Abstract
Symbiotic interactions between insects and bacteria are ubiquitous and form a continuum from loose facultative symbiosis to greatly intimate and stable obligate symbiosis. In blood-sucking insects living exclusively on vertebrate blood, obligate endosymbionts are essential for hosts and hypothesized to supplement B-vitamins and cofactors missing from their blood diet. The role and distribution of facultative endosymbionts and their evolutionary significance as seeds of obligate symbioses are much less understood. Here, using phylogenetic approaches, we focus on the Hippoboscidae phylogeny as well as the stability and dynamics of obligate symbioses within this bloodsucking group. In particular, we demonstrate a new potentially obligate lineage of Sodalis co-evolving with the Olfersini subclade of Hippoboscidae. We also show several likely facultative Sodalis lineages closely related to Sodalis praecaptivus (HS strain) and suggest repeated acquisition of novel symbionts from the environment. Similar to Sodalis, Arsenophonus endosymbionts also form both obligate endosymbiotic lineages co-evolving with their hosts (Ornithomyini and Ornithoica groups) as well as possibly facultative infections incongruent with the Hippoboscidae phylogeny. Finally, we reveal substantial diversity of Wolbachia strains detected in Hippoboscidae samples falling into three supergroups: A, B, and the most common F. Altogether, our results prove the associations between Hippoboscoidea and their symbiotic bacteria to undergo surprisingly dynamic, yet selective, evolutionary processes strongly shaped by repeated endosymbiont replacements. Interestingly, obligate symbionts only originate from two endosymbiont genera, Arsenophonus and Sodalis, suggesting that the host is either highly selective about its future obligate symbionts or that these two lineages are the most competitive when establishing symbioses in louse flies.
Keywords: Arsenophonus, Sodalis, Wolbachia, Louse flies, Replacements, Phylogeny
Background
Symbiotic associations are widespread among animals and bacteria and often considered to undergo a common evolution as a holobiont (Zilber-Rosenberg & Rosenberg, 2008). The host and symbiont are either fully dependent on each other for reproduction and survival (obligate symbiosis) or not (facultative symbiosis), but in reality there is a gradient of such interactions (Moran, McCutcheon & Nakabachi, 2008). Any establishment of a symbiotic association brings not only advantages, but also several challenges to both partners. Perhaps the most crucial is that after entering the host, the endosymbiont genome tends to decay due to population genetic processes affecting asexual organisms with small effective population sizes (Moran, 1996) and the host is becoming dependent on such a degenerating symbiont (Koga et al., 2007; Pais et al., 2008). Since symbionts are essential for the host, the host can try to escape from this evolutionary ‘rabbit hole’ by an acquisition of novel symbionts or via endosymbiont replacement and supplementation (Bennett & Moran, 2015). This phenomenon, known in almost all insect symbiotic groups, was especially studied in the sap-feeding group Hemiptera (Sudakaran, Kost & Kaltenpoth, 2017), while only few studies were performed from blood-sucking groups.
Blood-sucking insects, living exclusively on vertebrate blood, such as sucking lice (Allen et al., 2007; Hypša & Křížek, 2007; Fukatsu et al., 2009; Allen et al., 2016), bed bugs (Hypša & Aksoy, 1997; Hosokawa et al., 2010; Nikoh et al., 2014), kissing bugs (Ben-Yakir, 1987; Beard et al., 1992; Hypša & Dale, 1997; Šorfová, Škeříková & Hypša, 2008; Pachebat et al., 2013), tsetse flies (Aksoy, 1995; Dale & Maudlin, 1999), bat flies (Trowbridge, Dittmar & Whiting, 2006; Hosokawa et al., 2012; Wilkinson et al., 2016), and louse flies (Trowbridge, Dittmar & Whiting, 2006; Nováková & Hypša, 2007; Chrudimský et al., 2012) have established symbiotic associations with bacteria from different lineages, mostly α-proteobacteria (Hosokawa et al., 2010) and γ-proteobacteria (Aksoy, 1995; Hypša & Aksoy, 1997; Hypša & Dale, 1997; Dale et al., 2006; Allen et al., 2007; Hypša & Křížek, 2007; Nováková & Hypša, 2007; Chrudimský et al., 2012; Hosokawa et al., 2012; Wilkinson et al., 2016). Obligate symbionts of these blood-sucking hosts are hypothesized to supplement B-vitamins and cofactors missing from their blood diet or present at too low concentration (Akman et al., 2002; Kirkness et al., 2010; Rio et al., 2012; Nikoh et al., 2014; Nováková et al., 2015; Boyd et al., 2016; Boyd et al., 2017), but experimental evidence supporting this hypothesis is scarce (Hosokawa et al., 2010; Nikoh et al., 2014; Michalkova et al., 2014; Snyder & Rio, 2015). The role played by facultative bacteria in blood-sucking hosts is even less understood, with metabolic or protective function as the two main working hypotheses (Geiger et al., 2005; Geiger et al., 2007; Toh et al., 2006; Belda et al., 2010; Snyder et al., 2010; Weiss et al., 2013).
Due to their medical importance, tsetse flies (Diptera, Glossinidae) belong to the most frequently studied models of such symbioses (International Glossina Genome Initiative, 2014). They harbour three different symbiotic bacteria: obligate symbiont Wigglesworthia glossinidia which is essential for the host survival (Pais et al., 2008), facultative symbiont Sodalis glossinidius which was suggested to cooperate with Wigglesworthia on thiamine biosynthesis (Belda et al., 2010), and reproductive manipulator Wolbachia (Pais et al., 2011). Considerable amount of information has till now been accumulated on the distribution, genomics and functions of these bacteria (Akman et al., 2002; Toh et al., 2006; Rio et al., 2012; Balmand et al., 2013; Michalkova et al., 2014; Snyder & Rio, 2015). In contrast to our understanding of tsetse fly symbioses, only scarce data are available on the symbioses in its closely related groups. Apart from Glossinidae, the superfamily Hippoboscoidea includes additional three families of obligatory blood-sucking flies, tightly associated with endosymbionts, namely Nycteribiidae, Streblidae, and Hippoboscidae. Monophyly of Hippoboscoidea has been confirmed by numerous studies (Nirmala, Hypša & Žurovec, 2001; Dittmar et al., 2006; Petersen et al., 2007; Kutty et al., 2010), but its inner topology has not been fully resolved. The monophyletic family Glossinidae is considered to be a sister group to the three remaining families together designated as Pupipara (Petersen et al., 2007). The two groups associated with bats probably form one branch, where Nycteribiidae seems to be monophyletic while monophyly of Streblidae was not conclusively confirmed (Dittmar et al., 2006; Petersen et al., 2007; Kutty et al., 2010). According to several studies, Hippoboscidae is regarded to be a monophyletic group with not well-resolved exact position in the tree (Nirmala, Hypša & Žurovec, 2001; Dittmar et al., 2006; Petersen et al., 2007). However, louse flies were also shown to be paraphyletic in respect to bat flies (Dittmar et al., 2006; Kutty et al., 2010).
Nycteribiidae, Streblidae (bat flies), and Hippoboscidae (louse flies) are often associated with Arsenophonus bacteria (Trowbridge, Dittmar & Whiting, 2006; Dale et al., 2006; Nováková, Hypša & Moran, 2009; Morse et al., 2013; Duron et al., 2014). In some cases, these symbionts form clades of obligate lineages coevolving with their hosts, but some of Arsenophonus lineages are likely representing loosely associated facultative symbionts spread horizontally across the population (Nováková, Hypša & Moran, 2009; Morse et al., 2013; Duron et al., 2014). Bat flies and louse flies are also commonly infected with Bartonella spp. (Halos et al., 2004; Morse et al., 2012b). Wolbachia infection was found in all Hippoboscoidea groups (Pais et al., 2011; Hosokawa et al., 2012; Morse et al., 2012a; Nováková et al., 2015). Moreover, several Hippoboscidae species were also found to harbour distinct lineages of Sodalis-like bacteria (Dale et al., 2006; Nováková & Hypša, 2007; Chrudimský et al., 2012) likely representing similar facultative-obligatory gradient of symbioses as observed for Arsenophonus.
Hippoboscoidea thus represent a group of blood-sucking insects with strikingly dynamic symbioses. Obligate symbionts from Arsenophonus and Sodalis clades tend to come and go, disrupting the almost flawless host-symbiont co-phylogenies often seen in insect-bacteria systems. However, why are the endosymbiont replacements so common and what keeps the symbiont consortia limited to the specific bacterial clades remains unknown. Tsetse flies as medically important vectors of pathogens are undoubtedly the most studied Hippoboscoidea lineage. However, their low species diversity (22 species), sister relationship to all other clades, and host specificity to mammals, do not allow to draw any general conclusions about the evolution of symbiosis in Hippoboscoidea. To fully understand the symbiotic turn-over, more attention needs to be paid to the neglected Nycteriibidae, Streblidae, and Hippoboscidae lineages. Here, using gene sequencing and draft genome data from all involved partners, we present phylogenies of Hippoboscidae and their symbiont lineages and try to untangle their relationship to the host. In particular, we ask if these are obligate co-evolving lineages, facultative infections, or if they likely represent recent symbiont replacements just re-starting the obligate relationship.
