Abstract
Background
Diabetes impairs the cardioprotective effect of volatile anesthetics, yet the mechanisms are still murky. We examined the regulatory effect of isoflurane on microRNA-21, endothelial nitric oxide synthase, and mitochondrial respiratory complex I in type II diabetic mice.
Methods
Myocardial ischemia/reperfusion injury was produced in obese type 2 diabetic (db/db) and C57BL/6 control mice ex vivo in the presence or absence of isoflurane administered prior to ischemia. Cardiac microRNA-21 was quantified by real-time quantitative reverse transcriptional-polymerase chain reaction. The dimers and monomers of endothelial nitric oxide synthase were measured by Western blot analysis. Mitochondrial nicotinamide adenine dinucleotide fluorescence was determined in Langendorff-perfused hearts.
Results
Body weight and fasting blood glucose were greater in db/db than C57BL/6 mice. Isoflurane decreased left ventricular end-diastolic pressure from 35±8 mmHg in control to 23±9 mmHg (P=0.019, n=8 mice/group, mean ± SD) and elevated ±dP/dt 2 h after post-ischemic reperfusion in C57BL/6 mice. These beneficial effects of isoflurane were lost in db/db mice. Isoflurane elevated microRNA-21 and the ratio of endothelial nitric oxide synthase dimers/monomers and decreased mitochondrial nicotinamide adenine dinucleotide levels 5 min after ischemia in C57BL/6 but not db/db mice. MicroRNA-21 knockout blocked these favorable effects of isoflurane, whereas endothelial nitric oxide synthase knockout had no effect on the expression of microRNA-21 but blocked the inhibitory effect of isoflurane preconditioning on nicotinamide adenine dinucleotide.
Conclusions
Failure of isoflurane cardiac preconditioning in obese type II diabetic db/db mice is associated with aberrant regulation of microRNA-21, endothelial nitric oxide synthase, and mitochondrial respiratory complex I.
Obesity and type 2 diabetes mellitus are main health and economic problems with a dramatic increase in prevalence and incidence globally.1 Compared with individuals without obesity and diabetes, obese type 2 diabetic patients have a significantly higher incidence of coronary heart disease.2 Coronary artery bypass graft surgery can help restore blood flow to an area of the heart and thus is often used to treat coronary heart disease. At present, 15% to 30% of the patients who undergo coronary artery surgery are obese and diabetic.1,2 They have an increased mortality and poorer clinical recovery after cardiac surgery than non-obese nondiabetic patients.3 During coronary artery bypass graft surgery, coronary blood flow is interrupted, and the heart is put into electromechanical arrest. After the surgery has been completed, the blood flow is restored to the particular area of myocardium, causing ischemia/reperfusion injury. Attenuation or loss of cardioprotection against peri-operative ischemia/reperfusion injury in obesity and diabetes can be an important and direct cause of poorer clinical outcomes.
Volatile anesthetics, such as isoflurane and sevoflurane, are commonly used to maintain the state of general anesthesia during cardiac surgery.4,5 Brief periods of administration of volatile anesthetics prior to myocardial ischemia potently reduce myocardial ischemia/reperfusion injury in human studies and in a variety of animal models of myocardial ischemia/reperfusion injury.6 This phenomenon is similar to ischemic preconditioning, termed anesthetic preconditioning.7,8 However, a study from our laboratory found that the cardioprotective effect of isoflurane was markedly attenuated in streptozotocin-induced type 1 diabetes.9 Whether isoflurane-induced cardioprotection is attenuated in models of obesity and diabetes and the underlying mechanisms remain to be elucidated.
MicroRNAs are a class of single-stranded, non-protein-coding RNAs of approximately 22 nucleotides in length. By targeting specific coding mRNAs for degradation or translation repression, microRNA levels influence the magnitude of target gene repression.10 MicroRNA-21 is a highly expressed microRNA in cardiovascular system and is involved in divergent pathophysiological processes related to ischemia/reperfusion injury and ischemic conditioning, such as apoptosis, myocardial tolerance to ischemia/reperfusion injury, cardiac growth and differentiation, inflammation, and formation of myocardial fibrosis.11–14 The precise molecular pathways involved in the regulation of ischemia/reperfusion injury by microRNA-21 have not been fully elucidated. There is the evidence that the regulation of microRNA-21 impacts the expression and function of Akt, nitric oxide synthase, phosphatase and tensin homolog, programmed cell death protein 4, and the mitochondrial permeability transition pore, that are crucial for myocardial ischemia/reperfusion injury.11,15,16
Recently, we demonstrated that microRNA-21 mediates isoflurane-induced cardioprotection against ischemia/reperfusion injury through favorable regulation of endothelial nitric oxide synthase (eNOS) and suppression of the opening of the mitochondrial permeability transition pore.16 Multiple lines of evidence suggest that microRNA-21, eNOS, and mitochondria are aberrantly regulated in diabetes and obesity.17–19 How isoflurane regulates microRNA-21, eNOS, and mitochondria in obesity and type 2 diabetes mellitus remains unclear. In the present study, we examined the effects of isoflurane preconditioning on microRNA-21, eNOS, and mitochondrial respiratory complex I in the db/db mouse that is widely used as an animal model of type 2 diabetes mellitus with obesity.20
Materials and Methods
Expanded methods and results are described in the online supplement.
Animals
B6.BKS(D)-Leprdb/J (db/db), microRNA-21 knockout, and eNOS knockout mice on a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). The microRNA-21 knockout mice were mated with C57BL/6 mice for 7 generations at Medical College of Wisconsin (Milwaukee, WI). C57BL/6 wild-type mice were used as control. The experimental procedures were approved by the Animal Care and Use Committee of the Medical College of Wisconsin and conformed to the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Academy of Sciences, 8th edition, 2011).
Measurements of blood pressure and fasting blood glucose
Male C57BL/6 and db/db mice at 12–14 weeks of age were fasting for 12 h. The animals were anesthetized by intraperitoneal injection of 80 mg/kg pentobarbital sodium and ventilated with room air supplemented with 100% O2 at approximately 102 breaths/min. Body temperature was maintained between 36.8°C and 37.3°C throughout the experiment by using a heating pad (Model TC-1000, CWE Inc.; Ardmore, PA). The right carotid artery was cannulated with a Millar pressure catheter (Model SPR-1000, Millar Instruments, Houston, TX) inserted into right carotid artery, as described.18 The catheter was connected to an ADInstrument pressure transducer (ADInstruments, Sydney, Australia) and a Powerlab data acquisition system (ADInstruments). After a 30-minute of stabilization, blood pressure was continuously recorded for 20 minutes. A thoracotomy was performed, and the left ventricle was punctured with a 27 gauge needle. Blood glucose was measured with a blood gas analyzer (ABL-725 Radiometer, Radiometer America Inc., Westlake, OH). After mice were euthanized, the heart and the left ventricle were weighed. Heart weight and left ventricular weight was normalized to body weight.
Transthoracic echocardiography
Mice were sedated by the inhalation of 1.50% isoflurane and oxygen. Non-invasive transthoracic echocardiography was performed with a VisualSonics Vevo 770 High-resolution Imaging System (Toronto, Canada) equipped with a 30 MHz transducer (Scanhead RMV 707), as described previously.21,22 Measurements of left ventricular wall thickness and the internal diameters of left ventricular chamber were made by two-dimension guided M-mode according to the American Society of Echocardiography.23 Estimations of left ventricular volumes and ejection fraction were derived from Teichholz methods in diastole and systole.24 Pulsed Doppler waveforms recorded in the apical-4-chamber view were used for the measurements of the peak velocities of mitral E (early mitral inflow) and A (late mitral inflow) waves, isovolumic contraction time, ejection time, and isovolumic relaxation time of the left ventricle. Myocardial performance index was calculated with the following formula: myocardial performance index = (isovolumic contraction time + isovolumic relaxation time)/ejection time.
Myocardial ischemia/reperfusion injury ex vivo
Langendorff perfusion of mouse hearts
Mouse hearts were mounted on a Langendorff apparatus and perfused retrogradely through the aorta at a constant pressure of 80 mmHg with Krebs-Henseleit buffer at 37 °C, as described.21,25 The buffer was continuously bubbled with a mixture of 95% oxygen/5% carbon dioxide via in-line filter (5 μm pore size). A fluid-filled plastic balloon was inserted into the chamber of the left ventricle via the mitral valve, and connected to a pressure transducer for continuous measurement of left ventricular pressure. The hearts were immersed in perfusate maintained at 37.2 ± 0.3°C, and the balloon was inflated to a diastolic pressure of ~5 to 10 mmHg. Coronary flow was monitored by an in-line flow probe connected to a flow meter (Transonics Systems Inc., Ithaca, NY). The signal of the left ventricle was monitored to obtain heart rate and left ventricular dP/dt. Left ventricular developed pressure (LVDP) was calculated as the difference between the systolic and end-diastolic pressure of the left ventricle. Global ischemia/reperfusion was produced by cessation of perfusion followed by reperfusion at a designated time.
