Abstract
Bone tissue engineering requires the upregulation of several regenerative stages, including a critical early phase of angiogenesis. Previous studies have suggested that a sequential delivery of platelet-derived growth factor (PDGF) to bone morphogenetic protein-2 (BMP-2) could promote angiogenic tubule formation when delivered to in vitro cocultures of human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs). However, it was previously unclear that this PDGF to BMP-2 delivery schedule will result in cell migration into the scaffolding system and affect the later expression of bone markers. Additionally, a controlled delivery system had not yet been engineered for programmed sequential presentation of this particular growth factor. By combining alginate matrices with calcium phosphate scaffolding, a programmed growth factor delivery schedule was achieved. Specifically, a combination of alginate microspheres, alginate hydrogels, and a novel blend of resorbable calcium phosphate-based cement (ReCaPP) was used. PDGF and BMP-2 were sequentially released from this hybrid calcium phosphate/alginate scaffold with the desired 3-day overlap in PDGF to BMP-2 delivery. Using a three-dimensional coculture model, we observed that this sequence of PDGF to BMP-2 delivery influenced both cellular infiltration and alkaline phosphatase (ALP) expression. It was found that the presence of early PDGF delivery increased the distance of cell infiltration into the calcium phosphate/alginate scaffolding in comparison to early BMP-2 delivery and simultaneous PDGF+BMP-2 delivery. It was also observed that hMSCs expressed a greater amount of ALP+ staining in response to scaffolds delivering the sequential PDGF to BMP-2 schedule, when compared with scaffolds delivering no growth factor, or PDGF alone. Importantly, hMSCs cultured with scaffolds releasing the PDGF to BMP-2 schedule showed similar amounts of ALP staining to hMSCs cultured with BMP-2 alone, suggesting that the sequential schedule of PDGF to BMP-2 presentation promotes differentiation of hMSCs toward an osteoblast phenotype while also increasing cellular infiltration of the scaffold.
Keywords: : 3D coculture, angiogenesis, bone regeneration, controlled delivery, growth factors
Introduction
For the treatment of fracture nonunions, autografting is currently the gold standard of care.1 Autografts are taken from a patient's own body, serving as a nonimmunogenic, osteogenic, osteoconductive, osteoinductive graft while containing osteoblast precursors and natural growth factors that are presented to the site of healing.2,3 Notably, however, there are several drawbacks associated with autografts, including the introduction of secondary surgical sites, and an increased incidence of blood loss and infection from the associated surgical procedure.4,5 To overcome these limitations while still providing instructive cues in the form of growth factors to stimulate fracture repair, biomaterials (such as collagen sponges) loaded with bone morphogenetic protein 2 (BMP-2) have been approved for clinical use since 2004.6 Delivering exogeneous BMP-2 stimulates bone healing through the proliferation and differentiation of preosteoblasts.7 However, due to the short half-life of BMP-2 (∼6.7 min),6 the concentration of BMP-2 required to achieve robust bone formation is ∼1 million times higher than physiological concentrations.6 This may lead to complications, such as the presentation of BMP to neighboring tissues and ectopic bone formation.8 To mitigate these complications, a variety of methods for more sustainably delivering BMP-2 have been explored, including encapsulation in polymers.9
Controlled release techniques offer the ability to release continuous, lower levels of drugs or proteins to achieve therapeutic effect while minimizing adverse side effects.10 Indeed, the controlled delivery of BMP-2 from polymeric vehicles has shown to be effective for stimulating the proliferation and differentiation of preosteoblasts for the ultimate formation and mineralization of bone tissues11 while avoiding the aforementioned complications.
Despite these advantages, BMP-2-releasing biomaterial grafts have been shown in multiple studies to necrose and fail due to inadequate vascularization of the scaffolding material.12,13 This phenomenon is attributed to the limited capacity of BMP-2 alone to stimulate angiogenesis, a critical stage of fracture healing.14 In principle, if an angiogenic growth factor such as VEGF could be controllably delivered alongside BMP-2, this limitation may be overcome and lead to more successful healing outcomes.
However, recent evidence suggests that recreating the biological cues that orchestrate the regenerative process is not as simple as delivering two (or more) growth factors simultaneously. For example, although simultaneous release of either VEGF and BMP-2 or VEGF and platelet-derived growth factor (PDGF) results in improved bone formation versus relevant control scaffolds,15,16 vasculature infiltrating the scaffold appears to be immature and the resulting bone formation is characterized by imperfections such as fracturing or fibrous tissue formation.15,16 A similar study, where BMP-2 and PDGF were released from a scaffold to treat a rabbit femur defect, found weak synergistic effects at 4 weeks postimplantation and no effect at 12 weeks.17
An explanation for these suboptimal results may be derived from recent evidence suggesting that growth factor secretion in the bone healing microenvironment occurs in a stage-wise fashion, creating a sequence of instructions that first recruit various cell populations, and then direct their organization into the correct structure.18–20 In support of this idea, recently a study was performed wherein soluble VEGF was administered in tandem with an adenovirus encoding BMP-2.21 The delay in presentation of BMP-2 due to adenoviral administration appears to lead to significantly greater and more stable bone formation than simultaneous release of both proteins.17
These recent results suggest that the absence of a signal at a given point in time may be equally important to the presence of that signal at another time. Consequently, if it were possible to replicate the natural sequence of angiogenic–osteogenic instructions responsible for proper bone growth regeneration14,22,23 using a programmed, synthetic scaffold, it could begin to address some of the current deficiencies of bioactive scaffolds.
To this end, we employed three different, biomaterials-based components (in combination) to engineer a scaffold that is designed to controllably release a desired sequence of growth factors. As a foundation, by combining a calcium phosphate cement with poly-lactic-co-glycolic (PLGA) microspheres, we created a three-dimensional (3D) scaffold with an interconnected porous network. Alginate hydrogels and microspheres were then used to program a specific, predefined release schedule of PDGF and BMP-2 delivery that was previously found to effectively promote the formation of robust, organized, angiogenic tubules in vitro.24
In addition to characterizing this scaffold and its release properties, we further explored the capability of this scaffold system to stimulate cellular infiltration of the scaffold, induce tubule formation and organization, and promote osteogenic differentiation. We hypothesized that releasing a programmed schedule of PDGF and BMP-2 will have the potential to promote both the formation of angiogenic tubules by human umbilical vein endothelial cells (HUVECs) and the osteogenic differentiation of human mesenchymal stem cells (hMSCs).
The incorporation of this controlled release schedule from an osteoconductive calcium phosphate scaffold may encourage cell infiltration of the scaffold and promote osteogenic differentiation. The results of these studies, together with the previously shown promotion of angiogenic tubule formation, may be used in future work to guide the development of in vivo systems capable of orchestrating both the angiogenic and osteogenic phases of bone regeneration.
