Abstract
Objective
To determine the change in general health-related quality of life (HRQOL) after cochlear implantation and its association with speech recognition.
Study Design
Meta-analysis
Methods
Search was performed independently following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement by two authors using PubMed, Medline, Scopus, and CINAHL. Studies on adult cochlear implant (CI) patients measuring HRQOL before and after cochlear implantation were included. Standardized mean difference (SMD) for each measure and pooled effects were determined. Subset analyses of the Health Utilities Index -3 (HUI-3) and psychological measures were also conducted. A meta-analysis of correlations was also performed between all non-disease-specific patient-reported outcome measures (PROMs) and speech recognition after cochlear implantation.
Results
Twenty-two articles met criteria for meta-analysis of HRQOL improvement, but 15 (65%) were excluded due to incomplete statistical reporting. From the seven articles with 274 CI patients that met inclusion criteria, pooled analyses showed a medium positive effect of cochlear implantation on HRQOL (SMD = 0.79). Subset analysis of the HUI-3 measure showed a large effect (SMD = 0.84). Nine articles with 550 CI patients met inclusion criteria for meta-analysis of correlations between non-disease specific PROMs and speech recognition after cochlear implantation. Pooled analysis showed a low correlation between non-disease-specific PROMs and word recognition in quiet (r = 0.35), sentence recognition in quiet (r = 0.40), and sentence recognition in noise (r = 0.32).
Conclusion
Although regularly used, HRQOL measures are not intended to measure nor do they accurately reflect the complex difficulties facing CI patients. Accordingly, only a medium positive effect of cochlear implantation on HRQOL was observed along with a low correlation between non-disease-specific PROMs and speech recognition. The use of such instruments in this population may underestimate the benefit of cochlear implantation.
Keywords: cochlear implant, cochlear implantation, patient reported outcome measure, quality of life, speech recognition
Introduction
Cochlear implantation is the gold standard for treatment for individuals with severe to profound bilateral sensorineural hearing loss. With rising health care costs, much focus has been recently placed on the effectiveness of treatments, especially surgical procedures such as cochlear implantation. Since 2002, the National Institutes of Health has been focusing considerable attention towards patient-reported outcomes to ensure treatments are improving outcomes that are important to patients and, thus, providing significant benefit.1
Quality of life (QOL) patient-reported outcome measures (PROMs) are commonly used to determine the impact of an intervention on an individual's life. These PROMs can be subdivided into two major categories—general health and disease-specific. The former are generalizable instruments that are meant to be applied to large, diverse populations to evaluate overall QOL or an individual construct. In contrast, disease-specific instruments are typically validated for a particular population that share a common health condition or disability. General health-related quality of life (HRQOL) PROMs are the most commonly used instruments for economic analysis to determine the cost effectiveness of a particular treatment through measurement of total health.2,3 The importance of using QOL PROMs has been strengthened recently by an increased emphasis by the Center for Medicare and Medicaid Services (CMS) and the Food and Drug Administration (FDA)requirement to report these data.4,5
In contrast to the growing importance of patient-reported outcomes, open-set speech recognition scores measured in quiet and in noise continue to be the gold standard for assessing outcomes and benefit in adult CI recipients. In evaluating the literature, there does not appear to be a strong relationship between speech recognition ability and patient self-report.6-9 Reasons for these discrepancies are likely two-fold. First, the complex communication, social and emotional situations that CI users experience may not be fully represented by word or sentence recognition alone. Second, the manner in which cochlear implantation improves QOL likely extends well beyond improvements in speech recognition. Due to the routine use of word and sentence recognition scores in reporting CI outcomes and the increasing importance of reporting QOL PROMs, it is important to systematically determine the extent to which these measures correlate.
Our previous work has shown a very large effect size of cochlear implantation on QOL when measured with hearing and CI-specific PROMs10. For the current study, we sought to determine how this impact compares when using HRQOL PROMs that are routinely reported in the literature, but were not developed for or validated on individuals with hearing loss. This is an important comparison as the health utility and economic analyses of cochlear implantation are typically calculated using HRQOL PROMs . This study will help determine their utility in the CI population. A second meta-analysis assessed the association of HRQOL PROMs with speech recognition ability.
Materials and Methods
Search Methods
Literature search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.11 The PubMed, Scopus, and OVID/Medline databases were independently searched by two authors for the search terms: ‘cochlear implant,’ ‘cochlear implantation,’ ‘quality of life,’ and ‘patient-reported outcome measures’. This search identified 1,281 articles, of which, 591 were unique articles after eliminating duplicates (Figure 1). These 591 articles were first reviewed by abstract, which eliminated 360 articles. There were 231 articles remaining, which underwent full-text review for inclusion (one article satisfied criteria for both analyses). Disputes regarding the inclusion of a study were mediated with a third author to reach consensus.
