Abstract
Objective:
To evaluate the association between comorbidity and relapse rate in individuals with multiple sclerosis (MS).
Methods:
We recruited individuals with prevalent relapsing-onset MS from 4 Canadian MS Clinics to participate in a 2-year prospective multicenter cohort study involving cross-sectional assessment of comorbidities and relapses. Comorbidities were recorded using questionnaires, and relapses were captured from medical records at each visit. The association between comorbidities at baseline and relapse rate over the subsequent 2-year follow-up period was examined using Poisson regression, adjusting for age, sex, disability, disease duration, and treatment status.
Results:
Of 885 participants, 678 (76.6%) were women, averaging age 48.2 years at baseline. Anxiety (40.2%), depression (21.1%), hypertension (17.7%), migraine (18.1%), and hyperlipidemia (11.9%) were the most prevalent comorbidities. The frequency of participants experiencing relapses remained constant at 14.9% and 13.2% in years 1 and 2 post-baseline. After adjustment, participants reporting ≥3 baseline comorbidities (relative to none) had a higher relapse rate over the subsequent 2 years (adjusted rate ratio 1.45, 95% confidence interval [CI] 1.00–2.08). Migraine and hyperlipidemia were associated with increased relapse rate (adjusted rate ratio 1.38; 95% CI 1.01–1.89 and 1.67; 95% CI 1.07–2.61, respectively).
Conclusions:
Individuals with migraine, hyperlipidemia, or a high comorbidity burden (3 or more conditions) had an increased relapse rate over 2 years. These findings have potential implications for understanding the pathophysiology of MS relapses, and suggest that closer monitoring of individuals with specific or multiple comorbidities may be needed. Future research is needed to understand if the presence of comorbidity warrants a tailored approach to MS management.
Comorbid conditions, such as hypertension and diabetes, are frequent in multiple sclerosis (MS) and are associated with increased hospitalizations,1 greater disability progression,2 mortality risk,3 and contrast-enhancing lesions on brain MRI.4 Comorbid conditions are also associated with decreased quality of life in MS.5 Also, it has been suggested that comorbidity may explain part of the variability in clinical course between people with MS.2
Relapsing-onset MS is characterized by acute exacerbations, or relapses. These relapses adversely influence life activities,6 and the accumulation of residual neurologic deficits following relapses contributes to increasing disability.7 However, determinants of relapses remain incompletely understood.8 An emerging line of inquiry suggests a potential association between comorbidities and MS relapses but studies have been limited. Among people with MS recruited through social media who responded to an online questionnaire, those who had 3 or more comorbidities had 2-fold increased odds of reporting a relapse as compared to those reporting no comorbidities.9 Hyperlipidemia was not associated with MS relapse risk in 2 Australian studies,10,11 but was associated with gadolinium-enhancing lesions, a biomarker of disease activity.4 Rheumatoid arthritis and anemia were associated with a 2- to 3-fold increased risk of relapse in a cohort of 198 participants with MS.11
We aimed to evaluate the association between comorbidity and the risk of relapse in a prospective, multicenter study. We hypothesized that vascular comorbidities (diabetes, hypertension, and hyperlipidemia) would be associated with a higher risk of future relapse, compared to no vascular comorbidity.
METHODS
Study population.
Between July 2010 and March 2011, patients with MS attending a routine MS clinic visit at 1 of 4 MS clinics in the Canadian provinces of Nova Scotia, Manitoba, Alberta, or British Columbia were recruited.5 Patients were invited to participate in a 2-year prospective cohort study, which involved cross-sectional assessments at 3 time points (coinciding with clinic visits): baseline and at years 1 and 2. To maximize follow-up, participants unable to attend their annual MS clinic visit were offered follow-up questionnaires via telephone, mail, or e-mail. Inclusion criteria included a diagnosis of definite MS according to the predominant diagnostic criteria at the time of diagnosis,12–15 age ≥18 years, fluent in English, resident in the province where data collection was occurring, and ability to provide informed consent. Type of clinical course was not an inclusion criterion for the primary study. We restricted the present analyses to those with a relapsing-onset disease course at baseline (relapsing-remitting MS [RRMS], secondary progressive MS [SPMS], or clinically isolated syndrome [CIS]).
Standard protocol approvals, registrations, and patient consents.
In each province, we obtained ethics approval and participant consent.
Clinical and demographic information.