Experimental protocols
Langendorff-perfused hearts were used in the following three protocols. Protocol 1 determined the effect of isoflurane on cardiac function in C57BL/6 and db/db mice. Mouse hearts were assigned to the following 4 groups (n=10 C57BL/6 or 12 db/db mouse hearts/group): control, db/db, isoflurane, and db/db+isoflurane. All hearts were stabilized for 30 minutes and subjected to 30 minutes of no-flow global ischemia followed by 2 h of reperfusion. Isoflurane was bubbled into Krebs-Henseleit solution using an agent-specific vaporizer (Ohio Medical Instruments) placed in the 95% oxygen/5% carbon dioxide gas mixture line. Isoflurane concentrations in the coronary effluent were determined by a gas chromatography. Isoflurane at 1.4% produced 0.5 mM isoflurane in the coronary effluent (approximately 1.0 maximum alveolar concentration). In the isoflurane and db/db+isoflurane groups, the hearts were perfused with 2 cycles of 5-min Krebs-Henseleit solution containing 0.5 mM isoflurane/5-min Krebs-Henseleit solution without isoflurane followed by a 10-minute washout prior to ischemia. Heart rate, left ventricular end-diastolic pressure (LVEDP), LVDP, +dP/dt (maximum rate of LVDP increase), −dP/dt (maximum rate of LVDP decrease), and coronary flow rate at baseline, 10, 20, and 30 min after ischemia, and 10, 30, 60, 90, and 120 min after reperfusion were determined. Blinding methods were not used because db/db mice had different phenotypes from C57BL/6 control mice. Protocol 2 studied the effect of microRNA-21 knockout on isoflurane-induced improvement in cardiac function. MicroRNA-21 knockout and C57BL/6 mouse hearts were divided into the following 4 groups (n=10 hearts/group): control, microRNA-21 knockout, isoflurane, and microRNA-21 knockout+isoflurane. All hearts were stabilized for 30 minutes and subjected to 30 minutes of no-flow global ischemia followed by 2 h of reperfusion with or without 1.4% isoflurane administered prior to ischemia. The values of ±dP/dt were determined at baseline and 2 h after post-ischemic reperfusion. Protocol 3 determined the importance of eNOS in isoflurane-induced cardioprotection. C57BL/6 and eNOS knockout mouse hearts were divided into the following 4 groups (n=10 hearts/group): control, endothelial nitric oxide synthase knockout, isoflurane, and endothelial nitric oxide synthase knockout+isoflurane. All hearts were stabilized for 30 minutes and subjected to 30 minutes of no-flow global ischemia followed by 2 h of reperfusion with or without 1.4% isoflurane administered prior to ischemia. The values of ±dP/dt were determined at baseline and 2 h after post-ischemic reperfusion.
Quantitative reverse transcriptional-polymerase chain reaction analysis of microRNA-21
C57BL/6 and db/db mice at 12–14 weeks of age were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg) and ventilated with room air supplemented with 100% oxygen at a rate of ~102 breaths per minute with a tidal volume of ~225 μl using a rodent ventilator (Hugo Sachs Electronik, Harvard Apparatus, Germany). Under a dissecting microscope (Thermo Fisher Scientific Inc., Pittsburgh, PA), an 8-0 nylon suture is passed below the left main descending coronary artery 1–3 mm from tip of the normally positioned left auricle. Myocardial ischemia is induced by tying the suture over a piece of rolled wetted gauze for 30 min, and reperfusion is initiated by loosening the suture.25 Successful performance of coronary artery occlusion and reperfusion is verified by visual inspection (for example, by noting the development of a pale color in the distal myocardium upon occlusion and the return of a bright red color due to hyperemia after release) and by observing widening of QRS wave and changes of ST-segment (depressed ST-segment during ischemia and elevated ST-segment after reperfusion) on the electrocardiogram. Body temperature was maintained between 36.8 °C and 37.5 °C throughout the experiment by using a heating pad (Model TC-1000). The heart was excised, and the left ventricle was homogenized at 4 °C for real-time quantitative reverse transcriptional-polymerase chain reaction analysis of microRNA-21.26
MicroRNA-21 extraction and real-time quantitative reverse transcriptional-polymerase chain reaction analysis
Total RNA from the left ventricle was extracted using Qiazol reagent according to the protocol of the manufacturer (Qiagen, Valencia, CA). Chloroform was added and samples were centrifuged to facilitate phase separation. The aqueous phase was extracted and combined with ethanol in miRNeasy Mini spin columns (Qiagen). Total RNA was eluted in RNase-free water. The concentration of extracted total RNA was quantified by the Epoch spectrophotometer (Biotek, Winooski, VT). Samples were considered pure if the A260/280 ratio was between 1.9 and 2.0. One μg of total RNA from each sample was used to generate complementary DNA using miScript Reverse transcriptase mix, nucleics mix, and HiFlex Buffer (Qiagen). To analyze the microRNA-21 expression, a master mix (25 μL/well) containing the template cDNA (4.5 ng/well), RNase-free water, and miScript SYBR Green (Qiagen), and the primers (microRNA-21 or the housekeeping gene, Rnu-6) was prepared according to the manufacturer’s directions. Real-time quantitative reverse transcriptional-polymerase chain reaction was conducted using the BioRad iCycler Real-Time Polymerase Chain Reaction Detection System. Real-time quantitative reverse transcriptional-polymerase chain reaction for each sample was run in triplicate. Expression of microRNA-21 was normalized by expression of Rnu-6. The relative gene expressions were calculated in accordance with the ΔΔCt method. Relative microRNA levels were expressed as percentages compared to non-isoflurane exposed controls.
Immunoblotting
Pentobarbital-anesthetized C57BL/6 and db/db mice at 12–14 weeks of age were subjected to 30 minutes of coronary artery occlusion followed by 2 h of reperfusion in vivo with or without 1.4% isoflurane administered prior to coronary artery occlusion. The myocardium from the area at risk of mouse hearts was harvested and homogenized in a buffer containing 20.0 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 2.0 mM ethyleneglycol bis(2-aminoethyl ether)-N,N,N′,N′ tetraacetic acid (EGTA), 5.0 mM ethylenediaminetetraacetic acid (EDTA), protease inhibitor cocktail (1:100; Calbiochem, San Diego, CA), phosphatase inhibitors cocktail (1:100; Calbiochem), 0.5% detergent (Nonidet™ P-40 detergent pH 7.4, Sigma-Aldrich, St. Louis, MO). Immunoblots were performed using standard techniques, as described.27 Briefly, non-boiled protein lysate that contained 50 μg of protein was resolved by 6% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) at 4 °C overnight.26 Membranes were incubated with a 1:2000 dilution of mouse anti-eNOS monoclonal antibodies (BD Transduction Laboratories, San Jose, CA). The membrane was washed and then incubated with the appropriate anti-mouse secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence followed by densitometric analysis using image acquisition and analysis software (Image J, National Institutes of Health, Baltimore, MD).
Measurements of mitochondrial nicotinamide adenine dinucleotide levels in Langendorff-perfused hearts
Mitochondrial nicotinamide adenine dinucleotide (reduced form, NADH) fluorescence in mouse hearts was determined in a Langendorff apparatus placed within a light-proof Faraday cage to block the light, as described.16 A fiberoptic cable was placed against the left ventricle of Langendorff-prepared mouse hearts to excite and record transmyocardial fluorescence at a wavelength of 456 nm during ischemia and reperfusion. The two proximal ends of the fiberoptic cable were connected to a modified spectrophotofluorometer (Photon Technology International, London, Canada). Fluorescence (F) was excited with light at the appropriate wavelength (λ) from a xenon arc lamp at 75 W filtered through a monochromator (Delta RAM, Photon Technology International). NADH signal was recorded continuously using a Powerlab data acquisition system (ADInstruments) at baseline and during ischemia and reperfusion.
Statistical analysis
All data are expressed as mean ± SD. Two-way repeated measures analysis of variance (ANOVA) test was used to evaluate the differences in body weight, mean arterial blood pressure, heart weight, and the ratio of heart/body weight, and echocardiographic data. One-way analysis of variance followed by Bonferroni post hoc test was used to analyze LVEDP, LVDP, ±dP/dt, expression of microRNA-21, or the ratio of eNOS dimers/monomers. Repeated-measures analysis of variance followed by Bonferroni multiple comparison test was used to evaluate differences in NADH fluorescence. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc.). A value of P less than 0.05 (two tailed) was considered statistically significant.