Figure 1. Literature Review Process Flowchart.
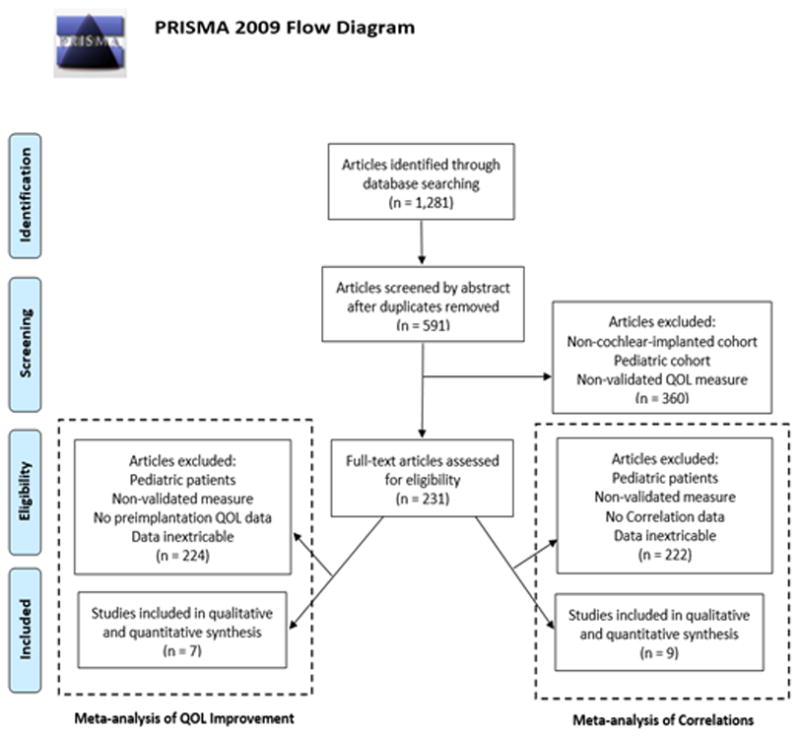
Literature review process utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) search method.
Letters to the editor, abstracts, book chapters, case reports and articles not published or translated in English were excluded. There were no date range limitations on date of publication. Studies with patients younger than 18 years old in the cohort were excluded. Analyzing PROMs in pediatric patients involves many different factors than adults, thus we limited the scope of this study to adult patients.12
The last time point available for each study was used for data collection. Data reported in graphical plots were not extracted for meta-analysis unless numerical data were available. We attempted to contact authors if we could not extract complete data from their publication; some authors provided additional data to allow inclusion of their study in our analysis.
Data Extraction
When selecting articles for meta-analysis of HRQOL improvement, studies meeting the following inclusion criteria were ultimately selected: assessment of HRQOL in an adult CI cohort before and after surgery (or in a post-treatment cohort versus a control cohort); sample size, mean, and standard deviation available for PROM data; and follow-up of at least 6 months. Two authors independently obtained data from articles including: year of publication, author, number of patients, patient demographics, and HRQOL PROM scores.
When selecting articles for meta-analysis of correlations, studies meeting the following inclusion criteria were used: correlation values of speech recognition scores versus any general PROM in an adult cohort after cochlear implantation; complete data available (sample size and Pearson or Spearman correlation values); and postoperative follow-up of at least 3 months. Two authors independently obtained data from articles including: author, year of publication, number of patients, patient demographics, speech recognition measure used, and correlation values. Studies included in this analysis did not require reporting of pre-implantation PROM data. Rather, this analysis aimed to evaluate the correlation of speech recognition ability and each respective PROM at the latest available time point after implantation.
Data reported in graphical plots were not extracted unless numerical values were published. We contacted authors to obtain complete details of results in the event of incomplete data in order to allow inclusion of their study. PROMs using a reverse scale (reduction in scores represent improved QOL) had standardized mean difference (SMD) values multiplied by negative one for analysis. Level of evidence for each selected article was evaluated with the Oxford Center for Evidence-Based Medicine.13
Meta-analysis of HRQOL Improvement Statistical Method
Meta-analysis of included studies evaluating the impact of cochlear implantation on HRQOL with a continuous measure (comparison of means and standard deviations between pre-implantation and post-implantation) was performed with Cochrane Review Manager (RevMan) version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, 2011, Copenhagen, Denmark). Fixed-effects and random- effects models were used in this study. Under the fixed-effects model, it is assumed that all studies come from a common population, and that the effect size as measured through SMD is not significantly different among the different trials. This assumption is tested by the heterogeneity test or I2 statistic. If this test yields a low probability value (p < 0.05), then there is a high likelihood the fixed-effects model is invalid and the random-effects model is more appropriate. The random-effects model incorporates both the random variation within the studies and the variation between the different studies.14 The random- effects model provides a more conservative estimate (i.e., a wider confidence interval), but the results from the two models typically agree when there is no heterogeneity. When heterogeneity was present, the random-effects model was the preferred model.
