Abstract
Meibum is a lipid-rich secretion that is produced by fully differentiated meibocytes in the holocrine Meibomian glands (MG) of humans and most mammals. The secretion is a part of a defense mechanism that protects the ocular surface from hazardous environmental factors, and from desiccation. Meibomian lipids that have been identified in meibum are very diverse and unique in nature. The lipid composition of meibum is different from virtually any other lipid pool found in the human body. In fact, meibum is quite different from sebum, which is the closest secretion that is produced by anatomically, physiologically, and biochemically related sebaceous glands. However, meibum of mice have been shown to closely resemble that of humans, implying similar biosynthetic mechanisms in MG of both species. By analyzing available genomic, immunohistochemical, and lipidomic data, we have envisioned a unifying network of enzymatic reactions that are responsible for biosynthesis of meibum, which we call meibogenesis. Our current theory is based on an assumption that most of the biosynthetic reactions of meibogenesis are catalyzed by known enzymes. However, the main features that make meibum unique – the ratio of identified classes of lipids, the extreme length of its components, extensive ω-hydroxylation of fatty acids and alcohols, iso- and anteiso-branching of meibomian lipids (e.g. waxes), and the presence of rather unique complex lipids with several ester bonds – make it possible that either the activity of known enzymes is altered in MG, or some unknown enzymes contribute to the processes of meibogenesis, or both. Studies are in progress to elucidate meibogenesis on molecular level.
INTRODUCTION
Meibomian glands (MG) – a part of the eye adnexa – were discovered and described for the first time in 1666 (Meibom, 1666). The glands are embedded in the tarsal plates of human and animal upper and lower eyelids perpendicularly to the eyelid margin (Fig. 1, upper panel). There are between 20 and 40 individual MG in the upper eyelid of humans, and 20 to 30 – in the lower on (Duke-Elder, 1961; Leeson, 1963); (Bron and Tiffany, 1998); (Greiner et al., 1998). The anatomical features of MG have been extensively studied (Knop et al., 2011; Obata, 2002). The anatomy and secretory mechanisms of MG modestly resemble those of sebaceous glands (Botek and Lookingbill, 2001), with major differences being the routine absence of hair follicles in MG, their greater size (5–7 mm or so; Fig. 1, upper panel) compared with facial sebaceous glands (typically, less than 1mm) (Plewig and Kligman, 1978), and a greater number of acini connected to a central duct in a grapevine-like manner (Fig. 1, middle and lower panels). The lipid-rich secretion (called meibum (Nicolaides et al., 1981)) is expressed from individual MG onto the ocular surface through a system of ductules and a central duct that ends with an orifice located at the eyelid margin. Meibum and aqueous tears mix during the blink to form the tear film. Currently, the predominant view is that the tear film is a rather stratified structure, the upper sublayer of which is formed mostly of meibum, and, possibly, smaller amounts of polar and nonpolar lipids of other origins (Butovich, 2008) (Butovich, 2013) (Pucker and Haworth, 2015), while the inner part that faces the ocular surface epithelium, is aqueous (Fig. 2) (Green-Church et al., 2011) (Butovich, 2011a). Between the blinks, the normal tear film preserves its integrity for up to 10–20 seconds, after which it starts to disintegrate due to collapsing of the lipid sublayer, accompanied by evaporation of the aqueous sublayer and its thinning (Bhamla et al., 2016). In dry eye patients, the integrity of the tear film is severely compromised, which results in desiccation of the ocular surface, its irritation, and inflammation. The increased instability of the tear film of dry eye patients has been linked to deficiencies in meibum production and/or alterations in the lipid [(Joffre et al., 2008) (Lam et al., 2014c) (Arita et al., 2015) (Bron et al, 2014)] and protein [(Soria et al., 2013) (Boehm et al., 2013) (Aluru et al., 2017) (Ong et al., 1991)] profiles of meibum and tears, though the causality of the changes has not been established yet. Therefore, chemical composition of meibum, mechanisms of its biosynthesis, and morphology and physiology of the Meibomian glands have recently gained considerable attention of ophthalmic researchers. In this review, we will discuss recent advances in lipidomics of meibomian gland secretions and present a hypothesis on the major pathways that lead to formation of meibum in normal conditions.
Figure 1.
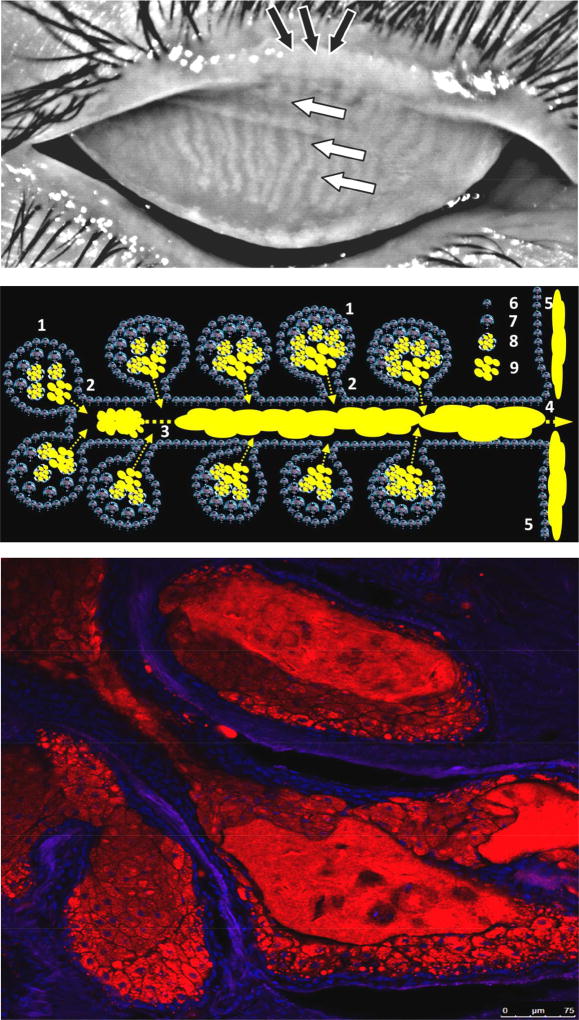
Human eyelids, meibomian glands, and ascini.
Upper panel. A high-contrast photography of a human upper eyelid with normal Meibomian glands that are visible as elongated whitish structures marked with white arrows (Wojtowicz and Butovich, unpublished).
Middle panel. A schematic representation of the Meibomian gland: 1 – ascini; 2 – ductules; 3 – central duct which is being filled with meibum; 4 – orifice; 5 – secreted meibum flowing onto the lid margin and then onto ocular surface; 6 – undifferentiated Meibomian gland epithelial cells; 7 – partially differentiated meibocytes; 8 – completely differentiated, mature meibocytes with lipid droplets (yellow); 9 – lipid content released by ruptured meibocytes at the last stage of their life cycle. Note that different types of progenitor cells may exist in the glands.
Lower panel. Lipid material, stored in lipid droplets, increases in amount within meibocytes as they move towards the center of the ascinus (McMahon, Wojtowicz, and Butovich, unpublished). Following cellular disintegration, the lipid (shown in red) is observed as a bulk mass. Lipids were stained with Nile Red and imaged to detect the signals from neutral lipids (excitation 488nm, emission 500–550nm). The nuclei were counterstained with DAPI (shown in blue). Note the virtual absence of neutral lipid staining in the basal layer of cells (only nuclei are visible) and a decreasing number of nuclei in the central parts of the ascinus (only lipid material is visible).
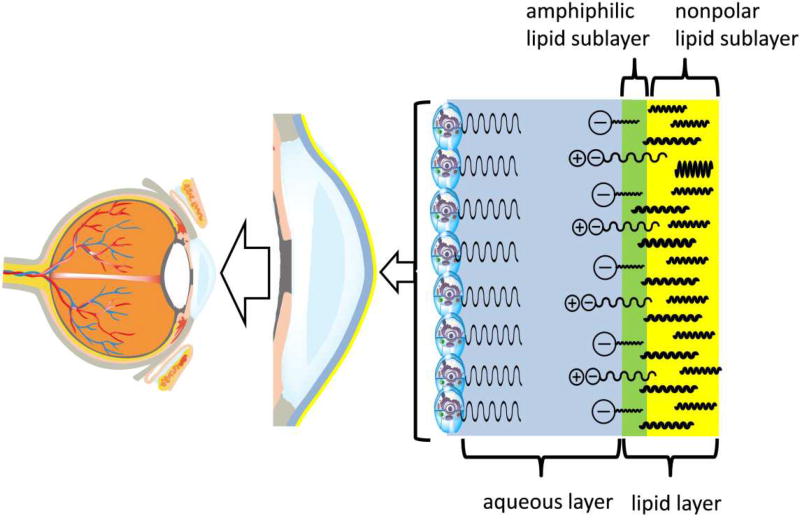
Figure 2.
Schematic representation of the human tear film and its layers and sublayers.
The inner aqueous layer is shown in blue. The layer is formed of a complex mixture of proteins, salts, and other low-molecular weight compounds. The aqueous layer covers the epithelial cells of cornea and conjunctiva, many of which express soluble (secreted, gel-forming) and bound (surface) mucins (Argueso, 2013; Hodges and Dartt, 2013; Mantelli and Argueso, 2008). The opposite side of the aqueous layer is covered with the tear film lipid layer (shown in green and yellow). The inner part of the lipid layer is enriched with amphiphilic lipids (anionic OAHFA and free fatty acids, and, possibly, zwitter-ionic species such as SM, PC, and lyso-PC). This layer is called amphiphilic (or "polar") lipid sublayer and is shown in green. The outer part of the lipid layer is formed mostly of nonpolar lipids and is called nonpolar lipid sublayer (shown in yellow). The actual tear film and tear film lipid layer are dynamic structures whose thickness and geometrical features change with time, blinking, aqueous tears in- and outflow, secretion of meibum, and tear evaporation.
LIPIDS OF MEIBUM
Initial efforts aimed at characterization of human meibomian lipids were undertaken by Pes (Pes, 1897), Linton (Linton et al., 1961), Ehlers (Ehlers, 1965); Cory (Cory et al., 1973)], Tiffany (Tiffany, 1978), and Nicolaides (Nicolaides et al., 1981), among others. Their findings, summarized in several recent reviews [(Green-Church et al., 2011), (Butovich, 2011a), (Pucker and Haworth, 2015), (Butovich, 2013), (Butovich, 2009c), and references cited therein], demonstrated that meibum is mostly comprised of neutral lipids, such as wax esters (WE), cholesteryl esters (CE), free cholesterol (Chl), and triacylglycerols (TAG), and smaller amounts of more polar compounds, such as free fatty acids (FFA), phospholipids (PL), sphingomyelins (SM), ceramides (Cer), and others. Most of the meibomian lipids are complex, featuring at least one or more ester bonds. In earlier experiments aimed at chemical characterization of meibum components, the lipids were mostly analyzed by using thin layer chromatography and/or gas chromatography (GC) with either flame ionization or (rarely) mass-spectrometric detectors. Note that complete hydrolysis of complex lipids and re-esterification of the resulting FA and fatty alcohols (FAl) were required, as the analysis of intact lipid species was not feasible [(Butovich, 2009c)] because of their low volatility and stability under the conditions of GC analyses. Notwithstanding these limitations, the ground-breaking work of Linton, Ehlers, Cory, Tiffany, and especially Nicolaides and co-authors, clearly identified many classes of meibomian lipids and demonstrated that FA and FAl found in complex lipids of meibum were of very long chain (VLC) and extremely long chain (ELC) nature, and often branched.
