Abstract
Of the thousands of natural product antibiotics discovered to date, only a handful have been developed for the treatment of bacterial infection. The clinically unexploited majority likely include compounds with untapped potential as antibacterial drugs, and in view of the ever-growing unmet medical need for such agents, warrant systematic re-evaluation. Here we revisit the actinorhodins, a class that was first reported 70 years ago, but which remains poorly characterized. We show that γ-actinorhodin possesses many of the requisite properties of an antibacterial drug, displaying potent and selective bactericidal activity against key Gram-positive pathogens (including Staphylococcus aureus and enterococci), a mode of action distinct from that of other agents in clinical use, an extremely low potential for the development of resistance, and a degree of in vivo efficacy in an invertebrate model of infection. Our findings underscore the utility of revisiting unexploited antibiotics as a source of novel antibacterial drug candidates.
Introduction
The growing prevalence of antibiotic resistance in pathogenic bacteria is severely eroding our ability to manage bacterial infection1. Central to an effective response to this problem will be the development of novel antibacterial drugs that display activity against bacteria resistant to existing antibiotics. Unfortunately, progress towards this end has been hindered by the extremely challenging nature of antibacterial drug discovery; the field is now 30 years into a ‘Discovery Void’, from which no novel drug class effective against the problematic ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) has emerged to reach the clinic2.
A strategy that we have been pursuing with a view to identifying new antibacterial drug candidates involves systematically revisiting known classes of natural product antibiotics that have not to date been exploited for the treatment of bacterial infection3. The underpinning hypothesis is that amongst the c. 3000 antibiotics discovered to date4 - of which only a handful of classes have been deployed clinically – there may reside a wealth of compounds with untapped potential as antibacterial drugs. By initiating the discovery process with compounds about which something is already known, including the fact that they possess antibacterial activity, this approach offers a potential fast-track through the challenging early stages of discovery. Corroborating the viability of such a strategy there are several examples of clinically-useful antibiotics that were dismissed as drug candidates following their discovery, but which were later successfully developed for therapeutic use (daptomycin, fidaxomicin, pleuromutilins)2.
Actinorhodin (ACT), a dimeric benzoisochromanequinone antibiotic produced by Streptomyces coelicolor A3(2), was first reported in the late 1940’s5. It was subsequently shown that S. coelicolor produces a series of closely-related compounds in addition to ACT itself 6, which are collectively referred to here as the actinorhodins (ACTs) or the ACT class. Most studies on this compound appear to have employed mixtures of ACTs, since the methods routinely used to purify and characterize ACT are insufficient to resolve it from its close analogues6–9. ACT and other members of the class exhibit some intriguing characteristics; they are strongly pigmented and demonstrate litmus-like properties, undergoing a reversible colour change from blue under alkaline conditions to red in acid8, and are capable of acting as organocatalysts to drive oxidation reactions in vitro 7. ACTs lend colonies of S. coelicolor their characteristic blue colour, and since the presence of these compounds is therefore easily detected by eye, ACT production has been widely employed as a phenotypic marker in streptomycete research10,11.
Only limited information exists regarding the antibacterial properties of ACTs. Weak antibacterial activity has been reported against some Gram-positive bacteria, with an estimated MIC against S. aureus of 25–30 µg/ml12. However, the experiments from which these results derive, involving measuring zones of inhibition around transplanted plugs of agar on which S. coelicolor had been grown, mean that these values must be considered approximate at best. There is also no clear understanding of the antibacterial mode of action of ACTs, although more than one hypothesis has been put forward. Naturally-occurring quinones, such as ACT, can function as bioreductive DNA-alkylating agents13,14, a property that could potentially explain ACT’s antibacterial action. Alternatively, a recent study proposed that ACT exerts its antibacterial effect by catalysing the production of toxic levels of hydrogen peroxide7.
Here we sought to more comprehensively investigate the antibacterial properties of this class, with a view to evaluating the potential of the ACT pharmacophore for use in antibacterial chemotherapy. In doing so, we elected to work with a single, defined species of ACT to avoid the potential confounding associated with studying a mixture of ACT analogues that might vary considerably in respect of their antibacterial properties. This strategy therefore required that we first define a particular species of ACT for study. Proceeding on the basis that S. coelicolor likely produces ACT as a defensive molecule, we identified and purified the predominant secreted (extracellular) form of ACT with the aim of maximising our chances of working with the congener exhibiting the greatest antibacterial potency. The studies on this compound (γ-actinorhodin) that we present here reveal that the ACT pharmacophore in fact displays potent antistaphylococcal activity, and possesses many of the requisite properties of a useful antibacterial drug.
Results
Isolation and purification of γ-actinorhodin (γ-ACT)
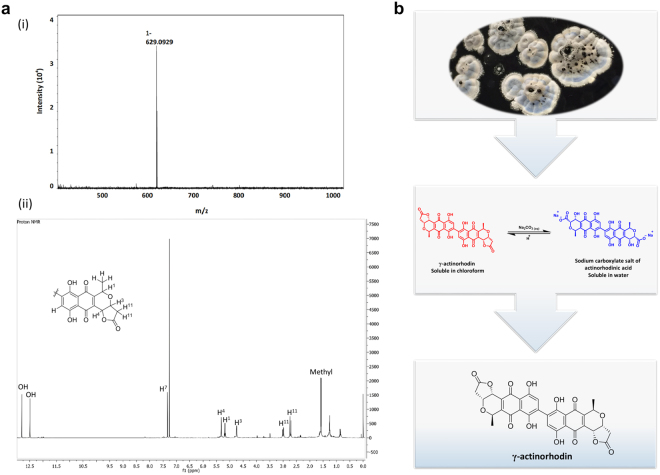
After 4–6 days’ growth on ISP 2 agar, S. coelicolor L646 turns the medium blue owing to ACT secretion. Preliminary TLC profiling of a crude extract of the agar revealed several pigments exhibiting the litmus-like properties of ACTs; the dominant, well-separated component at Rf 0.29 in dichloromethane-methanol 9:1 (v/v) was collected and characterized by 1H NMR spectroscopy and LC-MS, which identified it as γ-ACT (Fig. 1a). Subsequently, a scalable method was devised for the targeted isolation and purification of this compound (Fig. 1b). On the basis that the lactone substituent of γ-ACT makes this molecule substantially less polar compared with other ACTs, γ-ACT was extracted directly from agar using ethyl acetate, leaving behind the more polar congeners; 1H NMR analysis of the crude ethyl acetate extract confirmed the presence of γ-ACT alongside unknown impurities. We anticipated that at high pH (>10), γ-ACT would selectively ring-open to afford the hydroxycarboxylate (the salt of actinorhodinic acid), a compound with high water solubility. Washing the crude extract with aqueous sodium carbonate (pH 10.9) resulted in a blue aqueous layer, which was separated and then acidified with hydrochloric acid (6 M) to reform and precipitate the lactone. γ-ACT was triturated from methanol as a red solid in 19% yield. The identity and purity (>95%) of γ-ACT were confirmed using mass spectrometry, 1-D NMR, 2-D NMR, infrared spectroscopy and analytical HPLC (supplementary information).