Potential publication bias was evaluated by visual inspection of the funnel plot and Egger's regression test, which statistically examines the asymmetry of the funnel plot.15 For this analysis, the null hypothesis was that there is no difference between pre-implantation and post-implantation PROM scores. Analysis was performed on two subsets of PROMs: the Health Utilities Index 3 (HUI-3) HRQOL PROM and other HRQOL PROMs. Data are presented as SMD [95% confidence interval]. The following thresholds were used for subjective assessment of effect size: 0.2 - small effect, 0.5 – medium effect, and 0.8 – large effect.16
Meta-analysis of Correlations Statistical Methods
A meta-analysis of correlations was performed for correlations between speech recognition and HRQOL PROMs using MedCalc 17.2 (MedCalc Software, Ostend, Belgium). Heterogeneity testing was performed as previously described. Each study was weighted according to the number of patients included. MedCalc uses the Hedges-Olkin method for calculating the weighted summary correlation coefficient under the fixed-effects model, using a Fisher Z transformation of the correlation coefficients.17 Under the random-effects model, the heterogeneity statistic is incorporated in order to calculate the summary correlation coefficient.19 For this analysis, the null hypothesis was that speech recognition ability and PROM scores do not correlate. The following thresholds were used for subjective assessment of correlation values (r): 0 - 0.3, negligible; 0.3 – 0.5, low; 0.5 – 0.7, medium; 0.7 – 0.9, high; 0.9 – 1.0, very high.18,19
Results
Meta-analysis of HRQOL improvement
Twenty-two articles met many of the inclusion criteria for outcomes analysis, but 15 (68%) were excluded due to incomplete statistical reporting, leaving 7 articles for analysis (Table 1). From the 7 articles, 274 patients were included in the analysis with 100% of patients having published sex data (46% male, 54% female). The mean age across all study cohorts ranged from 49 – 62 years. Pooled analyses showed a medium positive effect of cochlear implantation on HRQOL (SMD = 0.79 [0.39 – 1.19]). Subset analysis of the HUI-3 showed a large positive effect (SMD = 0.84 [0.57 – 1.12]), whereas subset analysis of other HRQOL PROMs showed a medium effect (SMD = 0.76 [0.16 – 1.36]) (Figure 2). To investigate the presence of publication bias, inspection of the funnel plot of effects calculated from individual studies was performed. According to funnel plots and the Egger's test, there was no indication of publication bias (p = 0.272) among the set of studies included in this meta-analysis.
Table 1. Articles Included in Meta-analysis of HRQOL Improvement.
| Article | Level of Evidence | Cohort Age Mean ± SD (Range) | Male %/Female % | Follow-Up Time (Months) |
|---|---|---|---|---|
| Damen 20076 | 3 | 49.6 ± 10.9 | 54/46 | ≥ 12 |
| Arnoldner 201420 | 4 | 62 (18–89) | 51/49 | ≥ 11 |
| Klop 200821 | 4 | 54.7 ± 15.7 | 34/66 | 12 |
| Palmer 199922 | 3 | 56.0 ± 15.4 | 46/54 | 12 |
| Hawthorne 200425 | 4 | 49 ± 13 | 47/53 | 6 |
| Mo 200524 | 4 | 57.6 ± 14.5 (28-82) | 44/56 | 12 |
| Krabbe 200023 | 4 | 51 ± 16 | 47/53 | ≥ 12 |
Articles satisfying inclusion criteria for meta-analysis of HRQOL Improvement. Level of Evidence, cohort age mean, SD, and range (if available), male/female cohort ratio, and follow-up time in months.
Figure 2. Forest plot of meta-analysis of HRQOL Improvement.
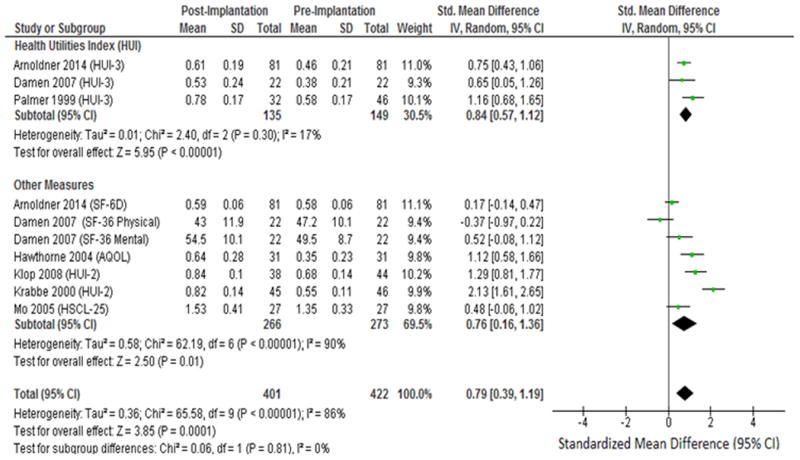
Forest plot of HRQOL PROMs including subset analysis of Health Utilities Index 3 (HUI-3) and other HRQOL measures. 36-Item Short Form (SF-36); 6-item descriptive system (SF-6D); Assessment of Quality of Life (AQOL); Hopkins Symptom Checklist-25 (HSCL-25); IV: Inverse Variance.
