The prevalence of small cell lung cancer has declined in the U.S. as tobacco use has declined; however, current and former smokers are at risk of developing small cell lung cancer. This article focuses on current and emerging treatment options for small cell lung cancer.
Keywords: Chemoradiotherapy, Immunotherapy, Antibody drug conjugated, Clinical trials, Rovalpituzumab tesirine, Prophylactic cranial radiation
Abstract
The prevalence of small cell lung cancer (SCLC) has declined in the U.S. as the prevalence of tobacco use has declined. However, a significant number of people in the U.S. are current or former smokers and are at risk of developing SCLC. Routine histological or cytological evaluation can reliably make the diagnosis of SCLC, and immunohistochemistry stains (thyroid transcription factor‐1, chromogranin, synaptophysin, and CD56) can be used if there is uncertainty about the diagnosis. Rarely do patients present with SCLC amendable to surgical resection, and evaluation requires a meticulous workup for extra‐thoracic metastases and invasive staging of the mediastinum. Resected patients require adjuvant chemotherapy and/or thoracic radiation therapy (TRT), and prophylactic cranial radiation (PCI) should be considered depending on the stage. For limited‐stage disease, concurrent platinum‐etoposide and TRT followed by PCI is the standard. Thoracic radiation therapy should be started early in treatment, and can be given twice daily to 45 Gy or once daily to 60–70 Gy. For extensive‐stage disease, platinum‐etoposide remains the standard first‐line therapy, and the standard second‐line therapy is topotecan. Preliminary studies have demonstrated the activity of immunotherapy, and the response rate is approximately 10–30% with some durable responses observed. Rovalpituzumab tesirine, an antibody drug conjugate, has shown promising activity in patients with high delta‐like protein 3 tumor expression (approximately 70% of patients with SCLC). The emergence of these and other promising agents has rekindled interest in drug development in SCLC. Several ongoing trials are investigating novel agents in the first‐line, maintenance, and second‐line settings.
Implications for Practice.
This review will provide an update on the standard therapies for surgically resected limited‐stage small cell lung cancer and extensive‐stage small cell lung cancer that have been investigated in recent clinical trials.
摘要
由于吸烟率的下降, 美国的小细胞肺癌(SCLC)发病率已开始下降。然而, 在美国, 相当多的人目前或以往是吸烟者, 因而有患SCLC的风险。常规组织学或细胞学评价能够可靠地做出SCLC的诊断, 如果诊断不确定, 可以采用免疫组织化学染色(甲状腺转录因子‐1、嗜铬粒蛋白、突触素和CD56)。很少有SCLC患者接受手术切除, 评估时需要对胸腔外转移和纵隔侵袭性分期进行细致检查。已进行切除术的患者需要接受辅助化疗和/或胸部放疗(TRT), 并应根据分期考虑是否进行预防性颅脑放疗(PCI)。对于局限期疾病, 同时使用铂类‐依托泊苷和TRT并且随后采用PCI是标准疗法。治疗时应早期开始胸部放疗, 每天可给予患者两次放疗(至45Gy)或一次放疗(60‐70 Gy)。对于广泛期疾病, 铂类联合依托泊苷仍然是标准的一线疗法, 标准的二线疗法是拓扑替康。初步研究已经证明了免疫治疗的活性, 缓解率约为10‐30%, 在一些患者中观察到持久的缓解。抗体偶联药物Rovalpituzumab tesirine在高δ‐样蛋白3肿瘤表达的患者(SCLC患者约占70%)中表现出不错的活性。这些和其它潜在药物的出现重新激起了治疗SCLC药物开发的兴趣。几项正在进行的临床试验正在一线、维持和二线治疗背景下研究新药。
对临床实践的提示:本篇综述将提供关于最近临床试验中用于经手术切除的局限期小细胞肺癌和广泛期小细胞肺癌的标准疗法的最新情况。
Introduction
In the U.S., lung cancer is the leading cause of cancer mortality and the second most common cancer diagnosis among both men and women [1]. Small cell lung cancer (SCLC) accounts for approximately 10%–15% of new lung cancer cases, and given the prevalence of lung cancer, this represents a substantial patient population [2], [3]. Small cell lung cancer is closely associated with tobacco use, and the prevalence of SCLC has declined as the rate of tobacco use has declined [4]. However, the prevalence of current and former regular use of cigarettes among adults in the U.S. is 18.1% and 20.1%, respectively [5]. This represents a large population at risk of developing SCLC in the future. Most often, the two‐stage system is used, in which limited‐stage SCLC (LS‐SCLC) is defined as disease confined to a single, tolerable radiation port, and extensive‐stage SCLC (ES‐SCLC) is disease that has extended beyond a single tolerable port.
Diagnosis
Routine histological and cytological evaluation can reliably make the diagnosis of SCLC. The characteristics of SCLC include a high rate of mitoses, monomorphic cells, a high nucleus/cytoplasm ratio, and the presence of necrosis. The higher rate of mitoses distinguishes SCLC from other neuroendocrine tumors such as atypical or typical carcinoids [6]. Immunohistochemistry (IHC) can be used when the histologic features are equivocal or the pathologist wants increased confidence in the diagnosis [7]. The commonly used IHC stains include thyroid transcription factor‐1 (TTF‐1) or neuroendocrine differentiation (chromogranin, synaptophysin, CD56). Thyroid transcription factor‐1 is positive in approximately 90% of SCLC, abundant membranous staining of CD56 is present in 98% of SCLC, and focal or diffuse staining of synaptophysin is present in 100% of SCLC. Ki‐67 staining can distinguish SCLC and large cell neuroendocrine from carcinoid tumors in the presence of crush artifact [7]. The use of IHC stains improves agreement among pathologists, and Thunnissen et al. have developed a flow diagram that incorporates cytoplasm nucleus, architecture, mitosis rate, and IHC to assist in the diagnosis of neuroendocrine lung cancers [7].
