Key Points
Flagellin activates TLR5 signaling in mouse bone marrow and induces hematopoietic progenitor cell proliferation.
Flagellin-induced MPP3 cells aid the survival of mice exposed to lethal irradiation.
Abstract
Administration of the bacterial protein flagellin to mice activates innate immune signaling that protects against an array of challenges, including ionizing radiation. Herein, we define the underlying mechanism for this protection. We report that flagellin treatment induces proliferation and mobilization of bone marrow cells that aid survival following irradiation. Specifically, treatment of mice or bone marrow cells ex vivo with flagellin induced Toll-like receptor 5 (TLR5)–dependent and NOD-like receptor C4–independent proliferation of Lin−Sca-1+Kit+ (LSK) cells, which includes both hematopoietic stem cells that provide long-term repopulation (LTR) and multipotent progenitor cells (MPPs) that transiently proliferate and differentiate into a range of blood cell types. TLR5 expression on bone marrow cells was necessary and sufficient for flagellin-induced LSK proliferation. Flagellin treatment stimulated LSK proliferation by inducing a 10-fold increase in type 3 MPP (MPP3) without a concomitant increase in LTR cells. Cotransfer of 5 × 103 fluorescence-activated cell sorted flagellin-induced MPP3 cells along with 1 × 105 whole bone marrow cells to lethally irradiated mice revealed that such cells predominantly repopulated the neutrophil compartment for up to 4 week, and dramatically increased the survival rate of the bone marrow transplantation procedure. Hence, we propose the administration of MPP3 cells, elicited by flagellin, as a novel approach to prevent life-threatening neutropenia that can accompany bone marrow transplant and other myeloablative therapeutic procedures.
Visual Abstract
Introduction
Bacterial flagellin is recognized via Toll-like receptor 5 (TLR5), which activates classic nuclear factor κB–mediated gene expression, and NOD-like receptor C4 (NLRC4), whose activation leads to inflammasome-mediated production of interleukin-1β (IL-1β) and IL-18. TLR5 has a cellular expression pattern, enriched on epithelial cells, mucosal CD11c+ phagocytes, and hepatocytes, that enables its activation to confer protection against a range of challenges, including infection, toxic chemicals, and γ-radiation.1-3 However, TLR5 is not functionally expressed on many populations of innate immune cells that express the lipopolysaccharide receptor TLR4.4 This explains why, in contrast to lipopolysaccharide, flagellin does not elicit systemic production of proinflammatory cytokines such as tumor necrosis factor α or its associated adverse events. However, multiple lines of evidence indicate that flagellin treatment has potent effects on immune cell proliferation and/or mobilization.3,5,6 Specifically, treatment with flagellin results in a very large increase in neutrophils in the intestine. The magnitude of this increase suggested that it did not only reflect neutrophil recruitment to the intestine but involved increased neutrophil production.3 Furthermore, bone marrow cells isolated from flagellin-treated mice had a greater capacity than untreated bone marrow to rescue lethally irradiated mice that had not been exposed to flagellin.1 This suggests that flagellin treatment had impacted hematopoietic precursors in bone marrow. Hence, we sought to further define the impact of flagellin treatment on bone marrow cells.
The majority of cells in murine bone marrow are differentiated cells, including neutrophils, monocytes, dendritic cells, T cells, and plasma cells.7-10 Undifferentiated bone marrow cells, including long-term (LT) hematopoietic stem cells (HSCs), short-term (ST) HSCs, and multipotent progenitor cells (MPPs), are lineage marker negative and express Sca-1 and c-Kit (or Kit), which constitute a population of cells referred to as Lin−Sca-1+Kit+ (LSK) cells.7,11 LSK cells only comprise ∼0.5% of total bone marrow cells.7,11-13 Among LSK cells, LT-HSC retain long-term renewal ability, while ST-HSC and MPPs only differentiate to various mature lineages.7,11,14 Repopulation of neutrophils in peripheral blood is critical for protection against infection after irradiation or bone marrow transplantation.15 Hence, we hypothesized that flagellin might activate bone marrow cells to induce either LT-HSC or hematopoietic progenitor cell proliferation and differentiation. To investigate these possibilities, we examined how flagellin treatment impacted bone marrow cells ex vivo and in mice treated with flagellin. We observed that flagellin treatment induced expansion of LSK cells, particularly ST-HSCs and MPPs, especially type 3 MPP (MPP3). Upon isolation of flagellin-induced MPP3 and their transfer into irradiated hosts, we found that these cells persisted for a few weeks, predominantly repopulated the neutrophil compartment, and, most importantly, greatly enhanced the ability of recipient mice to survive after a myeloablative dose of irradiation.
Materials and methods
Mice
Animal studies were approved by the Institutional Animal Care and Use Committee at Georgia State University (protocol A14033). Wild-type (WT) C57BL/6, RAG1−/−, and CD45.1+ mice (C57BL/6 background) were obtained from The Jackson Laboratory (Bar Harbor, ME). Swiss Webster mice were purchased from Taconic (Hudson, NY). IL-22−/− and NLRC4−/− mice were provided by Genentech (South San Francisco, CA). TLR5−/− mice were generated by Shizuo Akira (Osaka University, Japan) and backcrossed to C57BL/6 mice for 10 generations. Generation of TLR5−/−/NLRC4−/− mice was previously described.16 Albumin-Cre and TLR5-floxed mice were crossed to generate albumin-Cre-TLR5fl/fl mice, which have a >95% reduction in TLR5 messenger RNA levels in hepatocytes.17
Flagellin preparation
The FliC isoform of flagellin was purified by high-performance liquid chromatography and purity verified as previously described.18,19 Prepared flagellin did not activate gene expression (as assessed by reverse transcription polymerase chain reaction and microarray) in mice lacking both known flagellin receptors, indicating it did not contain levels of innate immune agonists beyond flagellin.
Whole bone marrow cell preparation
Bone marrow cells were eluted from the femurs of mice with RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (in complete RPMI), followed by incubation in red blood cell lysis buffer for 3 minutes at room temperature. The cells were then washed with fresh media and resuspended in cold phosphate-buffered saline (PBS) for further use.
