Abstract
The brain, which represents 2% of the body mass but consumes 20% of the body energy at rest, has a limited capacity to store energy and is therefore highly dependent on oxygen and glucose supply from the blood stream. Normal functioning of neural circuits thus relies on adequate matching between metabolic needs and blood supply. Moreover, not only does the brain need to be densely vascularized, it also requires a tightly controlled environment free of toxins and pathogens to provide the proper chemical composition for synaptic transmission and neuronal function. In this review, we will focus on three major factors that ensure optimal brain perfusion and function: the patterning of vascular networks to efficiently deliver blood and nutrients, the function of the blood-brain barrier to maintain brain homeostasis, and the regulation of cerebral blood flow to adequately couple energy supply to neural function.
Keywords: cerebrovascular patterning, neurovascular networks, blood-brain barrier, neurovascular unit, endothelial cells, cerebral blood flow
I- Vascular patterning in the nervous system
I.1. Control of vascular patterning by genetic programs during development
Vascular patterning and neural wiring by common guidance cues and receptors
Normal brain function relies heavily on the adequate matching between metabolic needs of neural cells and blood supply (Attwell & Laughlin 2001, Peters et al 2004). Nerves, in turn, control blood vessel tone as well as heart rate. The functional interdependence between the nervous and vascular systems is reflected in their close anatomical apposition throughout the organism. In the periphery, nerves and vessels often run in parallel, a phenomenon called ‘neurovascular congruency’ (Bates et al 2003, Lewis 1902, Martin & Lewis 1989). In the central nervous system (CNS), neural and vascular cells form a functionally integrated network, whereby neural activity and vascular dynamics are tightly coupled (Iadecola 2004), as discussed in the last section of this review. Moreover, both the nervous and vascular systems comprise highly branched and complex networks. The patterning of these networks is initiated during development in a highly stereotyped fashion that is controlled by genetic programs (Carmeliet & Tessier-Lavigne 2005). However, both networks exhibit a certain degree of plasticity and undergo dynamic remodeling postnatally.
Compared to the relatively well understood genetic programs and principles governing axon guidance and pathfinding (Huber et al 2003, O’Donnell et al 2009, Tessier-Lavigne & Goodman 1996), mechanisms underlying the elaboration of vascular networks remained mysterious until recent years. Hypoxia and hypoxia-induced vascular endothelial growth factor (VEGF) signalling are widely accepted as the main driving forces for vascular patterning during embryonic development (James et al 2009, Stone et al 1995). Whether intrinsic genetic programs are also needed and exist to control vascular patterning was not clear until a decade ago. Indeed, work from several studies showed that genetically engineered animals lacking traditional axon guidance cues and receptors display vascular patterning defects (Gitler et al 2004, Gu et al 2005, Lu et al 2004). Vascular-specific ablation of these guidance molecules recapitulates these defects, indicating that common cues are shared for wiring both the nervous and vascular systems (Adams & Eichmann 2010, Carmeliet & Tessier-Lavigne 2005). This molecular understanding of neural and vascular network patterning correlates with the structural and functional similarities between neuronal and vascular sprouts (growth cones and vascular tip cells, respectively), structures that allow neurons and vessels to sense and respond to their environments. Guidance receptors, typically expressed by neuronal growth cones and endothelial tip cells, initiate signalling upon binding to their correspondent environmental cues and control axon guidance and endothelial cell migration via regulation of cytoskeleton dynamics. While the specific molecules used within neurons and endothelial cells are often different, recent evidence suggests that similar intracellular signaling principles underlying cytoskeletal regulation are used to control both neural and vascular guidance (Gelfand et al 2009). The identification of traditional axon guidance cues and receptors as a new class of molecules controlling vascular patterning provides a new understanding of vascular network formation. Additionally, the realization that common guidance molecules are used to sculpt both neuronal and vascular networks provides conceptual insight into the coordinate development of both systems, a topic which has been widely reviewed previously (Adams & Eichmann 2010, Carmeliet & Tessier-Lavigne 2005, Melani & Weinstein 2010).
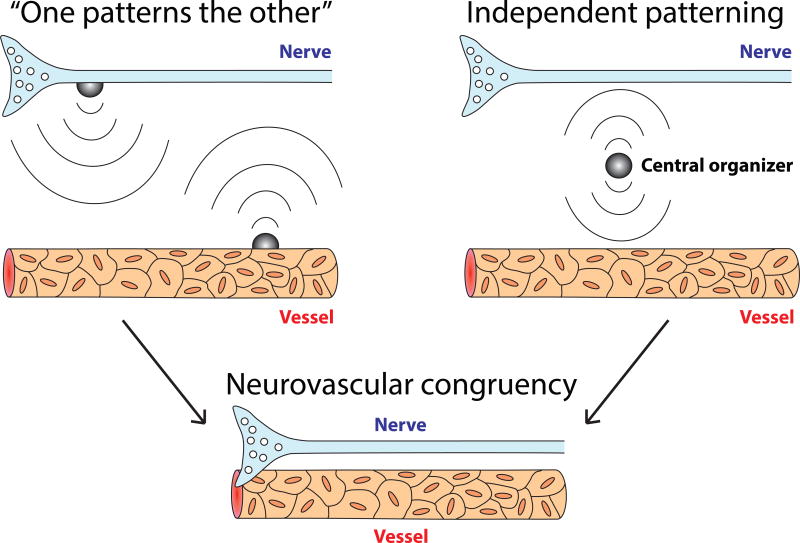
What are the basic principles underlying the establishment of neurovascular congruency?
While the existence of neurovascular congruency is widespread, so far the most studied example is within the vertebrate forelimb. During development when arterial differentiation and branching are occurring, in mice with genetic mutations resulting in misguided axons, arterial branches follow misrouted axons in forelimb skin, demonstrating that peripheral sensory nerves determine the pattern of arterial differentiation and blood vessel branching (Mukouyama et al 2005, Mukouyama et al 2002). These studies suggest that neurovascular congruency can be established by a “one patterns the other” model, in which either the nervous or vascular system precedes in development and then instructs the second system to form, using an already established architecture as a template. Consistent with this model, there is also evidence that vessels can express signals that attract axons. For example, artemin is expressed in smooth muscle cells surrounding vessels and attracts sympathetic axon fibers (Honma et al 2002). Similarly, vascular endothelins direct the extension of sympathetic axons from the superior cervical ganglion toward the external carotid artery (Makita et al 2008). Whether a “one patterns the other” model serves as a general mechanism to govern the establishment of neurovascular congruency in all tissues has been questionable until a recent study pointing to a different mechanism (Oh & Gu 2013). Oh and Gu found that in the mouse whisker pad, at the root of whiskers, nerves and vessels form a stereotypic ‘double ring’ structure around each follicle, with an inner nerve ring forming first, followed by an exterior vessel ring. A “one patterns the other” model would predict that the nerve rings attract surrounding blood vessels to establish the double ring structure. However, in mutant mice lacking trigeminal neurons and therefore lacking nerve rings, vessel rings form normally. Likewise, in mice with deformed vessel rings, nerve rings form normally, demonstrating that the neurovascular congruency in the whisker pad occurs via an independent patterning mechanism. In this particular case, nerves and vessels respond to a common guidance cue emanating from the center of each whisker follicle, with a differential response determining their inner vs. outer final position. This conclusion highlights previous findings that common guidance cues are used to forge networks of both the nervous and vascular systems, and are also contribute to their congruent patterning.