Methods
Sample collection and DNA isolation
Samples of louse flies were collected in seven countries (South Africa, Papua New Guinea, Ecuador—Galapagos, Vietnam, France, Slovakia, and the Czech Republic; see Table S1 for details), the single sample of bat fly was collected in the Czech Republic. All samples were stored in 96% ethanol at −20 °C. DNA was extracted using the QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA quality was verified using the Qubit High Sensitivity Kit (Invitrogen) and 1% agarose gel electrophoresis.
PCR, cloning, and sequencing
All DNA samples were used for amplification of three host genes (COI, 16S rRNA gene, EF) and symbiont screening with 16S rRNA gene primers (Table S2). Ten Wolbachia positive samples were used for MLST typing (coxA, fbpA, ftsZ, gatB, hcpA; see Table S2). PCR reaction was performed under standard conditions using High Fidelity PCR Enzyme Mix (Thermo Scientific, Waltham, MA, USA) and Hot Start Tag DNA Polymerase (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. PCR products were analysed using 1% agarose gel electrophoresis and all symbiont 16S rDNA products were cloned into pGEM®–T Easy vector (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Inserts from selected colonies were amplified using T7 and SP6 primers or isolated from plasmids using the Jetquick Plasmid Miniprep Spin Kit (Genomed GmbH, Löhne, Germany). Sanger sequencing was performed by an ABI Automatic Sequencer 3730XL (Macrogen Inc., Geumchun-gu-Seoul, Korea) or ABI Prism 310 Sequencer (SEQme, Dobříš, the Czech Republic).
In addition to sequencing, we also included in our analyses genomic data of Melophagus ovinus (Nováková et al., 2015), Lipoptena cervi (Nováková et al., 2016), Ornithomya biloba, and Crataerina pallida (Figshare: https://figshare.com/s/e1488900c5cb62af69ab) as well as their endosymbionts (see Table S1).
Although there is MLST available for Arsenophonus bacteria (Duron, Wilkes & Hurst, 2010), we were not successful in amplifying these genes.
Alignments and phylogenetic analyses
The assemblies of raw sequences were performed in Geneious v8.1.7 (Kearse et al., 2012). Datasets were composed of the assembled sequences, extracted genomic sequences, sequences downloaded from GenBank (see Table S4) or the Wolbachia MLST database. The sequences were aligned with Mafft v7.017 (Katoh, 2002; Katoh, Asimenos & Toh, 2009) implemented in Geneious using an E-INS-i algorithm with default parameters. The alignments were not trimmed as trimming resulted in massive loss of informative position. Phylogenetic analyses were carried out using maximum likelihood (ML) in PhyML v3.0 (Guindon & Gascuel, 2003; Guindon et al., 2009) and Bayesian inference (BI) in MrBayes v3.1.2 (Huelsenbeck & Ronquist, 2001). The GTR + I + Γ evolutionary model was selected in jModelTest (Posada, 2009) according to the Akaike Information Criterion (AIC). The subtree prunning and regrafting (SPR) tree search algorithm and 100 bootstrap pseudoreplicates were used in the ML analyses. BI runs were carried out for 10 million generations with default parameters, and Tracer v1.6 (http://tree.bio.ed.ac.uk/software/tracer/) was used for convergence and burn-in examination. Phylogenetic trees were visualised and rooted in FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/) and their final graphical adjustments were performed in Inkscape v0.91 (https://inkscape.org/en/).
Host phylogeny was reconstructed using single-gene analyses and a concatenated matrix of three genes (mitochondrial 16S rRNA, mitochondrial cytochrome oxidase I, and nuclear elongation factor). Concatenation of genes was performed in Phyutility 2.2.6 (Smith & Dunn, 2008). Phylogenetic trees were inferred for all species from the Hippoboscoidea superfamily, as well as for smaller datasets comprising only Hippoboscidae species. This approach was employed to reveal possible artefacts resulting from missing data and poor taxon-sampling (e.g., short, ∼360 bp, sequences of COI available for Streblidae and Nycteribiidae).
Mitochondrial genomes
Problems with reconstruction of host phylogeny based on mitochondrial genes (16S and COI) lead us to assemble mitochondrial genomes of four main louse fly lineages. Contigs of mitochondrial genomes were identified in genomic data of M. ovinus, L. cervi, O. biloba, and C. pallida using BLASTn and tBLASTn searches (Altschul et al., 1990). Open reading frame identification and preliminary annotations were performed using NCBI BlastSearch in Geneious. For identification of Numts, raw sequences were mapped to mitochondrial data using Bowtie v2.2.3 (Langmead & Salzberg, 2012). Web annotation server MITOS (http://mitos.bioinf.uni-leipzig.de/) was used for final annotation of proteins and rRNA/tRNA genes. We selected 15 mitochondrial genes (Table S4) present in all included taxa for phylogenetic inference as described above.
Results
Phylogenetic data
We obtained 138 host sequences: 31 sequences of 16S rRNA of 208–567 bp, 48 sequences of EF of 280–890 bp, and 59 sequences of COI of 299–1,491 bp; and 70 symbiont 16S rRNA sequences of 269–1,210 bp. We also assembled and annotated 4 host mitochondrial genomes of 15,975–16,445 bp. For more details see Table S3 . All raw sequences can be found online in Data S1 (their description is included in Table S6).
Hippoboscidae phylogeny
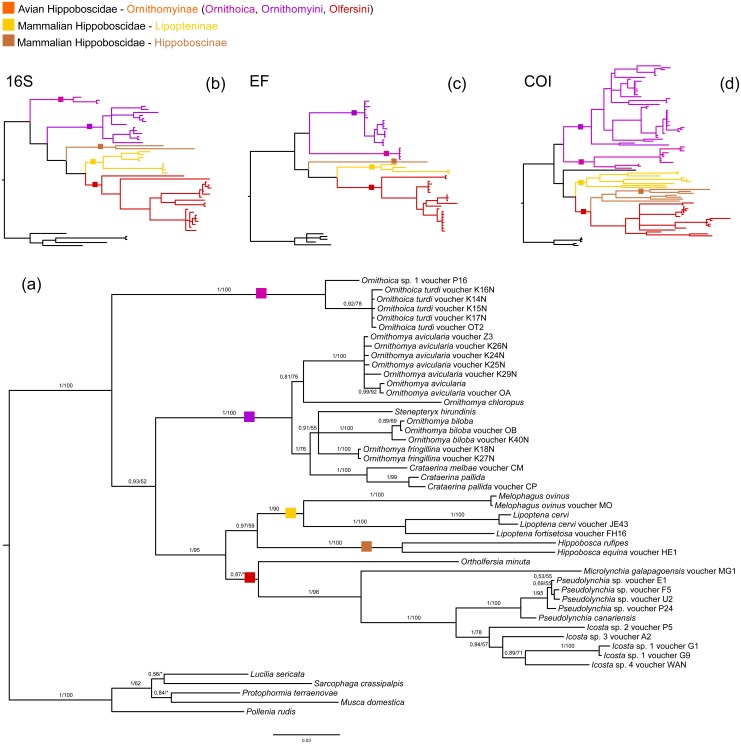
We reconstructed host phylogeny using three markers: 16S rRNA, EF and COI; as well as mitochondrial genomes. Our analyses of draft genome data revealed that all analysed mitochondrial genomes of louse flies are also present as Numts (nuclear mitochondrial DNA) on the host chromosomes, especially the COI gene often used for phylogenetic analyses. The taxonomically restricted mitochondrial genome matrix verified monophyly of Hippoboscoidea (Fig. S1). Our three-gene dataset yielded only partially resolved and unstable inner Hippoboscoidea phylogeny. Glossinidae and Nycteribiidae formed a well-defined monophyletic groups (only ML analysis of COI did not confirm monophyly of Nycteribiidae and also did not resolve its relationship to Streblidae), but monophyly of Hippoboscidae and Streblidae was not well supported and different genes/analyses frequently inferred contradictory topologies. Within Hippoboscidae, the position of the Hippoboscinae group and the genus Ornithoica were the most problematic (Fig. 1, Figs. S2–S8).
Figure 1. Host phylogeny.
(A) Host phylogeny derived from concatenation of three genes: 16S rRNA, EF, and COI. The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Three smaller trees on the top of the figure represent outlines of three separate phylogenetic trees based on BI analyses of 16S rRNA (B), EF (C), and COI (D) genes. Full versions of these phylogenies are included in Figs. S6–S8. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups. This labelling corresponds with labelling of branches at smaller outlines, which are in addition to this highlighted with the same colour. All host trees are included in Figs. S1–S8.