In vivo and ex vivo assay
For in vivo studies, mice were treated with PBS or 20 µg FliC by intraperitoneal (IP) injection. Fifteen hours later, mice were labeled with 1 mg 5-bromo-2′-deoxyuridine (BrdU) by IP injection. Mice were euthanized after 1 hour and whole bone marrow cells analyzed by flow cytometry. For ex vivo studies, whole bone marrow cells were isolated and incubated in 20 mL complete RPMI with or without 50 ng/mL FliC for 15 hours. The cells were then labeled with 10 µM BrdU for 1 hour before flow cytometry analysis. In some ex vivo cultures, granulocyte colony-stimulating factor (G-CSF) was neutralized with anti–G-CSF as previously described.20
Generation of bone marrow chimeras
Bone marrow chimeras were generated as previously described.21 Recipient mice were subjected to X-ray irradiation at a dose equivalent to 8.5 Gy using an RS-2000 irradiator (Rad Source Technologies) prior to being administered 1 × 107 donor bone marrow cells by IV injection. Mice were then maintained in sterile cages and supplied with drinking water containing 2 mg/mL neomycin. Mice were used for experiments 8 weeks later.
Repopulation assay
Measurement of short-term repopulation was performed as previously described.22-24 CD45.1+ WT B6 mice (recipient) were lethally irradiated as described in “Generation of bone marrow chimeras.” Then, 2 × 105 whole bone marrow cells from CD45.1+ WT B6 mice (competitor cells) plus a range of whole bone marrow cells from PBS- or FliC-treated CD45.2+ WT B6 mice (test donor cells) were cotransferred to recipient mice. Recipient mice were housed under sterile conditions with sterile food and sterile water containing 2 mg/mL neomycin. After 4 or 8 weeks, whole blood of these mice was analyzed for peripheral repopulation. The repopulation unit was determined by chimerism of donor whole blood cells based on CD45.1+ or CD45.2+ cells using the following formula: 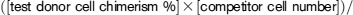
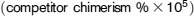 .22-24
.22-24
Fluorescence-activated cell sorting (FACS) and adoptive transfer
MPP3 cells were sorted on a Sony SH800 FACS sorter (Sony Biotechnology). Whole bone marrow cells from flagellin-treated mice were stained with fluorochrome-labeled antibodies. Lin−Sca-1+Kit+CD34+Flt3−CD48+CD150− (MPP3) cells were sorted. All cells were double sorted to ensure high purity (over 95% purity). In different experiments, indicated numbers of whole bone marrow cells with or without sorted MPP3 cells were injected into lethally irradiated mice.
Flow cytometry analysis
Mouse bone marrow cells or whole-blood leukocytes were isolated and blocked with 10 μg/mL anti-CD16/anti-CD32 (clone 2.4G2, ATCC) for 10 minutes in FACS buffer. Aliquots of cells were suspended in 0.1 mL BD FACS buffer on ice for 20 minutes with fluorescein isothiocyanate–, phycoerythrin-, PerCP-, allophycocyanin-, phycoerythrin-Cy7–, Alexa Fluor 700–, Pacific blue–, Brilliant Violet 650–, and Brilliant Violet 785–conjugated monoclonal antibodies to detect the following surface antigens: mouse lineage marker cocktail (CD3e, CD11b, B220, Gr-1, and Ter119), Sca-1, c-Kit (CD117), CD34, Flt3 (CD135), CD48, CD150, CD11b, CD45.1, CD45.2, and Ly-6G (BD Biosciences). Markers for LT-HSCs, ST-HSCs, and MPP1-MPP4 are previously described.14,25,26 In cell proliferation assays, intracellular staining of allophycocyanin-conjugated anti-BrdU and 7-amino-actinomycin D (7-AAD) were performed following surface staining. Stained cells were analyzed on a BD Fortessa flow cytometer. Data analysis was carried out using FlowJo (Tree Star, Ashland, OR).
Results
Flagellin treatment induces LSK cell expansion in vivo
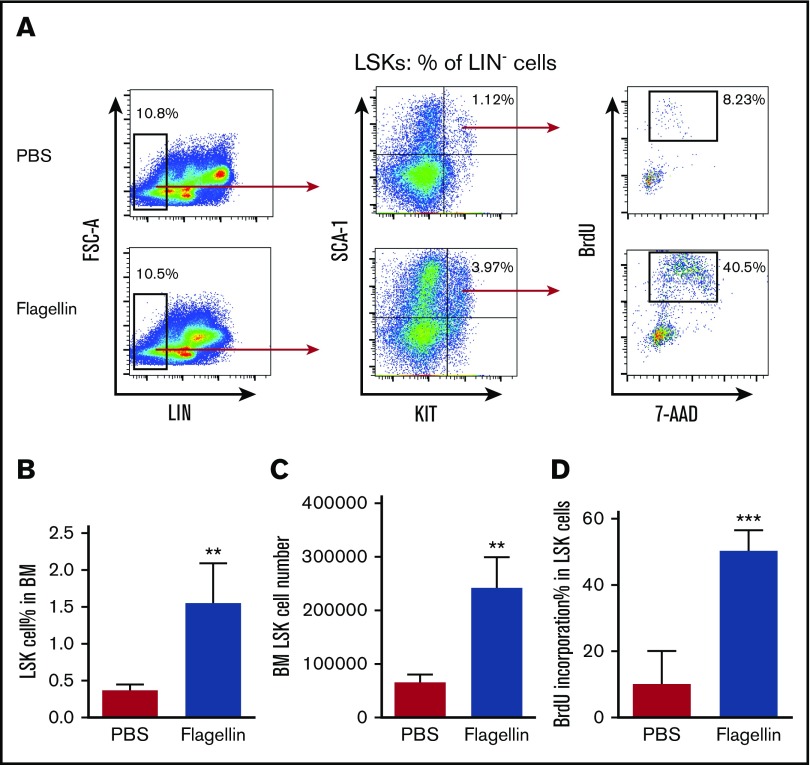
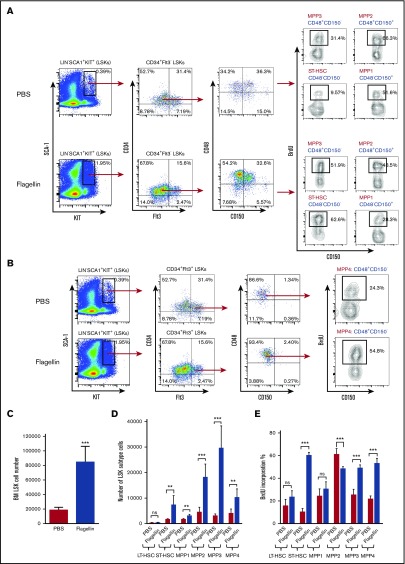
We previously observed that relative to bone marrow from untreated mice, bone marrow isolated from mice that had, 24 hours prior, been injected with flagellin had a greater capacity to rescue mice from radiation-induced death.1 We hypothesized that this might reflect the ability of bone marrow cells to sense flagellin and result in a proliferation of stem cells, enabling such marrow to more efficiently repopulate the hematopoietic compartment of irradiated mice. To investigate this possibility, we treated mice (C57BL/6) with flagellin (20 μg) by IP injection. Fifteen hours following treatment, mice were administered BrdU and euthanized 1 hour later. Bone marrow was then isolated and analyzed by flow cytometry. Such flagellin treatment resulted in an approximate fourfold increase in the number and percentage of LSK cells (Figure 1A-C), which contain both hematopoietic stem and progenitor cells. This increase in the number of LSK cells was associated with a fivefold increase in the percentage of LSK cells that were actively proliferating (Figure 1A,D). Together, these results indicate that flagellin induced the proliferation of LSK cells.