Figure 1.
Identification of γ-ACT as the predominant ACT species secreted by S. coelicolor L646 using LC-MS (ai) and 1H NMR (aii), and overview of the γ-ACT purification strategy (b).
In vitro antibacterial activity of γ-ACT
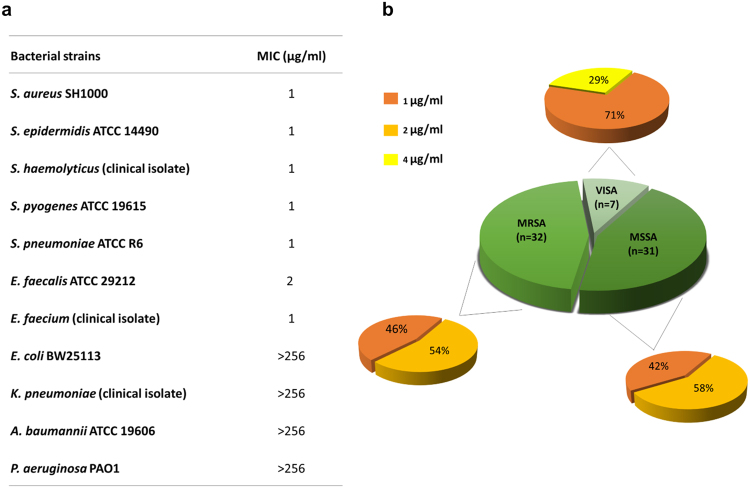
γ-ACT exhibited potent antibacterial activity against Gram-positive pathogens, displaying MIC values of 1–2 µg/ml against a cross-section of staphylococci, streptococci and enterococci (Fig. 2a). The antistaphylococcal activity of γ-ACT was further evaluated against a panel of 70 clinical isolates of S. aureus that included methicillin resistant S. aureus (MRSA) and vancomycin intermediate S. aureus (VISA) (Fig. 2b); the MIC90 of γ-ACT against these strains was 2 µg/ml (range of 1–4 µg/ml). Thus, the antistaphylococcal potency of γ-ACT is >10-fold greater than previously reported for compounds of this class12.
Figure 2.
In vitro antibacterial activity of γ-ACT. (a) MICs (µg/ml) of γ-ACT against a panel of Gram-positive and Gram-negative bacteria. (b) MICs (µg/ml) for γ-ACT against a collection (n = 70) of clinical isolates of S. aureus comprising strains that are methicillin sensitive (MSSA), methicillin resistant (MRSA), and vancomycin intermediate (VISA). The central pie chart shows a breakdown of these different strain types in the collection, whilst the outer charts indicate the proportion of isolates susceptible to different concentrations of γ-ACT.
γ-ACT did not demonstrate useful antibacterial activity against Gram-negative pathogens, displaying MICs of >256 µg/ml against representative Enterobacteriaceae and non-fermentative bacilli (Fig. 2). Antibiotics that lack activity against Gram-negative bacteria usually do so because they are unable to traverse the outer membrane (OM) and/or are subject to efflux15. To examine whether one or both of these phenomena account for the failure of γ-ACT to inhibit the growth of E. coli, γ-ACT susceptibility determinations were conducted against E. coli BW25113 in the presence of an OM-permeabilising agent (polymyxin B nonapeptide: PMBN)16, and against derivatives of BW25113 deleted for components of the major efflux transporter, AcrAB-TolC. Whilst no change in the antibacterial activity of γ-ACT was observed against strains independently deleted for acrA or tolC (MICs of >256 µg/ml), the γ-ACT MIC dropped to 16 µg/ml against BW25113 in the presence of PMBN. This result implies that the limited activity of γ-ACT against Gram-negative bacteria like E. coli is, at least in part, a consequence of limited ingress across the OM, and establishes that the target of γ-ACT is present in Gram-negative bacteria.
In time-kill experiments, γ-ACT was bactericidal at 4X MIC against rapidly growing cultures of S. aureus SH1000, mediating a reduction of ~3 log10 CFU/ml in viable cell numbers within an exponential phase population over 24 hours (Fig. 3). However, in common with most antibacterial drugs that exert a cidal action upon rapidly growing bacteria, γ-ACT showed negligible killing of non-growing (stationary phase) bacteria (data not shown).
Figure 3.

Time-dependent killing of exponential phase cultures of S. aureus SH1000 by γ-ACT and comparator agents at 4XMIC. The dotted line corresponds to a 3 log10 reduction in CFU/ml relative to cell density at time 0, and represents the threshold at which an antibiotic is considered to exhibit bactericidal activity. Values shown are the means of at least three independent experiments, with error bars representing standard deviations from the mean.
