Abstract
As the leading cause of cancer-related mortality, lung cancer is a worldwide health issue that is overwhelmingly caused by smoking. However, a substantial minority (~25%) of patients with non–small cell lung cancer (NSCLC) has never smoked. In these patients, activating mutations of the epidermal growth factor receptor (EGFR) are more likely, which render their tumors susceptible for a finite period to treatment with EGFR tyrosine kinase inhibitors (TKIs) and confer a better prognosis than EGFR wild-type NSCLC. On progression, due to the inevitable insurgence of resistance, TKIs are generally followed by second- or third-line salvage chemotherapy until treatment failure, after which no standard treatment options are available, resulting in a poor prognosis and a high risk of death. With the focus of clinical attention on treatment with TKIs, few studies on optimal salvage therapies, including cytotoxic chemotherapy, after failure of EGFR TKIs have been reported. Despite a paucity of available data, the aim of this review is to summarize the “no-man's land” of TKI-failed EGFR-mutated NSCLC and expand on alternative strategies as well as potential future directions.
Introduction
According to the GLOBOCAN 2012 database, the most recent year for which statistics are available, lung cancer is the most common cancer (1.8 million cases) and the leading cause of cancer-related death (1.59 million deaths) worldwide [1]. Tobacco smoking remains the dominant cause of all non–small cell lung cancers (NSCLCs) except in the case of adenocarcinomas with fusions or mutations of the kinase genes such as epidermal growth factor receptor (EGFR), which occur specifically in light or never-smokers [2]. These mutations, which have been identified in exons 18 to 21 of the TK domain and appear most commonly in exon 19 (del19) and in exon 21 (L858R), activate the receptor tyrosine kinase pathway (hence the term “activating mutations”), leading to overstimulation of downstream signaling prosurvival pathways including Ras-Raf-MAP-kinase and PI3K-Akt-mTOR that confer oncogenicity. Activating EGFR mutations, to which tumors are “addicted” for survival, are primarily associated with adenocarcinoma histology; frequently mutually exclusive with other activating tumor mutations; and only rarely found in large cell carcinomas, small cell carcinomas, and squamous carcinomas [3]. While deletions in exon 19 and L8585R point mutation in exon 21 are considered activating, other EGFR mutations such as exon 20 insertions are not activating and, therefore, do not tend to respond to EGFR tyrosine kinase inhibitor (TKI) treatment.
The frequency of EGFR driver mutations in lung adenocarcinomas differs according to ethnicity and sex, appearing at a rate of approximately 10% to 15% in North Americans and Europeans [4] and as high as 30% to 50% in East Asians [5], most commonly in East Asian women that have never smoked. Unlike SCLC, which is strongly linked to smoking behavior, a history of never or minimal smoking is the strongest predictor of harboring the EGFR mutation in NSCLC, which strongly suggests that EGFR mutagenesis arises from nontobacco carcinogens: in fact, the total number of pack-years smoked has been found in multiple studies to inversely correlate with the rate of EGFR TK mutations [6], [7]. In addition, the high mutation burden typically induced by carcinogens in cigarette smoke, which putatively leads to a higher quantity of neoantigens [8], may underlie the generally poorer response to PD-1 inhibition in EGFR+ NSCLC. According to the Surveillance, Epidemiology, and End Results database of the National Institutes of Health [9], lung cancer accounts for approximately 6.4% of all malignancies, and since ~10% to 15% of these are EGFR mutated, the lifetime prevalence of EGFR-mutated tumors is between 88,300 and 132,400, making it an orphan disease in the United States from a prevalence count perspective (<200,000 prevalent cases).
EGFR, a transmembrane glycoprotein, is part of the ErbB/HER family of receptor tyrosine kinases, which includes three other members: HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4) [10]. The presence of activating EGFR mutations in advanced NSCLC confers sensitivity to standard-of-care TKIs such as afatinib, gefitinib, erlotinib [11], dacomitinib, and osimertinib and predicts a better prognosis as compared to EGFR wild-type NSCLC. While the use of TKIs, which are superior to chemotherapy, has resulted in substantial benefits including an initial response rate of approximately 70% and an overall survival from 1 to nearly 3 years [12], the universality of treatment resistance limits the duration of their use.
