This article explores the characteristics of advanced gastric cancer patients receiving a third‐line chemotherapy, as well as factors that could be useful in identifying those patients who might benefit from a further line of treatment.
Keywords: Advanced gastric cancer, Third‐line chemotherapy, Prognostic factors, Overall survival, Progression‐free survival
Abstract
Background.
Second‐line therapy has consistently demonstrated survival benefit if compared with best supportive care; however, there is limited evidence whether further lines of treatment may improve the prognosis of advanced gastric cancer (AGC) patients.
Materials and Methods.
Starting from a real‐world cohort of 868 AGC patients, we retrospectively analyzed baseline parameters, tumor characteristics, and treatment data of those treated with at least three lines. Categorical features were described through cross‐tables and chi‐square test. We explored the impact of treatment intensity and progression‐free survival (PFS) experienced in previous lines on PFS and overall survival in third‐line by uni‐ and multivariate Cox regression models and described by Kaplan‐Meier estimator plot with log‐rank test.
Results.
Overall, 300 patients were included in the analysis. The most common site of primary tumor was gastric body; 45.3% of cancers had an intestinal histotype, 14% were human epidermal growth receptor 2 positive. In third‐line, 45.7% of patients received a single‐agent chemotherapy, 49.7% a combination regimen. Patients who had experienced a first‐line PFS ≥6.9 months had a better prognosis compared with those who had achieved a shorter one. Consistently, a second‐line PFS ≥3.5 months positively influenced the prognosis. Patients receiving a third‐line combination regimen had better outcomes compared with those treated with a single‐agent chemotherapy.
Conclusion.
Our real‐world study confirms that selected AGC patients may receive third‐line treatment. Longer PFS in previous lines or a more intense third‐line treatment positively influenced prognosis. Further efforts are warranted to define the best therapeutic sequences, and to identify the optimal candidate for treatment beyond second‐line.
Implications for Practice.
The benefit of third‐line treatment to advanced gastric cancer patients is controversial. This study depicts a real scenario of the clinical practice in Italy, confirming that a non‐negligible proportion of patients receive a third‐line therapy. Longer progression‐free survival in previous treatment lines or higher third‐line treatment intensity positively influenced prognosis. Including a large number of real‐world patients, this study provides information on third‐line treatment from the daily clinical practice; moreover, its results help in defining the best therapeutic sequence and offer some hints to select the optimal candidate for treatment beyond second‐line.
摘要
背景.研究一致表明二线治疗的生存获益大于最佳支持治疗;但鲜有证据表明进一步增加治疗线数可改善晚期胃癌(AGC)患者的预后。
材料与方法.本研究从含868例AGC患者的真实世界队列开始回顾性分析接受至少三线治疗患者的基线参数、肿瘤特征和治疗数据。通过交叉表和卡方检验描述分类特征。研究通过单因素和多因素Cox回归模型探索既往线数的治疗强度和无进展生存期(PFS)对三线治疗的PFS和总生存期影响, 并通过对数秩检验Kaplan‐Meier估计曲线描述。
结果.共分析300例患者。最常见的原发性肿瘤部位为胃体部, 肠组织型占45.3%, 人表皮生长因子受体2阳性占14%。在三线治疗中, 45.7%患者接受单药化疗, 49.7%采用联合给药方案。一线治疗PFS≥6.9个月的患者预后要好于PFS较短的患者。相应地, 二线治疗PFS≥3.5个月对预后有积极影响。接受三线联合给药方案的患者预后要好于单药化疗患者。
结论.此项真实世界研究证实经选的AGC患者可接受三线治疗。既往治疗线数的较长PFS或强度更大的三线治疗对预后有积极影响。但需进一步定义最佳治疗顺序并确定适合二线以上治疗的候选者。
对临床实践的提示:晚期胃癌患者能否从三线治疗中获益还存在争议。本研究描述意大利临床实践的真实情景, 证实有相当比例的患者接受三线治疗。既往治疗线数的较长PFS或强度更大的三线治疗对预后有积极影响。研究通过纳入大量的真实世界患者提供日常临床实践的三线治疗信息;而且, 其结果有助于定义最佳治疗顺序并为选择适合二线以上治疗的候选者提供一些线索
Introduction
Gastric cancer is one of the leading global causes of cancer death [1]. Despite the recent improvements in the knowledge of the underpinning biology and the significant advances in the therapeutic approaches, the prognosis of patients with metastatic gastric cancer remains largely disappointing [2]. Indeed, median overall survival (OS) is about 1 year in patients treated with standard chemotherapy regimens, usually represented by a combination of a platinum compound and a fluoropyrimidine with or without a third drug [3]. Even though a considerable proportion of patients may achieve a clinical remission or a disease stabilization through first‐line therapy, they eventually experience disease progression. After years of uncertainty [4], several randomized controlled trials have demonstrated the benefit of second‐line chemotherapy over best supportive care alone, providing significant improvements in OS and quality of life (QoL) amelioration. Actually, three chemotherapeutic drugs (docetaxel, paclitaxel, and irinotecan) [5], [6], [7] and three antiangiogenic compounds, namely ramucirumab [8], [9], apatinib [10], and regorafenib [11], were associated with a significant reduction in the risk of death in pretreated patients. The absolute survival gain of salvage therapy, however, is limited and ranges from 1.5 to 3 months. In the clinical practice, there are patients who still are in good clinical condition at the time of failure of second‐line chemotherapy. Although there is no clear evidence that further treatment lines may improve the prognosis, oncologists may sometimes choose to offer additional treatment to patients who still are in good performance status and are willing to receive subsequent active therapies. Given the increasing availability of active agents and the proportion of advanced gastric cancer (AGC) patients who benefit from second‐line therapies, it is conceivable that more patients could receive further active treatments beyond second‐line in the near future. The use of third‐line chemotherapy, however, raises controversial issues, including the need to identify the patient subset that may benefit most from a third‐line treatment, in order to avoid a potential use of costly and/or toxic agents in the last 3 months of life [12]. In this present study, we aimed at exploring the characteristics of AGC patients receiving a third‐line chemotherapy within our national practice, as well as detecting factors that could be useful in identifying those patients who might benefit in greater extent from a further line of treatment.