Arsenophonus and Sodalis phylogenies
In total, 70 endosymbiont 16S rRNA genes were sequenced in this study and six additional sequences of this gene were mined from our draft genomic data: four of Arsenophonus, one of Sodalis, and one of Wolbachia. Twenty-nine symbionts were identified as members of the genus Arsenophonus, 13 symbionts were the most similar to Sodalis-allied species, and 28 sequences were of Wolbachia origin. Despite cloning, we did not obtain any sequences of Bartonella reported to occur in some Hippoboscoidea. Moreover, using only phylogenetic approach, we would not be able to decide whether Bartonella-Hippoboscidae interaction is mutualistic or pathogenic, therefore Bartonella symbiosis is not in the scope of this manuscript. Putative assignment to the obligate or likely facultative symbiont categories was based on GC content of their 16S rRNA gene and genomic data available (Table S3), branch length, and the phylogenetic analyses.
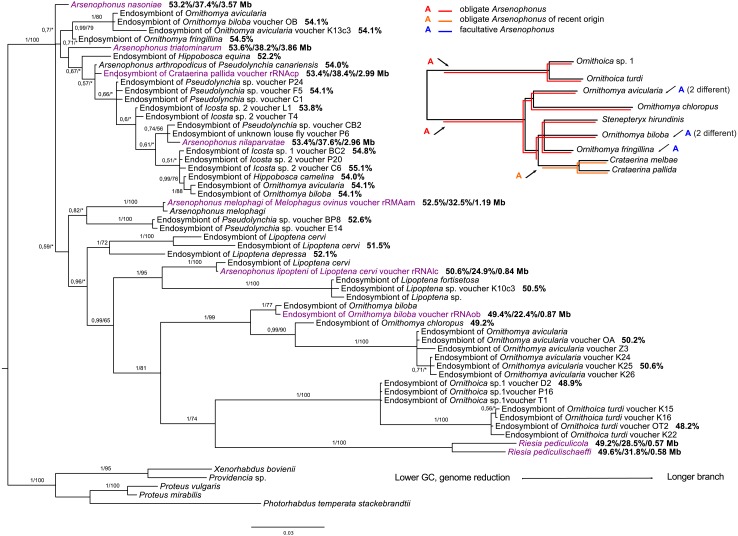
Phylogenetic analyses of the genus Arsenophonus based on 16S rDNA sequences revealed several distinct clades of likely obligate Arsenophonus species congruent with their host phylogeny, partially within the Nycteribiidae, Streblidae, and several Hippoboscidae lineages (Fig. 2, Figs. S9 and S10). However, it is important to note that these clades do not form a single monophyletic clade of co-diverging symbionts, but rather several separate lineages. Within Hippoboscidae, the Arsenophonus sequences from the Ornithomyini group form a monophyletic clade congruent with Ornithomyini phylogeny except Arsenophonus symbiont of Crataerina spp. which was probably recently replaced by another Arsenophonus bacteria. Other obligate Arsenophonus lineages were detected in the genera Lipoptena, Melophagus, and Ornithoica. All other Arsenophonus sequences from the Hippoboscidae either represent facultative symbionts or putatively obligate symbioses which are impossible to reliably detect by phylogenetic methods (but see the discussion for Hippobosca sp.).
Figure 2. 16S rRNA phylogeny of Arsenophonus in Hippoboscidae inferred by BI analysis.
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic sequences are labelled with rRNA. Taxa in dark purple represent Arsenophonus bacteria which genome was sequenced. Numbers behind these taxa correspond to their GC content of 16S rRNA, GC content of genome, and genome size, respectively. Numbers behind other taxa correspond to GC content of their 16S rRNA. The smaller picture on the right side represents host phylogeny to which symbiont phylogeny was compared. Red lineages correspond to obligate symbionts while orange lineage is symbiont of recent origin. The blue A represent likely facultative Arsenophonus infection. To achieve this, we also used the information available on groEL gene by Morse et al. (2013) and Duron et al. (2014). Phylogenetic reconstructions of Arsenophonus of entire Hippoboscoidea and all Arsenophonus bacteria are included in Figs. S9 and S10.
Most of the putatively facultative endosymbionts of the Hippoboscidae typically possess short branches and are also related with the previously described species Arsenophonus arthropodicus and Arsenophonus nasoniae. Interestingly, both obligate and likely facultative lineages were detected from several species, e.g., Ornithomya biloba, Ornithomya avicularia, and Ornithomya fringillina (Fig. 2). Phylogenetic analyses including symbionts from the genera Nycterophylia and Trichobius did not clearly place them into the Arsenophonus genus. Rather, they likely represent closely related lineages to the Arsenophonus clade as their position was unstable and changed with different taxon samplings and methods.
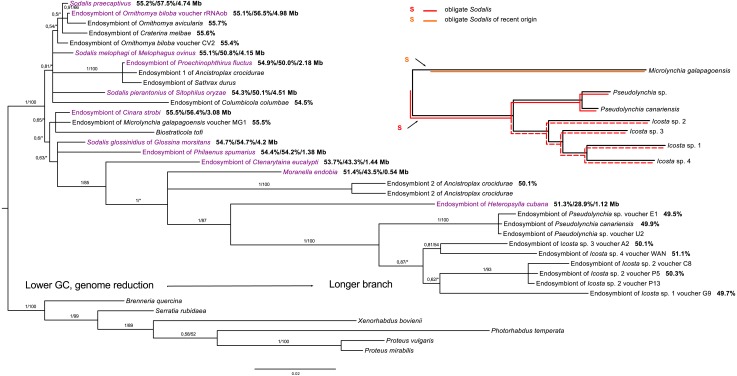
Within Sodalis, the phylogenetic reconstruction revealed a putatively obligate endosymbiont from the tribe Olfersini, including the genera Pseudolynchia and Icosta, and several facultative lineages. However, co-evolution with Icosta sp. seems to be imperfect and does not strictly follow the host phylogeny (Fig. 3).
Figure 3. 16S rRNA phylogeny of Sodalis in Hippoboscidae inferred by BI analysis.
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Taxa in dark purple represent Sodalis-like bacteria which genome was sequenced. Numbers behind these taxa correspond to their GC content of 16S rRNA, GC content of genome, and genome size, respectively. Numbers behind other taxa correspond to GC content of their 16S rRNA. Red lineages correspond to obligate symbionts while orange lineage is symbiont of recent origin. The red dashed line shows that co-evolution between Icosta spp. and their obligate endosymbiont imperfect.
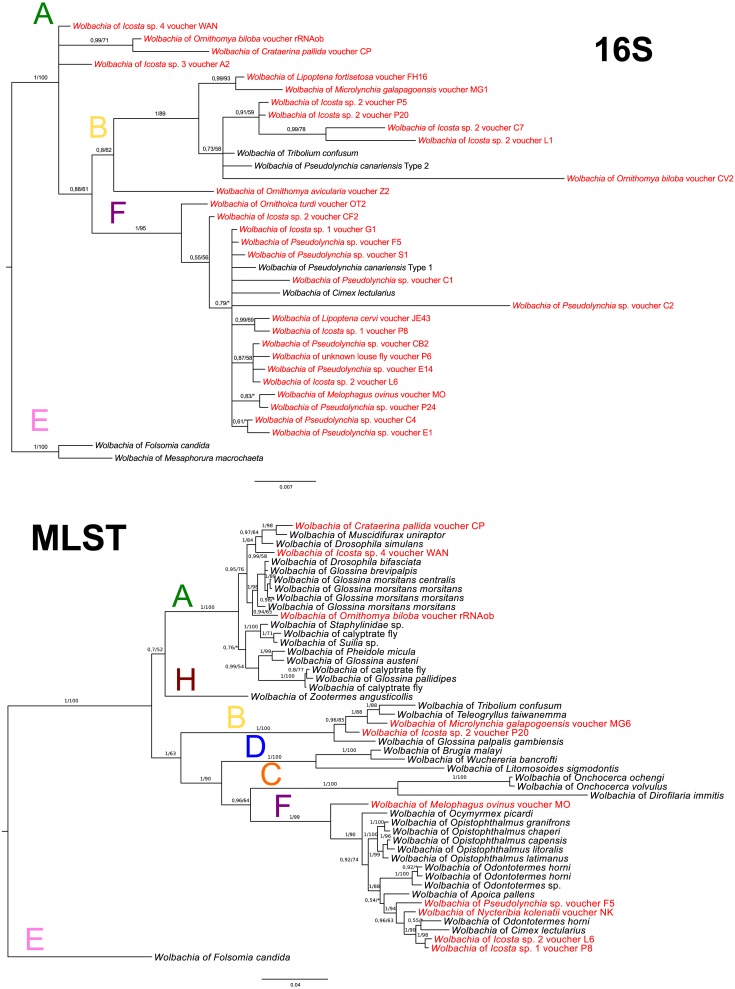
Wolbachia MLST analysis
In Wolbachia, the 16S rDNA sequences were used only for an approximate supergroup determination (Fig. 4A). The MLST analysis was performed with ten selected species (one of them was obtained from genomic data of O. biloba; see Table S3). Overall prevalence of Wolbachia in louse flies is 54.55%; 30 positive individuals out of 55 diagnosed. The supergroup A was detected from 4 species (4 individuals), the supergroup B from 5 species (9 individuals), and the supergroup F from 7 species (17 individuals) (Figs. 4A–4B). Additionally, Nycteribia kolenatii (one individual) was infected with the supergroup F.
