Abstract
Background
Although cardiac c-kit+ cells are being tested in clinical trials, the circumstances that determine lineage differentiation of c-kit+ cells in vivo are unknown. Recent findings suggest endogenous cardiac c-kit+ cells rarely contribute cardiomyocytes to the adult heart. We assessed whether various pathological stimuli differentially affect the eventual cell fates of c-kit+ cells.
Methods
We employed single cell sequencing and genetic lineage tracing of c-kit+ cells to determine whether various pathologic stimuli would result in different fates of c-kit+ cells.
Results
Single cell sequencing of cardiac CD45−c-kit+ cells showed innate heterogeneity, indicative of the existence of vascular and mesenchymal c-kit+ cells in normal hearts. Cardiac pressure overload resulted in a modest increase in c-kit derived cardiomyocytes with significant increases in the numbers of endothelial cells and fibroblasts. Doxorubicin-induced (DOX) acute cardiotoxicity did not increase c-kit derived endothelial cell fates but instead induced cardiomyocyte differentiation. Mechanistically, DOX induced DNA damage in c-kit+ cells resulted in expression of p53. Inhibition of p53 blocked cardiomyocyte differentiation in response to DOX, while the small molecule RITA induced stabilization of p53 was sufficient to increase c-kit derived cardiomyocyte differentiation.
Conclusion
These results demonstrate that different pathologic stimuli induce different cell fates of c-kit+ cells in vivo. Although the overall rate of cardiomyocyte formation from c-kit+ cells is still below clinically relevant levels, we show that p53 is central to the ability of c-kit+ cells to adopt cardiomyocyte fates, which could lead to the development of strategies to preferentially generate cardiomyocytes from c-kit+ cells.
Keywords: Cardiac Progenitor Cells, Anthracyclines, Regeneration, c-kit
INTRODUCTION
The discovery of resident cardiac progenitor cells (CPCs) over a decade ago has led to the expectation that the heart could be stimulated to regenerate.1 The most widely published CPC expresses the tyrosine kinase receptor c-kit, although other subtypes of CPCs can be identified based on marker gene expression.1 In cell culture, these primary cardiac cells, selected for expression of c-kit and the absence of the hematopoietic marker CD45, behave like stem cells, with the ability of clonal derivation, maintenance of an undifferentiated state over multiple population doublings, and multi-lineage potential.2 Initially, it was presumed that injection of cultured CPCs would lead to engraftment and differentiation of injected CPCs in situ into distinct cardiac lineages.3–5 Although early engraftment and differentiation can be observed, recent studies suggest that injected CPCs do not engraft long-term and instead stimulate endogenous regenerative processes.6, 7 If transdifferentiation is not the mechanism of action for the beneficiary effects of cell therapy, it is critically important to understand how cell therapy positively affects cardiac repair and function. Endogenous CPCs are probable targets of the injected CPCs through undefined paracrine effector molecules, and likely play an important role in the beneficial effects observed in response to cell therapy.8, 9 Therefore, it is important to understand which factors drive endogenous c-kit+ cells to adopt differentiated fates. Although cardiac c-kit+ cells have been identified over a decade ago, the role that cardiac c-kit+ cells play in cardiac homeostasis and regeneration remains controversial. A limiting factor for the field has been the lack of genetic tools that would allow the determination of cell fates of c-kit+ cells. We, and others, have recently published genetic mouse models that allow reliable genetic lineage tracing of c-kit+ cells.10, 11 We initially used these mouse models to determine whether myocardial infarction would lead to increased cardiomyocyte differentiation. However, the overall numbers of cardiomyocytes that were generated by c-kit+ cells were very low and considered of no physiologic relevance.10, 11
The goal of the current study was to determine whether different pathological stimuli induced different cellular fates of endogenous cardiac c-kit+ cells. Here, we identified different clusters of freshly isolated cardiac c-kit+ cells based on single cell sequencing, and demonstrate that cardiac pressure overload results in a balanced stimulation of cardiomyocyte, endothelial and fibroblast cell fates. We further report that anthracycline induced cardiomyopathy specifically enhances cardiomyocyte fates. Mechanistically, we identified p53 as a central regulator of cardiomyocyte fates in response to anthracyclines.
METHODS
An expanded Methods section is available in the Supplemental Material. All data, methods used in the analysis, and materials are available for purposes of reproducing the results by contacting the corresponding author.
Animals
Kit+/Cre-IRES-nGFP (Kit+/nGFP) mice and Kit+/MerCreMer (Kit+/MCM) as well as reporter mice were previously reported.10 Super p53 mice (B6;CBA-Tg(Trp53)1Srn/J) were purchased from the Jackson Laboratory.12 Cardiac pressure overload in mice was induced via transverse aortic constriction (TAC) as described previously.13 All animal procedures were performed in accordance with institutional guidelines, and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Pharmacological treatments
Details about dosing and administration of tamoxifen, doxorubicin, RITA and pifithrin-α are available in the Supplemental Material.
Histological analysis
Histological stains were performed on cryoembedded tissues according to well-established procedures. An overview of antibodies used is provided in Supplemental Table 1.
RNA sequencing analysis
Cardiac CD45−c-kit+ cells were isolated from adult C57Bl/6j mice following a published protocol with minor modifications.14 Cells were sorted by MACS, followed by PI staining and MoFlo sorting of live cells. Sorted cells were immediately stained for live/dead and used for single cell capture using the Fluidigm C1 capture system on an integrated fluidics circuit optimized for capture of cells sized 5–10μm. Based on acquired images, we selected single live cells to proceed with library preparation, according to the Fluidigm protocol.15 Raw single cell RNA sequencing data was plotted in a matrix consisting of 23425 genes and 405 cells. We used t-SNE to visualize the data and clustered cells into four clusters using Partitioning Around Medoids (PAM) clustering algorithm. The number of cluster groups is determined based on gap statistic.16 Further details on single cell and bulk RNA sequencing analyses are provided in the Supplemental Material. Sequencing data has been deposited at BioProject under Accession number PRJNA412028.
Statistics
Results are described as mean ± SEM. Student’s t-tests were performed when comparing 2 conditions, ANOVA followed by Tukey’s HSD post-hoc analysis was used for multiple condition comparison. A p-value less than 0.05 was considered significant.