The development of molecular profiling has revolutionized the treatment of non‐small cell lung cancer (NSCLC), and there has been interest in molecular profiling for SCLC. Comprehensive genomic profiling performed on 110 SCLC specimens revealed a very high rate of nonsynonymous mutations, and cytosine/guanine to adenine/thymine transversions were found in 28% of all mutations, a pattern consistent with prior history of heavy smoking [8]. Inactivating alterations of the tumor suppressor genes TP53 and RB1 were present in all but two cases. Alterations inactivating the NOTCH family of tumor suppressor genes occurred in 25% of the cases. The multiple simultaneous genomic alterations and the alterations that inactivate tumor suppressor genes make it unlikely a targeted therapy will be available for patients with SCLC in the near future.
The multiple simultaneous genomic alterations and the alterations that inactivate tumor suppressor genes make it unlikely a targeted therapy will be available for patients with SCLC in the near future.
Surgical Therapy
Patients with SCLC rarely present with disease amendable to surgical resection, and the principles of more advanced disease are applied to surgically resected patients. The premise is that SCLC is disseminated disease at the time of diagnosis and that adjuvant chemotherapy and/or radiation therapy have the potential to eradicate occult micrometastatic disease and improve distant and/or local control.
The management of patients with a known diagnosis of SCLC prior to surgical resection requires a staging workup for extrathoracic disease, and invasive mediational staging is recommended prior to considering surgical resection due to the variance between clinical and pathological staging [9], [10]. In the International Association for the Study of Lung Cancer staging program, 144 patients with SCLC were clinically node‐negative, and 14% were upstaged to N2 or greater after surgical resection [11]. Of cases with clinical N2 disease, 32% were downstaged to pathological N1 or less. In review of the National Cancer Data Base (NCDB), 477 patients were identified as clinical stage I SCLC, and pathological upstaging occurred in 25% of patients, the majority due to the detection of nodal involvement [12].
For patients seen after surgical resection, adjuvant chemotherapy and/or radiation therapy is recommended. A recent review of the NCDB identified 1,574 patients with pathologically stage T1–2N0M0 SCLC, and 954 patients underwent complete resection. The 5‐year overall survival (OS) for the entire cohort was 47% [13]. Of the resected patients, 354 (37.1%) received adjuvant chemotherapy and 190 received chemotherapy and radiation (19.9%). Compared with no adjuvant therapy, treatment with adjuvant chemotherapy with or without radiation was associated with improved 5‐year OS rate (52.7% vs. 40.4%, p < .01). After multivariable adjustment, analysis factors associated with improved OS were receipt of adjuvant chemotherapy (hazard ratio [HR] of 0.78, 95% confidence interval [CI], 0.63–0.95, p = .02) and chemotherapy with radiation to the brain (HR of 0.52, 95% CI, 0.36–0.70, p < .01). Retrospective analyses have revealed a survival to prophylactic cranial irradiation (PCI) in resected stage II and IV disease, but not in stage I disease [14], [15]. Importantly, a greater percentage of patients may be alive at 5–10 years and at risk for the late neurocognitive complications associated with PCI. Prophylactic cranial irradiation is at many times a clinical decision based on patient characteristics and preferences.
Limited‐Stage Small Cell Lung Cancer
Chemotherapy
Concurrent chemotherapy and thoracic radiation therapy (TRT) followed by PCI with curative intent is the standard therapy for LS‐SCLC. The standard chemotherapy combination is cisplatin and etoposide; however, some patients may not be candidates or be able to tolerate cisplatin. A retrospective analysis using the national Surveillance Epidemiology and End Results‐Medicare database of patients with LS‐SCLC revealed 565 patients; 219 (39%) received cisplatin and etoposide and 346 (61%) received carboplatin and etoposide [16]. The median age of patients was 72 years (range 66–80 years), reflecting the use of the Medicare database. In patients receiving cisplatin and etoposide, the median and 5‐year OS rate were 13.8 months and 10.2%, and for patients receiving carboplatin and etoposide, the median and 5‐year OS rate were 13.7 months and 10.9% (p = .51). A statistically significant difference in lung cancer‐specific survival was not observed (p = .91). Carboplatin and etoposide or cisplatin and etoposide are acceptable options for LS‐SCLC, and platinum and etoposide will remain the standard for the near future.
Thoracic Radiation Therapy
Most of the debate in treatment of LS‐SCLC is related to the timing and schedule of TRT. Several meta‐analyses have investigated the difference between early and late TRT, and the early initiation of thoracic radiation is associated with improved long‐term survival [17], [18], [19]. The benefit of early TRT is more pronounced when platinum‐based therapy is used, and when the radiotherapy is started and completed within 30 days. A prospective phase III trial investigated TRT starting with the first or third cycle of cisplatin and etoposide (n = 220), and the primary endpoint was noninferiority of the complete response [20], [21]. Patients assigned to the late initiation of TRT and those assigned to the early initiation of TRT had similar complete response rates (36.0% vs. 38.0%), which met the study's noninferiority criteria. When late TRT was compared with early TRT, a similar progression‐free survival (PFS; HR of 1.09, 95% CI, 0.80–1.48, p = .60; median 11.2 and 12.4 months, respectively) and a similar OS (HR of 0.93, 95% CI, 0.67–1.29, p = .69; median 24.1 and 26.8 months, respectively) were observed. The toxicity was similar between the two therapies, except patients assigned to the early compared with the late had a statistically significant higher rate or febrile neutropenia (21.6% vs. 10.2%, p = .02). For patients with limited symptoms, the goal should be to start chemotherapy and radiation therapy on the first cycle. However, many patients have significant symptoms or borderline performance status and treating with one or two cycles of chemotherapy and then integrating TRT into the second or third cycle is a reasonable treatment strategy.
Hyperfractionated TRT, defined as multiple doses of radiation therapy in a single day, continues to be an area of active investigation. There has been variability in the time TRT was initiated and in the radiation schedules in previous trials of hyperfractionated TRT. A phase III trial compared cisplatin and etoposide with TRT twice daily to 45 Gy over 3 weeks to cisplatin and etoposide with TRT once daily to 45 Gy over 5 weeks [22]. Patients assigned to the twice‐daily therapy had a statistically significant longer OS (Table 1). Compared with once‐daily therapy, twice‐daily therapy was associated with a higher rate of esophagitis and grade 3 esophagitis. The primary criticism of this trial is that the dose of TRT on the once‐daily arm was not an equivalent radiobiological dose to the dose on the twice‐daily arm. Another trial compared once daily TRT of 50.4 Gy with twice‐daily TRT of 48 Gy using a split‐course schedule with concurrent TRT (Table 1) [23]. Patients initiated cisplatin and etoposide, and after three cycles of cisplatin and etoposide, patients were randomized to either of the radiation schedules. This trial did not reveal a survival benefit for the twice‐daily TRT compared with the once‐daily TRT, and the twice‐daily TRT was associated with a statistically significant higher rate of grade ≥3 esophagitis. The criticisms of this trial have been the later initiation of the TRT and that the split‐course schedule on the twice‐daily arm is not optimal.
