 1.
Figure 1A–1D. Change in central systolic blood pressure (SBP) from baseline derived from carotid tonometry in modified intention to treat analysis (1A) and per protocol analysis including only subjects who met pre-specified adherence criteria (1B). Change in central systolic blood pressure from baseline derived from application of a generalized transfer function to radial tonometry in modified intention to treat analysis (1C) and per protocol analysis including only subjects who met pre-specified adherence criteria (1D). Error bars represent 95% confidence intervals.
Central systolic blood pressure assessed by radial tonometry in conjunction with a generalized transfer function revealed similar results. In these analyses, central systolic blood pressure was reduced significantly only in the combination arm (−7.0 mmHg, 95%CI: −12.0 to −2.1, P=0.006), without significant reductions detected in either the weight loss-only (−1.9 mmHg, 95%CI: −7.0 to 3.1, P= 0.45) or the CPAP-only (−3.5 mmHg, 95%CI: −8.5 to 1.5, P=0.17) arms (Figure 1C). Similarly, in the per-protocol compliant sub-sample, central systolic blood pressure decreased significantly in the combination arm (−9.9 mmHg, 95%CI: −16.7 to −3.2, P=0.004), without significant reductions detected in either the weight loss-only (−4.3 mmHg, 95%CI: −10.9 to 2.3, P=0.20) or the CPAP-only (−3.7 mmHg, 95%CI: −9.3 to 1.9, P=0.20) arms (Figure 1D).
We analyzed the relationship between central and peripheral SBP as well as between change in central SBP and change in peripheral SBP. On regression analysis, we found both the baseline and the change in central and peripheral systolic blood pressure to be highly correlated with correlation coefficient of 0.935 and 0.928 respectively.
Mean and diastolic blood pressure
Mean arterial pressure was reduced significantly in the combination therapy arm and the CPAP arm in intent to treat analysis (CPAP + WL: =−6.9 mmHg, 95% CI: −10.2 to −3.6, P <0.0001; CPAP: −3.7 mmHg, 95% CI: −6.9 to −0.5, P = 0.02; WL: −1.8 mmHg, 95% CI: −5.2 to 1.6, P =0.29) and in per-protocol analysis (CPAP+WL: −9.1 mmHg, 95% CI: −13.0 to −5.3, P <0.0001, CPAP: −3.1, 95%CI: −6.3 to −0.02, P=0.05, WL: −1.9, 95% CI: −5.8 to 2.0, P =0.34). After adjustment for heart rate, significant reductions in MAP were noted in the CPAP and CPAP + weight loss group in the intent to treat analysis (CPAP: −3.4, 95% CI: −6.6 to −0.2, P =0.04; CPAP + WL: −6.0, 95% CI: −9.1 to −3.0, P =0.0002; WL: −0.8, 95% CI: −4.0 to 2.4, P =0.62) and only in the combined therapy arm in per protocol analysis (CPAP: −2.8, 95% CI: −6.2 to 0.5, P =0.09; CPAP + WL: −8.8, 95% CI: −12.7 to −4.9, P <0.0001; WL: −1.9, 95% CI: −5.9 to 2.0, P =0.33).
The effect of the interventions in the three arms was similar with regards to change in diastolic blood pressure. In both intent to treat and per protocol analysis, diastolic BP decreased significantly in the combined CPAP + weight loss group but not in the CPAP and weight loss only arms. (ITT: CPAP: −3.4 mm Hg, 95% CI: −5.0 to 2.3, P=0.07; WL: −1.1, 95% CI: −3.6 to 1.8, P =0.43; CPAP+ WL: −5.2, 95% CI: −7.9 to −2.6, P =0.0001. Per protocol: CPAP: −2.3, 95% CI: −5.0 to 0.4, P = 0.09; WL: −1.3, 95% CI: −4.5 to 1.8, P =0.40; CPAP + WL: −6.9, 95% CI: −10.1 to −3.7, P <0.0001).