All data in this study were captured from the medical record or self-report measures. Information collected from each participant's medical record (which was recorded by the attending neurologist) using a standardized data collection form included study site, date of birth, sex, highest education level achieved, race, date of MS onset, MS clinical course (RRMS, SPMS, primary progressive MS [PPMS], or CIS), Expanded Disability Status Score (EDSS), and current disease-modifying therapy (DMT) use (current yes vs no; if yes, DMT product). All information was recorded on the day of study recruitment (baseline) and the latter 3 clinical characteristics were also collected at each follow-up visit from the medical chart, as recorded by the attending neurologist. Relapses since the last visit were recorded in the clinic chart by the attending neurologist at each clinic visit throughout the study; neurologists were not advised to adhere to a study-specific relapse definition.
Self-reported measures.
Participants completed validated questionnaires16,17 at baseline and at years 1 and 2. Comorbidities were assessed using the question “Have you ever been diagnosed with the following condition by a physician?”16 The conditions were chronic obstructive pulmonary disease, hyperlipidemia, hypertension, migraine, irritable bowel syndrome, inflammatory bowel disease, thyroid disease, osteoporosis, cataracts, diabetes mellitus, rheumatoid arthritis, fibromyalgia, heart disease, glaucoma, peripheral vascular disease, seizure disorder, and systemic lupus erythematosus. Participants also completed the Hospital Anxiety and Depression Scale (HADS), which assesses the severity of depression and anxiety over the previous week. A cutoff score of ≥8 on the HADS was used to classify the presence of depression or anxiety; as compared to a structured clinical interview, this threshold was previously found to be 90% sensitive and 87.3% specific in identifying depression, and 73.2% sensitive and 84.8% specific in identifying anxiety in MS.17 Operationalization of anxiety and depression using current symptom severity in this manner was considered more relevant to relapse risk at follow-up than past diagnoses of depression and anxiety given that these conditions can remit, and that depression and anxiety are often underdiagnosed and undertreated in MS. Specific comorbidities of interest for this analysis were diabetes, hypertension, hyperlipidemia, migraine, depression, and anxiety. These comorbidities were selected because they can be accurately captured by self-report,16 they occurred sufficiently frequently in the cohort (>10% affected) for meaningful analysis, and owing to their established high prevalence in the MS population; prior studies have reported that they are associated with relapses10,11 or disability progression.2 Five of these conditions were recently identified as the most relevant comorbidities to study with respect to MS outcomes.18
Statistical analysis.
Categorical variables were described as frequencies (%) and continuous variables were described using mean (SD) or median (interquartile range [IQR]). Missing data were reported but not imputed. The relapse count in the year preceding baseline and the number of relapses over the follow-up period were summarized. Comorbidity status was described as (1) presence of a specific condition and (2) a count of comorbidities; the latter was included for comparison with prior work,9 and achieved a balanced distribution of the sample population. All comorbidities, whether defined as physician-diagnosed or on the basis of the HADS, were included in the comorbidity count. We compared individuals with no relapse during the follow-up period and those with ≥1 relapses during the follow-up period with respect to demographic and clinical characteristics including comorbidity status using Pearson χ2 test, Fisher exact test, Student t test, or Mann-Whitney U test, as appropriate.
The association between the presence of comorbidity at enrollment (baseline) and the relapse rate over the follow-up period was examined using multivariable Poisson regression; annualized relapse rate is a common outcome in trials of the DMTs. The assumptions of the Poisson regression model were tested and met; there was no evidence of overdispersion as assessed by the deviance statistic divided by its degrees of freedom. To more accurately consider the follow-up time per participant, the log of follow-up time in days was included as an offset. We only considered baseline comorbidity status because the incidence of specific comorbidities was low over the 2-year follow-up period. Baseline comorbidities were included in the models as binary variables (yes or no). Covariates assessed at baseline included age (continuous), sex, disability status, DMT status (current, yes vs no), and number of relapses (continuous) in the previous year. These covariates were included either for clinical relevance (e.g., current DMT or sex) or on the basis of their association with the outcome from the baseline analysis. We repeated the regression analyses restricting them to participants with RRMS or CIS disease courses at baseline, as participants with SPMS would be expected to have fewer relapses. We report rate ratios (RRs) and 95% confidence intervals (CIs) for the associations.
Statistical significance was defined as p < 0.05. The p values were not corrected for multiple comparisons. All analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL).
RESULTS
Of 1,632 individuals who visited 1 of the 4 MS clinics between July 2010 and March 2011, 1,139 patients met the inclusion criteria for the primary study and were approached. Of these, 949 (82.6%) consented to participate. Of the 949 participants, 885 (93.3%) patients had a relapsing-onset disease course and were included in the present analyses. Overall, 93.7% of participants in the present analyses completed 2 years of follow-up, with an average (SD) follow-up time of 2.0 (0.24) years. Participants included in the present analyses (n = 885) did not differ from those excluded (n = 64) based on race, MS disease duration, baseline comorbidity count, or education level (all p > 0.1). As we excluded participants with a baseline PPMS course, sex (p = 0.004), age (p < 0.0001), age at MS onset (p < 0.0001), EDSS (p < 0.001), and current DMT use (p < 0.0001) differed between those included and excluded.