RESULTS
Single Cell RNA seq shows heterogeneity of c-kit+ cells in vivo
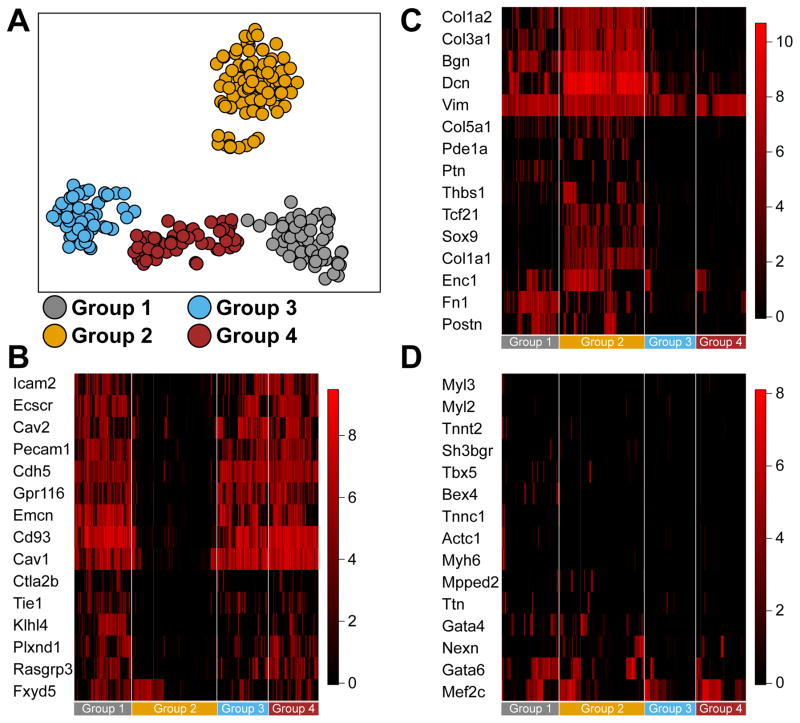
Expression of the tyrosine kinase receptor c-kit has been used extensively to select for cells that display characteristics of CPCs.4 Clonal assays in cell culture clearly show that CPCs can be identified from the adult heart that have the ability to differentiate into cardiomyocytes, endothelial cells and smooth muscle cells, although differentiation to beating cardiomyocytes is typically not observed in vitro.2 Moreover, recent genetic lineage tracing experiments showed a predominance of c-kit+ cells to become endothelial cells, while rarely giving rise to cardiomyocytes. The extent to which c-kit+ cells in vivo are uncommitted or lineage-dedicated is not clear. To determine to what extent c-kit+ cells are pre-committed, we performed single cell RNA seq on freshly isolated CD45−c-kit+ cardiac cells (Supplemental Figure 1), harvested from 3 different groups of mice. We isolated CD45−c-kit+ cardiac cells from 1-day old C57Bl/6 and adult C57Bl/6 mice, and from adult Kit+/MCM. After isolation, capture on microfluidic chips and verification of live single cells, we performed RNA seq on 405 single live cells (88 neonatal and 171 adult cells from C57Bl/6, and 146 adult cells from Kit+/MCM). Clustering analysis identified 4 main clusters, 3 in close proximity to each other, and 1 more distinct cluster (Figure 1A). We analyzed the gene expression that is indicative of lineage commitment within each of these clusters. This analysis defined an endothelial cell identity for cells in Group 1, 3 and 4, and a mesenchymal cell identity for cells in Group 2 (Figure 1B–C). We did not detect a specific cluster of cells that showed expression of cardiomyocyte characteristic genes (Figure 1D). We performed co-clustering with previously published RNA seq datasets of endothelial cells, fibroblasts and cardiomyocytes. This showed close proximity of cells in groups 1, 3 and 4 to endothelial cells, and proximity of group 2 to fibroblasts, while cardiomyocytes did not cluster together, and did not show a proximity to any of the identified clusters (Supplemental Figure 2A). Importantly, when we analyzed the extent that CD45−c-kit+ cells harvested from Kit+/MCM were different from those isolated from adult C57Bl/6 mice, we observed a similar assignment to the various clusters (Supplemental Figure 2B). Furthermore, to assess whether cells from Kit+/MCM would have reduced stem cell potency due to the targeting of 1 allele of the Kit gene, we analyzed expression of genes that were previously identified to be distinctly expressed in cardiac c-kit+ cells compared to other cardiogenic progenitor cells isolated from adult hearts.17 This analysis showed no differences between wild type and Kit+/MCM c-kit+ cells (Supplemental Figure 2C). These data show that targeting of 1 allele of the Kit gene did not affect the overall expression profile of c-kit+ cells, and further support the notion that this likely did not affect the ability of Kit+/MCM cells to give rise to cardiomyocytes. Although literature suggested that neonatal c-kit+ cells might have higher potency to generate cardiomyocytes, we did not detect a cardiogenic population of neonatal cardiac c-kit+ cells.18, 19 Furthermore, we plotted expression of cardiomyocyte specific genes onto clusters of cells, and still could not detect specific cells that were likely to give rise to cardiomyocytes (Supplemental Figure 3). These results suggest that under homeostatic conditions, CD45−c-kit+ cells are unlikely to give rise to cardiomyocytes, without any detectable differences between wild type or Kit+/MCM c-kit+ cells, in line with published genetic lineage-tracing studies.10, 20, 21
Figure 1. Single cell sequencing of cardiac CD45−c-kit+ cells.
A. Single cell sequencing and clustering of 281 cardiac CD45−c-kit+ cells. Clusters are identified by K-means clustering and indicated by different colors. Each circle represents a single cell. Clusters 1 through 4 are next analyzed for expression of genes indicative of different lineage commitment. B. Gene expression heatmap of 15 genes indicative of endothelial lineage commitment. Groups 1, 3 and 4 show high expression of endothelial lineage genes, while group 2 shows low expression. C. Gene expression heatmap of 15 genes indicative of fibroblast lineage commitment. Group 2 shows high expression of fibroblast lineage genes, while groups 1, 3 and 4 show low expression. D. Gene expression heatmap of 15 genes indicative of cardiomyocyte lineage commitment. No group of cells shows enrichment for cardiomyocyte lineage genes. The overall number of cells that express more than 2 cardiomyocyte markers is very low under baseline conditions.
Cardiac pressure overload stimulates endothelial and fibroblast fates
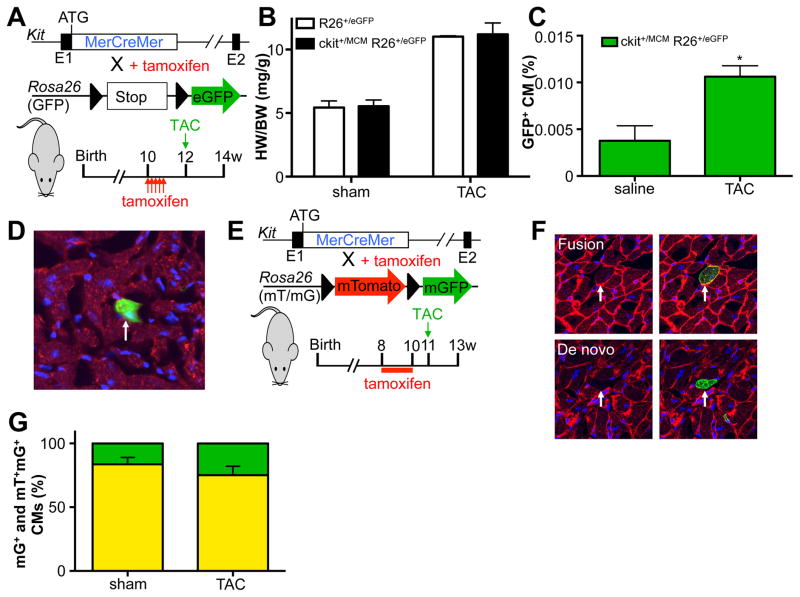
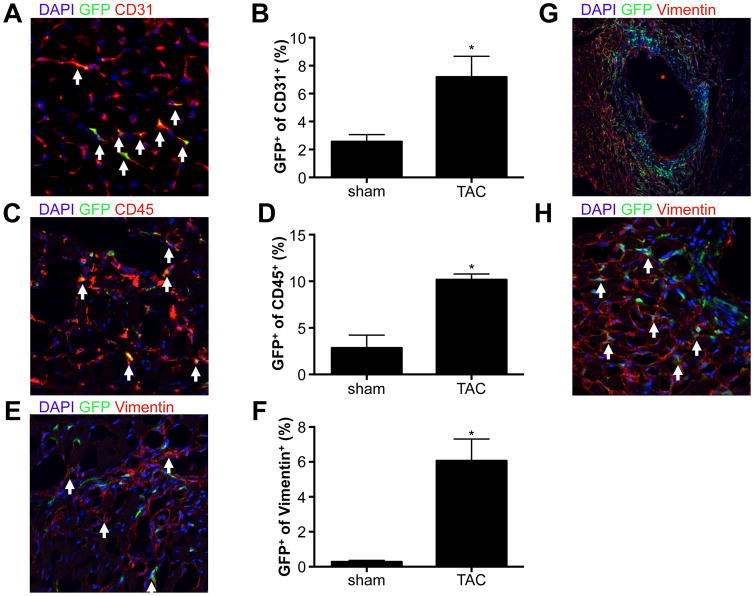