Pulse pressure
In the intent-to-treat analyses, the reduction in central (carotid) pulse pressure (Figure 2A) compared to baseline did not reach statistical significance in any of the three groups at 24 weeks (CPAP: −3.8 mmHg, 95%CI: −8.3 to 0.7, P=0.09; WL: −2.7 mmHg, 95%CI: −7.5 to 2.2, P=0.27; CPAP+WL: −4.1, 95%CI: −8.7 to 0.6, P=0.08). In per-protocol analyses (Figure 2B), subjects in the CPAP arm were found to have a significant reduction in central pulse pressure at 24 weeks compared to baseline (CPAP: −4.1 mmHg, 95%CI: −8.2 to −0.04, P=0.048; WL: −1.7, 95%CI: −6.8 to 3.4, P=0.50; CPAP+WL: −3.2 mmHg, 95%CI: −8.3 to 1.8, P=0.21), although no between-group differences were demonstrated. These results did not appreciably change after adjustment for MAP and heart rate (ITT: CPAP: −3.7, 95% CI: −8.0 to 0.6, P=0.09; CPAP + WL: −2.9, 95% CI:−7.2 to 1.5, P=0.19; WL: −3.7, 95% CI: −8.3 to 0.9, P=0.11; Per protocol analysis - CPAP: −4.2, 95% CI:−8.2 to −0.3, P=0.04; CPAP + WL: −1.8, 95% CI:−6.8 to 3.1, P=0.46; WL: −2.2, 95% CI:−7.1 to 2.7, P=0.37).
Figure 2.
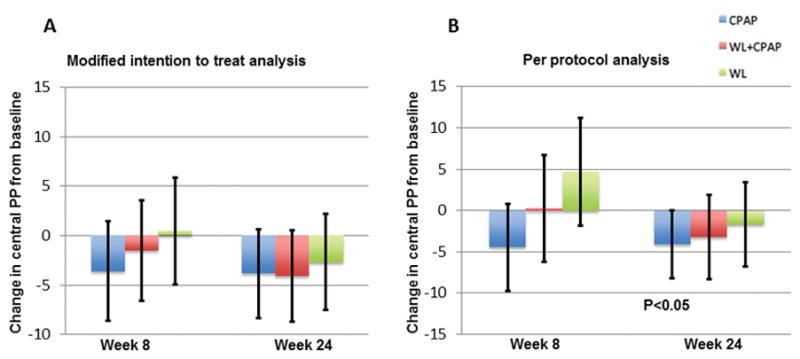
Figure 2A–2B. Change in central pulse pressure (PP) from baseline derived from carotid tonometry in modified intention to treat analysis (2A) and per protocol analysis including only subjects who met pre-specified adherence criteria (2B). Error bars represent 95% confidence intervals.
Pulse pressure amplification
Pulse pressure amplification, assessed as the ratio of carotid over brachial artery pressure, did not significantly change from baseline in any of the three arms in intention to treat analysis (Figure 3A; CPAP: −0.3 mmHg, 95%CI: −3.2 to 2.7, P=0.86; WL: −0.1 mmHg, 95%CI: −2.9 to 3.2, P=0.92; CPAP+WL: −2.4 mmHg, 95%CI: −5.4 to 0.6, P=0.11). Findings were very similar in per-protocol analysis (Figure 3B; CPAP: −0.5 mmHg, 95%CI: −3.9 to 2.8, P=0.76; WL: 0.1 mmHg, 95%CI: −3.8 to 4.1 mmHg, P=0.94; CPAP+WL: −2.7, 95%CI: −6.8 to 1.3, P=0.18). Results were similar with the use of radial tonometry and application of a generalized transfer function (not shown).
Figure 3.