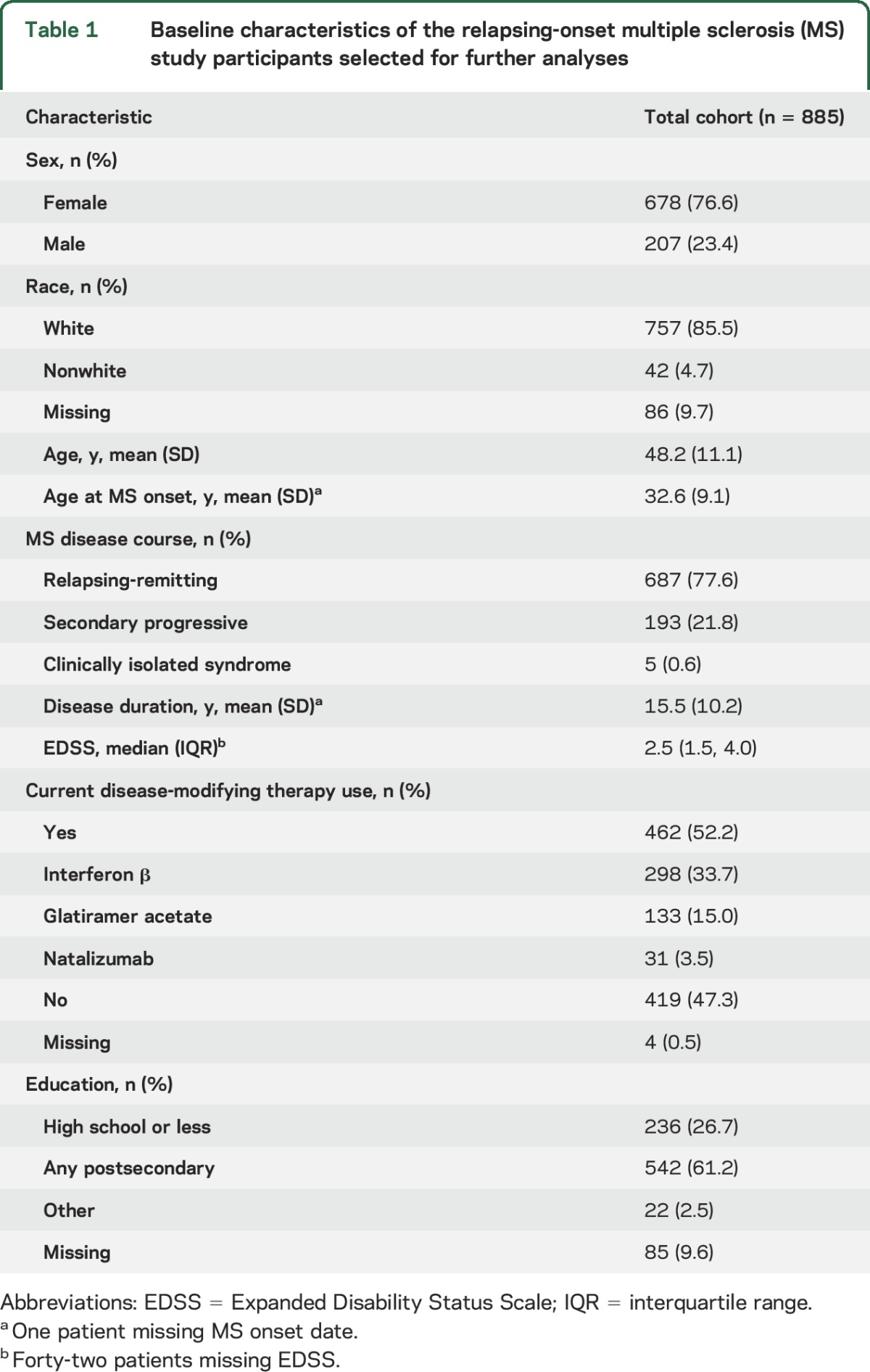
Most included participants had RRMS (687/885 [77.6%]), with the remainder having either SPMS (193/885 [21.8%]) or CIS (5/885 [0.6%]). Of the 4 Canadian recruitment sites, 377 (42.6%) participants were from Nova Scotia, 318 from British Columbia (35.9%), 109 from Manitoba (12.3%), and 81 from Alberta (9.2%). The mean (SD) age at the baseline visit was 48.2 (11.1) years and most participants were female (76.6%; table 1). The most prevalent comorbidities were depression and anxiety; more than 60% of the participants met the criteria for one of these (table e-1 at Neurology.org).
