Abstract
Hosts in free-living populations can experience substantial variation in the frequency and dose of pathogen exposure, which can alter disease progression and protection from future exposures. In the house finch-Mycoplasma gallisepticum (MG) system, the pathogen is primarily transmitted via bird feeders, and some birds may be exposed to frequent low doses of MG while foraging. Here we experimentally determined how low-dose, repeated exposures of house finches to MG influence host responses and protection from secondary high-dose challenge. MG-naive house finches were given priming exposures that varied in dose and total number. After quantifying host responses to priming exposures, all birds were given a secondary high-dose challenge to assess immunological protection. Dose, but not the number of exposures, significantly predicted both infection and disease severity following priming exposure. Furthermore, individuals given higher priming doses showed stronger protection upon secondary, high-dose challenge. However, even single low-dose exposures to MG, a proxy for what some birds likely experience in the wild while feeding, provided significant protection against a high dose challenge. Our results suggest that bird feeders, which serve as sources of infection in the wild, may in some cases act as “immunizers”, with important consequences for disease dynamics.
Keywords: host-pathogen interactions, dose response, Mycoplasma gallisepticum, house finch, Haemorhous mexicanus
Introduction
Infectious diseases pose a significant threat to human and animal health (Jones et al. 2008), but in any given population, there is significant heterogeneity among individuals in the likelihood or severity of infection (e.g, May and Anderson 1987; Grenfell et al. 1995; Woolhouse et al. 1997; Lloyd-Smith et al. 2005; Yates et al. 2006). While significant research has focused on genetic predictors of individual heterogeneity, environmental sources of variation such as exposure level are likely to be equally important in mediating heterogeneity in host responses (Johnson and Hoverman 2014; Gervasi et al. 2015). In most host-pathogen systems, individuals encounter pathogens at different doses and frequencies (e.g., Aiello et al. 2016). Exposure level to a pathogen has been shown to alter infectivity, disease progression, and an individual's susceptibility to reinfection across systems (e.g., McElroy et al. 1997; Timms et al. 2001; Spekreijse et al. 2010; Banyard et al. 2014; Song et al. 2015). Thus, understanding how exposure level mediates host responses can be critical for predicting population-level disease dynamics (Ben-Ami et al. 2010; Tidbury et al. 2012; Best et al. 2012; Pessoa et al. 2014).
In many well-studied host-pathogen systems, challenge exposures to a pathogen consist of single exposures at relatively high doses (e.g., Wu et al. 2003; Liu et al. 2009; McKinstry et al. 2009; May et al. 2012). Although high-dose exposures can maximize repeatability in experimental responses, this type of exposure is not always representative of natural systems and may eliminate variation in susceptibility and disease progression that exists with heterogeneous exposure (Regoes et al. 2005; Regoes 2012; Gomes et al. 2014; Song et al. 2015). Furthermore, high dose challenges can obscure the minimum degree of exposure necessary to elicit protective immunity to secondary infection, which is critical for estimating the proportion of susceptible hosts that can support pathogen spread and persistence in a population (Cellier-Holzem et al. 2010). In addition, low-level exposure to a pathogen may not cause disease but can sufficiently prime the host immune response, either via the generation of adaptive immune memory or innate immune priming, resulting in immunological protection (Tidbury et al. 2012; Murphy and Weaver 2017; Tate 2017). For example, the alteration of the within-host environment due to prior pathogen exposure in the absence of infection, akin to vaccination, has been found across taxa (e.g., Stranford et al. 1999; Pope et al. 2011; Tidbury et al. 2011; McTaggart et al. 2012), and in invertebrate populations, immune priming can have critical consequences for pathogen prevalence and population stability (Tidbury et al. 2012; Tate 2017). Thus, examining the effects of single time-point, high-dose challenges alone may not capture the full range of meaningful variation in host responses present in free-living populations.
The naturally-occurring host-pathogen system, house finches (Haemorhous mexicanus) and Mycoplasma gallisepticum (MG), provides an ecologically relevant model for studying how varying levels of exposure may influence host responses and protection upon reinfection (Dhondt et al. 2005; Sydenstricker et al. 2005). This bacterial pathogen, which causes conjunctivitis in houses finches, was first detected in finches in 1994 (Ley et al. 1996) and has continued to cause annual epidemics in eastern North American populations of house finches since its emergence (Altizer et al. 2004b; Dhondt et al. 2005). MG can be transmitted directly or environmentally at bird feeders while animals forage (Dhondt et al. 2007a), and the extent of time spent on bird feeders is the best predictor of individual risk of mycoplasmal conjunctivitis (Adelman et al. 2015), suggesting that fomite transmission is most important in free-living populations. However, MG is a cell-wall-less bacterium that appears to degrade quickly outside of its host, as it is only able to produce mild disease in naïve individuals exposed to contaminated feeders for up to 12 hours (Dhondt et al. 2007a). In addition, rates of deposition of MG onto a single available feeder port from experimentally infected finches show that only a very small percentage (∼0.3%) of the conjunctival burden of infectious birds is deposited onto feeders during foraging (Adelman et al. 2013a), resulting in mean deposition rates of 1.6 × 103 MG copies per day from hosts with the highest conjunctival pathogen loads (i.e., birds with a mean of 5.8 × 106 MG copies in the conjunctiva). Although it is extremely challenging to quantify MG exposure levels in natural populations, these lines of evidence suggest that free-living house finches are likely exposed to variable amounts of MG while feeding, with some individuals likely experiencing repeated exposure to relatively low doses of MG. However, to date, experimental exposures of house finches to MG have used relatively high infectious doses (≥104 color changing units[=CCU]/ml) and/or single time-point exposures (Dhondt et al. 2007b; Bouwman and Hawley 2010; Bonneaud et al. 2011; Hawley et al. 2011; Bonneaud et al. 2012; Adelman et al. 2013a), which are unlikely to be fully representative of the range of variation in MG exposure in the wild.
Here we experimentally examined how host variation in exposure to MG, which is likely characteristic of free-living populations, influences host responses and protection from subsequent infection. We used a direct inoculation approach, whereby we could directly control the exposure level each individual received, in order to maximize our power to detect effects of variation in exposure. Using wild-caught, first-year house finches with no prior exposure to MG, we assessed how variation in both dose and number of exposures influenced the probability of infection, the severity of infection and disease, and resulting protection from high dose secondary challenge. House finches were given varying number of priming inoculations (1, 3, or 6) at a range of MG doses (102-106 CCU/ml). We predicted that individuals given a single, low-dose priming exposure would develop mild or subclinical infection, as was found when house finches were given a single time-point exposure to an MG-contaminated feeder (Dhondt et al. 2007a). In contrast, we predicted that individuals given repeated low-dose priming exposures would show higher disease severity and pathogen loads similar to levels produced by single, high-dose experimental exposures. After clearance of all clinical signs from priming inoculations, animals were challenged with a single high dose (106 CCU/ml) of MG to assess the extent of secondary protection. We predicted that priming exposure level would mediate the extent of protection against secondary infection, with increased protection from priming exposures at higher dose and/or frequency.