Materials and Methods
Fabrication of PLGA particle as scaffold porogen
Blank PLGA microspheres (microspheres that do not contain growth factor) were fabricated using a double-emulsion–evaporation procedure. Specifically, a measure of 200 mg of PLGA polymer [poly(d,l-lactide-co-glycolide, 24–36 kDa, 50:50 lactide:glycolide, ester terminated; Sigma-Aldrich] was dissolved in 4 mL dichloromethane (Sigma-Aldrich). Dissolved PLGA was then sonicated with 200 μL of deionized water, then pipetted dropwise through a 250 μm sieve into a solution of 1% polyvinyl alcohol in deionized water (Polysciences, Inc.) spinning at 400 rpm. Following dichloromethane evaporation (3 h), microspheres were collected through centrifugation, washed four times with deionized water, frozen, and lyophilized.
Hydrogel fabrication for PDGF release
Alginate hydrogels were fabricated by mixing 30 mg of medium viscosity sodium alginate (MP Biomedicals, LLC) into 3 mL of deionized water until alginate was fully dissolved to constitute a 1% solution. A 30 μL aliquot from 200 μg/mL of soluble PDGF-BB (Thermo Fisher) was then gently, homogeneously stirred into each 200 μL of alginate solution. Once the aliquot of growth factor was homogenously mixed into the alginate solution, the hydrogel was chemically crosslinked with 1 mL of 700 mM calcium chloride (Fisher Scientific) for 10 min. Following crosslinking, the hydrogels were washed with deionized water to remove excess crosslinking solution.
To produce a hydrogel with a reasonably homogeneous thickness and shape, 200 μL aliquots of 1% alginate+PDGF were transferred to a flat-bottom 96-well plate (Corning). A rounded disk of medium porosity, medium flow rate filter paper (Fisher Scientific) was placed atop the alginate hydrogel, and 700 mM calcium chloride crosslinker was added dropwise on top of the filter paper to allow slow, even crosslinking of the hydrogel. Release behavior was assayed for three different conditions: from a 1% alginate hydrogel, from a 1% alginate hydrogel applied as a coating on the resorbable calcium phosphate-based cement (ReCaPP) scaffold, and from a 3% alginate hydrogel applied as a coating on the ReCaPP scaffold.
Microsphere fabrication for BMP-2 release
Single-emulsion alginate microspheres were fabricated by mixing 300 mg of sodium alginate in 10 mL of deionized water until alginate was fully dissolved to constitute a 3% alginate solution. A 200 μL aliquot of 100 μg/mL soluble BMP-2 (Shenandoah) in deionized water was homogeneously mixed into the alginate solution. The BMP-2-alginate solution was then placed into 5-mL syringes and left undisturbed for 30 min to remove air bubbles from the solution.
Once bubbles were removed from the alginate solution, alginate was added dropwise into 2.5% solution of Span 80 in iso-octane and homogenized at 5000 rpm. Next, 3 mL of 30% Tween 80 in iso-octane was added and homogenized at 5400 RPM. Then, an aliquot of 700 mM calcium chloride solution was used to crosslink the particles, which were further cured with 2-propanol. Following fabrication, the particles were collected through centrifugation, washed twice with 2-propanol, then washed three times with deionized water. Collected particles were then frozen and lyophilized.
Preparation of cement components
To produce ReCaPP cement powder, alpha-tricalcium phosphate (α-TCP, pure phase; Sigma-Aldrich), calcium carbonate (99%+; Acros Organics), calcium sulfate dihydrate (99%+; Acros Organics), and disodium hydrogen phosphate (99%+; Sigma-Aldrich) were mixed in an Agate mortar–pestle for 30 min and stored in 50-mL Falcon tubes for use. The composition of the cement powder consisted of ratios of 72.7% α-TCP, 9.1% calcium carbonate, 9.1% calcium sulfate dihydrate, and 9.1% disodium hydrogen phosphate, by weight.25,26
The liquid component of the cement (nano-CaPs) is a colloidal solution of nanosized calcium phosphate homogeneously dispersed in a buffer solution. On a per-milliliter basis, this solution is created by adding 500 μL of 0.75 M CaCl2 dropwise into 500 μL of phosphate precursor solution. The phosphate precursor solution consisted of 0.357 M NaCl (99.5%+; Fisher Scientific), 13 mM KCl (99%+; Fisher Scientific), 15 mM dextrose monohydrate (Fisher Scientific), 64 mM HEPES free acid (99.5%+; Sigma-Aldrich), and 1.91 mM Na3PO4.12H2O (98%+; Acros Organics).
Hybrid scaffold assembly
We aimed to fabricate hybrid, 3D scaffolding consisting of calcium phosphate cement, BMP-2-releasing microspheres. First, PDGF-BB-releasing hydrogel, first a porous cement scaffold was created by mixing 100 μm PLGA microspheres with ReCaPP cement powder in a ratio of 20 mg microspheres to 30 mg ReCaPP. When used in cell-culturing assays, the cement ReCaPP powder and microsphere mixture was sterilized through ultraviolet (UV) light for 30 min. Then, to form scaffolds 33 μL of liquid nano-CaPs was mixed into the ReCaPP-microsphere powder. This slurry was then compressed in a cylindrical mold to create disks. After initial setting, the resulting tablet was dried at 37°C overnight. The 50 mg tablet scaffolds were then placed in three successive washes of dichloromethane for 10 min each to completely dissolve PLGA microspheres from the scaffold. After the last wash of dichloromethane evaporated, scaffolds were rinsed in deionized water three times to remove residual dichloromethane, and dried at room temperature.
To infiltrate the ReCaPP cement scaffold with BMP-2-alginate microspheres, scaffolds were placed in a glass Pyrex dish with a solution of 10 mg of BMP-2-alginate microspheres in 500 μL deionized water. Pyrex dishes were then placed in the water bath of a Bronson 1510 sonication bath, and sonicated at full power for 30 min. Following BMP-2-alginate microsphere infiltration, scaffolds were frozen and lyophilized for 24 h.
A PDGF-BB-releasing hydrogel was applied to the scaffolds as an outer coating by submerging scaffolds for 10 min in a 1% alginate solution, which was fabricated as described previously in the Hydrogel Fabrication for PDGF Release section. For scaffolds used in cell culture, 10 μL of 200 μg/mL PDGF-BB was mixed into each 100 μL solution of alginate coating. Alginate solutions and calcium chloride crosslinking solutions were both UV sterilized before cell culture use. Once PDGF-BB alginate coatings were applied to porous ReCaPP scaffolds, they were crosslinked by submerging the scaffolds in a 700 mM calcium chloride solution for an additional 10 min. Completed, crosslinked scaffolds were rinsed in deionized water to remove residual calcium chloride.
Scaffold degradation analysis
To determine degradation rates of the scaffold, particularly the growth factor-containing alginate components, hybrid scaffolds were fabricated (as described in Hybrid scaffold assembly) by mixing 40 mg of PLGA microspheres with 60 mg ReCaPP cement powder to create porous scaffolds. These porous scaffolds were then infiltrated with 20 mg of alginate microspheres. For purposes of the degradation study, BMP-2 was not encapsulated in the alginate microspheres. The ReCaPP/alginate microsphere scaffold was allowed to dry overnight at 37°C.