Table 1.
Baseline characteristics of the relapsing-onset multiple sclerosis (MS) study participants selected for further analyses
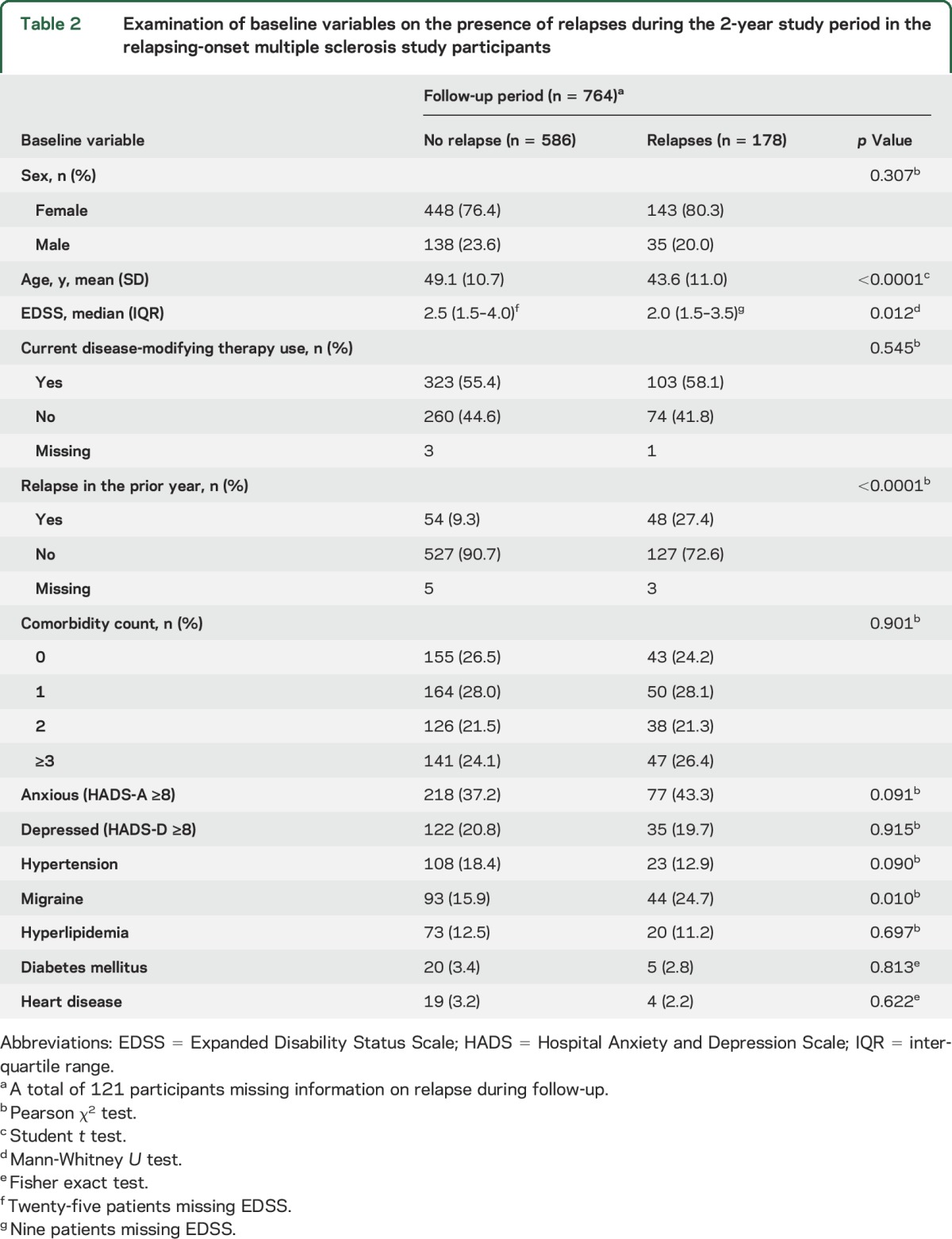
The number of participants experiencing relapses over the 3 assessments remained constant. Among the 874 participants with relapse information available, 115 (13.2%) experienced ≥1 relapse in the year preceding the baseline visit, 14.9% (122/821) in the first year, and 13.2% (103/780) in the second year. Of the participants with a relapse over the 2-year follow-up, the median number of relapses experienced was 1 (IQR 1.0–2.0), with most having had 1 relapse (119/178 [66.9%]), and the remainder having either 2 (48/178 [26.9%]) or ≥3 relapses (11/178 [6.2%]). During the 2-year follow-up period, those who experienced a relapse were younger, had a lower EDSS, and were more likely to have had a relapse in the year before baseline than those who had not (table 2).