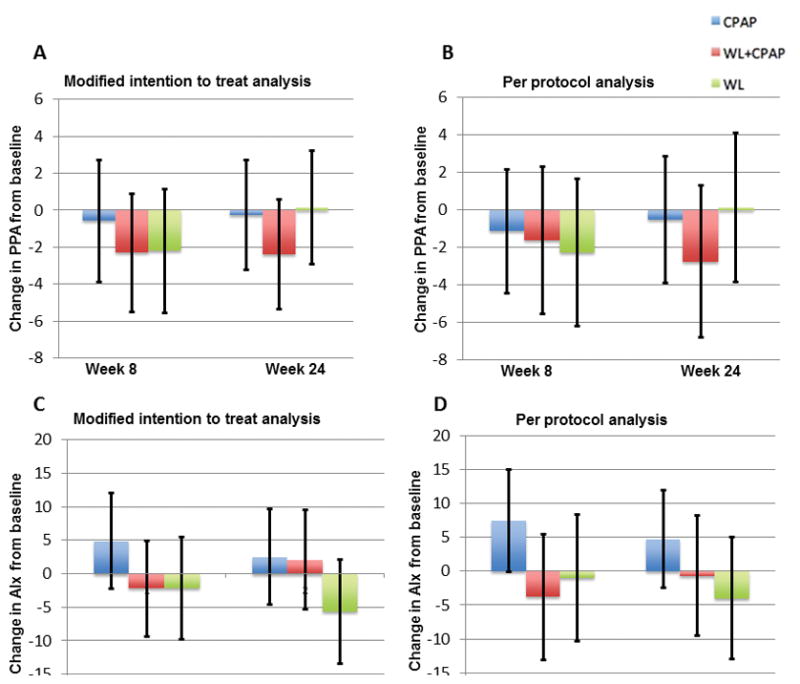
Figure 3A–3B. Change in pulse pressure amplification (PPA), ratio of central over radial artery pulse pressure from baseline in modified intention to treat analysis (3A) and per protocol analysis including only subjects who met pre-specified adherence criteria (3B). Error bars represent 95% confidence intervals.
Figure 3C–3D. Change in aortic augmentation index (AIx) from baseline in modified intention to treat analysis (3C) and per protocol analysis including only subjects who met pre-specified adherence criteria (3D). Error bars represent 95% confidence intervals.
Augmentation index (AIx)
The central augmentation index did not significantly change from baseline in any of the three arms in intention to treat analysis (Figure 3C; CPAP: 2.5, 95%CI: −4.7 to 9.7, P=0.48; WL: −5.7, 95%CI: −13.5 to 2.0, P=0.14; CPAP + WL: 2.1, 95%CI: −5.3 to 9.5, P=0.58). Similar findings were noted in per-protocol analysis (Figure 3D; CPAP: 4.7, 95%CI: −2.5 to 11.9, P=0.19; WL: −4.0, 95%CI: −13.0 to 5.0, P=0.38; CPAP+WL: −0.7, 95%CI: −10.0 to 8.2, P=0.88). Adjustment for MAP and heart rate did not reveal any significant changes in these results (ITT: CPAP: −3.1, 95% CI: −4.3 to 10.5, P=0.40; WL: −7.0, 95% CI: −14.9 to 0.9, P=0.09; CPAP + WL: 3.2, 95% CI:−4.4 to 10.7, P=0.41, Per protocol analysis - CPAP: 4.0, 95% CI:−3.1 to 11.2, P=0.26; WL: −4.3, 95% CI: −13.2 to 4.5, P=0.33; CPAP + WL: 1.6, 95% CI:−7.3 to 10.6, P=0.72). Results were also similar with the use of radial tonometry and application of generalized transfer function (not shown).
Carotid femoral pulse wave velocity (CF-PWV)
In intent to treat-analyses, no significant changes in CF-PWV were observed in any of the 3 study arms (CPAP: −0.13 m/s, 95%CI: −0.65 to 0.39; WL: −0.04 m/s, 95%CI: −0.55 to 0.47; WL+CPAP: 0.05 m/s, 95%CI=−0.45 to 0.54) (Figure 4A). There were no significant differences in the change in CF-PWV between the groups (P value for all pairwise comparisons >0.05). In pre-specified per-protocol analyses restricted to subjects who complied with randomized therapy, these results were not appreciably different (Figure 4B).
Figure 4.
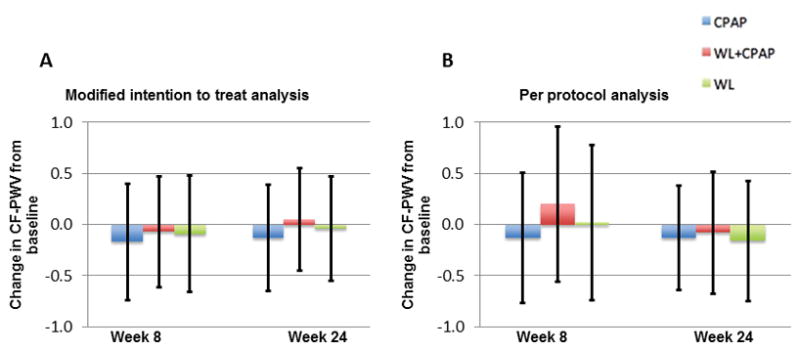
Figure 4A–4B. Change in carotid-femoral pulse wave velocity (CF-PWV) from baseline in modified intention to treat analysis (4A) and per protocol analysis including only subjects who met pre-specified adherence criteria (4B). Error bars represent 95% confidence intervals.