The following day, a 1% alginate hydrogel was applied as a coating to the scaffolds, as described in the Hybrid Scaffold Fabrication section. PDGF was not encapsulated in the outer alginate coating for the purposes of the degradation study. ReCaPP scaffolds containing alginate microspheres, fully coated with an outer layer of 1% alginate, were placed in 1.5-mL LoBind Eppendorf tubes with 1 mL phosphate-buffered saline (PBS) and subject to end-over-end rotation at 37°C. PBS solution was removed daily and replaced with fresh PBS. Scaffolds were removed from solution at experimental time points (days 1–10, 15, 20, 40), frozen in liquid nitrogen, and lyophilized for dry weight measurement.
Controlled growth factor release assay
To determine the release rate of BMP-2 from alginate microspheres, 10 mg of alginate microspheres were suspended in 1 mL of PBS in a LoBind Eppendorf tube and subject to end-over-end rotation at 37°C. At experimental time points, particle suspensions were centrifuged at 1000 rpm and supernatant was collected and frozen at −20°C. Fresh PBS was added, particles were resuspended, and again end-over-end rotated at 37°C. At the conclusion of the study, all frozen supernatant samples were thawed and assayed for BMP-2 concentration using a human BMP-2 enzyme-linked immunosorbent assay (ELISA; R&D Systems).
To determine the release rate of PDGF-BB from alginate hydrogels, 200 μL hydrogel samples were submerged in 1 mL of PBS release solution in a lo-bind Eppendorf tube and subject to end-over-end rotation at 37°C. PBS release solution consisted of PBS supplemented with sodium dodecyl sulfate (SDS; 50 mM) and 0.1% bovine serum albumin (BSA) to further prevent released PDGF-BB from sticking to the Eppendorf tube. At experimental time points, hydrogel samples were either removed and/or centrifuged to separate the samples from the PBS solution supernatant. Collected supernatant samples were frozen at −20°C until the conclusion of the experiment, whereupon they were thawed and analyzed for PDGF-BB content using a PDGF-BB ELISA (R&D Systems).
Tubule formation in response to controlled release growth factor delivery
Well inserts of 8 μm, 24-well transwell plates (Sigma-Aldrich) were filled with 250 μL of growth factor reduced, phenol-free Matrigel (LDEV free; Fisher Scientific). Human umbilical vascular endothelial cells (HUVECs; Lonza) and human bone marrow-derived human mesenchymal stem cells (BM hMSCs; Life Technologies) were seeded at a density of 1.5 × 105 cells per well, in a 1:1 (hMSC:HUVEC) ratio. The lower reservoirs of MSC–HUVEC cocultures were filled with 500 μL of a 1:1 ratio of endothelial basal media (Lonza) and minimum essential media (Life Technologies) supplemented with 1% penicillin–streptomycin (Life Technologies) and 1% antibiotic–antimycotic (Life Technologies) to provide hydration to Matrigel plugs.
For the positive control experimental group, soluble growth factors were delivered by daily hand dosing of PDGF-BB and BMP-2 concentrations to closely match growth factor release profiles of PDGF-BB and BMP-2 delivered by controlled release mechanisms. As a negative control, cells were seeded with Matrigel plugs and received no growth factor dosing.
For controlled release experimental groups, 100 μL alginate hydrogels were embedded within the Matrigel to release PDGF-BB, and 10 mg of alginate microspheres were embedded within the Matrigel to release BMP-2.
At the conclusion of the experimental timeframe (7 days), Matrigel plugs were removed from transwell inserts, embedded in TissueTek (Fisher Scientific), and snap frozen in liquid nitrogen. Frozen sections (10 μm) were incubated with primary antibody mouse anti-human CD31/PECAM-1 (R&D Systems) and secondary antibody donkey anti-mouse IgG (R&D Systems) to identify endothelial cell activity and vessel formation. Images of CD31-stained sections were taken at 20 × and 10 × using a Nikon inverted light microscope, then analyzed using WimTube software (Wimasis Image Analysis, GmbH). The 10 × images were used for analysis to gain a larger representative sample area. WIMASIS software was used for analysis of four parameters for every image: total area covered by vessels, total length of vessels, number of branching points, and number of closed loops.
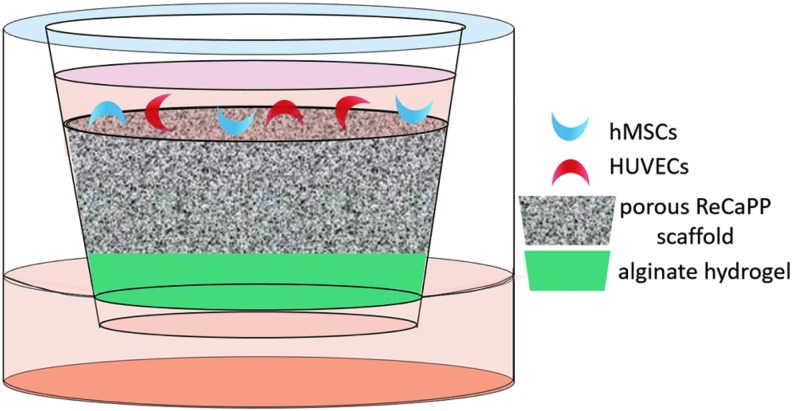
Cellular infiltration of scaffold in response to growth factor delivery
Alginate hydrogels were prepared as described in Hydrogel Fabrication for PDGF Release section. Four growth factor delivery groups were explored: hydrogels releasing no growth factor (No GF), hydrogels releasing PDGF alone (PDGF), hydrogels releasing BMP-2 alone (BMP-2), and hydrogels releasing both PDGF and BMP-2 at the same time (PDGF+BMP).
As shown in Figure 1, hydrogels were placed in transwell inserts of an 8 μm 24-well transwell plate (Sigma-Aldrich). Porous ReCaPP scaffolds were placed into each transwell insert, atop growth factor-eluting hydrogels, and snugly fit to prevent liquid leakage between the scaffold and transwell insert wall. Before fitting ReCaPP scaffolds in transwell inserts, scaffolds were soaked in a solution of 1% BSA in PBS to encourage cell attachment to scaffold surfaces. A coculture of HUVECs and hMSCs were then seeded atop porous ReCaPP scaffolds. Lower transwell reservoirs were filled with 500 μL of media to provide hydration to the alginate hydrogels. At the end of the experimental timeframe (7 days), scaffolds were removed from transwell inserts, snap frozen in liquid nitrogen and lyophilized. Subsequently, scaffolds were broken in half to image vertical cross-sections.
FIG. 1.
Schematic of transwell setup used to assay cell infiltration in response to growth factor releasing alginate hydrogels.