γ-ACT exhibits selective toxicity against bacteria
To provide a preliminary indication of prokaryotic selectivity, the activity of γ-ACT was evaluated against the eukaryotic microorganism, Candida albicans. Disk diffusion experiments revealed that γ-ACT exerts little activity against this organism when compared with that observed against S. aureus (Fig. 4a), and in MIC determinations, no inhibition of C. albicans was observed even at the highest concentration tested (256 μg/ml); these results imply that γ-ACT exhibits selectivity of action. Subsequently, we examined whether γ-ACT exerts any cytotoxic effects on mammalian cells by evaluating membrane integrity (monitored via release of lactate dehydrogenase) and viability (determined by measuring intracellular ATP levels) of human kidney-2 (HK-2) cells challenged with γ-ACT. γ-ACT exhibited no effect on HK-2 membrane integrity, even at the highest concentration tested (EC50 of >256 µg/ml) (Fig. 4b). However, a reduction in HK-2 viability was detected at higher concentrations of γ-ACT (EC50 of ~128 µg/ml) (Fig. 4c). Thus, whilst γ-ACT exhibited some cytotoxicity, this occurred at concentrations >50-fold higher than those required to achieve complete inhibition of bacterial growth, indicating that γ-ACT does indeed exhibit selective toxicity against bacteria.
Figure 4.
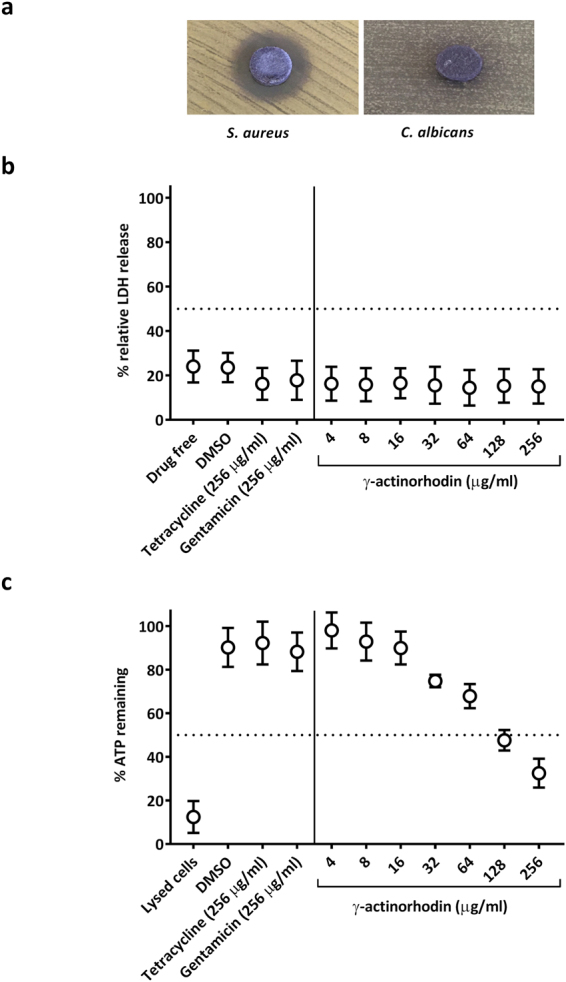
Prokaryotic selectivity and cytotoxicity of γ-ACT. γ-ACT inhibits the growth of the bacterium, S. aureus, but not that of the eukaryotic microorganism, C. albicans (a). The in vitro effect of γ-ACT and comparator agents on membrane integrity and viability of human kidney cells as determined by measuring release of lactate dehydrogenase (b) and cellular ATP levels (c), respectively. Values shown are the means of at least three independent experiments, with error bars representing standard deviations from the mean.
The mode of action (MOA) of γ-ACT involves collapse of the proton motive force and the generation of reactive oxygen species (ROS)
Most antibiotics in clinical use exert their antibacterial effects by inhibiting the biosynthesis of specific cellular macromolecules. To assess the effects of γ-ACT on macromolecular biosynthesis, we monitored incorporation of radiolabelled precursors into DNA, RNA, protein, fatty acid, and peptidoglycan, in S. aureus SH1000. At 4X MIC, γ-ACT caused substantial and comparable inhibition of all five biosynthetic pathways in 10 minutes (Fig. 5a). Non-specific inhibition of macromolecular biosynthesis is a characteristic of compounds that mediate their antibacterial effects through action on the cytoplasmic membrane17,18. We therefore sought to examine more directly whether γ-ACT exerts effects on the membrane of S. aureus SH1000. In experiments employing the fluorescent probe molecule, DiSC3 (5), γ-ACT at 4X MIC was found to cause substantial dissipation of the proton motive force (PMF) in 10 minutes, an effect comparable to that observed for the known membrane-active antibacterial agents, nisin and CTAB (Fig. 5b). In contrast to these control agents, however, γ-ACT did not cause physical perturbation of the membrane sufficient to permit leakage of ions (K+) from the cell (Fig. 5c).
Figure 5.
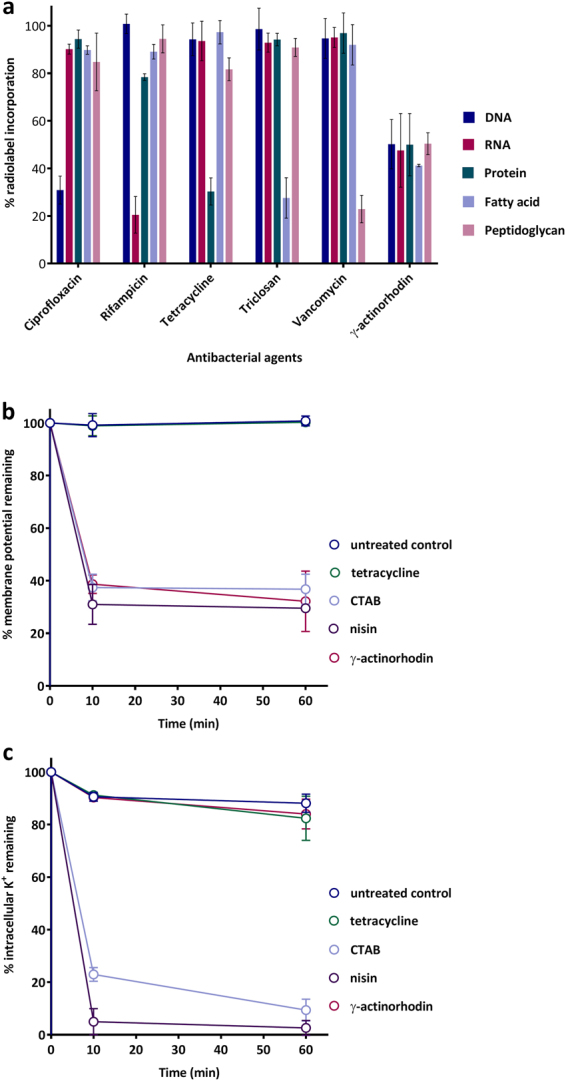
Antibacterial mode of action studies with γ-ACT. (a) Impact of a 10 minute challenge with γ-ACT and comparator agents at 4XMIC on macromolecular synthesis in S. aureus SH1000. (b and c) Effect of γ-ACT and comparator agents at 4XMIC on the function and integrity of the cytoplasmic membrane of S. aureus SH1000, as measured respectively in (b) with the PMF-responsive probe, DiSC3(5), and in (c) by monitoring leakage of K+ ions from cells. Values shown are the means of at least three independent experiments, with error bars representing standard deviations from the mean.