What is the logic for having two different principles establishing neurovascular congruency (Figure 1)? During pathfinding toward a target, or when a target tissue has a planar structure, a “one patterns the other” model allows for parallel nerve and vessel trajectories, independent of their position relative to their surroundings. However, in complex, three-dimensional target tissue structures, the relative orientation of nerves, vessels, and target tissues becomes functionally relevant. The independent or coordinate patterning model enables the target tissue to act as a central organizer to coordinate the development of multiple tissue subcomponents. Given the diversity of neurovascular structures in different tissues, local signals provided by a central organizer may be a critical mechanism used to establish neurovascular congruency. As it was the case in studies of the mouse whisker pad, genetic approaches used to selectively manipulate one system at a time could be a powerful approach to probe the neurovascular interactions in the CNS.
Figure 1.
Two models of Neurovascular congruency: (Left) “One patterns the other” model, in which either the nervous or vascular system precedes developmentally, and then instructs the other system to form using its established architecture as a template. In the case of a tissue with a planar structure such as skin, or during pathfinding before nerves and vessel reach their target tissue, this model allows for the development of parallel trajectories of nerves and vessels, independent of their position relative to their surroundings. (Right) Independent patterning model, in which balanced attractive and repulsive signals originate from a central organizer within a target tissue – this central organizer acts to pattern neurovascular congruency. When nerves and vessels reach a target tissue with a complex three-dimensional structure, the precise architecture of nerves, vessels and the target tissue becomes functionally relevant, as dictated by the unique requirements of the target tissue environment.
Cerebrovascular patterning during development
The physical relationships between neuronal and vascular networks in the brain are much more complex than the often congruent (parallel) structure observed in the periphery. The developing brain is vascularized via ingression of blood vessels from the outside. At embryonic day 10 (E10) in mice, new capillaries sprout from perineural vessels and invade the neuroectoderm. Relative hypoxia in the growing brain is a major driving force for the ingression and refinement of the complex vascular bed that serves it. Other angiogenic signalling pathways have also been shown to play important roles in shaping cerebrovascular networks, including VEGF, Notch, Wnt/ β-catenin, Semaphorins/neuropilins/plexins, BMPs, orphan G protein coupled adhesion receptor GPCR124, TGF-beta, and Nogo-A (Ruhrberg & Bautch 2013, Wittko-Schneider et al 2014). The neural environment plays a role in the initial ingression, elaboration, and pruning/stabilization of blood vessels. Multiple cell types including microglia, pericytes, neuroepithelial radial glia, neuroblasts and astrocytes are associated with blood vessels and influence their density/branching patterns (Arnold & Betsholtz 2013, Lee & McCarty 2014, Ma et al 2012, Ma et al 2013). Although the patterning of large brain arteries (external and surface arteries) is stereotyped, the question still remains whether smaller arteries, penetrating arterioles and capillaries also exhibit stereotyped patterns.
I.2. Control of cerebrovascular patterning by neural activity
Although the vascular network is initiated early during embryogenesis, expansion of vascular networks continues postnatally and vascular remodeling occurs in both physiological and pathological conditions. Whether neural activity influences the formation of cerebrovascular networks remains elusive and controversial. William T. Greenough and colleagues first postulated that during postnatal development, the brain adapts to increased metabolic demands by creating new vessels (Black et al 1990, Black et al 1987, Black et al 1991). These milestone studies introduced the concept of vascular remodeling during maturation of the brain, but the neuronal contribution to vascular patterning after birth is elusive.
The role of neural activity in cerebrovascular patterning during postnatal development and in pathological conditions
From studies in the rat cerebral cortex, the once prevailing view was that requirements of neural tissues (e.g. synaptogenesis, neuropil expansion) influence the maturation of underlying capillary networks (Black et al 1987, Sirevaag et al 1988), and that high metabolic activity correlates with higher vascular density (Riddle et al 1993). Moreover, several studies proposed the existence of anatomical “matching” relationships between neuronal and vascular modules within cortical columns in the rat somatosensory cortex (Cox et al 1993, Patel 1983). Such anatomical matching suggests that neuronal and vascular modules may instruct each other to build a precisely wired network for optimized local interactions, similar to the neurovascular congruency observed in the periphery. However, it was later demonstrated that cortical microvascular domains do not display any obvious topological matching relationship with underlying neuronal columns (Woolsey et al 1996). Consistent with this observation, recent studies using novel imaging and computational techniques, with three-dimensional reconstructions of cerebrovascular networks, further demonstrated that microvascular topology does not match neuroarchitecture in the mouse cerebral cortex (Blinder et al 2013, Lacoste et al 2014, Tsai et al 2009). Therefore, neuronal structure is not necessarily involved in vascular patterning, but rather neural activity may play a role.
The concept of activity-induced vascular plasticity during postnatal development was first introduced by studies postulating that sensory stimulation positively influenced brain angiogenesis (Argandona & Lafuente 1996, Argandona & Lafuente 2000, Black et al 1987, Sirevaag et al 1988). Thus, after birth, sensory-related neural activity may refine cerebrovascular networks into their mature form, much like it does for neuronal circuits (Katz & Shatz 1996, Zhang & Poo 2001). By simultaneously visualizing neuronal and vascular modules, the direct effect of sensory neural activity on cerebrovascular development during a critical postnatal period, under physiological conditions, was recently examined (Lacoste et al 2014). Lacoste et al found that vascular density and branching, as well as endothelial cell proliferation, were decreased in layer IV of the primary somatosensory cortex when sensory input was reduced by either a complete deafferentation, a genetic impairment of neurotransmitter release at thalamocortical synapses, or by a selective reduction of sensory-related neural activity. In contrast, increased sensory stimulation resulted in a vascular network with greater vessel density and branching. These findings suggest that, in addition to angiogenic programs that regulate the initial vascular patterning, sensory-related neural activity appears necessary for cerebrovascular refinement during early postnatal development, with changes in neural activity being sufficient to trigger changes in vascular networks.
Brain vascular structure may be regulated differently under pathological conditions in which neural activity is affected. Excessive neural activity following hyperactivation of sensorimotor systems was recently shown to impair cerebrovascular network formation during a critical postnatal window (Whiteus et al 2014). Whiteus et al found a severe reduction of angiogenesis in the cerebral cortex following either vigorous locomotor exercise, persistent auditory stimulation, or following chemically-induced seizures. In the adult rat brain however, previous studies using similar hyperactivation paradigms evidenced increased angiogenesis in the cerebellum following intense locomotor exercise (Isaacs et al 1992) or in the hippocampus after electroconvulsive seizures (Newton et al 2006), emphasizing the difference between the “immature” and the “mature” brain in terms of vascular plasticity. Importantly, this angiogenic capability of the adult brain might be relevant in ischemic conditions such as stroke. It has been shown that angiogenesis is increased in the penumbra of the ischemic adult mouse barrel cortex following enhancement of sensory-related neural activity by whisker stimulation (Whitaker et al 2007), an effect which involves VEGF/VEGFR2 signaling (Li et al 2011) and which can be amplified by inhibition of de novo cholesterol synthesis by statins (Zhang et al 2012).
How does neural activity control cerebrovascular patterning?