Table 1. Phase III trials of once‐daily thoracic radiation therapy compared towith twice daily in combination with cisplatin and etoposide.
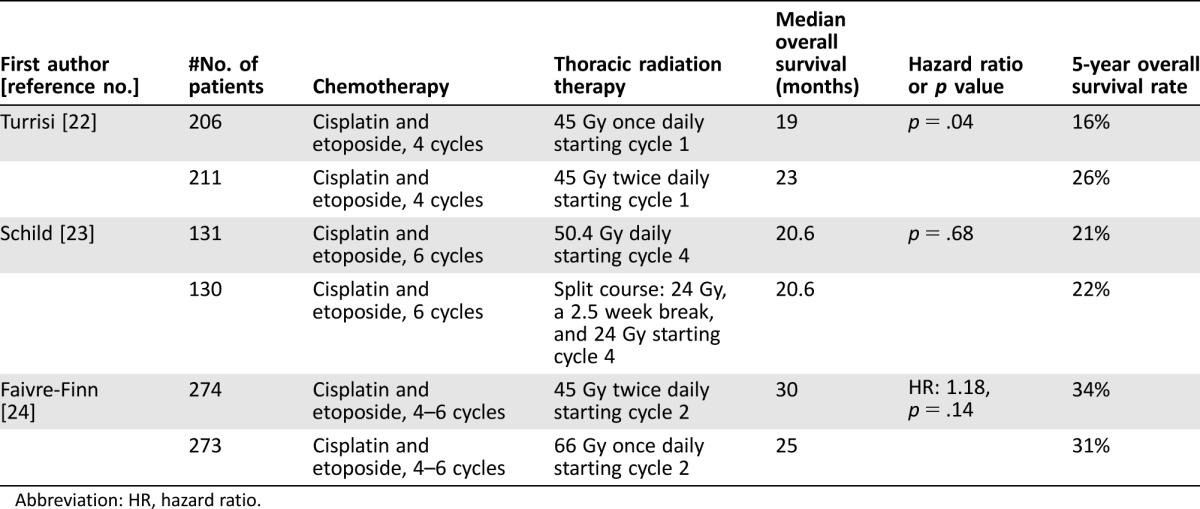
Abbreviation: HR, hazard ratio.
A recent phase III trial compared twice‐daily TRT of 45 Gy with once‐daily TRT of 66 Gy with concurrent cisplatin and etoposide. Thoracic radiation therapy started on the second cycle [24]. The primary endpoint was OS, and the trial did not reveal a statistically significant difference in OS (Table 1) or in the secondary endpoints of PFS, local progression, and distant progression. A statistically significant difference in the rate of grade 3 or 4 esophagitis and pneumonitis were not observed. The OS observed in both arms was longer than in previous studies, and the conclusion was that both treatment schedules are options. A phase III trial is investigating TRT of 45 Gy twice daily compared with 70 Gy once daily in combination with carboplatin or cisplatin and etoposide for four cycles (NCT00632853) [25]. The primary endpoint is OS and the trial will enroll 729 patients.
Outside the context of a clinical trial, the adoption of twice‐daily TRT has been low, in part due to logistical difficulties: an interval of at least 6 hours between fractions is required, and there were concerns about a higher rate of severe esophagitis. Currently, TRT schedules 45 Gy in 3 weeks (1.5 Gy twice daily) or once daily to 60–70 Gy are acceptable [25].
Extensive‐Stage Disease
First Line
The first‐line therapy for ES‐SCLC is platinum and etoposide, and carboplatin is the preferred agent based on similar efficacy and a better toxicity profile [26]. A phase III trial from Japan revealed a superior survival with cisplatin and irinotecan compared with cisplatin and etoposide, but subsequent U.S. trials did not reveal a survival benefit with cisplatin and irinotecan [27], [28], [29]. Phase III trials compared platinum and etoposide with platinum and topotecan, and these did not reveal a survival benefit with the combination of platinum and topotecan [30], [31]. A phase III trial compared cisplatin and amrubicin, an anthracycline derivative that is a topoisomerase II inhibitor, with cisplatin and irinotecan, and revealed noninferiority of the amrubicin arm [32]. Amurbicin is not available in the U.S. Platinum‐etoposide remains the standard first‐line chemotherapy for clinical trials and clinical practice.
Consolidation Radiotherapy
The use of radiation therapy on sites of residual disease to prevent or delay disease has been the focus of several clinical trials. A phase III trial compared TRT with no TRT in patients with ES‐SCLC who responded to first‐line chemotherapy [33]. Patients were assigned to TRT (30 Gy in 10 fractions) or no TRT; all patients received PCI. The primary endpoint was OS at 1 year. The OS at 1 year in the thoracic and no thoracic radiotherapy arms was 33% and 28%, respectively (HR of 0.84, 95% CI, 0.69–1.01, p = .066), and median OS was 8 months in both arms. In a secondary analysis of the OS rate at 2 years, the survival rate was higher in the TRT compared with the no TRT arm (13% and 3%, p = .004). In the TRT and no TRT arms, the median PFS rate was 4 and 3 months, respectively, and the 6‐month PFS rate was 24% and 20%, respectively (p = .001).
Patients in the TRT compared with the no TRT had a lower rate of intrathoracic disease progression. No significant differences in OS were observed in subgroups based on presence of intrathoracic disease at randomization, response to chemotherapy, performance status, or whether patients had ES‐SCLC due to distant metastases or intrathoracic disease. There were no significant differences in the rate of grade 3 or 4 toxicities in the two arms. A Quality of life (QOL) assessment was not included in the study. This study presents a clinical quandary because it appears that a small subset of patients benefit, but a method to identify that subset is lacking.