ACTs are redox-active agents that have recently been reported to catalyse oxidation reactions in vitro, an observation that led those who reported it to propose that the antibacterial properties of this class might result from production of toxic levels of hydrogen peroxide7. In potential support of this idea, these investigators described modest restoration of staphylococcal growth in the presence of ACT when cultures were supplemented with catalase7. We took an orthogonal approach to explore a potential role for reactive oxygen species (ROS) in the antibacterial MOA of γ-ACT, examining whether strains of S. aureus defective in key components of the ROS protection response exhibit greater susceptibility to the inhibitory action of the antibiotic (Table 1). No change in the MIC of γ-ACT was observed against a derivative of S. aureus SH1000 (KS100) lacking the major catalase enzyme, KatA, or against a strain (KC043) completely devoid of catalase activity as a consequence of lacking both KatA and the alkyl hydroperoxide reductase19. However, we observed a substantial (8-fold) increase in susceptibility to γ-ACT for a strain of SH1000 lacking the major superoxide dismutase (SOD) enzyme, SodA (Table 1), and a further 2-fold increase in γ-ACT susceptibility for a strain concurrently lacking the other staphylococcal SOD, SodM (Table 1). By contrast, none of these strains exhibited a significant (>2-fold) increase in susceptibility to several comparator antibacterial agents, including those whose MOA involves action on the membrane (Table 1). Our results imply that superoxide, but not hydrogen peroxide, plays an important role in mediating the antibacterial effect of γ-ACT.
Table 1.
Antibacterial activity of γ-ACT and comparator agents against S. aureus SH1000 and derivatives lacking enzymes important in the detoxification of ROS.
| Minimum inhibitory concentration (µg/ml) | ||||||
|---|---|---|---|---|---|---|
| γ-ACT | DAP | CTAB | VAN | CIP | RIF | |
| SH1000 | 1 | 1 | 1 | 1 | 0.25 | 0.016 |
| KS100 | 1 | 1 | 1 | 1 | 0.12 | 0.016 |
| KC043 | 1 | 1 | 1 | 1 | 0.25 | 0.016 |
| MHKA | 0.12 | 1 | 1 | 1 | 0.12 | 0.016 |
| MHKM | 1 | 1 | 1 | 1 | 0.12 | 0.016 |
| MHKAM | 0.06 | 0.5 | 1 | 1 | 0.12 | 0.008 |
γ-ACT: γ-actinorhodin, DAP: daptomycin, CTAB: cetyltrimethylammonium bromide, VAN: vancomycin, CIP: ciprofloxacin, RIF: rifampicin.
Inability to select resistance to γ-ACT in vitro
To evaluate the propensity for γ-ACT to select resistance, saturated cultures of S. aureus SH1000 were plated onto agar containing γ-ACT at multiples (4X-128X) of the (broth microdilution) MIC. Confluent growth was observed on agar containing up to 32X MIC γ-ACT, an observation that was subsequently explained by the finding that the antibacterial activity of γ-ACT becomes attenuated upon incorporation into agar (established by susceptibility testing using agar dilution; data not shown). A small number of colonies appeared on agar containing 64–128X MIC of γ-ACT after 48 hours’ incubation; however, these colonies were found to retain full susceptibility to γ-ACT in MIC determinations. We subsequently examined whether resistance to γ-ACT could be selected upon extended subculture in the presence of the compound. Serial passage of S. aureus SH1000 with γ-ACT was performed using the extended gradient MIC method20 that selects for resistance at both sub- and supra-MIC antibiotic concentrations in the same experiment, and may be considered to offer ‘optimal’ selection to favour the emergence of resistance. No reduction in γ-ACT susceptibility was observed in SH1000 over 20 days’ passage in the presence of the compound (Fig. 6). By contrast, over the same time period we recorded a substantial reduction (16-fold) in susceptibility to the comparator antibiotic, daptomycin, a drug that is generally considered to have low resistance potential.
Figure 6.
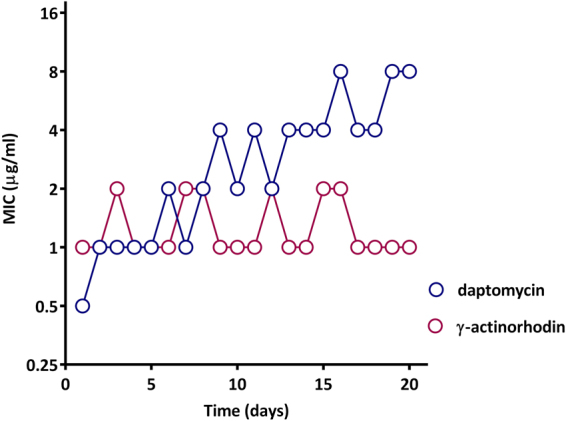
Resistance studies with γ-ACT. S. aureus SH1000 was serially-passaged in the presence of γ-ACT or the comparator compound (daptomycin) for 20 days. The data shown are representative of 3 independent experiments.