Clinical management of TKI-failed patients constitutes an uncertain “no man's land” since, to date, there are no clear guidelines, and new accepted strategies to improve outcomes are lacking [13], [14]. A retrospective analysis of 521 EGFR TKI-failed patients by Song et al. [15] highlights the poor prognosis in this population since of the 223 patients who previously received long-term TKI therapy (>6 months), overall survival was a dismal 5 months. In the absence of satisfactory therapeutic options and treatment guidelines after failure of subsequent salvage second- and third-line chemotherapy EGFR-positive NSCLC is an area of high unmet medical need. A systematic review of the available literature highlights a wealth of data from high-quality clinical trials on clinical outcomes with TKIs and a corresponding paucity of information and evidence pertaining to treatment of tumors that have exhausted TKI options. With the focus of attention on treatment with TKIs, a hotspot of clinical research, the question of how to manage patients that exhaust TKI and chemotherapy options is an open one. Despite the relative paucity of available data on this subject, this review aims to summarize current and alternative treatment strategies as well as potential future directions in advanced TKI-failed EGFR-mutated NSCLC.
Current treatment strategies
First-line TKIs
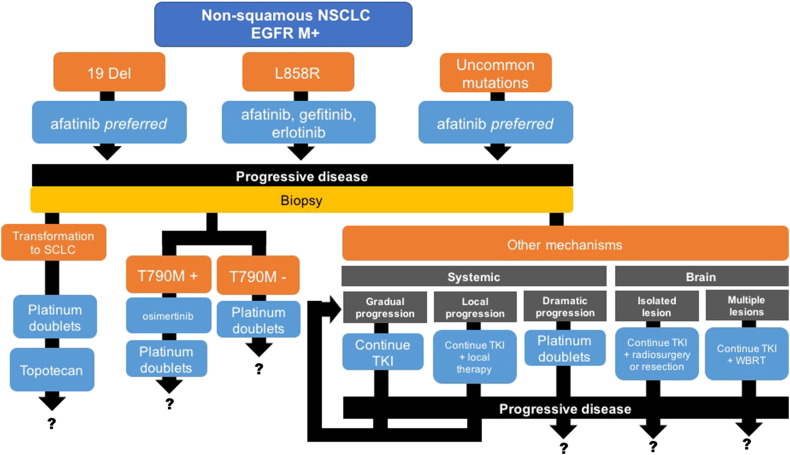
Several randomized trials including EURTAC [16] and OPTIMAL [17] for erlotinib; NEJGSG_ 002 [18], WJTOG 3405 [19], and IPASS [20] for gefitinib; and LUX LUNG 3 [21] and LUX LUNG 6 [22] for afatinib have demonstrated the superiority of EGFR TKIs to chemotherapy in terms of overall response rates and progression-free survival (PFS) for first-line therapy (see Figure 1).
Figure 1.
Patterns of clinical relapse and algorithm for EGFR mutation–positive NSCLC. Abbreviations: WBRT, whole brain radiotherapy; M+, mutation positive.
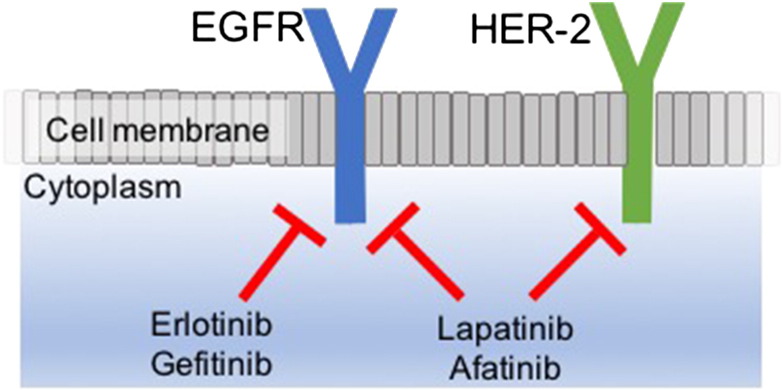
Erlotinib and gefitinib are first-generation reversible TKIs, while afatinib and lapatinib are second-generation irreversible pan-HER TKIs [23] that are reported to be superior to gefitinib in EGFR exon 19 deleted tumors and other less common mutations (see Figure 2).
Figure 2.
Mechanism of different EGFR TKIs. Abbreviations: HER-2, human epidermal growth factor receptor 2.
Despite strikingly high initial response rates with these TKIs, the development after 11 to 14 months of acquired resistance, as defined by the Jackman criteria [24] (disease progression after objective response or durable stable disease for >6 months on a TKI), is a virtual fait accompli [25]. In over 60% of patients that have progressed on first- and second-generation TKIs, the mechanism of resistance is an acquired threonine-to-methionine amino acid substitution at the gatekeeper position 790 of EGFR in exon 20 or T790M [26], which increases the affinity of the EGFR kinase domain for ATP and thereby outcompetes the binding of EGFR TKIs; the T790M-resistant tumor cells may arise from the selective pressure of a TKI or present de novo. Based on data from the Phase II AURA 2 trial and the AURA extension cohort, T790M-positive tumors are responsive to treatment with the third-generation TKI osimertinib [27]. However, secondary mutations have also been described as a mechanism of acquired resistance to osimertinib. In addition to T790M, the occurrence of other resistance mechanisms including activation of the MET pathway, HER2 amplification, mutations in Pi3kinase and BRAF, and transformation to small cell lung cancer supports rebiopsy at clinical progression to determine the molecular/morphologic changes and to personalize the therapy accordingly.