Five studies in our analysis utilized the HUI PROM.6,20-23 Both the HUI-2 and HUI-3 are used to evaluated QOL.3 The HUI-2 classification system utilizes 7 domains (sensation, mobility, emotion, cognitive, self-care, pain, fertility) whereas the HUI-3 classification utilizes nine alternative domains (vision, hearing, speech, ambulation, dexterity, emotion, cognition, pain). The HUI-3 hearing domain directly ascertains hearing function by asking patients if they can perceive what others are saying in various settings. For example, the HUI-3 asks patients if they are: “Able to hear what is said in a conversation with one other person in a quiet room, without a hearing aid, but unable to hear what is said in a group conversation with at least three other people even with a hearing aid.” The HUI-2 sensation domain collects similar but more basic information about hearing function while combining it with other senses. For example, the HUI-2 asks if patients can “See, hear, or speak with limitations even with equipment”. All five included studies utilizing the HUI found significant improvement in the hearing or sensation domain for CI patients. Only one study using the HIU-3 found improvement in the speech domain for CI patients.22 Additionally, 2 of the 5 studies of utilizing either HUI found significant improvement in the emotion domain for CI patients.20,23 No other domains except those mentioned above showed significant improvement in the five included studies utilizing the HUI.
Damen et al.6 utilized the SF-36 and found no significant change in the physical summary or mental summary for CI patients. Specifically, significant improvement for CI patients was found only in the mental health subdomain of the mental summary. Arnoldner et al.20 utilized the SF-6D conversion measure to find a total utility score of the SF-36 measure; this study found a significant improvement in the mental health and social functioning subdomains for CI patients, but overall there was no detectable effect of cochlear implantation on HRQOL (SMD = 0.17 [-0.14 – 0.47]).
Mo et al.24 utilized the 25-question Hopkins Symptom Checklist (HSCL-25), a HRQOL PROM that gauges anxiety and depression in patients, finding nearly twice the improvement in the depression domain compared to the anxiety domain for CI patients but overall had no detectable effect of cochlear implantation on HRQOL (SMD = 0.48 [-0.06 – 1.02]). The Assessment of QOL (AQOL) HRQOL PROM was utilized by one study,25 finding a large positive effect on HRQOL of cochlear implantation (SMD = 1.12 [0.58 – 1.66]); this 15-question measure has one question specifically asking about hearing function, whereas others questions ask about associated ailments of poor health including emotional and social problems.
Meta-Analysis of Correlations
Nine articles met criteria for inclusion in this analysis with none being excluded due to incomplete statistical reporting (Table 2).6,7,9,26-31 A total of 550 patients were included with 41% of the patients having published sex data (45% male, 55% female). The mean age across all studies ranged from 36.8 to 63.4 years.
Table 2. Articles Included in Meta-analysis of Correlations.
| Article | Level of Evidence | Speech Recognition Measure | Cohort Age Mean ± SD (Range) | Male %/Female % | Follow-Up Time (Months) |
|---|---|---|---|---|---|
| Calvino 201527 | 4 | NS | 52.8 ± 14.0 | 45 / 55 | ≥ 6 |
| Damen 20076 | 3 | Words in Quiet: Antwerp-Nijmegen, NVA | 49.6 ± 10.9 | 54/46 | ≥ 12 |
| Francis 20029 | 4 | Words in Quiet: NS Sentences in Quiet: CID |
63.4 ± 8.6 (50 - 80) | NA | ≥ 6 |
| Hirschfelder 200831 | 4 | Words in Quiet: Freiburg monosyllables Sentences in Noise: HSM Noise |
50.2 ± 14.4 (21 - 72) | 36/64 | ≥ 12 |
| Knutson 199826 | 4 | Words in Quiet: NU-6 Sentences in Quiet: Iowa Sentence Test |
51.8 ± 14.3 (24 - 70) | 46/54 | 54 |
| Kumar 20167 | 4 | Sentences in Quiet: BKB/CUNY | 36.8 (18 – 68) | NA | 12 |
| Sanchez-Cuadrado 201528 | 4 | NS | 60 (24-85) | 46/54 | ≥ 6 |
| Vermeire 200530 | 4 | Words in Quiet: NVA | 58 | NA | ≥ 4 |
| Vermeire 200629 | 4 | Words in Quiet: NVA | 62 (40-78) | NA | ≥ 3 |
Articles satisfying inclusion criteria for meta-analysis of correlations. Level of evidence, speech recognition measure used; patient age (mean, standard deviation, and range); male/female percentages; and follow-up time in months. NS: Not Specified; CID: Central Institute for the Deaf; NVA: Dutch Audiological Society; HSM - Hochmair Schulz Moser; NU-6: Northwestern University Auditory Test Number Six; CUNY: City University of New York; BKB: Bamford-Kowal-Bench.