Materials and Methods
This is a multicenter, retrospective study involving 19 Italian oncology departments, homogeneously located throughout the country. Medical records of approximately 2,200 AGC patients treated from May 2000 to February 2015 were reviewed: among 868 patients who received at least two lines of systemic therapy [13], we retrospectively analyzed baseline parameters, tumor characteristics, and treatment data of those treated with three lines. In particular, of 331 patients who had received a third‐line therapy, we conducted the analyses on 300 patients for whom we had information about treatment type received in third‐line. All the patients had a histologically confirmed diagnosis of metastatic gastric carcinoma. The inclusion criteria for the study included age >18 years, clinical or radiological confirmed disease progression after second‐line chemotherapy, and having received at least one cycle of a third‐line therapy for AGC. Baseline parameters, tumor characteristics, and treatment data were extracted from electronic medical records according to strict privacy standards and anonymized before analysis. Predefined endpoints of the study were progression‐free survival (PFS) in third‐line (PFS‐3), measured from the starting of third‐line chemotherapy to the date of evidence of progressive disease, death, or the last follow‐up visit (whichever occurred first), and OS in third‐line (OS‐3), calculated as the time interval between the beginning of third‐line chemotherapy to the date of death or the last follow‐up visit. Factors included in the univariate analyses were (a) age (≥70 vs. <70 years), (b) intensity of third‐line chemotherapy (combination vs. single‐agent chemotherapy), (c) PFS achieved in first‐line (PFS in first‐line ≥6.9 months vs. <6.9 months; median PFS in first‐line was 6.9 months in our series), and (d) PFS achieved in second‐line (PFS in second‐line ≥3.5 months vs. <3.5 months; median PFS in second‐line was 3.5 months in our series).
Patients’ clinical and pathological characteristics were summarized through descriptive analysis. Categorical features were described through cross‐tables and chi‐square test. The impact of treatment intensity and PFS experienced in previous lines (PFS in first‐line and PFS in second‐line) in terms of both PFS‐3 and OS‐3 was explored through uni‐ and multivariate Cox regression models and described by Kaplan‐Meier estimator plot with log‐rank test. A PFS in first‐line threshold capable of detecting a favorable outcome (defined as a PFS in second‐line and a PFS in third‐line of more than 6 months each) was identified by receiver operating characteristic (ROC) analysis on a randomly generated training set consisting of 150 patients. The results were verified on a validation set of 150 patients.
Statistical analyses were conducted using StataCorp 2016 Stata Statistical Software: Release 14 (StataCorp, College Station, TX, http://www.stata.com).
Results
Data from 300 AGC patients were entered from 19 participant sites and included in the analysis. Complete clinical and pathologic characteristics of the patients are shown in Table 1.
Table 1. Patients’ clinical and pathologic characteristics.