The question remains whether neural activity affects angiogenesis directly via neurotransmitter and/or growth factor release, for instance by thalamocortical axons, or indirectly via pathways that are activated following neural activation, which involve cortical interneurons and glial cells. Pyramidal (excitatory) neurons, inhibitory interneurons and astrocytes are recruited by somatosensory inputs (Lecrux et al 2011), and in turn release vasoactive mediators which control vascular tone and cerebral blood flow (CBF) (Cauli & Hamel 2010, Drake & Iadecola 2007). Whether these neural modules also release angiogenesis regulators upon neural activity changes is yet to be resolved. Among many possibilities, astrocytes might be involved. Astrocytes are in close contact with both neuronal synapses and cerebral microvessels, and are thus well positioned to couple neural activity to vascular growth. Indeed, in addition to their role in the control of CBF (Attwell et al 2010, Iadecola & Nedergaard 2007, Lind et al 2013), astrocytes respond to glutamate by releasing pro-angiogenic lipids (epoxy-eicosa-trienoic acids, or EETs) as potent as VEGF (Munzenmaier & Harder 2000, Potente et al 2003, Pozzi et al 2005, Zhang & Harder 2002). Moreover, it was recently demonstrated that astrocytes are essential for the normal postnatal development of cortical vasculature (Ma et al 2012). Future in vivo studies should investigate the precise mechanisms through which neural activity controls the release of astroglial angiogenesis modulators and their effects on cerebrovascular patterning. Finally, as we will discuss in the third chapter of this review, local hypoxia may be the trigger of activity-induced angiogenesis.
II- The blood-brain barrier
Historical perspectives: location and development of the blood-brain barrier
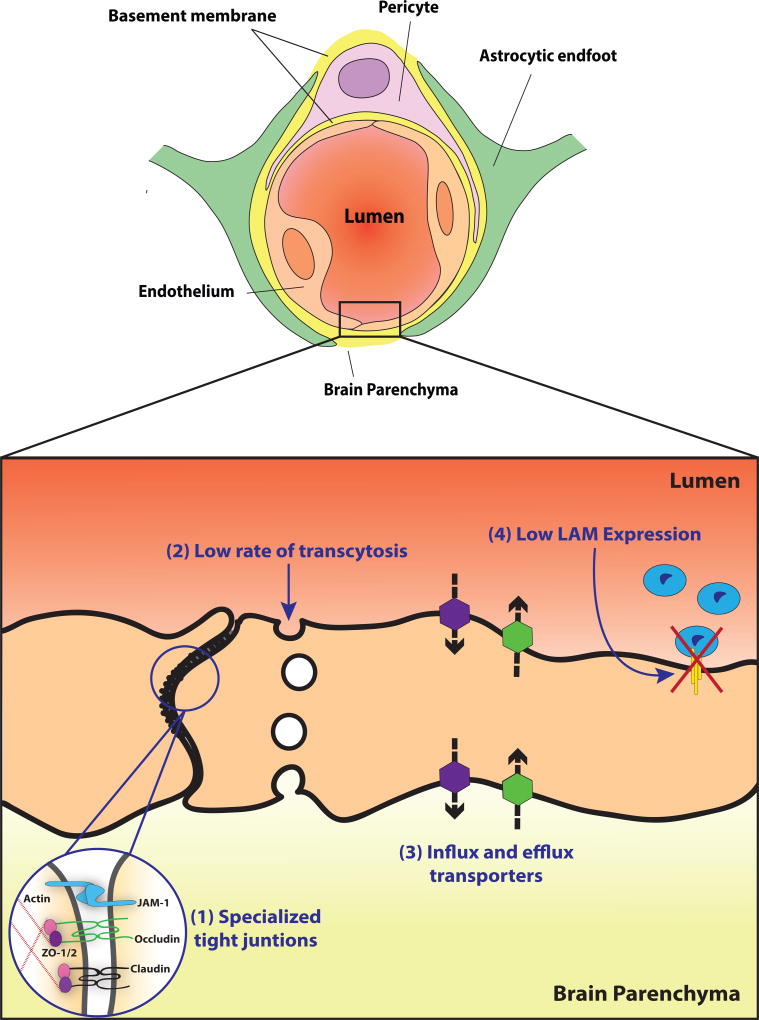
The blood-brain barrier (BBB) provides one of the best examples of how the neuronal-vascular interface functions to ensure a homeostatic environment for proper brain function. As opposed to the periphery, in which a fenestrated endothelium allows for the rapid transport of solutes and fluids to and from the blood (Aird 2007), the CNS requires a tightly controlled environment free of various toxins and pathogens to provide the proper chemical composition for synaptic transmission. This environment is maintained by the BBB, which is characterized by a thin layer of continuous, non-fenestrated endothelial cells that line the walls of the CNS blood vessels. This endothelial cell layer serves as the physiological barrier that seals the CNS and controls substance influx and efflux (Armulik et al 2010, Bell et al 2010, Daneman et al 2010b). Additionally, astrocytes, and pericytes provide functional support for the BBB, and together with endothelial cells are referred to as the ‘neurovascular unit’ (Figure 2, top) (Siegenthaler et al 2013).
Figure 2.
CNS endothelial cell properties contributing to BBB functionality: (Top) A cross-sectional view of the neurovascular unit at the level of a CNS capillary. Vessels are lined by a single-layer of non-fenestrated endothelial cells that exhibit barrier properties. Astrocytes and pericytes surround the abluminal surface of CNS endothelial cells and provide additional functional support for the establishment and maintenance of the BBB. (Bottom) A magnified view of the CNS endothelium, highlighting the four cellular properties that contribute to BBB integrity by stringently controlling the exchange of ions and nutrients between the blood and brain. (1) Specialized tight junctions prevent paracellular flux between endothelial cells. Cell-cell interactions are mediated by tight junction proteins, including junctional adhesion molecule-1 (JAM-1), occludin, and members of the claudin family. The cytoplasmic adaptor proteins ZO-1 and ZO-2 link these transmembrane proteins to the cytoskeleton. (2) Endothelial cells exhibit extremely low rates of transcytosis, as vesicular trafficking of ions and nutrients across cells is kept to a minimum. (3) Endothelial cells express influx (purple hexagons) and efflux (green hexagons) transporters that both shuttle specific nutrients into the brain and remove potentially harmful toxins and other small molecules from the brain, respectively. (4) The low expression of leukocyte adhesion molecules (LAM) aids in maintaining low levels of immune surveillance in the CNS.
Historically, three seminal lines of investigation established the existence of a barrier between the blood and brain. First, it was observed over a century ago that systemic injection of water-soluble trypan blue dye resulted in the staining of several tissues, notably excluding the brain (Ehrlich 1885). Second, the advent of electron microscopy (EM) allowed Reese and Karnovsky to identify CNS endothelial cells as the cell-type that possesses barrier properties. When horseradish peroxidase (HRP) was injected into the circulation as a subcellular tracer, no extravasation from vessel lumen to brain parenchyma was observed (Reese & Karnovsky 1967). Furthermore, pinocytotic vesicles were not present to transport tracer across cerebral endothelial cells, and the movement of tracer between cells was blocked by tight junctions. When HRP was injected directly into the brain, it diffused past astrocytic endfeet and the basement membrane but was again blocked at tight junctions between endothelial cells, indicating that these cells are the site of the BBB (Brightman & Reese 1969). Third, the uniqueness of the BBB to the CNS was investigated years later through the generation of quail-chick transplantation chimeras (Stewart & Wiley 1981). In these experiments, non-vascularized embryonic mesoderm engrafted into the brain, but not vice-versa, developed vasculature with barrier properties, demonstrating an inductive role for the CNS microenvironment in the development of the BBB.
