Abstract
Both big (BKCa) and small (SKCa) conductance Ca2+-sensitive K+ channels are present in mammalian cardiac cell mitochondria (m). We used pharmacological agonists and antagonists of BKCa and SKCa channels to examine the importance of endogenous opening of these channels and the relative contribution of either or both of these channels to protect against contractile dysfunction and reduce infarct size after ischemia reperfusion (IR) injury through a mitochondrial protective mechanism. Following global cardiac IR injury of ex vivo perfused guinea pig hearts we found the following: both agonists NS1619 (for BKCa) and DCEB (for SKCa) improved contractility; BKCa antagonist paxilline (PAX) alone or with SKCa antagonist NS8593 worsened contractility and enhanced infarct size; both antagonists PAX and NS8593 obliterated protection by their respective agonists; BKCa and SKCa antagonists did not block protection afforded by SKCa and BKCa agonists, respectively; and all protective effects by the agonists were blocked by scavenging superoxide anions (O2•−) with TBAP. Contractile function was inversely associated with global infarct size. In in vivo rats, infusion of NS8593, PAX, or both antagonists enhanced regional infarct size while infusion of either NS1619 or DCEB reduced infarct size. In cardiac mitochondria isolated from ex vivo hearts after IR, combined SKCa and BKCa agonists improved respiratory control index (RCI) and Ca2+ retention capacity (CRC) compared to IR alone, whereas the combined antagonists did not alter RCI but worsened CRC. Although the differential protective bioenergetics effects of endogenous or exogenous BKCa and SKCa channel opening remain unclear, each channel likely responds to different sensing Ca2+ concentrations and voltage gradients over time during oxidative stress-induced injury to individually or together protect cardiac mitochondria and myocytes.
Keywords: Cardiac mitochondria, inner mitochondrial membrane, cell signaling, ischemia reperfusion injury, oxidant stress, large and small conductance Ca2+ -sensitive K+ channels
Introduction
Altered mitochondrial (m) bioenergetics, excess reactive oxygen species (ROS) emission, and excess mCa2+ overload, are major factors that underlie ischemia and reperfusion (IR) injury.1 Prophylactic measures targeted to mitochondria that reduce cardiac IR injury2, 3 include ischemic preconditioning (IPC, i.e., brief pulses of ischemia and reperfusion before longer index ischemia) and pharmacologic pre-conditioning (PPC), i.e., cardiac protection elicited some time after the drug is washed out before index IR. Drugs that reduce cardiac metabolism or mitochondrial bioenergetics when given before and or after IR also afford protection.
The induction of mitochondrial (m) K+ influx is now recognized as an important underlying mechanism to reduce IR injury. We reported previously that activation by NS1619 of the large (big) conductance Ca2+-sensitive K+ channel (BKCa, KCa1.1, maxi-K), that is found in the cardiac myocyte inner mitochondrial membrane (IMM), induced pharmacologic preconditioning (PPC) and that this protection was blocked by the mitochondrial targeted superoxide anion (O2•−) scavenger TBAP.4 We subsequently reported that NS1619 had biphasic effects on mitochondrial respiration, membrane potential (ΔΨm), and O2•− emission in isolated mitochondria.5, 6 Due to our findings and many other reports on the protective effects of BKCa and putative KATP channel agonists in mitochondria, it is likely that opening of other mK+ channels might also be protective but rely on different ligands and conditions for activation.
KCa channels of intermediate or small conductance were first identified in non-cardiac cells7–10 as membrane bound, calmodulin (CaM)-dependent and activated by Ca2+. These channels have smaller unit conductances of 3–30 (small, SKCa) and 20–90 (intermediate IKCa) pS.11 The opening of SKCa channels is initiated by Ca2+ binding to calmodulin within the C terminus of the channel to form a dimer.12, 13 Of the four known genes encoding SKCa channel proteins, one of these, the KCa2.3 (aka SK3) isoform, was found in vascular endothelial cell membranes where it exerted a potent, tonic hyperpolarization that promoted reduced vascular smooth muscle tone.14 Later, the isoform KCa2.2 (aka SK2) was found in rat and human heart cell sarcolemma membranes by Western blot and RT-PCR.15 Amplification of the channel from total atrial and ventricular RNA showed much greater expression in atria than in ventricles, and electrophysiological recordings exhibited much greater atrial than ventricular sensitivity to AP repolarization by apamin, a selective SKCa antagonist.15, 16
Dolga et al.17 found that the SK2 channel was expressed also in mitochondria of neuronal HT-22 cells. At the same time, we furnished the first evidence for the presence of SK2 and SK3 isoforms in the IMM of guinea pig cardiac mitochondria.18 We tested the functionality of SKCa channel opening18 by perfusing isolated hearts transiently before ischemia with the KCa3.1 (IKCa1)7, 9, 19 and KCa2.2 and KCa2.320–23 opener DCEBIO (DCEB). We found18 that DCEB elicited PPC in a manner similar to that of the BKCa channel agonist NS1619.4 Either the SKCa antagonist NS859324, 25 or TBAP, which dismutates O2•− to H2O2, abolished the cardioprotection.18 DCEB also reduced the deleterious effects of IR injury on mitochondrial bioenergetics by attenuating cardiac mitochondrial Ca2+ overload and O2•− levels, and by preserving (more reduced redox state) cardiac mitochondrial NADH and FAD levels after IR.18 These studies established that KCa channel opening initiates a cascade of O2•−-dependent cardiac protective mechanisms that are based primarily in cardiac myocyte mitochondria. In a recent extension of this research,26 we: a) confirmed SKCa protein localization in cardiac ventricular mitochondria of rats and humans, in addition to guinea pigs; b) identified mitochondrial splice variants of SK3 in guinea pig and human cardiac mitochondria; and c) found that overexpression or silencing of SK3 reduced or enhanced, respectively, stress induced damage in two cardiac cell lines, HL-1 and H9c2.
Our aim in the present study was to determine if endogenous opening of the BKCa and or SKCa channel protected hearts and mitochondria from IR injury and if there were differential or additive cardioprotective effects of drug-induced opening of BKCa and SKCa channels. To test this we: a) conducted a pharmacological comparison of SKCa and BKCa agonists and antagonists given before and after ischemia on global ventricular infarct size and developed left ventricular pressure (LVP) in isolated ex vivo perfused guinea pig hearts; b) examined the effect of SKCa and BKCa agonists and antagonists on regional infarct size when infused in rats in vivo; c) assessed the role of mitochondrial-targeted O2•− scavenging by TBAP to block protection by the two agonists; and d) measured respiratory control index (RCI) and Ca2+ retention capacity (CRC) after IR injury to further assess the likely role of mitochondria in protecting or impairing cardiac function and damage by treating ex vivo perfused hearts with SKCa and BKCa agonists or antagonists before and after IR injury.
Materials and methods
Function and Infarction in Ex Vivo Guinea Pig Hearts after Global IR Injury
The investigation conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 1996); all animal protocols were reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. The hearts of adult guinea pigs of either sex were isolated and prepared as described previously in detail.4, 18, 27–33 Guinea pig hearts were chosen because of their more human-like cardiac action potentials and ion currents compared to rats.34 In brief, animals were injected intraperitoneally with heparin (1000 units) and ketamine (50 mg/kg). Once anesthetized, thoracotomy was performed, hearts were excised, and their aortas immediately perfused in retrograde manner with a cold modified Krebs-Ringer’s (KR) perfusate. After aortic cannulation, hearts were transferred to a Langendorff perfusion setup and instrumented with a saline filled balloon and transducer to measure isovolumetric, developed (systolic-diastolic) LVP and perfused at a constant aortic root pressure of 55 mmHg with the KR solution at 37°C. Heart rate was measured from right atrial and ventricular recording electrodes, and coronary (aortic) flow was measured with a flow transducer. Hearts were subjected to 35 min global ischemia and 120 min reperfusion; hearts not used for mitochondrial isolation were stained with 2,3,5-triphenyltetrazolium chloride (TTC) and infarct size was determined as a percentage of total ventricular heart weight.4, 18, 28, 32 Infarct size measurements were blinded to the drug administered.
Isolated guinea pig hearts (18 groups of 6–8 hearts each) were perfused with no drugs (control) or test drugs, i.e. BKCa or SKCa agonists or antagonists, or TBAP alone or in combination, before and after the 35 min period of global ischemia. Time control (TC) experiments were also conducted, i.e., perfusion of isolated hearts with vehicle (DMSO) but without drugs or IR for the duration of the IR protocol. Time control (TC, no IR) functional variables decreased less than 5% over a 2.5 h period. To assess endogenous cardioprotection mediated by IR-induced BKCa and SKCa channel activation, antagonists were given beginning 20 min before ischemia. To assess exogenous BKCa and or SKCa activation, agonists were given beginning 15 min before ischemia; all drugs were discontinued 20 min after the onset of reperfusion. Functional variables for display in figures were recorded at 25 min before ischemia (baseline) and at 120 min reperfusion. Concentrations of drugs were gauged to elicit maximal effects as reported in the literature.35–38 Drugs were dissolved in less than 0.1% DMSO in KR buffer (vehicle) and perfused into isolated hearts. They were: 3 μM DCEBIO (DCEB), a non-selective KCa2.2 and KCa2.3 channel agonist;7, 20–23 10 μM NS8593, a specific antagonist of SKCa channels;24, 25 3 μM NS1619, an agonist of BKCa channels; 40 μM paxilline (PAX), an antagonist of BKCa channels,39 and 20 μM TBAP, a chemical dismutator of O2•− that preferentially enters the mitochondrial matrix to convert O2•− to H2O2, which is eventually converted to H2O. NS8593 was selected as a specific antagonist of SKCa channels because DCEB can also open IKCa channels.7, 9, 19, 23 Generally, combinations of drugs would include an agonist with an antagonist, both agonists, both antagonists, or TBAP with either or both agonists. The protocols were designed to expose any protection afforded by endogenous BKCa vs. SKCa channel activation and any potential additive or potentiating cardiac protective or anti-protective effects of drug-induced opening or blocking of BKCa and SKCa channels.