The change in mean arterial pressure induced by the intervention was a significant predictor of the change in CF-PWV (Pearson R=0.31; P=0.008). Similarly, the change in heart rate was weakly related to the change in CF-PWV (Pearson R=0.23; P=0.027). After adjustment for mean arterial pressure and heart rate, no significant changes in CF-PWV were observed in either group, without significant between-group differences, in either intent-to-treat analyses or pre-specified analyses in subjects who met compliance criteria.
Discussion
In this study, we found that, in obese individuals with moderate to severe OSA, combination therapy with WL and CPAP (but not CPAP or WL monotherapy) reduced central systolic blood pressure. These results were consistently found when either carotid tonometry (without a transfer function) or radial tonometry (with the use of a generalized transfer function) was used. However, the reduction in central systolic blood pressure was largely the result of a larger reduction in mean arterial pressure in the combination arm, since central pulse pressure or large artery stiffness (measured as carotid-femoral PWV) were not significantly reduced in either arm in intent-to-treat analyses. Among participants who complied with therapy, a significant reduction in central pulse pressure in the CPAP-only arm was observed, which was not seen in either the weight loss arm or the combined intervention arm. Overall, however, pulse pressure amplification was not significantly changed by weight loss, CPAP, or combination therapy, suggesting that brachial systolic (and pulse) pressure remains a good surrogate of central pulse pressure changes in response to these interventions.
Previous randomized controlled trials have studied the effect of CPAP therapy on blood pressure in comparison to splinting devices, sham CPAP or supplemental nasal oxygen, with variable results. While most trials in patients with resistant hypertension have shown a significant improvement with CPAP, trials in normotensive subjects or those with nonresistant hypertension, similar to our population, have shown conflicting results.3–6,11,29–31 However the negative results have largely been from trials reporting effects of CPAP in subjects with mild OSA30 or shorter durations of therapy 29,31 and recent meta-analyses of RCT data have confirmed a small but statistically significant reduction in blood pressure with CPAP therapy.32,33 In our study, we noted a significant reduction in mean arterial pressure with CPAP in both intention to treat and per-protocol analyses, consistent with the notion that sleep apnea leads to hypertension, and that CPAP monotherapy can achieve a small blood pressure effect. Furthermore, since the combined CPAP and the weight loss intervention achieved a significantly higher reduction in MAP compared to CPAP alone, it follows that both obesity and OSA have independent effects on blood pressure and concurrent treatment of both achieves a larger blood pressure reduction. This is in concordance with the results of the primary study measuring peripheral pressures, where compliant subjects in all three arms achieved significant MAP reduction with the highest effect in the combination intervention arm.12
We report, for the first time, the effect of CPAP, WL, or the combination of CPAP and WL on central hemodynamics and large artery stiffness. We found a significant reduction in central systolic pressure only in the combined CPAP+WL arm. Interestingly, we also observed a reduction in central pulse pressure in compliant subjects randomized to CPAP therapy. This finding, although interesting, should be interpreted with caution. First, this is a finding in a subsample of individuals enrolled in the parent trial, who had both available tonometry measurements (for estimation of central pressures) and adequate compliance to therapy. Furthermore, the analyses overall suggest that pulse pressure amplification does not change with CPAP, WL or the combination, suggesting that peripheral pulse pressure is an adequate surrogate of central pulse pressure with regards to the response to these interventions. Of note, in the parent trial, pulse pressure was reduced in the combined intervention group in the intention to treat analysis, and in per-protocol analyses including compliant subjects, it was reduced in the combined CPAP + weight loss and the weight loss monotherapy arm.