The question of BBB development has been investigated by several groups and in diverse species with different experimental paradigms, the history of which has been extensively reviewed (Saunders et al 2012). Once a point of contention, the developmental timepoint at which the BBB becomes functional has been resolved in recent years. Several studies have used high-resolution imaging techniques to show that circulating tracers are completely excluded from the brain parenchyma at embryonic timepoints in rodent models, demonstrating that the BBB becomes non-leaky and thus functional before birth (Bauer et al 1995, Ben-Zvi et al 2014, Daneman et al 2010b).
What are the cellular and molecular underpinnings of BBB functionality?
Once an outstanding question in the neurovascular field, studies over the past several years have begun to shed light on the mechanisms whereby the neurovascular unit confers BBB properties upon CNS endothelium. Here, we will review the current understanding of the cellular and molecular pathways by which each neurovascular unit cell-type contributes to BBB development and functionality.
Endothelial Cells
As the cells forming the BBB, endothelial cells of the CNS have four specific properties that contribute to the integrity of the BBB (Figure 2, bottom). First, the tight junctions between endothelial cells prevent the passage of ions and nutrients within the paracellular space (Hawkins & Davis 2005). Although tight junctions are present in peripheral endothelial cells, they are “tighter” in CNS endothelium, as evidenced both by their ultrastructural characteristics under EM (Reese & Karnovsky 1967), and the observation that no water-soluble molecules can freely pass through tight junctions in the CNS. Second, compared to peripheral endothelium, which readily utilizes cargo-filled vesicles to transport macromolecules from the blood to underlying tissue, CNS endothelial cells display remarkably low rates of vesicular trafficking between the luminal and abluminal cell membranes, a process termed transcytosis (Reese & Karnovsky 1967, Siegenthaler et al 2013). Third, instead of utilizing vesicle trafficking, brain endothelial cells express numerous transporters to deliver nutrients, such as glucose, amino acids, and metabolically relevant ions to the brain, as well as remove potentially neurotoxic substances and drugs (Saunders et al 2013). Finally, CNS endothelial cells have low expression of leukocyte adhesion molecules (LAMs), and thus play a role in preventing the movement of immune cells into the immuno-privileged brain environment (Rossler et al 1992). In general, these four endothelial cell properties can be categorized into those that confer tightness to (tight junctions and transporter expression), and prevent leakiness of (low rates of transcytosis and LAM expression) the BBB.
Emerging evidence suggests that endothelial cells express molecules that are essential for the establishment of these CNS-specific properties. Much of this work has been guided by endothelial cell transcriptome studies that have produced lists of candidate genes that may be important for BBB function, some of which have been investigated in depth (Armulik et al 2010, Ben-Zvi et al 2014, Daneman et al 2010a, Tam et al 2012). Recently, major facilitator domain containing protein 2A (MFSD2A) was identified as a molecule expressed specifically in CNS endothelial cells that promotes BBB formation and function specifically by maintaining low rates of transcytosis (Ben-Zvi et al 2014), suggesting that CNS endothelial cells may promote BBB integrity by endogenously expressing machinery to suppress this process which readily occurs in the periphery. Additionally, this observation highlights the importance of the regulation of transcytosis, an often overlooked feature of CNS endothelial cells. Interestingly, a separate group identified MFSD2A as a transporter for fatty acids in brain endothelial cells (Nguyen et al 2014). Future studies will address whether MFSD2A serves a dual role in CNS endothelial cells, or if both functions are required to promote BBB integrity (Betsholtz 2014, Zhao & Zlokovic 2014).
Neural Progenitors
During embryonic development, neural progenitors secrete factors that regulate angiogenesis and the first steps of BBB formation. The most well characterized examples are the Wnt family of morphogens, including Wnt7a/7b, which are expressed by neural progenitors in ventral regions of the brain at the same time that nascent endothelial cells expressing β-catenin begin to ingress (Daneman et al 2009, Stenman et al 2008). If Wnt/β-catenin signaling is genetically abolished, severe defects in angiogenesis are observed specifically in the CNS, including loss of capillary beds and vascular malformations adjacent to the meninges. In addition, loss of the Wnt receptor Frizzled4 leads to BBB breakdown, specifically in the cerebellum (Wang et al 2012). With regards to the specific BBB properties that Wnt cues regulate, neuronally-derived Wnt7a induces the expression of BBB-specific transporters, most notably glucose transporter-1 (Glut-1/slc2a1). Temporal ablation of β-catenin in endothelial cells postnatally also results in decreased expression of tight junction component claudin-3 and a loss of BBB integrity (Liebner et al 2008), suggesting that neuron-endothelium interactions may facilitate the maintenance of BBB tight junctions. Together, these studies suggest that neuronally-derived Wnt cues contribute to the “tightness” properties (tight junctions and transporters) of the BBB in CNS endothelial cells. The canonical Wnt/β-catenin signaling pathway likely results in transcriptional regulation of effector proteins in endothelial cells with BBB function. For example, expression of downstream Wnt/β-catenin signaling targets DR6 and TROY, which are upregulated in endothelial cells with increased β-catenin levels, is necessary for BBB formation and vascular pattering (Tam et al 2012). Canonical β-catenin signaling, however, is not the only Wnt pathway relevant to BBB function. A recent study has shown that Wnt5a activates the planar cell polarity pathway in vitro to promote tight junction integrity in endothelial cells (Artus et al 2014), opening up the field for further study of how non-classical Wnt signaling regulates BBB integrity in vivo. One conceptual caveat to studying Wnt signaling in BBB development, however, is that disruption of this pathway leads to defects in vascular patterning, making it difficult to differentiate the processes of angiogenesis and barrier genesis in these conditions (Daneman et al 2009).
Astrocytes
It has long been known that astrocytes, a major glial subtype in the brain, ensheath CNS vessels and confer BBB properties to endothelial cells. For example, early experiments showed that transplantation of astrocytes can induce peripheral endothelial cells to form non-leaky vessels (Janzer & Raff 1987). Unlike the early role played by neural progenitors in BBB development, however, astrocytes do not appear at the neurovascular unit until after birth and are generally thought to aid in the maintenance of BBB functionality (Obermeier et al 2013, Siegenthaler et al 2013). For example, astrocytes express molecules that regulate properties of BBB integrity in endothelial cells. Most notably, astrocyte-secreted Sonic Hedgehog (Shh) improves barrier function both in vitro and in vivo (Alvarez et al 2011). Shh signaling in CNS endothelial cells increases the expression of tight junction components occludin and claudin-5, and decreases the expression of chemokine and cell adhesion molecule (CAM) expression, suggesting it has a role in both tight junction integrity and immune surveillance at the BBB. In addition to Shh, astrocyte-secreted angiopoietin-1 and angiotensinogen both signal to endothelial cells to promote tight junction integrity (Lee et al 2003, Wosik et al 2007). Together, these studies show that crosstalk between astrocytes and CNS endothelial cells functions to maintain the integrity of the BBB, primarily through the expression and regulation of inter-endothelial tight junctions. Recently, it was described that retinoic acid, produced by fetal astrocytes, plays an important role in the development of the BBB, calling into question the view that astrocytes are only required for BBB maintenance after birth (Mizee et al 2013). As retinoic acid has been shown to regulate both Shh and Wnt signaling in the CNS (Halilagic et al 2007), this study also illustrates the complex interactions between different cell types and signaling pathways in the establishment and maintenance of the BBB.