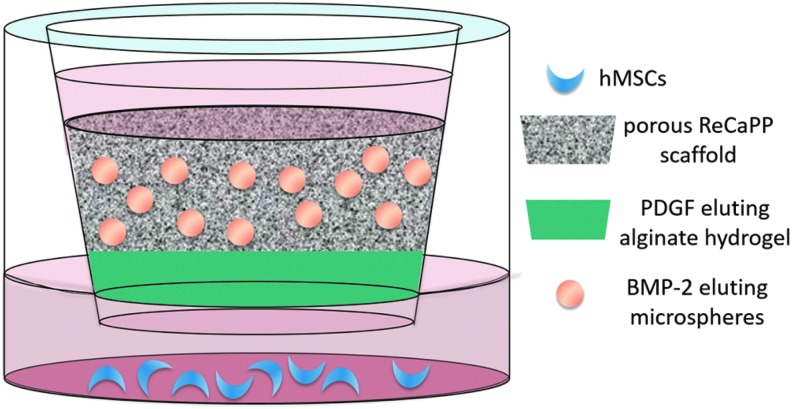
Alkaline phosphatase expression in response to growth factor delivery
Lower surfaces of an 8 μm 24-well transwell plate (Sigma-Aldrich) were seeded with BM hMSCs (Life Technologies) at a density of 1 × 104 cells per well. Each well was filled with 1 mL of α-MEM (Life Technologies) supplemented with 1% penicillin–streptomycin (Life Technologies), 1% antibiotic–antimycotic (Life Technologies), and 10% fetal bovine serum (Fisher Scientific), which was changed every 2–3 days throughout the experiment. Transwell inserts contained the following experimental scaffold groups: ReCaPP scaffold with no additional growth factor (No GF), ReCaPP scaffold with PDGF-BB-eluting alginate hydrogel (PDGF), ReCaPP scaffold with BMP-2-eluting alginate microspheres (BMP), and ReCaPP scaffold with PDGF-BB-eluting alginate hydrogel, and BMP-2-eluting alginate microspheres (PDGF to BMP). After scaffolds were placed in transwell inserts, 100 μL of medium was added to hydrate the scaffold and facilitate release of growth factors from hydrogels and microspheres (Fig. 2).
FIG. 2.
Schematic of transwell setup used to assay ALP expression in response to PDGF eluting alginates hydrogels and BMP-2-eluting alginate microspheres. ALP, alkaline phosphatase; BMP-2, bone morphogenetic protein-2; PDGF, platelet-derived growth factor.
At each experimental time point (7, 14, 21, 28 days), transwell inserts were removed, media was aspirated, and cells were assayed for alkaline phosphatase (ALP) expression using an AP Staining Kit (System Biosciences). Following staining, wells were imaged using a Nikon Inverted Light Microscope at 20 × magnification. To quantify staining, all representative images and their corresponding replicates were analyzed using ImageJ. Each image was processed to remove dark background artifacts, and converted into a binary image. Images were then analyzed using the “Measure” function in ImageJ to quantify the total area per image of positive ALP staining.
Scanning electron microscope analysis
To obtain scanning electron microscope (SEM) images of the scaffolding to visualize alginate coatings and cellular infiltration, scaffolds were snap frozen in liquid nitrogen and carefully cross-sectioned with a razor blade. Cross-sections were then lyophilized for 48 h to fully dehydrate the scaffold. These dehydrated cross-sections were mounted on copper tape and sputter coated with 5 mm layer of conductive gold alloy.
An a JEOL JSM 6335F SEM was utilized to capture images. Similar methods were used for preparing scaffold with cellular infiltration. Scaffolds were snap frozen in liquid nitrogen. To preserve the presence of cells on top of and throughout the scaffold, scaffolds were sectioned by applying gentle pressure to the center of the scaffold bottom while the outer edges were fixed in place. These cross-sections were lyophilized for 48 h, mounted on copper tape, and sputter coated with 3.5 mm of conductive gold alloy for image analysis on the JEOL JSM 6335 SEM.
Statistical analysis
Statistical analysis of tubule formation and ALP staining was performed using one-way analysis of variance (ANOVA) with Tukey's test for multiple comparisons.
Results
Hybrid scaffold fabrication
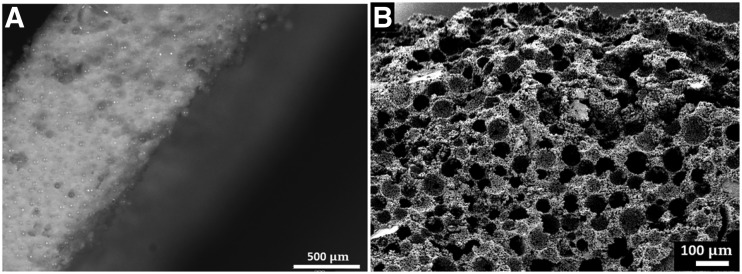
PLGA microspheres were fabricated to be morphologically spherical, with a smooth surface and approximate size of 90–100 μm in diameter. PLGA microspheres were used as a porogen and mixed with ReCaPP powders and liquids in a ratio of 40 mg microsphere to 60 mg ReCaPP powder by weight, compressed, and hardened overnight, resulting in a tablet-shaped ReCaPP scaffold with embedded PLGA microspheres. By dissolving away the PLGA microsphere porogen with dichloromethane, porous ReCaPP scaffolds were subsequently formed. (Fig. 3).
FIG. 3.
(A) ReCaPP/PLGA microsphere scaffold edge before PLGA microsphere dissolution showing distribution and density of PLGA microspheres embedded throughout the scaffold. (B) SEM image showing porosity of ReCaPP scaffold after PLGA microspheres have been dissolved. PLGA, poly-lactic-co-glycolic; ReCaPP, resorbable calcium phosphate-based cement; SEM, scanning electron microscope.
Using 50 mg total weight scaffolds (20 mg PLGA microspheres +30 mg ReCaPP powder), PLGA microspheres were dissolved from the scaffolds, and subsequently, the scaffolds were reinfiltrated with 10 mg of ∼20 μm alginate microspheres (Fig. 4) through sonication. Resulting scaffolds (Fig. 4) show that alginate microspheres fully infiltrated porous ReCaPP scaffolding, filling pores in the center of the cross-sectioned scaffold.
FIG. 4.
SEM of porous ReCaPP scaffold that has been reinfiltrated with BMP-2-alginate microspheres, showing that the BMP-2-alginate microspheres penetrate to the center of the scaffold.
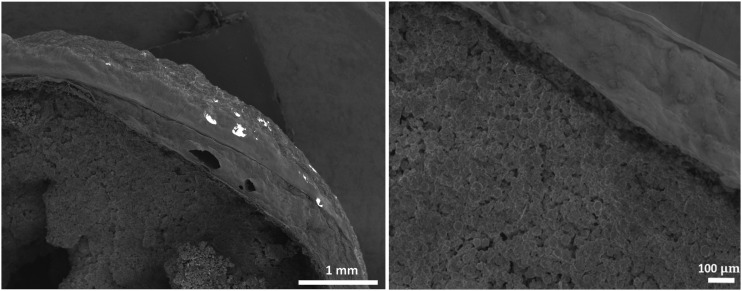
To incorporate PDGF release, PDGF-laden alginate hydrogels were prepared as described in Hybrid Scaffold Assembly section and applied to ReCaPP scaffolds as an outer coating (Fig. 5).
FIG. 5.
SEM images of cross-sectioned nonporous calcium phosphate scaffolding showing the application of PDGF-laden alginate hydrogel coating.