Infarction in In Vivo Rat Hearts after Regional IR Injury
Adult rats (250–300 g) of either sex were anesthetized with inactin 150 mg/kg i.p., intubated, and artificial respiration was initiated. The right carotid artery was cannulated to monitor blood pressure continuously. After thoracotomy a suture was placed around the proximal LAD to induce reversible coronary artery occlusion verified by regional epicardial cyanosis. Rats were chosen because the area-at-risk for cardiac infarction is more uniform than in guinea pigs.40 Hearts (6 groups of 4 each) were subjected to 35 min LAD occlusion by tightening the suture; releasing the suture was followed by 120 min reperfusion. Other hearts had the suture placed around the LAD but not tightened (sham, time control, (TC)). Inactin, 25 mg/kg i.p., was supplemented as needed. Infarct size was assessed after reperfusion using the Evan’s Blue area-at-risk and TTC staining techniques and expressed as percent ventricular area-at-risk. Drugs, dissolved in 0.1% DMSO and infused at 0.35 mL/h via the left jugular vein, were saline + 0.1% DMSO (vehicle control) or DCEB (30 μg/kg/min), NS1619 (30 μg/kg/min), NS8593 (100 μg/kg/min), or PAX (20 μg/kg/min). Total DMSO infused was less than 250 μg/h. Drug concentrations for infusion were obtained from the literature.35–38 PAX and NS8593, the antagonists for the BKCa and SKCa channels, respectively, were also perfused together to assess for any additive damaging effects. Drugs were infused 20 min before regional ischemia and for 20 min after release of the LAD occlusion. Infarct size measurements were blinded to the drug administered.
Respiration and Ca2+ Retention Capacity in Mitochondria Isolated after Global IR Injury
Ex vivo guinea pig hearts were subjected to global ischemia with or without drug treatment as described above. At 20 min of reperfusion some hearts (6 groups of approximately 5 each) that were not used to measure infarct size were removed from the perfusion apparatus and the ventricles were minced in cold mitochondrial isolation buffer on ice. Mitochondria were isolated by differential centrifugation as described previously5, 6, 41–46 and the mitochondria (0.5 mg protein/mL) were suspended in respiration buffer containing 130 mM KCl, 5 mM K2HPO4, 20 mM MOPS, 40 μM EGTA (carried over from the isolation buffer), 1 μM Na4P2O7, 0.1% BSA, pH 7.15 adjusted with KOH. To test mitochondrial viability and function, the respiratory control index (RCI, state 3/state 4) was determined under different substrate conditions: Na-pyruvate (P, 10 mM) or Na-succinate (S, 10 mM) or S + rotenone (R, 4 μM). State 3 respiration was determined after adding 250 μM ADP. In other experiments using the same mitochondrial pellet, Ca2+ retention capacity (CRC) was evaluated by measuring the decline in buffer [Ca2+] with Fura-4 penta-K+ using fluorescence spectrophotometry (QM-8, Horiba/Photon Technology International, PTI) as described previously44–48 during bolus additions of 20 μM CaCl2 every 90 s. Based on the similar improvement in cardiac function by individual or combined agonists, only the mitochondrial effects of the combined agonists NS1619 and DCEB were examined in the respiration and CRC experiments.
Statistical Analyses of Heart and Mitochondrial Data
Data obtained from hearts and mitochondria were expressed as means ± standard error of means. Appropriate comparisons were made among groups that differed by a variable at a given condition or time. Statistical differences were measured across groups using data comparisons at 25 min before global or regional ischemia vs. 120 of reperfusion (ex vivo and in vivo hearts). Mitochondria were isolated 20 min after reperfusion and data were collected within 2 h of isolation. Differences among variables were determined by two-way multiple ANOVA for repeated measures (Statview® and CLR anova® software programs for Macintosh®); if F tests were significant, appropriate post-hoc tests (e.g., Student-Newman-Keul’s, SNK) were used to compare means. Mean values were considered significant at P values (two-tailed) <0.05.
Results
Global Protection by BKCa and SKCa Agonists and Enhanced Damage by BKCa plus SKCa Antagonists after Cardiac IR Injury in Ex Vivo Guinea Pig Isolated Hearts
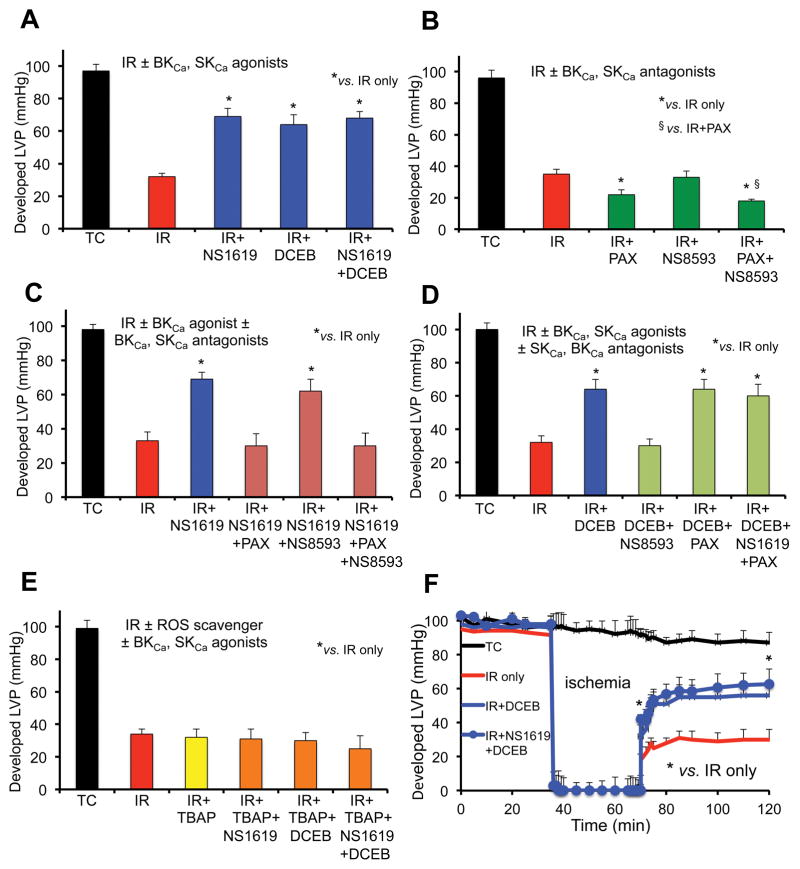
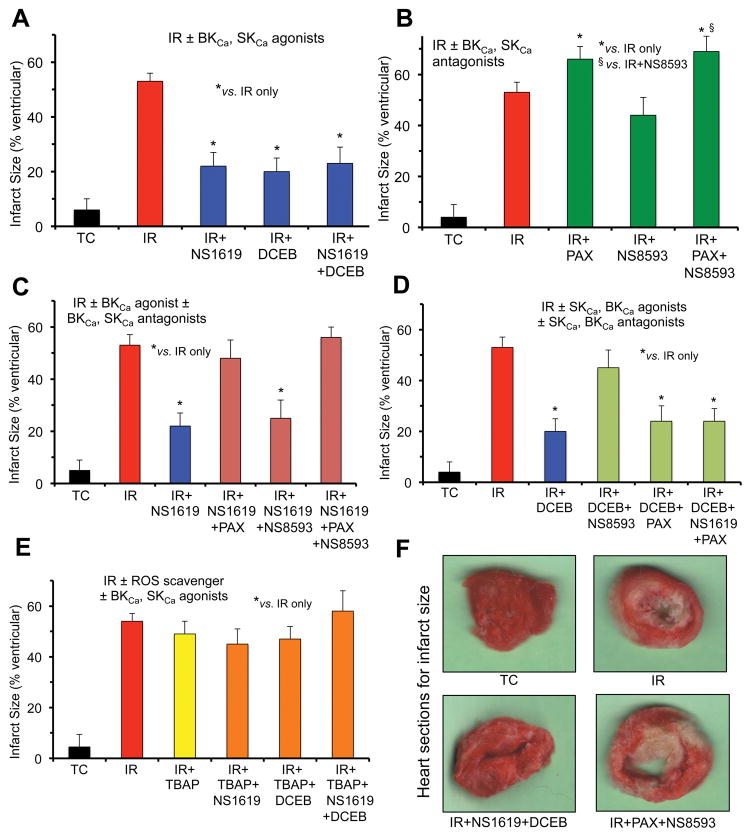
We conducted an extensive analyses of the interactions of SKCa and BKCa agonists and antagonists on cardiac function and tissue damage based on assessment in changes in developed LVP (Fig. 1A–F) and % infarct size (Fig. 2A–F) after perfusion of hearts with single or combined drugs. Developed LVP was markedly decreased after 35 min global ischemia and 120 min reperfusion compared to TC (Fig. 1, IR) whereas % infarct size was markedly increased (Fig. 2, IR). After perfusion of hearts with the agonists and antagonists we found that: a) DCEB or NS1619 alone was equally protective as was combining NS1619 + DCEB on developed LVP (Fig. 1A) and infarct size (Fig. 2A); b) NS8593 alone did not worsen cardiac function (Fig. 1B) or infarct size (Fig. 2B) when compared to IR injury alone, whereas PAX alone and PAX + NS8593 further worsened cardiac function and infarct size; c) protection by NS1619 (Figs. 1C, 2C) was blocked by PAX and PAX+NS8593 but not by NS8593 alone; d) protection by DCEB (Fig. 1D, Fig. 2D) was blocked by NS8593 but not altered by PAX alone or by NS1619 + PAX; e) TBAP completely blocked protection induced either by NS1619 or DCEB or combined NS1619 + DCEB (Figs. 1E, 2E). In general, the effects of the BKCa and SKCa agonists and antagonists on developed LVP (Fig. 1) were approximately inversely proportional to their effects on infarct size (Fig. 2). Spontaneous heart rate before ischemia was approximately 240 beats/min in TCs and before ischemia. Heart rate, LVP and infarct size were not affected by perfusion of any of the drugs before IR injury; heart rate and coronary flow returned to control values during the reperfusion phase in all groups.
Fig. 1.
Average developed (systolic-diastolic) left ventricular pressure (LVP) 120 min after global IR injury when isolated guinea pig hearts were perfused without ischemia (Time Controls, TC); with 35 min ischemia and 120 min reperfusion (IR); or with IR + BKCa and or SKCa channel agonists (A); IR + BKCa and or SKCa channel antagonists (B); IR + BKCa agonist and or BKCa, SKCa antagonists (C); IR + BKCa agonist and or SKCa or BKCa antagonist (D); and SOD dismutator + BKCa and or SKCa agonist (E). Developed LVP over time for the IR only, IR + SKCa agonist and SKCa + BKCa agonist groups are displayed (F). Note the worsened effects on developed LVP of BKCa and BKCa + SKCa antagonists (green bars) vs. IR only (red bars); the block of protection by the BKCa or SKCa agonists when the BKCa or SKCa antagonists, respectively, was present (green bars); the maintained protection by the BKCa and or SKCa agonists in the presence of either the SKCa or the BKCa antagonist, respectively, (blue bars); and the loss of protection by the SKCa and or BKCa agonists in the presence of SOD inhibition by TBAP (orange/green bars). For each treatment group n = 5–6 hearts; note that for 32 of these hearts’ mitochondria were isolated after 20 min reperfusion to assess RCI and CRC (Figs. 4,5). Data expressed as mean ± sem. *,§ P<0.05.
Fig. 2.