Pericytes
Although it is a more recent notion in the field, it is now well-established that pericytes are key regulators of BBB integrity, both during developmental and adult stages. Pericytes, contractile cells of the neurovascular unit at the capillary level, are recruited by release of platelet-derived growth factor-b (Pdgf-b) from nascent endothelial cells during embryonic development, which binds to platlet-derived growth factor receptor-β (Pdgfrβ) expressed on the pericyte cell surface (Hellstrom et al 1999, Lindahl et al 1997). The identification of Pdgfrβ as a marker for brain pericytes offered the first entry point into understanding the role of this cell type in BBB function, as mice lacking Pdgfrβ completely lack brain pericytes (Lindahl et al 1997). While they are embryonically lethal, pericyte-deficient mice are characterized by high vascular permeability at embryonic stages, indicating a defect in BBB development (Daneman et al 2010b). Specifically in these animals, endothelial cells display numerous membrane protrusions into the vessel lumen and an increased density of cytoplasmic vesicles, as visualized by EM, indicating increased rates of transcytosis. Additionally, loss of pericytes leads to the expression of several LAMs, while the expression of occludin, claudin-5, and several transporters remains unaltered. In a separate study, it was shown that adult mice with decreased pericyte coverage display BBB leakiness which correlates to the extent of pericyte loss (Armulik et al 2010). Moreover, leakiness in these animals occurs through a transcytotic route. Interestingly, MFSD2A expression is reduced in pericyte-deficient mice (Ben-Zvi et al 2014), pointing towards a possible link between pericyte loss and BBB permeability, a potential topic of future investigation (Betsholtz 2014).
The BBB in disease and neurodegeneration
Neuronal-vascular abnormalities, including BBB breakdown can have deleterious effects on neuronal function. For example, BBB leakiness has been shown to precede age-dependent neuronal loss, decreased dendritic spine density and length, memory impairment, and neuroinflammation (Bell et al 2010). BBB breakdown has also been implicated in numerous neurodegenerative diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and multiple sclerosis. The relationship between loss of BBB integrity and disease pathology has been extensively reviewed (Obermeier et al 2013, Zlokovic 2008).
Emerging therapeutic strategies for BBB manipulation for CNS drug delivery
The control of BBB integrity in health and disease is a double-edged sword: while an intact BBB is clearly essential for normal brain function, a sealed BBB is the major obstacle for CNS drug delivery. In fact, a large percentage of neurotherapeutic agents, including recombinant proteins and antibodies, do not cross the BBB under normal circumstances (Zlokovic 2008). Therefore, it is not surprising that a major research focus has been devoted to designing therapeutic methods of BBB manipulation for drug delivery to the CNS. Current approaches focus on altering the properties of CNS endothelial cells, allowing for the controlled penetration of therapeutic agents (Pardridge 2007, Pardridge 2012). As opposed to modifying intercellular tight junctions (which may damage the structural integrity of the neurovascular unit) or utilizing transporters (which requires designing drugs that mimic the molecular structure of endogenous substrates), hijacking transcytotic pathways in endothelial cells provides an appealing strategy for drug delivery to the CNS.
A subset of nutrients and macromolecules, such as insulin and iron, are known to cross the BBB by binding to surface receptors at the luminal endothelial cell plasma membrane with subsequent delivery to the brain parenchyma, a process termed “receptor-mediated transcytosis” (Tuma & Hubbard 2003). Manipulation of receptor-mediated transcytosis pathways through the use of molecular Trojan horse (MTH) technology is a favorable strategy for CNS drug delivery. To design these agents, neurotherapeutic agents are conjugated to an antibody IgG domain that targets an endogenous BBB receptor (Pardridge 2012, Xia et al 2009). Specifically, several groups have used the transferring receptor (TfR), which facilitates the delivery of transferrin-bound iron to the brain, as a target for MTH design to treat pathological conditions such as Alzheimer’s disease and brain tumors (Jones & Shusta 2007, Yu & Watts 2013). Although these strategies have had some success, an incomplete understanding of how TfR trafficking occurs in CNS endothelial cells confounds their interpretation. Indeed, studies have suggested that MTHs targeting the TfR remain trapped in the CNS endothelium and that this may relate to the affinity of the IgG to the TfR (Couch et al 2013, Manich et al 2013, Yu et al 2011), calling into question the ability of these tools to readily deliver neurotherapeutic agents to the brain.
To attempt to resolve these discrepancies, two recently published studies further investigated how MTHs targeting the TfR are trafficking in CNS endothelium (Bien-Ly et al 2014, Niewoehner et al 2014). First, Niewoehner et al generated a MTH that engages the TrR in a monovalent fashion, which they showed significantly increases the brain delivery of an anti-amyloid-β antibody. In contrast, a traditional bivalent version of the same MTH is sorted to the endothelial lysosomes, supporting a model in which the binding mode to the TfR is the critical factor that determines the efficiency of drug delivery. Bien-Ly et al propose that anti-TfR affinity is the key factor effecting MTH efficiency. The authors demonstrated that high-affinity TfR binding both reduces brain TfR levels and drives MTH lysosomal degradation, compared to a low-affinity MTH that allows more effective drug delivery to the brain.
While these studies provide novel insights guiding the future design of more effective MTH agents, the question remains whether targeting receptor-mediated transcytosis pathways is the most effective strategy of BBB manipulation for drug delivery. One major caveat to this strategy is that the targeted receptors, such as TfR, are expressed throughout the body, which leads to MTH clearance into peripheral tissues and reduces the amount of the agent available for brain delivery (Yu & Watts 2013). Targeting molecules that are expressed specifically in endothelial cells, such as MFSD2A, may provide an alternative strategy for CNS drug delivery. Also, the transcytotic pathway that is upregulated in the absence of MFSD2A has not been shown to place limits on the size or chemical properties of transported cargo (Armulik et al 2010, Ben-Zvi et al 2014), making this pathway particularly appealing from a drug delivery perspective (Betsholtz 2014).
What are the future directions in BBB research?
As the critical role of the BBB in both normal and pathological conditions has become increasingly apparent, the effort to develop new technologies to study its function is moving to the forefront of the field. While EM studies of endothelial cells and imaging of fixable injected tracers remain landmark techniques, they only provide a static snapshot and do not allow investigators to visualize the consequences of manipulating the properties of CNS endothelium in real time. Emerging techniques utilizing two-photon microscopy to visualize the molecular components of CNS endothelial cells are beginning to address these outstanding issues. For example, Knowland et al (2014) have used a transgenic-Claudin5-eGFP mouse (Evans et al 2000), which fluorescently labels tight junctions to show for the first time how tight junction dynamics are altered in vivo in response to stroke. Similar studies will become increasingly useful in the future to assess how endothelial cells respond to a variety of genetic and pharmacological assaults. In particular, it will be important to develop tools to allow the visualization of different transcytotic pathways, as the regulation of transcytosis is emerging as a critical mechanism of maintaining BBB integrity (Armulik et al 2010, Ben-Zvi et al 2014, Daneman et al 2010b).