Safety and antibacterial efficacy in vivo
In vivo studies employed larvae of the greater waxmoth (Galleria mellonella). In initial experiments, we established that γ-ACT does not exert overt toxic effects in vivo, with 80% (8/10) G. mellonella larva surviving for at least 48 hours’ post-administration of γ-ACT at 50 mg/kg (the highest concentration tested), and 100% (10/10) surviving following administration of γ-ACT at 20 mg/kg. Subsequently, we established infection in G. mellonella using the virulent MRSA USA300 strain, JE2, and used this model to evaluate the in vivo efficacy of γ-ACT. Following infection at an inoculum of 107 CFU, all of the insects died 96 hours’ post infection (pi) in the absence of antibiotic treatment. Analysis of survival curves (Fig. 7) using the Log-rank test for trend showed a global statistical significance (P < 0.0001). Subsequent pairwise analysis indicated that administration of vancomycin (20 mg/kg) 30 minutes pi significantly enhanced G. mellonella survival (P < 0.0001) when compared to the no-treatment control, with 50% of the insects alive at 120 hours pi. We observed modest but significant (P = 0.0003) protection following administration of γ-ACT compared to no-treatment control at low concentrations 30 minutes pi; γ-ACT at 1 mg/kg rescued 20% of the insects as judged 120 hours’ pi (Fig. 7). At a higher concentration of γ-ACT (5 mg/kg), the protective effect was not significant (P = 0.2365), with survival at 120 hours pi dropping to ~3% (Fig. 7). At higher concentrations still (20 and 50 mg/kg), administration of γ-ACT to infected G. mellonella not only failed to yield a protective effect, but actually appeared to act in concert with the infection to kill the insects; all infected insects treated with γ-ACT at these concentrations were dead by 72 hours (i.e. before those infected insects not treated with γ-ACT) (data not shown). Thus, whilst γ-ACT does display modest antibacterial efficacy in vivo when administered at low concentration, this effect is lost at higher concentrations.
Figure 7.
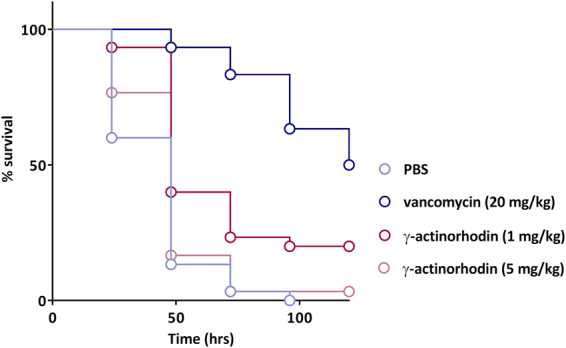
Effect of γ-ACT on survival of G. mellonella infected with MRSA. Groups of 30 larvae were challenged with S. aureus JE2 (1 × 107 CFU) and then treated with γ-ACT at concentrations of 1 or 5 mg/kg or with vancomycin at 20 mg/kg 30 mins post infection. The number of live vs dead larvae was determined every 24 h post-infection up to 120 h.
Discussion
We have described here the antibacterial properties of γ-ACT, and thereby provided the first evaluation of a member of the ACT class as a potential antibacterial drug candidate.
γ-ACT exhibits potent antibacterial activity that encompasses two of the ESKAPE pathogens (S. aureus and E. faecium), with MICs against members of these species comparable to those seen for antibacterial drugs in current clinical use18. As indicated above, the antibacterial potency of γ-ACT is substantially greater than that previously published for ACT; in contrast to the MIC of 1 µg/ml we recorded against our laboratory strain of S. aureus in a broth microdilution experiment, the original study that reported on the antibacterial activity of this class estimated an antistaphylococcal MIC of 25–30 µg/ml by agar diffusion12. Whilst there are several explanations that could account for this apparent discrepancy, we consider that it is likely predominantly due to the phenomenon we observed for γ-ACT, whereby the antibacterial activity becomes attenuated in agar. The weak activity originally reported for ACT12 probably explains why this class has not been further evaluated in respect of its therapeutic potential over the last 40 years, and it is intriguing to consider that other potentially interesting/useful antibiotic classes are languishing ‘obscured’ in the scientific literature owing to similar points of technicality.
The antibacterial mechanism of γ-ACT involves rapid dissipation of the PMF, prompting comprehensive shutdown of macromolecular biosynthesis, and ultimately, cell death. Several other antibacterial agents in use as drugs (daptomycin, telavancin), antiseptics (CTAB) or food preservatives (nisin) also possess an MOA that involves disruption of membrane energetics21–23. However, in contrast to γ-ACT, these agents additionally cause gross physical perturbation of the membrane, leading to detectable leakage of intracellular contents. Thus, the action of γ-ACT on the bacterial membrane is apparently distinct from that of other membrane-perturbing agents in use. Nor is the antibacterial action of γ-ACT restricted exclusively to effects at the membrane. The dramatic sensitization to γ-ACT observed in S. aureus strains lacking superoxide dismutases, enzymes that constitute the major cellular defence against superoxide, strongly implicates this radical in the MOA of γ-ACT. Sensitization to γ-ACT was predominantly associated with loss of SodA, an enzyme that has been linked specifically with protection against internally-generated oxidative stress in S. aureus 24, implying that γ-ACT prompts the generation of endogenous, rather than exogenous, superoxide. It seems probable that these two antibacterial mechanisms observed for γ-ACT (collapse of the PMF and the production of ROS) share a common root cause; both effects could be explained by γ-ACT-mediated interference with correct functioning of the electron transport chain, a process that ordinarily not only acts to generate the PMF, but which also represents the primary source of superoxide in the bacterial cell25. Thus, we speculate that γ-ACT mediates oxidative damage to one or more components of the electron transport chain, which in turn acts both to compromise the bacterium’s ability to maintain the PMF and yields a source of free electrons to drive the generation of superoxide.