In ex vivo experiments of myocardial ischemia/reperfusion injury, group size was determined by using a power analysis of means from published results as well as our experience to estimate the number of animals needed to test the null hypothesis. The value of +dP/dt in C57BL/6 mice 2 h after reperfusion without the treatment of isoflurane is typically 600±120 mmHg/s, and +dP/dt in C57BL/6 mice treated with 1.4% isoflurane is around 800±160 mmHg/s.16 Based upon an average standard deviation of 120 from our prior work with ischemia/reperfusion injury in C57BL/6 mice,21 an n = 8 per group will allow for detection of a difference between groups of up to 240 at P<0.05. Thus, 8 C57BL/6 or db/db mice per group were needed for these experiments.
Results
Characteristics of db/db mice
Db/db mice at 12–14 weeks of age displayed obesity and had an increased body weight compared with C57BL/6 mice (48.4±3.4 g in db/db and 27.4±2.4 g in C57BL/6 group, P<0.0001, n=10 mice/group) (Figure S1). Blood glucose was higher in db/db than C57BL/6 mice (380±59 mg/dl in db/db and 99±31 mg/dl in C57BL/6 group, P<0.0001, n=10 mice/group). Mean arterial blood pressure was comparable between db/db and C57BL/6 mice (114±35 mmHg in db/db and 91±33 mmHg in C57BL/6 group, n= 8 mice in db/db and 10 mice in C57BL/6 group, P=0.187). The ratios of heart weight/body weight and left ventricular weight/body weight were smaller in db/db than C57BL/6 mice (Heart weight/body weight: 0.0041±0.0004 in db/db and 0.0052±0.0006 in C57BL/6 group, P=0.005, n=10 mice/group; left ventricular weight/body weight: 0.0035±0.0003 in db/db and 0.0043±0.0006 in C57BL/6 group, P=0.001, n=10 mice/group).
Table 1 lists echocardiographic parameters of db/db and C57BL/6 mice. There were no significant differences between C57BL/6 and db/db mice in multiple parameters (P>0.05), including heart rate, the thickness of left ventricular anterior wall and posterior wall, left ventricular end-diastolic volume, the ratio of peak mitral E/A, isovolumic contraction time of the left ventricle, and myocardial performance index. Compared with C57BL/6 mice, ejection fraction was decreased (P=0.039, n=10 mice/group), whereas left ventricular end-systolic volume, ejection time, and isovolumic relaxation time of the left ventricle were increased in db/db mice (P=0.020, 0.032, and 0.032, n=10 mice/group). These results suggest that the systolic and diastolic functions of the left ventricle may be impaired in db/db mice.
Table 1.
Echocardiographic parameters of C57BL/6 and db/db mice
| C57BL/6 | db/db | |
|---|---|---|
| Heart rate, beats/min | 458±83 | 442±69 |
| Anterior wall at end diastole, mm | 0.78±0.12 | 0.82±0.19 |
| Anterior wall at end systole, mm | 1.20±0.26 | 1.22±0.31 |
| Posterior wall at end diastole, mm | 0.79±0.16 | 0.87±0.22 |
| Posterior wall at end systole, mm | 1.20±0.25 | 1.32±0.19 |
| Left ventricular end-diastolic volume, μl | 59±22 | 56±18 |
| Left ventricular end-systolic volume, μl | 13±6 | 28±16* |
| Ejection fraction, % | 71±22 | 52±14* |
| Peak mitral E, cm/s | 83±15 | 75±11 |
| Peak E/A ratio | 1.71±0.38 | 1.49±0.10 |
| Isovolumic contraction time, millisecond | 13.1±4.1 | 13.7±3.9 |
| Ejection time, millisecond | 42.6±3.7 | 47.0±4.7* |
| Isovolumic relaxation time, millisecond | 16.6±2.1 | 18.7±1.9* |
| Myocardial performance index | 0.70±0.13 | 0.69±0.09 |
P<0.05 versus C57BL/6 mice (mean ± SD, n=10 mice/group).
Isoflurane preconditioning improved cardiac function during reperfusion in C57BL/6 but not db/db mice
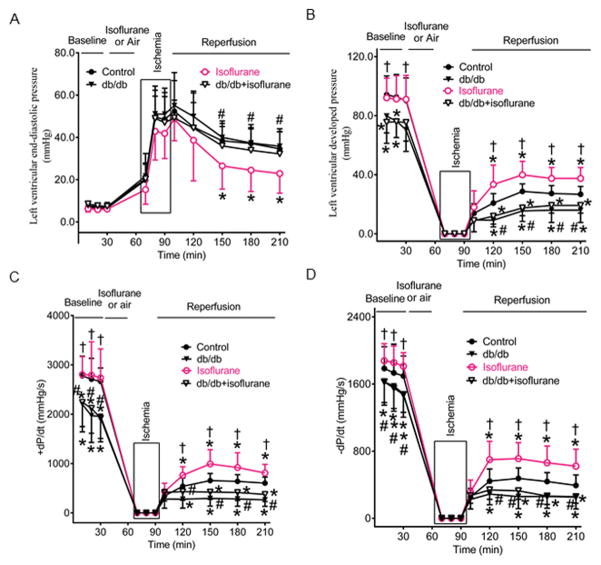
Figure 1 demonstrates cardiac function of Langendorff-perfused db/db and C57BL/6 mouse hearts subjected to ischemia/reperfusion injury in the presence or absence of isoflurane preconditioning. LVEDP at baseline was comparable among 4 groups (P>0.05) (Figure 1A), whereas the values of LVDP and ±dP/dt at baseline were smaller in db/db and db/db+isoflurane than control groups (P=0.031 in LVDP, 0.009 in +dP/dt, and 0.033 in −dP/dt between db/db and control groups; P=0.034 in LVDP, 0.024 in+dP/dt, and 0.028 in −dP/dt between db/db+isoflurane and control groups; n=8 mice/group) (Figures 1B, 1C, and 1D). Global ischemia for 30 minutes resulted in the cessation of the contraction and relaxation of the hearts and an increase in LVEDP. With reperfusion, contraction and relaxation were gradually restored in all mouse hearts. There were no significant differences in LVEDP between db/db and control groups during ischemia and reperfusion (P>0.05) (Figure 1A). Compared with control groups, LVEDP was significantly decreased in isoflurane groups 1 to 2 h (35±8 mmHg in control versus 23±9 mmHg in isoflurane, P=0.019, n=8 mice/group) after reperfusion but not in db/db+isoflurane groups. The values of LVDP and ±dP/dt were significantly smaller in db/db than control groups from 30 minutes to 2 h after reperfusion (P<0.05) (Figures 1B, 1C, and 1D). Compared with control groups, the values of LVDP and ±dP/dt were significantly increased in isoflurane groups from 30 minutes to 2 h after reperfusion and decreased in db/db+isoflurane groups (Figures 1B, 1C, and 1D). There were not significant differences between db/db+isoflurane and db/db groups in the values of ±dP/dt.
Figure 1.
Isoflurane preconditioning improved the recovery of cardiac fnction during post-ischemic reperfusion in Langendorff-perfused C57BL/6 mouse hearts but not db/db mouse hearts. A: left ventricular end-diastolic pressure (mean ± SD); B: left ventricular developed pressure; C: the maximum rate of developed pressure rise (+dp/dt); D: the maximum rate of developed presure decreases (−dP/dt). Control, C57BL/6 mouse hearts undergoing ischemia/reperfusion injury: db/db, db/db mouse hearts undergoing ischemia/reperfusion injury; isoflurane, C57BL/6 mouse hearts treated with 1.4% isoflurane before ischemia; db/db+isoflurane, db/db mouse hearts treated with 1.4% isoflurane before ischemia. *P<0.05 versus control groups, †P<0.05 versus db/db groups, #P<0.05 versus isoflurane groups (n=8 mice/group).
Failure of isoflurane preconditioning to up-regulate microRNA-21 in db/db mice contributed to attenuation of cardioprotection
Figure 2A shows the regulation of cardiac microRNA-21 by isoflurane in C57BL/6 and db/db mice. Compared with control group, the expression of microRNA-21 gene was significantly decreased in db/db mice (P=0.013, n=8 mice/group). Isoflurane increased microRNA-21 by 127±106% (P=0.011, n=8 mice) in C57BL/6 mice. In contrast, microRNA-21 expression was decreased by 27±33% (n=8 mice) by isoflurane in db/db mice (P=0.021 between db/db+isoflurane and db/db group).
Figure 2.