Scaffold degradation
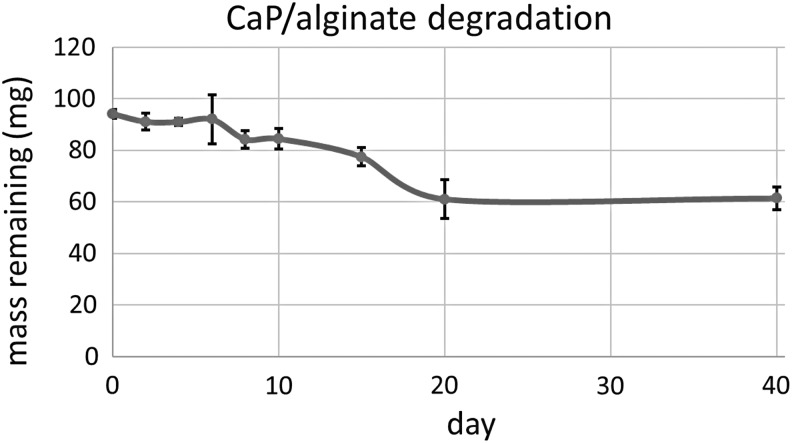
Degradation of scaffolds was measured over 40 days. Dry weights of three scaffolds comprised of ReCaPP cement, 3% alginate microspheres, and 1% alginate hydrogels were measured for each time point. It was observed that mass loss occurred primarily over the first 20 days of the assay timeframe, with no additional significant mass loss measured at day 40, as shown in Figure 6.
FIG. 6.
A graphical representation of the degradation of the alginate ReCaPP scaffold over 40 days, showing an approximate mass loss of 40 mg dry weight.
PDGF and BMP-2 release profiles
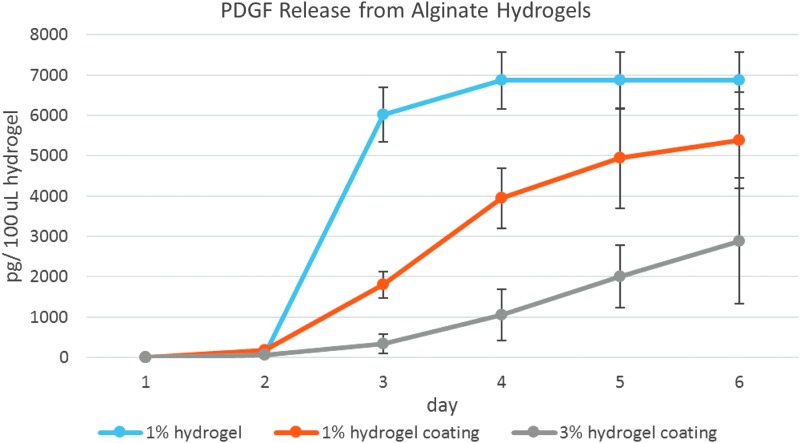
Three formulations of alginate hydrogels were assayed for PDGF release: a 1% hydrogel, a 1% hydrogel applied as a coating to ReCaPP scaffolding, and a 3% hydrogel applied to ReCaPP scaffolding (Fig. 7). Over the first 6 days of release, the 1% alginate hydrogel exhibited fast burst release of PDGF, which was complete by day 4. Both 1% and 3% hydrogels, when applied as a coating to scaffolds exhibited more steady, linear release of PDGF, with PDGF releasing more slowly from 3% alginate hydrogel coatings.
FIG. 7.
Release of PDGF from alginate hydrogel formulation showing burst release of PDGF from the 1% hydrogel. When hydrogels are applied as a coating to ReCaPP scaffolds, release profiles become linear over the first 6 days of release, indicating a more steady release of PDGF.
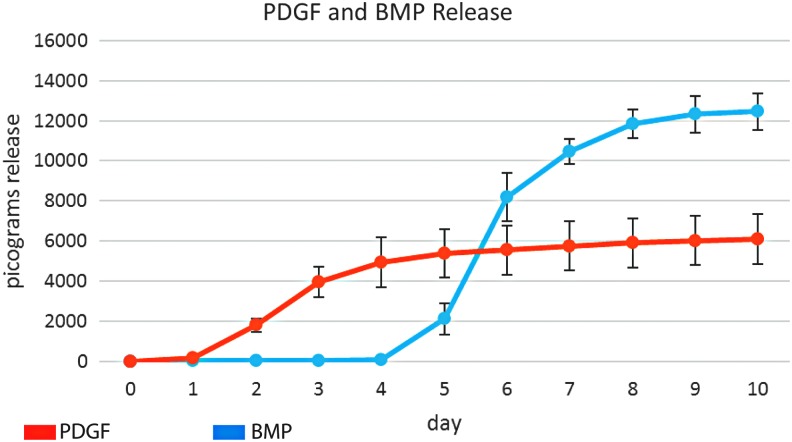
Release profiles of the PDGF from 1% alginate hydrogel coatings and BMP-2 from 3% alginate microspheres show early PDGF release, and later, delayed BMP-2 release (Fig. 8). BMP-2 release begins on day 5, overlapping with PDGF release for 3 days (day 5–7).
FIG. 8.
Release profiles of PDGF from 1% alginate hydrogels applied as a coating to ReCaPP scaffold, and BMP-2 from 3% alginate microspheres. PDGF release occurs over the first 7 days, whereas BMP-2 release subsequently begins on day 5, overlapping for 3 days with PDGF release.
Tubule formation in response to controlled growth factor delivery
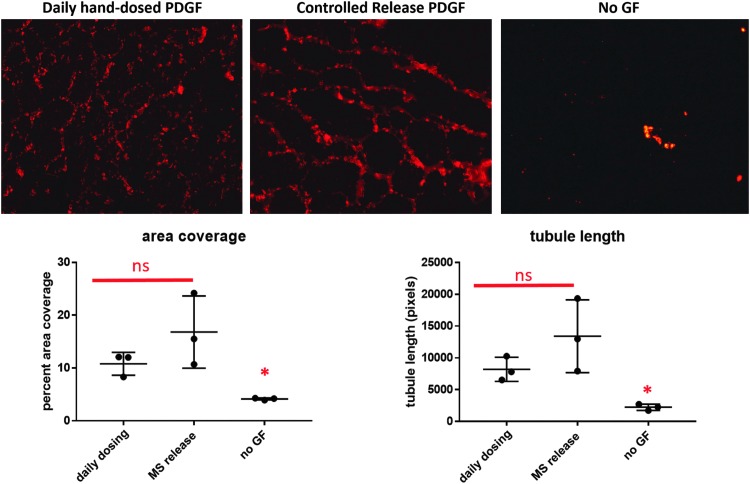
PDGF was delivered to a coculture of hMSCs and HUVECs in a Matrigel plug over the course of 7 days. Two methods of delivery were used: controlled delivery of PDGF from an alginate hydrogel and daily hand dosing of PDGF through pipetting soluble aliquots into the Matrigel plug. As seen in Figure 9, both methods of PDGF delivery resulted in statistically nonsignificant differences in tubule area coverage and length.
FIG. 9.
Representative images of tubule formation in response to PDGF dosing techniques showing comparable tubule formation area and tubule length in response to controlled release PDGF delivery with tubule formation in response to daily hand-dosed cell cultures. The negative control, receiving no delivered growth factors, shows significantly less tubule formation than either group receiving PDGF, as indicated by the asterisk.