Materials and Methods
Experimental Design and Timeline
We examined how repeated exposure to MG influences host responses by directly inoculating birds in the conjunctiva 1, 3 or 6 times with one of two low-doses of MG (102 or 103 CCU/ml; Table 1). These concentrations of pathogen were selected because they represent novel doses for this system and fall within the range of levels of MG copies deposited onto feeder ports by an infectious bird (Adelman et al. 2013a). Repeated “priming” exposures were given every other day and start dates were staggered such that the final priming exposure coincided for all individuals (Figure 1). To elucidate the effect of inoculation dose while holding exposure number constant, we also included single exposure treatments of an intermediate (104 CCU/ml) and high dose (106 CCU/ml). We selected the 104 concentration as an intermediate between the high-dose (106), standardly used in past studies with this isolate of MG (e.g., Grodio et al. 2012; Hawley et al. 2013; Adelman et al. 2013b; Grodio et al. 2013) and the novel lower dose exposures (102 and 103) first considered in this study. Our experimental design (Table 1) was not fully factorial in that the higher dose groups (104 and 106) only received a single priming exposure. Finally, our design included a negative control group which received sham priming exposures of media alone. Furthermore, during all priming time-points, all animals not receiving MG were given a sham inoculation of media (Fig. 1). For simplicity, priming treatment groups are referred to using the following format: [number of exposures (priming dose)]. Sex ratios were equal within treatment groups (Table 1).
Table 1.
Treatment groups and starting sample sizes for priming exposures of house finches to Mycoplasma gallisepticum.
| No. of Exposures | Dosage (CCU/mL) | ||||
|---|---|---|---|---|---|
| Low-Dose 102 | Low-Dose 103 | Intermediate-Dose 104 | High-Dose 106 | Sham | |
| 1 | N=8 | N=8 | N=6 | N=6 | |
| 3 | N=8 | N=8 | |||
| 6 | N=8 | N=8 | |||
| None | N=6 | ||||
Figure 1.
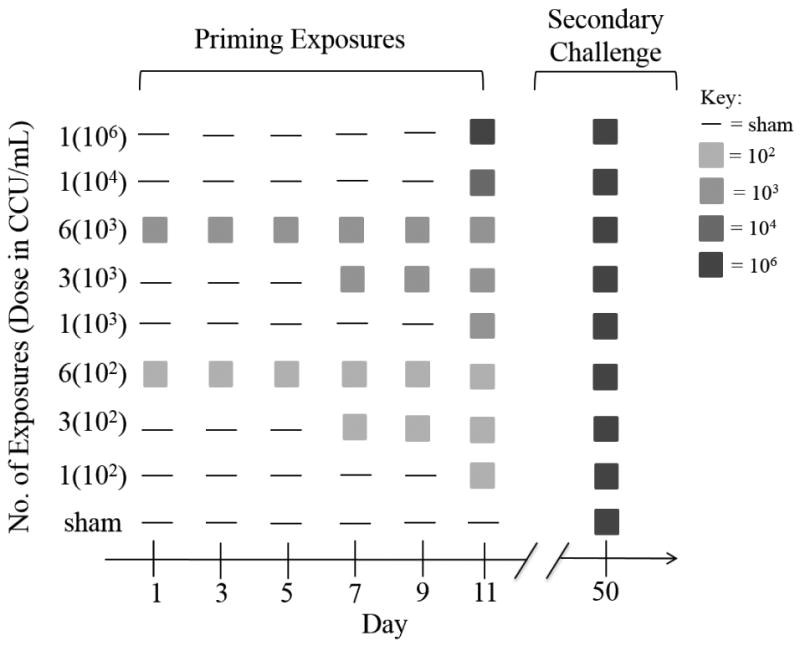
Experimental timeline for the nine priming inoculation treatments (y-axis). Dose (in parentheses) is expressed as color changing units (CCU) per ml. All priming treatment groups, including the sham priming group, were challenged with a high dose of pathogen 39 days after the last priming treatment.
Thirty-nine days after the final priming exposure, all treatment groups received a secondary high dose challenge (106 CCU/ml; Fig.1). Because the final priming inoculation for each group coincided, the time between final priming exposure and secondary challenge was equal across groups (Fig. 1). Animals were sampled for pathogen load and severity of clinical signs (see details below) on days 2, 4, 7, 11, 18, 25, and 32 after the final priming inoculation, and days 0, 4, 7, 14, and 21 post-secondary challenge.
Bird Capture and Housing
In June-July 2014, 66 juvenile house finches were captured using cage traps and mist nets in Montgomery County, VA and the city of Williamsburg, VA under permits from VDGIF (050352) and USFWS (MB158404-1). Birds captured in Williamsburg were housed temporarily (up to 3 days) in flocks in an outdoor aviary and transported to Virginia Tech by state vehicle. All finches were then housed in pairs at constant day length and temperature, and fed an ad libitum diet prior to and throughout the experiment (Daily Maintenance Diet, Roudybush Inc. Woodland, CA). Two weeks before the first inoculation, all birds were moved to individual cages for the duration of the experiment. All animals included in the experiment underwent a two-week quarantine protocol to ensure no previous exposure to MG. In brief, animals were monitored for two weeks for visible eye lesions, and were tested for MG-specific antibodies (as per Hawley et al. 2011) on day 14 post-capture to account for time for development of antibodies in case exposure to MG in the wild occurred on the day of capture. Only individuals that were seronegative, never showed clinical signs, and were never housed with a cagemate that showed clinical signs were used in this experiment. Additionally, all individuals were swabbed for pathogen load at a baseline sampling point four days prior to inoculation and all birds were either qPCR negative or had loads equivalent to background levels of contamination (see below under Primary Infection - Analysis). All protocols for animal housing and procedures were approved by Virginia Tech's and the College of William and Mary's Institutional Animal Care and Use Committees.
Inoculum
The MG isolate VA1994 (7994-1 7P 2/12/09; D. H. Ley, North Carolina State University, College of Veterinary Medicine, Raleigh, NC, USA), isolated from a free-living house finch in 1994 (Ley et al., 1996) was used for all inoculations. This isolate was selected because it has been used in the vast majority of past experimental studies to date (e.g., Dhondt et al. 2008; Grodio et al. 2012; Adelman et al. 2013a), thus facilitating comparisons to past work. The day of use, stock inocula were thawed and immediately diluted using Frey's broth media with 15% swine serum (FMS) to approximate dosage. Sham inoculations were done using FMS alone. All inoculations (MG or sham) consisted of a total volume of 70uL of inoculum which was distributed approximately equally into both conjunctivae of each bird using a 100uL micropipette.