Low pooled correlations were found between HRQOL PROMs and the three categories of speech recognition testing: word recognition in quiet (r = 0.35 [0.25 – 0.45]), sentence recognition in quiet (r = 0.40 [0.30 – 0.49]), and sentence recognition in noise (r = 0.32 [0.19 – 0.44]) (Table 3, Figure 3). Subset analysis of results with psychological PROMs showed low correlations with word recognition in quiet (r = 0.41 [0.28 – 0.53]) and sentence recognition in quiet (r = 0.45 [0.32 – 0.56]). Subset analysis of HRQOL PROMs showed low correlations with word recognition in quiet (r = 0.33 [0.19 – 0.46]), sentence recognition in quiet (r = 0.34 [0.18 – 0.48]), and sentence recognition in noise (r = 0.32 [0.19 – 0.44]).
Table 3. Meta-analysis of Correlations Results.
| r | 95% CI | I2 | p | |
|---|---|---|---|---|
| Subtotal: HRQOL | ||||
| Word recognition in quiet | 0.330 | 0.191 – 0.456 | 64.39% | 0.0027 |
| Sentence recognition in quiet | 0.335 | 0.180 – 0.475 | 57.53% | 0.0949 |
| Sentence recognition in noise | 0.323 | 0.193 – 0.442 | 0.00% | 0.4163 |
| Subtotal: Psychological | ||||
| Word recognition in quiet | 0.413 | 0.282 – 0.530 | 0.00% | 0.7943 |
| Sentence recognition in quiet | 0.445 | 0.316 – 0.557 | 0.00% | 0.9919 |
| Sentence recognition in noise | NA | NA | NA | NA |
| Total | ||||
| Word recognition in quiet | 0.353 | 0.252 – 0.445 | 51.46% | 0.0110 |
| Sentence recognition in quiet | 0.397 | 0.299 – 0.487 | 0.00% | 0.5126 |
| Sentence recognition in noise | 0.323 | 0.193 – 0.442 | 0.00% | 0.4163 |
Pooled correlation values (r and 95% confidence interval [CI]) and heterogeneity statistics (I2 and p) for meta-analysis of correlations. NA: Not Applicable.
Figure 3. Forest Plot of Meta-analysis of Correlations.
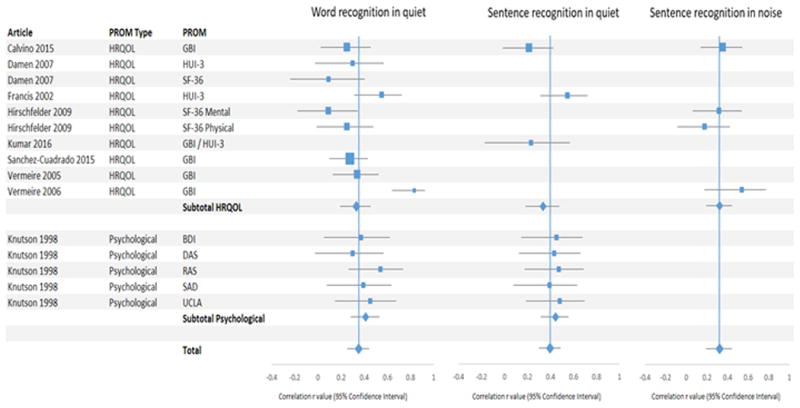
Forest plots pertaining to meta-analysis of correlations for articles reporting health-related QOL (HRQOL) (top) and psychological QOL (bottom) measures. Pooled correlations are represented by diamonds. PROM: patient-reported outcome measure; CI: cochlear implant
Hirschfelder et al.31 used the SF-36 and found negligible correlations of the mental (r = 0.09) and physical summaries (r = 0.25) with word recognition in quiet, a negligible correlation of SF-36 physical summary with sentence recognition in noise (r = 0.18), and a low correlation with SF-36 mental summary with sentence recognition in noise (r = 0.32). On analysis of the SF-36 subdomains, this study found low correlations between word recognition in quiet and physical functioning (r = 0.40) and vitality (r = 0.44), and sentence recognition in noise with vitality (r = 0.50) and mental health (r = 0.38) subdomains. No correlations with speech recognition scores were found with respect to emotional role functioning, social functioning, general health perception, pain, and physical role functioning.