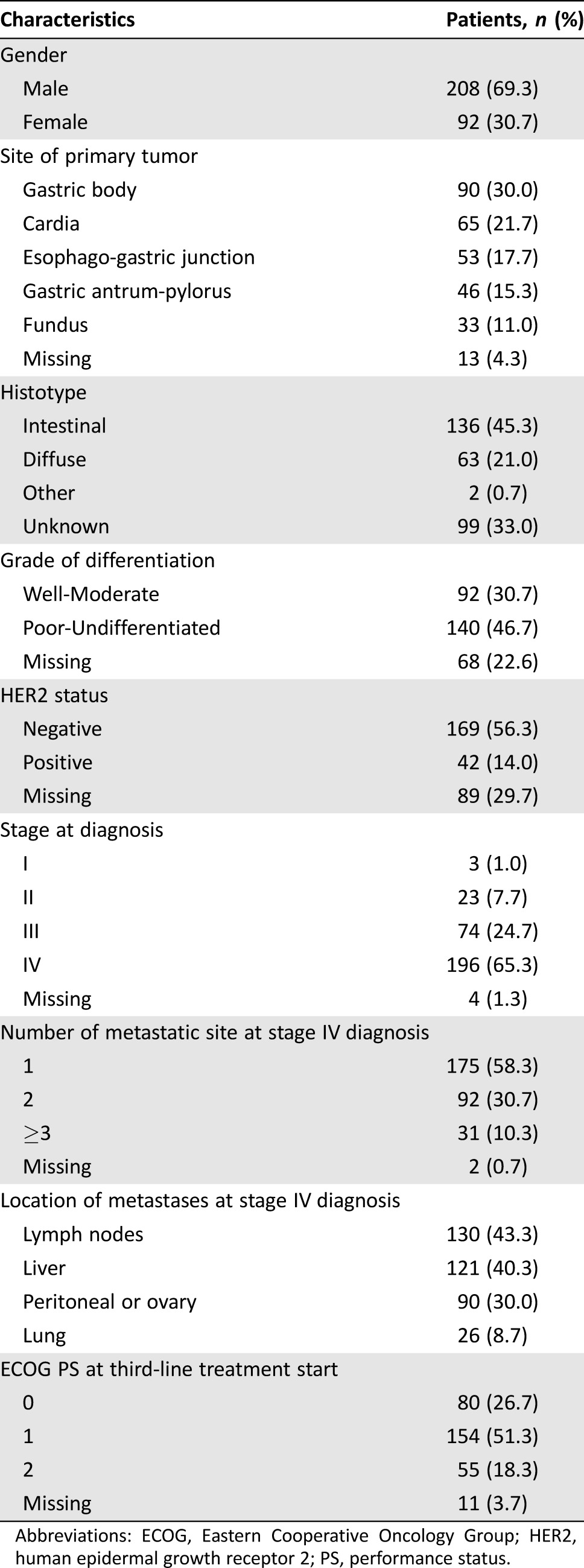
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth receptor 2; PS, performance status.
Median patient age at third‐line treatment start was 64.7 years (25th to 75th percentiles: 55.2–71.6 years). Two hundred eight patients (69.3%) were male. The most common site of primary tumor was gastric body (30%). Intestinal histotype accounted for 45.3% of cases, 46.7% of patients had poorly differentiated or undifferentiated tumors, and 14% had a human epidermal growth receptor 2 positive disease. Stage IV disease was diagnosed as initial presentation in 196 patients (65.3%). One hundred twenty‐three patients (41%) had multiple metastatic sites at the time of diagnosis of metastatic disease.
Types of treatment received are listed in Table 2. In first‐line setting, most patients received a combination regimen (94.3%); fluoropyrimidines were the most frequently used agents (94.7%), followed by platinum salts (86.7%). In 13.7% of cases, patients received a biological agent in first‐line therapy. A complete response to first‐line chemotherapy was achieved in 11 patients and a partial response in 118 patients, for an overall response rate (ORR) of 43%. In second‐line therapy, the majority of patients also received a combination regimen (58.3%), but the percentage of those who received a single‐agent chemotherapy increased (37.3% vs. 3.7%). Most patients received a fluoropyrimidine‐based, a taxane‐based, or an irinotecan‐based chemotherapy (52.7%, 39.7%, and 39%, respectively). A biological agent was used in 8.3% of patients in second‐line. A complete response to second‐line chemotherapy was achieved in 7 patients and a partial response in 42 patients, for an ORR of 16.3%. Detailed results of the whole cohort of nearly 900 patients who received a second‐line treatment have already been reported elsewhere [13]. Eastern Cooperative Oncology Group performance status at the start of the third‐line therapy was 0 or 1 in 234 patients (78%). Of note, in third‐line, near half of the study population received a combination regimen as well (49.7%). Most patients received a fluoropyrimidine‐based, a taxane‐based, or an irinotecan‐based chemotherapy (46.3%, 36%, and 34.3%, respectively). Unexpectedly, a complete response to third‐line chemotherapy was achieved in 2 patients and a partial response in 31 patients, for an ORR of 11%. With referral to treatment duration, the median number of cycles received was six in each line of treatment (around 4 months).
Table 2. Type of treatments received.