Disease and Infection Severity
To assess disease severity, clinical signs were scored for each eye on a scale of 0-3 based on the level of visible conjunctival inflammation, eversion and exudate as per Sydenstricker et al. 2006. In brief, a score of 0 indicates no detectable inflammation, 1 indicates minor swelling, 2 moderate swelling and eversion of the conjunctival tissue, and 3 indicates the eye is nearly hidden by swelling and exudate. Clinical severity scores (“eye scores”) were recorded at each sampling point and summed for both eyes. Scorers were blind to treatment and to limit potential variation, only one experienced individual scored all birds following primary infection while another experienced individual scored all birds following secondary infection. Because host responses to primary and secondary infection were analyzed separately and comparisons of interest were across treatments that were scored by the same individual, the change in scorer from primary to secondary infection should not influence our conclusions.
Pathogen load was determined by quantitative PCR (qPCR) using DNA extracted from conjunctival swabs as described in (Grodio et al. 2008; Hawley et al. 2013). In brief, conjunctival sacs were gently swabbed for 5 seconds using sterile swabs dipped in tryptose phosphate broth (TPB), and swabs were eluted in 300 uL of TPB. Swabs from both conjunctivae from an individual at a given sampling point were collected in the same vial. Samples were frozen at -20°C until DNA was extracted. DNA was extracted from swabs using Qiagen DNeasy 96 Blood and Tissue kits (Qiagen, Valencia, CA) and qPCR was performed using primers and a probe that target the Mgc2 gene of MG (Grodio et al. 2008). Each run included a standard curve of 2.98 × 101 to 2.98 × 108 copy numbers produced using a plasmid containing a 303 bp Mgc2 insert (Grodio et al. 2008). Output values of Mgc2 copies were log10 transformed before statistical analysis.
To account for potential contamination in our highly sensitive qPCR assay, we included a total of 39 extraction controls (blank samples that underwent the extraction and PCR process alongside actual samples) and 3 environmental controls (swabs waived in the air at the time of sampling and then eluted in buffer and treated like all other samples) dispersed throughout our samples of interest. These samples showed a very high rate of background contamination, with 14/42 (∼33%) coming up positive at largely low levels (median value log10 was 1.12, but maximum control value was log10 of 4.87). Thus, we used a conservative cut-off to define the probability of infection in our study (see below under Primary Infection - Analysis). Because samples from all treatments were treated identically and randomized within extraction plates, contamination was random with respect to treatment and is unlikely to influence our conclusions of interest.
Statistical Analyses
All analyses were done using R, version 3.2.5 (R Core team 2015).
Primary Infection
Final sample sizes
Final sample size for the [3(102)] group was reduced to 7 individuals due to unanticipated mortality. Two other birds (one from group [1(106)] and one from group [6(103)]) died during the course of primary infection but were included in the statistical analysis of primary infection due to sufficient data capturing the course and severity of infection in these individuals. All mortality events appeared random with respect to MG treatment, as individuals that died did not have high disease or infection severity at the time of mortality, and MG almost never causes direct mortality in captivity (Kollias et al. 2004; Hawley et al. 2013). Final sample sizes for each group are included in Figure 2a.
Figure 2.
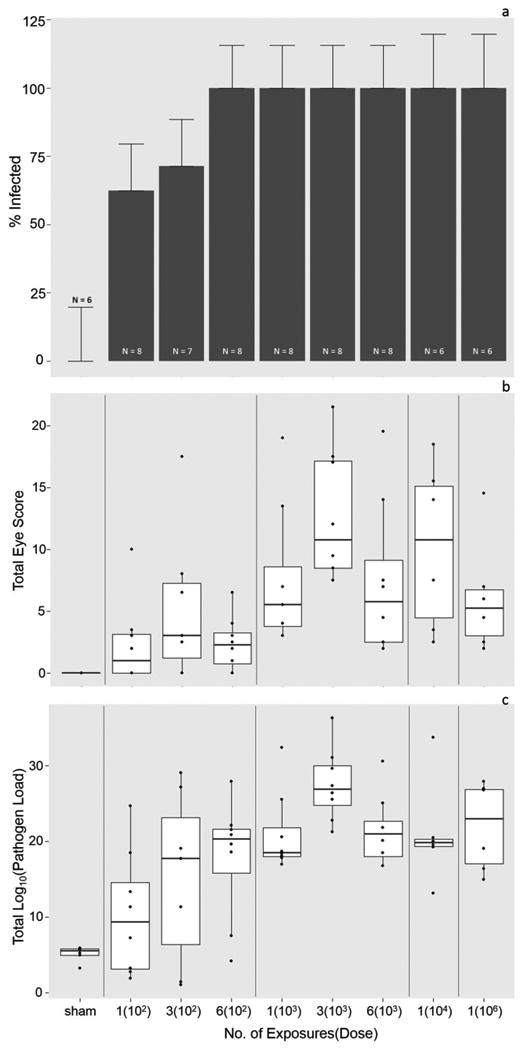
Probability of infection (a), disease severity (b) and infection severity (c) in response to priming exposures. a) The likelihood of successful infection (Y or N) of Mycoplasma gallisepticum in house finches varied with exposure level. Error bars represent standard error of the proportion infected, and the y-axis extends to 125% to visualize error bars. b) Disease severity increased with priming dose of Mycoplasma gallisepticum, but not with the number of exposures. For visual clarity, here scores (scale 0-3) for both eyes were summed across time-points for each individual to calculate total eye scores for the entire course of primary infection. Each point thus represents a single individual. c) Conjunctival pathogen load increased with priming dose of Mycoplasma gallisepticum, but not with the number of exposures. Again, for visual clarity, total pathogen load includes conjunctival loads summed across primary infection sampling time-points. Each point represents an individual.
Analysis
Probability of infection
We quantified successful infection (Y or N) as a host harboring a conjunctival pathogen load at any timepoint post-inoculation ≥ 3.1 log10 copies, a conservative threshold used to define infection for our selected isolate in past work (Adelman et al. 2015). Negative control animals (sham treatment) had conjunctival pathogen loads equivalent to those detected in our extraction controls (one-way ANOVA F1,91 = 0.0004, p = 0.98), and thus we used this conservative threshold to define infection. Probability of infection following priming treatments was analyzed using a chi-squared analysis with exposure treatment as the variable of interest. Due to low variation in the probability of infection (Fig. 2a), we did not have sufficient power to run a logistic regression model.