Percent infarct size assessed 120 min after global IR injury when the same (Fig. 1) isolated guinea pig hearts were perfused without ischemia (Time Controls, TC); with 35 min ischemia and 120 min reperfusion (IR); or with IR + BKCa and or SKCa channel agonists (A); IR + BKCa and or SKCa channel antagonists (B); IR + BKCa agonist and or BKCa, SKCa antagonists (C); IR + BKCa agonist and or SKCa or BKCa antagonist (D); and SOD dismutator + BKCa and or SKCa agonist (E). Representative hearts from 4 groups displaying normal and infarcted tissue are displayed (F). Note the enhanced infarct size after treatment with BKCa or BKCa + SKCa antagonists (green bars) vs. IR only (red bars); the block of tissue protection by the BKCa or SKCa agonists when the BKCa or SKCa antagonists, respectively, was present (green bars); the maintained reduction in infarct size by the BKCa and or SKCa agonists in the presence of either the SKCa or the BKCa antagonist, respectively, (blue bars); and the loss of protection by the SKCa and or BKCa agonists in the presence of SOD inhibition by TBAP (orange/green bars). For each treatment and control group n=4. *,§P<0.05.
Tissue Protection by BKCa and SKCa Agonists and Enhanced Tissue Damage by BKCa plus SKCa Antagonists after Regional IR Injury in in Vivo Rat Hearts
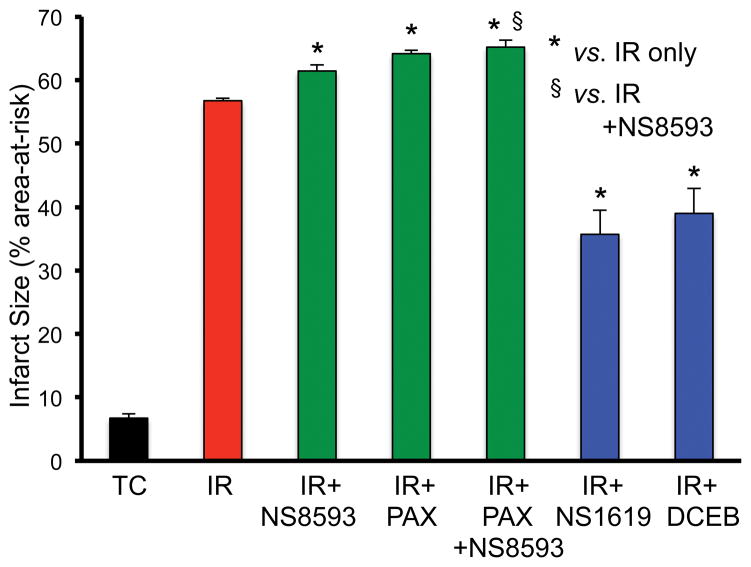
We then tested if the BKCa and SKCa agonists and antagonists had any effects on the heart when delivered intravenously before ischemia and during reperfusion. Rats were infused with vehicle, PAX, NS8593, or PAX+NS8593, or with NS1619 or DCEB, before and after regional ischemia (LAD occlusion). Infarct size (% area-at-risk) was assessed after 120 min reperfusion (Fig. 3). There was no significant infarction in the sham animals and the infusion of drugs did not alter LVP or heart rate (data not shown) before IR. NS8593 or PAX infused alone enhanced infarct size whereas simultaneous infusion of both NS8593 and PAX additionally enhanced infarct size; infusion of either NS1619 or DCEB reduced infarct size.
Fig. 3.
Infarct size (% area-at-risk) without (time control, TC) or after regional ischemia (LAD occlusion, IR) when 28 intact rats were infused i.v. with no drug (IR only), or with IR plus BKCa and or SKCa channel antagonists, or with the BKCa or SKCa channel agonist. Note that treatment either with the SKCa or the BKCa channel antagonist (green bars), or both together, increased infarct size vs. IR only (red bar), and that treatment with either the BKCa or SKCa agonist reduced infarct size (blue bars) vs. IR only. Average left ventricular area-at-risk was 39.2±2.1. For each treatment and control (sham) group n=4. *,§P<0.05.
Overall, these heart experiments demonstrated endogenous SKCa and BKCa channel activation in vivo rat hearts based on their antagonists’ effects to enhance regional infarct size as well as endogenous BKCa channel activation in ex vivo guinea pig hearts based on an additive effect of inhibiting both channels on contractile function. The experiments also established the selectivity of the respective antagonists to block their respective agonists, and the role of ROS in mediating the exogenous protection afforded by both BKCa and SKCa channel agonists.
Mitochondrial Respiration and mCa2+ Uptake after IR injury in Guinea Pig Ex Vivo Hearts Treated with BKCa and SKCa Agonists or Antagonists
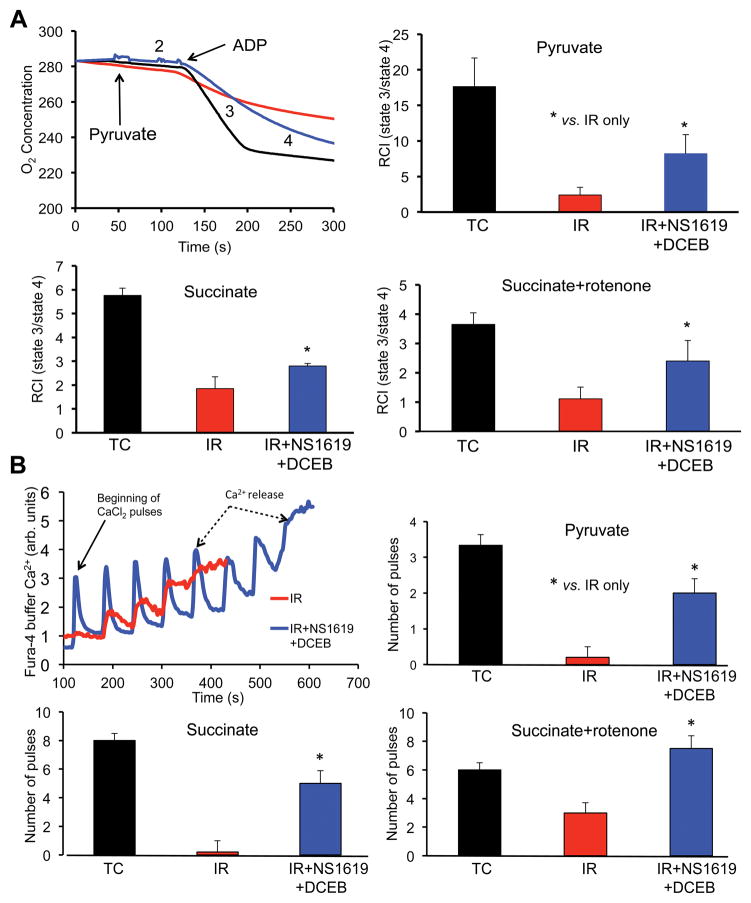
Cardiac protection due to BKCa and SKCa channel opening may be mediated largely by effects on mitochondrial bioenergetics. Thus we tested if combined BKCa and SKCa agonists improved oxidative-phosphorylation assessed by respiration rates and RCI in cardiac mitochondria isolated 20 min after reperfusion and 35 min global ischemia (Table 1 and Fig. 4). The agonists were given together because we observed equivalent effects with the single agonists in the hearts and because the agonists given together did not additionally improve cardiac function or reduce infarct size when compared to IR control (Figs. 1–3). Representative traces of O2 consumption (respiratory rate) in mitochondria energized with pyruvate (Fig. 4A upper left) are shown with mean RCI’s under the three substrate conditions (Fig. 4A). Under each substrate condition, states 2 and 4 respiration (Table 1) were increased, and state 3 respiration was decreased in mitochondria isolated after 20 min reperfusion and 35 min of ischemia (red tracing/bars) compared to no IR (TC, black tracing/bars) (Fig. 4A); thus RCI was markedly reduced by 2 to 6 fold indicating significant uncoupling of phosphorylation from oxidation (Table 1). In hearts treated during IR with the combined BKCa and SKCa agonists, NS1619 and DCEB (blue tracing/bars), RCI’s (state 3/state 4) improved under each substrate condition compared to IR alone (Fig. 4).
Table 1.
Modulation of respiration rate in isolated cardiac mitochondria after cardiac IR injury ±BKCa + SKCa channel agonists.
| Pyruvate | state 2 | state 3 | state 4 | RCI |
|---|---|---|---|---|
| TC | 6.7 ±0.7 | 222.4 ±23.5 | 11.6 ±3.8 | 17.6 ±2.6 |
| IR | 37.6 ±0.7§ | 125.3 ±53.2§ | 69.0 ±17.1§ | 2.4 ±1.1§ |
| IR+NS1619+DCEB | 7.3 ±4.2 | 221.8 ±31.3* | 33.4 ±4.4*§ | 8.2 ±2.7*§ |
| Succinate | state 2 | state 3 | state 4 | RCI |
|
| ||||
| TC | 65.0 ±0.7 | 457.6 ±38.6 | 79.7 ±15.4 | 5.8 ±0.4 |
| IR | 73.7 ±9.4 | 203.7 ±49.0§ | 114.6 ±7.7§ | 1.9 ±0.6§ |
| IR+NS1619+DCEB | 96.9 ±9.1*§ | 456.5 ±6.8* | 181.5 ±10.3*§ | 2.8 ±0.2*§ |
| Succinate+rotenone | state 2 | state 3 | state 4 | RCI |
|
| ||||
| TC | 63.0 ±10.7 | 281.4 ±57.0 | 77.1 ±0.7 | 3.7 ±0.5 |
| IR | 63.0 ±5.4 | 113.2 ±67.0§ | 100.5 ±17.7§ | 1.1 ±0.6§ |
| IR+ NS1619+DCEB | 82.7 ±13.7 | 312.0 ±72*§ | 138.0 ±4.9§ | 2.4 ±0.5*§ |
Respiration rate (O2 consumption, nmol/min/mg protein) was measured in cardiac mitochondria isolated at 20 min perfusion after 35 min global ischemia ± BKCa and SKCa channel agonists NS1619 and DCEB given 15 min before and for 20 min after ischemia. RCI (respiratory control index) = state 3/state 4. Time controls (TC) were not subject to ischemia but 75 min perfusion. Note that RCI’s were higher after hearts were treated with NS1619 + DCEB before IR vs. IR alone with pyruvate and succinate + rotenone, but not with succinate. N = hearts/group with 3–4 replicates within each mitochondrial pellet. P<0.05;
IR + drugs vs. IR only;
IR ± drugs vs. TC.
See also Fig. 3A.
Fig. 4.