Compounds that exert their antibacterial effects through action at the bacterial membrane – including those that act to interfere with membrane energetics - are a frequent occurrence in antibacterial drug discovery programmes18,26. Such agents are usually considered nuisance compounds in this context, since their action on biological membranes is almost invariably non-specific, and as a consequence, they lack prokaryotic selectivity2,18. γ-ACT, whilst exhibiting an effect on membrane energetics comparable to that mediated by such compounds, does however exhibit prokaryotic selectivity; the concentration of this compound required to produce a significant cytotoxic effect was at least 50X that required to completely inhibit bacterial growth. Future elucidation of the precise mechanism by which γ-ACT achieves this selectivity could potentially inform the generation of other membrane-active antibacterial drug candidates.
In view of the selective nature of γ-ACT, we evaluated the therapeutic potential of this compound in an invertebrate model of staphylococcal infection. γ-ACT at 1 mg/kg demonstrated a modest but significant protective effect in vivo, rescuing 20% of infected insects – a figure just less than half that seen (50%) for the established antibacterial drug, vancomycin. Whilst γ-ACT therefore appears to possess some therapeutic potential, further studies will be required to understand why the compound not only fails to improve survival of infected G. mellonella when administered at higher concentrations, but beyond a certain concentration, actually acts to reduce it. Potentially, whilst not overtly cytotoxic at the concentrations administered, γ-ACT may compromise the immune response in G. mellonella, which would in turn act to facilitate the progress of the infection. We note that several other compounds have been reported to increase the susceptibility of G. mellonella to infection, an effect that results from inhibition of haemocyte function27,28.
Whether such a phenomenon is also evident for γ-ACT in higher organisms remains to be established. However, even if that does transpire to be the case, that need not preclude γ-ACT from further consideration as a candidate topical agent to prevent and treat staphylococcal disease, or as a chemical starting point for the generation of derivatives with properties better suited for systemic application. This is particularly the case since γ-ACT otherwise possesses many of the requisite properties of a useful antibacterial drug18; in addition to potent and selective bactericidal activity, an MOA distinct from that of other antibacterial drugs in clinical use, and evidence for in vivo efficacy, γ-ACT exhibits extremely low resistance potential in vitro. We consider our findings to underscore the utility of revisiting unexploited antibiotics as a potential source of novel antibacterial drug candidates, and believe that a comprehensive re-evaluation of such compounds is now warranted.
Materials and Methods
Microorganisms and growth media
Laboratory strains of bacteria and yeast used in this study are listed in Table 2. Clinical isolates of S. aureus were part of a culture collection belonging to the Antimicrobial Research Centre, University of Leeds, UK. The ACT over-producer strain, Streptomyces coelicolor L646 (kindly provided by K. McDowall, University of Leeds), was used as the source of γ-ACT, and was propagated on ISP medium 2 (Difco) containing 50 µg/ml apramycin at 30 °C. Unless otherwise stated, experiments to evaluate the antibacterial properties of γ-ACT employed Staphylococcus aureus SH1000. Bacteria (with the exception of S. coelicolor) were routinely cultured in Mueller-Hinton broth (MHB) and on Mueller-Hinton agar (MHA) (Oxoid Ltd, Cambridge, UK) for 24 h at 37 °C, whilst Candida albicans was grown in lysogeny broth (LB) and on LB agar (Oxoid) for 48 hours at 35 °C.
Table 2.
Microorganisms used in this study.
| Strain | Description | References |
|---|---|---|
| Streptomyces coelicolor L646 | S. coelicolor M145 containing an integrated plasmid overexpressing wild-type atrA, which leads to hyper-production of actinorhodins | 33 |
| Staphylococcus aureus SH1000 | Standard laboratory strain - derivative of strain 8325-4, with a functional rsbU gene reinstated | 34,35 |
| S. aureus JE2 | Methicillin-resistant S. aureus USA300 strain | 36 |
| S. aureus KS100 | SH1000 deficient in catalase (katA::Tn917) | 19 |
| S. aureus KC043 | SH1000 deficient in alkyl hydroperoxide reductase and catalase (ahpC::tet, katA::Tn917) | 19 |
| S. aureus MHKA | SH1000 deficient in superoxide dismutase A (sodA::Tn917) | 37 |
| S. aureus MHKM | SH1000 deficient in superoxide dismutase M (sodM:: tet) | 37 |
| S. aureus MHKAM | SH1000 deficient in superoxide dismutases A and M (sodA::Tn917, sodM::tet) | 37 |
| Escherichia coli BW25113 | Derivative of E. coli K12 strain BD792 and parent strain of the Keio collection | 38,39 |
| E. coli BW25113ΔacrAB | BW25113 deleted for AcrAB | 38 |
| E. coli BW25113ΔtolC | BW25113 deleted for TolC | 38 |
| Candida albicans CA-6 | Clinical isolate | 40 |
Antibacterial agents and chemicals
Antibacterial agents were from Sigma-Aldrich (Poole, UK) with the following exceptions; ciprofloxacin (Alfa Aesar, Lancashire, UK), vancomycin (Cayman Chemical, Michigan, USA), daptomycin (Cubist Pharmaceuticals, Massachusetts, USA) and nisin (Duchefa Biochemie, Haarlem, Netherlands). Radiolabelled chemicals were from PerkinElmer (Waltham, Massachusetts, USA); unless otherwise stated, all other chemicals were from Sigma-Alrdich.
Extraction, purification and chemical characterization of γ-ACT
S. coelicolor L646 was grown on ISP 2 agar at 30 °C for 6 days. The agar was manually fragmented and the pigment extracted twice with ethyl acetate at 30 °C for an hour with vigorous shaking. The resulting extract was dried in vacuo, dissolved in chloroform (60 mL), and washed with aqueous sodium carbonate solution (pH 10.9, 60 ml × 3). The combined blue aqueous layer was acidified with hydrochloric acid (6 M), yielding a crude red pigment that was recovered by filtration. γ-ACT was then triturated from methanol (red precipitate, 125 mg, 19%). RF 0.29 (1% MeOH in dichloromethane). The identity and purity of γ-ACT were confirmed using mass spectrometry, 1-D NMR, 2-D NMR, infrared spectroscopy and analytical HPLC.