To assess whether cardiac pressure overload would stimulate specific cell fates of c-kit+ cells, we utilized our recently published tamoxifen-inducible lineage-tracing mouse model, Kit+/MCM X R-GFP (Figure 2A). For all tracing experiments we provided tamoxifen before the onset of subsequent treatments to reduce the potential of ectopic activation of lineage tracing within cardiomyocytes. We induced cardiac pressure overload by constricting the transverse aorta (TAC) between the brachiocephalic and left carotid artery as previously described.13 Two weeks after TAC, we observed a significant increase in heart weight to body weight ratios in both control and Kit+/MCM X R-GFP mice, indicative of cardiac hypertrophy and a mild reduction in cardiac function (Figure 2B). Next, we quantified the percentage of GFP+ cardiomyocytes in sham and TAC-treated Kit+/MCM X R-GFP mice and noted a significant 3-fold increase to 0.011% compared to experimental controls (Figure 2C). We verified the identity of GFP+ cardiomyocytes using immunohistochemistry and co-staining with the well-known cardiac marker desmin (Figure 2D). We next used dual fluorescent reporter mice Kit+/MCM R-mTmG, where membrane-targeted Tomato is expressed in all cells, but in response to Cre mediated recombination membrane-targeted GFP becomes expressed (Figure 2E). This mouse model was employed to test whether the increase in GFP+ cardiomyocytes was a result of fusion or de novo cardiomyocyte formation. When c-kit+ cells recombine in response to tamoxifen and then differentiate into cardiomyocytes, these cardiomyocytes will be mGFP+ only. However, when c-kit derived cells fuse with already existing cardiomyocytes, this would result in dual mTom and mGFP (yellow) expression. We again treated mice with tamoxifen before the onset of TAC. We could identify examples of both mGFP+ only cardiomyocytes as well as dual mGFP+ and mTom+ cardiomyocytes (Figure 2F), but did not see a statistically significant difference in the fraction of mGFP+ cardiomyocytes that was only mGFP+ vs dual mGFP+ and mTom+ (Figure 2G). We next tested the abundance of other cardiac lineages in response to TAC and found significant increases in the fraction of endothelial cells (CD31+), immune cells (CD45+) and fibroblasts (Vimentin+) that were c-kit derived (Figure 3A–H). Interestingly, both perivascular and interstitial areas of fibrosis showed extensive overlap between Vimentin staining and GFP signal. Clearly, TAC enhanced c-kit derived cell fates of cardiomyocytes, endothelial cells and fibroblasts. However, cardiomyocyte fates were only increased 3-fold to reach 0.011% of total cardiomyocytes, similar to our previously published response to experimental myocardial infarction.10 When fusion was taken into account, the overall addition of new cardiomyocytes from c-kit+ cells is likely closer to 0.0028% vs. 0.0006 in sham operated animals.
Figure 2. Pressure overload induced mild increase in c-kit derived cardiomyocytes.
A. Genetic model and experimental design. B. Pressure overload induces similar levels of cardiac hypertrophy in control and c-kit lineage tracing mice. C. Percentage GFP+ cardiomyocytes in response to sham or pressure overload (TAC) surgery. N=3 and 3 *p<0.05 D. Immunohistochemical staining of cardiac sections with DAPI (blue) and Desmin (red). Arrow indicates GFP+ cardiomyocyte. E. Genetic model and experimental design for panel F and G. F. Examples of fusion and de novo cardiomyocytes. G. Quantification of fraction of mGFP only (green) vs mGFP-mTomato dual positive (yellow) cardiomyocytes in response to sham or TAC surgery. N=7 and 6.
Figure 3. Non-cardiomyocyte lineages are enhanced in response to TAC.
A. Representative immunohistochemistry image of cardiac section stained for DAPI (blue) and CD31, showing overlap of GFP expressing cells with CD31. B. Quantification of c-kit derived CD31+ cells. N=3 and 3, *p<0.05 C. Representative immunohistochemistry image of cardiac section stained for DAPI (blue) and CD45, showing overlap of GFP expressing cells with CD45. D. Quantification of c-kit derived CD45+ cells. N=3 and 3, *p<0.05 E. Representative immunohistochemistry image of cardiac section stained for DAPI (blue) and vimentin, showing overlap of GFP expressing cells with vimentin. F. Quantification of c-kit derived vimentin+ cells (only interstitial cells are quantified). N=3 and 3, *p<0.05 G and H. Perivascular GFP+ (green) cells co-stained with vimentin (red) in low (G) and high (H) power magnification. A, C, E and H: white arrows show overlap between antibody stain and GFP expressing cells.
Anthracycline-induced cardiomyopathy stimulates cardiomyocyte differentiation
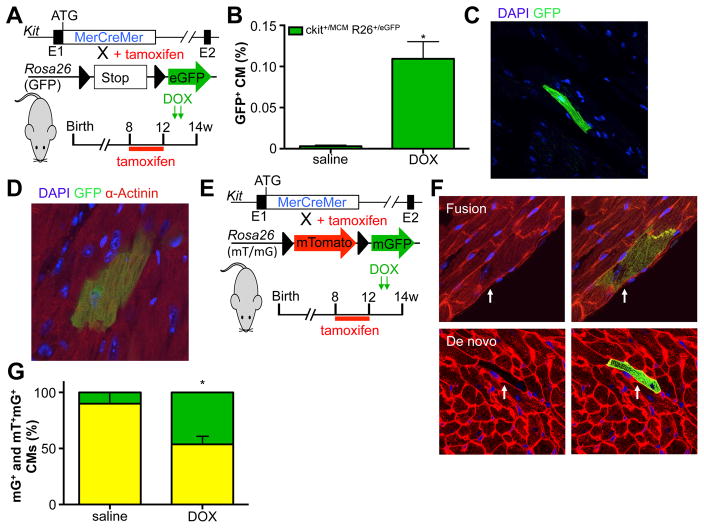
Next, we used the inducible Kit+/MCM X R-GFP mice to determine the effect of acute DOX-induced cardiomyopathy on c-kit+ dependent lineage commitment. Again, we treated mice with tamoxifen and withdrew the tamoxifen-laden chow well before the onset of injury to avoid potential activation of Kit expression in cardiomyocytes (Figure 4A). Interestingly, we found a significant increase (>30-fold) in GFP+ cardiomyocytes in response to DOX up to 0.11% of total cardiomyocytes (Figure 4B). GFP+ cardiomyocytes were identified by native GFP fluorescence (Figure 4C) and by co-staining with the cardiac marker α-actinin (Figure 4D). The GFP+ cardiomyocytes appeared to be well-connected with the surrounding myocardium, with proper Connexin 43 expression, and were essentially indistinguishable from GFP− cardiomyocytes (Supplemental Figure 4A). To rule out the possibility that DOX itself could activate nuclear translocation of the MerCreMer fusion protein or induce recombination of the reporter locus, we treated Kit+/MCM X R-GFP mice with DOX without prior tamoxifen treatment. Importantly, DOX alone could not activate R-GFP, as no GFP+ cells were observed in the hearts of mice treated with DOX. Next, we used the double fluorescent reporter mouse line, Kit+/MCM R-mTmG, to test whether DOX-induced new cardiomyocyte formation from c-kit+ cells (Figure 4E). We identified both mGFP+ only and dual mGFP+ and mTom+ cardiomyocytes, but now measured a significant increase in the fraction of mGFP+ only cardiomyocytes in response to DOX, indicative of newly differentiated cardiomyocytes from c-kit+ cells (Figure 4F–G and Supplemental Figure 4B–C). Based on these data we calculated new cardiomyocyte formation from c-kit+ cells to be around 0.00043% in saline treated mice and 0.046% in DOX treated mice.