Vermeire et al.29 found the highest correlations among HRQOL PROMs in our study (r= 0.83), correlating the Glasgow Benefit Inventory (GBI) and word recognition in quiet. This finding was an outlier as the three other studies correlating GBI versus word recognition in quiet found correlations of 0.25, 0.28, and 0.34. 28-30 The outlying study also utilized the smallest sample size (n = 24) resulting in a large confidence interval (0.64 – 0.92).
Only two other studies found an overall medium correlation or higher (r ≥ 0.50). Francis et al. found a correlation of 0.55 for both word recognition in quiet and sentence recognition in quiet with the HUI measure; when analyzing specific domains, this study found the highest correlations of the HUI emotion domain versus word recognition in quiet (r = 0.47) and sentence recognition in quiet (r = 0.48), but surprisingly found negligible correlations with the HUI hearing domain. Knutson et al.26 found a medium correlation (r = 0.54) between the Rathus Assertiveness Scale (RAS) and word recognition in quiet. The RAS attempts to determine a patient's social confidence and assertiveness. All other studies reviewed found negligible or low correlations with speech recognition abilities on review of PROM total score correlations or published PROM subdomain correlations.
Discussion
HRQOL PROMs are instruments that assess the patient's perspective of their health, illness, or the effects of interventions.32 Uses for PROMs include screening measures, effectiveness metrics, clinical trial endpoints, and economic assessments. The current study is a meta-analysis of HRQOL improvement following cochlear implantation and the first meta-analysis to assess the correlations between speech recognition and HRQOL PROMs in CI patients. We limited these analyses to HRQOL PROMs that are commonly used for a wide range of patients and conditions as we sought to understand the utility of these non-disease-specific PROMs in the CI population.
We found two previous studies performing reviews of the literature of PROM changes after CI; however, they utilized different inclusion criteria than our study.33,34 Gaylor et al.33 included many studies that reported preoperative QOL using a retrospective question format, that is, asking patients after implantation about their health status prior to implantation. This retrospective approach to data gathering is limited by recall bias and, therefore, studies that included data gathered in this manner were excluded from our analysis. In contrast to Gaylor et al., we did not include the GBI in our meta-analysis of HRQOL improvement, as this measure requires the patient to subjectively assess their improvement following cochlear implantation.35 The review by Loeffler et. al.34 included qualitative analyses of HRQOL improvement and did not perform a quantitative analysis. Our literature search found no quantitative meta-analyses of correlations of speech recognition ability and HRPROMs in CI patients.
In the current study, we found that cochlear implantation was associated with medium positive effect of cochlear implantation in HRQOL, which is a far smaller effect than we reported for hearing and CI-specific QOL PROMs (SMD = 1.82 and 1.69, respectively).10 This is expected as HRQOL PROMs do not typically include questions related to communication and instead focus on topics that may be unrelated to cochlear implantation, such as bodily pain, physical function, and vision. HRQOL PROMs, such as HUI-3 and SF-36, are often used to determine the economic utility of surgical interventions such as cochlear implantation. Thus, given the larger effect on QOL observed when using hearing/CI-specific, HRQOL measures may underestimate the quality-adjusted life years and other economic impacts of cochlear implantation. This difference in self-reported benefit may also render HRQOL PROMs insensitive to the economic benefits of a second cochlear implant, electroacoustic listening modalities, and auditory rehabilitation services.
SMDs from all HRQOL PROMs in our study ranged from -0.37 to 2.13 with a corresponding I2 value of 86%, indicating a high amount of heterogeneity. This heterogeneity may results from HRQOL PROMs measuring different domains of QOL and/or populations evaluated across studies were heterogeneous in the impact of cochlear implantation on their daily lives. It is important to note that these HRQOL measures have not been validated in individuals with hearing loss or patients with CIs. Therefore, these inconsistent results may represent individual differences in domains unrelated to cochlear implantation that may represent the diverse population that are implanted rather than changes related to implantation.
The above emphasizes the importance of using QOL PROMs developed and validated in the CI population. With improved communication abilities, we hypothesize that patients' social wellness and participation will also improve, and loneliness, isolation, and depression will decrease.26,36,37 However, the psychosocial changes that accompany an individual reentering the hearing world after cochlear implantation may be unique to the CI population and difficult to assess with more general HRQOL PROMs.