Figure 4. Wolbachia phylogeny.
(A) Wolbachia phylogeny inferred from 16S rRNA by BI analysis. (B) Wolbachia phylogeny inferred from MLST genes by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Colour letters upon branches correspond to Wolbachia supergroups. Taxa in red represent Wolbachia bacteria from Hippoboscidae and Nycteribidae which are newly sequenced in this study. Taxa labelled with # in the 16S tree represent taxa which were used for the MLST analysis. Wolbachia from O. biloba, which was obtained from genomic data, is labelled with rRNAob. Supergroup E was used for rooting both trees.
Discussion
Hippoboscidae phylogeny: an unfinished portrait
Although closely related to the medically important tsetse flies, the other hippoboscoids have only rarely been studied and their phylogeny is still unclear. Based on our concatenated matrix, we obtained the topology which to some extent resembles the one presented Petersen et al. (2007), although with slightly different taxon sampling (Fig. 1; Fig. S2). However, our three single-gene datasets implied only poor phylogenetic signal available carried by the hippoboscoid sequences. Therefore, we took an advantage of the four complete mitochondrial genomes reconstructed in this study to test the reliability of the previous phylogenetic reconstructions. The phylogenetic reconstruction based on the mitochondrial matrix correspond to the three-gene concatenated matrix phylogeny suggesting that mitochondrial genomes would be valuable for further phylogenetic analyses of this group (Fig. 1; Fig. S1). According to our results, Glossinidae, Nycteribiidae and Hippoboscidae were retained as monophyletic groups, but monophyly of Streblidae was not supported using the complete matrix (Fig. S2). Streblidae lineage appears to be paraphyletic with respect to Nycteribiidae and clusters into two groups, the Old World and the New World species, as previously reported (Dittmar et al., 2006; Kutty et al., 2010). Within Hippoboscidae, the groups Lipopteninae, Hippoboscinae, Ornithomyini and Olfersini (nomenclature was adopted from Petersen et al. (2007)) are well-defined and monophyletic, but their exact relationships are still not clear. The most problematic taxa are Hippoboscinae and also the genus Ornithoica with their positions depending on the used genes/analyses (Fig. 1; Figs. S2–S8). A possible explanation for these inconsistencies in the topologies can be a hypothetical rapid radiation from the ancestor of Hippoboscoidea group into main subfamilies of Hippoboscidae leaving in the sequences only very weak phylogenetic signal for this period of Hippoboscidae evolution. The most difficulties in reconstructing Hippoboscoidea phylogeny is caused by missing data (only short sequences of COI are available especially for Nycteribiidae and Streblidae in the GenBank; Fig. S3). Moreover, COI phylogenies are known to be affected by numerous pseudogenes called Numts (Black IV & Bernhardt, 2009). The Numts, we found to be common in louse fly genomes, can thus also contribute to the intricacy of presented phylogenies. On the other hand, EF seems to provide plausible phylogenetic information (Fig. S4). The biggest drawback of this marker however lies in the data availability in public databases, restricting an appropriate taxon sampling for the Hippoboscoidea superfamily.
Hidden endosymbiont diversity within the Hippoboscidae family
Among the three most commonly detected Hippoboscidae endosymbionts, attention has been predominantly paid to Arsenophonus as the supposedly most common obligate endosymbiont of this group. Our data show that several different lineages of Arsenophonus have established the symbiotic lifestyle within Hippoboscidae (Fig. 2). According to our results supported by genomic data, there are at least four lineages of likely obligate endosymbionts: Arsenophonus in Ornithomyini (genomes of Arsenophonus from Ornithomya biloba and Crataerina pallida will be published elsewhere), Arsenophonus in Ornithoica spp., previously described Arsenophonus melophagi (Nováková et al., 2015) and Arsenophonus lipopteni (Nováková et al., 2016). All these possess reduced genomes with low GC content as a typical feature of obligate endosymbionts (McCutcheon & Moran, 2012). Interestingly, within Ornithomyini, the original obligate Arsenophonus endosymbiont of Crataerina spp. was recently replaced by another Arsenophonus bacterium with ongoing genome reduction (Figshare: https://figshare.com/s/e1488900c5cb62af69ab). Apart from these potentially obligate lineages, there are other hippoboscid associated Arsenophonus bacteria distributed in the phylogenetic tree among Arsenophonus endosymbionts with likely facultative or free-living lifestyle (Fig. S10). This pattern suggests Arsenophonus is likely being repeatedly acquired from the environment. It has been hypothesized that obligate endosymbionts often evolve from facultative symbionts which are no longer capable of horizontal transmission between the hosts (Moran, McCutcheon & Nakabachi, 2008). Due to their recent change of lifestyle, endosymbionts with an ongoing genome reduction in many ways resemble facultative symbionts, e.g., their positions in phylogenetic trees are not stable and differ with the analysis method and taxon sampling (Fig. 2, Figs. S9 and S10). Such nascent stage of endosymbiosis was indicated for the obligate Arsenophonus endosymbiont of C. pallida (Figshare: https://figshare.com/s/e1488900c5cb62af69ab) and similar results can be expected for Arsenophonus endosymbionts of Hippobosca species.
Within bat flies, we found obligate Arsenophonus lineages in both Nycteribiidae and Streblidae as well as several presumably facultative Arsenophonus infections in both groups (Figs. S9 and S10). Similar results were reported in several previous studies (Morse et al., 2013; Duron et al., 2014; Wilkinson et al., 2016). Members of the Arsenophonus clade were also reported from Nycterophyliinae and Trichobiinae (Streblidae) (Morse et al., 2012a) and Cyclopodia dubia (Nycteribiidae) (Wilkinson et al., 2016). However, our results do not support their placement within the clade, as these sequences were attracted by the long branches in the ML analyses. The endosymbiont of Nycterophyliinae and Trichobiinae probably represents an ancient lineage closely related to Arsenophonus clade (Fig. S9) while the endosymbiont of Cyclopodia dubia is more likely related with Pectobacterium spp.; therefore, we excluded this bacterium from our further analyses. These findings indicate that bat flies established the endosymbiotic lifestyle several times independently with at least three bacterial genera.
In contrast to Arsenophonus, only a few studies reported Sodalis-like endosymbiotic bacteria from Hippoboscidae (Nováková & Hypša, 2007; Chrudimský et al., 2012; Nováková et al., 2015). Dale et al. (2006) detected a putative obligate endosymbiont from Pseudolynchia canariensis which was suggested to represent Sodalis bacterium. We detected this symbiont in several members of the Olfersini group and according to our results, it is obligate Sodalis-like endosymbiont forming a monophyletic clade, but its congruence with the Olfersini phylogeny is somewhat imperfect (Fig. 3). This incongruence might be a consequence of phylogenetic artefacts likely affecting long branches of Sodalis symbionts from Icosta. Similar to Arsenophonus, Sodalis bacteria also establish possible facultative associations, e.g., with Melophagus ovinus (Chrudimský et al., 2012; Nováková et al., 2015), Ornithomya avicularia (Chrudimský et al., 2012) or Ornithomya biloba (this study). Sodalis endosymbiont from Crataerina melbae was suggested to be obligate (Nováková & Hypša, 2007), but our study did not support this hypothesis since it clusters with free-living Sodalis praecaptivus. Interestingly, Sodalis endosymbiont of Microlynchia galapagoensis was inferred to be closely related to Sodalis-like co-symbiont of Cinara cedri, which underwent rapid genome deterioration after a replacement of former co-symbiont (Meseguer et al., 2017). These results suggest that there are several loosely associated lineages of Sodalis bacteria in louse flies. On one hand, the endosymbiont of Microlynchia galapagoensis probably represents a separate (or ancient) Sodalis infection, but on the other hand, other Sodalis infections seem to be repeatedly acquired from the environment as implied by their relationship to e.g., Sodalis praecaptivus (Clayton et al., 2012) (Fig. 3).