Effect of no treatment (TC, no I/R), IR only, or IR + BKCa channel agonist NS1619 + SKCa channel agonist DCEB, in 12 isolated guinea pig hearts on respiration (A) and Ca2+ retention capacity (CRC) (B) in mitochondria isolated after 20 min reperfusion from groups described in Figs. 1,2. The rate of O2 consumption (respiration) was measured when substrate was added (state 2), followed by ADP (state 3) and after the added ADP was consumed (state 4); substrates added were pyruvate, succinate, or succinate + rotenone (see also Table 1); state 3/state 4 ratio is defined as the respiratory control index (RCI). Uptake of external Ca2+ was assessed (Fura-4 fluorescence) in mitochondria energized under the same 3 substrate conditions; increased number of 20 μM CaCl2 injections (pulses) = increased CRC. Note increased RCI and retained Ca2+ (CRC) with combined BKCa and SKCa agonist treatment vs. IR only. For each group n = 4; RCI and CRC were measured from same mitochondrial pellet from each heart. *,§P<0.05.
By using the same mitochondrial pellets, we also monitored mitochondrial structural integrity by challenging isolated mitochondria with boluses of 20 μM CaCl2 added to the respiration buffer (Fig. 4B) until the capability to take up and sequester CaCl2 (CRC) was exceeded so that mitochondria extrude Ca2+ through the so-called mitochondrial permeability transition pore (mPTP). Representative traces of CRC in mitochondria energized under succinate + rotenone conditions are displayed (Fig. 4B, top left) with mean CRC’s under the 3 substrate conditions during state 2 respiration (Fig. 4B); cumulative mitochondrial Ca2+ uptake was reduced after IR injury because of early mPTP opening but was greater after hearts were treated with NS1619 and DCEB (Fig. 4B, red and blue tracings). Total average mitochondrial Ca2+ uptake before mPTP activation was greater when ex vivo hearts were perfused with NS1619 + DCEB before and after ischemia in mitochondria energized with any of the three substrate conditions (columns Fig. 4B). This indicated that relatively uncoupled mitochondria have elevated mitochondrial Ca2+ levels during and after IR as we have shown previously in intact hearts,27, 31, 49 or have less capacity to sequester the added CaCl2. The higher RCIs with BKCa and SKCa agonist treatment during IR coincided with greater mitochondrial CRCs.
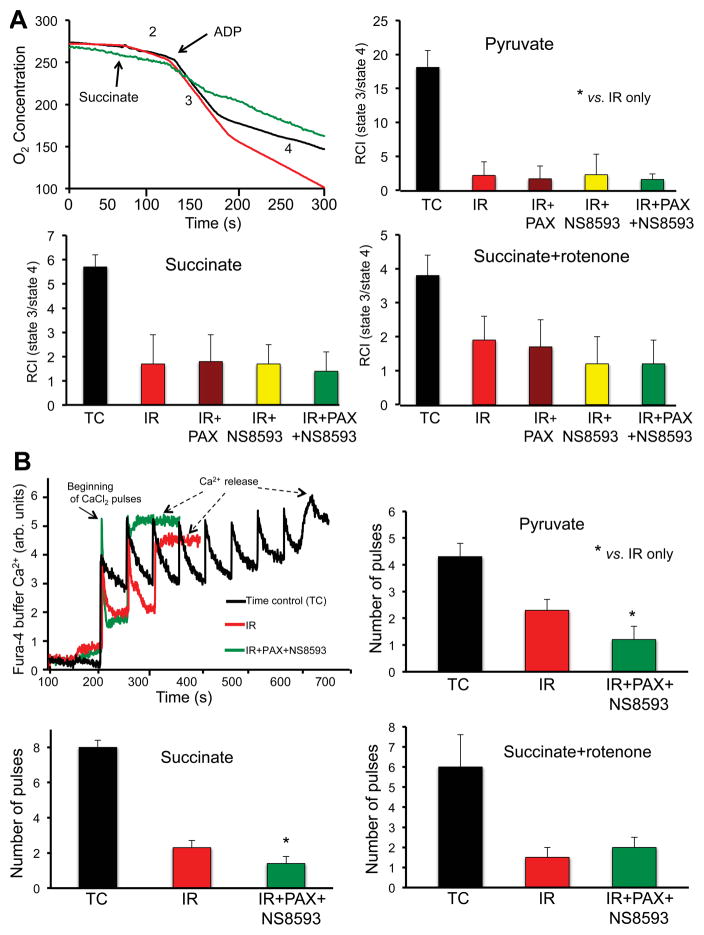
We also determined if BKCa and/or SKCa antagonism worsened oxidative-phosphorylation assessed by respiration rates and RCI in cardiac mitochondria isolated after 20 min reperfusion and 35 min global ischemia (Table 2, Fig. 5A). The antagonists were given separately and together because we observed different effects of NS8593 when given alone or with PAX on cardiac function and infarct size (Figs. 1–3). Representative traces of O2 consumption in mitochondria energized with succinate (Fig 5A upper left) are displayed with mean RCI’s under the three substrate conditions (Fig. 5A). Similar to data in Table 1 and Fig. 4A for the agonist experiments, states 2 and 4 respiratory rates were increased, and state 3 respiration was decreased in mitochondria isolated after ischemia and 20 min reperfusion (red tracing/bars) compared to no IR (TC, black tracing/bars) (Table 2, Fig. 5A). Treatment of hearts before and after IR with BKCa and or SKCa antagonists PAX and NS8593 (green tracing/bar) did not worsen average RCI’s (state 3/state 4) compared to IR alone under any substrate condition, which is different from the cardiac functional and tissue infarction results. This is not unexpected because the respiratory data is derived from an aggregate of functional and dysfunctional mitochondria retrieved during the isolation process and the variability of respiratory measurements may be insufficient to distinguish differences.
Table 2.
Modulation of respiration rate in isolated cardiac mitochondria after cardiac IR injury ±BKCa + SKCa and/or SKCa channel antagonists.
| Pyruvate | state 2 | state 3 | state 4 | RCI |
|---|---|---|---|---|
| TC | 6.8 ±0.7 | 210.2 ±22.4 | 11.6 ±3.8 | 18.1 ±2.5 |
| IR | 35.7 ±2.3§ | 112.0 ±33.9§ | 59.4 ±3.4§ | 2.2 ±2.0§ |
| IR+PAX | 12.0 ±2.6*§ | 92.3 ±25.2§ | 53.4 ±3.6§ | 1.7 ±1.9§ |
| IR+NS8593 | 30.9 ±2.1*§ | 145.1 ±37.0§ | 58.6 ±6.2§ | 2.3 ±3.0§ |
| IR+PAX+NS8593 | 20.6 ±1.1*§ | 96.0 ±20.1§ | 59.4 ±3.7§ | 1.6 ±0.8§ |
| Succinate | state 2 | state 3 | state 4 | RCI |
|
| ||||
| TC | 64.5 ±0.8 | 450.4 ±37.5 | 79.5 ±10.3 | 5.7 ±0.5 |
| IR | 72.0 ±3.2§ | 220.2 ±32.9§ | 129.0 ±12.0§ | 1.7 ±1.2§ |
| IR+PAX | 90.1 ±6.1*§ | 286.2 ±38.8§ | 154.1 ±14.2§ | 1.8 ±1.1§ |
| IR+NS8593 | 168.1 ±8.2*§ | 280.0 ±53.3§ | 161.1 ±18.2§ | 1.7 ±0.8§ |
| IR+PAX+NS8593 | 116.1 ±9.5*§ | 246.0 ±38.5§ | 178.2 ±15.2*§ | 1.4 ±0.8§ |
| Succinate+rotenone | state 2 | state 3 | state 4 | RCI |
|
| ||||
| TC | 62.4 ± 9.8 | 290.5 ±56.2 | 76.8 ±0.8 | 3.8 ±0.6 |
| IR | 87.2 ± 6.4 | 202.0 ±32.9 | 105.2 ±16.4§ | 1.9 ±0.7§ |
| IR+PAX | 77.4 ±11.6 | 234.2 ±55.1 | 136.0 ±33.7§ | 1.7 ±0.8§ |
| IR+NS8593 | 96.2 ±14.2§ | 312.0 ±48.6* | 261.3 ±38.2*§ | 1.2 ±0.8§ |
| IR+PAX+NS8593 | 108.2 ±26.0§ | 305.3 ±45.7* | 262.3 ±31.6*§ | 1.2 ±0.7§ |
Respiration rate (O2 consumption, nmol/min/mg protein) was measured in cardiac mitochondria isolated at 20 min perfusion after 35 min global ischemia ± BKCa and SKCa channel antagonists NS8593 ± PAX given 20 min before and for 20 min after ischemia. RCI (respiratory control index) = state 3/state 4. Time controls (TC) were not subject to ischemia but 75 min perfusion. Note that the lower RCI’s after IR were not significantly lower after hearts were treated with PAX ± NS8593 compared to IR alone with any substrate. N = hearts/group with 3–4 replicates within each mitochondrial pellet. P<0.05;
IR + drugs vs. IR only;
IR ± drugs vs. TC.
See also Fig. 4A.
Fig. 5.
Effect of no treatment (TC, no I/R), IR only, or IR + BKCa channel antagonist PAX ± SKCa channel antagonist NS8593, in 20 isolated guinea pig hearts on respiration (A) and Ca2+ retention capacity (CRC) (B) in mitochondria isolated after 20 min reperfusion from groups described in Figs. 1,2. The rate of O2 consumption (respiration) was measured when substrate was added (state 2), followed by ADP (state 3) and after the added ADP was consumed (state 4); substrates added were pyruvate, succinate, or succinate + rotenone (see also Table 2); state 3/state 4 ratio is defined as the respiratory control index (RCI). Uptake of external Ca2+ was assessed (Fura-4 fluorescence) in mitochondria energized under the same 3 substrate conditions; increased number of 20 μM CaCl2 injections (pulses) = increased CRC. Note hat the decreased RCI with IR only was not different after treatment with the BKCa and or SKCa antagonists; in contrast, CRC was reduced after treatment with the BKCa and or SKCa antagonists in the presence of pyruvate or succinate. For each group n = 4; RCI and CRC were measured from same mitochondrial pellet from each heart. * P<0.05.
Using the same mitochondrial pellets isolated after IR injury, we again monitored mitochondrial structural integrity by challenging isolated mitochondria with boluses of 20 μM CaCl2 (Fig. 5B). Treatment with the combination of PAX and NS8593 slightly reduced CRC in mitochondria energized with pyruvate or succinate, but not with succinate + rotenone, when compared to IR alone. The reduced CRC after combined BKCa and SKCa antagonist treatment during IR corroborates the results on cardiac functional and tissue infarction.