Table 2.
Examination of baseline variables on the presence of relapses during the 2-year study period in the relapsing-onset multiple sclerosis study participants
Participants who reported ≥3 comorbidities at baseline had a higher relapse rate over the follow-up period, when adjusting for baseline covariates (adjusted RR 1.45, 95% CI 1.00–2.08, model 1; table 3). Migraine was also associated with increased relapse rate, a finding that remained after adjustment for the baseline covariates, including age, sex, disease duration, EDSS, DMT use, and relapse history (adjusted RR 1.38, 95% CI 1.01–1.89, model 2; table 3).
Table 3.
Association between baseline comorbidity and the risk of relapses over subsequent 2 years (Poisson regression) in participants with relapsing-onset multiple sclerosis (MS)
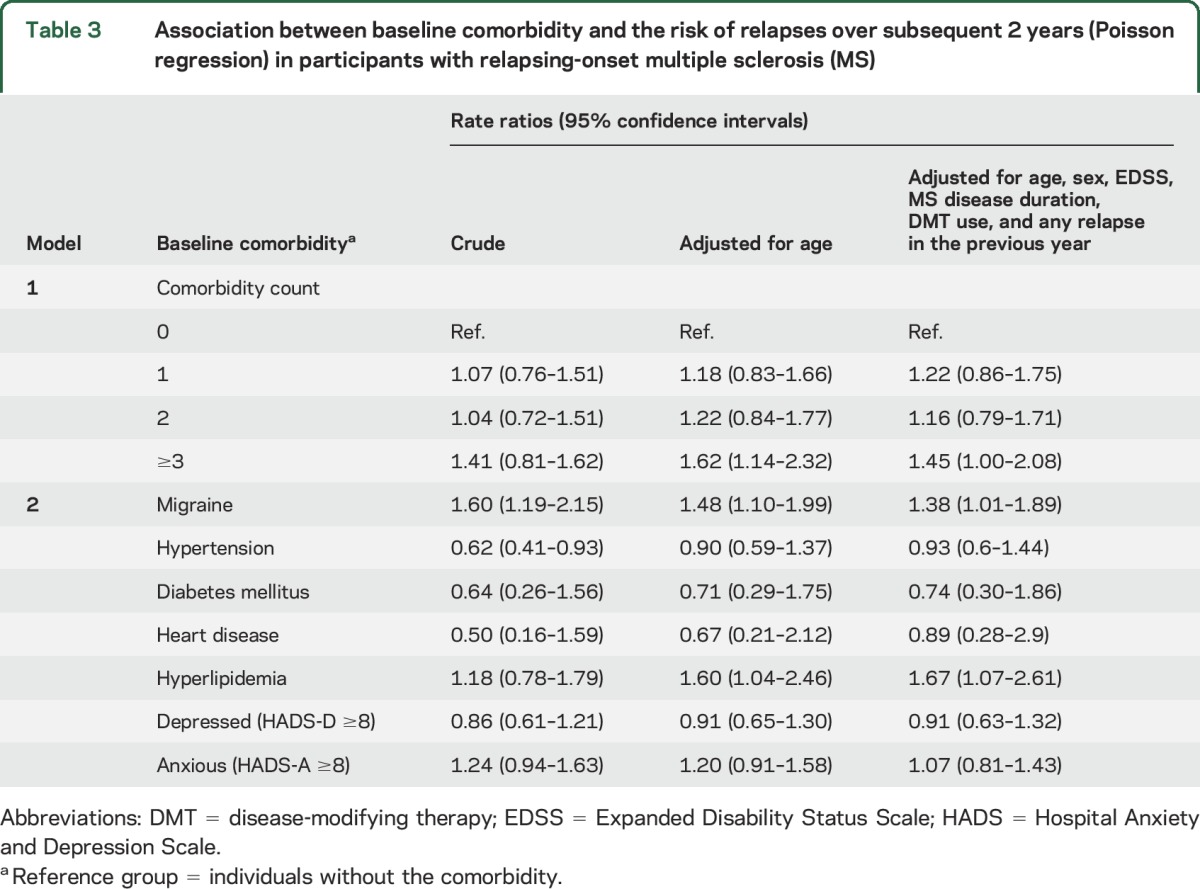
Patients with hypertension had a lower relapse rate compared to those with no hypertension (crude RR 0.60, 95% CI 0.40–0.90), but this association disappeared upon adjustment for age, indicating the crude protective association of hypertension was confounded by age (table 3). Hyperlipidemia and relapse rate were associated, when adjusting for age and other baseline covariates including DMT use and relapse history (RR 1.67, 95% CI 1.07–2.61).