Infection and disease severity
Because we had repeated measures from every individual and our response variables were not normally distributed, we used generalized linear mixed models (lme4 package Bates et al. 2015) to determine how infection and disease severity were altered by priming treatment. Although eye score and pathogen load positively covary (Hawley et al. 2013) and thus cannot be considered statistically independent, models for pathogen load (infection severity) and eye score (disease severity) were run independently because these metrics represent distinct biological responses (pathogen growth versus host inflammation) that may respond differently to exposure level. The model for pathogen load was run using a Gamma distribution with an inverse link function; eye score was run using a Poisson distribution with a log link function. All models included log10(dose+1) and number of priming exposures as fixed effects and individual ID as a random effect. In models where they significantly improved model fit, sex, post-inoculation day (PID), and log10(dose+1)2 were included as fixed effects. All pairwise interactions were tested, but were removed because they did not significantly improve model fit. Models were selected based on ΔAICc (> 2) values (Burnham and Anderson 2002). Dose, number of exposures and day post-infection were treated as continuous variables.
Secondary Infection
Final sample sizes
The three animals that died during primary infection (see above) reduced final sample sizes for secondary infection. One additional animal (group [1(106)]) died during secondary infection but was included in the analysis due to sufficient time points captured. Two animals (from groups [1(104)] and [1(103)]) were eliminated from secondary infection analysis due to chronic disease, assessed by continuation of visible eye lesions, produced from priming exposures. Finally, one animal in group [3(103)] was eliminated from secondary analysis due to an exceptional pre-challenge pathogen load equivalent to peak infection loads at secondary infection day 0. These animals were eliminated to allow us to detect distinct responses to secondary infection, as opposed to continued responses from primary infection. Final sample sizes for secondary infection are included in Figure 3a.
Figure 3.
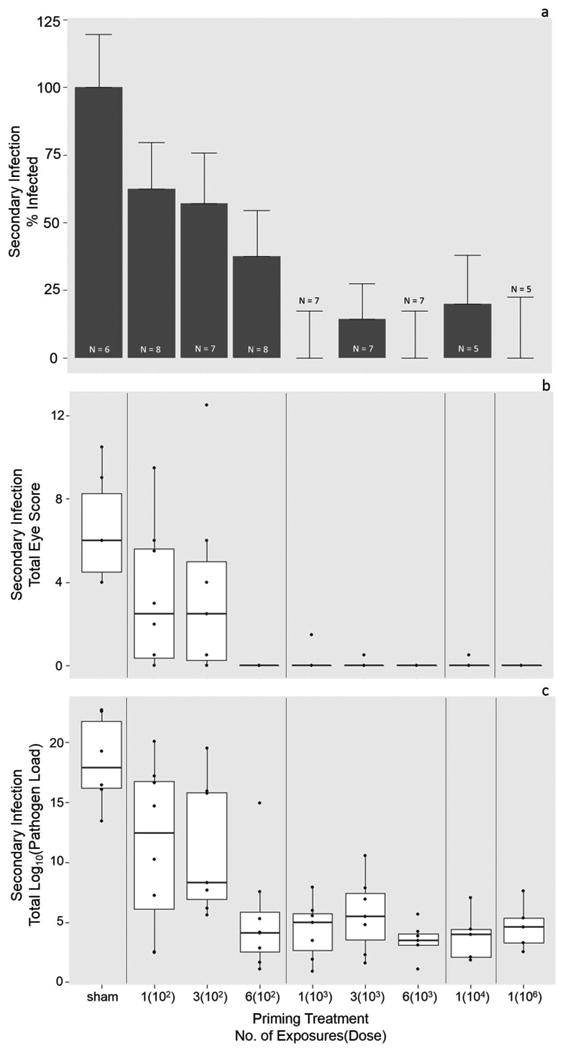
Probability of infection (a), disease severity (b) and infection severity (c) in response to a secondary high-dose challenge 39 days after priming exposures. a) House finches previously primed with higher doses of Mycoplasma gallisepticum showed a lower probability of infection (Y or N) in response to a secondary high dose challenge. Error bars represent standard error of the proportion infected, and the y-axis extends to 125% to visualize error bars. b) House finches primed with a greater number of exposures and higher doses of Mycoplasma gallisepticum showed significantly lower disease severity upon a high dose challenge. For visual clarity, eye scores (scale 0-3) for both eyes were summed across the course of secondary infection. Each point represents an individual. c) House finches previously primed with higher doses of Mycoplasma gallisepticum showed significantly higher levels of protection (i.e., significantly lower pathogen loads, y-axis) upon a high dose challenge. Again here, for visual clarity, conjunctival pathogen loads were summed across the course of secondary infection. Each point represents an individual.
Analysis
Probability of infection
Host infection (Y or N; defined as a pathogen load ≥ 3.1 log10 copies at any time point post infection; see above) was analyzed with a logistic regression model using a binomial distribution and probit link function with log10(dose+1) and number of priming exposures as continuous predictors and sex as a categorical predictor.
Infection and disease severity
Generalized linear mixed models were used to assess how priming exposures altered secondary infection and disease severity. As per primary infection models, models were run independently for pathogen load and eye score with priming log10(dose+1) and number of priming exposures as fixed effects and individual animal ID as a random effect. In models where they significantly improved model fit, sex, PID, and log10(dose+1)2 were also included as fixed effects. The model for pathogen load was run using a Gamma distribution with an inverse link function; eye score was run using a Poisson distribution with a log link function.
Results
Primary Infection
The likelihood of an individual becoming infected following priming inoculations was significantly altered by exposure treatment (chi-squared = 53.1, df = 16, P = <0.0001; Fig. 2a). Our lowest exposure treatment [1(102)] produced infection in 62.5% of individuals while all other treatments with the exception of [3(102)] produced 100% infection (Fig. 2a). Similarly, the severity of infection, measured via conjunctival pathogen load, was significantly affected by priming dose [individuals = 65, observations = 452, log10(dose) = -0.31 ± 0.12, P = 0.0083; log10(dose)2 = 0.043 ± 0.019, P = 0.028]. Generally, pathogen loads increased with priming inoculation dose (Fig. 2c), but contrary to our predictions, number of priming exposures did not significantly influence infection severity (no. of exposures = -0.021 ± 0.016, P = 0.19). As expected given the dynamics of this acute infection, post-inoculation day strongly predicted infection severity (PID = 0.019 ± 0.0049, P = <0.0001).
Priming dose and sex significantly influenced disease severity, measured via the extent of clinical signs following priming exposures (individuals = 65, observations = 452, log10(dose) = 2.6 ± 0.43 P = 1.7e-09; log10(dose)2 = -0.38 ± 0.071, P = <0.0001; sex[F] = 0.83 ± 0.0049, P = <0.0001). Disease severity generally increased with dose (Fig. 2b), and females had significantly higher eye scores than males. As with pathogen load, the number of exposures did not significantly affect disease severity (no. of exposures = -0.0029 ± 0.063, P = 0.96).