Radiation Therapy Oncology Group 0937 was a randomized phase II trial that enrolled patients who responded to chemotherapy and had one to four metastases and no brain metastases [34]. Patients were randomized to PCI only or PCI with consolidative radiation therapy, and the primary endpoint was 1‐year OS rate. A planned interim analysis revealed the study crossed the futility boundary after 97 patients had been randomized. The 1‐year OS rate in the PCI alone and the PCI and consolidative radiation therapy arms were 60.1% and 50.8%, respectively (p = .21). Time to any progression favored the PCI and consolidative arm (HR of 0.53, p = .01). Although this treatment strategy is appealing and appears to delay disease progression, it should not be used outside of a clinical trial.
Second Line
Patients who have disease progression after chemotherapy are classified as chemotherapy refractory, defined as disease progression within 60 or 90 days of completion of chemotherapy, or chemotherapy sensitive, defined as disease progression after 60 or 90 days of completion of chemotherapy. The historical purpose of these definitions is to select patients who are more likely to benefit from further chemotherapy. A phase III trial compared topotecan 1.5 mg/m2 daily for 5 days every 21 days with the combination of cyclophosphamide, adriamycin, and vincristine in patients with chemotherapy‐sensitive SCLC and revealed similar efficacy (Table 2) [35]. A subsequent phase III trial revealed similarity in the activity and tolerability of oral compared with intravenous topotecan, and another phase III trial compared oral topotecan to best supportive care alone and demonstrated the superiority of oral topotecan (Table 2) [36], [37]. A subset analysis of the phase III trial of oral topotecan compared with best supportive care in patients with disease progression ≤60 days revealed a survival benefit to topotecan. A phase III trial compared amrubicin with topotecan and revealed similar OS, ending the development of amrubicin in the U.S. [38].
Table 2. Selected phase III trials in second‐line small cell lung cancer.
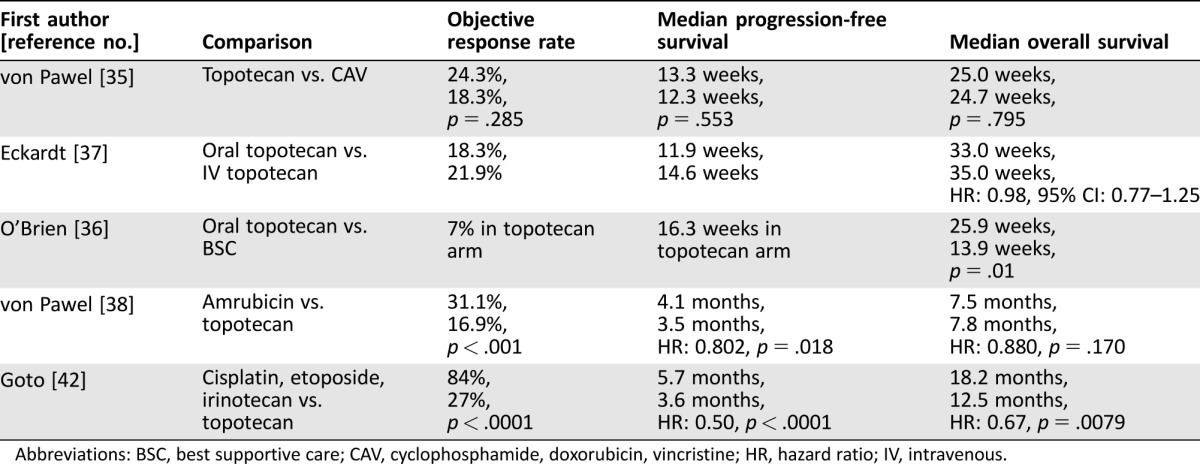
Abbreviations: BSC, best supportive care; CAV, cyclophosphamide, doxorubicin, vincristine; HR, hazard ratio; IV, intravenous.
These phase III trials established topotecan as the standard second‐line therapy, but there are concerns about the tolerability with the current schedule. Weekly topotecan at 4 mg/m2 on days 1, 8, and 15 every 28 days was investigated in a phase II trial; no responses were observed, and 27% of patients required a dose reduction [39]. A similar trial of weekly topotecan at 6 mg/m2 revealed a response rate of 8%, and high rate of hematologic toxicities [40]. These data suggest that weekly topotecan has limited activity and should not be used. The topotecan dose of 1.25 mg/m2 daily for 5 days has similar efficacy but lower grade 3 or 4 hematologic toxicity [41]. In clinical practice, many clinicians empirically reduce the daily dose of topotecan or reduce the schedule from 5 days to a shorter number of days.
A phase III study compared topotecan 1.0 mg/m2 on days 1–5 every 3 weeks with cisplatin (25 mg/m2 on days 1 and 8), etoposide (60 mg/m2 on days 1–3), and irinotecan (90 mg/m2 on day 8) with granulocyte colony factor stimulating support [42]. Patients were required to have disease progression ≥90 days after last chemotherapy. Overall survival was significantly longer in the combination chemotherapy compared with the topotecan arm (Table 2). Grade 3 or 4 febrile neutropenia was more common in the combination arm than in the topotecan arm (31% and 7%, respectively), as was grade 3 or 4 thrombocytopenia (41% and 28%, respectively). Dose reductions occurred in 11% of patients in the topotecan arm and in 50% of patients in the combination arm. The combination arm can be considered for good performance status patients with a prolonged treatment‐free interval. The rate of febrile neutropenia and need for frequent dose reductions with the combination arm are concerns.