Figure 1.
Flagellin induces LSK cell proliferation in vivo. Eight-week-old female C57BL/6 mice were treated with PBS only or with PBS containing 20 μg flagellin by IP injection. Fifteen hours following injection, the mice were labeled with 1 mg BrdU for 1 hour and then euthanized. Whole bone marrow cells were prepared and analyzed by flow cytometry. Representative flow cytometry plots comparing LSK cell proliferation (A), statistical analysis of LSK cell percentage in bone marrow (calculated by forward-side scatter gate % × single cell % × Lin− % × LSK % in Lin− cells) (B), LSK cell number (C), and BrdU incorporation in LSK cells (D) are shown. Statistics were analyzed by unpaired Student t test (**P < .01; ***P ≤ .001). Error bars represent standard error of the mean (SEM). BM, bone marrow; FSC, forward scatter.
Flagellin-induced LSK cell proliferation requires TLR5, but not NLRC4
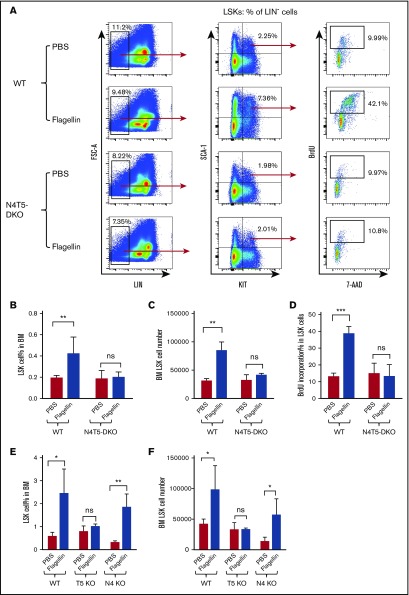
We next sought to elucidate how bone marrow cells recognized flagellin. We used mice deficient in both flagellin receptors, namely TLR5 and NLRC4. While the basal levels and proliferation rates of LSK cells in mice lacking these receptors (TLR5−/−/NLRC4−/− mice, hereafter referred to as N4T5-DKO) were variable (compare Figure 2A-D and supplemental Figure 1A-C), we consistently observed that these mice did not exhibit flagellin-induced proliferation based on the percentage or absolute number of LSK cells in the bone marrow or the percentage of such cells in S phase (Figure 2A-D; supplemental Figure 1A-C). Such absence of LSK cell proliferation in response to flagellin was also seen in mice lacking only TLR5 (Figure 2E-F). In contrast, although mice lacking NLRC4 had a modest reduction in the basal level of LSK cells, they exhibited a clear increase in LSK cell proliferation in response to flagellin (Figure 2E-F). Thus, flagellin-induced LSK proliferation is mediated by TLR5, but not NLRC4.
Figure 2.
Flagellin-mediated LSK cell proliferation is TLR5 dependent. WT B6 mice, TLR5-knockout (shown as T5 KO), NLRC4-knockout (shown as N4 KO), and N4T5-DKO mice were treated with flagellin as in Figure 1. Whole bone marrow cells from these mice were analyzed for LSK cell proliferation by flow cytometry. Representative flow cytometry plots comparing LSK proliferation (A), LSK cell % in bone marrow (B), LSK cell number (C), BrdU incorporation (D) in WT vs N4T5-DKO mice, LSK cell percentage (E), and LSK cell number in bone marrow (F) in WT, TLR5-knockout, and NLRC4-knockout mice are shown. Statistics were analyzed by unpaired Student t test (*P < .05; **P < .01; ***P ≤ .001). Error bars represent SEM. ns, not significant.