Secondary Infection
The probability of secondary infection in response to high dose challenge was significantly altered by priming dose and sex (priming log10(dose) = -1.3 ± 0.39; P = 0.001, sex[F] = -1.1 ± 0.47, P = 0.019). Increasing priming dose reduced the likelihood of infection upon high-dose challenge (Fig. 3a), and females had a significantly lower probability of secondary infection across treatments. Number of exposures did not significantly affect the likelihood of secondary infection (no. of exposures = -.019± 0.11, P = 0.077). Similarly, when protection was analyzed as infection severity (lower load = higher protection), priming dose and post-secondary inoculation day (SID) significantly influenced protection (individuals = 60, observations = 237, priming log10(dose) = 0.15 ± 0.060, P = 0.012; SID = 0.024 ± 0.010, P = 0.023; Fig. 3c). As with the probability of infection, number of exposures did not have a significant effect on protection (no. of exposures = 0.043 ± 0.032, P = 0.18).
Both dose (quadratic only) and number of priming exposures significantly influenced the severity of disease upon a high-dose secondary challenge (individuals = 60, observations = 237; priming log10(dose)2 = -1.4 ± 0.63; P = 0.023, no. of priming exposures = -0.72 ± 0.23, P = 0.0017). Overall, there was very little disease across previously exposed individuals, particularly in animals that received repeat exposures or a single high-dose (Fig. 3b).
Discussion
Our study aimed to experimentally mimic repeated exposure to low doses of MG, as may occur for some free-living house finches during foraging. Our findings suggest that variation in host exposure to Mycoplasma gallisepticum significantly alters the likelihood of infection, as well as the severity of both disease and infection. Additionally, low-level exposures significantly reduced the likelihood and intensity of secondary infection while, in some cases, producing little or no visible signs of disease. These results provide evidence that exposure level can be an important source of individual heterogeneity in this host-pathogen system, with critical implications for disease dynamics in wild house finch populations.
Individual responses to infection following a house finch's first encounter with MG (i.e., priming inoculations) were significantly influenced by inoculation dose, which had a positive effect on both infection and disease severity. These results are consistent with our predictions and past work across disparate systems, where infective dose has been found to be a significant driver of disease progression (e.g., Lillehoj 1988; Vandenberg et al. 1998; Havelaar et al. 2001; Timms et al. 2001; Hughes et al. 2002). Dose responses to MG have not been explicitly examined in house finches to date, but prior work demonstrated that naïve birds provided with an MG-contaminated feeder at a single time point had significantly lower clinical disease than birds directly inoculated in the conjunctiva (Dhondt et al. 2007a). In fact, disease outcomes of birds exposed to contaminated feeders in Dhondt et al. (2007) were roughly equivalent to those of our [1(102)] treatment group. Our results, in combination with those of Dhondt et al. (2007), which used the same MG isolate, suggest that birds foraging on a contaminated feeder used by a single infectious bird likely pick up a low infective dose of MG. However, further work is needed to determine the effective dose of MG that birds encounter when foraging at contaminated feeders in the wild, which is likely to vary with both local MG prevalence and host foraging behavior. Consistent with results in chickens (Ferguson-Noel et al. 2012), our results suggest that house finch MG is infectious at very low doses, with a 50% infectivity rate (ID50) of less than 102 CCU/ml, our lowest dose examined (Fig. 1). Because the ability of MG to infect birds at low doses has important consequences for maintenance of this pathogen in the wild, future experiments should assay an even lower range of doses to determine the minimum infectious dose for this pathogen in house finches.
Contrary to our predictions, the number of priming inoculations (1, 3, or 6) did not influence either infection or disease severity. Our findings are not unique, however, as no clear pattern has emerged with regard to the number of low-dose exposures and disease outcomes across systems (Regoes 2012; Banyard et al. 2014; Song et al. 2015). Although the number of exposures did not influence infection or disease severity, the probability of infection appeared to increase with the number of priming exposures in birds receiving our lowest infective dose: one or three priming exposures at the lowest dose (102 CCU/ml) produced infection in ∼60 and 70% of individuals respectively, while six exposures at the same dose produced 100% infection (Fig. 1). However, because the vast majority of birds in our study became infected following priming exposure, we did not have sufficient statistical power to examine how differences in the likelihood of infection may relate to dose and number of exposures. Future work should test whether repeated exposures at even lower doses and within shorter time intervals than those examined here (e.g., 3, 6, or 24 hours apart) result in more severe infection or disease than single time-point exposures.
A key objective of our study was to determine whether repeated exposure to low doses of MG, as may occur for some birds in the wild, can “immunize” house finches against a high challenge dose that would normally be infectious. We found priming dose to play a significant role in protection against secondary infection, with higher priming doses resulting in higher levels of protection. However, even low dose priming resulted in significant immunological protection: strikingly, even a single, low dose exposure (102), which only caused detectable infection for a subset of individuals (Fig 2a), resulted in partial protection against a high dose challenge (Fig. 3a,c). Severity of disease, however, was significantly altered by increases in both priming dose and number of exposures, which reduced conjunctival swelling and exudate upon high dose challenge (Fig. 3b). Because the severity of these clinical signs predicts the proportion of MG conjunctival load deposited onto bird feeders (Adelman et al. 2013a), effects of prior exposure on disease severity may have independent effects on transmission potential in this system. Overall, these results suggest that bird feeders, in addition to their role as infectious fomites, may in some cases act as “immunizers” if they serve as environmental reservoirs of low doses of MG. Unfortunately, we do not currently know the dose and frequency of MG exposure in free-living house finches, and thus further study is needed to confirm that our proxy for environmental exposure falls within the range of exposure levels in the wild.
Our study did not address the specific immunological mechanisms underlying protection from secondary infection, which likely resulted from the formation of immunological memory. House finches show significant increases in MG-specific IgY and IgA antibodies in plasma and lachrymal fluid, respectively, by day 14 post-inoculation, and these levels remain elevated at least until day 56 (Grodio et al. 2012). Because our secondary exposures occurred at day 39 post-inoculation, serum and lachrymal antibody levels from primary infection were likely still elevated at the time of high dose challenge. However, antibody levels measured by current assays available for this system are not predictive of protection against infection: in fact, in birds reexposed to high doses of VA1994 at varying time intervals after initial high dose exposure (9, 11, and 14 months), individual seropositivity just prior to reinoculation correlated with lower protection from reinoculation (Sydenstricker et al. 2005). Furthermore, during primary infections at high doses, antibody levels in both serum and lachrymal fluid correlate positively with disease severity across both individuals and MG isolates (Grodio et al. 2012). In contrast, work in chickens has demonstrated that early mucosal antibody responses are predictive of immune protection to MG infection (Javed et al. 2005). Given the sparsity of immunological assays and tools available for house finches, we did not attempt to measure the specific immunological mechanisms involved in protection in this study. However, further study of how the host immune system is altered by varying exposure levels would provide valuable insight into how protective immunity is formed in this system, and whether innate immune priming might play a role.