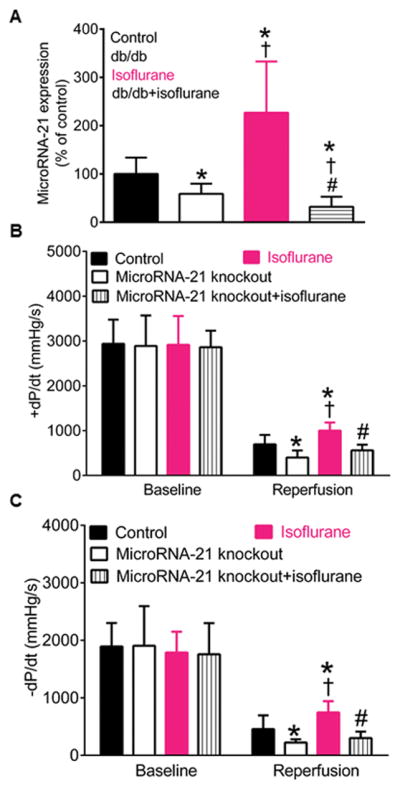
Isoflurane preconditioning elevated the expression of microRNA-21 associated with cardioprotective effect in C57BL/6 mice but decreased microRNA-21 in db/db mice. A: alterations in microRNA-21 by isoflurane in C57BL/6 and db/db mice (mean ± SD, n=8 mice/group); B: isoflurane improved +dP/dt (the maximum rate of left ventricular developed pressure increase) in C57BL/6 but not microRNA-21 knockout mice 2 h after reperfusion (reperfusion) (n=9 mice in control, 8 mice in microRNA-21 knockout group, 9 mice in isoflurane group, and 7 mice inmicroRNA-21 knockout+isoflurane group); C: isoflurane improved the values of -dP/dt (the maximum rate of left ventricular developed pressure decrease) in C57BL/6 but not microRNA-21 knockout mice 2 h min after reperfusion (n=9 mice in control, 8 mice in microRNA-21 knockout group, 9 mice in isoflurane group, and 7 mice inmicroRNA-21 knockout+isoflurane group). Control, C57BL/6 mouse hearts undergoing ischemia/reperfusion injury: db/db, db/db mouse hearts undergoing ischemia/reperfusion injury; microRNA-21 knockout, microRNA-21 knockout mouse hearts subjected to ischemia/reperfusion injury; isoflurane, C57BL/6 mouse hearts treated with 1.4% isoflurane before ischemia; db/db+isoflurane, C57BL/6 mouse hearts treated with isoflurane before ischemia; microRNA-21 knockout+isoflurane, microRNA-21 knockout mouse hearts treated with isoflurane prior to ischemia. *P<0.05 versus control groups, †P<0.05 versus db/db or microRNA-21 knockout groups, #P<0.05 versus isoflurane groups.
In Langendorff-perfused hearts, we examined whether microRNA-21 knockout impacted isoflurane preconditioning-elicited cardioprotection against ischemia/reperfusion injury. The values of ±dP/dt at baseline were comparable among 4 groups (P>0.05, n=7–9 mice/group) (Figures 2B and 2C). Compared with control groups, the values of ±dP/dt were significantly decreased in microRNA-21 knockout groups (P=0.005 in +dP/dt and 0.019 in −dP/dt, n=9 mice in control and 8 mice in microRNA-21 knockout group) and increased in isoflurane groups 2 h after post-ischemic reperfusion (P=0.005 in dP/dt and 0.014 in −dP/dt, n=9 mice/group). Interestingly, isoflurane failed to elevate ±dP/dt in microRNA-21 knockout mice 2 h after reperfusion (P=0.141 in +dP/dt and 0.107 in −dP/dt between microRNA-21 knockout+isoflurane and control groups; P=0.125 in +dP/dt and 0.143 in −dP/dt between microRNA-21 knockout+isoflurane and microRNA-21 knockout groups; n=7 mice in microRNA-21 knockout+isoflurane group, 9 mice in control, and 8 mice in microRNA-21 knockout group).
Failure of isoflurane preconditioning to increase eNOS dimerization in db/db mice was associated with reduction of cardioprotection
Figures 3A and 3B show the effect of isoflurane preconditioning on eNOS dimers (230 kDa, Figure S5) and monomers (130 kDa) in the myocardium of C57BL/6 and db/db mice subjected to ischemia/reperfusion injury. Compared with control groups, the ratio of eNOS dimers/monomers was decreased in db/db groups (P=0.006, n=5 mice/group). Isoflurane significantly increased the ratio of eNOS dimers/monomers in C57BL/6 (P=0.002 between isoflurane and control groups, n=5 mice/group) but not db/db mice subjected to ischemia/reperfusion injury (P=0.506 between db/db+isoflurane and control groups; P=0.057 between db/db+isoflurane and db/db groups; n=5 mice/group). There were significant decreases in the ratio of eNOS dimers/monomers in db/db+isoflurane group compared with isoflurane group (P=0.002, n=5 mice/group).
Figure 3.
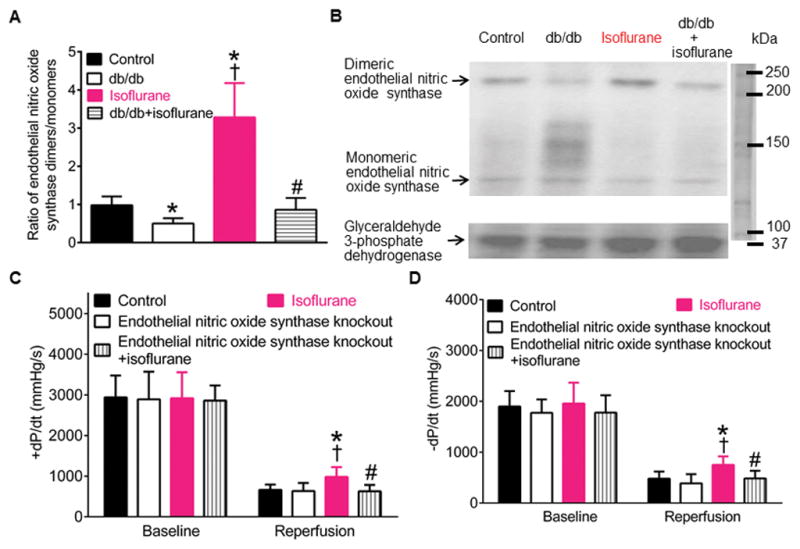
Isoflurane preconditioning increased the dimerization of endothelial nitric oxide synthase associated with cardioprotective effect in C57BL/6 but not db/db mice. A: alterations in the ratio of endothelial nitric oxide synthase dimers/monomers by isoflurane preconditioning (mean ± SD, n=5 mice/group); B: Western blot bands showing the expression of endothelial nitric oxide synthase dimers and monomers and glyceraldehyde 3-phosphate dehydrogenase as a loading control in mouse hearts (n=3 hearts/blot); C: isoflurane preconditioning increased +dP/dt in C57BL/6 but not endothelial nitric oxide synthase knockout mice 2 h after post-ischemic reperfusion (reperfusion) (n=9 mice in control and endothelial nitric oxide synthase knockout groups and 8 mice in isoflurane and endotheial nitric oxide synthase knockout+isoflurane groups); D: isoflurane preconditioning elevated the value of −dP/dt in C57BL/6 but not endothelial nitric oxide synthase knockout mice 2 h after reperfusion (reperfusion) (n=9 mice in control and endothelial nitric oxide synthase knockout groups and 8 mice in isoflurane and endotheial nitric oxide synthase knockout+isoflurane groups). Control, C57BL/5 mice subjected to ischemia/reperfusion injury; db/db, db/db mice undergoing ischemia/reperfusion injury; endothelial nitric oxide synthase knockout, endothelial nitric oxide synthase knockout mice undergoing ischemia/reperfusion injury; isoflurane, C57BL/6 mice undergoing ischemia/reperfusion injury; db/db+isoflurane, db/db mice treated with 1.4% isoflurane prior to ischemia/reperfusion injury; endothelial nitric oxide synthase knockout+isoflurane, endothelial nitric oxide synthase knockout mice treated with isoflurane before ischemia/reperfusion injury. *P<0.05 versus control groups, †P<0.05 versus db/db or endothelial nitric oxide synthase knockout groups, #P<0.05 versus isoflurane groups.
To study whether eNOS is associated with isoflurane preconditioning, we examined the effect of eNOS knockout on isoflurane preconditioning in Langendorff-perfused hearts. The values of ±dP/dt were comparable among 4 groups at baseline (+dP/dt: P=0.977 in endothelial nitric oxide synthase knockout group, 0.777 in isoflurane group, and 0.418 in endothelial nitric oxide synthase knockout+ isoflurane group versus control group; −dP/dt: P=0.751 in endothelial nitric oxide synthase knockout group, 0.358 in isoflurane group, and 0.452 in endothelial nitric oxide synthase knockout+ isoflurane group versus control; n=9 in control, 9 in endothelial nitric oxide knockout, 8 in isoflurane, and 8 hearts in endothelial nitric oxide synthase knockout+isoflurane groups) (Figures 3C and 3D). There were no significant differences in the values of ±dP/dt between endothelial nitric oxide synthase knockout and control groups. Compared with control goups, the values of ±dP/dt was significantly increased in isoflurane groups (P=0.007 in +dP/dt and 0.003 in −dP/dt, n=9 hearts in control and 8 hearts in isoflurane groups) but not in endothelial nitric oxide synthase knockout+isoflurane groups (P=0.656 in +dp/dt and 0.956 in −dP/dt, n=9 hearts in control and 8 hearts in endothelial nitric oxide synthase knockout+isoflurane groups) 2 h after post-ischemic reperfusion (Figures 3C and 3D). There were significant decreases in the values of ±dP/dt in endothelial nitric oxide synthase knockout+isoflurane groups compared with isoflurane groups (P=0.005 in ±dP/dt, n=8 hearts/group) (Figures 3C and 3D).