Flagellin activates TLR5 on bone marrow–derived cells to induce LSK cell proliferation
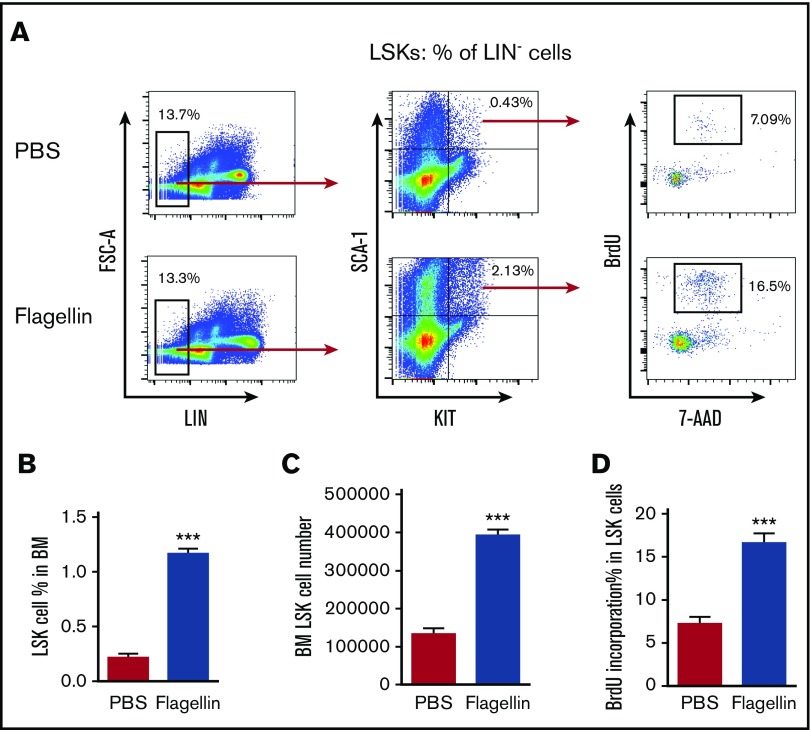
When administered systemically, a major portion of flagellin-induced cytokines is generated by nonhematopoietic cells, especially epithelial cells and hepatocytes (epithelial-like).21,27 Indeed, hepatocyte TLR5 is central to mediating the liver’s response to flagellin and is speculated to play a role in flagellin-mediated protection against radiation.28 Hence, we hypothesized that hepatocyte TLR5 might drive flagellin-induced LSK cell proliferation. However, we observed that the deletion of hepatocyte TLR5 in mice did not diminish flagellin-induced LSK cell proliferation, arguing against this possibility (supplemental Figure 2A-D). While several populations of bone marrow–derived cells are unresponsive to flagellin, key aspects of the flagellin-induced immune response (eg, activation of the IL-23/IL-22 axis) are mediated by TLR5-expressing dendritic cells.3,29,30 Hence, we hypothesized that flagellin-induced LSK cell proliferation might reflect its action on bone marrow–derived cells. To investigate this possibility, we generated bone marrow chimeric mice in which only hematopoietic or nonhematopoietic cells expressed TLR5. We observed that mice expressing TLR5 only in their hematopoietic cells displayed robust LSK cell proliferation in response to flagellin, whereas mice expressing TLR5 only on nonhematopoietic cells did not display this phenomenon (supplemental Figure 3A-B). To further identify the bone marrow cells being activated by flagellin, whole bone marrow cells were isolated and incubated in complete RPMI supplemented with flagellin or PBS for 15 hours, followed by 1-hour BrdU labeling and subsequent flow cytometry analysis. Such ex vivo exposure of bone marrow cells recapitulated the increase in LSK proliferation observed in bone marrow isolated from flagellin-treated mice (Figure 3A-D). Moreover, analogous to the in vivo studies described in Figure 2, ex vivo flagellin-induced LSK cell proliferation was dependent on flagellin receptors (supplemental Figure 4A-C), suggesting a direct impact of systemically administered flagellin upon bone marrow cells. Together, these results indicate that both ex vivo and in vivo, TLR5-mediated recognition of flagellin by bone marrow cells drives LSK cell proliferation.
Figure 3.
Flagellin induces LSK cell proliferation ex vivo. Whole bone marrow cells from WT B6 mice were incubated in complete RPMI with or without 50 ng/mL flagellin for 15 hours. The cells were then labeled with 10 µM BrdU for 1 hour and analyzed by flow cytometry. Representative flow cytometry plots comparing LSK proliferation (A), LSK cell percentage in bone marrow (B), LSK cell number in bone marrow (C), and BrdU incorporation (D) in PBS-treated vs flagellin-treated bone marrow cells are shown. Statistics were analyzed by unpaired Student t test (***P ≤ .001). Error bars represent SEM.
Flagellin induces LSK cell proliferation indirectly through other bone marrow cells
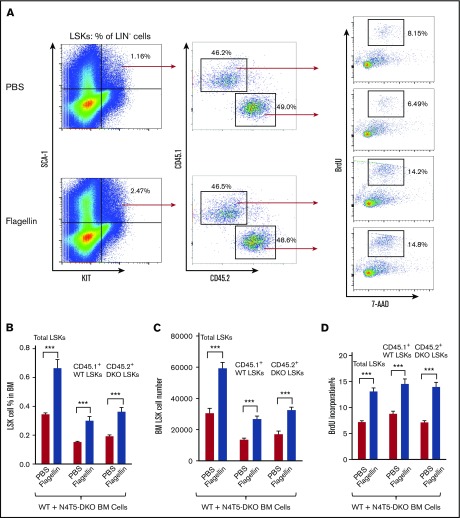
The key role of TLR5 in mediating bone marrow LSK cell proliferation in response to flagellin could be explained by activation of TLR5 on LSK cells themselves or result from another bone marrow cell population being activated via TLR5 in a manner that indirectly results in LSK cell proliferation. To investigate this question, we mixed at a 1:1 ratio bone marrow from CD45.1+ WT and CD45.2+ N4T5-DKO mice. The mixed bone marrow was then treated with flagellin in culture for 15 hours, and LSK cells were assayed as described above. The overall extent to which flagellin induced LSK cell proliferation tended to be modestly less in such mixed cultures, suggesting that the number of TLR5-expressing cells may be a limiting factor in this response. Nonetheless, we observed a similar increase in proliferation of LSK cells that originated in WT and N4T5-DKO mice, suggesting that activation of LSK cells by flagellin is indirect (Figure 4A-D). Analogous results were also seen in vivo. Specifically, we generated mixed chimeric mice in which ∼50% of donor bone marrow cells were from WT or N4T5-DKO mice and assayed for flagellin-induced LSK cell proliferation in vivo. Again, the extent of increase in LSK cell number and percentage of cells in S phase was reduced in these mice, which presumably had a 50% reduced number of cells capable of detecting flagellin. However, a similar increase in proliferation of LSK cells that originated from WT or N4T5-DKO mice was observed after flagellin treatment (supplemental Figure 5A-B). Hence, both ex vivo and in vivo approaches indicated that LSK cells were not directly sensing flagellin; rather, their proliferation was induced as a result of activation of another cell type in bone marrow by flagellin. To elucidate what bone marrow cell types might be driving flagellin-induced LSK cell proliferation, we sought to determine which bone marrow cell types express TLR5. Flow cytometry analysis indicated a significant expression of TLR5 on neutrophils (CD11b+Ly6G+ cells) (supplemental Figure 6), suggesting that flagellin may activate bone marrow neutrophils to mediate LSK cell proliferation.