After excluding participants with SPMS, the magnitude and direction of the associations were comparable to those observed in the full relapsing onset cohort, but CIs were broader in this smaller cohort (table e-2).
DISCUSSION
We investigated the association between baseline comorbidity status and the relapse rate in a prospective, multicenter study of individuals with MS. We found an association between the presence of 3 or more comorbidities at baseline and an increased relapse rate over the next 2 years, compared to those with no comorbidity. One other study exploring the burden of comorbidity as a count also reported an elevated risk of relapse associated with 3 or more comorbidities (compared to none), albeit with a larger risk estimate relative to our study (odds ratio 2.60, 95% CI 1.56–4.31).9 However, that study was essentially cross-sectional in design, with both comorbidity and relapse information collected at the same time. Further, all individuals were recruited via social media and all clinical data, including relapses, were self-reported through online questionnaires.9 In our study, relapses were confirmed by an MS specialist neurologist, as was the diagnosis of MS in individuals attending an MS clinic. The authors also reported a similar magnitude for the association between an increase in the number of comorbidities with more severe disability.9 A 2017 retrospective electronic medical record review investigating the influence of diabetes, hypertension, hyperlipidemia, and obstructive lung disease on MS disease course reported those with more than 3 comorbidities walked slower and performed worse on patient-reported outcomes compared to those with none of these comorbidities.19
We also found that specific comorbidities, including migraine, were associated with an increased relapse rate. Migraine is more common in individuals with MS than in individuals without MS20 and is frequent during MS relapses.21 A retrospective chart review of 1,113 Canadians with a discharge diagnosis of MS or followed at an MS clinic found 26 individuals presented with a vascular headache of migraine type during a relapse.21 Many of these individuals reported that the migraine preceded the relapse by hours or days.21 However, most of the participants were not established migraineurs, and thus the authors proposed the MS relapse mediated the release of vasoactive amines, bradykinin, and adenosine triphosphate that subsequently led to the migraine attack. However, elevated levels of the inflammatory marker C-reactive protein have been associated with migraine in a non-MS cohort22 and with MS relapse.23 Thus, it may be that the release of inflammatory and vasoactive compounds during a migraine can also trigger relapses, and account for the association of migraine and relapse found in our study.
In addition to migraine, hyperlipidemia was also specifically associated with an increased relapse rate in our cohort. Two previous studies found no association between hyperlipidemia and the hazard of relapse, although both included fewer than 200 participants, possibly limiting their power to detect an effect.10,11 The 2 studies also employed different measures for comorbidity: self-reported hyperlipidemia vs serum lipid and apolipoprotein levels.10,11 A study of 492 patients with MS assessing serum lipid profiles with disability and MRI outcomes found that higher levels of triglycerides, low-density lipoproteins, and cholesterol were all associated with greater worsening on EDSS over the 2-year follow-up period.4 In addition, the authors reported higher triglyceride levels were associated with increases in 2 measures of MS disease activity: gadolinium-enhancing lesions and gadolinium-enhancing lesion volume.4 This previous study did not distinguish between lesions related to hyperlipidemia or to MS, although they hypothesized that hyperlipidemia increases the risk of gadolinium-enhancing lesions by increasing the recruitment of leukocytes, risk of hypoperfusion, and endothelial dysfunction within the vascular endothelium at the blood–brain barrier.4
Age was a highly relevant factor in our study of the association between comorbidities and relapse, and highlights the complexity of the associations being evaluated (figure e-1). First, MS relapse rates decline with increasing age, therefore younger individuals tend to have more relapses than older individuals.24 Second, older individuals have an increased risk of vascular comorbidities, such as hypertension, hyperlipidemia, and diabetes, as well as for comorbidity in general.25 Beyond a theoretical basis for confounding by age, there was also evidence of confounding by the change in risk estimate for comorbidities following adjustment for age in our regression models. Hyperlipidemia and hypertension are more common in older adults; therefore the crude RR reflected an intermixing of the risk-elevating effect of comorbidity and the risk-lowering effect of older age. After adjusting for age, RRs were significantly elevated, indicating an independent association between these comorbidities and relapse rate.