Prophylactic Cranial Radiation
Prophylactic cranial radiation is the standard of care for patients with LS‐SCLC based on a meta‐analysis of individual patient data from 987 patients in 7 trials that revealed reduced risk of death with PCI compared with no PCI (HR of 0.84, 95% CI, 0.73–0.97, p = .01) [43]. This translated into an improvement in 3‐year OS from 15.3% to 20.7%. The cumulative risk of brain metastases at 3 years in the PCI and no PCI group was 33.3% and 58.6%, respectively. A phase III trial of 720 patients compared PCI at the standard dose of 25 Gy in 10 daily fractions with a higher dose of 36 Gy in 18 daily fractions of 2 Gy [44]. A significant difference in the rate of brain metastases at 2 years in the standard compared with higher dose PCI was not observed (HR of 0.80, 95% CI, 0.57–1.11, p = .18; rate of brain metastases of 29% and 23%, respectively). The 2‐year OS rate in the standard‐dose and higher‐dose arms was 37% and 42%, respectively (HR of 1.20, 95% CI, 1.00–1.44, p = .05). The lower OS in the higher dose group was related to increased cancer‐related mortality. The standard PCI dose is 25 Gy in 10 daily fractions. An analysis of the neurological and cognitive functions and QOL in the two groups over 3 years did not reveal a difference [45]. In both groups, a mild deterioration across time of communication deficit, weakness of legs, intellectual deficit, and memory was observed (all p < .005). At 3‐years, 44% of patients experienced a memory deficit of grade 1 and 8% experienced a memory deficit of grade 2 or more.
The role of PCI in patients with ES‐SCLC is more controversial, and two phase III trials have revealed contradictory results in OS. The first trial randomly enrolled patients with ES‐SCLC with a response to initial chemotherapy to PCI or no further therapy [46]. The primary endpoint was time to symptomatic brain metastases, and imaging was performed when predefined symptoms suggestive of brain metastases were present. Patients were not required to have brain imaging prior to randomization. Patients assigned to the PCI arm compared with no PCI arm had a lower risk of symptomatic brain metastases (Table 3). Patients assigned to the PCI arm compared with no PCI arm experienced a superior OS (HR of 0.68, 95% CI, 0.52–0.88, p = .003; median OS of 6.7 and 5.4 months, respectively). The 1‐year survival rate in the PCI and no PCI arms were 27.1% and 13.3%, respectively.
Table 3. Phase III trials of prophylactic cranial irradiation compared with no prophylactic cranial irradiation in extensive‐stage small cell lung cancer.
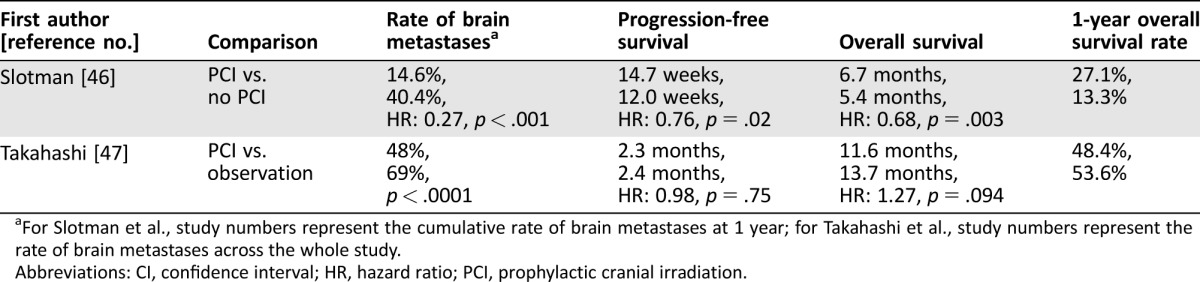
For Slotman et al., study numbers represent the cumulative rate of brain metastases at 1 year; for Takahashi et al., study numbers represent the rate of brain metastases across the whole study.
Abbreviations: CI, confidence interval; HR, hazard ratio; PCI, prophylactic cranial irradiation.
A second study investigated PCI compared with observation after initial response to chemotherapy, with the primary endpoint of OS [47]. This study required brain imaging prior to randomization and on regular intervals on the observation arm. This study was stopped after a planned interim analysis after 50% of patients had been enrolled (n = 163) because the trial had crossed the futility boundary. The OS inpatients assigned to the PCI and observation arm favored the observation arm (HR of 1.27, 95% CI, 0.96–1.68, p = .94; median OS of 11.6 and 13.7 months, respectively). In patients assigned to PCI and observation the PFS was similar, and the rate of brain metastases favored the PCI arm (Table 3).
In ES‐SCLC, PCI can reduce the rate of brain metastases, but the impact on PFS and OS is less clear. The second study, which required imaging prior to randomization and surveillance imaging for interval development of brain metastases, was similar to the practice patterns in the present study. Given the lack of OS or PFS benefit observed in this trial, the author does not recommend PCI for patients with ES‐SCLC.
Immunotherapy
Immunotherapy has revolutionized the care of patients with NSCLC, and preliminary studies in patients with ES‐SCLC are available. The biology and mechanism of action of immunotherapy in SCLC was recently reviewed in The Oncologist by Horn et al. [48]. A phase I/II multicohort investigated the activity of nivolumab alone and with ipilimumab in patients with ES‐SCLC that had progressed on chemotherapy [49]. Patients received nivolumab 3 mg/kg every 2 weeks or nivolumab 1 mg/kg and ipilimumab 3 mg/kg or nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks for four cycles followed by nivolumab 3 mg/kg every 2 weeks. The primary endpoint was objective response rate (ORR), and patients in the combination arms experienced a higher response rate and numerically longer PFS (Table 4). The Kaplan‐Meier PFS curves demonstrated a plateau similar to immunotherapy in other diseases. In the combination arms, three patients died from treatment‐related adverse events (myasthenia gravis, worsening renal failure, and pneumonitis). The rate of grade 3 or 4 treatment‐related adverse events in the nivolumab and combination arms was 13% and 19%–30%, respectively. When programmed death‐ligand 1 (PD‐L1) expression was assessed, 17% of sample had PD‐L1 expression of ≥1% and 5% had PD‐L1 expression of ≥5%. Responses were observed regardless of PD‐L1 expression, and in platinum‐sensitive and refractory disease. The National Comprehensive Cancer Network recommended nivolumab alone and with ipilimumab for patients who have relapsed 6 months or less from primary therapy (both category 2A) [10].
Table 4. Trials of immunotherapy in extensive‐stage small cell lung cancer [49], [50].