Evaluation of antimicrobial activity
Susceptibility testing with γ-ACT against bacteria was performed by broth microdilution in MHB according to CLSI guidelines29. Antifungal activity was assessed in essentially the same way, though used LB in place of MHB, and minimum inhibitory concentrations (MICs) were instead read after 48 hours’ incubation at 35 °C. In both antibacterial and antifungal susceptibility tests the MIC was defined as the lowest concentration of antibiotic that completely inhibited visible growth. To assess the role of the outer membrane in restricting entry of γ-ACT into E. coli, polymyxin B nonapeptide (PMBN; Sigma-Aldrich) was added to MIC determinations at a final concentration of 4 µg/ml16.
Time-kill studies on exponential-phase populations of S. aureus SH1000 were performed using standard methodology30, utilizing ~5 × 105 CFU/ml in MHB and antibiotics at 4X MIC. For time-kill studies on non-growing bacteria, stationary-phase cells were resuspended in spent medium at a density of ~5 × 105 CFU/ml. Following antibiotic challenge, bacterial viability was monitored by plating cultures onto MHA, and enumerating colonies after incubation at 37 °C for 18–24 h.
Cytotoxicity testing
Human Kidney 2 cells (ATCC CRL-2190) were obtained from the American Type Culture Collection and maintained in high-glucose Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 1% of penicillin-streptomycin combo solution and 10% fetal bovine serum (Sigma-Aldrich, Poole, UK). Cells were cultured at 37 °C in 5% CO2 saturated air, replacing the culture media every 48 hours. Cells were passaged upon reaching 80% confluency, and seeded in 96-well flat bottom plates at a density of 1.2 × 104 cells/well in 200 μl of DMEM medium without serum. After a further 24 hours’ culturing, cells were exposed to the antibiotic under test for 6 hours and cytotoxicity assessed by measuring lactate dehydrogenase leakage with the CytoTox-ONETM Homogenous Membrane Integrity Assay (Promega, USA), and by monitoring intracellular ATP levels using the Adenosine 5′-triphosphate bioluminescent somatic cell assay kit (Sigma-Aldrich, UK).
Mode of action studies
The effect of γ-ACT on macromolecular synthesis was determined in mid-exponential phase cultures (108 CFU/mL in LB) of S. aureus SH1000 by monitoring incorporation of radiolabelled precursors into TCA-precipitable material following a 10 minute challenge with the compound at 4X MIC17. The radioactive precursors [methyl-3H] thymidine (70–95 Ci/mmol), [5, 6–3H] uridine (31–56 Ci/mmol), [3,4-3H(N)] glutamine (20–50 Ci/mmol), [1,2-14C] acetic acid (45-60 mCi/mmol), and 14C (U)-glycine (>80 mCi/mmol) were used to monitor the synthesis of DNA, RNA, protein, fatty acid and peptidoglycan, respectively, and were added to bacterial cultures 10 minutes prior to the addition of antibiotics. The effect of γ-ACT on the proton motive force was assessed using 3, 3’-diprophlthiadicarbocyanine iodide [DiSC3(5)] (Invitrogen, Paisley, UK)23, whilst physical damage to the membrane was evaluated by measuring leakage of potassium ions, as previously described22.
Resistance studies
Initial attempts to select mutants exhibiting reduced susceptibility to γ-ACT involved plating saturated (>109 CFU/ml) cultures of S. aureus SH1000 onto MHA containing (4X-128X) MIC of γ-ACT, and incubation for 48 hours at 37 °C. To select resistance by serial passage, S. aureus SH1000 cells were continuously exposed to γ-ACT for 20 days using the extended gradient MIC method20.
Safety and efficacy studies in Galleria mellonella
G. mellonella (greater wax moth) larvae from Livefood UK Ltd (Rooks Bridge, Somerset, UK) were maintained on wood chips in the dark at 14 °C for up to 2 weeks. Bacterial infection and subsequent treatment of G. mellonella was performed on 30 larvae (3 groups of ten) per treatment using established methodology31, with antibiotic treatment of infection undertaken as described32. Briefly, 10 µl volumes containing 1 × 107 CFU of MRSA strain JE2 were injected into the bottom left proleg of each individual larva. Antibiotics were subsequently administered in phosphate-buffered saline (PBS) by injection 30 minutes post-infection, with PBS administered alone as a no-treatment control to account for the increase in liquid volume within the G. mellonella. Antibiotic concentrations achieved in G. mellonella were estimated on an average larva weight of 300 mg. The number of larvae alive/dead was calculated every 24 hours up to 120 hours when the experiment was halted, and the resulting data analysed using Prism Software Version 6 (Graphpad, San Diego, CA, USA). Kaplan-Meier curves were plotted and a global statistical comparison carried out using the Log-Rank test for trend; individual treatments were compared with the no-treatment (PBS) control using the Mantel-Cox (Log-Rank) statistical test. The effectiveness of a particular treatment was considered significant if P < 0.05.
Data availability
The majority of the data generated or analysed during this study are included in this published article and its Supplementary Information files; data not shown are available from the corresponding author upon request.
Electronic supplementary material
Acknowledgements
This work was supported by the Medical Research Council (grant number MR/L000369/1) awarded to A.J.O., and by sponsorship from the Saudi Government and the King Abdulaziz University in Jeddah to support N.M.N. S.F. was supported by the Engineering and Physical Sciences Research Council. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions
The study was directed by C.P.R., C.M.R. and A.J.O. S.F. and N.M.N. purified and characterized γ-ACT, with input from C.M.R. and R.F.S. N.M.N. performed the biological evaluation of γ-ACT, with the exception of the insect studies that were performed by C.H. and M.W. with guidance from J.M.S. The manuscript was drafted by N.M.N. and A.J.O., with contributions from all of the authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17232-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. https://amr-review.org/Publications.html (2016).