Cellular infiltration of scaffold in response to growth factor delivery
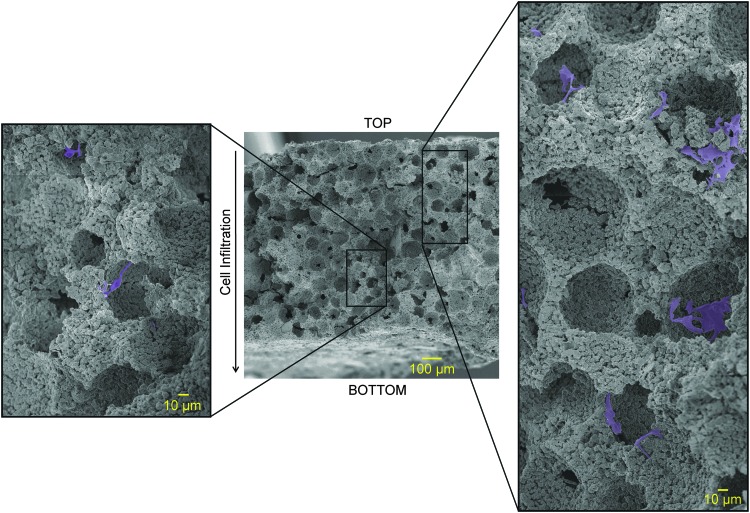
To evaluate the ability of PDGF to recruit cells to the scaffold, PDGF hydrogels were applied to one side of the scaffolding (creating a gradient of growth factor release across the scaffold), whereas a coculture of HUVECs and hMSCs were seeded on the opposite side of the scaffolding. At the experimental endpoint, scaffolds were broken in half to reveal a vertical cross-section and imaged with a scanning electron microscope to identify cellular locations within the scaffolding (Fig. 10).
FIG. 10.
SEM of a cross-section of porous ReCaPP scaffolding indicating depth of cell infiltration when PDGF is delivered. Inlays to the left and right of the full cross-section show examples of cells nested within ReCaPP scaffolding pores.
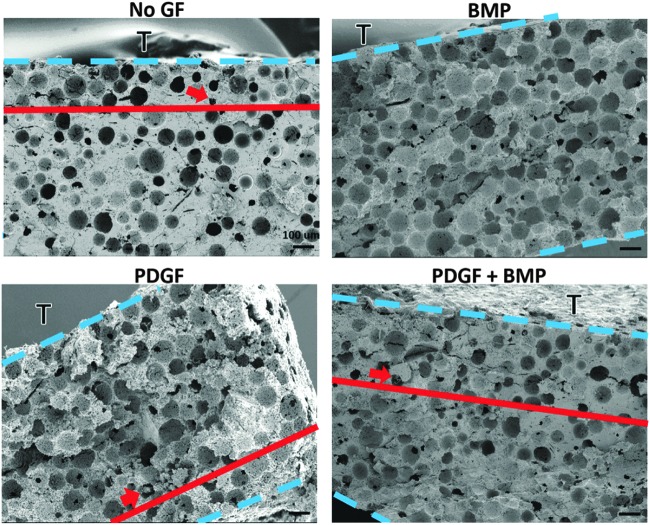
In response to PDGF release, cellular infiltration was observed through ∼75% of the 900 μm thick, 3D scaffold over the course of 7 days (Fig. 11). In comparison, when no growth factor was released from the hydrogel coatings, cell infiltration was only observed to penetrate ∼25% of the way into the scaffold. Interestingly, when BMP-2 was released from the hydrogel coating, no cells were observed to infiltrate the scaffold. When PDGF and BMP-2 were delivered simultaneously from the hydrogel, cells penetrated through ∼30% of thickness of the scaffold.
FIG. 11.
SEMs of porous ReCaPP scaffold cross-sections indicating penetration depth of infiltrating cells in response to growth factor gradients. Dotted lines demarcate upper and lower boundaries of the cross-sectional slice; the letter “T” identifies the top surface of the scaffold where cells were seeded. Solid lines and arrows indicate the furthest depth at which cells were found to penetrate the scaffold.
ALP expression in response to controlled growth factor delivery
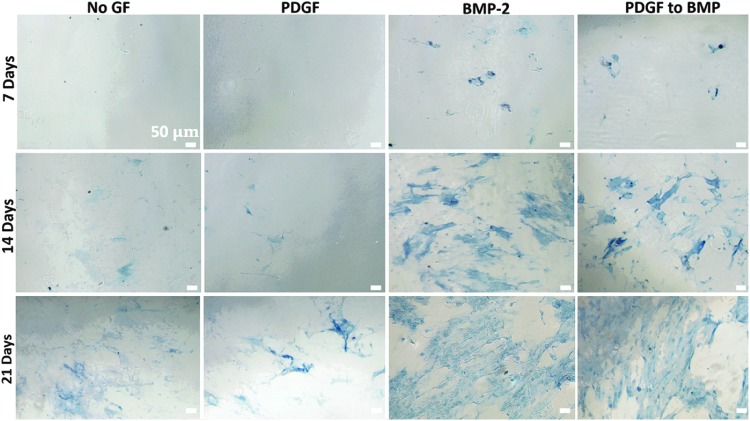
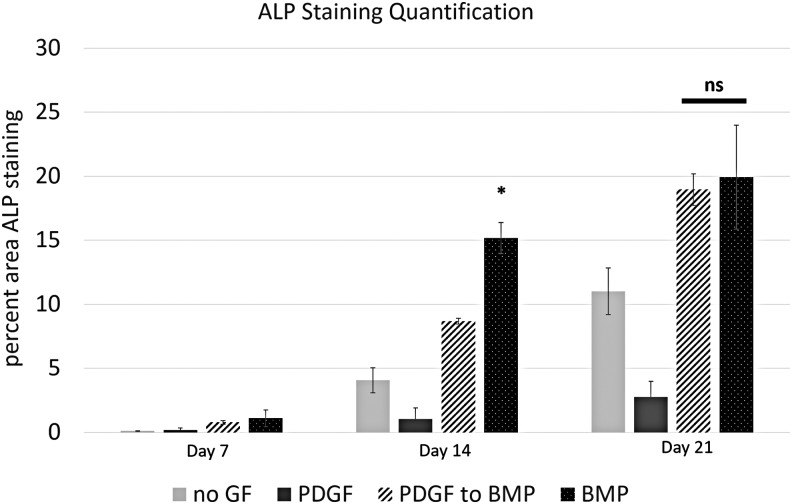
Programmed PDGF and BMP-2 delivery were presented to hMSC cultures as depicted in Figure 2. Cell cultures were assayed for ALP expression at three time points: 7, 14, and 21 days (Fig. 12). At the week 1 time point (7 days) hMSC cultures with PDGF delivery and no growth factor delivery did not stain for ALP. hMSC cultures with BMP-2 microspheres, as well as cultures receiving programmed PDGF to BMP-2 delivery stained positively for ALP expression. At day 14, cell cultures receiving BMP-2 showed significantly greater ALP staining compared with all other groups (Fig. 13). However, at day 21, cell cultures subject to BMP-2 release and PDGF to BMP-2 release, both showed an increase in ALP staining compared with previous time points. Additionally, there was no statistically significant difference in ALP readings for groups exposed to the PDGF to BMP-2 sequence versus BMP-2 alone at day 21 (Fig. 13). Interestingly, cell cultures receiving no growth factor treatment, as well as cultures exposed to PDGF release showed trace amounts of ALP staining at day 14, and increased ALP expression at 21 days (compared with the same treatment groups at earlier time points). Importantly, ALP staining in “No GF” and “PDGF” groups showed significantly less ALP staining than “BMP-2” and “PDGF to BMP” groups at the same time point.