Discussion
Our results demonstrate that intrinsic activation of BKCa and SKCa channels provides endogenous cardiac functional protection (Fig. 1B) in the ex vivo cardiac global IR model on the basis of lowered developed LVP in the presence of PAX alone, and more so in the presence of PAX+NS8593 vs. IR alone; however, only the presence of PAX enhanced global infarct size (Fig. 2B). In contrast, in the in vivo model of regional IR injury (Fig. 3), regional infarct size was larger in the presence of either PAX or NS8593 and even larger with infusion of both PAX and NS8593. The specific agonists for the BKCa and the SKCa channels, NS1619 and DCEB, provided equivalent protection against IR injury without additive effects. Our protocol for the isolated heart experiments was different from that used in our prior studies in which the BKCa and SKCa agonists were given only briefly to trigger pharmacologic preconditioning (PPC) and the antagonists were given to prevent PPC.4,18 The finding that NS8593 alone did not enhance infarct size in the ex vivo global ischemia model as it did in the in vivo LAD occlusion model could be related to many factors, including differences in species, anesthetics given, collateral flow, potential for ischemic or anesthetic preconditioning, methods of inducing ischemia and drug delivery. Nevertheless, the present results suggest that opening or preventing the opening of these K+ channels leads to protective or anti-protective effects, respectively, via a common downstream mediator that is likely mitochondrial generated superoxide (O2•−) as observed by the loss of protection in the presence of TBAP (Figs. 1E, 2E).
In combination, the BKCa and the SKCa channel agonists NS1619 and DCEB did not provide additional protection, unlike the combined antagonists that worsened function ex vivo, and enhanced infarct size in vivo. This may indicate that providing either agonist exogenously provides maximal protection, whereas both channels are endogenously important in reducing IR injury. Because the mitochondrial targeted O2•− scavenger, TBAP, given before and after ischemia, abrogated the functional recovery and the magnitude of infarction by both agonists alone or in combination, this suggested that the protection was mediated in large part by a mitochondrial effect to stimulate O2•− generation. This was further supported not only by effects of BKCa and SKCa agonists to improve oxidative phosphorylation (RCI) and retain excess mCa2+ (CRC) (Fig. 4) but also by effects of BKCa and SKCa antagonists to reduce Ca2+ loading after IR injury (Fig. 5). Overall, our novel observations suggest an important role for IR-induced BKCa and SKCa channel activation in cardiomyocyte mitochondria in providing endogenous protection against IR injury as suggested by treatment of hearts with either or both channel antagonists.
BKCa and SKCa Channel Agonists Reduce and Antagonists Enhance Cardiac IR Injury via a Mitochondrial Mechanism
Individually, BKCa and SKCa channels appear to be important for protecting against cardiac myocyte damage during oxidative stress.4, 18, 26, 50–53 Allowing for possible non-specific effects of the agonists and antagonists, our data furnish support that pharmacologic opening of either BKCa or SKCa channels is cardioprotective. Moreover, intrinsic activation of BKCa or SKCa channels, and especially when activated together, may be essential for endogenous protection against IR injury. In contrast, because there were no additive effects of the two agonists (Figs. 1A, 2A), protection by either of these agonists may have provided maximal protection via a mK+ uptake mechanism.
In our experiments the antagonists were given before, during and after ischemia to test for possible endogenous cardioprotection. Due to different K+ channel characteristics and endogenous protective effects we speculate that mSKCa channels open gradually under conditions in which ΔΨm becomes reduced, pHm becomes progressively lower, and matrix Ca2+ progressively rises, as occurs during ischemia. In contrast, mBKCa channel opening may occur when ΔΨm rebounds, cytosolic Ca2+ surges, and pHm is high, as during initial reperfusion. This purported timing of opening of these channels, although likely over-simplified, is based on our studies of changes in redox state (NAHD, FAD), diastolic, systolic, and mitochondrial matrix [Ca2+], and cytosolic [Na+] during the time course of IR injury.27, 54–58
Importantly, the BKCa and SKCa channel agonists and antagonists were bioactive when infused intravenously, as evidenced by the differences in cardiac infarct size after LAD occlusion in the in vivo rat model (Fig. 3). This suggests a translational value for potential intravenous treatment of KCa channel agonists during cardiac IR injury in humans. In fact, we have recently identified splice variants of the SKCa channel in human ventricular myocytes,26 which warrants development of SKCa-targeted therapeutic strategies for human cardiac IR injury.
The isolated heart and animal studies could not indicate specifically where the anti- and pro-cardioprotective effects of BKCa and SKCa antagonist and agonists, respectively, occur, i.e. in mitochondria vs. other cellular sites such as the sarcolemma. However, pharmacologic evidence for a role of mitochondrial BKCa and SKCa channels is given by the improved or worsened RCI’s and CRC’s (Fig. 4A, B vs. Fig. 5A, B) in isolated guinea pig mitochondria after ex vivo IR injury in the presence of SKCa and BKCa agonists/antagonists. That the protection is mediated at the mitochondrial level is further supported by the effect of the free radical scavenger TBAP to abolish cardioprotection (Figs. 1E, 2E). This is because the respiratory complexes of the mitochondrial electron transport chain are most likely the major source of signaling ROS for cytoprotection and deleterious excess ROS production during IR injury.1 Indeed, both BKCa50 and SKCa18, 26 channels are located in cardiac cell IMM rather than in the sarcolemmal membrane, and drug-induced opening of the BKCa channel in isolated mitochondria leads to mild ROS production without significantly affecting ΔΨm.6 We believe that this small amount of O2•− emitted is important for signaling mechanisms that confer protection during treatment with a BKCa or SKCa agonist.
Distribution and Function of Ca2+-Sensitive K+ Channels
The cell membranes of vascular smooth muscle, neural, and secretory cells contain large conductance (200–300 pS, i.e. Big Ca2+-sensitive K+ (BKCa, aka maxi-KCa) channels that when activated produce vasodilation, hyperpolarization, and secretion in these cells, respectively. BKCa channel opening is activated by increased cytosolic [Ca2+] and by a large voltage gradient.59 Activation of BKCa channels over a range of [Ca2+] is mediated at several binding sites within the channel60 so that there is a wide range of [Ca2+] responsiveness (Ka 10–1000 μM).61 As K+ exits the cell with BKCa channel opening, this elicits cell membrane repolarization or hyperpolarization, which in turn reduces cell Ca2+ entry by closing voltage-dependent Ca2+ channels. The finding of altered redox potential in smooth muscle cells62 suggested there was a mitochondrial involvement. Indeed, Xu et al.50 provided the first evidence that BKCa channels are prominently located in cardiac mitochondria. In contrast, SKCa channels are not voltage dependent and their agonists interact with their Ca2+ sensor calmodulin in the C-terminus region; however, at least for NS8593, the SKCa-mediated gating modulation occurs via interaction with gating structures near the selectivity filter within the inner pore vestibule.24
Studies of effects of BKCa and SKCa channels in cell membranes might help to assess the mechanisms of protection by their counterparts that reside in the IMM. For example, BKCa channels are proposed to regulate cell membrane potential in neurons.64 SKCa channels in neurons lie adjacent to Ca2+ stores and Ca2+ channels to sense Ca2+ activated K+ flux.65 Neuronal SKCa channels appear to play a role in setting the intrinsic firing frequency, whereas neuronal BKCa channels may regulate action potential shape.15 SKCa channels are also located in neuronal (hippocampus-derived cells) mitochondria where they appear to provide protection against excitotoxicity.17
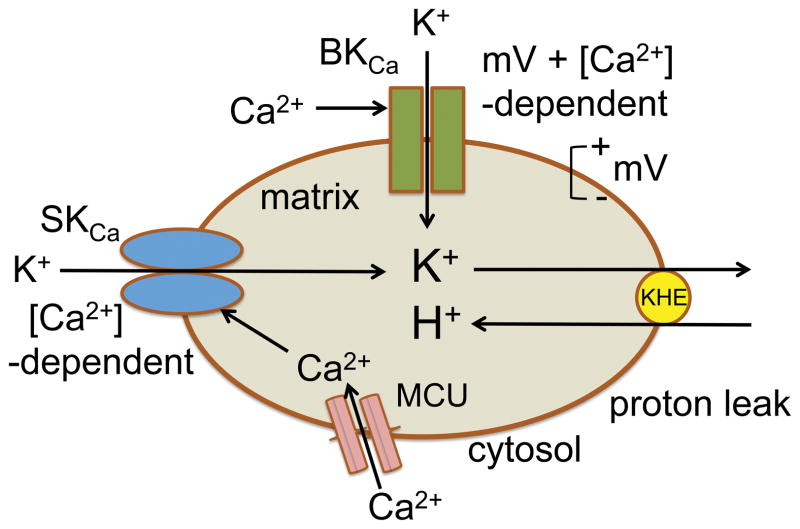
BKCa and SKCa-mediated K+ flux in mitochondria may be differentiated by the number of channels/surface area, sensitivity to Ca2+, and dependence on ΔΨm during mitochondrial respiration. Because there are several differences in the triggers for activating these channels, the functional effects of opening these channel may be different; e.g., unlike for BKCa, SKCa channel opening may “fine tune” matrix K+ influx due to changing matrix Ca2+ levels independent of changes in ΔΨm during the variable rate of oxidative phosphorylation. The presence of the SKCa channels specifically in the IMM18 probably indicate that they have an important function in modulating mitochondrial bioenergetics in response to high matrix [Ca2+], perhaps via modulation of K+-mediated mitochondrial volume that leads to an uncoupling effect63 due to K+/H+ exchange (KHE) (cartoon, Fig. 6). In contrast, the voltage and Ca2+-dependent BKCa channels may open primarily when ΔΨm and pHm are restored and excess cytosolic [Ca2+] leads to high matrix [Ca2+] during reperfusion after ischemia.
Fig. 6.
Schema of proposed interaction of BKCa and SKCa channels on regulation of mitochondrial K+ influx stimulated by increases in cytosolic and mitochondrial [Ca2+] and on reciprocal mitochondrial H+ influx with K+ influx via mitochondrial K+/H+ exchange (KHE).
Protection by mK+ Channel Types Likely Requires Superoxide Release
There is ample evidence that O2•− is necessary to trigger protection by a variety of mK+ channels;1, 3 however, the mechanism of O2•−, and its downstream mediators, in mediating protection remain unresolved. An increase in redox state (increased NADH, decreased FAD) at a given [O2] can result in a small increase in signaling O2•− generation.66 This is different than the much larger amount of deleterious O2•− “bursts” that occur during reperfusion when excess O2 is available but mitochondrial respiration is compromised. Our group4, 28, 29, 67 and others68, 69, 70 have shown, moreover, that O2•− and its products are also formed in excess during ischemia despite lower tissue O2 tension.