Figure 4. DOX induced increase in c-kit derived cardiomyocytes.
A. Genetic model and experimental design used in panels B–D. B. Percentage of DOX (2×10mg/kg) induced GFP+ cardiomyocytes. N=7 and 7, *p<0.05 C. Histological section showing native fluorescence of a representative GFP+ cardiomyocyte. D. Representative GFP+ cardiomyocytes in cardiac section stained for α-actinin (red), GFP (green) and DAPI (blue). E. Genetic model and experimental design used in panels F–G. F. Examples of fusion and de novo cardiomyocytes. G Quantification of mGFP only (green) cardiomyocytes shows significant increase in response to DOX in comparison to mGFP-mTomato dual positive (yellow) cardiomyocytes. N=5 and 6, *p<0.05
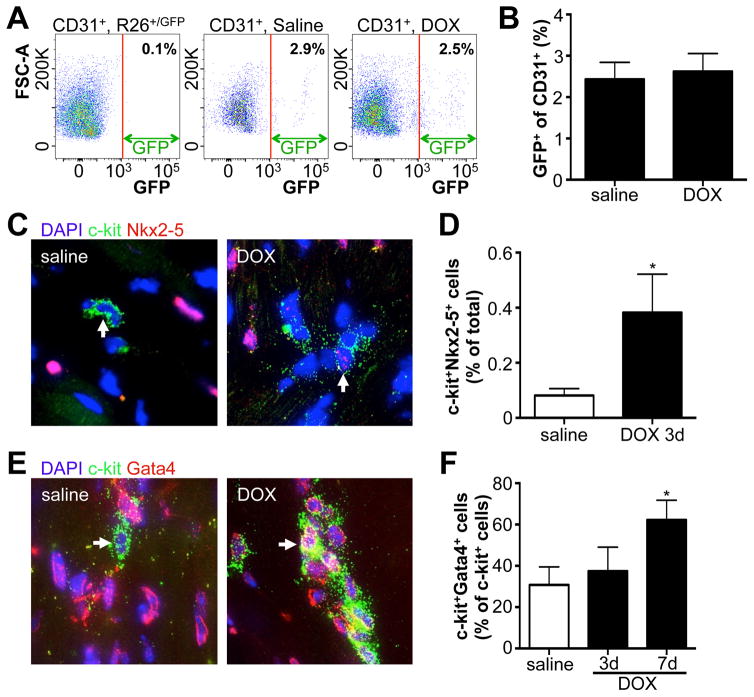
To assess whether DOX also enhanced commitment of c-kit+ cells toward the endothelial lineage, we used a separate mouse line, Kit+/nGFP, where Cre recombinase followed by a nuclear-targeted GFP cDNA was inserted into the murine Kit locus (Supplemental Figure 5A). The nuclear localized GFP provides a real-time readout of active c-kit expression. We treated these mice with DOX and found no difference in the fraction of c-kit expressing cells that co-stained with the vascular differentiation marker Kdr (Supplemental Figure 5B–C), suggesting that DOX does not enhance the endothelial fate of c-kit+ cells. Indeed, when we measured the fraction of endothelial cells that were GFP+ in Kit+/MCM X R-GFP mice (c-kit+ derived endothelial cells), we observed similar percentages in both saline and DOX treated mice (Figure 5A–B). To assess whether DOX stimulated expression of cardiac transcription factors in c-kit+ cells, we treated C57Bl/6 mice with a single dose of DOX and stained for the cardiac transcription factor Nkx2–5 three days later (Figure 5C). Although the level of Nkx2–5 expression is clearly lower than that in adult cardiomyocytes, there is a significant increase in the abundance of c-kit+Nkx2–5+ double positive cells in response to DOX (Figure 5D). We further examined the expression of GATA4, which showed an increase by day 7 after DOX treatment, which was confirmed in Kit+/nGFP mice (Figure 5E–F and Supplemental Figure 5D–E). These data indicate that DOX stimulates c-kit+ cells to preferentially differentiate toward cardiomyocyte fates while maintaining the levels of endothelial cell fates.
Figure 5. DOX induced expression of cardiac transcription factors in c-kit+ cells.
A. Flow cytometry of CD31+ cardiac cells for GFP fluorescence. Left panel uses CD31+ cells isolated from R-GFP control mice to set the gate. Middle and right panels show CD31+ cells from Kit+/MCM X R-GFP after saline or DOX treatment respectively. B. Quantification of GFP+CD31+ cardiac cells. N=4 and 8 C. Representative immunohistochemistry of cardiac sections for DAPI (blue), C-kit (green) and Nkx2–5 (red) 3 days after saline or DOX injection. D. Quantification of percentage of cells that is positive for both Nkx2–5 and c-kit 3 days after saline or DOX. N=3 and 4, *p<0.05 E. Representative immunohistochemistry of cardiac sections for DAPI (blue), C-kit (green) and Gata4 (red) 7 days after saline or DOX injection. F. Quantification of percentage of c-kit+ cells that is positive for Gata4, three and seven days after saline or DOX. N=4, 4 and 4, *p<0.05
Anthracycline stimulates p53 activation, which mediates differentiation
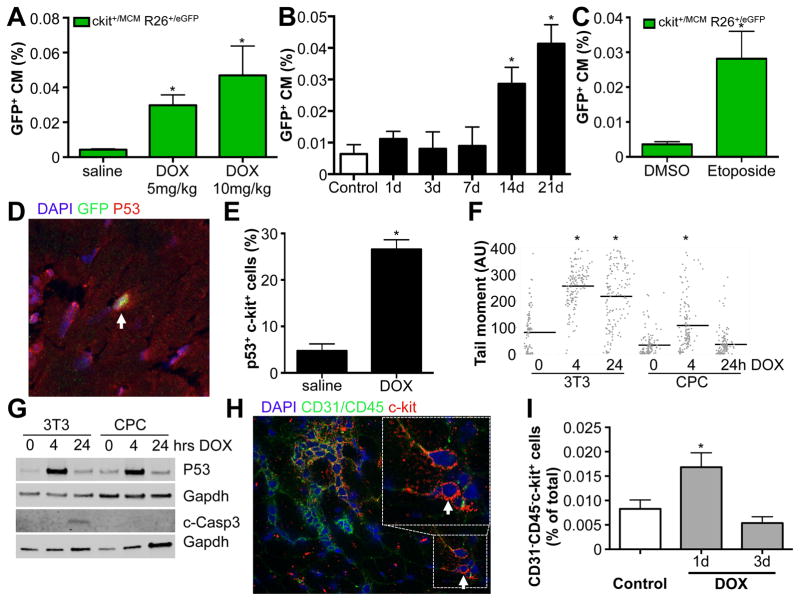
Anthracyclines are well-known for their cardiotoxicity, and we indeed showed significant cardiac dysfunction after a cumulative dose of 20mg/kg DOX (Supplemental Table 2). Since it was possible that paracrine effectors, released due to extensive cardiac damage, exerted stimulatory effects on c-kit+ cells to differentiate into cardiomyocytes, we next tested whether the effects of DOX on c-kit+ cells were independent of cardiac dysfunction. We treated Kit+/MCM X R-GFP mice with a single low dose DOX at 5 or 10 mg/kg and measured c-kit derived GFP+ cardiomyocytes 4 weeks later. We observed a significant dose-dependent increase in c-kit derived cardiomyocytes in response to these lower dosages, which did not cause significant changes in left ventricular fractional shortening (FS) (Figure 6A and Supplemental Table 2). To further rule out spurious activation of the Kit promoter within cardiomyocytes as a potential reason for increased GFP+ cardiomyocytes, we assessed the percentage of GFP+ cardiomyocytes at multiple time-points after DOX administration. After a single dose of DOX at 10mg/kg, we measured the percentage of GFP+ cardiomyocytes in different mice for each time point after DOX injection, and noticed a significant increase starting 2 weeks after DOX administration (Figure 6B). These data are consistent with differentiation of c-kit+ cells rather than Kit expression within cardiomyocytes as the mechanism for the appearance of GFP+ cardiomyocytes.