OSA has also been linked to increased arterial stiffness and a recent meta-analysis summarizing 18 studies confirmed this association.25 Evidence from prior studies on the impact of CPAP therapy in reducing arterial stiffness is less concrete with only a few randomized controlled trials evaluating this effect,7,34–36 of which three studied the gold standard measure of large artery stiffness, CF-PWV.7,35,36 Contrary to our results, Drager et al and Litvin et al described striking reductions in CF-PWV with 4 months and 3 weeks of CPAP therapy respectively, whereas Jones et al did not find a significant difference in CF-PWV with 12 weeks of CPAP therapy in patients with mild OSA. In our study, we did not observe any significant reduction in arterial stiffness with CPAP, weight loss or both interventions combined even in subjects who demonstrated compliance with therapy. Of note, both the reduction in MAP and heart rate were significant predictors of the change in CF-PWV (as is expected from physiologic principles).19 However, none of the interventions reduced CF-PWV independently of MAP or heart rate, indicating that they do not modify the material properties of the aortic wall. It remains to be determined whether greater weight loss and/or more prolonged periods of CPAP therapy exert favorable effects on large artery stiffness.
Our study should be interpreted in the context of its strengths and limitations. Strengths of our study include its prospective randomized experimental design, the assessment of central hemodynamics with high-fidelity carotid tonometry, and the design to assess the effects of weight loss, CPAP, and their combination, thus separating the effect of CPAP versus OSA treatment on the study endpoints. There are also limitations to our study. The study was not blinded for treatment assignment or outcome assessment. We did not include a sham CPAP or placebo control group; however both sham CPAP and absence of CPAP therapy are accepted as adequate controls for an active CPAP intervention37. We did not assess ambulatory blood pressure and included a non-diabetic population with significant obesity and moderate-to-severe OSA. Our results are therefore not necessarily generalizable to patients with milder OSA, milder obesity, or those with diabetes mellitus. The magnitude of BP reduction in the monotherapy arms was relatively small. Although it is possible that this was partially due to the inclusion of both hypertensive and normotensive participants in the study, the mean magnitude of BP reduction with CPAP observed in our study is comparable to the BP reduction seen in previous trials with CPAP in hypertensive populations.32,38 We performed single, rather than duplicate arterial tonometry measurements at each visit. Despite the randomized nature of the study, we encountered imbalances in baseline characteristics which may have influenced the results. Furthermore, this analysis represented a subsample of the parent trial, with a lower representation of African-American participants.
Perspectives
Our study provides evidence that both OSA and obesity have independent causal relationship with elevated central systolic blood pressure, and that, among obese subjects with OSA, combination therapy with WL and CPAP is effective in reducing central systolic pressure. However, this effect is largely mediated by changes in mean, rather than central pulse pressure. WL and CPAP, alone or in combination, did not reduce large artery stiffness in this population. Our study adds support to the concept that in obese patients with OSA, combination therapy with CPAP and weight loss is required to address the increased cardiovascular risk factor burden of this population.
Supplementary Material
Novelty and Significance.
What’s new?
This is the first randomized controlled trial to examine the effect of CPAP, weight loss or the combination of CPAP and weight loss on central hemodynamics and large artery stiffness.
What’s relevant?
Among obese subjects with OSA, combination therapy with weight loss and CPAP is effective in reducing central systolic pressure.
Reduction in central systolic pressure is largely mediated by changes in mean, rather than central pulse pressure.
Weight loss and CPAP, alone or in combination, did not reduce large artery stiffness in this population.
Summary
In obese individuals with moderate to severe OSA, combination therapy with weight loss and CPAP but not CPAP or weight loss monotherapy reduced central systolic blood pressure.
Acknowledgments
None
Sources of Funding: Supported by grants from the American Heart Association Award #0885031N (Chirinos) and National Heart, Lung, and Blood Institute HL-R01080076 (Chirinos), 01 HL094307 (Pack). Dr. Chirinos is also supported by NIH grants R01 HL-121510-01A1 and R56 HL-124073-01A.
Footnotes
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT00371293
Disclosures: J.A.C. has received consulting honoraria from Bristol Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft and Merck. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol Myers Squibb, Microsoft and CVRx Inc., and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. STK receives grant support from Philips Respironics, Inc. Other authors have no disclosures.