Also needed in the field is an in vitro system used for high-throughput screening of drugs that can alter BBB permeability – one that is simple, widely acceptable, and reliably recapitulates the in vivo barrier. Ideally, such a cellular model would express the molecular constituents of CNS endothelial cells (transporters and tight junction molecules), recapitulate endothelial cell architecture and polarity, and most importantly, possess highly restrictive paracellular and transcellular barriers. As multiple neurovascular unit cell types maintain BBB functionality in vivo, however, CNS endothelial cells readily lose barrier properties in culture. For example, transendothelial electrical resistance across CNS endothelial monolayers, a commonly used metric of tight junction integrity, is severely reduced in vitro (Grant et al 1998), indicating a non-restrictive barrier (Wilhelm et al 2011).
Several groups have recently made strides to improve in vitro BBB models. In an attempt to recapitulate the neurovascular unit in vitro, endothelial cells are often co- and tri-cultured with astrocytes and pericytes, resulting in increased transendothelial electrical resistance measurements (Hatherell et al 2011). Paolinelli et al (2013) demonstrated that activating the Wnt/β-catenin pathway in vitro increases the restrictiveness of endothelial cell monolayers without the need to co-culture with additional cell types, raising the interesting notion that stimulating the cellular signaling pathways necessary for BBB function in culture may be sufficient to form a restrictive barrier. One recurring discrepancy among the various in vitro BBB models relates to cell type, as isolated primary endothelial cells and immortalized lines from various species have been used to varying degrees of success (Gumbleton & Audus 2001). Recent evidence has demonstrated that endothelial cells with barrier properties can be generated from human pluripotent stem cells (Lippmann et al 2014, Lippmann et al 2012). In vitro BBB models from stem cell sources may provide an ideal platform for future work, as they circumvent the variability generated by the use of isolated primary cells and can be propagated in vitro from all-human cell sources.
Despite all the progress since the discovery of the BBB, the field is in many ways still in its infancy, with many fundamental questions remaining to be answered: What are the key molecular regulators and pathways essential for the development of the BBB? How do these core components work together to ensure BBB functionality? Are endothelial cell tight junctions regulated by unique mechanisms in the CNS? How is transcytosis, the most promising pathway to manipulate for therapeutic purposes, regulated in CNS endothelial cells? These topics deserve to be a major focus of future research in neuroscience.
III- Neurovascular coupling
The first evidence of a spatio-temporal coupling between brain activity and CBF (or ‘neurovascular coupling’) was provided in 1890 by Roy and Sherrington, who challenged the doctrine that active changes in brain vessels diameter were impossible (Friedland & Iadecola 1991, Roy & Sherrington 1890). A key conclusion of that work remains valid today: “(…) the brain possesses an intrinsic mechanism by which its vascular supply can be varied locally in correspondence with local variations of functional activity”. Since then, tremendous effort has been made to investigate mechanisms governing the regulation of CBF by neural activity, at various levels along the vascular tree from arterioles to capillaries. Hence, studies pertaining to the “vasomotor function of nerves”, proposed in the late 1920’s by Talbott and colleagues (Talbott et al 1929), involving various neurotransmitters and signaling molecules (Cauli & Hamel 2010), gave birth to a fast-evolving field in neurobiology with far-reaching implications in both health and disease. Indeed, thorough investigation about the relationships between neural activity and CBF is essential for adequate analysis of Blood Oxygenation Level Dependent (BOLD) functional brain imaging (Hillman 2014) and for fundamental understanding of vascular dysfunctions (Iadecola 2004, Zlokovic 2010).
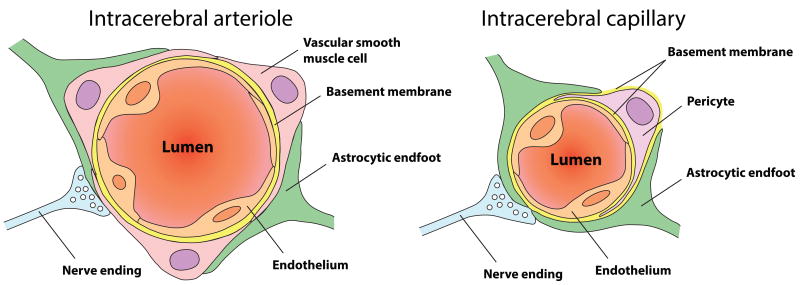
The neurovascular unit is the anatomical substrate of neurovascular interactions in the brain (Lecrux & Hamel 2011, Lo & Rosenberg 2009). Along the vascular tree, cellular components of the neurovascular unit integrate messages conveyed by neural activity to modify the diameter of brain vessels and regulate CBF. In the control of vessel diameter, the main difference between intracerebral arterioles and capillaries is the nature, position and abundance of contractile cells surrounding the external (abluminal) vessel surface (Figure 3). Arterioles are fully covered by a single layer of vascular smooth muscle cells (VSMC), whereas capillaries, which by definition lack VSMCs, are partly covered by contractile pericytes with the highest density in the central nervous system (Armulik et al 2005, Cipolla 2009). In the neocortex, the regulation of CBF by neural activity is thought to happen mainly at the arteriole level (Fergus & Lee 1997, Hillman et al 2007), although the involvement of pericytes at the capillary level was recently established, opening a new debate about the contribution of each vascular compartment to the regulation of CBF, as well as to the generation of the BOLD signal used as a readout for neural activity in functional brain imaging.
Figure 3.
Schematic representation of the neurovascular unit: Inside the brain, endothelial cells are organized into a multicellular complex together with contractile and glial cells, an assembly called the neurovascular unit. The main difference between intracerebral arterioles and capillaries is the nature, position and abundance of contractile cells that surround the external vessel surface. (Left) At the level of intracerebral arterioles, the endothelium is fully covered by a single layer of vascular smooth muscle cells which provide contractile properties to the arteriole. Astrocytes send their processes called “end-feet” around the arteriole, providing further support as well as a functional connection to surrounding neural tissues. (Right) Intracerebral capillaries lack vascular smooth muscle cells but are partly covered by contractile pericytes, with a higher density in the central nervous system. Recent findings provide new evidence about the importance of pericytes contractile properties in neurovascular coupling.
Control of cerebrovascular function at the level of arterioles
The net effect of neural activity on arteriole diameter, the induction of either vasodilation or vasonconstriction, reflects the integration by the neurovascular unit of signals controlling the electric state and contractile properties of VSMCs (Hamel 2006, Hillman 2014). The major players in cortical neurovascular coupling are i) projection neurons sending fibers to the neocortex from subcortical centers (Cauli et al 2004, Elhusseiny & Hamel 2000, Zhang et al 1995), ii) local excitatory and inhibitory neurons (Cauli & Hamel 2010, Lecrux et al 2011), and iii) astrocytes (Attwell et al 2010, Howarth 2014, Zonta et al 2003), which all release vasoactive substances. More details about the regulation of CBF at the level of cerebral arterioles can be found in recent reviews (Cauli & Hamel 2010, Hillman 2014).