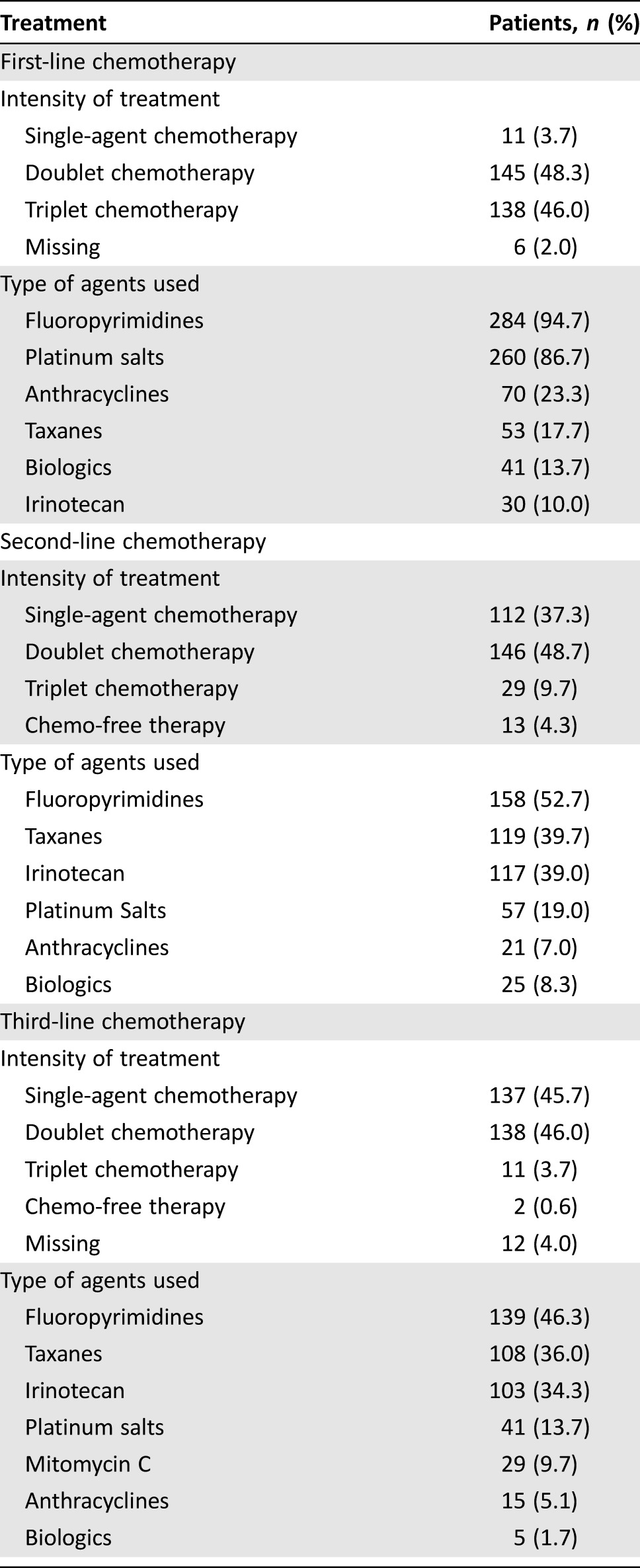
At the time of analysis, 275 (91.7%) patients had died, mainly because of tumor progression. Median OS from the beginning of the first‐line chemotherapy for the whole cohort was 19.9 months (25th to 75th percentiles: 14.1–29.4 months). Median PFS in first‐line was 6.9 months (25th to 75th percentiles: 4.4–11.1 months), whereas median PFS in second‐line was 3.5 months (25th to 75th percentiles: 2.4–6.5 months). Median PFS‐3 was 2.9 months (25th to 75th percentiles: 1.8–5.2 months) and median OS‐3 was 5.6 months (25th to 75th percentiles: 3.1–10.2 months).
At univariate analysis (Table 3), three variables were significantly associated with longer PFS‐3 and OS‐3: a PFS in first‐line ≥6.9 months, a PFS in second‐line ≥3.5 months, and the intensity of third‐line treatment. About one third of the population (91 patients) was ≥70 years. Interestingly, survival benefits were very similar, regardless of age. Median PFS‐3 was 3.3 months (25th to 75th percentiles: 2.0–6.7) for those aged ≥70 and 2.8 months (25th to 75th percentiles: 1.7–5.0) for those <70 years (hazard ratio [HR] 0.83; 95% confidence interval [CI] 0.64–1.07; p = .15). Accordingly, median OS‐3 was 6.6 months (25th to 75th percentiles: 3.3–11.4) and 5.2 months (25th to 75th percentiles: 2.6–9.5), respectively (HR 0.82; 95% CI 0.63–1.06; p = .13), suggesting that age should not limit the use of a third‐line treatment.
Table 3. Univariate and multivariate analyses.
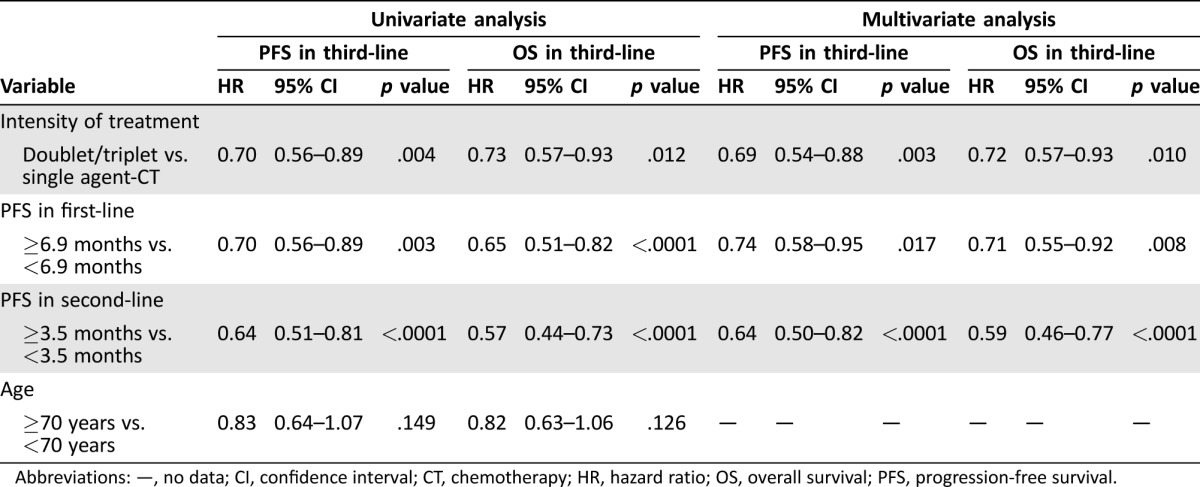
Abbreviations: —, no data; CI, confidence interval; CT, chemotherapy; HR, hazard ratio; OS, overall survival; PFS, progression‐free survival.