Chemotherapy
After progression on osimertinib, despite a lack of strong supporting evidence (no prospective trials comparing the efficacy of chemotherapy regimens have been conducted), platinum doublets, e.g., cisplatin/carboplatin plus paclitaxel, nab-paclitaxel or pemetrexed × 4 to 6 cycles +/− bevacizumab, are routinely recommended for patients without any targetable mutations followed by the option of single-agent pemetrexe, docetaxel, or erlotinib. For so-called T790M-negative tumors, second-line therapy is a chemotherapy doublet. Options for patients with T790M mutation–negative tumors that progress on chemotherapy are limited.
EGFR Inhibition Beyond Progression
Based on the concept of “oncogene addiction” in which tumors, dependent on oncogenes such as EGFR for their continued proliferation and survival, experience accelerated regrowth or “flare” after stopping TKI therapy, progressive disease on a TKI does not necessarily equate with exhausted benefit. Accordingly, one option is to continue TKIs beyond progression in asymptomatic patients. In a 2013 study, Yang et al. [28], having stratified TKI-failed patients according to three types of progression (dramatic, gradual, and local), demonstrated that the continuation of EGFR TKI is feasible for gradual and local progression (+ local modalities such as surgical resection, stereotactic radiotherapy, and cryotherapy); continued sensitivity to therapy is suggested. For dramatic progression, chemotherapy, not EGFR TKI continuation, is indicated as well as a biopsy to rule out histologic transformation from NSCLC to small cell lung cancer, which constitutes an additional resistance mechanism to TKI therapy [29].
The effect of TKI continuation past true progression in EGFR+ NSCLC was also explored in the ASPIRATION “Asian Pacific Trial of Tarceva as First-Line in EGFR Mutation” and IMPRESS “IRESSA Mutation-Positive Multicenter Treatment Beyond Progression Study.” In the nonrandomized ASPIRATION (Asian Pacific trial of Tarceva as first‐line in EGFR mutation) study, patients with indolent, small-volume asymptomatic growth, i.e., gradual progressors and oligometastatic progressors, selected at investigators' discretion, that were continued on single-agent erlotinib beyond RECIST progression showed an improvement in PFS by 3.1 months [30] from 11.0 to 14.1.
However, in the randomized Phase 3 IMPRESS (IRESSA Mutation‐Positive Multicenter Treatment Beyond Progression Study), patients that remained on gefitinib during doublet chemotherapy did not experience improved response rates or PFS, which established doublet chemotherapy as the standard of care after progression on EGFR TKIs [31]. Nevertheless, the overall consensus is that while dramatic progression mandates a change in treatment strategy, EGFR TKI continuation in the setting of oligometastatic disease is a feasible option [32] to delay the time to progression and the need for subsequent platinum-based treatments, eventually with the addition of local therapy.
Continuation of EGFR TKI with Brain Metastases
The incidence of brain metastases in NSCLC is 25% to 30%, and that incidence is increased in EGFR-mutated NSCLC to 44% to 63% [33], possibly because patients live longer on TKIs and the risk of brain metastases increases with lifespan or possibly because the propensity to metastasize is greater in EGFR-mutated cancers. For patients with oligometastatic progression in the brain after EGFR TKI therapy, the consensus is to continue EGFR TKI with local therapies such as radiotherapy, surgery, and stereotactic ablative radiotherapy on the suspicion that poor blood brain barrier penetration of the TKI rather than cellular resistance [34] is to blame; in this case, EGFR TKI continuation may serve to maintain systemic remission. Jackman et al. [24]. demonstrated that high-dose gefitinib penetrates the blood-brain barrier more effectively, and likewise, pulsatile high-dose erlotinib was found to overcome acquired resistance to standard-dose erlotinib [35].
Treatment-Free Interval (Drug Holiday)
Despite the potential for disease flare during an EGFR TKI–free period, restoration of TKI sensitivity, which may be epigenetically mediated, has been documented after a drug holiday. In a study of 23 patients who were rechallenged with gefitinib after a median 7-month break, during which time they received cytotoxic anticancer therapy, Tomizawa et al. [36] reported a partial response rate of 22% and a disease control rate of 65%. In a retrospective study of 14 patients [37], reintroduction of erlotinib after a median 9.5-month holiday resulted in 36% (n = 5) partial response, 50% stable disease (n = 7) ,and 14% progressive disease (n = 2). However, these were small-scale studies, and further investigation from larger prospective trials is required to confirm these outcomes.