Control of cerebrovascular function at the level of capillaries
CNS capillaries lack VSMCs, and at least 80% of their abluminal surface is covered by pericytes (Armulik et al 2005, Peppiatt et al 2006). These contractile cells contribute to the capillary neurovascular unit. Pericytes were long considered as ‘support’ cells for the endothelium, involved in the formation and maintenance of the BBB (Armulik et al 2010, Daneman et al 2010b, Mae et al 2011). The regulation of capillary diameter by pericytes in response to neural activity has been clarified over the past decade (Hamilton et al 2010, Itoh & Suzuki 2012, Peppiatt et al 2006). Most recently, the contribution of pericytes to CBF regulation was reevaluated in more physiological conditions (Fernandez-Klett et al 2010, Hall et al 2014). Using two-photon microscopy in mice with genetically labeled pericytes, Fernandez-Klett et al demonstrated in real time that pericytes are effective regulators of capillary diameter and capillary flow, without contributing significantly to global CBF regulation. In contrast, Hall et al demonstrated that pericytes increase blood flow in the somatosensory cortex following whisker pad stimulation, through relaxation (vasodilation) initiated at the capillary level. Overall, the activity-induced control of capillary diameter (and flow) by pericytes must also be considered as an integrative process in the context of cerebrovascular anatomy. Indeed, the population of pericytes is heterogenous along the vascular tree. Subtypes of pericytes respond differentially to neurotransmitters or vasomodulators (Fernandez-Klett et al 2010, Hall et al 2014, Peppiatt et al 2006), and pericytes adjacent to small capillaries are most probably non-contractile since they are negative for smooth muscle α-actin (Nehls & Drenckhahn 1991). This suggests that activity-induced CBF regulation by pericytes might happen locally within defined microvascular segments, with contractile signals possibly propagating between pericytes and spreading back to upstream arterioles (Hall et al 2014, Peppiatt et al 2006).
A new concept for neurovascular interactions in the early postnatal brain
Recent studies in rodents and humans have shown that the phenomenon of neurovascular coupling is not functional until a few weeks after birth (three weeks in rats, seven to eight weeks in humans). While sensory stimulation in adults leads to a positive BOLD signal, reflecting a local increase in CBF, the identical stimulus in newborn infants or rat pups was shown to result in an inverted response with negative BOLD signals (Anderson et al 2001, Born et al 2002, Kozberg et al 2013, Muramoto et al 2002, Yamada et al 2000). In these studies, negative BOLD signals were suggested to result from either decreased perfusion or increased oxygen consumption, in response to sensory stimulation. The absence of a neurovascular coupling response to neuronal activation implies that, during early postnatal development, the immature brain must rely on alternative mechanisms to adequately match oxygen and nutrients supply to increasing energy demands. One potential mechanism during postnatal development could be the control of cerebrovascular patterning by neural activity, which we discussed in the first chapter of this review. Indeed, it was recently demonstrated that during postnatal development, sensory-related neural activity promotes the formation of vascular networks in the mouse barrel cortex (Lacoste et al 2014).
Could hypoxia be involved in the control of cerebrovascular patterning by neural activity? After birth, the maturation of neuronal networks involves energy-consuming processes such as neurogenesis, synaptogenesis, maturation of astrocytes, and changes brain cytoarchitecture. Thus, in the early postnatal brain, in the absence of neurovascular coupling, neuronal network activation might generate a local and transient hypoxic state. Hypoxia-induced growth factors represent the main driving force of vascular development not only during embryogenesis (Haigh et al 2003, Provis et al 1997, Raab et al 2004, Stone et al 1995) but also during postnatal cerebrovascular remodeling (Rey & Semenza 2010), and particularly in pathological conditions such as ischemia and cancer (Silpanisong & Pearce 2013). Local reduction in oxygen tension leads to activation of the transcription factor ‘hypoxia-inducible factor 1’ (HIF-1). Activated HIF-1 regulates the expression of virtually all the key angiogenic factors, including VEGF, angiopoietin-2 and placental growth factor upon binding to their hypoxia response elements (Jiang et al 1997, Rey & Semenza 2010, Semenza et al 1997). Future studies could investigate whether sensory stimulation, which leads to increased vascular density and branching in the cerebral cortex (Lacoste et al 2014), is due to a local and transient hypoxic state that in turn triggers hypoxia-induced proangiogenic pathways (Yuen et al 2014).
IV- Conclusion
In this review, we have highlighted the current understanding of how the nervous and vascular systems interact in order to ensure the proper function of the brain. Neuronal and vascular networks are established during development by utilizing a common set of guidance cues, providing the closely juxtaposed anatomical framework for the delivery of oxygen and nutrients from the blood to underlying neuronal tissue. This framework can be refined during postnatal development by neural activity in response to the demands of the environment. Additionally, the control of cerebrovascular function at different levels along the vascular tree is crucial in regulating CBF and ensuring that the metabolic needs of neurons are met within the brain. Yet, the regulation of CBF is not functional until several weeks after birth. This implies that the early postnatal brain relies on alternative mechanisms, such as neural activity-driven angiogenesis, for efficient delivery of oxygen and glucose. Finally, the integrity of the BBB is critical in maintaining the safe and homeostatic environment necessary for the function of neural circuits. As neuroscience research progresses, the functional relevance of a proper neuronal-vascular interface within the brain becomes increasingly clear. A fundamental understanding of neuronal and vascular interactions has far-reaching benefits in developing strategies to treat psychological and neurodegenerative diseases, brain tumors, and stroke, highlighting the importance of future research in this field of neuroscience.
Acknowledgments
We thank Drs. Edith Hamel, Christer Betsholtz, and Jonathan Cohen for helpful comments on the manuscript. We apologize to our colleagues whose research we could not cite or discuss due to space limitations.