We unexpectedly found sex to have significant effects on host responses in our study. Specifically, female house finches had more severe eye lesions than males after priming exposures, and had significantly lower infection rates than males in response to secondary high-dose challenge. A past large-scale observational field study also found more severe clinical signs of Mycoplasmal conjunctivitis in female versus male house finches, but only for juvenile birds (Altizer et al. 2004a), which were also used in this study. However, past experimental studies using high doses of MG (and juvenile house finches) have not found evidence that sex alters house finch responses to MG (Kollias et al. 2004). It is possible that differences in disease severity are more pronounced at lower dose exposures where there is greater variation in disease outcome across individuals. We did not detect a significant pairwise interaction between sex and dose in our study, suggesting that sex effects were largely equivalent across doses, but low statistical power may have limited our ability to detect pairwise interactions. Intriguingly, we recently documented sex differences in the healthy ocular microbiome of a subset of house finches used in this experiment (Thomason et al. 2017), and these microbiome differences may have played a role in generating the observed sex effects on disease severity and probability of secondary infection. Further study is needed to evaluate potential sex effects on disease severity in this system, and the way in which these effects may interact with exposure level.
Together, our findings suggest that within-host dynamics in this wildlife disease system are strongly influenced by the level of initial exposure to the pathogen. Furthermore, prior exposure to low and repeated doses of pathogen, which may occur for some free-living finches in the wild, can result in significant levels of protection against infection and/or disease during high dose challenge. These results suggest a potential context-dependent role for environmental fomites as either “transmission hubs” or “immunizers” in this system. Furthermore, our results indicate that low priming doses produce largely incomplete protection, whereby individuals can still be successfully infected but have significantly lower infection and disease severity compared to naïve individuals. This incomplete protection could have important evolutionary consequences for pathogen virulence if more virulent pathogen strains are better able to colonize incompletely immune individuals in a population, as has been shown for incomplete vaccination (Gandon et al. 2001; Read et al. 2015). Virulence has been found to be increasing since MG emerged in house finch populations (Hawley et al. 2013), and low dose environmental exposure leading to incomplete host immunity in a population may be one potential mechanism contributing to this pathogen's evolution. More broadly, our results indicate that heterogeneous exposure to a pathogen is an important mediator of within-host responses, with critical implications for both the ecology and evolution of host-pathogen interactions.
Acknowledgments
This work was funded by NIH grant 5R01GM105245 as part of the joint NIH-NSF-USDA Ecology and Evolution of Infectious Diseases program. Additional fellowship support for A. Leon provided by the Virginia Tech IMSD program funded through NIH-NIGMS grant R25GM072767-09. We thank members of the House Finch Project Group for useful discussion. We especially thank Laila Kirkpatrick for running all qPCR assays, David Ley for providing inoculum, and Andre Dhondt, Wesley Hochachka, James Adelman, Sahnzi Moyers, Skylar Hopkins, Arietta Fleming-Davies, Courtney Thomason, Joel McGlothlin, Rami Dalloul and Liwu Li for valuable feedback. We thank Dan Cristol and Trevor Sleight for their assistance in trapping house finches in Williamsburg, VA. We thank Catherine Beach, Johanel Caceres, Dorian Jackson, David Vasquez, and Courtney Youngbar for assistance in data collection.
References
- Adelman JS, Carter AW, Hopkins WA, Hawley DM. Deposition of pathogenic Mycoplasma gallisepticum onto bird feeders: host pathology is more important than temperature-driven increases in food intake. Biology Letters. 2013a;9:20130594–20130594. doi: 10.1098/rsbl.2013.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM. House Finch Populations Differ in Early Inflammatory Signaling and Pathogen Tolerance at the Peak of Mycoplasma gallisepticum Infection. The American Naturalist. 2013b;181:674–689. doi: 10.1086/670024. [DOI] [PubMed] [Google Scholar]
- Adelman JS, Moyers SC, Farine DR, Hawley DM. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20151429. doi: 10.1098/rspb.2015.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello CM, Nussear KE, Esque TC, Emblidge PG, Sah P, Bansal S, Hudson PJ. Host contact and shedding patterns clarify variation in pathogen exposure and transmission in threatened tortoise Gopherus agassizii : implications for disease modelling and management. Journal of Animal Ecology. 2016;85:829–842. doi: 10.1111/1365-2656.12511. [DOI] [PubMed] [Google Scholar]
- Altizer S, Davis AK, Cook KC, Cherry JJ. Age, sex, and season affect the risk of mycoplasmal conjunctivitis in a southeastern house finch population. Canadian Journal of Zoology. 2004a;82:755–763. doi: 10.1139/z04-050. [DOI] [Google Scholar]
- Altizer S, Hochachka WM, Dhondt AA. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. Journal of Animal Ecology. 2004b;73:309–322. doi: 10.1111/j.0021-8790.2004.00807.x. [DOI] [Google Scholar]
- Banyard AC, Healy DM, Brookes SM, Voller K, Hicks DJ, Núñez A, Fooks AR. Lyssavirus infection: “Low dose, multiple exposure” in the mouse model. Virus Research. 2014;181:35–42. doi: 10.1016/j.virusres.2013.12.029. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Package lme4. Journal Of Statistical Software. 2015;67:1–91. http://lme4.r-forge.r-project.org. [Google Scholar]
- Ben-Ami F, Ebert D, Regoes RR. Pathogen Dose Infectivity Curves as a Method to Analyze the Distribution of Host Susceptibility: A Quantitative Assessment of Maternal Effects after Food Stress and Pathogen Exposure. The American Naturalist. 2010;175:106–115. doi: 10.1086/648672. [DOI] [PubMed] [Google Scholar]