Coinfections of obligate and facultative Arsenophonus strains in Hippoboscidae (or potentially Sodalis in Olfersini) are extremely difficult to recognize using only PCR-acquired 16S rRNA gene. Facultative endosymbionts retain several copies of this gene and thus their 16S rRNA tend to be amplified more likely in PCR than from reduced obligate endosymbionts due to its higher copy number and lower frequency of mutations in primer binding sites. Even though there is a MLST available for Arsenophonus bacteria (Duron, Wilkes & Hurst, 2010), it was shown that it is effective only partially (Duron et al., 2014). Since our data are probably also influenced by this setback, we do not speculate which of the detected potentially facultative Arsenophonus lineages represent source of ‘ancestors’ for several distinct obligate lineages or which of them were involved in the recent replacement scenario. However, the replacement/independent-origin scenario is well illustrated by endosymbionts from Olfersini (Figs. 2 and 3).
To complement the picture of Hippoboscidae endosymbiosis, we also reconstructed Wolbachia evolution. We found three different supergroups: A, B and F (see Table S3). Apparently, there is no coevolution between Wolbachia and Hippoboscidae hosts suggesting horizontal transmission between species (Figs. 4A–4B) as common for this bacterium (Schilthuizen & Stouthamer, 1997; Gerth et al., 2014). Since Wolbachia seems to be one of the most common donors of genes horizontally transferred to insect genomes, including tsetse flies (Husník et al., 2013; Brelsfoard et al., 2014; Sloan et al., 2014), we cannot rule out that some of Wolbachia sequences detected in this study represent HGT insertions into the respective host genomes. The biological role of Wolbachia in Hippoboscidae was never examined in spite of its relatively high prevalence in this host group (55%). The F supergroup was detected as the most frequent lineage in Hippoboscidae which is congruent with its common presence in blood-sucking insects such as Streblidae (Morse et al., 2012a), Nycteribiidae (Hosokawa et al., 2012), Amblycera (Covacin & Barker, 2007), and Cimicidae (Hosokawa et al., 2010; Nikoh et al., 2014).
Besides the three main Hippoboscidae symbionts we paid attention to, Bartonella spp. that are also widespread among louse flies and bat flies. The infection seems to be fixed only in Melophagus ovinus suggesting a mutualistic relationship (Halos et al., 2004), but additional functional data are needed to confirm this hypothesis (Nováková et al., 2015). Nevertheless, deer ked and sheep ked are also suspected of vectoring bartonellosis (Maggi et al., 2009; De Bruin et al., 2015). According to the recent findings, Bartonella spp. used to be originally gut symbionts which adapted to pathogenicity (Segers et al., 2016; Neuvonen et al., 2016).
What is behind dynamics of Hippoboscidae-symbiont associations?
According to our results, symbiosis in the Hippoboscidae group is very dynamic and influenced by frequent symbiont replacements. Arsenophonus and Sodalis infections seem to be the best resources for endosymbiotic counterparts, but it remains unclear why just these two genera. Both are endowed with several features of free-living/pathogenic bacteria enabling them to enter new host which can be crucial in establishing novel symbiotic association. Sodalis glossinidius possesses modified outer membrane protein (OmpA) which is playing an important role in the interaction with the host immune system (Weiss et al., 2008; Weiss, Maltz & Aksoy, 2012). Both Sodalis and Arsenophonus bacteria retain genes for the type III secretion system (Dale et al., 2001; Wilkes et al., 2010; Chrudimský et al., 2012; Oakeson et al., 2014) allowing pathogenic bacteria to invade eukaryotic cells. Moreover, several strains of these bacteria are cultivable under laboratory conditions (Hypša & Dale, 1997; Dale & Maudlin, 1999; Dale et al., 2006; Darby et al., 2010; Chrudimský et al., 2012; Chari et al., 2015) suggesting that they should be able to survive horizontal transmission. For instance, Arsenophonus nasoniae is able to spread by horizontal transfer between species (Duron, Wilkes & Hurst, 2010), while Sodalis-allied bacteria have several times successfully replaced ancient symbionts (Conord et al., 2008; Koga et al., 2013; Meseguer et al., 2017).
Whereas the facultative endosymbionts of Hippoboscoidea are widespread in numerous types of tissues such as milk glands, bacteriome, haemolymph, gut, fat body, and reproductive organs (Dale & Maudlin, 1999; Dale et al., 2006; Balmand et al., 2013; Nováková et al., 2015), the obligate endosymbionts are restricted to the bacteriome and milk glands (Aksoy, 1995; Attardo et al., 2008; Balmand et al., 2013; Morse et al., 2013; Nováková et al., 2015). Entering the milk glands ensures vertical transmission of facultative endosymbiont to progeny and better establishment of the infection. Vertical transmission also enables the endosymbiont to hitch-hike with the obligate endosymbiont and because the obligate endosymbiont is inevitably degenerating (Moran, 1996; Wernegreen, 2002), the new co-symbiont can eventually replace it if needed. For instance, Sodalis melophagi was shown to appear in both milk glands and bacteriome and to code for the same full set of B-vitamin pathways (including in addition the thiamine pathway) as the obligate endosymbiont Arsenophonus melophagi (Nováková et al., 2015). This suggests that it could be potentially capable of shifting from facultative to obligatory lifestyle and replace the Arsenophonus melophagi endosymbiont.
We suggest that the complex taxonomic structure of the symbiosis in Hippoboscoidea can be result of multiple replacements, similar to that already suggested for the evolution of symbiosis in Columbicola lice (Smith et al., 2013) or mealybugs (Husník & McCutcheon, 2016). Based on the arrangement of the current symbioses in various species of Pupipara, the ancestral endosymbiont was likely either an Arsenophonus or Sodalis bacterium (given our finding of the potential obligate Sodalis lineage in Olfersini). In the course of Pupipara evolution and speciation, this symbiont was repeatedly replaced by different Arsenophonus (or Sodalis in Olfersini if not ancestral) lineages, as indicated by the lack of phylogenetic congruence and differences in genome reduction, gene order, and GC content in separate Arsenophonus lineages (Nováková et al., 2015; Nováková et al., 2016; Figshare: https://figshare.com/s/e1488900c5cb62af69ab). This genomic diversity across the Arsenophonus bacteria from distinct Hippoboscidae thus likely reflects their different age correlating with the level of genome reduction in symbiotic bacteria.
Conclusions
Despite the considerable ecological and geographical variability, the Hippoboscoidea families surprisingly share some aspects of their association with symbiotic bacteria. Particularly, they show high affinity to two bacterial genera, Arsenophonus and Sodalis. This affinity is not only reflected by frequent occurrence of the bacteria but mainly by their multiple independent acquisitions. Comparisons between the hippoboscid and bacterial phylogenies indicate several independent origins of the symbiosis, although more precise evolutionary reconstruction is still hampered by the uncertainties in hippoboscid phylogenies.
Supplemental Information
File includes five tables. Table 1 provides information about samples. Table 2 provides information about Primer names, sequences and products used for PCR amplification and sequencing. Table 3 summarizes results: detected endosymbionts, GC content of their 16S rDNA, and sequences acquired from their host. Table 4 summarizes mitochondrial genes used for phylogeny reconstruction in this study. Table 5 provides accession numbers of GenBank sequences used in this study.
It provides information about raw sequences acquired in this study and their accession numbers.
File includes raw sequences acquired in this study: symbiont 16S rRNA, Wolbachia MLST, host 16S rRNA, host elongation factor, and host cytochrome oxidase subunit I.
Phylogeny of Hippoboscoidea based on 15 mitochondrial genes. It includes figures of four mitochondrial genomes assembled and annotated in this study which were also used for phylogeny reconstruction.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic sequences are labelled with rRNA. Dark blue boxes represent Arsenophonus distribution into groups suggested by Morse et al. (2013) and Duron et al. (2014).
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic sequences are labelled with rRNA.
Acknowledgments
Ali Halajian thanks Prof. Wilmien J. Luus-Powell (Biodiversity Research Chair, University of Limpopo) for support and Prof. Derek Engelbrecht for help with bird catching and identifications.
Funding Statement
This work was supported by the Grant Agency of the Czech Republic (grant 13-01878S to Václav Hypša). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Eva Šochová performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Filip Husník and Václav Hypša conceived and designed the experiments, reviewed drafts of the paper.
Eva Nováková performed the experiments, reviewed drafts of the paper.
Ali Halajian collected samples of African louse flies.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
Raw sequences are included in Data S1. Matrices and genomic data of four bacteria used for our analyses are deposited at Figshare (https://figshare.com/s/e1488900c5cb62af69ab).