Reduction of mitochondrial NADH levels by isoflurane preconditioning in ischemia in C57BL/6 mice and aberrant regulation of NADH in db/db mice
Mitochondrial NADH levels from Langendorff-perfused hearts at baseline were higher in db/db and db/db+isoflurane groups than control groups (P=0.0006 in db/db and 0.0005 in db/db+isoflurane group versus control group, n=8 hearts in db/db and db/db+isoflurane groups and 9 hearts in control groups) (Figure 4). During ischemia, the NADH signal initially increased and peaked 5 minutes after ischemia followed by a gradual decline in control groups. NADH fluorescece was significantly lower in isoflurane group than in control group 3 to 5 minutes after ischemia and greater in either db/db or db/db+isoflurane groups during a period of 30 minutes than in control group (P<0.05, n=8–9 hearts/group). At all time points, no significant differences existed between the db/db+isoflurabe and db/db groups (P>0.05, n=8–9 hearts/group). During reperfusion, the NADH signal remained relatively stable in the 4 experimental groups. NADH levels were higher in either db/db or db/db+isoflurane group than in control groups during a period of 2-h reperfusion.
Figure 4.
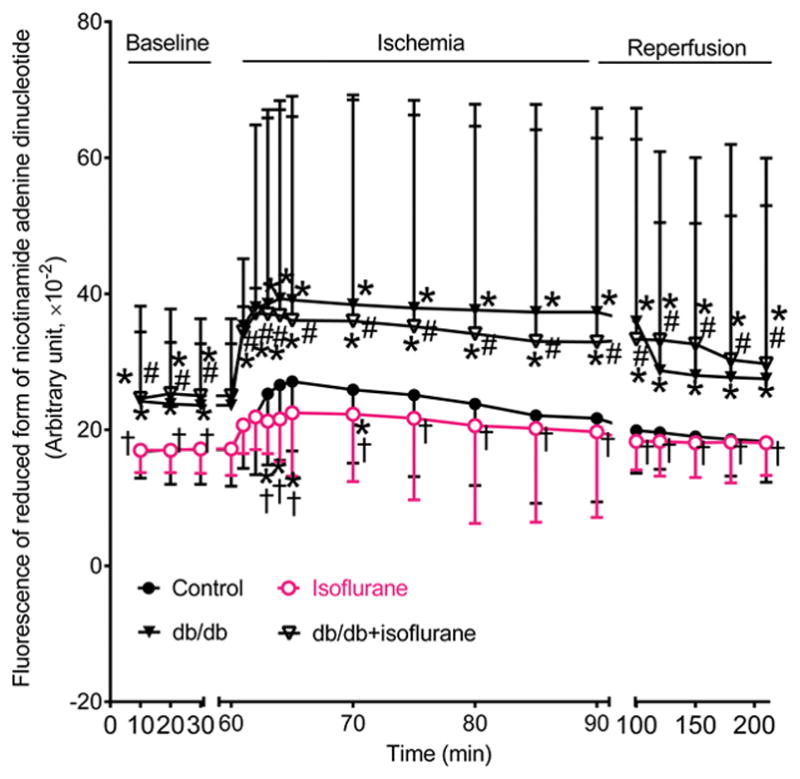
Effects of diabetes and isoflurane treatment on the levels of reduced form of nicotinamide adenine dinucleotide during ischemia and reperfusion. All hearts were stabilized in Langendorff apparatus for 30 minutes (baseline) and perfused with the buffer with or without isoflurane prior to 30 minutes of global ischemia (ischemia) followed by 2 h of reperfusion (reperfusion). Control, C57BL/6 mouse hearts undergoing ischemia/reperfusion injury: db/db, C57BL/6 mouse hearts undergoing ischemia/reperfusion injury; isoflurane, C57BL/6 mouse hearts treated with 1.4% isoflurane before ischemia; db/db+isoflurane, db/db mouse hearts treated with 1.4% isoflurane before ischemia. *P<0.05 versus control groups, †P<0.05 versus db/db groups, #P<0.05 versus isoflurane groups (n=9 mice in control, 8 mice in db/db group, 9 mice in isoflurane, and 8 mice in db/db+isoflurane group).
Genetic disruption of microRNA-21 blocked the regulation of mitochondrial NADH by isoflurane preconditioning via eNOS
Figures 5A, 5B, and 5C show the effects of microRNA-21 knockout on eNOS dimers and monomers and mitochondrial NADH levels in isoflurane preconditioned hearts 5 minutes after ischemia. The ratio of eNOS dimers/monomers was comparable between microRNA-21 knockout and control groups (P=0.224, n=5 hearts/group) (Figures 5A and 5B). Compare with control group, the ratio of eNOS dimers/monomers was significantly increased in isoflurane group (P=0.007, n=5 hearts/group) but not microRNA-21 knockout+isoflurane groups (Figures 5A and 5B). There were significant differences in the ratio of eNOS dimers/monomers between microRNA-21 knockout+isoflurane and isoflurane groups (P=0.006, n=5 hearts/group). Mitochondrial NADH levels were comparable among 4 groups at baseline and were elevated in early ischemia (Figure 5C). Compred with control group, mitochondrial NADH levels were elevated in microRNA-21 knockout group (P=0.008, n=9 hearts/group) and decreased in isoflurane group 5 min after post-ischemic reperfusion (P=0.008, n=9 hearts/group) (Figure 5C). There were significant differences in NADH levels between microRNA-21 knockout+isoflurane and isoflurane groups. These results suggest that eNOS and mitochondrial respiratory complex I are the target of microRNA-21 in isoflurane-induced cardioprotection.
Figure 5.
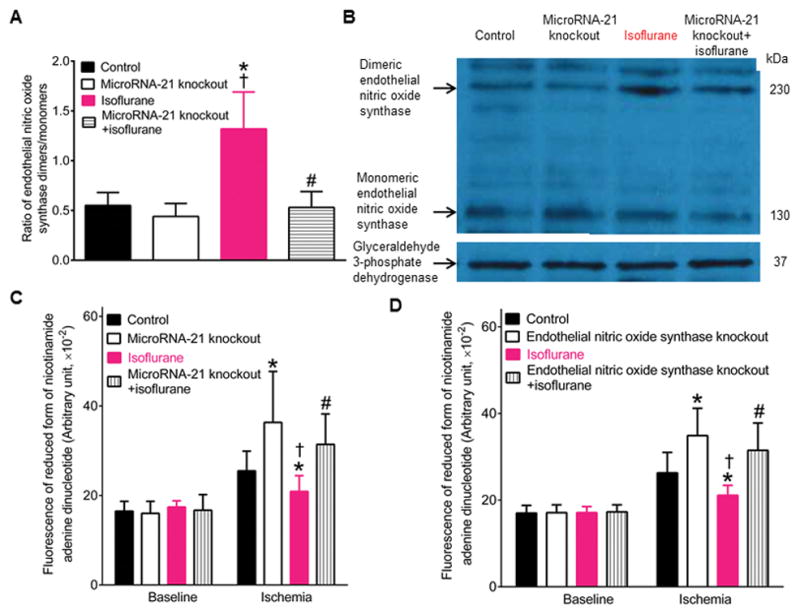
MicroRNA-21 knockout blocked the regulatory effects of isoflurane preconditioning on endothelial nitric oxide synthase and mitochontrial nicotinamide adenine dinucleotide in ischemic myocardium. A: microRNA-21 knockout blocked isoflurane-induced increases in the ratio of endothelial nitric oxide synthase dimers/monomers in myocardium 5 min after ischemia (mean ± SD, n=5 hearts/group); B: representativive Western blot bands showing the expression of endothelial nitric oxide synthase dimers (230 KDa) and monomers (130 KDa) and glyceraldehyde 3-phosphate dehydrogenase (37 KDa) as a loading control in myocardium 5 min after ischemia (n=3 hearts/blot); C: microRNA-21 knockout blocked isoflurane-induced decreases in mitochondrial nicotinamide adenine dinucleotide levels 5 min after ischemia (n=9 hearts in control, microRNA-21 knockout, and isoflurane groups and 8 hearts in microRNA-21 knockout+isoflurane group). All hearts were stabilized for 30 minutes in a Langendorff apparatus and subjected to 5 minutes of global ischemia. Control, C57BL/6 mouse hearts underlying 5 minutes of ischemia; microRNA-21 knockout, microRNA-21 knockout hearts subjected to 5 minutes of ischemia; isoflurane, C57BL/6 mouse hearts treated with isoflurane and subsequently underlying 5 minutes of ischemia; microRNA-21 knockout+isoflurane, microRNA-21 knockout hearts treated with isoflurane and subsequently subjected to 5 minutes of ischemia. *P<0.05 versus control groups, †P<0.05 versus microRNA-21 knockout groups, #P<0.05 versus isoflurane groups. D: endothelial nitric oxide synthase knockout blocked isoflurane-induced decreases in mitochondrial nicotinamide adenine dinucleotide levels 5 minutes after ischemia (n=10 hearts in control, 9 hearts in endothelial nitric oxide synthase knockout and isoflurane groups, and 8 hearts in endothelial nitric oxide synthase knockout+isoflurane group). Control, C57BL/6 mouse hearts underlying 5 minutes of ischemia; endothelial nitric oxide synthase knockout, endothelial nitric oxide synthase knockout hearts subjected to 5 minutes of ischemia; isoflurane, C57BL/6 mouse hearts treated with isoflurane and subsequently subjected to 5 minutes of ischemia; endothelial nitric oxide synthase knockout+isoflurane, endothelial nitric oxide synthase knockout hearts treated with isoflurane and subsequently subjected to 5 minutes of ischemia. *P<0.05 versus control groups, †P<0.05 versus endothelial nitric oxide synthase knockout groups, #P<0.05 versus isoflurane groups.