Figure 6. DOX induced p53 expression.
A. Dose-dependent increase in GFP+ cardiomyocytes in response to low dose of DOX in the absence of cardiac dysfunction. N=11, 9 and 9, *p<0.05 B. Quantification of percentage GFP+ CMs in response to 10mg/kg DOX. Hearts of mice were harvested at indicated time points after DOX administration. N=4, 3, 4, 4, 4 and 3, *p<0.05. C. Etoposide increases the percentage of GFP+ cardiomyocytes. N= 4 and 5, *p<0.05 D. Representative immunohistochemistry for p53 (red), GFP (green), DAPI (blue) showing p53 expression in c-kit+ cells (green). E. Quantification of p53 expressing c-kit+ cells. N=3 and 4, *p<0.05 F. Quantification of Tail Moment using Comet assays for DNA damage in response to DOX in 3T3 cells and neonatal c-kit+ CPCs. G. Western blotting for P53 and cleaved Caspase 3 expression in response to DOX stimulation in 3T3 cells and cultured c-kit+ cells. H. Representative immunohistochemical staining for c-kit (red), CD45 (green) and CD31 (green) and DAPI (blue). I. Quantification of percentage of CD31−CD45−c-kit+ cells from histological sections harvested from control mice, or 1 or 3 days after DOX treatment. N=5, 6 and 5, *p<0.05
Next, we began to address the underlying mechanism for DOX induced cardiomyocyte differentiation of c-kit+ cells. The main effect of DOX as a chemotherapeutic agent is to cause DNA damage, which in turn activates p53 to repair this damage, or alternatively, induces apoptosis. To further rule out that cardiomyocyte differentiation in response to DOX was not the consequence of extensive cardiac damage, we tested the effect of Etoposide, a different genotoxic chemotherapeutic that also induces DNA damage and p53 activation, but is not known to cause cardiac dysfunction. Indeed, Etoposide also significantly enhanced c-kit derived cardiomyocyte formation (Figure 6C). These results suggested that the activation of DNA repair mechanisms in response to DNA damage might be important in mediating cardiomyocyte differentiation. To test this hypothesis, we used Kit+/nGFP mice and treated them with a single dose of 10mg/kg DOX. Twenty-four hours after DOX treatment, we harvested hearts and stained them for p53, a critical upstream response gene that is activated in following DNA damage. In response to DOX, there was a significant increase in the fraction of c-kit expressing cells that co-expressed p53 (Figure 6D–E). We also observed activation of DNA repair mechanisms within c-kit expressing cells, by co-staining with γ-H2Ax and 53BP1, mediators of the DNA repair machinery (Supplemental Figure 6A–B). Especially in rapidly dividing cancer cells, the DNA damage induced by Etoposide or DOX stimulates apoptotic cell death. Since DNA damage might also lead to apoptosis, we wanted to rule out this possibility in the c-kit expressing cells. We used cultured primary c-kit+ cells and compared them to 3T3 fibroblasts. We treated both cell types with DOX and measured the presence of DNA damage 4 and 24 hrs after addition of DOX using Comet assays (Figure 6F). As expected, DOX induced DNA damage in both 3T3 cells and c-kit+ cells at 4 hrs. However, c-kit+ cells appeared to be able to repair the DNA damage by 24 hrs, while 3T3 cells remained positive for the presence of DNA damage at 24hrs. Western blotting showed that both 3T3 and c-kit+ cells activate p53 in response to DOX, but only 3T3 cells went on to express cleaved caspase 3, showing activation of apoptosis, while c-kit+ cells did not activate caspase 3 (Figure 6G). We further confirmed these findings with freshly isolated non-cardiomyocytes that were depleted from c-kit+ cells vs adult c-kit+ cells (Supplemental Figure 6C). Moreover, we verified that endogenous c-kit+ cells were not depleted from the heart in response to DOX. We stained cardiac sections with antibodies against CD45, CD31 and c-kit and quantified the number of CD31−CD45−c-kit+ cells in response to saline or DOX, and observed a significant increase 24 hrs post-DOX with no decrease in the overall number of c-kit+ cells (Figure 6H–I and Supplemental Figure 6D).