References
- Akman et al. (2002).Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nature Genetics. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- Aksoy (1995).Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. International Journal of Systematic Bacteriology. 1995;45:848–851. doi: 10.1099/00207713-45-4-848. [DOI] [PubMed] [Google Scholar]
- Allen et al. (2016).Allen JM, Burleigh JG, Light JE, Reed DL. Effects of 16S rDNA sampling on estimates of the number of endosymbiont lineages in sucking lice. PeerJ. 2016;4:e2187. doi: 10.7717/peerj.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen et al. (2007).Allen JM, Reed DL, Perotti MA, Braig HR. Evolutionary relationships of “Candidatus Riesia spp.”, endosymbiotic enterobacteriaceae living within hematophagous primate lice. Applied and Environmental Microbiology. 2007;73:1659–1664. doi: 10.1128/AEM.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Attardo et al. (2008).Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. Journal of Insect Physiology. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmand et al. (2013).Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. Journal of Invertebrate Pathology. 2013;112:S116–S122. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard et al. (1992).Beard CB, Mason PW, Aksoy S, Tesh RB, Richards FF. Transformation of an insect symbiont and expression of a foreign gene in the Chagas’ disease vector Rhodnius prolixus. The American Journal of Tropical Medicine and Hygiene. 1992;46:195–200. doi: 10.4269/ajtmh.1992.46.195. [DOI] [PubMed] [Google Scholar]
- Belda et al. (2010).Belda E, Moya A, Bentley S, Silva FJ. Mobile genetic element proliferation and gene inactivation impact over the genome structure and metabolic capabilities of Sodalis glossinidius, the secondary endosymbiont of tsetse flies. BMC Genomics. 2010;11:449. doi: 10.1186/1471-2164-11-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yakir (1987).Ben-Yakir D. Growth retardation of Rhodnius prolixus symbionts by immunizing host against Nocardia (Rhodococcus) rhodnii. Journal of Insect Physiology. 1987;33:379–383. doi: 10.1016/0022-1910(87)90015-1. [DOI] [Google Scholar]
- Bennett & Moran (2015).Bennett GM, Moran NA. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10169–10176. doi: 10.1073/pnas.1421388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black IV & Bernhardt (2009).Black IV WC, Bernhardt SA. Abundant nuclear copies of mitochondrial origin (NUMTs) in the Aedes aegypti genome. Insect Molecular Biology. 2009;18:705–713. doi: 10.1111/j.1365-2583.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Boyd et al. (2016).Boyd BM, Allen JM, Koga R, Fukatsu T, Sweet AD, Johnson KP, Reed DL. Two bacterial genera, Sodalis and Rickettsia, associated with the seal louse Proechinophthirus fluctus (Phthiraptera: Anoplura) Applied and Environmental Microbiology. 2016;82:3185–3197. doi: 10.1128/AEM.00282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd et al. (2017).Boyd BM, Allen JM, Nguyen N-P, Vachaspati P, Quicksall ZS, Warnow T, Mugisha L, Johnson KP, Reed DL. Primates, lice and bacteria: speciation and genome evolution in the symbionts of hominid lice. Molecular Biology and Evolution. 2017;34:1743–1757. doi: 10.1093/molbev/msx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard et al. (2014).Brelsfoard C, Tsiamis G, Falchetto M, Gomulski LM, Telleria E, Alam U, Doudoumis V, Scolari F, Benoit JB, Swain M, Takac P, Malacrida AR, Bourtzis K, Aksoy S. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina morsitans morsitans. PLOS Neglected Tropical Diseases. 2014;8:e2728. doi: 10.1371/journal.pntd.0002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari et al. (2015).Chari A, Oakeson KF, Enomoto S, Jackson DG, Fisher MA, Dale C. Phenotypic characterization of Sodalis praecaptivus sp. nov., a close non-insect-associated member of the Sodalis-allied lineage of insect endosymbionts. International Journal of Systematic and Evolutionary Microbiology. 2015;65:1400–1405. doi: 10.1099/ijs.0.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrudimský et al. (2012).Chrudimský T, Husník F, Nováková E, Hypša V. Candidatus Sodalis melophagi sp. nov.: phylogenetically independent comparative model to the tsetse fly symbiont Sodalis glossinidius. PLOS ONE. 2012;7:e40354. doi: 10.1371/journal.pone.0040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton et al. (2012).Clayton AL, Oakeson KF, Gutin M, Pontes A, Dunn DM, Von Niederhausern AC, Weiss RB, Fisher M, Dale C. A novel human-infection-derived bacterium provides insights into the evolutionary origins of mutualistic insect-bacterial symbioses. PLOS Genetics. 2012;8:e1002990. doi: 10.1371/journal.pgen.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conord et al. (2008).Conord C, Despres L, Vallier A, Balmand S, Miquel C, Zundel S, Lemperiere G, Heddi A. Long-term evolutionary stability of bacterial endosymbiosis in curculionoidea: additional evidence of symbiont replacement in the dryophthoridae family. Molecular Biology and Evolution. 2008;25:859–868. doi: 10.1093/molbev/msn027. [DOI] [PubMed] [Google Scholar]
- Covacin & Barker (2007).Covacin C, Barker SC. Supergroup F Wolbachia bacteria parasitise lice (Insecta: Phthiraptera) Parasitology Research. 2007;100:479–485. doi: 10.1007/s00436-006-0309-6. [DOI] [PubMed] [Google Scholar]
- Dale et al. (2006).Dale C, Beeton M, Harbison C, Jones T, Pontes M. Isolation, pure culture, and characterization of “Candidatus Arsenophonus arthropodicus”, an intracellular secondary endosymbiont from the hippoboscid louse fly Pseudolynchia canariensis. Applied and Environmental Microbiology. 2006;72:2997–3004. doi: 10.1128/AEM.72.4.2997-3004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale & Maudlin (1999).Dale C, Maudlin I. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. International Journal of Systematic Bacteriology. 1999;49(Pt 1):267–275. doi: 10.1099/00207713-49-1-267. [DOI] [PubMed] [Google Scholar]
- Dale et al. (2001).Dale C, Young SA, Haydon DT, Welburn SC. The insect endosymbiont Sodalis glossinidius utilizes a type III secretion system for cell invasion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1883–1888. doi: 10.1073/pnas.98.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby et al. (2010).Darby AC, Choi J-H, Wilkes T, Hughes MA, Werren JH, Hurst GDD, Colbourne JK. Characteristics of the genome of Arsenophonus nasoniae, son-killer bacterium of the wasp Nasonia. Insect Molecular Biology. 2010;19(Suppl 1):75–89. doi: 10.1111/j.1365-2583.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- De Bruin et al. (2015).De Bruin A, Van Leeuwen AD, Jahfari S, Takken W, Földvári M, Dremmel L, Sprong H, Földvári G. Vertical transmission of Bartonella schoenbuchensis in Lipoptena cervi. Parasites & Vectors. 2015;8:176–181. doi: 10.1186/s13071-015-0764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar et al. (2006).Dittmar K, Porter ML, Murray S, Whiting MF. Molecular phylogenetic analysis of nycteribiid and streblid bat flies (Diptera: Brachycera, Calyptratae): implications for host associations and phylogeographic origins. Molecular Phylogenetics and Evolution. 2006;38:155–170. doi: 10.1016/j.ympev.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Duron et al. (2014).Duron O, Schneppat UE, Berthomieu A, Goodman SM, Droz B, Paupy C, Nkoghe JO, Rahola N, Tortosa P. Origin, acquisition and diversification of heritable bacterial endosymbionts in louse flies and bat flies. Molecular Ecology. 2014;23:2105–2117. doi: 10.1111/mec.12704. [DOI] [PubMed] [Google Scholar]
- Duron, Wilkes & Hurst (2010).Duron O, Wilkes TE, Hurst GDD. Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecology Letters. 2010;13:1139–1148. doi: 10.1111/j.1461-0248.2010.01502.x. [DOI] [PubMed] [Google Scholar]
- Fukatsu et al. (2009).Fukatsu T, Hosokawa T, Koga R, Nikoh N, Kato T, Hayama S, Takefushi H, Tanaka I. Intestinal endocellular symbiotic bacterium of the macaque louse Pedicinus obtusus: distinct endosymbiont origins in anthropoid primate lice and the old world monkey louse. Applied and Environmental Microbiology. 2009;75:3796–3799. doi: 10.1128/AEM.00226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger et al. (2005).Geiger A, Ravel S, Frutos R, Cuny G. Sodalis glossinidius (Enterobacteriaceae) and vectorial competence of Glossina palpalis gambiensis and Glossina morsitans morsitans for Trypanosoma congolense savannah type. Current Microbiology. 2005;51:35–40. doi: 10.1007/s00284-005-4525-6. [DOI] [PubMed] [Google Scholar]
- Geiger et al. (2007).Geiger A, Ravel S, Mateille T, Janelle J, Patrel D, Cuny G, Frutos R. Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei s.l. and genetic diversity of the symbiont Sodalis glossinidius. Molecular Biology and Evolution. 2007;24:102–109. doi: 10.1093/molbev/msl135. [DOI] [PubMed] [Google Scholar]
- Gerth et al. (2014).Gerth M, Gansauge M-T, Weigert A, Bleidorn C. Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nature Communications. 2014;5:5117–5123. doi: 10.1038/ncomms6117. [DOI] [PubMed] [Google Scholar]