Figure 5D shows the effect of eNOS knockout on mitochondrial NADH levels in isoflurane preconditioned hearts 5 minutes after ischemia. Genetic disruption of eNOS did not change the expression of cardiac microRNA-21 in the presence and absence of isoflurane preconditioning (data not shown). Mitochondrial NADH levels were comparable among 4 groups at baseline (P=0.876 in endothelial nitric oxide synthase knockout, 0.916 in isoflurane, and 0.709 in endothelial nitric oxide synthase knockout+isoflurane group versus control groups, n=9 hearts in endothelial nitric oxide synthase knockout and isoflurane groups, 8 hearts in endothelial nitric oxide synthase knockout+isoflurane, and 10 hearts in control groups). Conpared with control group, mitochondrial NADH levels were significantly elevated in eNOS knockout groups (P=0.005, n=10 hearts in control group and 9 hearts in endothelial nitric oxide synthase knockout group) and decreased in isoflurane group 5 minutes after ischemia (P=0.008, n=10 hearts in control and 9 hearts isoflurane group). There were significant differences in NADH levels between eNOS knockout and eNOS knockout+isoflurane groups 5 minutes after ischemia. These results suggest that mitochondrial respiratory complex I is the target of eNOS, and that microRNA-21 is not the target of eNOS in isoflurane-induced cardioprotection against ischemia/reperfusion injury.
Exclusion criteria
Landgendorff-perfused heart experiments used 60 C57BL/6, 40 db/db, 20 microRNA-21 knockout, and 20 eNOS knockout mice. Eight C57BL/6, 8 db/db, 5 microRNA-21 knockout, and 3 eNOS knockout mouse hearts were excluded from datum analysis due to any of the following undesirable situations: 1) time delay in aortic cannulation (>3 minutes); 2) aortic damage during the cannulation process; and 3) sustained arrhythmia during the 30 minutes of stabilization period.
Discussion
The results of the present study demonstrate that isoflurane preconditioning fails to protect obese type 2 diabetic hearts against ischemia/reperfusion injury and causes aberrant regulation of microRNA-21, eNOS, and mitochondrial NADH in obese type 2 diabetic mice. In C57BL/6 wild-type mice subjected to ischemia/reperfusion injury, isoflurane preconditioning not only reduces myocardial infarct size (Figures S2 and S3) but also improves the recovery of cardiac function during post-ischemic reperfusion. The favorable effect of isoflurane are attributed to increases in cardiac microRNA-21 and eNOS dimerization and decreases in mitochondrial NADH levels during early ischemia, as supported by our previous studies.16,28 However, isoflurane preconditioning fails to produce these beneficial effects in db/db mice. These results suggest that abnormal regulation of microRNA-21, eNOS, and mitochondrial complex I might play important roles in the diminished cardioprotective effect of isoflurane preconditioning in obesity and type 2 diabetes mellitus.
The db/db mouse at 12–14 weeks of age had increased body weight and blood glucose. Left ventricular systolic (ejection fraction) function was significantly depressed, and left ventricular end-systolic volume and isovolumic relaxation time of the left ventricle were elevated in db/db mice. These results suggest that cardiac contractility and relaxation function are impaired in db/db mice. Since systemic blood pressure was not higher in db/db than C57BL/6 mice, cardiac dysfunction cannot be attributed to hypertension. Previous studies showed that stroke volume and heart rate were decreased in db/db mice compared with non-diabetic control mice.20 Diabetes results in increases in atherosclerosis, arterial calcification, and vascular inflammation, leading to elevated systemic vascular resistance. It is possible that cardiac dysfunction in db/db mice may be caused by both decreased stroke volume and increased systemic vascular resistance. Clinical manifestation of coronary heart disease may present as ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, or unstable angina in patients.29 In the experiment of in vivo ischemia/reperfusion injury, ST-segment and T wave from electrocardiogram were normal before coronary artery occlusion (data not shown). Whether the db/db mice at 12–14 weeks of age had non-ST-segment elevation myocardial ischemia is not known. Diabetic cardiomyopathy refers to diabetes-associated changes in the structure and function of the myocardium that is not directly attributable to other confounding factors such as coronary heart disease or hypertension.30 Possibly, the db/db mouse develops diabetic cardiomyopathy at 12–14 weeks of age.
Although clinical studies show diabetes exacerbates myocardial ischemia/reperfusion injury,31,32 previous studies report that diabetes can reduce, increase, or have no effect on myocardial infarct size in experimental models of animals.33,34 In the present study, the values of LVDP and ±dP/dt during post-ischemic reperfusion were significantly decreased in isolated hearts of db/db mice compared with age-matched C57BL/6 mice, suggesting exacerbation of ischemia/reperfusion injury in db/db mice. The db/db mouse develops obesity, hyperglycemia, atherosclerosis, insulin resistance, etc.35 Mounting evidence suggests that cardiovascular risk factors such as diabetes, obesity, hypertension, atherosclerosis, etc. interact to exacerbate lethal tissue injury.36 It is likely that interaction of these risk factors in db/db mice elevates the susceptibility of myocardium to ischemia/reperfusion injury.
In clinical setting, the important goal of myocardial protection is to maintain cardiac function after cardiac surgery. Isoflurane preconditioning decreased LVEDP and elevated the values of LVDP and ±dP/dt during reperfusion in C57BL/6 mice. However, these favorable effects of isoflurane were lost in db/db mice. Clinical studies have shown that obese, type 2 diabetic patients undergoing cardiac surgery have poorer clinical outcome than non-obese, non-diabetic patients.37 We believe that impaired cardioprotective effect of volatile anesthetics may contribute to increased mortality and poorer prognosis in obese type 2 diabetic patients undergoing cardiac surgery.
Mounting evidence suggests that short period of microRNA-21 up-regulation protects the heart against ischemia/reperfusion injury and contributes to the cardioprotective effect of ischemic preconditioning.12,14,15 Isoflurane up-regulated cardiac microRNA-21 in C57BL/6 mice subjected to ischemia/reperfusion injury, and genetic disruption of microRNA-21 abrogated the cardioprotective effect of isoflurane. These results indicate the crucial role of microRNA-21 in isoflurane-induced cardioprotection against ischemia/reperfusion injury. In contrast to C57BL/6 mice, isoflurane down-regulated cardiac microRNA-21 in db/db mice subjected to ischemia/reperfusion injury. Taken together, abnormal regulation of microRNA-21 by isoflurane in db/db mice might in part contribute to the failure of isoflurane cardiac preconditioning.