Figure 4.
Flagellin activates LSK cell proliferation indirectly. Whole bone marrow cells from CD45.1+ WT and CD45.2+ N4T5-DKO mice were mixed in a 1:1 ratio and incubated in complete RPMI or RPMI containing 50 ng/mL flagellin for 15 hours, followed by 1-hour labeling with 10 µM BrdU. Cells were analyzed by flow cytometry as in Figure 1. Representative flow cytometry plots comparing LSK proliferation (A), LSK cell percentage in BM (B), LSK cell number in bone marrow (C), and BrdU incorporation (D) for PBS vs flagellin-treated mixed cell culture. Statistics were analyzed by unpaired Student t-test, error bars depict SEM. ***P ≤ .001.
Flagellin treatment preferentially induces hematopoietic progenitor cells in bone marrow
In addition to LT-HSCs, the LSK population also contains ST-HSCs and a range of multipotent hematopoietic progenitor populations (MPP1-MPP4).7,11,13,14 In order to better understand which of these subtypes were impacted by flagellin treatment, we performed flow cytometry analysis on LSK cells from flagellin- or PBS-treated WT B6 mice using the recently defined signaling lymphocytic activation molecule (SLAM) code, which is capable of distinguishing LT-HSC, ST-HSC, and MPP subtypes.14,25,26 This cellular identification protocol indicated that flagellin did not increase the level or alter the proliferative status of LT-HSCs (supplemental Figure 7A-C). Rather, flagellin treatment drove the proliferation of ST-HSCs and myeloid precursors MPP2 and especially MPP3, whose levels increased 10-fold following flagellin treatment (Figure 5A-E). This suggests that flagellin was not uniformly increasing the ability of bone marrow to repopulate the hematopoietic compartment but rather preferentially promoting proliferation of myeloid precursors.14 As a functional approach to complement this analysis, we also performed a classic competitive repopulation assay that compared the repopulation ability of bone marrow from flagellin-treated or control (PBS-treated) mice, wherein these donor marrows were distinguished by using mice with different CD45 antigen (CD45.1 or CD45.2). In accord with the SLAM-code flow cytometry analysis, this approach indicated that flagellin treatment did not alter the bone marrow’s long-term repopulation ability; rather, it preferentially enhanced repopulation of myeloid (CD11b+) lineages (supplemental Figure 8A-B).
Figure 5.
Flagellin treatment preferentially induces hematopoietic progenitor cell proliferation in bone marrow. Bone marrow cells from WT B6 mice treated with flagellin for 15 hours were analyzed by flow cytometry using antibodies including SLAM-code CD48 and CD150 to determine hematopoietic stem cell and hematopoietic progenitor cell proliferation. The following markers were used: LT-HSCs, CD34−Flt3−CD150+CD48−; ST-HSCs, CD34+Flt3−CD150−CD48−; MPP1, CD34+Flt3-CD150+CD48−; MPP2, CD34+Flt3−CD150+CD48+; MPP3, CD34+Flt3−CD150−CD48+; and MPP4, CD34+Flt3+CD150−CD48+. Shown are BrdU incorporation of MPP1, MPP2, MPP3, ST-HSCs (A), and MPP4 (B) in PBS- vs flagellin-treated mice, statistics of total LSK cell number (C); LT-HSC, ST-HSC, MPP1, MPP2, MPP3, and MPP4 cell numbers (D); and BrdU incorporation (E) in PBS- vs flagellin-treated mice. Statistics were analyzed by unpaired Student t test (**P < .01; ***P ≤ .001). Error bars represent SEM. BM, bone marrow.
Flagellin-induced MPP3 cells are myeloid biased and predominantly differentiate to neutrophils
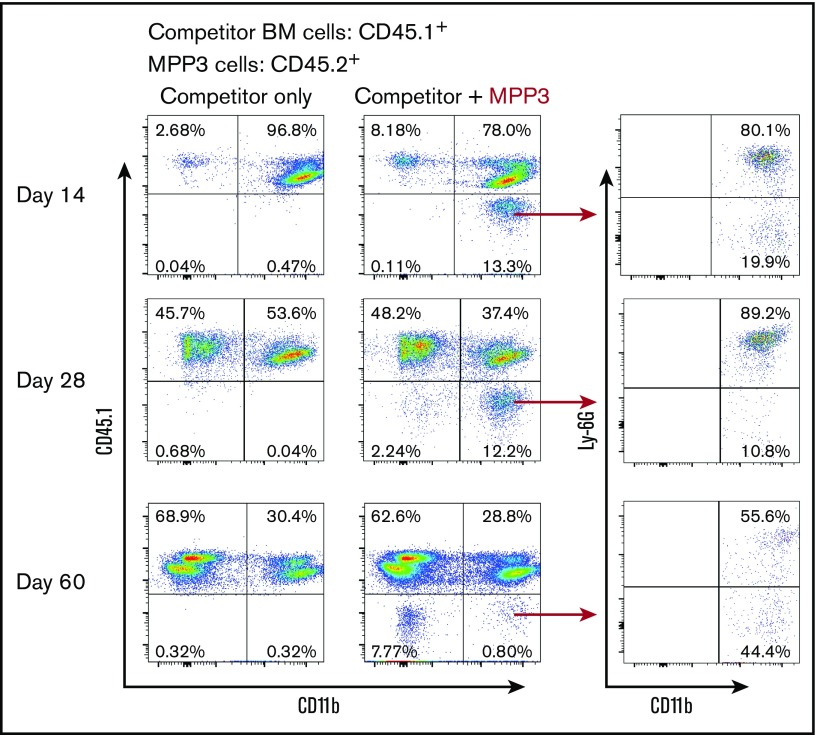
Next, we sought to better understand the functional capacity of the bone marrow cell types whose proliferation is promoted by flagellin, particularly MPP3 cells. FACS-sorted MPP3 cells isolated from the bone marrow of flagellin-treated CD45.2+ mice were adoptively transferred, along with whole bone marrow cells isolated from untreated CD45.1+ mice, to lethally irradiated mice. Peripheral blood was then collected on days 14, 28, and 60 and assayed by flow cytometry. On days 14 and 28, almost all detectable cells of MPP3 origin (CD45.2) were CD11b+ cells, accounting for 99.2% and 84.5% of the population, respectively, and these cells were predominantly neutrophils (Figure 6). During this period, ∼20% of all myeloid cells were of MPP3 origin, despite MPP3 comprising <1% of the administered cells. In contrast, by 60 days, the myeloid compartment was virtually devoid of cells of MPP3 origin (Figure 6). Thus, MPP3 have a high capacity to temporarily repopulate the myeloid compartment. In accord with this notion and earlier work, we observed that simply administering flagellin to an otherwise untreated WT mouse resulted in a rapid robust increase in blood neutrophils (supplemental Figure 9). Next, we sought to define whether flagellin was altering the relative ability of MPP3 to populate the myeloid compartment or was primarily functioning upstream to increase numbers of MPP3. In accord with the latter notion, we did not observe a significant difference in the ability of equal numbers of MPP3 cells from naive and flagellin-treated mice to develop along the myeloid lineage, nor did we observe a difference in neutrophil production (supplemental Figure 10A-B). However, we observed a linear relationship between input MPP3 cell number and the number of MPP3-originated neutrophils (supplemental Figure 11).