FIG. 12.
ALP expression (blue staining) at day 7, 14, and 21 showing that hMSCs with scaffolds releasing only BMP-2, as well as scaffolds releasing a schedule of PDGF to BMP-2 stimulate ALP expression. hMSCs receiving no growth factor or PDGF show less ALP expression at days 7, 14, and 21 versus groups receiving BMP-2 or PDGF to BMP-2. hMSCs, human mesenchymal stem cells.
FIG. 13.
Quantification of ALP staining showing significantly greater ALP staining in cell cultures exposed to controlled release BMP-2 on day 14. On day 21, there is no significant difference in ALP staining for groups exposed to the PDGF to BMP delivery sequence, compared with the positive control of BMP delivery alone.
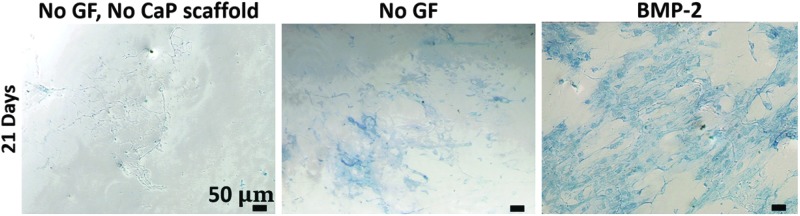
In Figure 14, ALP expression was assayed at day 21 under three conditions: cells exposed to BMP-2 releasing ReCaPP scaffolding, cells exposed to ReCaPP scaffolding with no growth factor, and cells cultured in the absence of ReCaPP scaffolding and growth factors. Cells showed no positive ALP staining in the absence of ReCaPP scaffolding and growth factors, a moderate amount of ALP expression when cultured in the presence of ReCaPP scaffolding without growth factors, and the greatest amount of ALP staining when subject to ReCaPP scaffolding releasing BMP-2 (Fig. 14).
FIG. 14.
ALP expression at day 21 for cells cultured with no additional growth factor and no calcium phosphate scaffolding, cells cultured with calcium phosphate scaffolding releasing no growth factor, and cells cultured with calcium phosphate scaffolding releasing BMP-2. Scaffolds releasing BMP-2 result in the most ALP staining, whereas scaffolds releasing no growth factor result in a moderate amount of ALP staining. Cells cultured in the absence of calcium phosphate scaffolding show no ALP staining.
Discussion
Calcium phosphate cements have been widely investigated for use as synthetic bone scaffolding material due to their similarities in composition to bone mineral, biocompatibility, and tunable degradation/resorption rates.27–30 Bioactive components, such as antibiotics and growth factors can also be released from calcium phosphate scaffolds, either by adsorbing the proteins or antibiotics to the surface of the scaffolding, or through the inclusion of a second biomaterial phase, such as synthetic or natural polymers to encapsulate bioactive agents.26,27 For example, by incorporating vancomycin-loaded PLGA microspheres into calcium phosphate scaffolding, it was shown that vancomycin could be released over the course of 100+ days from the scaffold.26
Calcium phosphate cements can also be engineered to be highly porous through fabrication methods (such as sintering or salt leaching31) or by including degrading polymers,26 to form interconnected micro- and macroporous networks. In this study, we aimed to design a controlled release system incorporated into a calcium phosphate template to create a highly porous, osteoconductive scaffold capable of delivering a programmed schedule of growth factors. For the purposes of this study, we engineered a controlled delivery system capable of releasing a schedule of PDGF and BMP-2 that has shown to be effective in generating a robust, organized network of angiogenic tubules.24
While some group have found great success in optimizing the bioactivity of PDGF and BMP-2 for bone repair by delivering these growth factors in a fibrin hydrogel matrix,32 this hydrogel formulation results in growth factor release profiles with a great degree of overlap.33 Because the aim of our study was to deliver a sequence of PDGF to BMP-2 release, we have chosen to encapsulate these growth factors in alginate hydrogel formulations, which gave us greater control over the timing of release. We have incorporated this controlled delivery system with a calcium phosphate matrix, and characterized the degradation, release profiles, and cellular responses to the resulting hybrid scaffold.
A matrix of medium viscosity, sodium alginate (crosslinked with calcium chloride), served as the base for both PDGF- and BMP-2-controlled release systems. Alginate was chosen as the polymer for PDGF release, as other polymers, such as PLGA, have been shown to be adherent with PDGF due to their opposing electronegative (PLGA) and electropositive (PDGF) charges, leading to unpredictable and hindered PDGF release.34 Additionally, because PDGF is a heparin-binding protein, it may reversibly bind to alginate hydrogels, enabling its sustained release, a phenomenon that has been demonstrated in studies investigating the release of other heparin-binding growth factors (including VEGF and FGF) from alginate hydrogels.35–37 Furthermore, this binding behavior of PDGF with alginate hydrogels could serve to mimic in situ PDGF surface binding with extracellular matrix, a property that is important to the biological function of PDGF.38 In this study, alginate hydrogels of 1% and 3% were investigated for PDGF release. When released from a 1% bulk hydrogel, PDGF exhibited burst release in the first 48 h of the release assay. When the same 1% alginate hydrogel formulation was applied as a coating to ReCaPP scaffolding, linear release over 6 days was observed. By applying the alginate hydrogel as a coating, we expose more surface area of the hydrogel, possibly allowing a greater amount of the hydrogel to be more strongly, homogeneously crosslinked, in turn delaying PDGF release. This hypothesis is consistent with known release behaviors of alginate hydrogels in the literature, with a greater amount of crosslinking generally serving to slow protein release from the hydrogel.36,39 Similarly, when a 3% alginate hydrogel was applied as a coating to ReCaPP scaffolding, linear release was observed over the first 6 days, at a slower rate of release compared with 1% alginate hydrogels. This difference can be explained by the concentration of alginate used in the hydrogels; as the percentage of alginate increases, total release is correspondingly extended.39 In addition to generating the desired PDGF release profile, alginate hydrogels were also chosen for this application because of their ability to be applied as a coating. Segregating PDGF to the outside of the ReCaPP prevents potential adsorption (and potentially inactivation) of PDGF to the interior of ReCaPP scaffolding.