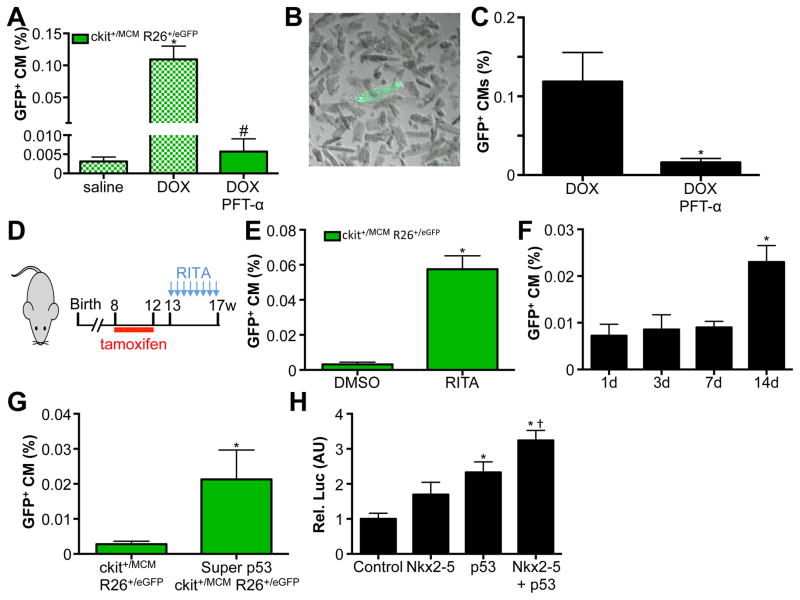
Since both 3T3 cells and c-kit+ cells activated p53 in response to DOX, we next tested whether p53 was critical in mediating cardiomyocyte differentiation from endogenous c-kit+ cells. To examine this possibility, we used the specific p53 inhibitor pifithrin-α and injected this inhibitor daily in DOX treated mice.22 Importantly, blocking p53 mediated gene transcription completely blocked the appearance of GFP+ cardiomyocytes and reverted the numbers back to saline treated controls (Figure 7A). To further confirm that we were correctly counting adult cardiomyocytes from histological sections, we performed adult cardiomyocyte isolations from mice treated with DOX alone or DOX in combination with daily pifithrin-α injections. In response to DOX, we quantified 0.1% of isolated adult cardiomyocytes to be GFP+, while pifithrin-α treated mice showed significantly lower levels, entirely consistent with our quantifications from histological sections (Figure 7B–C). These findings show that activation of p53 is required for cardiomyocyte differentiation of c-kit+ cells in response to DOX. If indeed p53 was mediating the differentiation of c-kit+ cells to cardiomyocytes, then we hypothesized that stabilization of p53 might be sufficient to induce this differentiation event. To test this hypothesis, we used the small molecule RITA, which blocks the interaction between p53 and MDM-2, thereby stabilizing p53.23 We again verified that RITA alone, without prior tamoxifen treatment, was unable to activate the genetic lineage-tracing system. We injected RITA twice per week in Kit+/MCM X R-GFP mice for 4 weeks and found a significant 12-fold increase in the numbers of GFP+ cardiomyocytes, indicating that stabilization of p53 is sufficient to drive cardiomyocyte differentiation of c-kit+ cells (Figure 7D–E). Importantly, RITA stimulation by itself does not cause DNA damage, indicating that p53, and not DNA damage, is the main mediator of cardiomyocyte differentiation from c-kit+ cells (Supplemental Figure 6E). We again assessed the percentage of GFP+ cardiomyocytes in response to RITA administration at multiple time points, to verify that RITA treatment results in differentiation of c-kit+ cells, as opposed to activation of the Kit gene within cardiomyocytes (Figure 7F). To further assess a role for p53 in differentiation of c-kit+ cells into cardiomyocytes, we cross-bred mice that express 1 additional copy of p53 from a transgenic construct (super p53) with Kit+/MCM X R-GFP mice. Interestingly, 1 additional copy of p53 was sufficient to increase the number of GFP+ cardiomyocytes derived from c-kit+ cells (Figure 7G). To begin to understand how p53 might be responsible for the cardiogenic potential of c-kit+ cells, we performed luciferase assays using the ANF promoter. We transfected HEK cells with this promoter, and either Nkx2–5, p53, or Nkx2–5 and p53 expression plasmids. Combined transfection of half the amount of Nkx2–5 and p53 together potentiated the transcriptional activation of the ANF promoter (Figure 7H). These results clearly establish a central role for p53 in the differentiation of c-kit+ cells toward cardiomyocytes.
Figure 7. p53 mediates c-kit+ CPC derived cardiomyocyte formation.
A. GFP+ cardiomyocytes are completely blocked by injection of Pifithrin-α in conjunction with DOX. Blocked bars represent the same data as presented in Figure 4B. B. Adult cardiomyocyte isolation showing representative GFP+ adult cardiomyocyte. C. Quantification of isolated GFP+ adult cardiomyocytes shows pifithrin-α reduces abundance of GFP+ cardiomyocytes. N=4 and 3, *p<0.05 D. Experimental design used in panel E. E. RITA injection is sufficient to induce GFP+ cardiomyocytes. N=4 and 6, *p<0.05 F. Quantification of percentage GFP+ CMs in response to RITA administration. Hearts of mice were harvested at indicated time points after administration. N=3, 4, 4 and 4, *p<0.05. G. Quantification of percentage GFP+ CMs from Kit+/MCM X R-GFP and Super p53 Kit+/MCM X R-GFP mice. N=5 and 3, *p<0.05. H. Relative luciferase activity in HEK cells transfection with ANF promoter luciferase construct with control plasmid, 1μg Nkx2–5 expression plasmid, 1μg p53 expression plasmid and 0.5μg of both Nkx2–5 and p53 expression plasmid. N=3 each, *p<0.05 vs control, † p<0.05 vs Nkx2–5 and p53 alone.
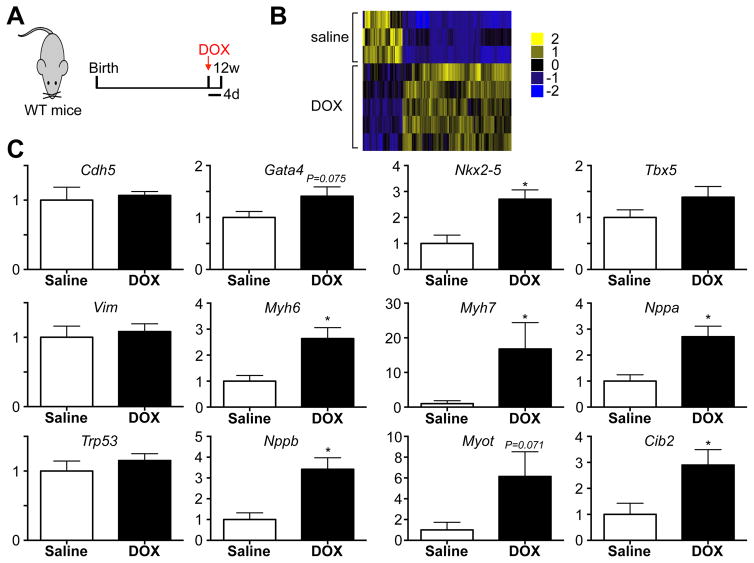
Finally, to explore how DOX treatment affects gene expression within c-kit+ cells, we performed RNA sequencing on freshly isolated CD45−c-kit+ cells four days after saline or DOX injection (Figure 8A–B). We identified 220 significant differences in gene expression between CD45−c-kit+ cells harvested from saline and DOX treated mice, including cardiac genes Myotilin and Nppb (Supplemental Table 3). We performed qPCR on freshly isolated CD45−c-kit+ cells four days after saline or DOX treatment for a number of cardiogenic and other candidate genes. Overall, these results show consistent upregulation of cardiogenic genes, such as the transcription factor Nkx2–5, fetal genes Nppa and Nppb, as well as cardiac myosins Myh6 and Myh7, indicative of expression of cardiomyocyte specific genes and ongoing differentiation towards a cardiomyocyte fate (Figure 8C and Supplemental Table 4).
Figure 8. c-kit+ cells express cardiac genes in response to DOX.