However, it was not until a decade ago that the advancements in high performance liquid chromatography and mass spectrometry (HPLC-MS) made it possible to characterize and quantitate individual intact lipid species, bringing meibomian lipid studies to the general field of lipidomics. More than a hundred major individual lipid species that belong to a dozen or more lipid classes were found in meibum of humans and many mammals and described in detail (Butovich et al., 2007b) (Butovich et al., 2007a) (Butovich, 2008, 2009a, 2010a; Butovich et al., 2012a; Butovich et al., 2011; Butovich et al., 2012b; Butovich et al., 2009; Chen et al., 2010; Lam et al., 2011) (Saville et al., 2011) (Campbell et al., 2011) (Brown et al., 2013) (Lam et al., 2014c) (Mori et al., 2014). Despite the continuing search for progressively minor new members of the already identified families of meibomian lipids (Chen et al., 2010; Chen et al., 2013, 2015), major components of meibum, and their average abundances, are now known (Table 1). It seems that further efforts of this nature, without addressing specific unanswered questions, would produce progressively less new information due to the universal law of diminishing returns. For comparison purposes, the lipid composition of a related secretion, namely sebum (Butovich et al., 2016) (Thiboutot, 2004) (Picardo et al., 2009) (Nakahara et al., 2015), is also presented in Table 1, from which one can conclude that sebum and meibum substantially differ from each other.
Table 1.
Chemical composition of normal human meibum and sebum
| Component | Human meibum (Butovich et al., 2016) | Human sebum (Nakahara et al., 2015; Picardo et al., 2009; Thiboutot, 2004) |
|---|---|---|
| Percentage of total lipids, % (w/w) | ||
| (O)-Acylated ω-hydroxy fatty acids (OAHFA) | 1–5 | none reported |
| Cholesteryl esters | 30–40 | 3–6 |
| Ceramides | traces | none reported |
| Free cholesterol | <0.5 | 1–3 |
| Cholesteryl esters of OAHFA | 3 | none reported |
| Diacylated diols | Reported, not quantitated | none reported |
| Free fatty acids | ~0.1–1 | 6–40 |
| Phospholipids and sphingomyelins | <<0.1 | none reported |
| Squalene | traces | 10–20 |
| Glycerides | 1 (mostly, triolein) | 20–80 |
| Wax esters | 30–48 | 20–30 |
Structures of the most abundant representatives of each class of meibomian lipids are shown in Fig. 3. For example, individual unsaturated extremely long chain WE found in human meibum were originally characterized in ((Butovich et al., 2007b); (Butovich et al., 2007a)), followed by several publications by independent groups ((Chen et al., 2010); (Chen et al., 2015); (Lam et al., 2011)), while saturated WE were characterized in [(Butovich et al., 2012a; Chen et al., 2015)]. Individual human meibomian CE, also of extremely long chain nature, were described in (Butovich, 2009a, 2010a), and, later, in (Chen et al., 2010; Chen et al., 2013). Another group of lipids – diacylated α,ω-diols (DiAD) – were initially reported by Nicolaides et al. (Nicolaides et al., 1981; Nicolaides et al., 1984), but have been characterized as intact species in human and animal specimens only recently (Butovich et al., 2011; Butovich et al., 2012b; Chen et al., 2010). By making a first chemical standard of DiAD, the overall nature and structures of DiAD have been confirmed a few years ago (Butovich et al., 2012b).
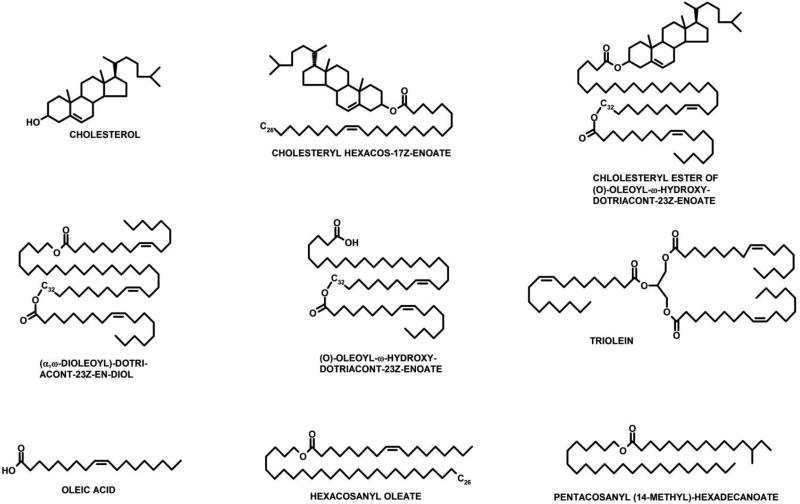
Figure 3.
Typical lipids of human meibum. The most common representatives of the corresponding classes of lipids are shown.
While the overall dominance of WE and CE, and low abundances of squalene (Sql) [(Butovich, 2008; Ivanova et al., 2015)], FFA [(Arita et al., 2015) (Butovich, 2010b, 2011b)], TAG and Chl [(Butovich, 2008; Butovich et al., 2007a; Butovich et al., 2007b)] in healthy meibum have never been seriously questioned, the presence and nature of amphiphilic (often called "polar") lipids have been debated for decades. Initially, it was proposed that uncharacterized polar lipids may comprise up to 13%–16% of meibum (Nicolaides et al., 1981). In earlier studies, polar lipids were tentatively identified as PL and/or SM (for a comprehensive discussion on this topic see earlier reviews, for example (Bron and Tiffany, 1998; Butovich, 2009c; Butovich et al., 2008; Wojtowicz et al., 2009)). However, these assignments were not confirmed in later experiments, where PL and SM were observed in normal human meibum only in minute quantities (Butovich, 2009b; Butovich et al., 2007a; Butovich et al., 2007b; Chen et al., 2010; Lam et al., 2011; Saville et al., 2011), specifically <0.2% (weight/weight) per Lam et al., <0.05% (weight/weight) per our data, and <0.005% per Saville et al., which all are low numbers. Interestingly, the presence of a novel (for human meibum) amphiphilic lipid species – cholesteryl sulfate – was reported by Lam et al. (Lam et al., 2014b), but has not been verified in independent studies yet.
Concurrently, other, previously unreported, classes of amphiphilic lipids [such as (O)-acylated ω-hydroxy fatty acids, OAHFA] were found (Butovich, 2011b; Butovich et al., 2011; Butovich et al., 2012b; Butovich et al., 2009). Several independent reports [Chen et al., 2010, 2011; Lam et al., 2011; Mori et al., 2014) corroborated those observations. Lam et al. estimated OAHFA to comprise approximately 3% (w/w) of normal human meibum in Asian population [(Lam et al., 2011)]. Importantly, OAHFA were detected not only in human meibum, but in meibum samples of all tested species, such as canines, rabbits, and mice, as well (Butovich et al., 2011; Butovich et al., 2012b). One of the envisioned physiological roles for OAHFA was to form an interfacial layer that stabilizes the tear film (Fig. 2) (Butovich, 2011a, 2013; Butovich et al., 2007b). Later, this theory was tested in vitro (Schuett and Millar, 2013). Recent publications (Lam et al., 2014a) (Lam et al., 2011) demonstrated that OAHFA are diminished in the tear film of dry eye patients, which is in line with their proposed physiological roles and the lower stability of the tear film of dry eye patients.
Furthermore, compounds of the OAHFA family serve as precursors of even more complex lipids, such as cholesteryl esters of OAHFA (Chl-OAHFA) (Butovich, 2011a; Butovich et al., 2011; Butovich et al., 2012b; Chen et al., 2010), which comprise up to 3% (w/w) of normal meibum [Table 1 and (Arciniega et al., 2013; Butovich, 2013)]. Chl-OAHFA – an interesting amalgam of CE and OAHFA, – belong to the broad class of di- and tri-esters envisioned by Nicolaides et al., but not characterized as individual species (Nicolaides and Santos, 1985). Recently, Chl-OAHFA were repeatedly observed in human tears and animal meibum (Arciniega et al., 2013; Butovich et al., 2011; Butovich et al., 2012b).
The intriguing and defining feature of all these major classes of complex meibomian lipids is the extreme length (up to, at least, C32-C36) of one of their components – alcohol chains in WE, fatty acids in CE, ω-hydroxy fatty acids in OAHFA, and α,ω-diols in DiAD. Free fatty acids of VLC and ELC nature have also been reported in human meibum (Arita et al., 2015; Butovich, 2010b). This is in sharp contrast with human serum and plasma, where the entire pool of FA residues (both esterified and free) are 14 to 22 carbons long, while ELC-FA with more than 26 carbons are virtually absent (Bakkeren et al., 1984; Hsu et al., 2010; Johnson et al., 2003; Takemoto et al., 2003). On the other hand, the main fatty acid chains in human skin acyl-Cer can be as long as C36 (Farwanah et al., 2005), which is on par with lipids of meibum. However, the overall lipid profile of meibum (Butovich et al., 2016) is very different from that of skin (Farwanah et al., 2005; McMahon et al., 2007): Cer and acyl-Cer (including those of the ELC nature) are major constituents of epidermis of both humans and mice, while being virtually nonexistent in normal human meibum and sebum. Sebum lipids are also different from meibum: the former, unlike meibum, are dominated by four lipid classes – acylglycerols (mostly TAG), WE, FFA, and Sql. Of those, TAG do not typically have FA residues longer than C16-C20. Major sebaceous WE have molecular masses ranging from C30H58O2 (450.4 Da) to about C44H86O2 (646.66 Da), reaching a summit at about C37H72O2 (or 548.55 Da; Fig. 4A). The FA moieties of the sebaceous WE are typically in the C12-C18 range, with the rest of the length coming from the FAl components (Butovich, unpublished data). Note that human meibomian WE are, on average, six to seven methylene groups longer than their sebaceous counterparts (Fig. 4B), spreading from C30H58O2 to C52H102O2 and peaking out at C42H82O2–C44H86O2 for the monounsaturated series of WE, with the saturated ones being two mass units heavier, and di-unsaturated ones – two mass units lighter. Most of human meibomian WE are based on C14-C20 fatty acids, with FAl residues reaching at least C32 (Butovich et al., 2012a; Butovich et al., 2007b; Butovich et al., 2009). It should be noted that though both shorter and longer WE might be present in sebum and meibum, those components were not attempted to be identified due to their extremely low abundance.