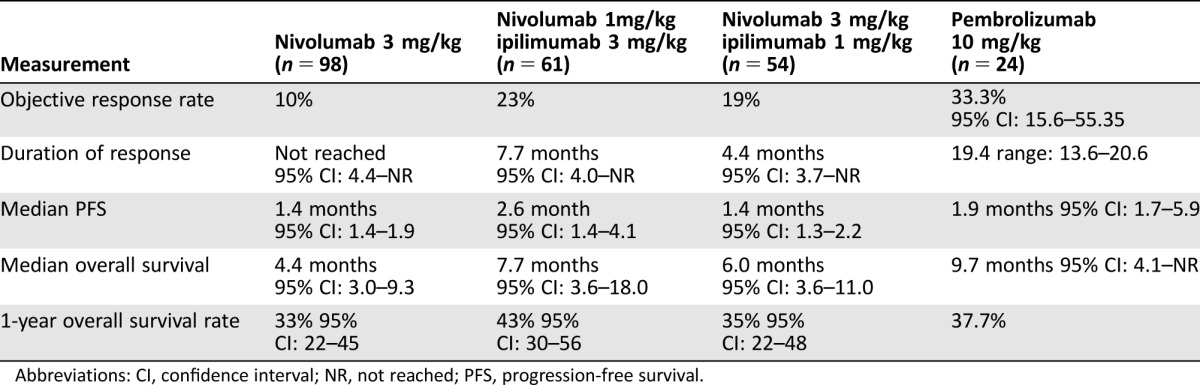
Abbreviations: CI, confidence interval; NR, not reached; PFS, progression‐free survival.
A single‐arm phase II trial investigated pembrolizumab 10 mg/kg every 2 weeks in patients with ES‐SCLC with PD‐L1 expression (defined as PD‐L1 ≥1%) [50]. Of the 147 evaluable tumor samples, 42 were PD‐L1 positive (28.6%), and 24 patients were treated on trial. The ORR and PFS are presented in Table 4. The median PFS is modest, but the duration of response was 19.4 months (95% CI, 3.6–20.0+). Of 24 patients, 2 experienced a grade 3–5 event, and autoimmune events observed were colitis, infusion site reaction, thyroiditis, and cytokine release syndrome. A phase III trial compared carboplatin and etoposide with ipilimumab or placebo in treatment‐naïve patients with ES‐SCLC, and the primary endpoint was OS [51]. Patients assigned to the ipilimumab‐containing arm compared with the placebo arm experienced a similar OS and ORR in both arms, and further investigation of chemotherapy in combination with cytotoxic T‐lymphocyte‐associated protein 4 agents is not warranted.
The lower rate of PD‐L1 expression and lack of an association with benefit to date suggest that PD‐L1 expression is unlikely to be a predictive marker. Immunotherapy is an option to consider for patients with a good performance status without significant comorbidities, especially if they experienced disease progression within 60 or 90 days given the limited efficacy of topotecan in this patient population. These patients should be encouraged to participate in ongoing trials.
Although the results of preliminary phase II studies are promising, the ongoing phase III studies will define the role of immunotherapy in ES‐SCLC as second‐line therapy. The development of biomarkers for selection of SCLC patients who are most likely to benefit from immunotherapy would benefit the field. The lower rate of PD‐L1 expression and lack of an association with benefit to date suggest that PD‐L1 expression is unlikely to be a predictive marker. Immunotherapy is an option to consider for patients with a good performance status without significant comorbidities, especially if they experienced disease progression within 60 or 90 days given the limited efficacy of topotecan in this patient population. These patients should be encouraged to participate in ongoing trials.
Rovalpituzumab Tesirine
Rovalpituzumab tesirine is an antibody drug conjugated with a humanized antibody against delta‐like protein 3 (DLL3) and a DNA cross‐linking agent [52]. A phase I trial investigated this agent in patients with SCLC and large‐cell neuroendocrine carcinoma. The dose‐limiting toxicities were grade 4 thrombocytopenia and liver test elevation, and the recommended dose for phase II trials is 0.3 mg/kg every 6 weeks. Seventy‐four previously treated SCLC patients were enrolled, and the most common grade ≥3 adverse events were thrombocytopenia (11%), pleural effusion (8%), and increased lipase (7%). In the intent‐to‐treat patient population, the response rate was 18%. Patients were assessed for DLL‐3 tumor overexpression by IHC using the cutoff ≥50%, and 67% had high expression. In patients with high DLL‐3 expression (n = 26), the ORR and median PFS by central radiologic review were 31% and 4.6 months, respectively (95% CI, 4.0–5.7). In contrast, in patients with DLL‐3 low expression, the ORR and median PFS were 0% and 2.2 months, respectively (95% CI, 4.0–5.7).
Conclusion
Ongoing Clinical Trials
The preliminary evidence of activity with immunotherapy and rovalpituzumab tesirine has led to a resurgence of interest in clinical trials in SCLC. Some of the trials are investigating the role of immunotherapy in combination with platinum and etoposide as first‐line therapy, after platinum and etoposide as maintenance therapy, and in the second‐line setting (Table 5). The activity of rovalpituzumab tesirine is being investigated in a third‐line patient population, a patient population without any approved therapies. The future development of rovalpituzumab tesirine will most likely be as part of combination therapy. The results of the current trials will define the role of immunotherapy and rovalpituzumab tesirine in ES‐SCLC, and trials of immunotherapy in LS‐SCLC have been initiated.
Table 5. Ongoing trials in extensive‐stage small cell lung cancer [23].