In the present study, evidence that the protective effects of DCEB and NS1619 are mediated by signaling O2•− is again indicated by reversal of the protection in the presence of TBAP as shown previously for SKCa18, 26 and BKCa4 channel agonists. Enhanced transfer of electrons by uncoupling before ischemia may minimize respiratory inefficiency; i.e., by reducing matrix contraction and improving respiration on reperfusion. mSKCa channel opening, like opening of any mK+ channel, could induce protection by a small increase in O2•− generation to stimulate enzymatic pathways that help to protect cells from IR injury. In any case, we propose that TBAP accelerates O2•− dismutation to H2O2 and thus blocks the signaling O2•− that leads to protection by either NS1619 or DCEB. We have reported in isolated cardiac mitochondria that low, but not high, concentrations of the BKCa channel opener NS1619 increased resting state 4 respiration and mildly enhanced H2O2 levels while maintaining ΔΨm.6
We have suggested, moreover,63 that an increase in intra-matrix K+ is replaced over time with H+ via mKHE and that at low concentrations, these openers produce a transient increase in matrix acidity, i.e., in which the proton leak stimulates respiration but maintains ΔΨm so that signaling O2•− is generated at mitochondrial respiratory complexes due to accelerated electron transport. Therefore, during the periods of ischemia and reperfusion, mK+ influx, due to condition-dependent activation of SKCa and BKCa channels, and, in turn, mKHE may trigger a signaling amount of O2•−, due to enhanced respiration and electron leak, that then stimulates downstream signaling pathways. The net effect of improved mitochondrial bioenergetics during late ischemia and reperfusion, i.e., higher redox state, lower mCa2+, and reduced deleterious O2•− emission contributes to improved contractile and relaxant function.29, 31, 57, 71
Ca2+-induced mK+ Influx Causes Mitochondrial Swelling and Changes in Respiration
We have reported that cytosolic and mCa2+ both increase steadily during the period of cardiac ischemia and that cytosolic Ca2+ peaks on initial reperfusion.27, 49 During IR injury the rise in cytosolic and in mCa2+ likely triggers mK+ influx via mBKCa and mSKCa channel activation to initially induce matrix swelling as a potential protective mechanism. Alternatively, or in addition, H+ influx via mKHE may enhance respiration to maintain ΔΨm but also induce signaling levels of O2•− (cartoon, Fig. 6). We observed that PAX, NS1619 and PAX+NS8593 treatments reduced the increase in pyruvate-mediated state two respiration found after IR alone, but increased the succinate-mediated state 2 respiration compared to IR alone (Table 2). This implies that endogenous mKCa channel activation does lead to mild uncoupling via mKHE during succinate but not during pyruvate consumption. It is also possible that K+ is required for optimal functioning of oxidative phosphorylation because mK+ flux largely regulates mitochondrial volume that in turn may modulate bioenergetics.72–74 Thus mSKCa channels, like mBKCa channels,50, 75 may also act to modulate mitochondrial volume during the increase in matrix Ca2+ loading that occurs during ischemia and more so on reperfusion.27, 31 Xu et al.50 suggested that opening mBKCa channels to enhance matrix K+ influx mitigates IR injury in a manner similar to putative mKATP channel opening. They proposed50 that the function of mBKCa channels was to improve the efficiency of mitochondrial energy production. In addition to mBKCa and mSKCa channels, and the putative mKATP channels,72, 75–79 another K+ channel, the Na+-activated K+ channel, SLO2,80, 81 has been reported to be located in mitochondria; this channel is also cytoprotective, at least in part due to activation of mK+ influx. Thus, although the mK+ channel sensing conditions may be different, the net effect of mK+ influx, increases in mitochondrial volume and or mH+ via mKHE, appears to be the same.
Because mKCa channels likely play a major role in regulating mitochondrial bioenergetics by modulating ion flux, it is unclear exactly how opening of these channels leads to higher NADH/FAD ratios and decreases in excess ROS and in Ca2+ overload during IR. For the presumptive mKATP channel, it was proposed that its opening depolarizes the IMM and decreases mCa2+ overload.75, 76, 82 It should be noted that Garlid’s group72, 83 has proposed that the physiological role of mK+ channel opening is control of mitochondrial volume rather than dissipation of ΔΨm and uncoupling, because swelling induced by mK+ uptake (without mKHE) would occur with concomitant uptake of Cl− and subsequently water by osmosis. They suggested that subsequent activation of mKHE would only slightly dissipate the proton motive gradient (ΔμH) by increasing matrix acidity (H+ leak) without significantly altering ΔΨm.73, 83
We found that NS1619 resulted in an increase in mK+ uptake, but only in the presence of quinine, a non-specific KHE blocker;63 this suggested that mKHE promoted mK+ efflux over time. However, our results also indicated that mpH was lower when quinine was present; this led to the suggestion that mH+ influxy induced by NS1619 was not exclusively via mKHE and that NS1619 may also directly or indirectly act as a protonophore. Moreover, valinomycin-induced mK+ influx exerted a biphasic effect, i.e. to alkalinize and then to acidify the matrix.63 Thus the mechanism and timing remain unclear. What does appear apparent is that either agonist of mKCa channels leads to improved mitochondrial bioenergetics, with reduced mCa2+ overload and ROS production, which thereby provides overall substantial mitochondrial and myocyte protective effects during cardiac IR injury ex vivo and reduced infarct size ex vivo and in vivo.
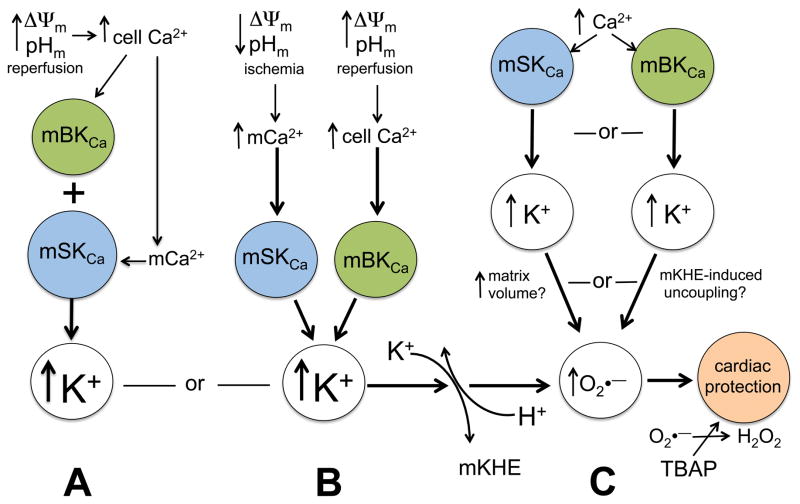
Based on limited experimental observations and some speculation, there are several possible scenarios of potential mechanisms for mBKCa and mSKCa activation and effectors that lead to cardiac protection (cartoon, Fig. 7). In scenario A, as reperfusion restores cytosolic pH, this promotes excess cytosolic Ca2+ loading by sarcolemal NHE and NCE, which then causes excess mCa2+ loading with ΔΨm repolarization; in turn this activates mK+ influx predominately via mBKCa but also via mSKCa channels due to increased mCa2+. In scenario B, as ischemia ensues, an increase in the IMM chemical gradient for Ca2+ enhances mCa2+ uptake when ΔΨm and cell pH are lower; this induces mK+ influx predominately via SKCa channels but also via BKCa channels due to increased cyctosolic Ca2+; via either pathway mK+ influx is countered by activation of mKHE that acidifies the matrix and promotes uncoupling (higher respiration). In scenario C, independent increases of either cell or mCa2+, induced under conditions of ischemia or reperfusion, stimulates mK+ influx independently via mBKCa and mSKCa channels. In each scenario, uncoupling via mKHE leads to reversal of the increase in mitochondrial volume and generation of small amounts of signaling O2•−. In each scenario, if the matrix signaling O2•− generated by uncoupling were rapidly dismutated to H2O2 by TBAP, the protection would be lost because downstream ROS-dependent protective pathways would not be activated. We suggest that scenario B may best explain the utility of independent activation of either type of mKCa channel during ischemia and during reperfusion that together protect against cardiac IR injury. This is because we observed that the antagonists together exerted some additive endogenous anti-protective effects that likely depend on the different biochemical conditions that occur during ischemia and during reperfusion.
Fig. 7.
Cartoon summarizes possible scenarios for the interactions of mSKCa and mBKCa channels and the effects in mediating protection against IR injury. BKCa channels might be rapidly activated during reperfusion just after ischemia when ΔΨm rapidly becomes fully charged and there is a surge in cytosolic and concomitantly an increase in mitochondrial [Ca2+] (A). Independently, SKCa channels might be activated more slowly during ischemia when mitochondrial matrix [Ca2+] progressively rises in association to the lower ΔΨm and pHm during ischemia (B); in this scenario, BKCa and SKCa channels might function rather independently to enhance K+ influx and trigger K+/H+ exchange (KHE), cause mild uncoupling, and induce small amounts of signaling ROS. In another alternative pathway, net mK+ influx due to SKCa channel opening might be affected by simultaneous BKCa channel opening to alter either the matrix volume or the degree of mKHE-induced uncoupling (C). In all possibilities, signaling levels of ROS would need to be produced as a result of mK+ influx to initiate cardiac protection. A = additive or single pathway, B = parallel pathways with convergence, C = parallel pathways with different mechanisms.
Summary and Limitations
We have furnished comparative evidence for cardiac protective effects of BKCa and SKCa channel agonists and counter protective effects of BKCa and SKCa channel antagonists with demonstrated specificity of the antagonists to hinder protection by the agonists. Moreover, systemic application of the agonists and antagonists appears efficacious and suggests therapeutic potential in humans. The end effect of mK+ channel opening in cardiac mitochondria appears to be a respiration dependent pathway that stimulates a small amount of signaling mitochondrial O2•− that leads to cardioprotection by downstream and local mitochondrial mechanisms as shown by drug treatments on mitochondrial respiration and mCa2+ uptake. However, there is as yet little delineation of the individual stages and timing of triggering, activation, and end-effects of mK+ channel opening. Unraveling this apparently complicated, and potentially redundant, mechanism that culminates in cardiac protection could lead to therapeutic approaches for protection, specifically via a mitochondrial-targeted mechanism. However, an efficacious therapy would likely require targeting of specific cardiac mitochondrial splice variants of mKCa channels, so that the function of other BKCa and SKCa channels in other organs and subcellular sites would not be impinged.
Summary.
Big and small conductance Ca2+ -sensitive K+ channel isoforms are present in the inner mitochondrial membrane of cardiac ventricular mitochondria. These channels appear to have a differential protective role against cardiac ischemia reperfusion injury.
Acknowledgments
Acknowledgements and Disclosures
The authors thank David Schwabe, Wai-Meng Kwok and Mohammed Aldakkak and for their valuable contributions to this research study. The authors have nothing to disclose concerning any conflict of interest.
This work was supported in part by the Veterans Administration (BX-002539-01, DFS) and the National Institutes of Health (P01 GM066730-11, AKSC).