Given that speech recognition ability has traditionally been the primary outcome measure in cochlear implantation, it was important to understand the correlation between speech recognition scores and patient self-report through HRQOL PROMs. The narrow range of pooled correlation values (r = 0.32 – 0.45) demonstrates that PROMs have a low correlation with all categories of speech recognition testing. These correlation values are similar to and slightly higher than the correlations between hearing/CI-specific QOL measures and speech recognition scores (0.20 – 0.28 and 0.21 – 0.26, respectively).10 Moreover, the coefficient of determination (or the proportion of variance attributable to the independent variable) reveals that only 10.2% to 20.3% of the variation in HRQOL can be attributed to speech recognition abilities, as measured by word and sentence recognition scores. Interestingly, our prior work showed that speech recognition ability accounts for even less of the variation in QOL when measured using hearing and CI-specific QOL instrument (4.8-5.8%).10 This weak association between patient reported improvement and measured speech recognition abilities underscores the importance of using disease-specific PROMs to evaluate the benefits of cochlear implantation for individual patients. How individuals listen, communicate, and interact with their environment is likely far more complex than conventional speech recognition tasks, even when performed in background noise.
The National Institutes of Health established the Patient-Reported Outcomes Measurement Information System (PROMIS) in 2004 to improve the assessment of patient-reported outcomes and development of instrument with the goals of reliability, precision, and construct validity.38 PROMIS has created QOL instruments that have undergone rigorous validity testing for cross-sectional, content, and clinical validity.39-41 Creating these instruments also consist of including patients in the question development process to specifically address the QOL-related challenges facing the population of interest. No CI-specific PROM has been developed using this rigorous validation methodology. A PROM specifically developed for and validated on the CI population, would expand our ability to understand and report the communication, social, emotional, and other experiences of CI users.
Our study is limited by biases inherent to many systematic reviews of the literature, particularly publication bias. In addition, the speech recognition measures used by the included studies varied widely. We performed separate analyses for word recognition in quiet, sentence recognition in quiet, and sentence recognition in noise in an attempt to minimize these differences. Pooled estimates account for these differences, as study differences such as this are common in meta-analyses. We were unable to perform a multivariate analysis that might account for population differences as individual patient data such as age, sex, and hearing and CI related information were unavailable. With respect to correlation data, many studies excluded from our analysis stated that no significant correlations were found, but did not cite numerical data; therefore, these studies could not be included in our analysis. Additionally, many studies had incomplete statistical data, particularly standard deviation, and had to be excluded.
Conclusion
A meta-analysis of HRQOL improvement showed a medium positive effect of cochlear implantation on HRQOL. In contrast, a meta-analysis of correlations showed negligible pooled correlations between speech recognition scores and HRQOL PROMs. Disease-specific measures that focus on domains of significance to QOL in CI patients have greater utility in the CI population.
Acknowledgments
This publication was supported by a K12 award through the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number UL1TR001450 and a grant from the Doris Duke Foundation.
Footnotes
The authors have no conflicts of interest to declare.
The authors have no financial disclosures to make.
References
- 1.O'Leary TJ, Slutsky JR, Bernard MA. Comparative effectiveness research priorities at federal agencies: the view from the Department of Veterans Affairs, National Institute on Aging, and Agency for Healthcare Research and Quality. J Am Geriatr Soc. 2010;58(6):1187–1192. doi: 10.1111/j.1532-5415.2010.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 3.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125–137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services: Quality Strategy. 2016 https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/Downloads/CMS-Quality-Strategy.pdf.
- 6.Damen GW, Beynon AJ, Krabbe PF, Mulder JJ, Mylanus EA. Cochlear implantation and quality of life in postlingually deaf adults: long-term follow-up. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2007;136(4):597–604. doi: 10.1016/j.otohns.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Kumar RS, Mawman D, Sankaran D, et al. Cochlear implantation in early deafened, late implanted adults: Do they benefit? Cochlear implants international. 2016;17(Suppl 1):22–25. doi: 10.1080/14670100.2016.1161142. [DOI] [PubMed] [Google Scholar]
- 8.Luxford WM, Ad Hoc Subcommittee of the Committee on H, Equilibrium of the American Academy of O-H. Neck S. Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124(2):125–126. doi: 10.1067/mhn.2001.113035. [DOI] [PubMed] [Google Scholar]
- 9.Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. The Laryngoscope. 2002;112(8 Pt 1):1482–1488. doi: 10.1097/00005537-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 10.McRackan TR, Bauschard MJ, Hatch JL, et al. Meta-analysis of quality of life in cochlear implant patients and its association with speech recognition abilities. Laryngoscope. 2017 doi: 10.1002/lary.26738. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. 2009 doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Lin FR, Niparko JK. Measuring health-related quality of life after pediatric cochlear implantation: a systematic review. International journal of pediatric otorhinolaryngology. 2006;70(10):1695–1706. doi: 10.1016/j.ijporl.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Group OLoEW. The Oxford Levels of Evidence 2. [Accessed 3/9/2017];Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653.