Alternative Treatment Strategies/Future Directions
Immunotherapy + TKI
Four randomized phase III trials have reported a statistically significant improvement in response rate (RR) and overall survival (OS) with immune checkpoint inhibitors such as nivolumab and pembrolizumab over standard second-line docetaxel chemotherapy in platinum-refractory NSCLC [38]. By contrast, EGFR-mutant advanced NSCLC patients gained no overall survival (OS) benefit from immune checkpoint inhibitors over docetaxel [39], which may be related to the general lack of tobacco use in this population since smoking is associated with a higher mutational burden, a biomarker of response to immunotherapy [40].
Nonetheless, PD-L1 expression has been associated with EGRF mutation [41], which has been reported to correlate with a higher likelihood of response to PD-1 blockade, suggesting that immunotherapy in EGFR-mutant NSCLC may still hold promise. However, despite evidence of clinical activity, combinations of nivolumab plus erlotinib, gefitinib plus durvalumab, and osimertinib plus durvalumab (anti–PD-L1 monoclonal antibody) resulted in a relatively high incidence of treatment-related grade 3 to 4 toxicities [42], and in fact, two trials with the osimertinib plus durvalumab combination were halted due to an excess of pulmonary toxicity. However, many new immunotherapy agents are under development including the inhibitory molecules TIM-3, LAG-3, IDO (indoleamine-pyrrole 2,3-dioxygenase), BTLA (B- and T-lymphocyte attenuator), adenosine, and VISTA (V-domain immunoglobulin containing suppressor of T cell activation) and the stimulatory molecules 4-1BB, OX40, CD40, and CD27. Therefore, the “jury is still out” on the clinical feasibility of immunotherapy in this population.
Mesenchymal-Epidermal Transition (MET) Inhibition
Amplification (but probably not mutation) of MET, a receptor tyrosine kinase, occurs in up to 20% of tumors [43] with acquired resistance to EGFR TKIs, which suggests the potential for synergistic benefit from combination treatment with an EGFR and MET inhibitor [44]. MET amplification and T790M mutation are not mutually exclusive; both may coexist, or MET amplification may occur on its own. Therefore, concomitant inhibition of MET and EGFR is likely required. Several published case reports with a TKI + crizotinib, a known MET and ALK inhibitor that has demonstrated activity in MET-amplified patients, and other clinical data [26] support this assumption [45], [46]. In a Phase 2 study with erlotinib + tivatinib, a selective c-MET inhibitor, in a TKI-failed EGFR-positive population, a subgroup of patients with high MET expression responded to the combination therapy, although the study as a whole did not meet its endpoint [47]. In a Phase 1b/2 study (NCT01610336), the combination of capmatinib (INC280), another MET inhibitor, and gefitinib in 65 high-MET EGFR TKI-resistant NSCLC patients yielded a disease control rate of 80% (overall response rate 18%; stable disease 62%) [48]. In a Phase 1 study of tepotinib (MSC2156119J) plus gefitinib, 18 patients were treated, and 5 had a partial response [49]. While the combination of MET inhibitors and EGFR TKIs seems promising, the potential for overlapping or additive toxicities based on the mechanisms of action of these agents is an important consideration, which requires testing in large clinical trials.
Oncolytic Virotherapy
Since nonsmoking EGFR-mutated NSCLC patients may resist checkpoint therapy due to a low mutational load, treatments like radiotherapy or virotherapy that induce the release of tumor antigens may augment the activity of PD-1/L1 inhibitors. As cancer-specific lytic agents, with or without therapeutic transgenes to augment the immune response, locally injected replicating oncolytic viruses that liberate nonself neoantigens for T-cell priming may elicit abscopal effects at distant metastatic sites; however, unlike radiotherapy, which also is associated with abscopal effects but may induce pneumonitis and scarring in the lungs, oncolytic viruses have been genetically engineered for attenuation and nonpathogenicity in normal cells and are, hence, in general, minimally host toxic.
The mechanism by which oncolytic viruses even in the absence of therapeutic transgenes kill tumor cells and elicit a systemic immune effect involves direct lysis of tumor cells and cell death via synthesis of new viral particles with subsequent spread of tumor-associated antigens and epitopes after the cell lysis [50]. When the virus is armed with therapeutic immunomodulatory transgenes, which theoretically enhance the immune response, the infected tumor cell serves as a “factory” for protein synthesis such that the beneficial transgene is overproduced and may spread to distant tumor sites via the circulation.