Multivariate regression analysis (Table 3) included all the variables that showed prognostic significance in univariate analysis, which resulted to be all independently associated with longer survival.
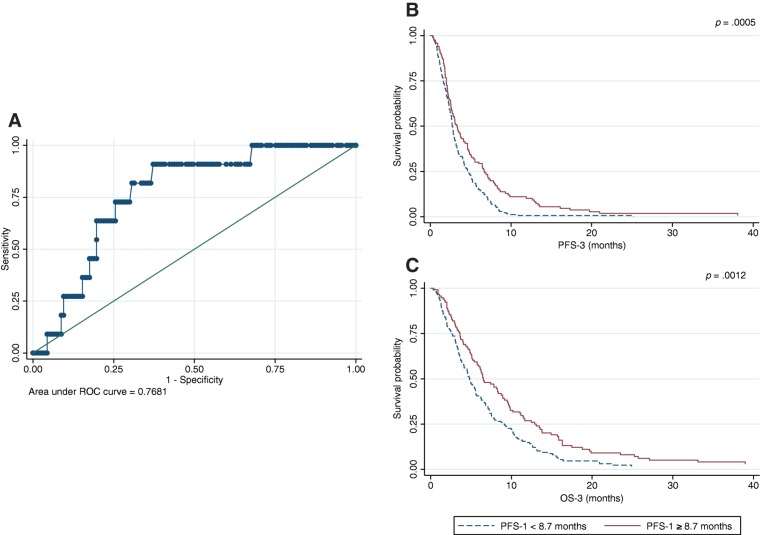
Through a ROC analysis on a randomly generated training set consisting of 150 patients, we found that a PFS in first‐line ≥8.7 months was able to detect a PFS in both second‐ and third‐line of more than 6 months each (ROC area under the curve [AUC] = 0.7681; Fig. 1A). Moreover, a PFS in first‐line ≥8.7 months was associated with better PFS‐3 (HR 0.59; 95% CI 0.41–0.83; p = .0029) and OS‐3 (HR 0.69; 95% CI 0.49–0.98; p = .0383; Figs. 1B and 1C). Both results were confirmed in the validation set (HR 0.64; 95% CI 0.45–0.91; p = .0134 and HR 0.59; 95% CI 0.50–0.99; p = .0473, respectively).
Figure 1.
ROC curve (A) and survival curve for progression‐free survival in third‐line (B) and overall survival in third‐line (C) according to progression free‐survival in first‐line identified through the ROC analysis.
Abbreviations: OS, overall survival; PFS, progression‐free survival; ROC, receiver operating characteristic.
Discussion
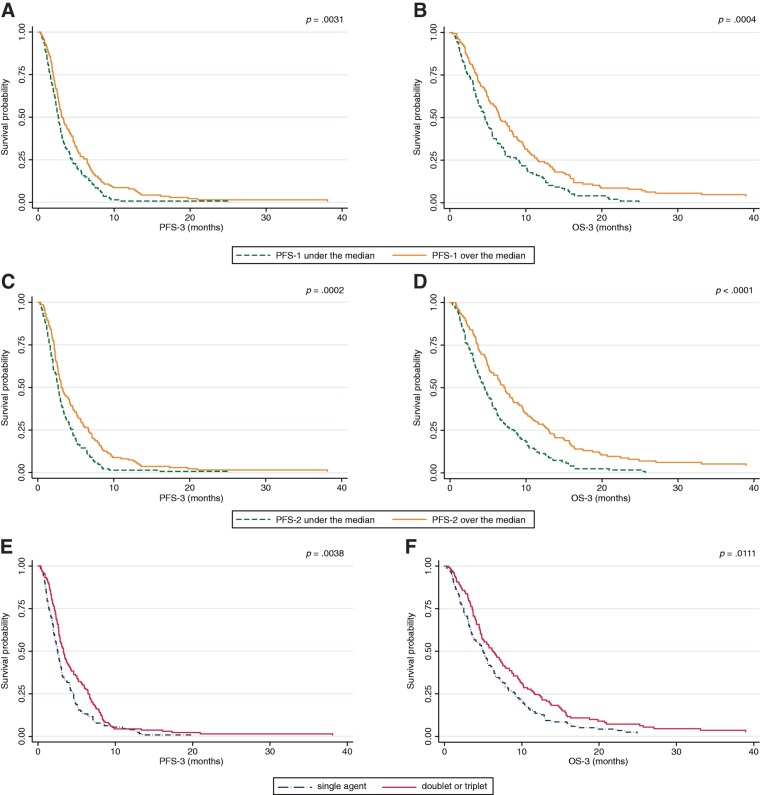
Systemic treatment represents the most important treatment option for unresectable AGC patients. Based on the results of several randomized clinical trials [5], [6], [7], [8], [9], second‐line treatment is now considered the standard of care for AGC patients who are still in good general condition. After the failure of second‐line chemotherapy, some patients continue to have good performance status and therefore may receive third‐ or subsequent lines of therapy on a personalized basis. Literature data focusing on third‐line chemotherapy in AGC is mainly retrospective or based on small phase II studies; authors investigated the activity of monotherapy, such as irinotecan, paclitaxel, or docetaxel [14], [15], [16], as well as combinations [17], [18], [19], [20] in patients pretreated with fluoropyrimidines and platinum salts. Some studies have also investigated the role of possible prognostic factors in third‐line therapy for AGC patients [17], [18], [21], [22]. To our knowledge, our retrospective report is one of the largest studies on third‐line palliative chemotherapy in AGC patients. Median PFS‐3 and median OS‐3 are in line with previous reports and with the most recent data on third‐line with apatinib [10]. Patients who had experienced longer PFS in previous lines of treatment had better outcomes. More specifically, a first‐line PFS ≥6.9 months was associated with longer median PFS‐3 (3.2 