Figure 6.
Flagellin-induced MPP3 cells are myeloid biased and predominantly differentiate into neutrophils. Two thousand MPP3 cells were FACS-sorted from whole bone marrow cells of flagellin-treated CD45.2+ mice and transferred to irradiated CD45.1+ WT B6 mice along with 4 × 105 whole bone marrow cells from CD45.1+ mice. Whole blood cells of recipient mice were analyzed at indicated time points by flow cytometry using antibody to CD45.1, CD11b, and Ly6G to show peripheral blood cells differentiated from MPP3. Representative flow cytometry plots of MPP3-originated myeloid cells (CD45.1−CD11b+) and neutrophils (CD45.1−CD11b+Ly6G+) are shown.
Flagellin-induced MPP3 cells increase survival following irradiation/bone marrow transplantation
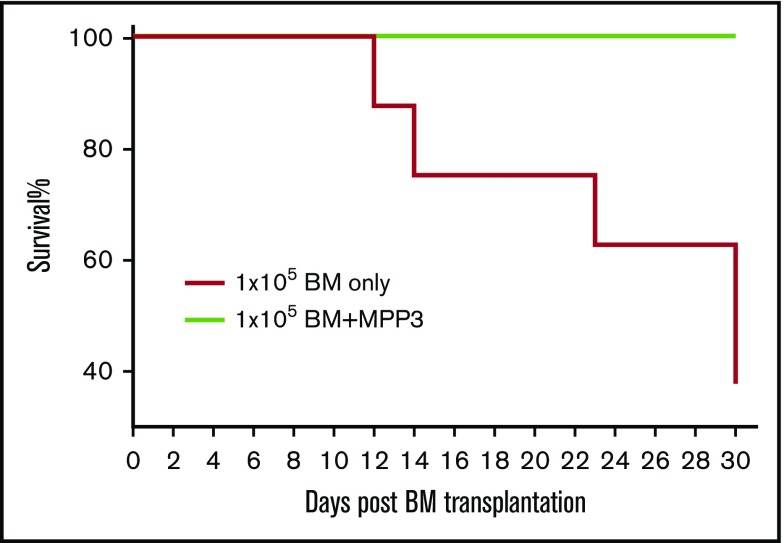
MPP3 cells are the main source of neutrophils, which play a critical role in survival after bone marrow transplantation or chemotherapy.14,31-35 We hypothesized that flagellin’s induction of MPP3 cell proliferation may underlie its radioprotective effect and, hence, that administration of exogenous purified MPP3 cells might have the ability to aid survival in a scenario in which administered bone marrow was insufficient for this purpose. To test this hypothesis, we lethally irradiated C57BL/6 mice and transferred 1 × 105 whole bone marrow cells (the number of cells needed, but not sufficient, to rescue them), whereas giving a higher number of such bone marrow cells allowed them survive.1 To test whether MPP3 cells could aid survival, we lethally irradiated mice (n = 8 per condition) and administered 1 × 105 whole bone marrow cells with or without 5 × 103 FACS-sorted MPP3 cells isolated from flagellin-treated mice. The majority of mice receiving only 1 × 105 bone marrow cells died within 3 weeks of the procedure. In contrast, all irradiated mice receiving bone marrow cells plus MPP3 remained alive throughout the 30-day period (Figure 7). A complete blood count indicated that mice receiving bone marrow plus MPP3 showed an approximate twofold increase in the number of white blood cells and a fourfold increase in the number of red blood cells at 3 weeks posttransfer (the average white and red blood cell counts for mice receiving bone marrow plus MPP3 or bone marrow only were 1.6 × 108/L vs 0.8 × 108 /L and 8.0 × 1011/L vs 2 × 1011/L, respectively). We next performed a similar experiment using outbred Swiss-Webster mice, which like human donor-recipient pairs that rarely have highly precise major histocompatibility complex matching (n = 10 per condition) and observed a similar degree of protection by addition of MPP3 cells (supplemental Figure 12). That temporary repopulation of the myeloid compartment via administration of small numbers MPP3 cells greatly improved survival after irradiation suggests that these cells might have the potential to treat a range of conditions in which neutropenia is problematic.
Figure 7.
Flagellin-induced MPP3 cells significantly increase mouse survival after bone marrow transplantation. Five thousand MPP3 cells were FACS-sorted from whole bone marrow cells from flagellin-treated mice and transferred to irradiated mice along with 1 × 105 whole bone marrow cells from the same species. In parallel, additional group of mice were irradiated and given whole bone marrow cells only. After bone marrow transplantation, mice were housed under sterile conditions, and survival was observed for 30 days. Both donor and recipients were C57BL/6 mice.