References
- 1.Bauters F, Rietzschel ER, Hertegonne KB, Chirinos JA. The Link Between Obstructive Sleep Apnea and Cardiovascular Disease. Curr Atheroscler Rep. 2016;18:1. doi: 10.1007/s11883-015-0556-z. [DOI] [PubMed] [Google Scholar]
- 2.Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–1437. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 3.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, Davies RJ. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 4.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 5.Norman D, Loredo JS, Nelesen RA, Ancoli-Israel S, Mills PJ, Ziegler MG, Dimsdale JE. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–845. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 8.Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med. 2008;178:984–988. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- 9.Seetho IW, Asher R, Parker RJ, Craig S, Duffy N, Hardy KJ, Wilding JP. Effect of CPAP on arterial stiffness in severely obese patients with obstructive sleep apnoea. Sleep Breath. 2015;19:1155–1165. doi: 10.1007/s11325-015-1131-0. [DOI] [PubMed] [Google Scholar]
- 10.Phillips CL, Yee BJ, Trenell MI, Magnussen JS, Wang D, Banerjee D, Berend N, Grunstein RR. Changes in regional adiposity and cardio-metabolic function following a weight loss program with sibutramine in obese men with obstructive sleep apnea. J Clin Sleep Med. 2009;5:416–421. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyos CM, Yee BJ, Wong KK, Grunstein RR, Phillips CL. Treatment of Sleep Apnea With CPAP Lowers Central and Peripheral Blood Pressure Independent of the Time-of-Day: A Randomized Controlled Study. Am J Hypertens. 2015;28:1222–1228. doi: 10.1093/ajh/hpv023. [DOI] [PubMed] [Google Scholar]
- 12.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend RR, Rosendorff C, Nichols WW, Edwards DG, Chirinos JA, Fernhall B, Cushman WC. American Society of Hypertension position paper: central blood pressure waveforms in health and disease. J Am Soc Hypertens. 2016;10:22–33. doi: 10.1016/j.jash.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the Determinants of Elevated Forward Wave Amplitude Inflates the Role of the Proximal Aorta. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend RR, Black HR, Chirinos JA, Feig PU, Ferdinand KC, Germain M, Rosendorff C, Steigerwalt SP, Stepanek JA. Clinical Use of Pulse Wave Analysis: Proceedings From a Symposium Sponsored by North American Artery. J Clin Hypertens (Greenwich) 2015;17:503–513. doi: 10.1111/jch.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 18.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 19.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on H. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirinos JA, Segers P, Duprez DA, Brumback L, Bluemke DA, Zamani P, Kronmal R, Vaidya D, Ouyang P, Townsend RR, Jacobs DR., Jr Late systolic central hypertension as a predictor of incident heart failure: the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e001335. doi: 10.1161/JAHA.114.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelic S, Bartels MN, Mateika JH, Ngai P, DeMeersman RE, Basner RC. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25:850–855. [PubMed] [Google Scholar]
- 22.Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28:604–609. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- 23.Seetho IW, Parker RJ, Craig S, Duffy N, Hardy KJ, Wilding JP. Obstructive sleep apnea is associated with increased arterial stiffness in severe obesity. J Sleep Res. 2014;23:700–708. doi: 10.1111/jsr.12156. [DOI] [PubMed] [Google Scholar]
- 24.Jones A, Vennelle M, Connell M, McKillop G, Newby DE, Douglas NJ, Riha RL. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013;14:428–432. doi: 10.1016/j.sleep.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, Wei Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher D, Adji A, O’Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens. 2004;17:1059–1067. doi: 10.1016/j.amjhyper.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 29.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 30.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 31.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, Almeida-Gonzalez C. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 32.Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, Narkiewicz K, Minuz P. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145:762–771. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 33.Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32:1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 34.Kohler M, Craig S, Pepperell JC, Nicoll D, Bratton DJ, Nunn AJ, Leeson P, Stradling JR. CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest. 2013;144:896–902. doi: 10.1378/chest.13-0179. [DOI] [PubMed] [Google Scholar]
- 35.Jones A, Vennelle M, Connell M, McKillop G, Newby DE, Douglas NJ, Riha RL. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep Med. 2013;14:1260–1265. doi: 10.1016/j.sleep.2013.08.786. [DOI] [PubMed] [Google Scholar]
- 36.Litvin AY, Sukmarova ZN, Elfimova EM, Aksenova AV, Galitsin PV, Rogoza AN, Chazova IE. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc Health Risk Manag. 2013;9:229–235. doi: 10.2147/VHRM.S40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown DL, Anderson CS, Chervin RD, Kushida CA, Lewin DS, Malow BA, Redline S, Goldman EB. Ethical issues in the conduct of clinical trials in obstructive sleep apnea. J Clin Sleep Med. 2011;7:103–108. [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest. 2013;144:1487–1494. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.