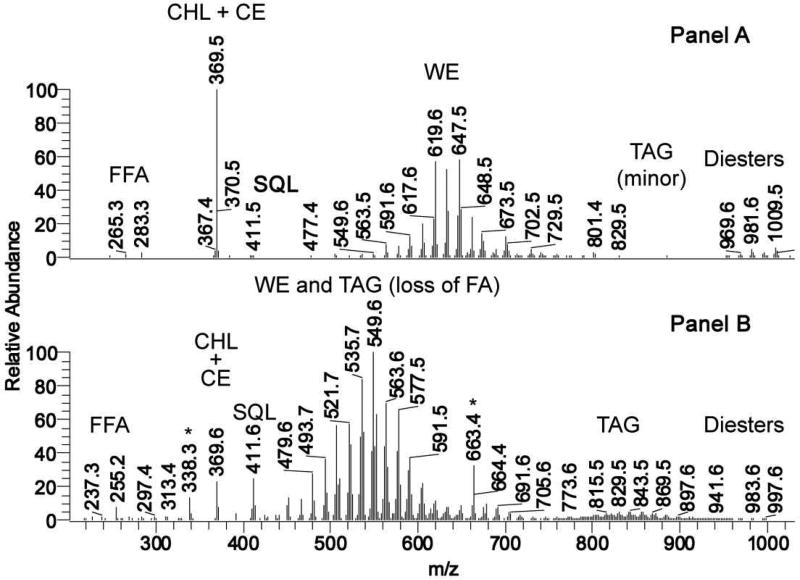
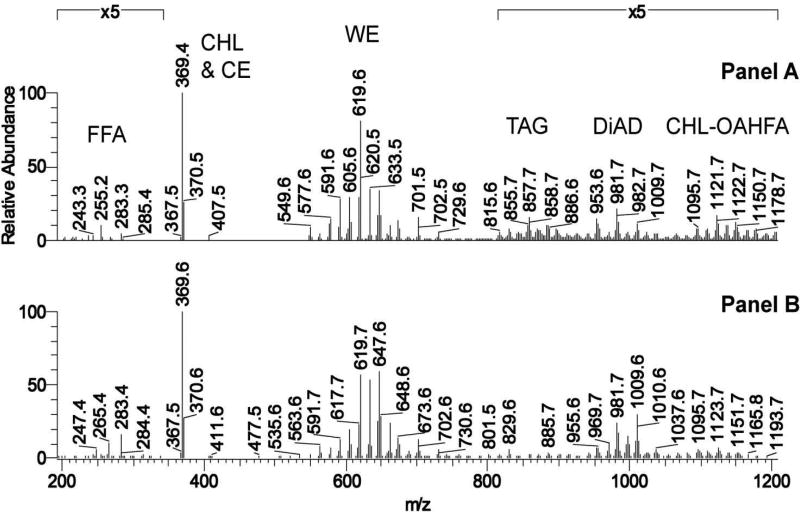
Figure 4.
Mass-spectrometric comparison of human meibum and sebum. Most of ions depicted in Panels A and B are (M+H)+ adducts of the corresponding lipid species.
Panel A. A representative APCI mass spectrum of a human meibum sample taken in the positive ion mode.
Panel B. A representative APCI mass spectrum of a human sebum sample taken in the positive ion mode Adopted from (Butovich et al., 2016).
The most abundant homolog of OAHFA in human and canine meibum has a ω-hydroxy-C32:1-FA moiety (Butovich et al., 2009) (Butovich et al., 2011) (Chen et al., 2010), a ω-hydroxy-C32:0-FA moiety – in rabbits, and a ω-hydroxy-C34:2-FA moiety – in mice (Butovich et al., 2012b). The most common α,ω-diol moieties found in DiAD are also in the C30-C34 range. While the overall lengths of OAHFA and DiAD species are almost identical in all tested species, they differ in their degrees of saturation, changing from predominantly fully saturated lipids (i.e. without double bonds in their FA and FAl chains) in rabbits, to predominantly mono- and di-unsaturated FA and FAl chains in humans and other species (Butovich et al., 2011; Butovich et al., 2012b; Butovich et al., 2009). Again, similar results were obtained in independent studies with human samples (Chen et al., 2010; Lam et al., 2011).
The initial observations on the high presence of branched lipids in human and animal meibum, originally reported by Andrews (Andrews, 1970), Tiffany (Tiffany, 1978), Harvey and Tiffany (Harvey and Tiffany, 1984; Harvey et al., 1987), and Nicolaides et al. (Kolattukudy et al., 1985; McFadden et al., 1979; Nicolaides et al., 1981; Nicolaides and Santos, 1985; Nicolaides et al., 1984). Because of a rather rudimentary state of the mass spectrometric and HPLC techniques at the time, Tiffany et al. and Nicolaides et al. could not characterize individual intact lipid species, and had to resort to the analyses of products of their saponification/transesterification, to produce volatile derivatives of FA, FAl, and sterols that can be analyzed by GC. This made it impossible to come up with exact initial lipid profiles of meibum. Still, the results of Tiffany evidenced that a large fraction of FA and FAl moieties in human meibomian lipids was iso- and anteiso-branched (Tiffany, 1978), and of long and very long chain nature (up to C32, for both FA and FAl). Data of Nicolaides et al. (Nicolaides et al., 1981; Nicolaides et al., 1984) further advanced our understanding of FA and FAl found in meibomian lipids as very long chain and/or branched moieties. These observations were later corroborated in the studies of individual intact WE (Butovich et al., 2012a) and whole human meibum [(Butovich et al., 2016)].
A CONCEPT OF MEIBOGENESIS
Considering the extremely diverse and complex nature of meibomian lipids, in a recent publication (Butovich et al., 2016) we have summarized major features that, from our standpoint, define meibum as a unique fusion of various compounds whose composition is, apparently, not duplicated in other secretions, cells, and tissues. These features are shown in Table 2. Taking the lipidomics data as a starting point, one can conclude that the network of biosynthetic pathways that are responsible for making meibum (which we call meibogenesis) should be either 1) quite different from those that have been described in other cells, tissues, and organs, or 2) altered in a way that ensures generation of final lipid products with distinctively unique combination of structural features, or 3) both. The latter two possibilities do not necessarily mean that novel, previously unidentified enzymes have to be present in meibocytes. Instead, we hypothesized that the activity levels of known enzymes may be up- or down-regulated through alterations in gene expression patterns, posttranslational modifications of enzymes, and/or by other regulatory mechanisms. At the same time, the substrate and product specificities of known enzymes can also be altered as a result of post-translational modifications, or due to differential expression of isozymes. Finally, it is worth noting that, in many instances, known enzymes with documented activity toward specific substrates, are later on found to be able to catalyze other types of reactions. For example, it was argued that the enzyme elongase of very long chain fatty acids-4 (ELOVL4) is involved in making polyunsaturated ELC FA (Agbaga et al., 2010), while other reports described that ELOVL4 inactivation had led to a virtual disappearance of ELC acyl-Cer, which had only mono- and di-unsaturated FA chains in their structures (McMahon et al., 2011; McMahon et al., 2007). Also, enzymes of hydroxysteroid dehydrogenase family (HSD), in addition to fulfilling the implied functions of the dehydrogenation of pregnenolone, cortisone, progesterone and other sex hormones, also catalyze red-ox conversions of FA (HSD17B4, HSD19B10, HSD17B12). Such substrate and/or product promiscuity of certain poorly characterized enzymes makes any definitive statements with regard to their specific roles in meibogenesis challenging, and requires additional verification on the mechanistic level.
Table 2.
Major characteristic features of meibum and meibomian lipids*
| Feature | Class of lipids with the feature |
|---|---|
|
| |
| High levels of CE in meibum | saturated and unsaturated CE, Chl-OAHFA |
| High levels of WE in meibum | saturated and unsaturated WE |
| Low degree of unsaturation of fatty chains (mostly, one, wo, or three double bonds per FA and FAl chain) | All complex lipids |
| Branching of FA and FAl (predominantly "iso" and "anteiso") | WE (both FA and FAl moieties), FA, FAl, and CE |
| High levels of odd-numbered FA residues (mostly C17 and C15) | WE, CE, FFA |
| High levels of VLC and ELC FA residues (mostly C24–C34) | WE, CE, OAHFA, Chl-OAHFA, FFA |
| High levels of ELC FAl residues in lipids (mostly C24–C34) | WE, DiAD |
| High levels of ω– and a,ω-hydroxylation of FA and FAl residues, respectively | OAHFA, Chl-OAHFA, DiAD |
|
| |
| Low levels of Chl, Sql, Cer, acyl-Cer, PL and SM | whole meibum |
Modified from (Butovich et al., 2016)
However, enzymological aspects of meibogenesis, with a few exclusions, have not been elucidated yet. In 2001, Miyazaki, Man, and Ntambi reported the results of disruption of stearoyl-CoA desaturase-1 (SCD1) on sebaceous and meibomian glands of mice (Miyazaki et al., 2001b). The SCD1-null mice had severely reduced levels of WE, CE, and TAG in their eyelids, increased levels of Chl, and, generally, atrophic sebaceous and meibomian glands. Importantly, saturated and polyunsaturated FA were either unaffected, or increased in the SCD1−/− mice. In direct experiments, Miyazaki et al. demonstrated that SCD was capable of making C16:1 and C18:1 FA (Miyazaki et al., 2001a).
In a two-paper installment published in 2004, Cheng and Russell reported results of a study in which three enzymes implicated in making meibomian WE, namely fatty acyl reductases 1 and 2 (FAR1 and FAR2), and a wax ester synthase (WES), had been characterized (Cheng and Russell, 2004a, b). The authors demonstrated that FAR1/FAR2 and WES, when expressed in transfected HEK 293 cells, were required for making, correspondingly, FAl and WE, albeit only relatively short-chain substrates (ranging from C10 to C20) were tested and/or reported in the study.
A massive effort to elucidate regulation of MG functions has been undertaken by Sullivan and colleagues since 1998 (Sullivan et al., 1998). Initially, the project has been centered on the putative role of sex hormones in physiology and pathophysiology of MG. The effects of androgens, estrogen, and progesterone were evaluated in MG of humans, mice, and rabbits (Cermak et al., 2003; Darabad et al., 2013; Krenzer et al., 2000; Liu et al., 2016b; Richards et al., 2006; Rocha et al., 2000; Schirra et al., 2006a; Schirra et al., 2007; Schirra et al., 2006b; Schirra et al., 2005; Steagall et al., 2002; Sullivan et al., 2002a; Sullivan et al., 2006; Sullivan et al., 2000a; Sullivan et al., 2009; Sullivan et al., 1999a; Sullivan et al., 2002b; Sullivan et al., 2000b; Sullivan et al., 1999b; Suzuki et al., 2008; Wickham et al., 2000; Yamagami et al., 2002). It was reported that sex hormones induced rather convoluted changes in the gene expression patterns and lipid profiles of tested species. Apparently, androgens (such as testosterone) upregulated the mRNA levels of enzymes ATP-citrate lyase, acetyl-CoA synthase, acetyl-CoA-carboxylase, acetoacetyl-CoA-synthase, HMG-CoA synthase 1, HMG-CoA reductase, and also increased the expression of SREBP1 and SREBP2 (Schirra et al., 2006b).
However, questions remained about the actual nature of the observed lipid species, and their possible changes in response to sex hormones. Interestingly, Schirra and colleagues proposed the existence of a lipogenic pathway in MG (Fig. 1 in (Schirra et al., 2006a)) that did not reflect any of the quintessential meibomian lipids such as very, and extremely, long chain WE, CE, OAHFA, Chl-OAHFA, and DiAD (Figs. 3–5 and Table 1 of this manuscript). In fact, the diagram of Schirra et al. reflects initial stages of typical lipogenic reactions that produce short- and medium-length FA (such as palmitate and stearate) and cholesterol. Also notable is the fact that the reactions illustrated in the diagram lead to TAG and PL – all minor meibomian lipids, per current views. Thus, accelerating these early steps of lipid biosynthesis may not necessarily induce meibogenesis per se. Also, the results of lipid analyses were not reported in full, experiments with lipid standards were not illustrated and discussed, the chromatograms and raw mass spectra of meibomian lipid samples were not shown, and the algorithms for identification of the analytes were not explained. It seems that some of the observed MS signals were inadvertently miscategorized. For example, the abundant group of lipids with mass-to-charge ratios (m/z) between 500 and 650, initially described as genuine diacyl glycerols (DAG) (Krenzer et al., 2000; Sullivan et al., 2000a), could be in fact products of inadvertent spontaneous in-source fragmentation of TAG in mass spectrometric experiments, or a different class of lipids altogether. The latter statement was corroborated in direct HPLC-MS experiments with human meibum (Butovich, 2009b; Butovich et al., 2007a; Butovich et al., 2007b), in which only exceedingly small amounts of TAG, and no true DAG, have been observed. Also, the m/z values reported by Sullivan et al. (Krenzer et al., 2000; Sullivan et al., 2002a) as those of major lipid analytes were too small to be the signals of actual meibomian lipids: contrary to the authors' conclusions, there are no lipids in existence with m/z values between 100 and 200 such as those reported in Tables 2 and 3 and in Fig. 3 in (Sullivan et al., 2006), and in Tables 1 and 2 in (Sullivan et al., 2002a). With all likelihood, those signals were either spontaneously formed fragments of actual lipids as a result of in-source fragmentation, or chemical or electronic noise generated by the instrument. Thus, it is not clear at this juncture what, or even if, androgens caused qualitative and/or quantitative changes in lipid metabolism in MG of tested species.