- 14.Borenstein M. Introduction to meta-analysis. Chichester, U.K: John Wiley & Sons; 2009. [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 17.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 18.Lee Rodgers J, Nicewander WA. Thirteen ways to look at the correlation coefficient. The American Statistician. 1988;42(1):59–66. [Google Scholar]
- 19.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 20.Arnoldner C, Lin VY, Bresler R, et al. Quality of life in cochlear implantees: comparing utility values obtained through the Medical Outcome Study Short-Form Survey-6D and the Health Utility Index Mark 3. The Laryngoscope. 2014;124(11):2586–2590. doi: 10.1002/lary.24648. [DOI] [PubMed] [Google Scholar]
- 21.Klop WM, Boermans PP, Ferrier MB, van den Hout WB, Stiggelbout AM, Frijns JH. Clinical relevance of quality of life outcome in cochlear implantation in postlingually deafened adults. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29(5):615–621. doi: 10.1097/MAO.0b013e318172cfac. [DOI] [PubMed] [Google Scholar]
- 22.Palmer CS, Niparko JK, Wyatt JR, Rothman M, de Lissovoy G. A prospective study of the cost-utility of the multichannel cochlear implant. Archives of otolaryngology--head & neck surgery. 1999;125(11):1221–1228. doi: 10.1001/archotol.125.11.1221. [DOI] [PubMed] [Google Scholar]
- 23.Krabbe PF, Hinderink JB, van den Broek P. The effect of cochlear implant use in postlingually deaf adults. International journal of technology assessment in health care. 2000;16(3):864–873. doi: 10.1017/s0266462300102132. [DOI] [PubMed] [Google Scholar]
- 24.Mo B, Lindbaek M, Harris S. Cochlear implants and quality of life: a prospective study. Ear Hear. 2005;26(2):186–194. doi: 10.1097/00003446-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Hawthorne G, Hogan A, Giles E, et al. Evaluating the health-related quality of life effects of cochlear implants: A prospective study of an adult cochlear implant program. Int J Audiol. 2004;43(4):183–192. doi: 10.1080/14992020400050026. [DOI] [PubMed] [Google Scholar]
- 26.Knutson JF, Murray KT, Husarek S, et al. Psychological change over 54 months of cochlear implant use. Ear & Hearing (01960202) 1998;19(3):191–201 111p. doi: 10.1097/00003446-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Calvino M, Gavilan J, Sanchez-Cuadrado I, et al. Using the HISQUI to assess the sound quality levels of Spanish adults with unilateral cochlear implants and no contralateral hearing. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2015 doi: 10.1007/s00405-015-3789-0. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Cuadrado I, Lassaletta L, Perez-Mora R, Munoz E, Gavilan J. Reliability and validity of the Spanish Glasgow Benefit Inventory after cochlear implant surgery in adults. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2015;272(2):333–336. doi: 10.1007/s00405-013-2844-y. [DOI] [PubMed] [Google Scholar]
- 29.Vermeire K, Brokx JP, Wuyts FL, et al. Good speech recognition and quality-of-life scores after cochlear implantation in patients with DFNA9. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27(1):44–49. doi: 10.1097/01.mao.0000187240.33712.01. [DOI] [PubMed] [Google Scholar]
- 30.Vermeire K, Brokx JP, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2005;26(2):188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Hirschfelder A, Grabel S, Olze H. The impact of cochlear implantation on quality of life: the role of audiologic performance and variables. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2008;138(3):357–362. doi: 10.1016/j.otohns.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess. 1998;2(14):i–iv. 1–74. [PubMed] [Google Scholar]
- 33.Gaylor JM, Raman G, Chung M, et al. Cochlear implantation in adults: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(3):265–272. doi: 10.1001/jamaoto.2013.1744. [DOI] [PubMed] [Google Scholar]
- 34.Loeffler C, Aschendorff A, Burger T, Kroeger S, Laszig R, Arndt S. Quality of life measurements after cochlear implantation. The Open Otorhinolaryngology Journal. 2010;4:47–54. [Google Scholar]
- 35.Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann Otol Rhinol Laryngol. 1996;105(6):415–422. doi: 10.1177/000348949610500601. [DOI] [PubMed] [Google Scholar]
- 36.Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10(3):e0119616. doi: 10.1371/journal.pone.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: results from the Nord-Trondelag Hearing Loss Study. Psychosom Med. 2004;66(5):776–782. doi: 10.1097/01.psy.0000133328.03596.fb. [DOI] [PubMed] [Google Scholar]
- 38.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Cella D, Gershon R, et al. Representativeness of the Patient-Reported Outcomes Measurement Information System Internet panel. J Clin Epidemiol. 2010;63(11):1169–1178. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riley WT, Rothrock N, Bruce B, et al. Patient-reported outcomes measurement information system (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual Life Res. 2010;19(9):1311–1321. doi: 10.1007/s11136-010-9694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