Strengths of this study include the prospective, longitudinal design, and involvement of multiple sites to generate a relatively large sample, representative of the Canadian clinic-attending MS population.26,27 The use of a questionnaire to assess comorbidities that has been previously validated in an MS population,16 a comparative follow-up time,10,11 and the assessment of relapses by neurologists were additional strengths. This study further highlights the potential importance of identifying and controlling comorbidities in MS, but should not be interpreted to mean that treating specific comorbidities, such as migraines or hyperlipidemia, will lead to a reduction in future relapses. Future investigations should evaluate whether treatment of comorbidity affects relapse rates. However, this study also had limitations. First, we were unable to explore the effects of all comorbidities on relapse risk due to the low prevalence in our population, nor could we distinguish between subtypes of comorbidities, such as type 1 vs type 2 diabetes. Second, unmeasured factors such as vitamin D status and use of corticosteroids could have confounded the observed associations. While body mass index has been associated with the risk of developing MS and of comorbidities, previous studies demonstrated that body mass index is not associated with relapses,10 nor did it confound the association between comorbidities and relapse risk in another study.9 Third, the point estimates were not high, and the lower bound for some of the CIs included 1, suggesting the estimates are too imprecise to rule out a trivial effect at the 95% confidence level. Fourth, as this was an observational study, it was potentially susceptible to bias, including possible selection, information, or surveillance biases. Potential participants who chose not to participate may have differed from those who chose to participate in meaningful ways, although our high participation rate (82.6%) reduces this risk. Participants who self-reported comorbidities may be more likely to report relapses to their neurologist. However, the finite duration of the study and consistent assessment schedule for participants over the study period reduces the likelihood of surveillance bias. Relapses were recorded by neurologists specializing in MS, but misclassification due to pseudorelapses, minor cerebrovascular events, and other conditions that can mimic an MS relapse could still occur. Self-reported comorbidities are susceptible to misclassification as well, but we focused on conditions that can be accurately self-reported,16 and expect that the misclassification of comorbidities themselves would be nondifferential. Future prospective studies assessing the risk of relapse in MS that employ additional laboratory measures of comorbidity such as serum lipid levels, rely on formal diagnostic criteria for comorbidity where available, such as the International Classification of Headache Disorders criteria, and capture details regarding treatment status are needed. Finally, some comorbidities may affect adherence to DMT, and poor adherence may confound our findings; this should be addressed in future studies.
We found that individuals with MS and multiple comorbidities and specific comorbidities (migraine or hyperlipidemia) had an increased relapse rate over the subsequent 2 years. These findings were independent of the effects of age and sex. Many of the comorbidities studied here can be managed either pharmacologically or with lifestyle interventions. Our findings raise future areas for research, including whether individuals with specific comorbidities such as migraine or hyperlipidemia should be treated more aggressively or differently from individuals without these conditions.
ACKNOWLEDGMENT
The authors thank the research participants who volunteered their time, the neurologists and staff in all 4 clinics who contributed to this study through patient examination and facilitated access to clinical data, and the research assistants who coordinated data collection (Anna-Marie Bueno, Stacey Tkachuk, Nicholas Hall, Karen Turpin, Leanne Bergsma, Amy Cuthbertson, Alexandra Nelson, Beth DeCoste) and facilitated data manipulation (Tom Duggan, Karen Stadnyk).
GLOSSARY
- CI
confidence interval
- CIS
clinically isolated syndrome
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Score
- HADS
Hospital Anxiety and Depression Scale
- IQR
interquartile range
- MS
multiple sclerosis
- PPMS
primary progressive multiple sclerosis
- RR
rate ratio
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: CIHR Team in Epidemiology and Impact of Comorbidity on Multiple Sclerosis (ECoMS), James Blanchard, Lawrence Elliott, Bo Nancy Yu, Joanne Profetto-McGrath, Sharon Warren, Larry Svenson, Nathalie Jette, Virender Bhan, and Christina Wolfson
AUTHOR CONTRIBUTIONS
Kaarina Kowalec: analysis and interpretation of data, drafting of the manuscript. Kyla McKay, Charity Evans: interpretation of data, revision of the manuscript for intellectual content. Scott B. Patten, John Fisk, Helen Tremlett: conceptualization and design of the study, interpretation of data, revision of the manuscript for intellectual content. Ruth Ann Marrie: study concept and design, interpretation of data, revision of the manuscript for intellectual content.
STUDY FUNDING
Study funded by the Canadian Institutes of Health Research (CBG 101829).