Talimogene laherparepvec (Imlygic), commonly referred to as T-VEC, an attenuated herpes simplex virus type 1 virus armed with a GM-CSF transgene that is approved for the treatment of melanoma [51], has the potential to overcome tumor-induced T-cell anergy and thereby enhance the activity of anti-CTLA and PD-1 antibodies in EGFR-mutated NSCLC. Several oncolytic adenoviruses carrying different immunomodulatory [52] transgenes and theoretically safer than herpes virus appear poised to enter the clinic in early 2018 as potential treatments for multiple tumor types including EGFR+ NSCLC.
Epigenetic Resensitization
The term “epigenetics” refers to reversible modifications that influence gene expression without directly changing or affecting the DNA coding sequence [53]. Epigenetic alterations, which include DNA methylation, microRNA regulation, and histone/nucleosome modifications, influence multiple cellular processes such as transcription, replication, and repair and are implicated in tumorigenesis and chemotherapy resistance [54], [55]. Epigenetic mechanisms have been proposed to explain the reversion to TKI sensitivity after a drug holiday. Epigenetic inhibitors such as histone deacetylators and DNA methyltransferases, which dynamically reprogram the epigenome, the collective name for chromatin and DNA modification patterns, have been associated with resensitization of several refractory tumor types including EGFR+ NSCLC to conventional therapies such as cisplatin and carboplatin. One such agent is the experimental tumor-associated macrophage and neutrophil-repolarizing agent RRx-001, which in Phase 2 clinical trials has been shown to resensitize refractory TKI- and chemotherapy-failed EGFR-positive NSCLC patients [27] to subsequently administered platinum doublets. If successful, the potential to reverse resistance to chemotherapy and possibly TKIs as well might significantly improve overall survival in this last-line population.
Conclusion and discussion
While EGFR TKI inhibitors yield impressive and durable responses, prolonged PFS, and improved quality of life when compared to chemotherapy, inevitable resistance to them as well as to subsequent platinum doublets is a “no man's land” of unmet medical need, as patients at this terminal stage have exhausted all available lines of treatment. Careful analysis of resistance mechanisms and mutation status at the time of clinically significant disease progression may guide the selection of subsequent treatment, if one exists; but if not, the only options that remain to patients are local ablative therapies, clinical trials, or hospice with poor quality of life and death at the end of their palliative care. New strategies to prevent or reverse the development of resistance are therefore urgently needed and include immunotherapy, concomitant EGFR + MET inhibition, oncolytic virotherapy, epigenetic resensitization with subsequent administration of previously failed chemotherapies, and potentially the discovery of currently unknown driver mutations that synergize with all of the above especially immunotherapy.
The key advantage of immunotherapy in NSCLC is the durability of responses. Where resistance development is a near-universal feature of chemotherapy and targeted agents, the tumor-specific memory function of the immune system has the potential to confer lasting remission/regression of metastatic disease. However, as stated earlier in this review, EGFR-mutated tumors seem not to benefit from checkpoint inhibitors possibly due to a relatively low mutation burden despite higher levels of PD-L1 expression, which has been shown to correlate with response. Since it is infeasible to increase the mutational load in EGFR+ cancers, one strategy to refocus the immune response is to selectively increase the exposure/release of tumor neoantigens, inflammatory cytokines, and particular damage-associated molecular patterns that can stimulate the immune system and potentiate the efficacy of formerly inactive checkpoint inhibitors. If successful, targeted therapies such as dual EGFR + MET inhibition and particularly oncolytic virotherapy will mediate release of tumor neoantigens from lysed cells along with cytotoxics in epigenetically resensitized tumors.
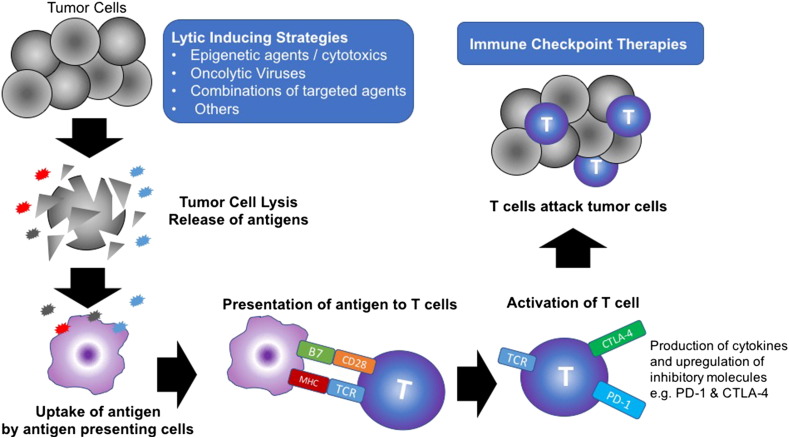
On this basis, potentially optimal sequential immunotherapeutic priming strategies to trigger the release of immunogenic antigens from dying cancer cells in TKI- and chemotherapy-failed tumors might include 1) pretreatment of a non–MET-amplified tumor for several weeks with an epigenetic inhibitor before starting a cytotoxic like cisplatin or carboplatin for four to six cycles followed by checkpoint inhibition and 2) administration of oncolytic viruses in combination with EGFR TKI + MET targeted therapy in MET-amplified tumors followed by checkpoint inhibition (see Figure 3).