- Guindon et al. (2009).Guindon S, Delsuc F, Dufayard J-F, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. In: Posada D, editor. Bioinformatics for DNA sequence analysis. Humana Press; Totowa: 2009. pp. 113–137. [DOI] [PubMed] [Google Scholar]
- Guindon & Gascuel (2003).Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Halos et al. (2004).Halos L, Jamal T, Maillard R, Girard B, Guillot J, Chomel B, Vayssier-Taussat M, Boulouis H-J. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Applied and Environmental Microbiology. 2004;70:6302–6305. doi: 10.1128/AEM.70.10.6302-6305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa et al. (2010).Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa et al. (2012).Hosokawa T, Nikoh N, Koga R, Satô M, Tanahashi M, Meng X-Y, Fukatsu T. Reductive genome evolution, host-symbiont co-speciation and uterine transmission of endosymbiotic bacteria in bat flies. The ISME Journal. 2012;6:577–587. doi: 10.1038/ismej.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck & Ronquist (2001).Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Husník & McCutcheon (2016).Husník F, McCutcheon JP. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5416–E5424. doi: 10.1073/pnas.1603910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husník et al. (2013).Husník F, Nikoh N, Koga R, Ross L, Duncan RP, Fujie M, Tanaka M, Satoh N, Bachtrog D, Wilson ACC, Von Dohlen CD, Fukatsu T, McCutcheon JP. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. CELL. 2013;153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Hypša & Aksoy (1997).Hypša V, Aksoy S. Phylogenetic characterization of two transovarially transmitted endosymbionts of the bedbug Cimex lectularius (Heteroptera: Cimicidae) Insect Molecular Biology. 1997;6:301–304. doi: 10.1046/j.1365-2583.1997.00178.x. [DOI] [PubMed] [Google Scholar]
- Hypša & Dale (1997).Hypša V, Dale C. In vitro culture and phylogenetic analysis of “Candidatus Arsenophonus triatominarum”, an intracellular bacterium from the triatomine bug, Triatoma infestans. International Journal of Systematic Bacteriology. 1997;47:1140–1144. doi: 10.1099/00207713-47-4-1140. [DOI] [PubMed] [Google Scholar]
- Hypša & Křížek (2007).Hypša V, Křížek J. Molecular evidence for polyphyletic origin of the primary symbionts of sucking lice (Phthiraptera, Anoplura) Microbial Ecology. 2007;54:242–251. doi: 10.1007/s00248-006-9194-x. [DOI] [PubMed] [Google Scholar]
- International Glossina Genome Initiative (2014).International Glossina Genome Initiative IGG Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh (2002).Katoh K. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, Asimenos & Toh (2009).Katoh K, Asimenos G, Toh H. Multiple alignment of DNA sequences with MAFFT. In: Posada D, editor. Bioinformatics for DNA sequence analysis. Humana Press; Totowa: 2009. pp. 39–64. [DOI] [PubMed] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness et al. (2010).Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, Lee SH, Robertson HM, Kennedy RC, Elhaik E, Gerlach D, Kriventseva EV, Elsik CG, Graur D, Hill CA, Veenstra JA, Walenz B, Tubío JMC, Ribeiro JMC, Rozas J, Johnston JS, Reese JT, Popadic A, Tojo M, Raoult D, Reed DL, Tomoyasu Y, Kraus E, Mittapalli O, Margam VM, Li H-M, Meyer JM, Johnson RM, Romero-Severson J, Vanzee JP, Alvarez-Ponce D, Vieira FG, Aguadé M, Guirao-Rico S, Anzola JM, Yoon KS, Strycharz JP, Unger MF, Christley S, Lobo NF, Seufferheld MJ, Wang N, Dasch GA, Struchiner CJ, Madey G, Hannick LI, Bidwell S, Joardar V, Caler E, Shao R, Barker SC, Cameron S, Bruggner RV, Regier A, Johnson J, Viswanathan L, Utterback TR, Sutton GG, Lawson D, Waterhouse RM, Venter JC, Strausberg RL, Berenbaum MR, Collins FH, Zdobnov EM, Pittendrigh BR. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12168–12173. doi: 10.1073/pnas.1003379107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga et al. (2013).Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environmental Microbiology. 2013;15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- Koga et al. (2007).Koga R, Tsuchida T, Sakurai M, Fukatsu T. Selective elimination of aphid endosymbionts: effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiology Ecology. 2007;60:229–239. doi: 10.1111/j.1574-6941.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- Kutty et al. (2010).Kutty SN, Pape T, Wiegmann BM, Meier R. Molecular phylogeny of the Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine’s fly. Systematic Entomology. 2010;35:614–635. doi: 10.1111/j.1365-3113.2010.00536.x. [DOI] [Google Scholar]
- Langmead & Salzberg (2012).Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi et al. (2009).Maggi RG, Kosoy M, Mintzer M, Breitschwerdt EB. Isolation of Candidatus Bartonella melophagi from human blood. Emerging Infectious Diseases. 2009;15:66–68. doi: 10.3201/eid1501.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon & Moran (2012).McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nature Reviews. Microbiology. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- Meseguer et al. (2017).Meseguer AS, Manzano-Marín A, Coeur d’Acier A, Clamens A-L, Godefroid M, Jousselin E. Buchnera has changed flatmate but the repeated replacement of co-obligate symbionts is not associated with the ecological expansions of their aphid hosts. Molecular Ecology. 2017;26:2363–2378. doi: 10.1111/mec.13910. [DOI] [PubMed] [Google Scholar]
- Michalkova et al. (2014).Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Applied and Environmental Microbiology. 2014;80:5844–5853. doi: 10.1128/AEM.01150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran (1996).Moran NA. Accelerated evolution and Muller’s rachet in endosymbiotic bacteria. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2873–2878. doi: 10.1073/pnas.93.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, McCutcheon & Nakabachi (2008).Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annual Review of Genetics. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- Morse et al. (2013).Morse SF, Bush SE, Patterson BD, Dick CW, Gruwell ME, Dittmar K. Evolution, multiple acquisition, and localization of endosymbionts in bat flies (Diptera: Hippoboscoidea: Streblidae and Nycteribiidae) Applied and Environmental Microbiology. 2013;79:2952–2961. doi: 10.1128/AEM.03814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse et al. (2012a).Morse SF, Dick CW, Patterson BD, Dittmar K. Some like it hot: evolution and ecology of novel endosymbionts in bat flies of cave-roosting bats (Hippoboscoidea, Nycterophiliinae) Applied and Environmental Microbiology. 2012a;78:8639–8649. doi: 10.1128/AEM.02455-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse et al. (2012b).Morse SF, Olival KJ, Kosoy M, Billeter S, Patterson BD, Dick CW, Dittmar K. Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae) Infection, Genetics and Evolution. 2012b;12:1717–1723. doi: 10.1016/j.meegid.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Neuvonen et al. (2016).Neuvonen M-M, Tamarit D, Näslund K, Liebig J, Feldhaar H, Moran NA, Guy L, Andersson SGE. The genome of Rhizobiales bacteria in predatory ants reveals urease gene functions but no genes for nitrogen fixation. Scientific Reports. 2016;6:39197. doi: 10.1038/srep39197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh et al. (2014).Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, Fukatsu T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmala, Hypša & Žurovec (2001).Nirmala X, Hypša V, Žurovec M. Molecular phylogeny of Calyptratae (Diptera: Brachycera): the evolution of 18S and 16S ribosomal rDNAs in higher dipterans and their use in phylogenetic inference. Insect Molecular Biology. 2001;10:475–485. [PubMed] [Google Scholar]