Discussion
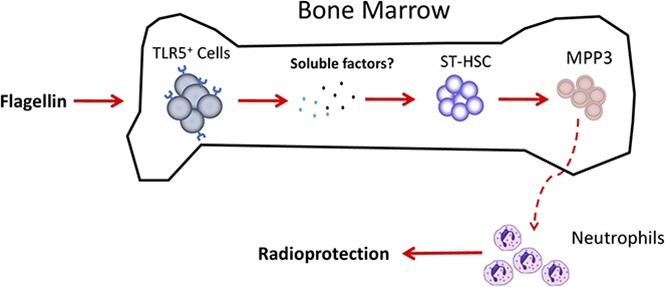
This study sought to define the extent to which systemic treatment with bacterial flagellin impacts populations of cells in the bone marrow. We hypothesized that attaining such knowledge would elucidate the mechanism by which bacterial flagellin administration protects against ionizing radiation. Herein, we report that such flagellin treatment activated TLR5 on bone marrow cells and drove the rapid expansion of hematopoietic progenitor cells, providing an ample neutrophil reserve in the bone marrow. We view this mechanism as one that would be robustly activated in a major infectious or injurious event wherein it provides a means to couple the emergency recruitment of neutrophils to the acceleration of the production pipeline. However, some toxic insults such as radiation and chemotherapy that might create a need for rapid neutrophil production would tend to destroy rapidly dividing progenitor cells. Our results indicate that this problem can be surmounted by direct administration of neutrophil precursors following radiation. Such temporary boost in neutrophils, conferred by flagellin-elicited MPP3 cells in particular, afforded a clear survival advantage following irradiation. We propose that transfer of such MPP3 cells may be developable as a novel strategy to treat a variety of diseases associated with neutropenia, especially those in which the disease or its treatment has impaired endogenous hematopoiesis. Given that each transferred MPP3 cell produces hundreds of millions of daughter cells in its weeks-long lifetime in its new host,14 we envision it might provide a greater and more lasting benefit than granulocyte transfers, which are currently used to treat severely neutropenic patients. Conversely, the temporary nature of these cells and their minimal tendency to develop along lymphocytic lineages should minimize the likelihood of graft-versus-host disease or other long-term adverse events associated with stem cell transplants. In accord with this notion, we note that, in fact flagellin treatment of mice has been shown to reduce disease severity in a mouse model of graft-versus-host disease.36
Ability to drive the remodeling of the bone marrow compartment is not unique to flagellin. Rather, emergency hematopoiesis has been described in a number of infectious events and in response to other TLR agonists.37-41 One important difference between flagellin and such agonists is that flagellin does not induce large amounts of master proinflammatory cytokines such as tumor necrosis factor α and IL-12 p70, enabling it to be administered repeatedly at doses similar to those used here without significant clinical-type adverse events. Another difference is that some TLR agonists are reported to directly act upon LSK cells, which was not the case in our study.41 There also appears to be a difference between hematopoiesis induced by flagellin and that described for other agents in that the hematopoiesis induced by flagellin seems particularly biased toward neutrophil production. Such bias is in accord with observations that the most numerous and highly flagellin-responsive cell type, namely epithelial cells, produces copious amounts of neutrophil-attracting chemokines in response to flagellin.6,21,42 Thus, a systemic exposure to flagellin would at once mobilize neutrophils to mucosal surfaces and activate production of these cells.3 This explains our previous observation that repeated treatment of mice with flagellin over a 10-day period resulted in an enormous load of neutrophils in the intestine.3 The production phase of this process can be initiated quickly, as we observed a sixfold increase in blood neutrophils 15 hours following flagellin treatment (supplemental Figure 9).
The requirement for TLR5, but not NLRC4, in mediating flagellin-induced hematopoiesis is not surprising, as the binding of these receptors leads to very different signal pathways: nuclear factor κB activation by TLR5 and caspase-1 activation by NLRC4.42-44 Surprising to us, however, is that such hematopoiesis was driven by TLR5-expressing cells in the bone marrow compartment (Figures 3A-D and 4A-D). Given that, relative to other TLR agonists, flagellin preferentially activates hepatocytes and cells at mucosal surfaces (epithelial cells and mucosal CD11c+ phagocytes), we had initially presumed that flagellin’s impact on the bone marrow compartment was driven by its systemic induction of cytokines.21,45 Indeed, recent studies strongly suggested that flagellin-induced liver cytokines impact the bone marrow compartment, resulting in leukocyte egress that plays a role in radioprotection.2,28 However, use of mice that lack TLR5 in hepatocytes,17 which account for ∼98% of the TLR5 messenger RNA in this organ, indicates such TLR5 was not needed for activation of flagellin-induced hematopoiesis (supplemental Figure 2A-D). Rather, a very similar pattern of results using WT/TLR5-knockout bone marrow chimeras and ex vivo study with culture of WT or N4T5-DKO bone marrow cells argued that flagellin-induced hematopoiesis was mediated by activation of TLR5 on bone marrow cells (Figure 3A-D; supplemental Figure 3A-B). Given that we have not observed TLR5 expression on LSK cells (data not shown) or MPP3 cells (supplemental Figure 13), the activation of LSK cell proliferation and MPP3 cell expansion by flagellin was likely not direct but rather driven by a locally produced mediator, possibly a cytokine or chemokine. To date, we have, via gene-deletion approaches and antibody neutralizations, ruled out possible roles for G-CSF and IL-22, both of which are abundantly induced by flagellin in a TLR5-dependent manner (supplemental Figures 14A-B and 15A-D). We continue to consider other candidate cytokines and possible cell contact–mediated signals and nonproteinaceous mediators. For example, in equine neutrophils, flagellin activates generation of reactive oxygen species, which are increasingly appreciated to serve as signal transducers in a broad range of cell types.46 In any case, we anticipate that future studies will more precisely reveal how activating neutrophils or other bone marrow cells with flagellin drive neutrophil progenitor proliferation, and such knowledge will pave the way for new strategies to treat neutropenia in a range of disease states.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK099071 and DK083890.