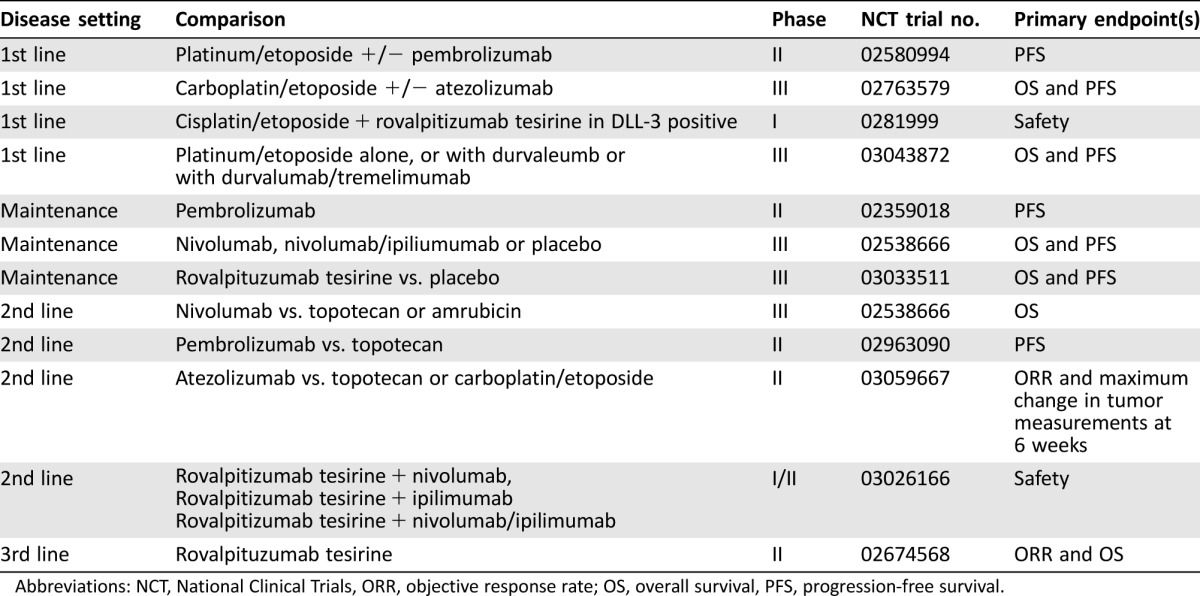
Abbreviations: NCT, National Clinical Trials, ORR, objective response rate; OS, overall survival, PFS, progression‐free survival.
Disclosures
The author indicated no financial relationships.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Govindan R, Page N, Morgensztern D et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 3. Houston KA, Henley SJ, Li J et al. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer 2014;86:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis DR, Check DP, Caporaso NE et al. US lung cancer trends by histologic type. Cancer 2014;120:2883–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kasza KA, Ambrose BK, Conway KP et al. Tobacco‐product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 2017;376:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Travis WD, Brambilla E, Nicholson AG et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–1260. [DOI] [PubMed] [Google Scholar]
- 7. Thunnissen E, Borczuk AC, Flieder DB et al. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. An international reproducibility study in a demanding set of cases. J Thorac Oncol 2017;12:334–346. [DOI] [PubMed] [Google Scholar]
- 8. George J, Lim JS, Jang SJ et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jett JR, Schild SE, Kesler KA et al: Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013;143(suppl 5):e400S–e419S. [DOI] [PubMed] [Google Scholar]
- 10.Available at https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed February 28, 2017.
- 11. Vallieres E, Shepherd FA, Crowley J et al. The IASLC Lung Cancer Staging Project: Proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049–1059. [DOI] [PubMed] [Google Scholar]
- 12. Thomas DC, Arnold BN, Rosen JE et al. Defining outcomes of patients with clinical stage I small cell lung cancer upstaged at surgery. Lung Cancer 2017;103:75–81. [DOI] [PubMed] [Google Scholar]
- 13. Yang CF, Chan DY, Speicher PJ et al. Role of adjuvant therapy in a population‐based cohort of patients with early‐stage small‐cell lung cancer. J Clin Oncol 2016;34:1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Yang H, Fu X et al: Prophylactic cranial irradiation for patients with surgically resected small cell lung cancer. J Thor Oncol 2017;12:347–353. [DOI] [PubMed] [Google Scholar]
- 15. Zhu H, Guo H, Shi F et al. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer 2014;86:334–338. [DOI] [PubMed] [Google Scholar]
- 16. Kim E, Biswas T, Bakaki P et al. Comparison of cisplatin/etoposide versus carboplatin/etoposide concurrent chemoradiation therapy for limited‐stage small cell lung cancer (LS‐SCLC) in the elderly population (age >65 years) using national SEER‐Medicare data. Pract Radiat Oncol 2016;6:e163–e169. [DOI] [PubMed] [Google Scholar]
- 17. Pijls‐Johannesma MC, De Ruysscher D, Lambin P et al. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev: 2005:CD004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried DB, Morris DE, Poole C et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited‐stage small‐cell lung cancer. J Clin Oncol 2004;22:4837–4845. [DOI] [PubMed] [Google Scholar]
- 19. De Ruysscher D, Pijls‐ Johannesma MC, Vansteenkiste J et al. Systematic review and meta‐analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited‐stage, small‐cell lung cancer. Ann Oncol 2006;17:543–552. [DOI] [PubMed] [Google Scholar]
- 20. Sun JM, Ahn YC, Choi EK et al. Phase III trial of concurrent thoracic radiotherapy with either first‐ or third‐cycle chemotherapy for limited‐disease small‐cell lung cancer. Ann Oncol 2013;24:2088–2092. [DOI] [PubMed] [Google Scholar]
- 21. Sun JM, Ahn YC, Choi EK et al. Phase III trial of concurrent thoracic radiotherapy with either first‐ or third‐cycle chemotherapy for limited‐disease small‐cell lung cancer (correction). Ann Oncol 2014;25:1672. [DOI] [PubMed] [Google Scholar]
- 22. Turrisi AT 3rd, Kim K, Blum R et al. Twice‐daily compared with once‐daily thoracic radiotherapy in limited small‐cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–271. [DOI] [PubMed] [Google Scholar]
- 23. Schild SE, Bonner JA, Shanahan TG et al. Long‐term results of a phase III trial comparing once‐daily radiotherapy with twice‐daily radiotherapy in limited‐stage small‐cell lung cancer. Int J Radiat Oncol Biol Phys 2004;59:943–951. [DOI] [PubMed] [Google Scholar]
- 24. Faivre‐Finn C, Snee M, Ashcroft L et al. Concurrent once‐daily versus twice‐daily chemoradiotherapy in patients with limited‐stage small‐cell lung cancer (CONVERT): An open‐label, phase 3, randomised, superiority trial. Lancet Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Available at https://clinicaltrials.gov/. Acccessed March 13, 2017.