Figure 3.
Lytic-inducing strategies in EGFR+ NSCLC are anticipated to result in the release of multiple tumor neoantigens. Tumor neoantigens are engulfed by antigen-presenting cells and processed and presented to T cells in the context of B7 costimulatory molecules and major histocompatibility complex. T cells express checkpoint inhibitory molecules such as PD-1 and CTLA-4, which prevent full activation. Immune checkpoint blockade relieves immune suppression, effectively taking the brakes off the T cells and restoring their effector function to effectively attack the tumor. Abbreviations: CTLA-4, cytotoxic T lymphocyte–associated protein 4; MCH, major histocompatibility complex; PD1, programmed cell death protein 1; PD-L1, PD1 ligand; TCR, T-cell receptor. Adapted from Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205-14.
The cancer-immunity cycle proposed by Chen and Mellman consists of seven steps [56]: 1) release of cancer antigens, 2) cancer antigen presentation, 3) priming and activation, 4) T-cell trafficking to tumors, 5) T-cell tumor infiltration, 6) recognition of cancer cells by T cells, and 7) killing of cancer cells.
In this cycle, the induction of an immune response begins when professional APCs, such as dendritic cells, engulf apoptotic or necrotic tumor cells and present tumor-associated antigens on their surface to their cognate T cells. A prerequisite for immune cell–mediated tumor cell destruction is interaction of the antigen-specific T cells with MHC-I-peptide complexes. Since NSCLC, like melanoma, kidney, and urothelial cancers, typically (although not always) presents as inflamed or T-cell rich, steps 1, 2, and 3 (i.e., release of cancer antigens, cancer antigen presentation, and priming and activation) arguably play the most important role in EGFR-mutated NSCLC.
In summary, while possibly an oversimplification of complex oncoimmunology, the overall low mutation load in EGFR+ NSCLC may render these tumors preferentially susceptible to lytic-inducing strategies that amplify intratumoral T cells against undefined antigens prior to administration of checkpoint inhibitors. Besides viruses, targeted therapies, epigenetic inhibitors, and cytotoxic chemotherapies other potentially lytic-inducing agents may include adoptive cell transfer therapies such as CAR-T, cytokines such as IL-2 and IL-15, bacteria and yeast vectors, and personalized polyepitope DNA vaccines that are selected on the basis of mutation status and MHC binding affinity.
The opposite of no man's land is a fertile plain. It is hoped that, in the near future, new, innovative strategies will be available to overcome resistance mechanisms, especially immunologically based resistance mechanisms, and thereby transform the no man's land of TKI- and chemotherapy-failed EGFR+ NSCLC into a vast fertile plain, rich with promising therapeutic options that result in meaningful improvements to quality of life and survival.
Funding
None.
Ethical Approval
This review article does not contain any studies with human participants or animals.