Figure 5.
Observation MS profiles of mouse and human meibum.
Panel A. Mass spectrum of mouse meibum in the m/z range of 200 to 1200. Normal phase HPLC-atmospheric pressure chemical ionization MS, positive ion mode. Averaged spectrum of meibomian lipids is shown.
Panel B. Mass spectrum of human meibum. Same conditions as in Panel A. (from (Butovich et al., 2016)). Most of ions depicted in Panels A and B are (M+H)+ adducts of the corresponding lipid species.
Another approach undertaken by Sullivan et al. was to immortalize human MG epithelial cells (HMGEC) (Liu et al., 2010) and induce their differentiation and lipogenesis by various factors. The authors reported an increase in lipid production by immortalized HMGEC after their treatment with serum (Liu et al., 2010; Liu et al., 2013), omega-3/omega-6 (Liu et al., 2016a), insulin and glucose (Ding et al., 2015), azithromycin (Liu et al., 2014), but not with insulin-like growth factor [(Ding and Sullivan, 2014)], cis-retinoic acid (Ding et al., 2013), tetracyclines other than azithromycin (Liu et al., 2015), and other effectors (Kam et al., 2016; Kam and Sullivan, 2011), all of which did not stimulate lipogenesis in HMGEC cultures.
Notably, the lipid components detected in the HMGEC (Kam et al., 2016; Liu et al., 2015) did not resemble human meibomian lipidome (studied as expressed meibum), while closely matching the lipidome of a generic cell, comprising predominantly of PL, Chl, and TAG. Importantly, no similarities between human meibomian lipidome and lipidome of immortalized HMGEC were found in an independent study conducted using the same line of immortalized HMGEC, either (Hampel et al., 2015). Moreover, no effects of stimulation of HMGEC with sex hormones on the lipid production or cell morphology and proliferation have been found (Schroder et al., 2016). In the latter publication, the authors concluded that the immortalized HMGEC "do not reflect the biology of meibomian gland epithelial cells in vivo exactly". It seems that a simple induction of lipogenesis in the HMGEC cultures does not automatically lead to meibogenesis – the generation of meibum-like lipids by HMGEC.
Lastly, it is worthwhile to note a paper by Knop et al. (Knop et al., 2011) in which the authors provided, along with other information, a description of the basic mechanisms of the lipid biosynthesis in general, and undertook an effort to connect them with the biosynthesis of lipids in sebaceous and meibomian glands. Also, possible regulatory roles of sex hormones and other factors on the MG regulation were discussed. The paper of Knop et al., however, was primarily concerned with the initial steps of lipogenesis (such as biosynthesis of TAG, FA, FAl, and sterols), which are largely generic and not exclusive to the Meibomian gland, meibocytes, and meibum. Thus, possible networks of biosynthetic reactions that define meibogenesis remained untangled, because the key features of meibomian lipids that differentiate them from other lipid pools found in human and animal bodies, and corresponding genes and proteins, were not particularized and analyzed. Moreover, based on extensive data published by Sullivan et al. in 1998–2016 and discussed above, Knop et al. summarized possible regulatory roles of sex hormones in regulation of the MG functions in general, and lipogenesis specifically. The regulatory roles of the hormones in physiology of sebaceous glands, and the presence of sex steroid receptors in sebocytes, are well known (Pelletier and Ren, 2004; Thiboutot, 2004). However, their effects on Meibomian glands and meibum are not clear yet because of the deficiencies in the initial characterization of affected lipids by Sullivan et al. (discussed above), and recent data of Hampel et al. (Hampel et al., 2015) and Schroder et al. (Schroder et al., 2016), who demonstrated no appreciable effects of the hormonal treatments on the lipid accumulation in, and lipid profiles of, treated HMGEC: the lipids of HMGEC remained to be drastically different from those found in meibum.
A different approach was undertaken by Jester and colleagues who tested the role of PPARγ in modulating the lipid metabolism in cultured mouse meibocytes and mice (Hwang et al., 2017; Jester et al., 2016). It was observed that the amount of lipid produced by the cells was dependent on the serum levels in the culture media: low serum concentrations (0% to 2%) led to a marked reduction in the lipid production, as evidenced by HCS LipidTox staining. Simultaneously, cells underwent major alterations in the intracellular localization of PPARγ which showed a significant loss of its cytoplasmic fraction. The lipids produced by the cells were characterized using coherent anti-Stokes Raman scattering (CARS) and Raman microspectroscopy, and the results were compared to those obtained for lipid vacuoles within acini of MG. The authors reported that cultured meibocytes produced neutral lipid containing equal amounts of WE and CE. Considering the low specificity of employed analytical techniques – CARS and Raman microspectroscopy, – this observation needs to be verified by GC-MS and/or HPLC-MS to find out if those lipids were indeed similar to the lipids of mouse meibum. It seems that, for now, the actual mechanisms of differentiation of HMGEC into mature meibocytes, and the factors that can induce meibogenesis in HMGEC, remain to be determined.
To facilitate the progress in the area of meibogenesis studies, we initiated a pilot project which was to integrate our knowledge of human and mouse meibomian lipidomes with newly obtained data on gene and protein expression patterns in human and mouse meibomian glands (Butovich et al., 2016). Human and mouse meibomian lipids were found to be very similar to each other, in terms of lipid classes and individual lipid species. The molecular masses of individual nonpolar lipids detected in HPLC-MS experiments were virtually indistinguishable (Fig. 5), with a major difference being the relative ratios of their FA and FAl moieties: the human WE were predominantly based on C18:1-FA (oleic acid), while the mouse WE were mostly based on C16:1-FA (palmitoleic acid and, possibly, sapienic acid, with the latter being a common FA found in sebum (Destaillats et al., 2011; Ge et al., 2003; Marzouki et al., 1988; Picardo et al., 2009)). Notably, the two-carbon decrease in the FA length in WE of mice was compensated by a simultaneous two-carbon increase in the length of their FAl moieties, which resulted in the mixture of WE with the same total masses as in humans. Other major lipid classes, including CE, OAHFA, and Chl-OAHFA were found to be surprisingly similar in both species, as well.
These facts are consistent with a hypothesis that the molecular machinery of meibogenesis should be quite similar in humans and mice. Indeed, the expression patterns of major lipid biosynthesis-related genes in the tarsal plates of humans and mice were found to be very close to each other (Butovich et al., 2016).
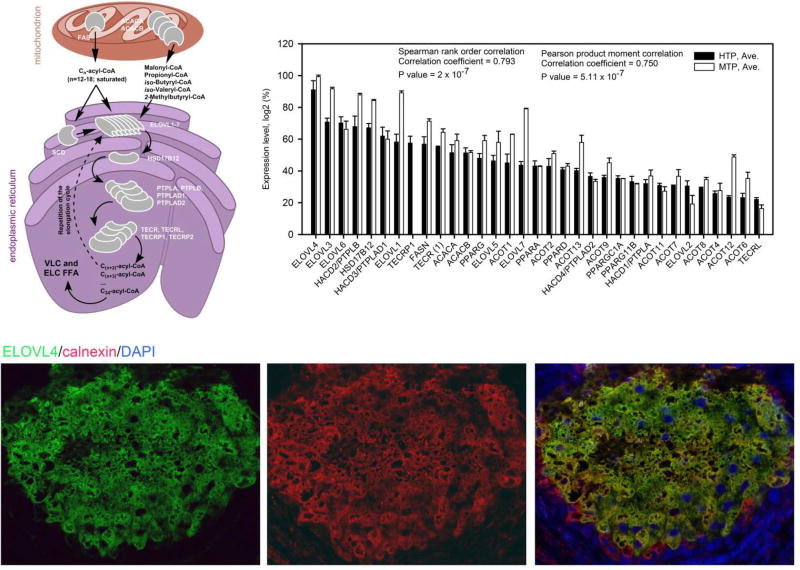
As an example, key genes and proteins related to the FA elongation cycle – a characteristic feature of meibomian lipids – are shown in Fig. 6. Notably, FAS/FASN and ACACA/ACACB (encoding key enzymes of the biosynthesis of short and medium chain FA), and ELOVL1-ELOVL7, HSD17B12, PTPLB/PTPLAD1/PTPLAD2, and TECR/TECRP (encoding key enzymes of the very and extremely long chain fatty acid elongation cycle) are highly expressed in the tarsal plates of both species (Fig. 6 and (Butovich et al., 2016)). Note that the presence of the ELOVL4 protein in the tarsal plates has been verified using immunohistochemical staining with ELOVL4-specific antibodies, and so was the expression of ELOVL3. Detailed descriptions of putative mechanisms of the FA elongation cycle can be found in several reviews for example (Guillou et al., 2010; Jakobsson et al., 2006; Kihara, 2012; Zhang et al., 2016).
Figure 6.
Elongation of fatty acids in Meibomian glands of humans and mice.
Upper left panel. Proposed sequence of FA elongation steps occurring in mitochondria and endoplasmic reticulum of meibocytes.
Upper right panel. Expression patterns of major fatty acid elongation genes in tarsal plates of humans (4 donors, one sample of tarsal plate from each) and mice (12 mice; two pooled samples, 6 mice × 4 tarsal plates each) (Butovich et al., unpublished). Expression levels (Log2 values of SST-RMA-Gene-Full-Signals, varying from ~20 to ~2) were calculated using the Expression Console (built 1.4.1.46) from Affymetrix, then ranked from high to low, normalized from 100% (the highest expression level) to about 10% (the lowest expression level which was considered to be just above the noise level), and plotted as shown in the Panel (Butovich et al., 2016).
Bottom panels. Immunohistochemical anti-ELOVL4 (green)/anti-calnexin (red)/DAPI (blue) co-staining of meibocytes in the human tarsal plates acini [from (Butovich et al., 2016)].
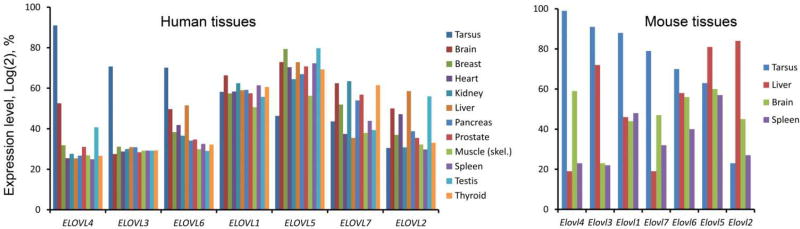
Notably, the genes of the ELOVL family are differentially expressed in those mouse and human tissues that are known for their active lipid metabolism (Fig. 7). The microarray data demonstrated a modest expression of ELOVL2 (averaging 20% in the tarsal plates of mice and 30% – in humans), but high expression levels of the gene in the livers of mice (about 80–85%) and humans (about 60%). The ELOVL2 enzyme catalyzes elongation of C20-C22 polyunsaturated n-6 series FA with three or more double bonds, such as C20 and C22 polyunsaturated FA (Gregory et al., 2011); (Gregory et al., 2013a); (Okuda et al., 2010), but is also fairly active with C14-C24 saturated substrates (Kitazawa et al., 2009). The low expression levels of ELOVL2/Elovl2 in TP of both species are consistent with our previous reports on the very small presence of its products in human and mouse meibum (Butovich, 2008; Butovich et al., 2012a; Butovich et al., 2007b; Butovich et al., 2009).