Previous studies show that up-regulation of microRNA-21 by either ischemic preconditioning or isoflurane preconditioning elevates the activity of eNOS.15,16 In the current study, we demonstrated that isoflurane elevated the ratio of eNOS dimers/monomers in C57BL/6 but not db/db mice subjected to ischemia/reperfusion injury. The mechanisms underlying elevated eNOS dimerization by isoflurane remain elusive. It is evident that tetrahydrobiopterin bioavailability is crucial for eNOS dimerization.38 A recent study shows that isoflurane elevated the expression of cardiac GTP cyclohydrolase 1, which is the first and rate-limiting enzyme in de novo biosynthesis of tetrahydrobiopterin in rats subjected to ischemia/reperfusion injury.39 It is likely that isoflurane increases GTP cyclohydrolase 1 and tetrahydrobiopterin bioavailability, leading to eNOS dimerization. Isoflurane preconditioning decreased LVEDP and improved the values of LVDP and ±dP/dt in C57BL/6 mice 30 minutes to 2 h after reperfusion. These beneficial effects of isoflurane preconditioning were lost in eNOS-null mice, suggesting that eNOS is required for isoflurane preconditioning-elicited improvements in cardiac function in mice. However, isoflurane failed to elevate the ratio of eNOS dimers/monomers in db/db mice. Dimeric eNOS enzyme consists of a heme-containing oxygenase domain that binds the essential co-factor tetrahydrobiopterin, molecular oxygen, and the substrate L-arginine; and a reductase domain that transfers electrons from the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) to flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN.)40 In the presence of tetrahydrobiopterin and L-arginine, heme and oxygen reduction are coupled to the synthesis of nitric oxide.40 Moreover, superoxide levels are increased in db/db mice.41 It is evident that nitric oxide interacts with superoxide to form peroxynitrite, a stronger oxidants than superoxide.42 It is likely that nitric oxide bioavailability is decreased in db/db mice following isoflurane treatment. Previous studies have identified eNOS-derived nitric oxide as both the trigger and mediator of isoflurane preconditioning.7 Therefore, decreased dimerization of eNOS may be crucial for failure of isoflurane cardiac preconditioning in db/db mice.
NADH is a substrate for the enzymatic activity of dehydrogenase that form part of the respiratory chain and reside in the inner membrane of the mitochondria. In mitochondria, acetyl-CoA entering the citric acid cycle produces NADH and hydroquinone form of flavin adenine dinucleotide (FADH2). Mitochondrial NADH is oxidized upon donating its electrons to respiratory Complex I (NADH:ubiquinone oxidoreductase) of the electron transport chain in mitochondria.43 These electrons are sequentially relayed from Complex I to ubiquinone (Coenzyme 10), Complex II (coenzyme Q-cytochrome c oxidoreductase), cytochrome c, and Complex IV (cytochrome c oxidase), resulting in the formation of oxygen to water. During electron transfer, superoxide (O2·−) is generated, causing oxidative stress and potential induction of NF-E2-related factor 2 (NRF2), and activation of antioxidant response elements to decreases oxidative stress levels. In type 2 diabetes mellitus, high levels of glucose can induce glucose oxidation, thereby generating pyruvate and NADH, and NADH:ubiquinone oxidoreductase is inhibited.44,45 They together cause the accumulation of NADH. Furthermore, reactive oxygen species are released from mitochondrial Complex I and III.45 Thus, the monitoring of mitochondrial NADH levels provides the important information on the metabolic state of the mitochondria in terms of energy production and intracellular oxygen levels.46 In the present study, mitochondrial NADH levels were elevated in db/db mouse hearts at baseline and in early ischemia. These results suggest that mitochondrial respiratory chain is impaired in db/db mouse hearts. It is evident that dysfunction of electron chain transportation results in the reduction of adenosine triphosphate (ATP) synthesis and increases in superoxide, that contributes to exacerbation of myocardial ischemia/reperfusion injury.47 Isoflurane significantly decreased NADH levels during early ischemia in C57BL/6 but not db/db mice. It is possible that impaired electron transfer in mitochondrial respiratory chain by diabetes also contributes to failure of isoflurane cardiac preconditioning in db/db mice.
In the present study, genetic disruption of microRNA-21 blocked isoflurane preconditioning-elicited increase in the ratio of eNOS dimers/monomers, whereas genetic disruption of eNOS did not change the regulatory effect of isoflurane on microRNA-21. Previous studies showed that up-regulation of microRNA-21 elevated the activity of eNOS, whereas microRNA-21 knockout reduced the production of the eNOS-derived nitric oxide.48,49. Thus, in isoflurane-induced cardioprotection against ischemia/reperfusion injury, eNOS acts as an important target of microRNA-21. Genetic disruption of either microRNA-21 or eNOS blocked isoflurane preconditioning-induced decrease in mitochondrial NADH levels in early ischemia. Studies have shown that modulation of eNOS activity negatively impacts the NADH contents.50 We speculate that isoflurane preconditioning-elicited up-regulation of microRNA-21 functionally suppresses the production of mitochondrial NADH in early ischemia through inducing eNOS dimerization (Figure 6).
Figure 6.
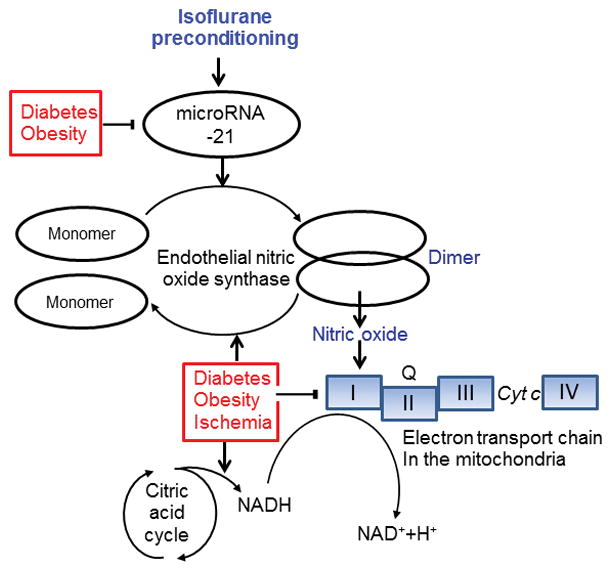
Proposed mechanisms responsible for failure of isoflurane preconditioning to protect the heart against ischemia/reperfusion injury in obese type 2 diabetic mice. InC57BL/6 control mice, isoflurane preconditioning up-reglates microRNA-21, leading to the dimerization of endothelial nitric oxide synthase through direct and/or indirect mechanisms. Dimeric endothelial nitric oxide synthase produces nitric oxide, that acts on respiratory chain Complex I [reduced form of nicotinamide adenine dinucleotide (NADH):ubiquinone] in the mitochondria and facilitaes NADH to release electrons. The electrons removed from respiratory chain Complex I are subsequently transferred to coenzyme Q, Complex III, cytochrome c (Cyt c), and Complex IV, which uses the electrons and hydrogen ions to reduce molecular oxygen to water. Type 2 diabetes mellitus with obesity down-regulates microRNA-21 and prevents the up-regulation of microRNA-21 by isoflurane. Moreover, diabetes, obesity, and ischemia together facilitates the transfer of endothelial nitric oxide synthase dimers to monomers, inhibits respiratory chain Complex I, and elevates the production of NADH though multiple pathways including citric acid cycle. These changes elicited by diabetes and obesity contribute to failure of isoflurane cardiac preconditioning.
In summary, the present study demonstrates that the failure of isoflurane cardiac preconditioning in db/db mice is associated with aberrant regulation of the microRNA-21, eNOS, mitochondrial respiratory complex I. The findings reveal the importance of preservation of these signaling molecules for isoflurane to protect the heart against ischemia/reperfusion injury. To find therapeutic targets for restoration of isoflurane-induced cardioprotection in obesity and diabetes, future studies will examine the effect of cardiomyocyte-specific overexpression of microRNA-21 and increased dimerization of eNOS by pharmacological approaches (e.g., tetrahydrobiopterin supplementation) on isoflurane-elicited cardioprotection against ischemia/reperfusion injury in db/db mice.
Supplementary Material
Summary Statement.
Failure of isoflurane to precondition the heart in obese type 2 diabetic db/db mice is associated with aberrant regulation of microRNA-21, endothelial nitric oxide synthase, mitochondrial respiratory complex I.
Acknowledgments
We thank Drs. John A. Auchampach (Ph.D., Professor of Pharmacology and Toxicology), Tina C. Wan (Ph.D., Research Scientist), and Garrett J. Gross (Ph.D., Emeritus Professor) (all from Department of Pharmacology and Toxicology, Medical College of Wisconsin) for their equipment, Mark Paterson (B.S., Ph.D. candidate) (from Department of Physiology, Medical College of Wisconsin), and David Schwabe (B.S., Engineer) and John Tessmer (B.S., Lab Manager) (both from Department of Anesthesiology, Medical College of Wisconsin) for their excellent technical assistance.
Footnotes
Competing Interests
The authors declare no competing interests.
Disclosure of Funding
This work was supported, in part, by National Institutes of Health research grants P01GM 066730 (to Drs. Liang and Bosnjak) and HL 063705 (to Dr. Kersten) from the United States Public Health Services, Bethesda, Maryland.