months [25th to 75th percentiles: 2.0–6.4] vs. 2.7 months [25th to 75th percentiles: 1.6–4.5]; HR 0.74; 95% CI 0.58–0.95; p = .017) and a 2‐month longer median OS‐3 (6.5 months [25th to 75th percentiles: 3.6–11.5] vs. 4.6 months [25th to 75th percentiles: 2.5–8.9]; HR 0.71; 95% CI 0.55–0.92; p = .008; Figs. 2A and 2B). Similarly, patients who experienced a PFS in second‐line ≥3.5 months also had longer median PFS‐3 (3.2 months [25th to 75th percentiles: 2.2–6.7] vs. 2.7 months [25th to 75th percentiles: 1.6–4.6]; HR 0.64; 95% CI 0.50–0.82; p < .0001) and a 2.5 month‐longer median OS‐3 (7.3 months [25th to 75th percentiles: 3.7–13.0] vs. 4.6 months [25th to 75th percentiles: 2.3–8.4]; HR 0.59; 95% CI 0.46–0.77; p < .0001; Figs. 2C and 2D). These findings are also in parallel with those noted in previous reports [21], [22], confirming that gastric cancer is a heterogeneous disease; patients who achieved longer PFS in previous treatment lines might have an indolent disease, a more chemosensitive tumor, or a slower tumor growth rate compared with those who experienced shorter PFS.
Figure 2.
Survival curves for progression‐free survival in third‐line and overall survival in third‐line according to progression free‐survival achieved in first‐line (A, B), progression‐free survival achieved in second‐line (C, D), and intensity of third‐line treatment (E, F).
Abbreviations: OS, overall survival; PFS, progression‐free survival.
In our series, patients treated with a combination regimen experienced better outcomes compared with those who received single‐agent treatment. In particular, these patients had a longer median PFS‐3 (3.3 months [25th to 75th percentiles: 2.1–6.5] vs. 2.6 months [25th to 75th percentiles: 1.4–4.6]; HR 0.69; 95% CI 0.54–0.88; p = .003) and median OS‐3 (6.3 months [25th to 75th percentiles: 3.6–11.7] vs. 5.0 months [25th to 75th percentiles: 2.5–9.1]; HR 0.72; 95% CI 0.57–0.93; p = .010; Figs. 2E and 2F). Indeed, the absolute increase in median OS is limited to approximately 1 month, and the higher response rate obtained with combination chemotherapy does not necessarily translate into a survival benefit [23]. This might be even more realistic in subsequent lines, where the treatment choice depends on previous therapies and patient's performance status. Moreover, part of the survival benefit might lean on the sequence rather than on the treatment intensity.
Importantly, no differences in PFS‐3 nor in OS‐3 were observed between patients younger or older than 70 years, suggesting that chronological age should not be considered as the main determinant in the decision‐making process.