A. Experimental design of RNA-seq experiment performed on RNA extracted from CD45−c-kit+ cells isolated 4 days after saline or DOX. N=3 and 5. B. Heat map of gene expression differences in CD45−c-kit+ cells harvested from saline or DOX treated mice. C. Real-time PCR of indicated genes on RNA from CD45−c-kit+ cells harvested 4 days after saline or DOX treatment. N=8 and 8, *p<0.05
DISCUSSION
Cardiac progenitor cells likely play important roles in normal homeostasis of the heart, and the response to cardiac injury, although their precise function in homeostasis or repair is unclear, and their contribution to cardiomyocytes has been questioned.10, 11, 20, 21, 24–26 Our results showed that endogenous c-kit expressing cells can be stimulated to generate cardiomyocytes, albeit at relatively low rates. Surprisingly, we found that anthracycline-induced cardiomyopathy produced the most conducive stimulus to enhance lineage-traced cardiomyocytes more than 30-fold to reach 0.11% of total cardiomyocytes. To begin to assess why anthracyclines stimulate c-kit+ cells towards cardiomyocyte fates, we defined p53 as not only a central regulator in the DNA damage response program27, but also an important mediator of the differentiation of c-kit+ cells towards a cardiomyocyte fate. This was evident from blocking p53 mediated gene transcription in the setting of DOX-induced cardiomyopathy, where p53 appeared to be necessary for the increase in new cardiomyocyte formation. Moreover, when we used the small molecule RITA to stabilize p53, this was sufficient to cause a 12-fold increase in c-kit+ cell-derived cardiomyocytes. Importantly, stabilization of p53 using RITA did not give rise to DNA damage, indicating that p53 by itself is sufficient to enhance cardiomyocyte differentiation from c-kit+ cells. This was further substantiated by cross-breeding to super p53 transgenic mice, in which 1 additional copy of p53 was sufficient to increase the number of lineage-traced cardiomyocytes.12 Moreover, we showed that p53 could potentiate the ability of Nkx2–5 to activate the ANF promoter. These results showed that p53 plays a crucial role in the differentiation of c-kit+ cells towards cardiomyocytes. Although p53 is typically viewed as a regulator of cell death, its role in differentiation has become evident over the past couple of years.28, 29 P53 null mice develop normally, to succumb to malignancies by 3–4 months of age.30 Interestingly, a number of recent findings have shown a critical role for p53 in regulating differentiation. It plays an initiating step in suppressing pluripotency by inhibiting factors, such as Nanog.31 In addition, p53 can regulate miR-34a and miR-145, resulting in downregulation of pluripotency factors Oct4, Klf4, Lin28A and Sox2.29 Furthermore, p53 plays a crucial role in maintaining hematopoietic stem cell quiescence.32 Further evidence for an important role of p53 in the heart comes from the identification of p53 as an inhibitor of Hif1 activity.33 Increased expression of p53 resulted in reduced angiogenesis after pressure overload, ultimately leading to heart failure. Interestingly, DOX has been shown to result in impaired vascularization, entirely consistent with our findings that endothelial fates are not enhanced in response to DOX.34 However, increased expression of p53 in fibroblasts actually increased vascularization in response to myocardial infarction by converting fibroblasts to endothelial cells.35 These results clearly showed that p53 plays important roles in regulating the response of the heart to stimuli, although the cellular context is likely to be important for the precise outcome and for the regulation of new cell formation. Moreover, the precise role of p53 in mediating cardiomyocyte differentiation of c-kit+ cells, as well as downstream pathways remains to be determined.
It is intriguing that different stimuli, such as pressure overload stimulation or anthracycline stimulation, have such divergent effects on the eventual cell fate of c-kit+ cells. This suggests that there must be specific stimuli that promote cardiomyocyte fates, such as p53. One potential caveat for our findings however, comes from a recent publication suggesting that the c-kit gene could be activated within cardiomyocytes.19, 21 If this were indeed the case, it could potentially lead to overestimation of the levels of new cardiomyocyte formation from c-kit+ cells. The basis for claiming c-kit could be expressed in cardiomyocytes came from the early activation of recombination in cardiomyocytes in response to tamoxifen addition. Only 24 hours after tamoxifen addition, GFP expression in cardiomyocytes was already observed, a time window that is clearly insufficient to allow for active differentiation, although fusion might explain such timing.21 Nevertheless, expression of c-kit within cardiomyocytes was deemed a more likely explanation. However, it is noteworthy that additional knock-in mouse lines that express fluorescently tagged histone proteins whenever the Kit promoter is activated never show cardiomyocytes to express these fluorescent histone proteins.20 One critical strategy we followed to avoid this potential bias was to consistently withdraw tamoxifen before the onset of the experiments. We waited at least 72 hours between withdrawing tamoxifen and initiation of the experiment, which is more than 5 times the elimination half-life in mice.36 Further convincing evidence comes from our experiment where we precisely quantified the fraction of GFP+ cardiomyocytes at multiple time points after a single administration of DOX. Up to 7 days after DOX administration, we see no difference between DOX and saline treated numbers of GFP+ cardiomyocytes. We only began to observe a significant increase between 1 and 2 weeks after DOX administration, which is inconsistent with activation of the Kit promoter in cardiomyocytes in our experiments, and clearly suggests differentiation of c-kit+ cells. Therefore, we are confident that our findings show actual c-kit+ cell dependent differentiation, and not activation of the Kit promoter in cardiomyocytes. Importantly, our single cell sequencing data shows no differences between c-kit+ cells harvested from wild type mice or lineage tracing mice, showing that the lineage-tracing mouse does not have some sort of inherent genetic defect to explain why so few c-kit+ cell derived cardiomyocytes are seen under homeostatic conditions.10, 11, 20, 21
Single cell sequencing is revolutionizing our understanding of the precise cellular content of organs. Here, we performed single cell sequencing on c-kit+ cells harvested from adult hearts to show a distribution into 2 main clusters of endothelial-like and mesenchymal-like cells. We did not detect a specific dedicated cardiomyocyte cardiac progenitor cell, further supporting the notion that c-kit+ cells don’t contribute large numbers of cardiomyocytes under homeostatic conditions. Although expression of transcription factors that are likely indicative of lineage commitment in partially differentiated c-kit+ cells is often reported, it remained unclear whether these cells actually progressed to differentiate and give rise to new cardiac cells.4, 37 We used genetic lineage-tracing to show that under specific circumstances, c-kit+ cells can give rise to cardiomyocytes. Whether these cardiomyocytes were derived from specific subgroups of c-kit+ cells is unclear at this point. Future single cell studies, performed after specific stimuli, might provide additional insight. Although these results are encouraging for the possibility of generating new cardiomyocytes from c-kit+ cells, the precise roles of p53 and DNA damage herein need to be further studied. A DNA damage checkpoint, involving Batf expression, independent of p53-mediated gene transcription, which determines self-renewal vs. differentiation has been proposed in hematopoietic stem cells.38 Whether a similar mechanism is operational in the heart is unclear. However, we showed that RITA is sufficient to induce cardiomyocyte differentiation without causing DNA damage, indicating that p53 is more important than DNA damage in the differentiation process. Furthermore, precise quantification of the number of presumed multipotent c-kit+ cells (CD45−CD31−c-kit+) does not show an acute reduction in the first 3 days after DOX stimulation. This was somewhat surprising given a previous report that showed reduced numbers of c-kit+ cells after DOX treatment.27 Important differences between that study and our study include the dose of DOX, and the time point when c-kit+ cells were studied. Future studies will be necessary to determine how different pathological stimuli affect the overall abundance, turnover and differentiation of c-kit+ cells. Furthermore, it will be important to determine the precise mechanisms that favor specific lineage determination within c-kit+ cells. One potential factor influencing the decision of c-kit+ cells to become endothelial cells or cardiomyocytes might be wall-stress mediated factors, although additional factors, such as paracrine mediators may also be involved.27, 39 Moreover, how these initiating factors in turn stimulate c-kit+ CPCs to differentiate will be important to identify specific differentiation pathways that are relevant for adult CPCs. Identification of these pathways may further improve the efficacy of cell therapy, which is likely mediated by paracrine effectors that stimulate endogenous cells, such as c-kit+ cells.8 Importantly, although these results are promising for the role of p53 as an important mediator of cardiomyocyte commitment of c-kit+ cells, the overall effect size is probably too small to be therapeutically relevant.