References
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harbor perspectives in biology. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magnetic resonance imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Argandona EG, Lafuente JV. Effects of dark-rearing on the vascularization of the developmental rat visual cortex. Brain Res. 1996;732:43–51. doi: 10.1016/0006-8993(96)00485-4. [DOI] [PubMed] [Google Scholar]
- Argandona EG, Lafuente JV. Influence of visual experience deprivation on the postnatal development of the microvascular bed in layer IV of the rat visual cortex. Brain Res. 2000;855:137–42. doi: 10.1016/s0006-8993(99)02361-6. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vascular cell. 2013;5:4. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus C, Glacial F, Ganeshamoorthy K, Ziegler N, Godet M, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34:433–40. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Developmental biology. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 1995;86:317–25. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–27. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–11. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C. Physiology: Double function at the blood-brain barrier. Nature. 2014;509:432–3. doi: 10.1038/nature13339. [DOI] [PubMed] [Google Scholar]
- Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med. 2014;211:233–44. doi: 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–5. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Black JE, Zelazny AM, Greenough WT. Capillary and mitochondrial support of neural plasticity in adult rat visual cortex. Exp Neurol. 1991;111:204–9. doi: 10.1016/0014-4886(91)90008-z. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–97. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born AP, Rostrup E, Miranda MJ, Larsson HB, Lou HC. Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR) Magnetic resonance imaging. 2002;20:199–205. doi: 10.1016/s0730-725x(02)00469-1. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. The Journal of cell biology. 1969;40:648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–9. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. Chapter 2, Anatomy and Ultrastructure. [PubMed] [Google Scholar]
- Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005338. 183ra57, 1–12. [DOI] [PubMed] [Google Scholar]
- Cox SB, Woolsey TA, Rovainen CM. Localized dynamic changes in cortical blood flow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. J Cereb Blood Flow Metab. 1993;13:899–913. doi: 10.1038/jcbfm.1993.113. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010a;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010b;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–52. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Das Sauerstoff-Bedürfniss des Organismus : eine farbenanalytische Studie. Berlin: Hirschwald; 1885. [Google Scholar]
- Elhusseiny A, Hamel E. Muscarinic--but not nicotinic--acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Evans V, Hatzopoulos A, Aird WC, Rayburn HB, Rosenberg RD, Kuivenhoven JA. Targeting the Hprt locus in mice reveals differential regulation of Tie2 gene expression in the endothelium. Physiological genomics. 2000;2:67–75. doi: 10.1152/physiolgenomics.2000.2.2.67. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997;754:35–45. doi: 10.1016/s0006-8993(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–5. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland RP, Iadecola C. Roy and Sherrington (1890): a centennial reexamination of “On the regulation of the blood-supply of the brain”. Neurology. 1991;41:10–4. doi: 10.1212/wnl.41.1.10. [DOI] [PubMed] [Google Scholar]
- Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends in cell biology. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Developmental cell. 2004;7:107–16. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grant GA, Abbott NJ, Janigro D. Understanding the Physiology of the Blood-Brain Barrier: In Vitro Models. News Physiol Sci. 1998;13:287–93. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–8. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood-brain barrier. J Pharm Sci. 2001;90:1681–98. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Developmental biology. 2003;262:225–41. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Developmental biology. 2007;303:362–75. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006;100:1059–64. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199:223–9. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annual review of neuroscience. 2014;37:161–81. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–82. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Frontiers in neuroscience. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annual review of neuroscience. 2003;26:509–63. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab. 2012;32:1167–76. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–41. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–7. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–60. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharmaceutical research. 2007;24:1759–71. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kozberg MG, Chen BR, DeLeo SE, Bouchard MB, Hillman EM. Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc Natl Acad Sci USA. 2013;110:4380–5. doi: 10.1073/pnas.1212785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, et al. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–30. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, et al. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–47. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, McCarty JH. Inducible gene deletion in glial cells to study angiogenesis in the central nervous system. Methods Mol Biol. 2014;1135:261–74. doi: 10.1007/978-1-4939-0320-7_22. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–6. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Lewis BM. The development of the arm in man. Am J Anat. 1902;1:145–85. [Google Scholar]
- Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214:503–13. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. The Journal of cell biology. 2008;183:409–17. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA. 2013;110:E4678–87. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Scientific reports. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature biotechnology. 2012;30:783–91. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40:S2–3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012;7:e48001. doi: 10.1371/journal.pone.0048001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS biology. 2013;11:e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae M, Armulik A, Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17:2750–4. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–63. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manich G, Cabezon I, del Valle J, Duran-Vilaregut J, Camins A, et al. Study of the transcytosis of an anti-transferrin receptor antibody with a Fab’ cargo across the blood-brain barrier in mice. Eur J Pharm Sci. 2013;49:556–64. doi: 10.1016/j.ejps.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Martin P, Lewis J. Origins of the neurovascular bundle: interactions between developing nerves and blood vessels in embryonic chick skin. The International journal of developmental biology. 1989;33:379–87. [PubMed] [Google Scholar]
- Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annual review of cell and developmental biology. 2010;26:639–65. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, et al. Retinoic acid induces blood-brain barrier development. J Neurosci. 2013;33:1660–71. doi: 10.1523/JNEUROSCI.1338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–52. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–7. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Muramoto S, Yamada H, Sadato N, Kimura H, Konishi Y, et al. Age-dependent change in metabolic response to photic stimulation of the primary visual cortex in infants: functional magnetic resonance imaging study. Journal of computer assisted tomography. 2002;26:894–901. doi: 10.1097/00004728-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. The Journal of cell biology. 1991;113:147–54. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Girgenti MJ, Collier EF, Duman RS. Electroconvulsive seizure increases adult hippocampal angiogenesis in rats. Eur J Neurosci. 2006;24:819–28. doi: 10.1111/j.1460-9568.2006.04958.x. [DOI] [PubMed] [Google Scholar]
- Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–6. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annual review of neuroscience. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WJ, Gu C. Establishment of neurovascular congruency in the mouse whisker system by an independent patterning mechanism. Neuron. 2013;80:458–69. doi: 10.1016/j.neuron.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug discovery today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U. Non-random distribution of blood vessels in the posterior region of the rat somatosensory cortex. Brain Res. 1983;289:65–70. doi: 10.1016/0006-8993(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, et al. The selfish brain: competition for energy resources. Neuroscience and biobehavioral reviews. 2004;28:143–80. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–25. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–46. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Provis JM, Leech J, Diaz CM, Penfold PL, Stone J, Keshet E. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res. 1997;65:555–68. doi: 10.1006/exer.1997.0365. [DOI] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. The Journal of cell biology. 1967;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler K, Neuchrist C, Kitz K, Scheiner O, Kraft D, Lassmann H. Expression of leucocyte adhesion molecules at the human blood-brain barrier (BBB) J Neurosci Res. 1992;31:365–74. doi: 10.1002/jnr.490310219. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Bautch VL. Neurovascular development and links to disease. Cell Mol Life Sci. 2013;70:1675–84. doi: 10.1007/s00018-013-1277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA. Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med. 2013;34:742–52. doi: 10.1016/j.mam.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Frontiers in pharmacology. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, et al. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553–5. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol. 2013;23:1057–64. doi: 10.1016/j.conb.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silpanisong J, Pearce WJ. Vasotrophic regulation of age-dependent hypoxic cerebrovascular remodeling. Current vascular pharmacology. 2013;11:544–63. doi: 10.2174/1570161111311050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Res. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–50. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Developmental biology. 1981;84:183–92. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott JH, Wolff HG, Cobb S. The cerebral circulation: VII. Changes in cerebral capillary bed following cervical sympathectomy. Arch Neur Psych. 1929;21:1102–06. [Google Scholar]
- Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, et al. Death receptors DR6 and TROY regulate brain vascular development. Developmental cell. 2012;22:403–17. doi: 10.1016/j.devcel.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–70. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma P, Hubbard AL. Transcytosis: crossing cellular barriers. Physiological reviews. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–44. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–11. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta neurobiologiae experimentalis. 2011;71:113–28. doi: 10.55782/ane-2011-1828. [DOI] [PubMed] [Google Scholar]
- Wittko-Schneider IM, Schneider FT, Plate KH. Cerebral angiogenesis during development: who is conducting the orchestra? Methods Mol Biol. 2014;1135:3–20. doi: 10.1007/978-1-4939-0320-7_1. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–60. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Wosik K, Cayrol R, Dodelet-Devillers A, Berthelet F, Bernard M, et al. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27:9032–42. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Boado RJ, Pardridge WM. Antibody-mediated targeting of siRNA via the human insulin receptor using avidin-biotin technology. Molecular pharmaceutics. 2009;6:747–51. doi: 10.1021/mp800194y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Sadato N, Konishi Y, Muramoto S, Kimura K, et al. A milestone for normal development of the infantile brain detected by functional MRI. Neurology. 2000;55:218–23. doi: 10.1212/wnl.55.2.218. [DOI] [PubMed] [Google Scholar]
- Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10:459–72. doi: 10.1007/s13311-013-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002230. 84ra44. [DOI] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–96. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–64. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;(4 Suppl):1207–14. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang S, Wang B, Sun B, Li W, et al. Atorvastatin and whisker stimulation synergistically enhance angiogenesis in the barrel cortex of rats following focal ischemia. Neurosci Lett. 2012;525:135–9. doi: 10.1016/j.neulet.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zlokovic BV. Blood-brain barrier: a dual life of MFSD2A? Neuron. 2014;82:728–30. doi: 10.1016/j.neuron.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]