Figure 7.
Differential expression profiles of the genes of the ELOVL family in human and mouse tissues.
Left panel. Human tissues. Data for human tarsal plates is from (Butovich et al., 2016). Data for other tissues is recalculated from Affymetrix (file Human_Exon_tissues_AGCC_ARR_CHP, obtained using Affymetrix GeneChip® Human Exon 1.9 ST Array).
Right panel. Mouse tissues. Data for tarsal plates of mice is from (Butovich et al., 2016).
Another ELOVL that is known for elongating long-chain mono- and polyunsaturated FA is ELOVL5. This enzyme was shown to be active toward C14-C20 mono-, di- and polyunsaturated FA (Kitazawa et al., 2009). The enzyme elongates C18 and C20 PUFA typically by two carbons to make C20 and C22 products (Gregory and James, 2014). The chicken enzyme elongated docosapentaenoic acid and synthesized a C24:5(n-3) product (Gregory et al., 2013b). ELOVL5/Elovl5 is moderatly expressed in the tarsi of human and mice (at about 50–60% level), but low levels of its potential polyunsaturated lipid products possibly imply that the enzymatic activity of ELOVL5 is either insignificant, or channeled mostly toward the elongation of mono- and di-unsaturated substrates.
On the contrary, ELOVL4/Elovl4, at average expression levels of 92% and 98%, dominates the transcriptome of, respectively, human and mouse tarsal plates, while being only marginally present in liver, which is consistent with the dominance of ELC FA and FAl in meibum, and the absence of FA longer than C24 in liver (Zadravec et al., 2011). The latter team has demonstrated that the enzyme participates in the biosynthesis of a variety of ELC mono-, di- and polyunsaturated FA up to C38 in length, specifically eicosapentaenoic and docosahexaenoic acids. Conversely, Okuda et al. (Okuda et al., 2010) reported that ELOVL4 expressed in HEK 293T cells did not elongate eicosa- and docosapentaenoic acids, while being active with saturated C24:0 and C26:0-acyl-CoA. Earlier, we found that disruptive mutations in Elovl4 gene resulted in the termination of the biosynthesis of acyl-Cer with extremely long main chains in the epidermis of mice (McMahon et al., 2011; McMahon et al., 2014). The same conclusion was independently made by Li et al. (Li et al., 2007). Thus, the actual substrate/product specificity of ELOVL4 remains unclear as the results vary based on studied species and used experimental conditions. Still, because of the apparently broad specificity of ELOVL4, many of its putative products can, and are, present in meibum as components of various classes of complex lipids (Table 2 and Figs. 3–5). Importantly, the high expression levels of ELOVL4 protein in the mouse and human eyelids has also been reported (Butovich et al., 2016; McMahon et al., 2014), and shown to be localized to the endoplasmic reticulum of meibocytes.
Also highly expressed in tarsal plates is SCD, a gene that encodes a major enzyme of fatty acid desaturation, namely stearoyl-CoA desaturase. In human and mouse TP, SCD/Scd is always one of the most highly expressed genes, routinely present at 95% to 100% levels (Butovich et al., 2016)). Importantly, the presence (but not the expression levels) of Scd in mouse tarsal plates was reported by Schirra et al. (Schirra et al., 2005). As the results of that study were available through the NIH-supported Gene Expression Omnibus database (accession ## GDS 1009, 1136, 1832), we calculated actual expression levels of Scd and compared them with our recent results presented in our recent publication (Butovich et al., 2016). Importantly, Parfitt et al. also listed Scd as one of the most highly expressed in MG of mice [Appendix 1 in (Parfitt et al., 2016)]. This enzyme converts C16:0 (palmitic) and C18:0 (stearic) acids in, correspondingly, C16:1 (palmitoleic) and C18:1 (oleic) acids (Miyazaki et al., 2001a; Miyazaki et al., 2001b), both of which are the top two unsaturated FA residues of meibomian lipids of humans and mice. The distant third and fourth places belong to linoleic (C18:2) and linolenic (C18:3) acids. Conversely, FADS2/Fads2 – a gene that encodes a fatty acid desaturase-2 (FADS2) – is only modestly expressed in TP of humans and mice [(Butovich et al., 2016)]. FADS2 [(Guillou et al., 2010; Nakamura and Nara, 2004; Tosi et al., 2014)] catalyzes Δ4- and Δ6-desaturation of C20-C24 polyunsaturated FA, with four or more double bonds making, for example, docosahexaenoic acid in humans and rodents (Zhang et al., 2016). Again, its modest expression level (45% or so) in tarsal plates is consistent with a low abundance of its expected products – PUFA with three or more double bonds.
It is known that a large portion of meibomian lipids is branched. Branching was confirmed for a number of FA and FAl residues of complex lipids analyzed after their complete hydrolysis/transesterification (Andrews, 1970; Harvey and Tiffany, 1984; Harvey et al., 1987; Nicolaides et al., 1981; Nicolaides and Ruth, 1982; Tiffany, 1978), and also for intact WE (Butovich et al., 2012a; Butovich et al., 2016). The extensive branching of meibomian lipids was also verified in 13C-NMR studies of human meibum (Butovich et al., 2016). A group of genes that are encoding enzymes that biosynthesize branched lipids include BCKDHA/BCKDHB (encoding alpha and beta subunits of the branched chain keto acid dehydrogenase E1; EC 1.2.4.4), DBT (encoding dihydrolipoamide branched chain transacylase E2; EC 2.3.1.168) and DLD (encoding dihydrolipoyl dehydrogenase E3, EC 1.8.1.4), among others proteins that are assembled in a very large branched-chain alpha-keto acid dehydrogenase complex (BCKD) (Jia et al., 2016). Note that BCKDHB and DBT proteins have been detected in Meibomian glands using immunohistochemical approaches (Butovich et al., 2016).
Related to the FA biosynthesis is formation of FAl, which are essential parts of meibomian WE and DiAD (Table 1). Fatty alcohols are known to be produced from fatty acyl-CoA via a reduction reaction catalyzed by enzymes FAR1 and FAR2 (Cheng and Russell, 2004a). Their genes – FAR1/Far1 and FAR2/Far2, respectively, – are highly expressed in MG of mice (Cheng and Russell, 2004a); (Butovich et al., 2016) and humans (Butovich et al., 2016). For example, FAR1 expression levels in human TP are slightly above 50%, while AWAT2 is expressed at about 90% level (Butovich et al., 2016). It is worth noting that the mouse enzymes that were expressed in transfected insect Sf9 or HEK 239 cells, were active with relatively short (for meibomian lipids) substrates of C16-C20 nature (Cheng and Russell, 2004a, b) and are currently classified as short chain dehydrogenase/reductase family 10E, Members 1 and 2 (2017a, b). As meibomian FAl are typically much longer than that [(being, primarily, in the C24-C32 range (Butovich et al., 2012a; Butovich et al., 2007b; Butovich et al., 2009)], it is conceivable that either FAR1/FAR2 have a high, but unreported, activity toward VLC and ELC acyl-CoAs, or there might be other, unknown enzymes that are active with these substrates in Meibomian glands.
In meibocytes, FA and FAl are typically conjugated by an enzyme wax ester synthase (WES) to form esters, such as WE. The enzyme (also known as acyl-CoA:long-chain-alcohol O-acyltransferase and WES) exists in two forms – AWAT1 and AWAT2, encoded, respectively, by genes AWAT1/Awat1 and AWAT2/Awat2. Both genes are abundantly present in human and mouse Meibomian glands, typically at 70–80% (for AWAT1/Awat1) to 80–90% (for AWAT2/Awat2) expression levels (Butovich et al., 2016). The human enzymes, expressed in yeast cells, were reported to differ in their substrate specificities: AWAT1 preferred shorter FAl (with a highest activity with decyl alcohol), while AWAT2 strongly favored C16 and C18 FAl. Interestingly, both enzymes had only residual activity with a saturated arachidyl alcohol (C20:0) (Turkish et al., 2005). In that study, AWAT1 had highest activity with stearoyl-CoA, and slightly lower activities with palmitoyl-, palmitoleoyl- and oleoyl-CoAs, while AWAT2 was more active with C16:1 and C18:1–acyl-CoAs. The last observation aligns well with the fact that oleic and palmitoleic (and, possibly, sapienic) acids are the predominant FA species in meibomian WE (Butovich et al., 2012b). However, as the major meibomian FAl are much longer than C18, it is not clear at this time whether it is AWAT1, AWAT2, or some other enzyme with wax ester synthase activity that is the actual enzyme that produces ELC WE in meibocytes.
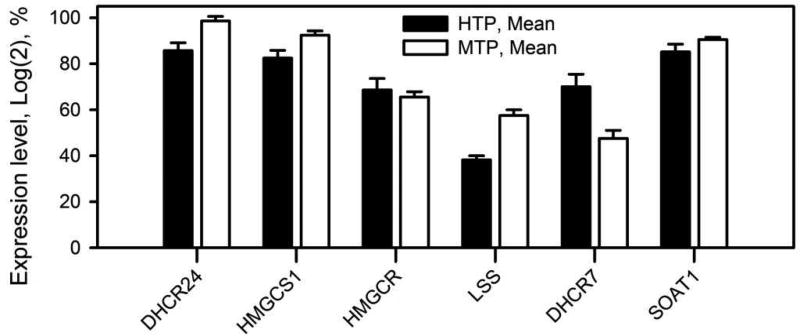
Yet another major class of meibomian lipids is CE. Their high abundance in meibum (~30% w/w), and the extremely long chain nature of their FA residues, approaching at least C32 (Butovich, 2009a, 2010a), imply that they are also generated in situ by meibocytes. Therefore, it was feasible that the tarsal plates would demonstrate high expression levels of genes that are related to biosynthesis of cholesterol and its esters. Indeed, major genes that are critical for the biosynthesis of cholesterol (2017c) were found to be expressed in the tarsal plates of humans and mice. Importantly, HMGCR/Hmgcr genes that encode 3-hydroxy-3-methylglutaryl-CoA reductase – a catalyst of a rate-limiting step in cholesterol biosynthesis – is expressed at a high level of up to 70%, while SOAT1/Soat1 that encodes sterol O-acyltransferase 1 – one of the key enzymes in the biosynthesis of CE – at a very high level of 85%–90% (SOAT1 has an alias ACAT1) (Fig. 8). These high levels of expression of HMGCR/Hmgcr and SOAT1/Soat1 support the idea of the in situ formation of Chl and CE in meibocytes, and are in line with a very high abundance of CE, and a very low presence of free Chl, in meibum.
Figure 8.
Expression profiles of key genes of the biosynthesis of cholesterol and cholesteryl esters in Meibomian glands of humans and mice. HTP – human tarsal plates; MTP – mouse tarsal plates (recalculated from (Butovich et al., 2016)).
Enzymological details of formation of other, more complex meibomian lipids, such as OAHFA, Chl- OAHFA, DiAD, and others, currently remain unknown. Still, it is feasible that one of the enzymes of cytochrome P450 family is responsible for making ω-hydroxylated derivatives of FA and FAl that are needed for biosynthesis of OAHFA, Chl-OAHFA, and DiAD. Recently, CYP4F22 has been shown to participate in making O-acyl-Cer (Ohno et al., 2015), the deficiency of which is associated with ichtyosis (Gruber et al., 2016). Esterification of OAHFA with Chl may or may not be catalyzed by SOAT1/SOAT2 or LCAT, of which SOAT1/Soat1 gene is expressed at a very high 85% to 90% level in human and mouse tarsal plates (Fig. 8). However, it remains to be seen if these enzymes are capable of esterification of Chl with FA other than their more typical straight chain substrates of C14-C20 length.