Finally, it is well known that anthracyclines can cause both acute and late-onset cardiotoxicity, in part due to progressive loss of cardiomyocytes. We also observed significant cardiac dysfunction in response to DOX treatment. The overall increase in c-kit-derived cardiomyocytes in response to DOX is not sufficient to counteract the substantial loss of cardiomyocytes in the setting of acute cardiotoxicity. However, our study provides a platform for future studies to enhance the potential to generate new cardiomyocytes. By building on our results, future studies might further stimulate new cardiomyocyte formation without inducing substantial damage to the myocardium, to add physiologically meaningful numbers of cardiomyocytes over the course of months rather than weeks.
There are some limitations to our study. Most importantly, although we show that p53 is important in the differentiation of c-kit+ cells toward cardiomyocytes, the observed effects fall short of a therapeutic benefit of cardiomyogenesis from c-kit+ cell differentiation. The numbers of cardiomyocytes generated by c-kit+ cells, as quantified from histological quantifications, or from isolated cardiomyocytes (<0.1%), are too low to give rise to meaningful regeneration.40 A second limitation to our study is the lack of identifying a specific subpopulation of c-kit+ cells that is pre-destined to become cardiomyocytes. If less than 1% of c-kit+ cells are predestined to become cardiomyocytes, we may have missed identification of this small population of c-kit+ cells by sequencing ‘only’ 400 cells, or potentially due to size exclusion based on single cell capture chip. However, other reports suggested that negative selection for CD45 enriches for cardiac cells that exhibit stem/progenitor characteristics.14 This is exactly the approach we used, and despite this approach we failed to identify a specific population of c-kit+ cells that expressed cardiomyocyte characteristic genes under homeostatic conditions. Although our approach of single cell sequencing may have missed a small subpopulation of cells that is pre-destined to become cardiomyocytes, the results further substantiate the limited cardiomyogenic potential of c-kit+ cells under homeostatic conditions.41
In conclusion, we have identified p53 as a critical mediator of cardiomyocyte differentiation by endogenous c-kit+ cells. Our findings are promising since they begin to show the circumstances under which endogenous cardiac c-kit+ cells can generate cardiomyocytes, although the magnitude of new cardiomyocyte formation is currently too low to constitute a therapeutic effect of cardiomyogenesis from c-kit+ cell differentiation. Nevertheless, this is an initial step towards the long-term goal of identifying strategies that enhance endogenous cardiac regeneration.
Supplementary Material
Clinical Perspective.
What is new?
This is one of the first studies to show that cardiac c-kit+ cells behave differently in response to various stimuli, and to show that doxorubicin stimulates them to adopt a cardiomyocyte fate
This study used single cell sequencing to show innate heterogeneity within c-kit+ cells, where these have either endothelial, or mesenchymal identity.
This study showed an important role for p53 in the differentiation of c-kit+ cells to cardiomyocyte fates.
What are the clinical implications?
This study shows that endogenous cardiac cells can give rise to cardiomyocytes under specific circumstances, such as in response to doxorubicin, which may ultimately lead to the discovery of specific drugs or treatments that could enhance new cardiomyocyte formation in the adult heart to combat heart failure.
Although we show that cardiomyocytes can be derived from endogenous c-kit+ cells, the rates are currently too low to be therapeutically meaningful.
Acknowledgments
We thank Yi Ren and the Lillehei Heart Institute FACS Core for help with flow cytometry.
Funding Sources
This work was supported by the NIH (HL112852 and HL 130072 to JHvB, HL095077 to JZ and HL122576 to DJG. AY was supported by NIH MSTP grant T32GM008244).
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med. 2014;20:1386–1393. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 6.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated Administrations of Cardiac Progenitor Cells Are Markedly More Effective Than a Single Administration: A New Paradigm in Cell Therapy. Circ Res. 2016;119:635–651. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM. cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci U S A. 2015;112:13051–13056. doi: 10.1073/pnas.1517201112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Cao I, García-Cao M, Martín-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Berlo JH, Elrod JW, van den Hoogenhof MM, York AJ, Aronow BJ, Duncan SA, Molkentin JD. The transcription factor GATA-6 regulates pathological cardiac hypertrophy. Circ Res. 2010;107:1032–1040. doi: 10.1161/CIRCRESAHA.110.220764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AJ, Lewis FC, Aquila I, Waring CD, Nocera A, Agosti V, Nadal-Ginard B, Torella D, Ellison GM. Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9:1662–1681. doi: 10.1038/nprot.2014.113. [DOI] [PubMed] [Google Scholar]
- 15.Gong W, Rasmussen TL, Singh BN, Koyano-Nakagawa N, Pan W, Garry DJ. Dpath software reveals hierarchical haemato-endothelial lineages of Etv2 progenitors based on single-cell transcriptome analysis. Nat Commun. 2017;8:14362. doi: 10.1038/ncomms14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Statist Soc B. 2001;63:411–423. [Google Scholar]
- 17.Dey D, Han L, Bauer M, Sanada F, Oikonomopoulos A, Hosoda T, Unno K, De Almeida P, Leri A, Wu JC. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res. 2013;112:1253–1262. doi: 10.1161/CIRCRESAHA.112.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Qiao Z, Wang QD, Lui KO, Zhou B. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26:119–130. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 23.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 24.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108:1226–1237. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M, Mohsin S, Avitabile D, Siddiqi S, Nguyen J, Wallach K, Quijada P, McGregor M, Gude N, Alvarez R, Tilley DG, Koch WJ, Sussman MA. β-Adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ Res. 2013;112:476–486. doi: 10.1161/CIRCRESAHA.112.280735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-Term Outcome of Administration of c-kit(POS) Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ Res. 2016;118:1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, Wu J, Ding M, Deng H. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–5852. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- 29.Jain AK, Allton K, Iacovino M, Mahen E, Milczarek RJ, Zwaka TP, Kyba M, Barton MC. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012;10:e1001268. doi: 10.1371/journal.pbio.1001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 31.Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Elf SE, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4:37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, Gude NA, Thistlethwaite PA, Sussman MA, Gottlieb RA, Gustafsson AB. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121:675–683. doi: 10.1161/CIRCULATIONAHA.109.902221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- 37.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, Schambach A, Wuestefeld T, Dauch D, Schrezenmeier H, Hofmann WK, Nakauchi H, Ju Z, Kestler HA, Zender L, Rudolph KL. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Hatzistergos KE, Saur D, Seidler B, Balkan W, Breton M, Valasaki K, Takeuchi LM, Landin AM, Khan A, Hare JM. Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circ Res. 2016;119:921–930. doi: 10.1161/CIRCRESAHA.116.309281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, Marbán E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Berlo JH, Molkentin JD. Most of the Dust Has Settled: cKit+ Progenitor Cells Are an Irrelevant Source of Cardiac Myocytes In Vivo. Circ Res. 2016;118:17–19. doi: 10.1161/CIRCRESAHA.115.307934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.