Our study also had notable limits. Firstly, it is retrospective in its nature and this precludes us from making strong statements regarding the benefits of these treatments. Secondly, the study population was not homogeneous regarding first‐ and second‐line therapies administered (as for type of treatment received, cycles administered, and therapeutic sequence); hence, definitive conclusions on the clinical management based on our data are not appropriated. A detailed description of the agents used and the order in which they were received are beyond the scope of our study. Thirdly, a selection bias may be considered because patients included were all treated with three lines of therapy and none of them received apatinib, which significantly improved survival with an acceptable safety profile in heavily pretreated AGC patients [10]. Similarly, according to the time span considered, no patient in third‐line received immunotherapy, which is revolutionizing the treatment paradigm of many solid tumors, including esophagogastric cancers [24]. Whereas anti‐CTLA4 compounds (i.e., tremelimumab and ipilimumab) have produced unsatisfactory results [25], programmed cell death protein 1/programmed death‐ligand 1 (PD‐L1) inhibitors are showing more promising effects [24]. A recent phase III trial (NCT02267343) involving 493 Asian patients evaluated the efficacy and safety of nivolumab as salvage treatment after the failure of two or more previous chemotherapy regimens. This study demonstrated that nivolumab significantly improved OS compared with placebo (5.32 vs. 4.14 months; HR 0.63; 95% CI 0.50–0.78; p < .0001) [26]. In line with these findings were the results of the phase I/II CheckMate 032 study that enrolled 160 heavily pretreated patients (79% had ≥2 prior treatment lines). This trial showed favorable clinical activity of nivolumab +/− ipilimumab in Western patients with advanced/metastatic chemotherapy‐refractory gastric, esophageal, or gastroesophageal junction cancer [27]. Again, the phase II KEYNOTE‐059 study explored the efficacy and safety of pembrolizumab monotherapy in patients with previously treated AGC. The ORR, the primary trial endpoint, was 14.9% (95% CI 9.4–22.1) in third‐line patients; in particular, in patients with PD‐L1 positive tumors, ORR was 21.3% (95% CI 12.7–32.3), whereas in patients with PD‐L1 negative tumors, ORR was 6.9% (95% CI 1.9–16.7) [28]. Even if Epstein‐Barr virus and microsatellite instability gastric cancer molecular subtypes raise the highest interest because of their peculiar immunogenicity [29], the optimal candidate for immunotherapy has not yet been identified. It would be interesting to verify if longer PFS in previous treatment lines might affect third‐line outcomes even in patients treated with immunotherapy. Despite the aforementioned limits, our study included a large number of real‐world AGC patients, providing information on third‐line treatment from the daily clinical practice.
Conclusion
Our real‐world study confirms that a not‐negligible proportion of patients receive third‐line treatment in daily clinical practice, and it provides detailed information on treatment type and useful data of survival outcome. Having achieved longer PFS in previous treatment lines may help in selecting patients who are more likely to benefit from a further therapy after progressive disease. In addition, treatment intensity may have a role in improving clinical outcomes. Further efforts are warranted to define the best therapeutic sequences and to identify the optimal candidate for a third‐line chemotherapy.
Acknowledgments
This work was supported in part by the Investigator Grant number 19111 through the Associazione Italiana per la Ricerca sul Cancro (AIRC), and by the Basic Research Project 2015 through the University of Verona to DM. Approval for this study was obtained from local scientific review boards.
Author Contributions
Conception/design: Valentina Fanotto, Caterina Vivaldi, Giuseppe Aprile
Provision of study material or patients: Valentina Fanotto, Mario Uccello, Irene Pecora, Lorenza Rimassa, Francesco Leone, Gerardo Rosati, Daniele Santini, Riccardo Giampieri, Samantha Di Donato, Gianluca Tomasello, Nicola Silvestris, Filippo Pietrantonio, Francesca Battaglin, Antonio Avallone, Mario Scartozzi, Eufemia Stefania Lutrino, Davide Melisi, Lorenzo Antonuzzo, Antonio Pellegrino, Laura Ferrari, Roberto Bordonaro, Caterina Vivaldi, Lorenzo Gerratana, Silvia Bozzarelli, Roberto Filippi, Domenico Bilancia, Marco Russano, Giuseppe Aprile
Collection and/or assembly of data: Valentina Fanotto, Mario Uccello, Irene Pecora, Lorenza Rimassa, Francesco Leone, Gerardo Rosati, Daniele Santini, Riccardo Giampieri, Samantha Di Donato, Gianluca Tomasello, Nicola Silvestris, Filippo Pietrantonio, Francesca Battaglin, Antonio Avallone, Mario Scartozzi, Eufemia Stefania Lutrino, Davide Melisi, Lorenzo Antonuzzo, Antonio Pellegrino, Laura Ferrari, Roberto Bordonaro, Caterina Vivaldi, Lorenzo Gerratana, Silvia Bozzarelli, Roberto Filippi, Domenico Bilancia, Marco Russano, Giuseppe Aprile
Data analysis and interpretation: Valentina Fanotto, Lorenzo Gerratana, Giuseppe Aprile
Manuscript writing: Valentina Fanotto, Giuseppe Aprile
Final approval of manuscript: Valentina Fanotto, Mario Uccello, Irene Pecora, Lorenza Rimassa, Francesco Leone, Gerardo Rosati, Daniele Santini, Riccardo Giampieri, Samantha Di Donato, Gianluca Tomasello, Nicola Silvestris, Filippo Pietrantonio, Francesca Battaglin, Antonio Avallone, Mario Scartozzi, Eufemia Stefania Lutrino, Davide Melisi, Lorenzo Antonuzzo, Antonio Pellegrino, Laura Ferrari, Roberto Bordonaro, Caterina Vivaldi, Lorenzo Gerratana, Silvia Bozzarelli, Roberto Filippi, Domenico Bilancia, Marco Russano, Giuseppe Aprile