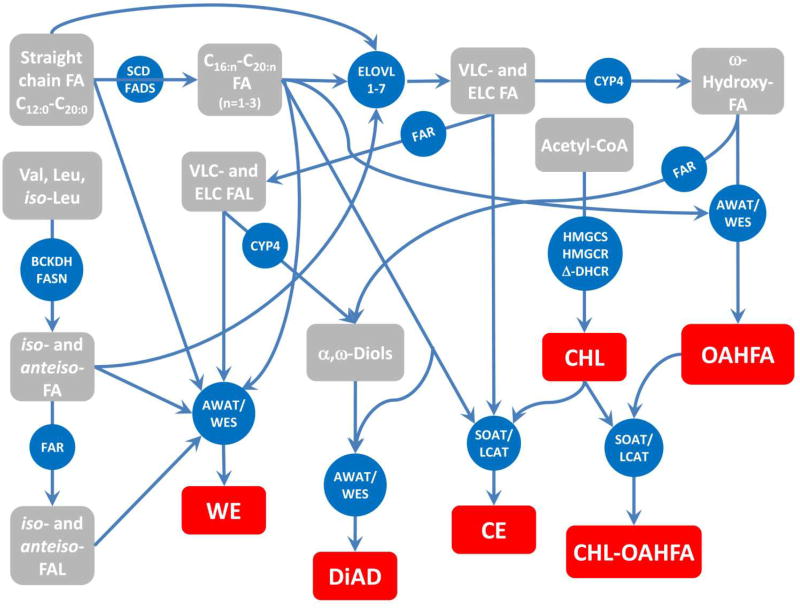
These and other considerations prompted us to propose a generalized scheme of meibogenesis (Butovich et al., 2016) which outlines the major steps in the biosynthesis of meibum by meibocytes (Fig. 9). Note that even more complex lipids of triester nature were reported by Nicolaides et al. before (Nicolaides and Santos, 1985). However, their complexity precludes us from speculating on the mechanisms of their biosynthesis. Also, the diagram does not include minor components of normal meibum such as FFA, Sql, TAG, PL, SM, and Cer (Butovich, 2009c, 2011a, 2013), because some of these lipids (such as FFA and Chl) can be considered as building blocks for more complex meibomian lipids, or products of their degradation, while Sql is presumably an intermediate product of the in situ biosynthesis of Chl. The latter differentiates meibum from sebum, where Sql is, apparently, one of the major end product with a distinctive physiological role(s) as it is present in very large quantities. Other minor lipids of meibum – TAG, diacylglycerols and monoacylglycerols – are known sources of energy and carbon for living cells. Thus, they are expected to be present in rupturing meibocytes and meibum, but most likely in highly variable amounts. Additionally, PL, SM, Cer, and acyl-Cer may originate from incompletely catabolyzed cell membranes and cytosol of meibocytes rupturing at their last stage of their life cycle. Incomplete catabolism of these lipids can be one of the contributing factors to the often observed variability of the lipid composition of human and animal meibum. However, their presence in trace amounts is normal and expected of metabolically active cells. Conversely, other compounds, such as homologous hydrocarbons and fatty acid amides (including oleamide), if detected in meibum samples, should be recognized as exogenous contaminants and labeled as such.
Figure 9.
Meibogenesis – a network of biosynthetic reactions in meibomian glands of humans and mice that lead to biosynthesis of meibum [modified from (Butovich et al., 2016)].
Major confirmed final lipid products of meibogenesis are shown in red. Intermediate products are shown in grey. Key enzymes are shown in blue. The presence and nature of meibomian lipids have been established in GC-MS and HPLC-MS experiments. The nature of enzymes has been predicted from their respective gene expression patterns, and confirmed (for some of them) using immunohistochemical and immunocytochemical approaches [(Butovich et al., 2016) and references cited therein].
The major challenge in studying meibogenesis is the fact that meibomian lipids are based on VLC and ELC FA and FAl. Additionally, many of these molecules are branched, and the final products are of high molecular weight (especially compounds of di- and triester families). It is not known how the extreme length of FA and FAl, and their branching, affect activity and substrate/product specificity of known enzymes which typically metabolize much shorter, and usually straight chain, molecules in other tissues. The prominent exception is the ELOVL family, whose activity toward VLC and ELC FA is well documented. Most of studies of other related enzymes, such as FAR1/FAR2, WES, SOAT etc., have been performed with much shorter substrates, typically not exceeding C20-C22. Thus, the future progress in the area of meibogenesis largely depends on the progress in studying chemistry, enzymology, genomics, and in vivo regulation of the biosynthesis of extremely long chain and/or branched lipids.
The data discussed in this paper, and the mechanism shown in Fig. 9, are intended to narrow the gaps in our views on meibogenesis, and propose a unified mechanism that connects rather complex biosynthetic reactions downstream of generic lipogenesis and cholesterogenesis which are not unique to the Meibomian gland and meibocytes, but rather occur in a wide variety of cells and tissues. For the lack of available information and/or space, more complex lipids of meibum (such as triesters) have been left out of discussion, and so were intermediate steps of the reactions shown in Fig. 9. There is no doubt that on-going and future studies related to the MG physiology in general, and meibogenesis specifically, will answer these questions.
In this review, recent advances in the Meibomian gland studies are summarized
Main features of the lipid biosynthesis in Meibomian glands are discussed
A concept of meibogenesis is presented and explained
Acknowledgments
The author would like to thank Dr. Anne McMahon and Dr. Jadwiga Wojtowicz for fruitful discussions of meibogenesis. The project has been supported by NIH grants R01 EY019480, R01 EY024324, P30 EY020799, and an unrestricted grant from Research to Prevent Blindness (New York, NY).
ABBREVIATIONS
- CE
cholesteryl ester
- Cer
ceramide
- Chl
free (unesterified) cholesterol
- Chl-OAHFA
cholesteryl ester of (O)-acyl-ω-hydroxy fatty acid
- DiAD
diacylated α,ω-diol
- ELC
extremely long chain
- FFA
free fatty acid
- GC
gas chromatography
- HPLC
high pressure liquid chromatography
- MG
Meibomian gland
- MS
mass spectrometry
- OAHFA
(O)-acyl-w-hydroxy fatty acid
- PL
phospholipid
- SM
sphingomyelins
- TAG
triacylglycerol
- VLC
very long chain
- WE
wax ester
- WES
wax ester synthase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- FAR1 Gene (Protein Coding) Fatty Acyl-CoA Reductase 1. 2017a GeneCards.org.
- FAR2 Gene (Protein Coding) Fatty Acyl-CoA Reductase 2. 2017b GeneCards.org.
- Steroid Biosynthesis - Reference Pathway. Kyoto Encyclopedia of Genes and Genomes 2017c [Google Scholar]
- Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. Journal of lipid research. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru SV, Shweta A, Bhaskar S, Geetha K, Sivakumar RM, Utpal T, Padmanabhan P, Angayarkanni N. Tear Fluid Protein Changes in Dry Eye Syndrome Associated with Rheumatoid Arthritis: A Proteomic Approach. The ocular surface. 2017;15:112–129. doi: 10.1016/j.jtos.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Experimental eye research. 1970;10:223–227. doi: 10.1016/s0014-4835(70)80032-x. [DOI] [PubMed] [Google Scholar]
- Arciniega JC, Uchiyama E, Butovich IA. Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Investigative ophthalmology & visual science. 2013;54:1352–1360. doi: 10.1167/iovs.12-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P. Glycobiology of the ocular surface: mucins and lectins. Japanese journal of ophthalmology. 2013;57:150–155. doi: 10.1007/s10384-012-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita R, Mori N, Shirakawa R, Asai K, Imanaka T, Fukano Y, Nakamura M, Amano S. Meibum Color and Free Fatty Acid Composition in Patients With Meibomian Gland Dysfunction. Investigative ophthalmology & visual science. 2015;56:4403–4412. doi: 10.1167/iovs.14-16254. [DOI] [PubMed] [Google Scholar]
- Bakkeren JA, Monnens LA, Trijbels JM, Maas JM. Serum very long chain fatty acid pattern in Zellweger syndrome. Clinica chimica acta; international journal of clinical chemistry. 1984;138:325–331. doi: 10.1016/0009-8981(84)90140-2. [DOI] [PubMed] [Google Scholar]
- Bhamla MS, Chai C, Rabiah NI, Frostad JM, Fuller GG. Instability and Breakup of Model Tear Films. Investigative ophthalmology & visual science. 2016;57:949–958. doi: 10.1167/iovs.15-18064. [DOI] [PubMed] [Google Scholar]
- Boehm N, Funke S, Wiegand M, Wehrwein N, Pfeiffer N, Grus FH. Alterations in the tear proteome of dry eye patients--a matter of the clinical phenotype. Investigative ophthalmology & visual science. 2013;54:2385–2392. doi: 10.1167/iovs.11-8751. [DOI] [PubMed] [Google Scholar]
- Botek AA, Lookingbill DP. The Structure and Function of Sebaceous Gland. In: Freinkel RK, Woodley DT, editors. The Biology of the Skin. The Parthenon Publishing Group; New York, London: 2001. pp. 87–100. [Google Scholar]
- Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Advances in experimental medicine and biology. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tomlinson A, Foulks GN, Pepose JS, Baudouin C, Geerling G, Nichols KK, Lemp MA. Rethinking dry eye disease: a perspective on clinical implications. Ocular Surf. 2014;12(2 Suppl):S1–31. doi: 10.1016/j.jtos.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Brown SH, Kunnen CM, Duchoslav E, Dolla NK, Kelso MJ, Papas EB, Lazon de la Jara P, Willcox MD, Blanksby SJ, Mitchell TW. A comparison of patient matched meibum and tear lipidomes. Investigative ophthalmology & visual science. 2013;54:7417–7424. doi: 10.1167/iovs.13-12916. [DOI] [PubMed] [Google Scholar]
- Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Investigative ophthalmology & visual science. 2008;49:3779–3789. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. Journal of lipid research. 2009a;50:501–513. doi: 10.1194/jlr.M800426-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods in molecular biology. 2009b;579:221–246. doi: 10.1007/978-1-60761-322-0_11. [DOI] [PubMed] [Google Scholar]
- Butovich IA. The Meibomian puzzle: combining pieces together. Progress in retinal and eye research. 2009c;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010a;75:726–733. doi: 10.1016/j.steroids.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the presence and role of polar lipids in meibum. Investigative ophthalmology & visual science. 2010b;51:6908–6910. doi: 10.1167/iovs.10-6328. author reply 6910–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Lipidomics of human Meibomian gland secretions: Chemistry, biophysics, and physiological role of Meibomian lipids. Progress in lipid research. 2011a;50:278–301. doi: 10.1016/j.plipres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the presence of (O-acyl)-omega-hydroxy fatty acids and of their esters in human meibomian gland secretions. Investigative ophthalmology & visual science. 2011b;52:639–641. doi: 10.1167/iovs.10-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Tear film lipids. Experimental eye research. 2013;117:4–27. doi: 10.1016/j.exer.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Arciniega JC, Lu H, Molai M. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Investigative ophthalmology & visual science. 2012a;53:3766–3781. doi: 10.1167/iovs.11-9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Borowiak AM, Eule JC. Comparative HPLC-MS analysis of canine and human meibomian lipidomes: many similarities, a few differences. Scientific reports. 2011;1:24. doi: 10.1038/srep00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Lu H, McMahon A, Eule JC. Toward an animal model of the human tear film: biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investigative ophthalmology & visual science. 2012b;53:6881–6896. doi: 10.1167/iovs.12-10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Lu H, McMahon A, Ketelson H, Senchyna M, Meadows D, Campbell E, Molai M, Linsenbardt E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Investigative ophthalmology & visual science. 2014;55:87–101. doi: 10.1167/iovs.13-13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, McMahon A, Wojtowicz JC, Lin F, Mancini R, Itani K. Dissecting lipid metabolism in meibomian glands of humans and mice: An integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochimica et biophysica acta. 2016;1861:538–553. doi: 10.1016/j.bbalip.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids--a review. Current eye research. 2008;33:405–420. doi: 10.1080/02713680802018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007a;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. Journal of lipid research. 2007b;48:2220–2235. doi: 10.1194/jlr.M700237-JLR200. [DOI] [PubMed] [Google Scholar]
- Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. Journal of lipid research. 2009;50:2471–2485. doi: 10.1194/jlr.M900252-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Griffiths G, Tighe BJ. Tear analysis and lens-tear interactions: part II. Ocular lipids-nature and fate of meibomian gland phospholipids. Cornea. 2011;30:325–332. doi: 10.1097/ICO.0b013e3181eae239. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Krenzer KL, Sullivan RM, Dana MR, Sullivan DA. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea. 2003;22:516–521. doi: 10.1097/00003226-200308000-00006. [DOI] [PubMed] [Google Scholar]
- Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Investigative ophthalmology & visual science. 2010;51:6220–6231. doi: 10.1167/iovs.10-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Investigative ophthalmology & visual science. 2013;54:5730–5753. doi: 10.1167/iovs.12-10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Green KB, Nichols KK. Characterization of Wax Esters by Electrospray Ionization Tandem Mass Spectrometry: Double Bond Effect and Unusual Product Ions. Lipids. 2015;50:821–836. doi: 10.1007/s11745-015-4044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, Russell DW. Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. The Journal of biological chemistry. 2004a;279:37789–37797. doi: 10.1074/jbc.M406225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, Russell DW. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. The Journal of biological chemistry. 2004b;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. The British journal of dermatology. 1973;89:25–27. doi: 10.1111/j.1365-2133.1973.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Darabad RR, Suzuki T, Richards SM, Jensen RV, Jakobiec FA, Zakka FR, Liu S, Sullivan DA. Influence of aromatase absence on the gene expression and histology of the mouse meibomian gland. Investigative ophthalmology & visual science. 2013;54:987–998. doi: 10.1167/iovs.12-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats F, Guitard M, Cruz-Hernandez C. Identification of Delta6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography-mass-spectrometry using ionic-liquid coated capillary column. Journal of chromatography. A. 2011;1218:9384–9389. doi: 10.1016/j.chroma.2011.10.095. [DOI] [PubMed] [Google Scholar]
- Ding J, Kam WR, Dieckow J, Sullivan DA. The influence of 13-cis retinoic acid on human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2013;54:4341–4350. doi: 10.1167/iovs.13-11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Liu Y, Sullivan DA. Effects of Insulin and High Glucose on Human Meibomian Gland Epithelial Cells. Investigative ophthalmology & visual science. 2015;56:7814–7820. doi: 10.1167/iovs.15-18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Sullivan DA. The effects of insulin-like growth factor 1 and growth hormone on human meibomian gland epithelial cells. JAMA ophthalmology. 2014;132:593–599. doi: 10.1001/jamaophthalmol.2013.8295. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S. The Anatomy of the Visual System. Henry Kimpton; London: 1961. System of Ophthalmology. Vol. II. [Google Scholar]
- Ehlers N. The Precorneal Film. Biomicroscopical, Histological and Chemical Investigations. Acta ophthalmologica. Supplementum. 1965;(SUPPL 81):81–134. [PubMed] [Google Scholar]
- Farwanah H, Wohlrab J, Neubert RH, Raith K. Profiling of human stratum corneum ceramides by means of normal phase LC/APCI-MS. Analytical and bioanalytical chemistry. 2005;383:632–637. doi: 10.1007/s00216-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Ge L, Gordon JS, Hsuan C, Stenn K, Prouty SM. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. The Journal of investigative dermatology. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, Glasgow BJ. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investigative ophthalmology & visual science. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MK, Cleland LG, James MJ. Molecular basis for differential elongation of omega-3 docosapentaenoic acid by the rat Elovl5 and Elovl2. Journal of lipid research. 2013a;54:2851–2857. doi: 10.1194/jlr.M041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MK, Geier MS, Gibson RA, James MJ. Functional characterization of the chicken fatty acid elongases. The Journal of nutrition. 2013b;143:12–16. doi: 10.3945/jn.112.170290. [DOI] [PubMed] [Google Scholar]
- Gregory MK, Gibson RA, Cook-Johnson RJ, Cleland LG, James MJ. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PloS one. 2011;6:e29662. doi: 10.1371/journal.pone.0029662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MK, James MJ. Functional characterization of the duck and turkey fatty acyl elongase enzymes ELOVL5 and ELOVL2. The Journal of nutrition. 2014;144:1234–1239. doi: 10.3945/jn.114.194159. [DOI] [PubMed] [Google Scholar]
- Greiner JV, Glonek T, Korb DR, Whalen AC, Hebert E, Hearn SL, Esway JE, Leahy CD. Volume of the human and rabbit meibomian gland system. Advances in experimental medicine and biology. 1998;438:339–343. doi: 10.1007/978-1-4615-5359-5_48. [DOI] [PubMed] [Google Scholar]
- Gruber R, Rainer G, Weiss A, Udvardi A, Thiele H, Eckl KM, Schupart R, Nurnberg P, Zschocke J, Schmuth M, Volc-Platzer B, Hennies HC. Morphological alterations in two siblings with autosomal recessive congenital ichthyosis associated with CYP4F22 mutations. The British journal of dermatology. 2016 doi: 10.1111/bjd.14860. [DOI] [PubMed] [Google Scholar]
- Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Progress in lipid research. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Hampel U, Schroder A, Mitchell T, Brown S, Snikeris P, Garreis F, Kunnen C, Willcox M, Paulsen F. Serum-induced keratinization processes in an immortalized human meibomian gland epithelial cell line. PloS one. 2015;10:e0128096. doi: 10.1371/journal.pone.0128096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DJ, Tiffany JM. Identification of meibomian gland lipids by gas chromatography-mass spectrometry: application to the meibomian lipids of the mouse. Journal of chromatography. 1984;301:173–187. doi: 10.1016/s0021-9673(01)89187-1. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. Journal of chromatography. 1987;414:253–263. doi: 10.1016/0378-4347(87)80051-8. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Dartt DA. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Experimental eye research. 2013;117:62–78. doi: 10.1016/j.exer.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WY, Lin WD, Hwu WL, Lai CC, Tsai FJ. Screening assay of very long chain fatty acids in human plasma with multiwalled carbon nanotube-based surface-assisted laser desorption/ionization mass spectrometry. Analytical chemistry. 2010;82:6814–6820. doi: 10.1021/ac100772j. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD) Experimental eye research. 2017 doi: 10.1016/j.exer.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova S, Tonchev V, Yokoi N, Yappert MC, Borchman D, Georgiev GA. Surface Properties of Squalene/Meibum Films and NMR Confirmation of Squalene in Tears. International journal of molecular sciences. 2015;16:21813–21831. doi: 10.3390/ijms160921813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Progress in lipid research. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Jester JV, Potma E, Brown DJ. PPARgamma Regulates Mouse Meibocyte Differentiation and Lipid Synthesis. The ocular surface. 2016;14:484–494. doi: 10.1016/j.jtos.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Cui M, Than MT, Han M. Developmental Defects of Caenorhabditis elegans Lacking Branched-chain alpha-Ketoacid Dehydrogenase Are Mainly Caused by Monomethyl Branched-chain Fatty Acid Deficiency. The Journal of biological chemistry. 2016;291:2967–2973. doi: 10.1074/jbc.M115.676650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre C, Souchier M, Gregoire S, Viau S, Bretillon L, Acar N, Bron AM, Creuzot-Garcher C. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. The British journal of ophthalmology. 2008;92:116–119. doi: 10.1136/bjo.2007.126144. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Trinh MU, Oe T. Measurement of plasma pristanic, phytanic and very long chain fatty acids by liquid chromatography-electrospray tandem mass spectrometry for the diagnosis of peroxisomal disorders. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2003;798:159–162. doi: 10.1016/j.jchromb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Kam WR, Liu Y, Ding J, Sullivan DA. Do Cyclosporine A, an IL-1 Receptor Antagonist, Uridine Triphosphate, Rebamipide, and/or Bimatoprost Regulate Human Meibomian Gland Epithelial Cells? Investigative ophthalmology & visual science. 2016;57:4287–4294. doi: 10.1167/iovs.16-19937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam WR, Sullivan DA. Neurotransmitter influence on human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2011;52:8543–8548. doi: 10.1167/iovs.11-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. Journal of biochemistry. 2012;152:387–395. doi: 10.1093/jb/mvs105. [DOI] [PubMed] [Google Scholar]
- Kitazawa H, Miyamoto Y, Shimamura K, Nagumo A, Tokita S. Development of a high-density assay for long-chain fatty acyl-CoA elongases. Lipids. 2009;44:765–773. doi: 10.1007/s11745-009-3320-8. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative ophthalmology & visual science. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE, Rogers LM, Nicolaides N. Biosynthesis of lipids by bovine meibomian glands. Lipids. 1985;20:468–474. doi: 10.1007/BF02534238. [DOI] [PubMed] [Google Scholar]
- Krenzer KL, Dana MR, Ullman MD, Cermak JM, Tolls DB, Evans JE, Sullivan DA. Effect of androgen deficiency on the human meibomian gland and ocular surface. The Journal of clinical endocrinology and metabolism. 2000;85:4874–4882. doi: 10.1210/jcem.85.12.7072. [DOI] [PubMed] [Google Scholar]
- Lam SM, Tong L, Duan X, Acharya UR, Tan JH, Petznick A, Wenk MR, Shui G. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. Journal of lipid research. 2014a;55:1959–1969. doi: 10.1194/jlr.P051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. Journal of lipid research. 2014b;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SM, Tong L, Reux B, Duan X, Petznick A, Yong SS, Khee CB, Lear MJ, Wenk MR, Shui G. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. Journal of lipid research. 2014c;55:299–306. doi: 10.1194/jlr.P041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, Wenk MR. Meibum lipid composition in Asians with dry eye disease. PloS one. 2011;6:e24339. doi: 10.1371/journal.pone.0024339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson TS. Tarsal (Meigomian) Glands of the Rat. The British journal of ophthalmology. 1963;47:222–231. doi: 10.1136/bjo.47.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sandhoff R, Kono M, Zerfas P, Hoffmann V, Ding BC, Proia RL, Deng CX. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. International journal of biological sciences. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton RG, Curnow DH, Riley WJ. The Meibomian Glands: An Investigation into the Secretion and Some Aspects of the Physiology. The British journal of ophthalmology. 1961;45:718–723. doi: 10.1136/bjo.45.11.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hatton MP, Khandelwal P, Sullivan DA. Culture, immortalization, and characterization of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2010;51:3993–4005. doi: 10.1167/iovs.09-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Investigative ophthalmology & visual science. 2013;54:2541–2550. doi: 10.1167/iovs.12-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kam WR, Ding J, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA ophthalmology. 2014;132:226–228. doi: 10.1001/jamaophthalmol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kam WR, Ding J, Sullivan DA. Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. 2015;34:342–346. doi: 10.1097/ICO.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kam WR, Sullivan DA. Influence of Omega 3 and 6 Fatty Acids on Human Meibomian Gland Epithelial Cells. Cornea. 2016a;35:1122–1126. doi: 10.1097/ICO.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Knop E, Knop N, Sullivan DA, List EO, Kopchick JJ, Kam WR, Ding J. Growth Hormone Influence on the Morphology and Size of the Mouse Meibomian Gland. Journal of ophthalmology. 2016b;2016:5728071. doi: 10.1155/2016/5728071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Current opinion in allergy and clinical immunology. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouki ZM, Taha AM, Gomaa KS. Fatty acid profiles of sebaceous triglycerides by capillary gas chromatography with mass-selective detection. Journal of chromatography. 1988;425:11–24. doi: 10.1016/0378-4347(88)80002-1. [DOI] [PubMed] [Google Scholar]