- Best A, Tidbury H, White A, Boots M. The evolutionary dynamics of within-generation immune priming in invertebrate hosts. Journal of The Royal Society Interface. 2012;10:20120887–20120887. doi: 10.1098/rsif.2012.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Balenger SL, Russell AF, Zhang J, Hill GE, Edwards SV. Rapid evolution of disease resistance is accompanied by functional changes in gene expression in a wild bird. Proceedings of the National Academy of Sciences. 2011;108:7866–7871. doi: 10.1073/pnas.101858010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Balenger SL, Zhang J, Edwards SV, Hill GE. Innate immunity and the evolution of resistance to an emerging infectious disease in a wild bird. Molecular ecology. 2012;21:2628–39. doi: 10.1111/j.1365-294X.2012.05551.x. [DOI] [PubMed] [Google Scholar]
- Bouwman KM, Hawley DM. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biology Letters. 2010;6:462–465. doi: 10.1098/rsbl.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KKP, Anderson DRD. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2002 [Google Scholar]
- Cellier-Holzem E, Esparza-Salas R, Garnier S, Sorci G. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. International Journal for Parasitology. 2010;40:1447–1453. doi: 10.1016/j.ijpara.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Dhondt AA, Altizer S, Cooch EG, Davis AK, Dobson A, Driscoll MJL, Hartup BK, Hawley DM, Hochachka WM, Hosseini PR, Jennelle CS, Kollias GV, Ley DH, Swarthout ECH, Sydenstricker KV. Dynamics of a novel pathogen in an avian host: Mycoplasmal conjunctivitis in house finches. Acta Tropica. 2005;94:77–93. doi: 10.1073/pnas.101858010. [DOI] [PubMed] [Google Scholar]
- Dhondt AA, Dhondt KV, Hawley DM, Jennelle CS. Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathology. 2007a;36:205–208. doi: 10.1080/03079450701286277. [DOI] [PubMed] [Google Scholar]
- Dhondt AA, Dhondt KV, McCleery BV. Comparative infectiousness of three passerine bird species after experimental inoculation with Mycoplasma gallisepticum. Avian Pathology. 2008;37:635–640. doi: 10.1073/pnas.101858010. [DOI] [PubMed] [Google Scholar]
- Dhondt KV, Dhondt AA, Ley DH. Effects of route of inoculation on Mycoplasma gallisepticum infection in captive house finches. Avian Pathology. 2007b;36:475–479. doi: 10.1080/03079450701642016. [DOI] [PubMed] [Google Scholar]
- Ferguson-Noel NM, Laibinis VA, Kleven SH. Evaluation of Mycoplasma gallisepticum K-Strain as a Live Vaccine in Chickens. Avian Diseases. 2012;56:44–50. doi: 10.1637/9833-061411-Reg.1. [DOI] [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Civitello DJ, Kilvitis HJ, Martin LB. The context of host competence: a role for plasticity in host–parasite dynamics. Trends in Parasitology. 2015;31:419–425. doi: 10.1016/j.pt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MGM, Lipsitch M, Wargo AR, Kurath G, Rebelo C, Medley GF, Coutinho A. A Missing Dimension in Measures of Vaccination Impacts. PLoS Pathogens. 2014;10:e1003849. doi: 10.1371/journal.ppat.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell BT, Wilson K, Isham VS, Boyd HEG, Dietz K. Modelling patterns of parasite aggregation in natural populations: trichostrongylid nematode–ruminant interactions as a case study. Parasitology. 1995;111:S135. doi: 10.1017/S0031182000075867. [DOI] [PubMed] [Google Scholar]
- Grodio JL, Dhondt KV, O'Connell PH, Schat KA. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathology. 2008;37:385–391. doi: 10.1080/03079450802216629. [DOI] [PubMed] [Google Scholar]
- Grodio JL, Hawley DM, Osnas EE, Ley DH, Dhondt KV, Dhondt AA, Schat KA. Pathogenicity and immunogenicity of three Mycoplasma gallisepticum isolates in house finches (Carpodacus mexicanus) Veterinary Microbiology. 2012;155:53–61. doi: 10.1016/j.vetmic.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Grodio JL, Ley DH, Schat KA, Hawley DM. Chronic Mycoplasma conjunctivitis in house finches: Host antibody response and M. gallisepticum VlhA expression. Veterinary Immunology and Immunopathology. 2013;154:129–137. doi: 10.1016/j.vetimm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Garssen J, Takumi K, Koedam MA, Dufrenne JB, van Leusden FM, de la Fonteyne L, Bousema JT, Vos JG. A rat model for dose-response relationships of Salmonella Enteritidis infection. Journal of Applied Microbiology. 2001;91:442–452. doi: 10.1046/j.1365-2672.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- Hawley DM, Grodio J, Frasca S, Kirkpatrick L, Ley DH. Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum : a new model system for a wildlife disease. Avian Pathology. 2011;40:321–327. doi: 10.1080/03079457.2011.571660. [DOI] [PubMed] [Google Scholar]
- Hawley DM, Osnas EE, Dobson AP, Hochachka WM, Ley DH, Dhondt AA. Parallel patterns of increased virulence in a recently emerged wildlife pathogen. PLoS biology. 2013;11:e1001570. doi: 10.1371/journal.pbio.1001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GJ, Kitching RP, Woolhouse MEJ. Dose-dependent Responses of Sheep Inoculated Intranasally with a Type O Foot-and-mouth Disease Virus. Journal of Comparative Pathology. 2002;127:22–29. doi: 10.1053/jcpa.2002.0560. [DOI] [PubMed] [Google Scholar]
- Javed MA, Frasca S, Rood D, Cecchini K, Gladd M, Geary SJ, Silbart LK. Correlates of Immune Protection in Chickens Vaccinated with Mycoplasma gallisepticum Strain GT5 following Challenge with Pathogenic M. gallisepticum Strain Rlow. Infection and Immunity. 2005;73:5410–5419. doi: 10.1128/IAI.73.9.5410-5419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Hoverman JT. Heterogeneous hosts: how variation in host size, behaviour and immunity affects parasite aggregation. The Journal of animal ecology. 2014:1–10. doi: 10.1073/pnas.101858010. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias GV, Sydenstricker KV, Kollias HW, Ley DH, Hosseini PR, Connolly V, Dhondt AA. Experimental infection of house finches with Mycoplasma gallisepticum. Journal of Wildlife Diseases. 2004;40:79–86. doi: 10.7589/0090-3558-40.1.79. [DOI] [PubMed] [Google Scholar]
- Ley DH, Berkhoff JE, McLaren JM. Mycoplasma gallisepticum Isolated from House Finches (Carpodacus mexicanus) with Conjunctivitis. Avian Diseases. 1996;40:480. doi: 10.2307/1592250. [DOI] [PubMed] [Google Scholar]
- Lillehoj HS. Influence of inoculation dose, inoculation schedule, chicken age, and host genetics on disease susceptibility and development of resistance to Eimeria tenella infection. Avian diseases. 1988;32:437–44. [PubMed] [Google Scholar]