- Nováková et al. (2015).Nováková E, Husník F, Šochová E, Hypša V. Arsenophonus and Sodalis symbionts in louse flies: an analogy to the Wigglesworthia and Sodalis system in tsetse flies. Applied and Environmental Microbiology. 2015;81:6189–6199. doi: 10.1128/AEM.01487-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nováková & Hypša (2007).Nováková E, Hypša V. A new Sodalis lineage from bloodsucking fly Craterina melbae (Diptera, Hippoboscoidea) originated independently of the tsetse flies symbiont Sodalis glossinidius. FEMS Microbiology Letters. 2007;269:131–135. doi: 10.1111/j.1574-6968.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- Nováková, Hypša & Moran (2009).Nováková E, Hypša V, Moran NA. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiology. 2009;9:143. doi: 10.1186/1471-2180-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nováková et al. (2016).Nováková E, Hypša V, Nguyen P, Husník F, Darby AC. Genome sequence of Candidatus Arsenophonus lipopteni, the exclusive symbiont of a blood sucking fly Lipoptena cervi (Diptera: Hippoboscidae) StandArds in Genomic Sciences. 2016;11:1–7. doi: 10.1186/s40793-016-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeson et al. (2014).Oakeson KF, Gil R, Clayton AL, Dunn DM, Von Niederhausern AC, Hamil C, Aoyagi A, Duval B, Baca A, Silva FJ, Vallier A, Jackson DG, Latorre A, Weiss RB, Heddi A, Moya A, Dale C. Genome degeneration and adaptation in a nascent stage of symbiosis. Genome Biology and Evolution. 2014;6:76–93. doi: 10.1093/gbe/evt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachebat et al. (2013).Pachebat JA, Van Keulen G, Whitten MMA, Girdwood S, Del Sol R, Dyson PJ, Facey PD. Draft genome sequence of Rhodococcus rhodnii strain LMG5362, a symbiont of Rhodnius prolixus (Hemiptera, Reduviidae, Triatominae), the principle vector of Trypanosoma cruzi. Genome Announcements. 2013;1:e00329-13. doi: 10.1128/genomeA.00329-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais et al. (2011).Pais R, Balmand S, Takac P, Alam U, Carnogursky J, Brelsfoard C, Galvani A, Aksoy S, Medlock J, Heddi A, Lohs C. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLOS Pathogens. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais et al. (2008).Pais R, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Applied and Environmental Microbiology. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen et al. (2007).Petersen FT, Meier R, Kutty SN, Wiegmann BM. The phylogeny and evolution of host choice in the Hippoboscoidea (Diptera) as reconstructed using four molecular markers. Molecular Phylogenetics and Evolution. 2007;45:111–122. doi: 10.1016/j.ympev.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Posada (2009).Posada D. Selection of models of DNA evolution with jModelTest. In: Posada D, editor. Bioinformatics for DNA sequence analysis. Humana Press; Totowa: 2009. pp. 93–112. [DOI] [PubMed] [Google Scholar]
- Rio et al. (2012).Rio RVM, Symula RE, Wang J, Lohs C, Wu Y, Snyder AK, Bjornson RD, Oshima K, Biehl BS, Perna NT, Hattori M, Aksoy S. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: glossinidae) obligate symbiont Wigglesworthia. mBio. 2012;3:e00240-11. doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilthuizen & Stouthamer (1997).Schilthuizen M, Stouthamer R. Horizontal transmission of parthenogenesis-inducing microbes in Trichogramma wasps. Proceedings. Biological Sciences/the Royal Society. 1997;264:361–366. doi: 10.1098/rspb.1997.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers et al. (2016).Segers FH, Kešnerová L, Kosoy M, Engel P. Genomic changes associated with the evolutionary transition of an insect gut symbiont into a blood-borne pathogen. The ISME Journal. 2016;11(5):1232–1244. doi: 10.1038/ismej.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan et al. (2014).Sloan DB, Nakabachi A, Richards S, Qu J, Murali SC, Gibbs RA, Moran NA. Parallel histories of horizontal gene transfer facilitated extreme reduction of endosymbiont genomes in sap-feeding insects. Molecular Biology and Evolution. 2014;31:857–871. doi: 10.1093/molbev/msu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith & Dunn (2008).Smith SA, Dunn CW. Phyutility: a phyloinformatics tool for trees, alignments and molecular data. Bioinformatics. 2008;24:715–716. doi: 10.1093/bioinformatics/btm619. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2013).Smith WA, Oakeson KF, Johnson KP, Reed DL, Carter T, Smith KL, Koga R, Fukatsu T, Clayton DH, Dale C. Phylogenetic analysis of symbionts in feather-feeding lice of the genus Columbicola: evidence for repeated symbiont replacements. BMC Evolutionary Biology. 2013;13:1. doi: 10.1186/1471-2148-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder et al. (2010).Snyder AK, Deberry JW, Runyen-Janecky L, Rio RVM. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2389–2397. doi: 10.1098/rspb.2010.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder & Rio (2015).Snyder AK, Rio RVM. “Wigglesworthia morsitans” folate (Vitamin B9) biosynthesis contributes to tsetse host fitness. Applied and Environmental Microbiology. 2015;81:5375–5386. doi: 10.1128/AEM.00553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakaran, Kost & Kaltenpoth (2017).Sudakaran S, Kost C, Kaltenpoth M. Symbiont acquisition and replacement as a source of ecological innovation. Trends in Microbiology. 2017;25:375–390. doi: 10.1016/j.tim.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Toh et al. (2006).Toh H, Weiss BL, Perkin S a H, Yamashita A, Oshima K, Hattori M, Aksoy S. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Research. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge, Dittmar & Whiting (2006).Trowbridge RE, Dittmar K, Whiting MF. Identification and phylogenetic analysis of Arsenophonus- and Photorhabdus-type bacteria from adult Hippoboscidae and Streblidae (Hippoboscoidea) Journal of Invertebrate Pathology. 2006;91:64–68. doi: 10.1016/j.jip.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Šorfová, Škeříková & Hypša (2008).Šorfová P, Škeříková A, Hypša V. An effect of 16S rRNA intercistronic variability on coevolutionary analysis in symbiotic bacteria: molecular phylogeny of Arsenophonus triatominarum. Systematic and Applied Microbiology. 2008;31:88–100. doi: 10.1016/j.syapm.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Weiss, Maltz & Aksoy (2012).Weiss BL, Maltz MA, Aksoy S. Obligate symbionts activate immune system development in the tsetse fly. Journal of Immunology. 2012;188:3395–3403. doi: 10.4049/jimmunol.1103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss et al. (2013).Weiss BL, Wang J, Maltz MA, Wu Y, Aksoy S. Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLOS Pathogens. 2013;9:e1003318. doi: 10.1371/journal.ppat.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss et al. (2008).Weiss BL, Wu Y, Schwank JJ, Tolwinski NS, Aksoy S. An insect symbiosis is influenced by bacterium-specific polymorphisms in outer-membrane protein A. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15088–15093. doi: 10.1073/pnas.0805666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen (2002).Wernegreen JJ. Genome evolution in bacterial endosymbionts of insects. Nature Reviews. Genetics. 2002;3:850–861. doi: 10.1038/nrg931. [DOI] [PubMed] [Google Scholar]
- Wilkes et al. (2010).Wilkes TE, Darby AC, Choi J-H, Colbourne JK, Werren JH, Hurst GDD. The draft genome sequence of Arsenophonus nasoniae, son-killer bacterium of Nasonia vitripennis, reveals genes associated with virulence and symbiosis. Insect Molecular Biology. 2010;19(Suppl 1):59–73. doi: 10.1111/j.1365-2583.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson et al. (2016).Wilkinson DA, Duron O, Cordonin C, Gomard Y, Ramasindrazana B, Mavingui P, Goodman SM, Tortosa P. The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. Applied and Environmental Microbiology. 2016;82:1778–1788. doi: 10.1128/AEM.03505-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber-Rosenberg & Rosenberg (2008).Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiology Reviews. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File includes five tables. Table 1 provides information about samples. Table 2 provides information about Primer names, sequences and products used for PCR amplification and sequencing. Table 3 summarizes results: detected endosymbionts, GC content of their 16S rDNA, and sequences acquired from their host. Table 4 summarizes mitochondrial genes used for phylogeny reconstruction in this study. Table 5 provides accession numbers of GenBank sequences used in this study.
It provides information about raw sequences acquired in this study and their accession numbers.
File includes raw sequences acquired in this study: symbiont 16S rRNA, Wolbachia MLST, host 16S rRNA, host elongation factor, and host cytochrome oxidase subunit I.
Phylogeny of Hippoboscoidea based on 15 mitochondrial genes. It includes figures of four mitochondrial genomes assembled and annotated in this study which were also used for phylogeny reconstruction.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Four main Hippoboscidae groups are colour coded: blue for Glossinidae, light green for Streblidae, dark green for Nycteribiidae, and red for Hippoboscidae.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic COI sequences are labelled with rRNA. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
The phylogeny was reconstructed by BI analysis. Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Three main families of Hippoboscidae are colour coded: yellow for Lipopteninae (one group), brown for Hippoboscinae (one group), and orange for Ornithomiinae (three groups). Colour squares label branches where are placed main Hippoboscidae groups.
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic sequences are labelled with rRNA. Dark blue boxes represent Arsenophonus distribution into groups suggested by Morse et al. (2013) and Duron et al. (2014).
Posterior probabilities and bootstrap support are printed upon branches, respectively (asterisk was used for very low or missing bootstrap branch support). Taxa labelled with voucher are newly sequenced in this study. Genomic sequences are labelled with rRNA.