Abbreviations
- IR
ischemia reperfusion
- SKCa
small conductance Ca2+ -sensitive K+ channel
- BKCa
big conductance Ca2+ -sensitive K+ channel
- FAD
flavin adenine dinucleotide
- KHE
K+/H+ exchange
- NCE
Na+/Ca2+ exchange
- NHE
N+/H+ exchange
- KATP
ATP -sensitive K+ channel
- DCEBIO
DCEB, 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one
- NADH
nicotinamide adenine dinucleotide
- NS1619
(1-(2′-hydroxy-5′-trifluoromethylphenyl)-5-trifluoromethyl-2(3H) benzimid-axolone)
- IMM
inner mitochondrial membrane
- ΔΨm
mitochondrial membrane potential
- TBAP
Mn(III) tetrakis (4-benzoic acid) porphyrin
- IPC
ischemic preconditioning
- PPC
pharmacological preconditioning
- PAX
paxilline
- NS8593
N-[(1R)-1,2,3,4-tetrahydro-1-naphthalenyl]-1H-benzimidazol-2-amine hydrochloride
- RT-PCR
reverse transcription -polymerase chain reaction
- RCI
respiratory control index
- TTC
2,3,5-triphenyltetrazolium chloride
- CRC
Ca2+ retention capacity
- CaM
calmodulin
References
- 1.Stowe DF, Camara AK. Mitochondrial Reactive Oxygen Species Production in Excitable Cells: Modulators of Mitochondrial and Cell Function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camara AK, Lesnefsky EJ, Stowe DF. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid Redox Signal. 2010;13:279–347. doi: 10.1089/ars.2009.2788. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camara AK, Bienengraeber M, Stowe DF. Mitochondrial Approaches to Protect against Cardiac Ischemia and Reperfusion Injury. Front Physiol. 2011;2:1–34. doi: 10.3389/fphys.2011.00013. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac Mitochondrial Preconditioning by Big Ca2+-Sensitive K+ Channel Opening Requires Superoxide Radical Generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- 5.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse Electron Flow-Induced ROS Production Is Attenuated by Activation of Mitochondrial Ca2+-Sensitive K+ Channels. Am J Physiol Heart Circ Physiol. 2007;293:H1400–1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 6.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-Induced K+ Influx Increases Respiration and Enhances ROS Production While Maintaining Membrane Potential. Am J Physiol Cell Physiol. 2007;292:C148–156. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone Activators of Chloride Secretion: Potential Therapeutics for Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. J Pharmacol Exp Ther. 2001;296:600–611. [PubMed] [Google Scholar]
- 8.Syme CA, Gerlach AC, Singh AK, Devor DC. Pharmacological Activation of Cloned Intermediate- and Small- Conductance Ca2+-Activated K+ Channels. Am J Physiol Cell Physiol. 2000;278:C570–581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- 9.Wulff H, Miller MJ, Haensel W, Grissmer S, Cahalan MD, Chandy KG. Design of a Potent and Selective Inhibitor of the Intermediate -Conductance Ca2+-Activated K+ Channel, IKCa1: A Potential Immunosupressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stocker M. Ca2+-Activated K+ Channels: Molecular Determinants and Function of the SK Family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 11.Hille B. Ion Channels in Excitable Membranes. Sunderland: Sinauer Asociates, Inc; 2001. p. 814. [Google Scholar]
- 12.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the Gating Domain of a Ca2+-Activated K+ Channel Complexed with Ca2+/Calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 13.Bruening-Wright A, Schumacher MA, Adelman JP, Maylie J. Localization of the Activation Gate for Small Conductance Ca2+-Activated K+ Channels. J Neurosci. 2002;22:6499–6506. doi: 10.1523/JNEUROSCI.22-15-06499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered Expression of Small-Conductance Ca2+-Activated K+ (SK3) Channels Modulates Arterial Tone and Blood Pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N. Molecular Identification and Functional Roles of a Ca2+-Activated K+ Channel in Human and Mouse Hearts. J Biol Chem. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 16.Weatherall KL, Seutin V, Liegeois JF, Marrion NV. Crucial Role of a Shared Extracellular Loop in Apamin Sensitivity and Maintenance of Pore Shape of Small-Conductance Calcium-Activated Potassium (SK) Channels. Proc Natl Acad Sci U S A. 2011;108:18494–18499. doi: 10.1073/pnas.1110724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolga AM, Netter MF, Perocchi F, Doti N, Meissner L, Tobaben S, Grohm J, Zischka H, Plesnila N, Decher N, Culmsee C. Mitochondrial Small Conductance SK2 Channels Prevent Glutamate-Induced Oxytosis and Mitochondrial Dysfunction. J Biol Chem. 2013;288:10792–10804. doi: 10.1074/jbc.M113.453522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowe DF, Gadicherla AK, Zhou Y, Aldakkak M, Cheng Q, Kwok WM, Jiang MT, Heisner JS, Yang M, Camara AK. Protection against Cardiac Injury by Small Ca2+-Sensitive K+ Channels Identified in Guinea Pig Cardiac Inner Mitochondrial Membrane. Biochim Biophys Acta. 2013;1828:427–442. doi: 10.1016/j.bbamem.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa Channels Enhance Agonist-Evoked Endothelial Nitric Oxide Synthesis and Arteriolar Vasodilation. FASEB J. 2009;23:1138–1145. doi: 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. Activation of Human IK and SK Ca2+-Activated K+ Channels by NS309 (6,7-Dichloro-1h-Indole-2,3-Dione 3-Oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific Enhancement of Sk Channel Activity Selectively Potentiates the Afterhyperpolarizing Current IAHP and Modulates the Firing Properties of Hippocampal Pyramidal Neurons. J Biol Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- 22.Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels and Their Therapeutic Indications. Curr Med Chem. 2007;14:1437–1457. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 23.Pedarzani P, Stocker M. Molecular and Cellular Basis of Small--and Intermediate-Conductance, Calcium-Activated Potassium Channel Function in the Brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins DP, Strobaek D, Hougaard C, Jensen ML, Hummel R, Sorensen US, Christophersen P, Wulff H. Negative Gating Modulation by (R)-N-(Benzimidazol-2-Yl)-1,2,3,4-Tetrahydro-1-Naphthylamine (NS8593) Depends on Residues in the Inner Pore Vestibule: Pharmacological Evidence of Deep-Pore Gating of KCa2 Channels. Mol Pharmacol. 2011;79:899–909. doi: 10.1124/mol.110.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of Small-Conductance Ca2+-Activated K+ Channels Terminates and Protects against Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, Camara AK, Aldakkak M, Kwok WM, Stowe DF. Identity and Function of a Cardiac Mitochondrial Small Conductance Ca2+-Activated K+ Channel Splice Variant. Biochim Biophys Acta. 2017;1858:442–458. doi: 10.1016/j.bbabio.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varadarajan SG, An JZ, Novalija E, Smart SC, Stowe DF. Changes in [Na+]I, Compartmental [Ca2+], and NADH with Dysfunction after Global Ischemia in Intact Hearts. Am J Physiol Heart Circ Physiol. 2001;280:H280–293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 28.Kevin L, Camara AKS, Riess MR, Novalija E, Stowe DF. Ischemic Preconditioning Alters Real-Time Measure of O2 Radicals in Intact Hearts with Ischemia and Reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 29.Kevin L, Novalija E, Riess MR, Camara AKS, Rhodes SS, Stowe DF. Sevoflurane Exposure Generates Superoxide but Leads to Decreased Superoxide During Ischemia and Reperfusion in Isolated Hearts. Anesth Analg. 2003;96:945–959. doi: 10.1213/01.ANE.0000052515.25465.35. [DOI] [PubMed] [Google Scholar]
- 30.Riess ML, Camara AKS, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and Improved Function by Anesthetic and Ischemic Preconditioning in Guinea Pig Intact Hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 31.Riess ML, Camara AK, Novalija E, Chen Q, Rhodes SS, Stowe DF. Anesthetic Preconditioning Attenuates Mitochondrial Ca2+ Overload During Ischemia in Guinea Pig Intact Hearts: Reversal by 5- Hydroxydecanoic Acid. Anesth Analg. 2002;95:1540–1546. doi: 10.1097/00000539-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 32.An JZ, Camara AK, Rhodes SS, Riess ML, Stowe DF. Warm Ischemic Preconditioning Improves Mitochondrial Redox Balance During and after Mild Hypothermic Ischemia in Guinea Pig Isolated Hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2620–2627. doi: 10.1152/ajpheart.01124.2004. [DOI] [PubMed] [Google Scholar]
- 33.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia Augments Reactive Oxygen Species Detected in the Guinea Pig Isolated Perfused Heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 34.Attwell D, Cohen I, Eisner DA. The Effects of Heart Rate on the Action Potential of Guinea-Pig and Human Ventricular Muscle. J Physiol. 1981;313:439–461. doi: 10.1113/jphysiol.1981.sp013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai RP, Xue YX, Huang J, Wang JH, Wang JH, Zhao SY, Guan TT, Zhang Z, Gu YT. Ns1619 Regulates the Expression of Caveolin-1 Protein in a Time-Dependent Manner Via ROS/PI3K/PKB/FOXO1 Signaling Pathway in Brain Tumor Microvascular Endothelial Cells. J Neurol Sci. 2016;369:109–118. doi: 10.1016/j.jns.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Criado-Marrero M, Santini E, Porter JT. Modulating Fear Extinction Memory by Manipulating Sk Potassium Channels in the Infralimbic Cortex. Front Behav Neurosci. 2014;8:96. doi: 10.3389/fnbeh.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugaard MM, Hesselkilde EZ, Pehrson S, Carstensen H, Flethoj M, Praestegaard KF, Sorensen US, Diness JG, Grunnet M, Buhl R, Jespersen T. Pharmacologic Inhibition of Small-Conductance Calcium-Activated Potassium (SK) Channels by NS8593 Reveals Atrial Antiarrhythmic Potential in Horses. Heart Rhythm. 2015;12:825–835. doi: 10.1016/j.hrthm.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 38.Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-Mediated FAHP Is Modulated by Learning a Hippocampus-Dependent Task. Proc Natl Acad Sci U S A. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez M, McManus OB. Paxilline Inhibition of the Alpha-Subunit of the High-Conductance Calcium-Activated Potassium Channel. Neuropharmacology. 1996;35:963–968. doi: 10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 40.Maxwell MP, Hearse DJ, Yellon DM. Species Variation in the Coronary Collateral Circulation During Regional Myocardial Ischaemia: A Critical Determinant of the Rate of Evolution and Extent of Myocardial Infarction. Cardiovasc Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 41.Riess ML, Camara AK, Heinen A, Eells JT, Henry MM, Stowe DF. KATP Channel Openers Have Opposite Effects on Mitochondrial Respiration under Different Energetic Conditions. J Cardiovasc Pharmacol. 