- Liu G, Kahan SM, Jia Y, Karst SM. Primary high-dose murine norovirus 1 infection fails to protect from secondary challenge with homologous virus. Journal of virology. 2009;83:6963–8. doi: 10.1128/JVI.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M, Szczepanek SM, Frasca S, Gates AE, Demcovitz DL, Moneypenny CG, Brown DR, Geary SJ. Effects of sialidase knockout and complementation on virulence of Mycoplasma gallisepticum. Veterinary microbiology. 2012;157:91–5. doi: 10.1016/j.vetmic.2011.12.004. [DOI] [PubMed] [Google Scholar]
- May RM, Anderson RM. Transmission dynamics of HIV infection. Nature. 1987;326:137–142. doi: 10.1038/326137a0. [DOI] [PubMed] [Google Scholar]
- McElroy PD, Beier JC, Oster CN, Onyango FK, Oloo AJ, Lin X, Beadle C, Hoffman SL. Dose- and time-dependent relations between infective Anopheles inoculation and outcomes of Plasmodium falciparum parasitemia among children in western Kenya. American journal of epidemiology. 1997;145:945–56. doi: 10.1093/oxfordjournals.aje.a009054. [DOI] [PubMed] [Google Scholar]
- McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 Deficiency Unleashes an Influenza-Specific Th17 Response and Enhances Survival against High-Dose Challenge. The Journal of Immunology. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart SJ, Wilson PJ, Little TJ. Daphnia magna shows reduced infection upon secondary exposure to a pathogen. Biology Letters. 2012;8:972–975. doi: 10.1098/rsbl.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Weaver C. Janeway's Immunobiology. 9th. Garland Science/Taylor & Francis Group, LLC; 2017. [Google Scholar]
- Pessoa D, Souto-Maior C, Gjini E, Lopes JS, Ceña B, Codeço CT, Gomes MGM. Unveiling Time in Dose-Response Models to Infer Host Susceptibility to Pathogens. PLoS Computational Biology. 2014;10:e1003773. doi: 10.1371/journal.pcbi.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope EC, Powell A, Roberts EC, Shields RJ, Wardle R, Rowley AF. Enhanced Cellular Immunity in Shrimp (Litopenaeus vannamei) after “Vaccination”. PLoS ONE. 2011;6:e20960. doi: 10.1371/journal.pone.0020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vol. 55. R Foundation for Statistical Computing; Vienna, Austria: 2015. pp. 275–286. R Core Team. URL http://www.R-project.org/ [Google Scholar]
- Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, Smith LP, Kennedy DA, Walkden-Brown SW, Nair VK. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLOS Biology. 2015;13:e1002198. doi: 10.1371/journal.pbio.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoes RR. The Role of Exposure History on HIV Acquisition: Insights from Repeated Low-dose Challenge Studies. PLoS Computational Biology. 2012;8:e1002767. doi: 10.1371/journal.pcbi.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoes RR, Longini IM, Feinberg MB, Staprans SI. Preclinical Assessment of HIV Vaccines and Microbicides by Repeated Low-Dose Virus Challenges. PLoS Medicine. 2005;2:e249. doi: 10.1371/journal.pmed.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wang X, Zhang H, Tang X, Li M, Yao J, Jin X, Ertl HCJ, Zhou D. Repeated Low-Dose Influenza Virus Infection Causes Severe Disease in Mice: a Model for Vaccine Evaluation. Journal of Virology. 2015;89:7841–7851. doi: 10.1128/JVI.00976-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekreijse D, Bouma A, Stegeman JA, Koch G, de Jong MCM. The effect of inoculation dose of a highly pathogenic avian influenza virus strain H5N1 on the infectiousness of chickens. Veterinary Microbiology. 2011;147:59–66. doi: 10.1016/j.vetmic.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Stranford SA, Skurnick J, Louria D, Osmond D, Chang SY, Sninsky J, Ferrari G, Weinhold K, Lindquist C, Levy JA. Lack of infection in HIV-exposed individuals is associated with a strong CD8+ cell noncytotoxic anti-HIV response. Proceedings of the National Academy of Sciences. 1999;96:1030–1035. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydenstricker KV, Dhondt AA, Hawley DM, Jennelle CS, Kollias HW, Kollias GV. Characterization of Experimental Mycoplasma gallisepticum Infection in Captive House Finch Flocks. Avian Diseases. 2006;50:39–44. doi: 10.1073/pnas.101858010. [DOI] [PubMed] [Google Scholar]
- Sydenstricker KV, Dhondt AA, Ley DH, Kollias GV. Re-Exposure of Captive House Finches that Recovered from Mycoplasma Gallisepticum Infection. Journal of Wildlife Diseases. 2005;41:326–333. doi: 10.1073/pnas.101858010. [DOI] [PubMed] [Google Scholar]
- Tate AT. A general model for the influence of immune priming on disease prevalence. Oikos. 2017;126:350–360. doi: 10.1111/oik.03274. [DOI] [Google Scholar]
- Thomason CA, Leon A, Kirkpatrick LT, Belden LK, Hawley DM. Eye of the Finch: characterization of the ocular microbiome of house finches in relation to mycoplasmal conjunctivitis. Environmental Microbiology. 2017 doi: 10.1073/pnas.101858010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidbury HJ, Best A, Boots M. The epidemiological consequences of immune priming. Proceedings Biological sciences / The Royal Society. 2012;279:4505–12. doi: 10.1098/rspb.2012.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidbury HJ, Pedersen AB, Boots M. Within and transgenerational immune priming in an insect to a DNA virus. Proceedings Biological sciences / The Royal Society. 2011;278:871–6. doi: 10.1098/rspb.2010.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms R, Colegrave N, Chan BH, Read AF. The effect of parasite dose on disease severity in the rodent malaria Plasmodium chabaudi. Parasitology. 2001;123:1–11. doi: 10.1017/S0031182001008083. [DOI] [PubMed] [Google Scholar]
- Vandenberg JD, Ramos M, Altre JA. Dose-Response and Age- and Temperature-Related Susceptibility of the Diamondback Moth (Lepidoptera: Plutellidae) to Two Isolates of Beauveria bassiana (Hyphomycetes: Moniliaceae) Environmental Entomology. 1998;27:1017–1021. doi: 10.1093/ee/27.4.1017. [DOI] [Google Scholar]
- Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:338–42. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Prince JE, Brayton CF, Shah C, Zeve D, Gregory SH, Smith CW, Ballantyne CM. Host Resistance of CD18 Knockout Mice against Systemic Infection with Listeria monocytogenes. Infection and Immunity. 2003;71:5986–5993. doi: 10.1128/IAI.71.10.5986-5993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A, Antia R, Regoes RR. How do pathogen evolution and host heterogeneity interact in disease emergence? Proceedings of the Royal Society B: Biological Sciences. 2006;273:3075–3083. doi: 10.1098/rspb.2006.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]