References
- 1.Benedetto U, Caputo M, Vohra H, Davies A, Hillier J, Bryan A, Angelini GD. Off-pump versus on-pump coronary artery bypass surgery in patients with actively treated diabetes and multivessel coronary disease. J Thorac Cardiovasc Surg. 2016;152:1321–30. doi: 10.1016/j.jtcvs.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5:e003921. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzmann MJ, Rathsman B, Eliasson B, Kuhl J, Svensson AM, Nystrom T, Sartipy U. Long-term prognosis in patients with type 1 and 2 diabetes mellitus after coronary artery bypass grafting. J Am Coll Cardiol. 2015;65:1644–52. doi: 10.1016/j.jacc.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 4.Lee MC, Chen CH, Kuo MC, Kang PL, Lo A, Liu K. Isoflurane preconditioning-induced cardio-protection in patients undergoing coronary artery bypass grafting. Eur J Anaesthesiol. 2006;23:841–7. doi: 10.1017/S0265021506000354. [DOI] [PubMed] [Google Scholar]
- 5.Freiermuth D, Mets B, Bolliger D, Reuthebuch O, Doebele T, Scholz M, Gregor M, Haschke M, Seeberger MD, Fassl J. Sevoflurane and isoflurane-pharmacokinetics, hemodynamic stability, and cardioprotective effects during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30:1494–1501. doi: 10.1053/j.jvca.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Lotz C, Kehl F. Volatile anesthetic-induced cardiac protection: molecular mechanisms, clinical aspects, and interactions with nonvolatile agents. J Cardiothorac Vasc Anesth. 2015;29:749–60. doi: 10.1053/j.jvca.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Chiari PC, Bienengraeber MW, Weihrauch D, Krolikowski JG, Kersten JR, Warltier DC, Pagel PS. Role of endothelial nitric oxide synthase as a trigger and mediator of isoflurane-induced delayed preconditioning in rabbit myocardium. Anesthesiology. 2005;103:74–83. doi: 10.1097/00000542-200507000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Deng B, Zhao X, Gao C, Yang L, Zhao H, Yu D, Zhang F, Xu L, Chen L, Sun X. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci Rep. 2015;5:11445. doi: 10.1038/srep11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka K, Kehl F, Gu W, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Isoflurane-induced preconditioning is attenuated by diabetes. Am J Physiol Heart Circ Physiol. 2002;282:H2018–23. doi: 10.1152/ajpheart.01130.2001. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Clark AG. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012;22:1243–54. doi: 10.1101/gr.132514.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–5. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–25. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469:336–42. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, Liang M, Ding X. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics. 2014;46:789–97. doi: 10.1152/physiolgenomics.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–5. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao S, Olson JM, Paterson M, Yan Y, Zaja I, Liu Y, Riess ML, Kersten JR, Liang M, Warltier DC, Bosnjak ZJ, Ge ZD. MicroRNA-21 mediates isoflurane-induced cardioprotection against ischemia-reperfusion injury via Akt/nitric oxide synthase/mitochondrial permeability transition pore pathway. Anesthesiology. 2015;123:786–98. doi: 10.1097/ALN.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Sundquist J, Zoller B, Memon AA, Palmer K, Sundquist K, Bennet L. Determination of 14 circulating microRNAs in Swedes and Iraqis with and without diabetes mellitus type 2. PLoS One. 2014;9:e86792. doi: 10.1371/journal.pone.0086792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgardt SL, Paterson M, Leucker TM, Fang J, Zhang DX, Bosnjak ZJ, Warltier DC, Kersten JR, Ge ZD. Chronic co-administration of sepiapterin and L-citrulline ameliorates diabetic cardiomyopathy and myocardial ischemia/reperfusion injury in obese type 2 diabetic mice. Circ Heart Fail. 2016;9:e002424. doi: 10.1161/CIRCHEARTFAILURE.115.002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459:977–94. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 20.Panagia M, Schneider JE, Brown B, Cole MA, Clarke K. Abnormal function and glucose metabolism in the type-2 diabetic db/db mouse heart. Can J Physiol Pharmacol. 2007;85:289–94. doi: 10.1139/y07-028. [DOI] [PubMed] [Google Scholar]
- 21.Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Jr, Auchampach JA, Gross GJ, Kersten JR, Warltier DC. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85. doi: 10.1097/ALN.0b013e3181c4a607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge ZD, Ionova IA, Vladic N, Pravdic D, Hirata N, Vasquez-Vivar J, Pratt PF, Jr, Warltier DC, Pieper GM, Kersten JR. Cardiac-specific overexpression of GTP cyclohydrolase 1 restores ischaemic preconditioning during hyperglycaemia. Cardiovasc Res. 2011;91:340–9. doi: 10.1093/cvr/cvr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 24.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 25.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, Auchampach JA. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319:1200–10. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Baumgardt SL, Fang J, Shi Y, Qiao S, Bosnjak ZJ, Vasquez-Vivar J, Xia Z, Warltier DC, Kersten JR, Ge ZD. Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling. Sci Rep. 2017;7:3093. doi: 10.1038/s41598-017-03234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vladic N, Ge ZD, Leucker T, Brzezinska AK, Du JH, Shi Y, Warltier DC, Pratt PF, Jr, Kersten JR. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2011;301:H2130–39. doi: 10.1152/ajpheart.01078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson JM, Yan Y, Bai X, Ge ZD, Liang M, Kriegel AJ, Twaroski DM, Bosnjak ZJ. Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology. 2015;122:795–805. doi: 10.1097/ALN.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144–53. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanke P, Naoum C, Ahmadi A, Cheruvu C, Soon J, Arepalli C, Gransar H, Achenbach S, Berman DS, Budoff MJ, Callister TQ, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Rubinshtein R, Marquez H, DeLago A, Villines TC, Hadamitzky M, Hausleiter J, Shaw LJ, Kaufmann PA, Cury RC, Feuchtner G, Kim YJ, Maffei E, Raff G, Pontone G, Andreini D, Chang HJ, Chow BW, Min J, Leipsic J. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imaging. 2016;9:1280–88. doi: 10.1016/j.jcmg.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Kang SH, Park GM, Lee SW, Yun SC, Kim YH, Cho YR, Park HW, Suh J, Yang DH, Kang JW, Lim TH, Jung CH, Koh EH, Lee WJ, Kim MS, Lee KU, Park JY. Long-term prognostic value of coronary CT angiography in asymptomatic type 2 diabetes mellitus. JACC Cardiovasc Imaging. 2016;9:1292–1300. doi: 10.1016/j.jcmg.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol. 2012;11:67. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Povlsen JA, Lofgren B, Dalgas C, Birkler RI, Johannsen M, Stottrup NB, Botker HE. Protection against myocardial ischemia-reperfusion injury at onset of type 2 diabetes in Zucker diabetic fatty rats is associated with altered glucose oxidation. PLoS One. 2013;8:e64093. doi: 10.1371/journal.pone.0064093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–94. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 37.Pan W, Hindler K, Lee VV, Vaughn WK, Collard CD. Obesity in diabetic patients undergoing coronary artery bypass graft surgery is associated with increased postoperative morbidity. Anesthesiology. 2006;104:441–7. doi: 10.1097/00000542-200603000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20:3040–77. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baotic I, Weihrauch D, Procknow J, Vasquez-Vivar J, Ge ZD, Sudhakaran S, Warltier DC, Kersten JR. Isoflurane favorably modulates guanosine triphosphate cyclohydrolase-1 and endothelial nitric oxide synthase during myocardial ischemia and reperfusion injury in rats. Anesthesiology. 2015;123:582–9. doi: 10.1097/ALN.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vecoli C, Cao J, Neglia D, Inoue K, Sodhi K, Vanella L, Gabrielson KK, Bedja D, Paolocci N, L’Abbate A, Abraham NG. Apolipoprotein A-I mimetic peptide L-4F prevents myocardial and coronary dysfunction in diabetic mice. J Cell Biochem. 2011;112:2616–26. doi: 10.1002/jcb.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weidinger A, Kozlov AV. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–84. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo X, Li R, Yan LJ. Roles of pyruvate, NADH, and mitochondrial complex I in redox balance and imbalance in beta cell function and dysfunction. J Diabetes Res. 2015;2015:512618. doi: 10.1155/2015/512618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4:R1–R15. doi: 10.1530/EC-14-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayevsky A, Barbiro-Michaely E. Use of NADH fluorescence to determine mitochondrial function in vivo. Int J Biochem Cell Biol. 2009;41:1977–88. doi: 10.1016/j.biocel.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang XY, Shen BR, Zhang YC, Wan XJ, Yao QP, Wu GL, Wang JY, Chen SG, Yan ZQ, Jiang ZL. Induction of thoracic aortic remodeling by endothelial-specific deletion of microRNA-21 in mice. PLoS One. 2013;8:e59002. doi: 10.1371/journal.pone.0059002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–8. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YH, Hwang JH, Noh JR, Gang GT, Kim DH, Son HY, Kwak TH, Shong M, Lee IK, Lee CH. Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res. 2011;91:519–27. doi: 10.1093/cvr/cvr110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.