- McFadden WH, Bradford DC, Eglinton G, Hajlbrahim SK, Nicolaides N. Application of combined liquid chromatography/mass spectrometry (LC/MS): analysis of petroporphyrins and meibomian gland waxes. Journal of chromatographic science. 1979;17:518–522. doi: 10.1093/chromsci/17.9.518. [DOI] [PubMed] [Google Scholar]
- McMahon A, Butovich IA, Kedzierski W. Epidermal expression of an Elovl4 transgene rescues neonatal lethality of homozygous Stargardt disease-3 mice. Journal of lipid research. 2011;52:1128–1138. doi: 10.1194/jlr.M014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Butovich IA, Mata NL, Klein M, Ritter R, 3rd, Richardson J, Birch DG, Edwards AO, Kedzierski W. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Molecular vision. 2007;13:258–272. [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Lu H, Butovich IA. A role for ELOVL4 in the mouse meibomian gland and sebocyte cell biology. Investigative ophthalmology & visual science. 2014;55:2832–2840. doi: 10.1167/iovs.13-13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom H. De Vasis Palpebrarum Novis Epistola. Henningi Mulleri, Helmstadt; Germany: 1666. [Google Scholar]
- Miyazaki M, Kim HJ, Man WC, Ntambi JM. Oleoyl-CoA is the major de novo product of stearoyl- CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. The Journal of biological chemistry. 2001a;276:39455–39461. doi: 10.1074/jbc.M106442200. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. The Journal of nutrition. 2001b;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- Mori N, Fukano Y, Arita R, Shirakawa R, Kawazu K, Nakamura M, Amano S. Rapid identification of fatty acids and (O-acyl)-omega-hydroxy fatty acids in human meibum by liquid chromatography/high-resolution mass spectrometry. Journal of chromatography. A. 2014;1347:129–136. doi: 10.1016/j.chroma.2014.04.082. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Takayama K, Nakanishi Y, Furue M. Analysis of sebum lipid composition and the development of acneiform rash before and after administration of egfr inhibitor. Current oncology. 2015;22:e124–127. doi: 10.3747/co.22.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annual review of nutrition. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Investigative ophthalmology & visual science. 1981;20:522–536. [PubMed] [Google Scholar]
- Nicolaides N, Ruth EC. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Current eye research. 1982;2:93–98. doi: 10.3109/02713688208997682. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Santos EC. The di- and triesters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467. doi: 10.1007/BF02534237. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Santos EC, Papadakis K, Ruth EC, Muller L. The occurrence of long chain alpha, omega-diols in the lipids of steer and human meibomian glands. Lipids. 1984;19:990–993. doi: 10.1007/BF02534741. [DOI] [PubMed] [Google Scholar]
- Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70–74. doi: 10.1097/01.ico.0000263122.45898.09. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Nakamichi S, Ohkuni A, Kamiyama N, Naoe A, Tsujimura H, Yokose U, Sugiura K, Ishikawa J, Akiyama M, Kihara A. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7707–7712. doi: 10.1073/pnas.1503491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda A, Naganuma T, Ohno Y, Abe K, Yamagata M, Igarashi Y, Kihara A. Hetero-oligomeric interactions of an ELOVL4 mutant protein: implications in the molecular mechanism of Stargardt-3 macular dystrophy. Molecular vision. 2010;16:2438–2445. [PMC free article] [PubMed] [Google Scholar]
- Ong BL, Hodson SA, Wigham T, Miller F, Larke JR. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Current eye research. 1991;10:1113–1119. doi: 10.3109/02713689109024128. [DOI] [PubMed] [Google Scholar]
- Parfitt GJ, Brown DJ, Jester JV. Transcriptome analysis of aging mouse meibomian glands. Molecular vision. 2016;22:518–527. [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histology and histopathology. 2004;19:629–636. doi: 10.14670/HH-19.629. [DOI] [PubMed] [Google Scholar]
- Pes O. Ricerche microchimiche sulla secrezione delle ghiandole sebaceepalpebrali. Archivio Di Ottalmologia. 1897:82–91. [Google Scholar]
- Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinology. 2009;1:68–71. doi: 10.4161/derm.1.2.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewig G, Kligman AM. Proliferative activity of the sebaceous glands of the aged. The Journal of investigative dermatology. 1978;70:314–317. doi: 10.1111/1523-1747.ep12543478. [DOI] [PubMed] [Google Scholar]
- Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. The ocular surface. 2015;13:26–42. doi: 10.1016/j.jtos.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Richards SM, Yamagami H, Schirra F, Suzuki T, Jensen RV, Sullivan DA. Sex-related effect on gene expression in the mouse meibomian gland. Current eye research. 2006;31:119–128. doi: 10.1080/02713680500514644. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Wickham LA, da Silveira LA, Krenzer KL, Yu FS, Toda I, Sullivan BD, Sullivan DA. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. The British journal of ophthalmology. 2000;84:76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville JT, Zhao Z, Willcox MD, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Experimental eye research. 2011;92:238–240. doi: 10.1016/j.exer.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Experimental eye research. 2006a;83:291–296. doi: 10.1016/j.exer.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Schirra F, Richards SM, Sullivan DA. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Current eye research. 2007;32:393–398. doi: 10.1080/02713680701316674. [DOI] [PubMed] [Google Scholar]
- Schirra F, Suzuki T, Dickinson DP, Townsend DJ, Gipson IK, Sullivan DA. Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea. 2006b;25:438–442. doi: 10.1097/01.ico.0000183664.80004.44. [DOI] [PubMed] [Google Scholar]
- Schirra F, Suzuki T, Richards SM, Jensen RV, Liu M, Lombardi MJ, Rowley P, Treister NS, Sullivan DA. Androgen control of gene expression in the mouse meibomian gland. Investigative ophthalmology & visual science. 2005;46:3666–3675. doi: 10.1167/iovs.05-0426. [DOI] [PubMed] [Google Scholar]
- Schroder A, Abrar DB, Hampel U, Schicht M, Paulsen F, Garreis F. In vitro effects of sex hormones in human meibomian gland epithelial cells. Experimental eye research. 2016;151:190–202. doi: 10.1016/j.exer.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Schuett BS, Millar TJ. An investigation of the likely role of (O-acyl) omega-hydroxy fatty acids in meibomian lipid films using (O-oleyl) omega-hydroxy palmitic acid as a model. Experimental eye research. 2013;115:57–64. doi: 10.1016/j.exer.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Soria J, Duran JA, Etxebarria J, Merayo J, Gonzalez N, Reigada R, Garcia I, Acera A, Suarez T. Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. Journal of proteomics. 2013;78:94–112. doi: 10.1016/j.jprot.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Steagall RJ, Yamagami H, Wickham LA, Sullivan DA. Androgen control of gene expression in the rabbit meibomian gland. Advances in experimental medicine and biology. 2002;506:465–476. doi: 10.1007/978-1-4615-0717-8_65. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Archives of ophthalmology. 2002a;120:1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Archives of ophthalmology. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. The Journal of clinical endocrinology and metabolism. 2000a;85:4866–4873. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Jensen RV, Suzuki T, Richards SM. Do sex steroids exert sex-specific and/or opposite effects on gene expression in lacrimal and meibomian glands? Molecular vision. 2009;15:1553–1572. [PMC free article] [PubMed] [Google Scholar]
- Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Investigative ophthalmology & visual science. 1999a;40:1261–1265. [PubMed] [Google Scholar]
- Sullivan DA, Rocha EM, Ullman MD, Krenzer KL, Gao J, Toda I, Dana MR, Bazzinotti D, da Silveira LA, Wickham LA. Androgen regulation of the meibomian gland. Advances in experimental medicine and biology. 1998;438:327–331. doi: 10.1007/978-1-4615-5359-5_46. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Sullivan BD, Evans JE, Schirra F, Yamagami H, Liu M, Richards SM, Suzuki T, Schaumberg DA, Sullivan RM, Dana MR. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Annals of the New York Academy of Sciences. 2002b;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Sullivan BD, Ullman MD, Rocha EM, Krenzer KL, Cermak JM, Toda I, Doane MG, Evans JE, Wickham LA. Androgen influence on the meibomian gland. Investigative ophthalmology & visual science. 2000b;41:3732–3742. [PubMed] [Google Scholar]
- Sullivan DA, Wickham LA, Rocha EM, Krenzer KL, Sullivan BD, Steagall R, Cermak JM, Dana MR, Ullman MD, Sato EH, Gao J, Rocha FJ, Ono M, Silveira LA, Lambert RW, Kelleher RS, Tolls DB, Toda I. Androgens and dry eye in Sjogren's syndrome. Annals of the New York Academy of Sciences. 1999b;876:312–324. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Schirra F, Richards SM, Jensen RV, Sullivan DA. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Investigative ophthalmology & visual science. 2008;49:1797–1808. doi: 10.1167/iovs.07-1458. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Suzuki Y, Horibe R, Shimozawa N, Wanders RJ, Kondo N. Gas chromatography/mass spectrometry analysis of very long chain fatty acids, docosahexaenoic acid, phytanic acid and plasmalogen for the screening of peroxisomal disorders. Brain & development. 2003;25:481–487. doi: 10.1016/s0387-7604(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Thiboutot D. Regulation of human sebaceous glands. The Journal of investigative dermatology. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- Tiffany JM. Individual variations in human meibomian lipid composition. Experimental eye research. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- Tosi F, Sartori F, Guarini P, Olivieri O, Martinelli N. Delta-5 and delta-6 desaturases: crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Advances in experimental medicine and biology. 2014;824:61–81. doi: 10.1007/978-3-319-07320-0_7. [DOI] [PubMed] [Google Scholar]
- Turkish AR, Henneberry AL, Cromley D, Padamsee M, Oelkers P, Bazzi H, Christiano AM, Billheimer JT, Sturley SL. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. The Journal of biological chemistry. 2005;280:14755–14764. doi: 10.1074/jbc.M500025200. [DOI] [PubMed] [Google Scholar]
- Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta ophthalmologica Scandinavica. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JC, Butovich IA, McCulley JP. Historical brief on composition of human meibum lipids. The ocular surface. 2009;7:145–153. doi: 10.1016/s1542-0124(12)70309-9. [DOI] [PubMed] [Google Scholar]
- Yamagami H, Richards SM, Sullivan BD, Liu M, Steagall RJ, Sullivan DA. Gender-associated differences in gene expression of the meibomian gland. Advances in experimental medicine and biology. 2002;506:459–463. doi: 10.1007/978-1-4615-0717-8_64. [DOI] [PubMed] [Google Scholar]
- Zadravec D, Tvrdik P, Guillou H, Haslam R, Kobayashi T, Napier JA, Capecchi MR, Jacobsson A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. Journal of lipid research. 2011;52:245–255. doi: 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Kothapalli KS, Brenna JT. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Current opinion in clinical nutrition and metabolic care. 2016;19:103–110. doi: 10.1097/MCO.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]