- 2.Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ooi N, O’Neill AJ. Revisiting unexploited antibiotics in search of new antibacterial drug candidates: the case of MSD-819 (6-chloro-2-quinoxalinecarboxylic acid 1,4-dioxide) J Antibiot (Tokyo) 2017;70:317–319. doi: 10.1038/ja.2016.140. [DOI] [PubMed] [Google Scholar]
- 4.Lewis K. Platforms for antibiotic discovery. Nature Reviews Drug Discovery. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 5.Brockmann H, Pini H. Actinorhodin, ein roter Farbstoff aus Actinomyceten. Naturwissenschaften. 1947;34:190. doi: 10.1007/BF00602581. [DOI] [PubMed] [Google Scholar]
- 6.Bystrykh LV, et al. Production of actinorhodin-related “blue pigments” by Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:2238–2244. doi: 10.1128/jb.178.8.2238-2244.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiyama T, Hashimoto Y, Kusakabe H, Kumano T, Kobayashi M. Natural low-molecular mass organic compounds with oxidase activity as organocatalysts. Proc Natl Acad Sci USA. 2014;111:17152–17157. doi: 10.1073/pnas.1417941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockmann H, Hieronymous E. Uber Actinomycetenfarbstoffe, V: Zur Konstitution des Actinorhodins. Chem Ber. 1955;88:1379–1390. doi: 10.1002/cber.19550880908. [DOI] [Google Scholar]
- 9.Brockmann H, Loeschcke V. Actinomycetenfarbstoffe IV: Uber die Konstitution des Actinorhodins und die Isolierung des Proto-actinorhodins. Chem Ber. 1955;88:778–788. doi: 10.1002/cber.19550880607. [DOI] [Google Scholar]
- 10.Sherman DH, Kim ES, Bibb MJ, Hopwood DA. Functional replacement of genes for individual polyketide synthase components in Streptomyces coelicolor A3(2) by heterologous genes from a different polyketide pathway. J Bacteriol. 1992;174:6184–6190. doi: 10.1128/jb.174.19.6184-6190.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosla C, Ebert-Khosla S, Hopwood DA. Targeted gene replacements in a Streptomyces polyketide synthase gene cluster: role for the acyl carrier protein. Mol Microbiol. 1992;6:3237–3249. doi: 10.1111/j.1365-2958.1992.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 12.Wright LF, Hopwood DA. Actinorhodin is a chromosomally-determined antibiotic in Streptomyces coelicolor A3(2) J Gen Microbiol. 1976;96:289–297. doi: 10.1099/00221287-96-2-289. [DOI] [PubMed] [Google Scholar]
- 13.Moore HW. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977;197:527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- 14.Moore HW, Czerniak R. Naturally occurring quinones as potential bioreductive alkylating agents. Med Res Rev. 1981;1:249–280. doi: 10.1002/med.2610010303. [DOI] [PubMed] [Google Scholar]
- 15.Randall CP, Mariner KR, Chopra I, O’Neill AJ. The target of daptomycin is absent from Escherichia coli and other Gram-negative pathogens. Antimicrobial Agents & Chemotherapy. 2013;57:637–639. doi: 10.1128/AAC.02005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva B, O’Neill AJ, Miller K, Stubbings W, Chopra I. Antistaphylococcal activity and mode of action of clofazimine. J. Antimicrob. Chemother. 2004;53:435–440. doi: 10.1093/jac/dkh114. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill AJ, Chopra I. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opinion on Investigational Drugs. 2004;13:1045–1063. doi: 10.1517/13543784.13.8.1045. [DOI] [PubMed] [Google Scholar]
- 19.Cosgrove K, et al. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:2137–2145. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill AJ, Miller K, Oliva B, Chopra I. Comparison of assays for detection of agents causing membrane damage in Staphylococcus aureus. J Antimicrob Chemother. 2004;54:1127–1129. doi: 10.1093/jac/dkh476. [DOI] [PubMed] [Google Scholar]
- 22.Higgins DL, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1127–1134. doi: 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs JK, Miller K, O’Neill AJ, Chopra I. Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J Antimicrob Chemother. 2008;62:1003–1008. doi: 10.1093/jac/dkn321. [DOI] [PubMed] [Google Scholar]
- 24.Clements MO, Watson SP, Foster SJ. Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J Bacteriol. 1999;181:3898–3903. doi: 10.1128/jb.181.13.3898-3903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imlay JA, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 26.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 27.Banville N, Fallon J, McLoughlin K, Kavanagh K. Disruption of haemocyte function by exposure to cytochalasin b or nocodazole increases the susceptibility of Galleria mellonella larvae to infection. Microbes Infect. 2011;13:1191–1198. doi: 10.1016/j.micinf.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Fallon JP, Reeves EP, Kavanagh K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011;157:1481–1488. doi: 10.1099/mic.0.043786-0. [DOI] [PubMed] [Google Scholar]
- 29.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A9 (2012).
- 30.CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. CLSI Document M26-A (1999).
- 31.Wand ME, Muller CM, Titball RW, Michell SL. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol. 2011;11:11. doi: 10.1186/1471-2180-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peleg AY, et al. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother. 2009;53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towle, J. E. AtrA-mediated transcriptional regulation in Streptomyces secondary metabolite production. Ph.D. thesis, University of Leeds (unpublished) (2007).
- 34.Horsburgh MJ, et al. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill AJ. Staphylococcus aureus SH1000 and 8325-4: comparative genome sequences of key laboratory strains in staphylococcal research. Lett Appl Microbiol. 2010;51:358–361. doi: 10.1111/j.1472-765X.2010.02885.x. [DOI] [PubMed] [Google Scholar]
- 36.Fey PD, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4:e00537–00512. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149:2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 38.Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol2, 2006 0008 (2006). [DOI] [PMC free article] [PubMed]
- 39.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martel CM, et al. Identification and characterization of four azole-resistanterg3 mutants of Candida albicans. Antimicrob Agents Chemother. 2010;54:4527–4533. doi: 10.1128/AAC.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The majority of the data generated or analysed during this study are included in this published article and its Supplementary Information files; data not shown are available from the corresponding author upon request.