- 26. Rossi A, Di Maio M, Chiodini P et al. Carboplatin‐ or cisplatin‐based chemotherapy in first‐line treatment of small‐cell lung cancer: The COCIS meta‐analysis of individual patient data. J Clin Oncol 2012;30:1692–1698. [DOI] [PubMed] [Google Scholar]
- 27. Noda K, Nishiwaki Y, Kawahara M et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002;346:85–91. [DOI] [PubMed] [Google Scholar]
- 28. Lara PN Jr, Natale R, Crowley J et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive‐stage small‐cell lung cancer: Clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanna N, Bunn PA Jr, Langer C et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive‐stage disease small‐cell lung cancer. J Clin Oncol 2006;24:2038–2043. [DOI] [PubMed] [Google Scholar]
- 30. Eckardt JR, von Pawel J, Papai Z et al. Open‐label, multicenter, randomized, phase III study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy‐naive patients with extensive‐disease small‐cell lung cancer. J Clin Oncol 2006;24:2044–2051. [DOI] [PubMed] [Google Scholar]
- 31. Fink TH, Huber RM, Heigener DF et al. Topotecan/cisplatin compared with cisplatin/etoposide as first‐line treatment for patients with extensive disease small‐cell lung cancer: Final results of a randomized phase III trial. J Thorac Oncol 2012;7:1432–1439. [DOI] [PubMed] [Google Scholar]
- 32. Satouchi M, Kotani Y, Shibata T et al. Phase III study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive‐disease small‐cell lung cancer: JCOG 0509. J Clin Oncol 2014;32:1262–1268. [DOI] [PubMed] [Google Scholar]
- 33. Slotman BJ, van Tinteren H, Praag JO et al. Use of thoracic radiotherapy for extensive stage small‐cell lung cancer: A phase 3 randomised controlled trial. Lancet 2015;385:36–42. [DOI] [PubMed] [Google Scholar]
- 34. Gore EM, Hu C, Sun A et al. NRG Oncology/RTOG 0937: Randomized phase 2 study comparing prophylactic cranial irradiation (PCI) alone to PCI and consolidative extracranial irradiation for extensive disease small cell lung cancer (ED‐SCLC). Int Journal Radiat Oncol Biol Phys 2016;94:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Pawel J, Schiller JH, Shepherd FA et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small‐cell lung cancer. J Clin Oncol 1999;17:658–667. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien ME, Ciuleanu TE, Tsekov H et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small‐cell lung cancer. J Clin Oncol 2006;24:5441–5447. [DOI] [PubMed] [Google Scholar]
- 37. Eckardt JR, von Pawel J, Pujol JL et al. Phase III study of oral compared with intravenous topotecan as second‐line therapy in small‐cell lung cancer. J Clin Oncol 2007;25:2086–2092. [DOI] [PubMed] [Google Scholar]
- 38. von Pawel J, Jotte R, Spigel DR et al. Randomized phase III trial of amrubicin versus topotecan as second‐line treatment for patients with small‐cell lung cancer. J Clin Oncol 2014;32:4012–4019. [DOI] [PubMed] [Google Scholar]
- 39. Shah C, Ready N, Perry M et al. A multi‐center phase II study of weekly topotecan as second‐line therapy for small cell lung cancer. Lung Cancer 2007;57:84–88. [DOI] [PubMed] [Google Scholar]
- 40. Spigel DR, Greco FA, Burris HA 3rd et al. A phase II study of higher dose weekly topotecan in relapsed small‐cell lung cancer. Clin Lung Cancer 2011;12:187–191. [DOI] [PubMed] [Google Scholar]
- 41. Huber RM, Reck M, Gosse H et al. Efficacy of a toxicity‐adjusted topotecan therapy in recurrent small cell lung cancer. Eur Respir J 2006;27:1183–1189. [DOI] [PubMed] [Google Scholar]
- 42. Goto K, Ohe Y, Shibata T et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second‐line treatment for patients with sensitive relapsed small‐cell lung cancer (JCOG0605): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2016;17:1147–1157. [DOI] [PubMed] [Google Scholar]
- 43. Aupérin A, Arriagada R, Pignon JP et al. Prophylactic cranial irradiation for patients with small‐cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–484. [DOI] [PubMed] [Google Scholar]
- 44. Le Péchoux C, Dunant A, Senan S et al. Standard‐dose versus higher‐dose prophylactic cranial irradiation (PCI) in patients with limited‐stage small‐cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99‐01, EORTC 22003‐08004, RTOG 0212, and IFCT 99‐01): A randomised clinical trial. Lancet Oncol 2009;10:467–474. [DOI] [PubMed] [Google Scholar]
- 45. Le Péchoux C, Laplanche A, Faivre‐Finn C et al. Clinical neurological outcome and quality of life among patients with limited small‐cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99‐01, EORTC 22003‐08004, RTOG 0212 and IFCT 99‐01). Ann Oncol 2011;22:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slotman B, Faivre‐Finn C, Kramer G et al. Prophylactic cranial irradiation in extensive small‐cell lung cancer. N Engl J Med 2007;357:664–672. [DOI] [PubMed] [Google Scholar]
- 47. Takahashi T, Yamanaka T, Seto T et al. Prophylactic cranial irradiation versus observation in patients with extensive‐disease small‐cell lung cancer: A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2017;18:663–671. [DOI] [PubMed] [Google Scholar]
- 48. Horn L, Reck M, Spigel DR. The future of immunotherapy in the treatment of small cell lung cancer. The Oncologist 2016;21:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonia SJ, Lopez‐Martin JA, Bendell J et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small‐cell lung cancer (CheckMate 032): A multicentre, open‐label, phase 1/2 trial. Lancet Oncol 2016;17:883–895. [DOI] [PubMed] [Google Scholar]
- 50. Ott P, Felip E, Hiret S et al. OA05.01 pembrolizumab in patients with extensive‐stage small cell lung cancer: Updated survival results from KEYNOTE‐028. J Thorac Oncol 2017;12:S259. [DOI] [PubMed] [Google Scholar]
- 51. Reck M, Luft A, Szczesna A et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive‐stage small‐cell lung cancer. J Clin Oncol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52. Rudin CM, Pietanza MC, Bauer TM et al. Rovalpituzumab tesirine, a DLL3‐targeted antibody‐drug conjugate, in recurrent small‐cell lung cancer: A first‐in‐human, first‐in‐class, open‐label, phase 1 study. Lancet Oncol 2017;18:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