Disclosures
The authors indicated no financial relationships.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Wagner AD, Grothe W, Haerting J et al. Chemotherapy in advanced gastric cancer: A systematic review and meta‐analysis based on aggregate data. J Clin Oncol 2006;24:2903–2909. [DOI] [PubMed] [Google Scholar]
- 4. Wesolowski R, Lee C, Kim R. Is there a role for second‐line chemotherapy in advanced gastric cancer? Lancet Oncol 2009;10:903–912. [DOI] [PubMed] [Google Scholar]
- 5. Thuss‐Patience PC, Kretzschmar A, Bichev D et al. Survival advantage for irinotecan versus best supportive care as second‐line chemotherapy in gastric cancer–A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306–2314. [DOI] [PubMed] [Google Scholar]
- 6. Kang JH, Lee SI, Lim DH et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513–1518. [DOI] [PubMed] [Google Scholar]
- 7. Ford HE, Marshall A, Bridgewater JA et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR‐02): An open‐label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78–86. [DOI] [PubMed] [Google Scholar]
- 8. Fuchs CS, Tomasek J, Yong CJ et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo‐controlled, phase 3 trial. Lancet 2014;383:31–39. [DOI] [PubMed] [Google Scholar]
- 9. Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 10. Li J, Qin S, Xu J et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–1454. [DOI] [PubMed] [Google Scholar]
- 11. Pavlakis N, Sjoquist KM, Martin AJ et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): A multinational placebo‐controlled phase II trial. J Clin Oncol 2016;34:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SM, Park SH. Chemotherapy beyond second‐line in advanced gastric cancer. World J Gastroenterol 2015;21:8811–8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fanotto V, Cordio S, Pasquini G et al. Prognostic factors in 868 advanced gastric cancer patients treated with second‐line chemotherapy in the real world. Gastric Cancer 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Shimoyama R, Yasui H, Boku N et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer 2009;12:206–211. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Kim SH, Oh SY et al. Third‐line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med 2013;28:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawakami T, Machida N, Yasui H et al. Efficacy and safety of irinotecan monotherapy as third‐line treatment for advanced gastric cancer. Cancer Chemother Pharmacol 2016;78:809–814. [DOI] [PubMed] [Google Scholar]
- 17. Lee MJ, Hwang IG, Jang JS et al. Outcomes of third‐line docetaxel‐based chemotherapy in advanced gastric cancer who failed previous oxaliplatin‐based and irinotecan‐based chemotherapies. Cancer Res Treat 2012;44:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang EJ, Im SA, Oh DY et al. Irinotecan combined with 5‐fluorouracil and leucovorin third‐line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: Treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013;16:581–589. [DOI] [PubMed] [Google Scholar]
- 19. Fushida S, Kinoshita J, Kaji M et al. Paclitaxel plus valproic acid versus paclitaxel alone as second‐ or third‐line therapy for advanced gastric cancer: A randomized phase II trial. Drug Des Devel Ther 2016;10:2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquini G, Vasile E, Caparello C et al. Third‐Line chemotherapy with irinotecan plus 5‐fluorouracil in Caucasian metastatic gastric cancer patients. Oncology 2016;91:311–316. [DOI] [PubMed] [Google Scholar]
- 21. Park JS, Lim JY, Park SK et al. Prognostic factors of second and third line chemotherapy using 5‐fu with platinum, irinotecan, and taxane for advanced gastric cancer. Cancer Res Treat 2011;43:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shim HJ, Yun JY, Hwang JE et al. Prognostic factor analysis of third‐line chemotherapy in patients with advanced gastric cancer. Gastric Cancer 2011;14:249–256. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Ma B, Huang XT et al. Doublet versus single agent as second‐line treatment for advanced gastric cancer: A meta‐analysis of 10 randomized controlled trials. Medicine (Baltimore) 2016;95:e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonotto M, Garattini SK, Basile D et al. Immunotherapy for gastric cancers: Emerging role and future perspectives. Expert Rev Clin Pharmacol 2017;10:609–619. [DOI] [PubMed] [Google Scholar]
- 25. Bang YJ, Cho JY, Kim YH et al. Efficacy of sequential ipilimumab monotherapy vs best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Kang YK, Satoh T, Ryu MH et al. Nivolumab (ONO‐4538/BMS‐936558) as salvage treatment after second or later‐line chemotherapy for advanced gastric or gastro‐esophageal junction cancer (AGC): A double‐blinded, randomized, phase III trial. J Clin Oncol 2017;35(suppl 4S):2a. [Google Scholar]
- 27. Janjigian YY, Ott PA, Calvo E et al. Nivolumab ± ipilimumab in pts with advanced (adv)/metastatic chemotherapy‐refractory (CTx‐R) gastric (G), esophageal (E), or gastroesophageal junction (GEJ) cancer: CheckMate 032 study. J Clin Oncol 2017;35:4014a. [Google Scholar]
- 28. Fuchs CS, Doi T, Jang RW et al. KEYNOTE‐059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:4003a. [Google Scholar]
- 29. Garattini SK, Basile D, Cattaneo M et al. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J Gastrointest Oncol 2017;9:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]