DISCLOSURE
K. Kowalec reports no disclosures relevant to the manuscript. K. McKay is funded by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award. S. Patten reports no disclosures relevant to the manuscript. J. Fisk receives research funding from the CIHR, MS Society of Canada, National MS Society, and Dalhousie Medical Research Foundation, consultation and distribution royalties from MAPI Research Trust, and has received speaker honoraria and travel expenses from EMD Serono (2014). C. Evans reports no disclosures relevant to the manuscript. H. Tremlett is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. She currently receives research support from the National MS Society, the CIHR, the MS Society of Canada and the MS Scientific Research Foundation. In addition, in the last 5 years she has received research support from the MS Society of Canada (Don Paty Career Development Award), the Michael Smith Foundation for Health Research (Scholar Award), and the UK MS Trust; and speaker honoraria and/or travel expenses to attend conferences from the Consortium of MS Centres (2013), the National MS Society (2012, 2014, 2016), ECTRIMS (2012, 2013, 2014, 2015, 2016), the Chesapeake Health Education Program, US Veterans Affairs (2012), Novartis Canada (2012), Biogen Idec (2014), and American Academy of Neurology (2013, 2014, 2015, 2016). All speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by her research group. R. Marrie has conducted clinical trials for Sanofi-Aventis and receives research funding from CIHR, the National MS Society, the MS Society of Canada, the MS Scientific Research Foundation, Research Manitoba, the Consortium of MS Centers, Crohn's and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis. She serves on the Editorial Board for Neurology. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marrie RA, Elliott L, Marriott J, Cossoy M, Tennakoon A, Yu N. Comorbidity increases the risk of hospitalizations in multiple sclerosis. Neurology 2015;84:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;74:1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology 2015;85:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstock-Guttman B, Zivadinov R, Mahfooz N, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation 2011;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrigan LI, Fisk JD, Patten SB, et al. Health-related quality of life in multiple sclerosis: direct and indirect effects of comorbidity. Neurology 2016;86:1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickerson M, Cofield SS, Tyry T, Salter AR, Cutter GR, Marrie RA. Impact of multiple sclerosis relapse: the NARCOMS participant perspective. Mult Scler Relat Disord 2015;4:234–240. [DOI] [PubMed] [Google Scholar]
- 7.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003;61:1528–1532. [DOI] [PubMed] [Google Scholar]
- 8.McKay KA, Jahanfar S, Duggan T, Tkachuk S, Tremlett H. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology 2017;61:189–212. [DOI] [PubMed] [Google Scholar]
- 9.Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA. Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One 2016;11:e0148573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tettey P, Simpson S Jr, Taylor B, et al. Adverse lipid profile is not associated with relapse risk in MS: results from an observational cohort study. J Neurol Sci 2014;340:230–232. [DOI] [PubMed] [Google Scholar]
- 11.Tettey P, Siejka D, Simpson S Jr, et al. Frequency of comorbidities and their association with clinical disability and relapse in multiple sclerosis. Neuroepidemiology 2016;46:106–113. [DOI] [PubMed] [Google Scholar]
- 12.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 13.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton M, Rudick RA, Hara-Cleaver C, Marrie RA. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010;35:83–90. [DOI] [PubMed] [Google Scholar]
- 17.Honarmand K, Feinstein A. Validation of the hospital anxiety and depression scale for use with multiple sclerosis patients. Mult Scler 2009;15:1518–1524. [DOI] [PubMed] [Google Scholar]
- 18.Marrie RA, Miller A, Sormani MP, et al. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology 2016;86:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult Scler 2017;23:277–285. [DOI] [PubMed] [Google Scholar]
- 20.Pakpoor J, Handel AE, Giovannoni G, Dobson R, Ramagopalan SV. Meta-analysis of the relationship between multiple sclerosis and migraine. PLoS One 2012;7:e45295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman MS, Gray TA. Vascular headache: a presenting symptom of multiple sclerosis. Can J Neurol Sci 1989;16:63–66. [DOI] [PubMed] [Google Scholar]
- 22.Avci AY, Lakadamyali H, Arikan S, Benli US, Kilinc M. High sensitivity C-reactive protein and cerebral white matter hyperintensities on magnetic resonance imaging in migraine patients. J Headache Pain 2015;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannoni G, Thorpe JW, Kidd D, et al. Soluble E-selectin in multiple sclerosis: raised concentrations in patients with primary progressive disease. J Neurol Neurosurg Psychiatry 1996;60:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremlett H, Zhao Y, Joseph J, Devonshire V, Neurologists UC. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008;79:1368–1374. [DOI] [PubMed] [Google Scholar]
- 25.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler 2008;14:1091–1098. [DOI] [PubMed] [Google Scholar]
- 26.McKay KA, Tremlett H, Zhu F, Kastrukoff L, Marrie RA, Kingwell E. A population-based study comparing multiple sclerosis clinic users and non-users in British Columbia. Can Eur J Neurol 2016;23:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 2016;86:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]