References
- 1.International Agency For Research On Cancer (IARC), Globocan 2012 . 2012. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012, IARC. [ http://globocan.iarc.fr/old/FactSheets/cancers/lung-new.asp. Accessed May 1st 2017] [Google Scholar]
- 2.Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–3234. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 3.Toyooka S, Yatabe Y, Tokumo M, Ichimura K, Asano H, Tomii K, Aoe M, Yanai H, Date H, Mitsudomi T. Mutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lung. Int J Cancer. 2006;118:1588–1590. doi: 10.1002/ijc.21500. [DOI] [PubMed] [Google Scholar]
- 4.Dogan S, Shen R, Ang DC, Johnson ML, D'Angelo SP, Paik PK, Brzostowski EB, Riely GJ, Kris MG, Zakowski MF. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non–small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosaka T, Yatabe Y, Endoh H. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 7.Pham D, Kris MG, Riely GJ. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol. 2006;24:1700–1704. doi: 10.1200/JCO.2005.04.3224. [DOI] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute SEER Cancer Statistics Review 1975-2013. https://seer.cancer.gov/archive/csr/1975_2013/results_merged/topic_prevcounts.pdf
- 10.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Schmid-Bindert G, Zhou C. Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther Adv Med Oncol. 2012;4(1):19–29. doi: 10.1177/1758834011427927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiroshige Y, Tetsuya M, Satoshi M, Yasushi Y, Shunichi N, Isamu O., Takashi S, Miyako S, Hirohito T, Tomonori H. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non–small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR) J Clin Oncol. 2014;32:8117. [Google Scholar]
- 13.Zhong WZ, Zhou Q, Wu YL. The resistance mechanisms and treatment strategies for EGFR mutant advanced non–small cell lung cancer. Oncotarget. 2017;8(41):71358–71370. doi: 10.18632/oncotarget.20311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L, Lei D, Zhang H. Clinical strategies for acquired epidermal growth factor receptor tyrosine kinase inhibitor resistance in non–small-cell lung cancer patients. Oncotarget. 2017;8(38):64600–64606. doi: 10.18632/oncotarget.19925. [eCollection 2017 Sep 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Z, Zhang Y. Treatment and prognosis after progression in long-term responders to EGFR-tyrosine kinase inhibitor in advanced non–small cell lung cancer. Arch Med Sci. 2016;12(1):107–111. doi: 10.5114/aoms.2016.57586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez J.M. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S., Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 18.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A., Harada M., Yoshizawa H., Kinoshita I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 19.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T., Satouchi M, Tada H, Hirashima T. Gefitinib versus cisplatin plus docetaxel in patients with non–small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 20.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B., Margono B., Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 21.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai C.M., Boyer M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 22.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C., Hu CP, O'Byrne K., Feng J. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 23.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1(3):e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackman D, Pao W, Riely GJ, Engelman JA, Kris M.G., Jänne PA, Lynch T, Johnson B.E., Miller VA. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancer. J Clin Oncol. 2010;28:357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalczyk A, Klüter S, Rode HB, Simard JR, Grütter C, Rabiller M, Rauh D. Structural insights into how irreversible inhibitors can overcome drug resistance in EGFR. Bioorg Med Chem. 2008;16:3482–3488. doi: 10.1016/j.bmc.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 26.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M., Riely G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter CA, Oronsky B, Caroen S, Scicinski J, Cabrales P, Degesys A, Brzezniak C. Partial response to carboplatin in an RRx-001 pretreated patient with EGFR-inhibitor-resistance and T790M-negative NSCLC. Respir Med Case Rep. 2016;18:62–65. doi: 10.1016/j.rmcr.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, Wang Z, Xu CR, Huang Y.S., Wang B.C. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non–small cell lung cancer. Lung Cancer. 2013;79:33–39. doi: 10.1016/j.lungcan.2012.09.016. [PMID: 23079155] [DOI] [PubMed] [Google Scholar]
- 29.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K., Shaw AT, Gettinger S, Cosper AK. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park K, Ahn M, Yu C, Kim S, Lin M., Sriuranpong V, Tsai C, Lee J, Kang J, Perez-Moreno P. Aspiration: first-line erlotinib until and beyond RECIST progression in Asian patients with EGFR mutation-positive NSCLC. Ann Oncol. 2014;25:iv426–iv427. [Google Scholar]
- 31.Mok TSK, Wu Y, Nakagawa K, Kim S, Yang J, Ahn M, Wang J, Yang JC, Lu Y, Atagi S. Gefitinib/chemotherapy vs chemotherapy in epidermal growth factor receptor (EGFR) mutation-positive non–small cell lung cancer (NSCLC) after progression on first-line gefitinib: the phase III, randomized IMPRESS study [abstract] Ann Oncol. 2014;25:1–41. [Abstract LBA2_PR] [Google Scholar]