Authorship
Contribution: B.Z. and A.T.G. designed the study and wrote the manuscript; B.Z., D.O.-S., and J.Z. performed the studies and collected and analyzed the data; I.R.W. helped design the research; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benyue Zhang, Center for Inflammation, Immunity, and Infection, Institute for Biomedical Science, Georgia State University, 100 Piedmont Ave SE, RM732, Atlanta, GA 30303; e-mail: bzhang8@gsu.edu.
References
- 1.Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008;180(12):8280-8285. [DOI] [PubMed] [Google Scholar]
- 2.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Chassaing B, Shi Z, et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346(6211):861-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14(9):1065-1074. [DOI] [PubMed] [Google Scholar]
- 5.Tsujimoto H, Uchida T, Efron PA, et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005;78(4):888-897. [DOI] [PubMed] [Google Scholar]
- 6.Tallant T, Deb A, Kar N, Lupica J, de Veer MJ, DiDonato JA. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93-106. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29(4):776-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitnicka E. From the bone marrow to the thymus: the road map of early stages of T-cell development. Crit Rev Immunol. 2009;29(6):487-530. [DOI] [PubMed] [Google Scholar]
- 10.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26(7):360-366. [DOI] [PubMed] [Google Scholar]
- 11.Sureshkumar P, Srinivasan SP, Natarajan K, Gaspar JA, Hescheler J, Sachinidis A. Stem cells and differentiation--a synoptic review of patents granted since 2009. Expert Opin Ther Pat. 2015;25(6):663-673. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg LR, Dooner MS, Johnson KW, et al. The murine long-term multi-lineage renewal marrow stem cell is a cycling cell. Leukemia. 2014;28(4):813-822. [DOI] [PubMed] [Google Scholar]
- 13.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):640-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietras EM, Reynaud D, Kang YA, et al. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17(1):35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheretakis C, Leung R, Sun CX, Dror Y, Glogauer M. Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood. 2006;108(8):2821-2826. [DOI] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol. 2010;40(12):3528-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-like receptor 5 promotes bacterial clearance and protects mice against high-fat diet-induced liver disease. Cell Mol Gastroenterol Hepatol. 2016;2(5):584-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167(4):1882-1885. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz AT, Simon PO Jr, Schmitt CK, et al. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107(1):99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scapini P, Nardelli B, Nadali G, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197(3):297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders CJ, Moore DA III, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180(11):7184-7192. [DOI] [PubMed] [Google Scholar]
- 22.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126(4):1614-1619. [PubMed] [Google Scholar]
- 23.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1(3):263-270. [DOI] [PubMed] [Google Scholar]
- 24.Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci USA. 1990;87(22):8736-8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabezas-Wallscheid N, Klimmeck D, Hansson J, et al. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15(4):507-522. [DOI] [PubMed] [Google Scholar]
- 26.Napier RJ, Norris BA, Swimm A, et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015;11(3):e1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Maele L, Carnoy C, Cayet D, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185(2):1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdelya LG, Brackett CM, Kojouharov B, et al. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc Natl Acad Sci USA. 2013;110(20):E1857-E1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnebrew MA, Buffie CG, Diehl GE, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147(6):1363-1377. [DOI] [PMC free article] [PubMed]
- 31.Ramaprasad C, Pouch S, Pitrak DL. Neutrophil function after bone marrow and hematopoietic stem cell transplant. Leuk Lymphoma. 2010;51(5):756-767. [DOI] [PubMed] [Google Scholar]
- 32.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 33.Garelli S, Schieppati G, Banfi L, Meroni P. Neutrophil function during cyclic chemotherapy for cancer disease. Tumori. 1983;69(5):409-415. [DOI] [PubMed] [Google Scholar]
- 34.Gandossini M, Souhami RL, Babbage J, Addison IE, Johnson AL, Berenbaum MC. Neutrophil function during chemotherapy for Hodgkin’s disease. Br J Cancer. 1981;44(6):863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerli W, Zarth A, Gratwohl A, Speck B. Neutrophil function and pyogenic infections in bone marrow transplant recipients. Blood. 1991;77(2):393-399. [PubMed] [Google Scholar]
- 36.Hossain MS, Jaye DL, Pollack BP, et al. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. J Immunol. 2011;187(10):5130-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vega-Letter AM, Kurte M, Fernández-O’Ryan C, et al. Differential TLR activation of murine mesenchymal stem cells generates distinct immunomodulatory effects in EAE. Stem Cell Res Ther. 2016;7(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, Lin J, Cui J, et al. Radioprotection of bone marrow hematopoiesis by CpG-oligodeoxynucleotides administered to mice after total-body irradiation. J Radiat Res (Tokyo). 2011;52(6):828-833. [DOI] [PubMed] [Google Scholar]
- 39.Pascutti MF, Erkelens MN, Nolte MA. Impact of viral infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;7:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glatman Zaretsky A, Engiles JB, Hunter CA. Infection-induced changes in hematopoiesis. J Immunol. 2014;192(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yáñez A, Goodridge HS, Gozalbo D, Gil ML. TLRs control hematopoiesis during infection. Eur J Immunol. 2013;43(10):2526-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vijay-Kumar M, Gewirtz AT. Flagellin: key target of mucosal innate immunity. Mucosal Immunol. 2009;2(3):197-205. [DOI] [PubMed] [Google Scholar]
- 43.Muñoz-Wolf N, Rial A, Fougeron D, Tabareau J, Sirard JC, Chabalgoity JA. Sublingual flagellin protects against acute pneumococcal pneumonia in a TLR5-dependent and NLRC4-independent fashion. Future Microbiol. 2016;11:1167-1177. [DOI] [PubMed] [Google Scholar]
- 44.Gewirtz AT. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol. 2006;22(1):8-12. [DOI] [PubMed] [Google Scholar]
- 45.Strindelius L, Filler M, Sjöholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22(27-28):3797-3808. [DOI] [PubMed] [Google Scholar]
- 46.Kwon S, Gewirtz AT, Hurley DJ, Robertson TP, Moore JN, Vandenplas ML. Disparities in TLR5 expression and responsiveness to flagellin in equine neutrophils and mononuclear phagocytes. J Immunol. 2011;186(11):6263-6270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.