2008;51:483–491. doi: 10.1097/FJC.0b013e31816bf4a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riess ML, Eells JT, Kevin LG, Camara AKS, Henry MM, Stowe DF. Attenuation of Mitochondrial Respiration by Sevoflurane in Isolated Cardiac Mitochondrial Is Mediated in Part by Reactive Oxygen Species. Anesthesiology. 2004;100:498–505. doi: 10.1097/00000542-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Huang M, Camara AK, Stowe DF, Qi F, Beard DA. Mitochondrial Inner Membrane Electrophysiology Assessed by Rhodamine-123 Transport and Fluorescence. Ann Biomed Eng. 2007;35:1276–1285. doi: 10.1007/s10439-007-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blomeyer CA, Bazil JN, Stowe DF, Pradhan RK, Dash RK, Camara AK. Dynamic Buffering of Mitochondrial Ca2+ During Ca2+ Uptake and Na+-Induced Ca2+ Release. J Bioenerg Biomembr. 2013;45:189–202. doi: 10.1007/s10863-012-9483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boelens AD, Pradhan RK, Blomeyer CA, Camara AK, Dash RK, Stowe DF. Extra-Matrix Mg2+ Limits Ca2+ Uptake and Modulates Ca2+ Uptake-Independent Respiration and Redox State in Cardiac Isolated Mitochondria. J Bioenerg Biomembr. 2013;45:203–218. doi: 10.1007/s10863-013-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blomeyer CA, Bazil JN, Stowe DF, Dash RK, Camara AK. Mg2+ Differentially Regulates Two Modes of Mitochondrial Ca2+ Uptake in Isolated Cardiac Mitochondria: Implications for Mitochondrial Ca2+ Sequestration. J Bioenerg Biomembr. 2016;48:175–188. [Google Scholar]
- 47.Lindsay DP, Camara AK, Stowe DF, Lubbe R, Aldakkak M. Differential Effects of Buffer pH on Ca2+-Induced ROS Emission with Inhibited Mitochondrial Complexes I and III. Front Physiol. 2015;6:58. doi: 10.3389/fphys.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aldakkak M, Stowe DF, Dash RK, Camara AK. Mitochondrial Handling of Excess Ca2+ Is Substrate-Dependent with Implications for Reactive Oxygen Species Generation. Free Radic Biol Med. 2013;56:193–203. doi: 10.1016/j.freeradbiomed.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhodes SS, Camara AK, Heisner JS, Riess ML, Aldakkak M, Stowe DF. Reduced Mitochondrial Ca2+ Loading and Improved Functional Recovery after Ischemia-Reperfusion Injury in Old vs. Young Guinea Pig Hearts. Am J Physiol Heart Circ Physiol. 2012;302:H855–863. doi: 10.1152/ajpheart.00533.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective Role of Ca2+- Activated K+ Channels in the Cardiac Inner Mitochondrial Membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 51.Clements RT, Terentyev D, Sellke FW. Ca2+-Activated K+ Channels as Therapeutic Targets for Myocardial and Vascular Protection. Circ J. 2015;79:455–462. doi: 10.1253/circj.CJ-15-0015. [DOI] [PubMed] [Google Scholar]
- 52.Borchert GH, Yang C, Kolar F. Mitochondrial Bkca Channels Contribute to Protection of Cardiomyocytes Isolated from Chronically Hypoxic Rats. Am J Physiol Heart Circ Physiol. 2011;300:H507–513. doi: 10.1152/ajpheart.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang SH, Park WS, Kim N, Youm JB, Warda M, Ko JH, Ko EA, Han J. Mitochondrial Ca2+-Activated K+ Channels More Efficiently Reduce Mitochondrial Ca2+ Overload in Rat Ventricular Myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H307–313. doi: 10.1152/ajpheart.00789.2006. [DOI] [PubMed] [Google Scholar]
- 54.Riess M, Rhodes SS, Novalija E, Jiang MT, Stowe DF. Reduced Cytosolic [Ca2+] During Preconditioning with Sevoflurane Is Associated with Improved Function and Reduced Infarct Size after Global Ischemia in Intact Hearts. Anesthesiology. 2001;95:A497. [Google Scholar]
- 55.An JZ, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na+/H + Exchange Reduces [Na+]I and [Ca2+]I Load after Ischemia and Improves Function in Intact Hearts. Am J Physiol Heart Circ Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 56.Riess ML, Varadarajan SG, Chen Q, Camara AKS, Novalija E, Rhodes SS, Stowe DF. Anesthetic Preconditioning Decreases Mitochondrial [Ca2+] Overload During Global Ischemia in Isolated Guinea Pig Hearts. FASEB J. 2002;16:A488. [Google Scholar]
- 57.Camara AK, Chen Q, An J, Novalija E, Riess ML, Rhodes SS, Stowe DF. Comparison of Hyperkalemic Cardioplegia with Altered [CaCl2] and [MgCl2] on [Ca2+]I Transients and Function after Warm Global Ischemia in Isolated Hearts. J Cardiovasc Surg (Torino) 2004;45:1–13. [PubMed] [Google Scholar]
- 58.Aldakkak M, Camara AK, Heisner JS, Yang M, Stowe DF. Ranolazine Reduces Ca2+ Overload and Oxidative Stress and Improves Mitochondrial Integrity to Protect against Ischemia Reperfusion Injury in Isolated Hearts. Pharmacol Res. 2011;64:381–392. doi: 10.1016/j.phrs.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-Controlled Gating in a Large Conductance Ca2+-Sensitive K+ Channel (Hslo) Proc Natl Acad Sci U S A. 1997;94:5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia XM, Zeng X, Lingle CJ. Multiple Regulatory Sites in Large-Conductance Calcium-Activated Potassium Channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 61.Sheng JZ, Weljie A, Sy L, Ling S, Vogel HJ, Braun AP. Homology Modeling Identifies C-Terminal Residues That Contribute to the Ca2+ Sensitivity of a BKCa Channel. Biophys J. 2005;89:3079–3092. doi: 10.1529/biophysj.105.063610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang ZW, Nara M, Wang YX, Kotlikoff MI. Redox Regulation of Large Conductance Ca2+-Activated K+ Channels in Smooth Muscle Cells. J Gen Physiol. 1997;110:35–44. doi: 10.1085/jgp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AK. Mitochondrial Matrix K+ Flux Independent of Large-Conductance Ca2+-Activated K+ Channel Opening. Am J Physiol Cell Physiol. 2010;298:C530–541. doi: 10.1152/ajpcell.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett JN, Magleby KL, Pallotta BS. Properties of Single Calcium-Activated Potassium Channels in Cultured Rat Muscle. J Physiol. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millar ID, Bruce J, Brown PD. Ion Channel Diversity, Channel Expression and Function in the Choroid Plexuses. Cerebrospinal Fluid Res. 2007;4:8. doi: 10.1186/1743-8454-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular Energetics and the Oxygen Dependence of Respiration in Cardiac Myocytes Isolated from Adult Rat. J Biol Chem. 1990;265:15392–15402. [PubMed] [Google Scholar]
- 67.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced Reactive O2 Species Formation and Preserved Mitochondrial NADH and [Ca2+] Levels During Short-Term 17°C Ischemia in Intact Hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 68.Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of Superoxide in Cardiomyocytes During Ischemia before Reperfusion. Am J Physiol Heart Circ Physiol. 1999;277:H2240–2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 69.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant Levels of Oxidants Are Generated by Isolated Cardiomyocytes During Ischemia Prior to Reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 70.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular Signaling by Reactive Oxygen Species During Hypoxia in Cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 71.Camara AK, An JZ, Chen Q, Novalija E, Varadarajan SG, Schelling P, Stowe DF. Na+/H+ Exchange Inhibition with Cardioplegia Reduces Cytosolic [Ca2+] and Damage after 4 Hour Cold Ischemia. J Cardiovasc Pharmacol. 2003;41:689–698. doi: 10.1097/00005344-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic Consequences of Opening the ATP-Sensitive K+ Channel of Heart Mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 73.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by Which Opening the Mitochondrial ATP-Sensitive K+ Channel Protects the Ischemic Heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 74.Korge P, Honda HM, Weiss JN. K+-Dependent Regulation of Matrix Volume Improves Mitochondrial Function under Conditions Mimicking Ischemia-Reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H66–77. doi: 10.1152/ajpheart.01296.2004. [DOI] [PubMed] [Google Scholar]
- 75.O’Rourke B. Pathophysiological and Protective Roles of Mitochondrial Ion Channels. [review] J Physiol. 2000;529:23–36. doi: 10.1111/j.1469-7793.2000.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akao M, O’Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically Distinct Steps in the Mitochondrial Death Pathway Triggered by Oxidative Stress in Cardiac Myocytes. Circ Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- 77.Das M, Parker JE, Halestrap AP. Matrix Volume Measurements Challenge the Existence of Diazoxide/Glibencamide-Sensitive KATP Channels in Rat Mitochondria. J Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murata M, Akao M, O’Rourke B, Marban E. Mitochondrial ATP-Sensitive Potassium Channels Attenuate Matrix Ca2+ Overload During Simulated Ischemia and Reperfusion: Possible Mechanism of Cardioprotection. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 79.Drose S, Brandt U, Hanley PJ. K+-Independent Actions of Diazoxide Question the Role of Inner Membrane KATP Channels in Mitochondrial Cytoprotective Signaling. J Biol Chem. 2006;281:23733–23739. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 80.Wojtovich AP, Smith CO, Urciuoli WR, Wang YT, Xia XM, Brookes PS, Nehrke K. Cardiac Slo2.1 Is Required for Volatile Anesthetic Stimulation of K+ Transport and Anesthetic Preconditioning. Anesthesiology. 2016;124:1065–1076. doi: 10.1097/ALN.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wojtovich AP, Sherman TA, Nadtochiy SM, Urciuoli WR, Brookes PS, Nehrke K. Slo-2 Is Cytoprotective and Contributes to Mitochondrial Potassium Transport. Plos One. 2011:6. doi: 10.1371/journal.pone.0028287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-Sensitive K+ Channels Modulate Cardiac Mitochondrial Function. Am J Physiol. 1998;275:H1567–1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- 83.Garlid KD. Opening Mitochondrial KATP in the Heart--What Happens, and What Does Not Happen. Basic Res Cardiol. 2000;95:275–279. doi: 10.1007/s003950070046. [DOI] [PubMed] [Google Scholar]
- 84.Halestrap AP. Regulation of Mitochondrial Metabolism through Changes in Matrix Volume. Biochem Soc Trans. 1994;22:522–529. doi: 10.1042/bst0220522. [review] [DOI] [PubMed] [Google Scholar]
- 85.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP Channel-Independent Targets of Diazoxide and 5-Hydroxydecanoate in the Heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]