- 32.Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, Yung RC, Wistuba II., Yatabe Y., Unger M. The international association for the study of lung cancer consensus statement on optimizing management of EGFR mutation-positive non–small cell lung cancer: status in 2016. J Thorac Oncol. 2016;11(7):946–963. doi: 10.1016/j.jtho.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt VR, Kedia S, Kessinger A, Ganti AK. Brain metastasis in patients with non–small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol. 2013;31(25):3162–3164. doi: 10.1200/JCO.2013.49.8915. [DOI] [PubMed] [Google Scholar]
- 34.Gandara DR, Li T, Lara PN, Kelly K., Riess JW, Redman M.W., Mack P.C. Acquired resistance to targeted therapies against oncogene-driven non–small-cell lung cancer: approach tosubtyping progressive disease and clinical implications. Clin Lung Cancer. 2014;15:1–6. doi: 10.1016/j.cllc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, Clarke JL, Lassman AB. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non–small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomizawa Y, Fujita Y, Tamura A, Shirai M, Shibata S, Kawabata T, Shibayama T, Fukai S, Kawahra M, Saito R. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer. 2010;68:269–272. doi: 10.1016/j.lungcan.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Becker A, Crombag L, Heideman D.A., Thunissen F.B., van Wijk AW, Postmus P.E., Smit E.F. Retreatment with erlotinib: regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011;47:2603–2606. doi: 10.1016/j.ejca.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 38.Remon J, Besse B, Soria JC. Successes and failures: what did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med. 2017;15:55. doi: 10.1186/s12916-017-0819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CK, Man J, Lord S., Links M, Gebski V., Mok T., Yang J.C. Checkpoint inhibitors in metastatic EGFR-mutated non–small cell lung cancer—a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P, Ho TS. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C., Minuti G, Salvini J, Coppi E. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]
- 43.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich MF, Yan SX, Schiller JH. Response to crizotinib/erlotinib combination in a patient with a primary EGFR-mutant adenocarcinoma and a primary c-met–amplified adenocarcinoma of the lung. J Thorac Oncol. 2015;10(5):e23–25. doi: 10.1097/JTO.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gainor JF, Niederst MJ, Lennerz JK, Dagogo-Jack I, Stevens S, Shaw AT, Sequist LV, Engelman JA. Dramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-mutant lung cancer harboring de novo MET amplification. J Thorac Oncol. 2016;11(7):e83–e85. doi: 10.1016/j.jtho.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J. MET Amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 47.Azuma K, Hirashima T, Yamamoto N, Okamoto I, Takahashi T, Nishio M, Hirata T, Kubota K, Kasahara K, Hida T. Phase II study of erlotinib plus tivantinib (ARQ 197) in patients with locally advanced or metastatic EGFR mutation-positive non–small-cell lung cancer just after progression on EGFR-TKI, gefitinib or erlotinib. ESMO Open. 2016;1(4):e000063. doi: 10.1136/esmoopen-2016-000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y-L, Kim D-W, Felip E, Zhang L, Liu X, Zhou CC, Lee DH, Han J-Y, Krohn A, Lebouteiller R. Phase (Ph) II safety and efficacy results of a single-arm ph ib/II study of capmatinib (INC280) + gefitinib in patients (pts) with EGFR-mutated (mut), cMET-positive (cMET+) non–small cell lung cancer (NSCLC) ASCO Meet Abstr. 2016;34(15 Suppl):9020. [Google Scholar]
- 49.Wu Y-L, Soo RA, Kim D-W, Yang JC-H, Stammberger UM, Chen W, Johne A, Park K. Tolerability, efficacy and recommended phase II dose (RP2D) of tepotinib plus gefitinib in Asian patients with c-Met–positive/EGFR-mutant NSCLC: Phase Ib data. ASCO Meet Abstr. 2016;34(15 Suppl):e20501. [Google Scholar]
- 50.Larson C, Oronsky B, Scicinski J, Fanger GR, Stirn M, Oronsky A, Reid TR. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015;6(24):19976–19989. doi: 10.18632/oncotarget.5116. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puzanov I, Milhem MM, Andtbacka RHI, Minor DR, Hamid O, Li A, Chastain M, Gorski K, Anderson A, VanderWalde A. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage 3B-IV melanoma. J Clin Oncol. 2014;32(Suppl. 5) [abstr 9029] [Google Scholar]
- 52.Hedjran F, Shantanu K, Tony R. Deletion analysis of Ad5 E1a transcriptional control region: impact on tumor-selective expression of E1a and E1b. Cancer Gene Ther. 2011;18:717–723. doi: 10.1038/cgt.2011.41. [DOI] [PubMed] [Google Scholar]
- 53.Oronsky B, Oronsky N, Scicinski J, Fanger G, Lybeck M, Reid T. Rewriting the epigenetic code for tumor resensitization: a review. Transl Oncol. 2014;7(5):626–631. doi: 10.1016/j.tranon.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oronsky B, Oronsky N, Knox S, Fanger G, Scicinski J. Episensitization: therapeutic tumor resensitization by epigenetic agents: a review and reassessment. Anticancer Agents Med Chem. 2014;14(8):1121–1127. doi: 10.2174/1871520614666140418144610. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter CA, Zeman K, Day RM, Richard P, Oronsky A, Oronsky N, Lybeck M, Scicinski J, Oronsky B. Addressing the elephant in the room, therapeutic resistance in non–small cell lung cancer, with epigenetic therapies. Oncotarget. 2016;7(26):40781–40791. doi: 10.18632/oncotarget.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]