To generate sequential delivery of PDGF and BMP-2 with a moderate window of overlap (3 days), BMP-2 delivery must be delayed 7 days following the start of PDGF delivery. This BMP-2 release behavior was achieved by encapsulating the protein in 3% alginate microspheres. In addition to achieving delayed, sequential release of BMP-2, the use of small, 20 μm alginate microspheres allowed for the local distribution of BMP-2-laden microspheres throughout the pores of the calcium phosphate scaffolding. As noted by Mitchell et al., supraphysiological doses of BMP-2 are often used in growth factor-based therapies, due to its rapid proteolysis and fast outward tissue diffusion.40 By encapsulating BMP-2 in alginate microspheres, we are able to sustain the slow release of small amounts of BMP-2, and by embedding these microspheres inside the calcium phosphate scaffold, the release of BMP-2 is localized. Additionally, while a fraction of released BMP-2 may also bind to calcium phosphate surfaces through electrostatic attraction and hydrogen bonding, there is evidence that bound BMP-2 may retain biological activity and promote osteoblastic differentiation within the scaffolding.41 For these reasons, we did not expect to see measurable release of BMP-2 from the entire hybrid scaffolding construct, and instead performed release studies on BMP-laden microspheres to approximate its local presentation to cells which have infiltrated the scaffold.
To evaluate the efficacy of PDGF to recruit cells into the scaffold, PDGF hydrogels were applied to one side of the scaffolding (creating a gradient of growth factor release across the scaffolding), whereas a coculture of HUVECs and hMSCs were seeded on the opposite side of the scaffolding. When PDGF and BMP-2 were delivered simultaneously (dual delivery) from the hydrogel, cells penetrated through ∼30% of thickness of the scaffold. Comparatively, cells penetrated down through more than double this length (∼75% of the scaffold) when PDGF alone was presented. Importantly, these results indicate that the dual presentation of BMP-2 with PDGF may send conflicting signals to the cells and potentially inhibit their migration into the scaffold, giving further support to the idea that early PDGF release followed by later BMP-2 release is beneficial for the ordered regenerative stages of bone formation.
Another indicator that early, simultaneous PDGF and BMP-2 presentation may be biologically nonsensical is the presence of BMP-2-activity inhibitors during early phases of cellular recruitment. In previously published evidence, it has been found that the in situ osteoblast differentiation mediated by BMP-2 is blocked by BMP-4 and noggin expression during early phases when cellular recruitment to the fracture site is most active.42 Additionally, as Martino et al. have noted in a recent review, while BMPs are present in various stages of bone repair, the presence of PDGF is restricted to earlier stages of inflammation and soft callus formation.43
While there are no alginate microspheres present in the cell infiltration, ReCaPP/alginate scaffolds were designed with consideration to provide adequate porosity for cellular infiltration. Because PLGA and alginate microspheres were engineered to have a predictable, repeatable size, and shape, calculations could be made to design pore space in the scaffolding by using defined ratios of 100 μm PLGA microspheres and ReCaPP cement. In depth details of these calculations and methods can be found in previously done work, which characterizes the ReCaPP scaffolding with a porous network created by PLGA microspheres using mercury intrusion porosimetry and BET surface area analyses.26
Briefly, using the equation: Pρ = [1 − ρe/ρt] × 100 from surface area analysis, where Pρ = percentage porosity, ρe = envelope density of the scaffolds, and ρt = true density values of the scaffold as well as the relation, PHg = [ρe·VHg·100] from mercury intrusion porosimetry, where ρe is the envelope density and VHg is the total mercury intrusion volume per gram, wherein the volume encompasses all of the different pore size regimes, the total porosity of the scaffold following complete degradation of PLGA microspheres was determined to be 73.7%.
The total volume of this pore space taken up by alginate particles is thus calculated to be at a maximum of 20% (given an approximate alginate microsphere density of 1.102 g/cm3,44 10 mg of alginate particles infiltrated, and a scaffold volume of 46.2 mm3), creating a final scaffold structure with 54% porosity. As shown in a similar study of calcium phosphate scaffolding with comparable total porosity (57.2%), cells were able to significantly infiltrate and proliferate within the scaffold.45
To assay for the early tendency of hMSCs toward an osteogenic phenotype, fully assembled scaffolds of porous ReCaPP cement with PDGF-releasing alginate hydrogels and BMP-2-releasing alginate microspheres were assessed using a transwell system (Fig. 2). By day 14, greater amounts of ALP staining were observed in cell cultures that received BMP-2 and PDGF to BMP-2 growth factors. Cell cultures with no released growth factors, and those receiving only PDGF showed relatively small amounts of positive ALP staining, which may be attributed to the osteoconductive (and potentially osteoinductive) effects of the calcium phosphate scaffolding.25,46
In support of this hypothesis, when cells were cultured in the absence of ReCaPP scaffolding and growth factors, no ALP expression was detected at day 21. Importantly, these results indicate that while PDGF does not induce the expression of ALP (or the differentiation of hMSCs toward an osteoblastic phenotype), its early presentation does not inhibit the ability of later BMP-2 presentation to promote osteoblastic differentiation. These results are supported by evidence that PDGF is involved not only during the angiogenic phase of bone repair, but also plays an important role in stimulating the cellular events preceding osteogenesis, such as recruiting MSCs and stabilizing the blood vessels which guide the orientation of osteoblast sheets.47
Our current investigation of programmed growth factor delivery suggests that sequential PDGF to BMP-2 release does indeed influence angiogenesis, cellular infiltration of scaffolding, and expression of the osteogenic marker ALP. While the models used in this study provide valuable in vitro systems to analyze cellular responses, the full complexity of in vivo bone healing is not limited to the growth factors and cell types selected for this study. Importantly, in vivo validation studies must be performed to confirm these results.
Conclusions
The present study demonstrates the use of degradable PLGA microspheres along with calcium phosphate cements to form scaffolds with interconnecting porous networks following the dissolution of PLGA microspheres with DCM. Furthermore, these scaffolds can be reinfiltrated with smaller, BMP-2-releasing alginate microspheres and coated with PDGF-releasing alginate hydrogels to deliver a programmed schedule of these growth factors to cell cultures.
The chosen delivery schedule of PDGF and BMP-2 has shown to be effective in stimulating cell infiltration of the scaffold, generating angiogenic tubule network formation, and promoting ALP expression, indicating the potential for this hybrid scaffold system to supply the cues to orchestrate vascularized bone regeneration. With further in vivo studies of these materials, the translation of this technology from a proof-of-concept model to the development of a new approach for fracture healing may be realized.
Disclosure Statement
No competing financial interests exist.
References
- 1.Stevens M.M. Biomaterials for bone tissue engineering. Mater Today 11, 18, 2008 [Google Scholar]
- 2.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84-A, 454, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Stevenson S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res 10, S239, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Reichert J.C., Saifzadeh S., Wullschleger M.E., Epari D.R., Schütz M.A., Duda G.N., Schell H., van Griensven M., Redl H., and Hutmacher D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30, 2149, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Moghadam H.G., Sándor G.K., Holmes H.H., and Clokie C.M. Histomorphometric evaluation of bone regeneration using allogeneic and alloplastic bone substitutes. J Oral Maxillofac Surg 62, 202, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Lo K.W., Ulery B.D., Ashe K.M., and Laurencin C.T. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev 64, 1277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampath T.K., Maliakal J.C., Hauschka P.V., Jones W.K., Sasak H., Tucker R.F., White K.H., Coughlin J.E., Tucker M.M., and Pang R.H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem 267, 20352, 1992 [PubMed] [Google Scholar]
- 8.Lissenberg-Thunnissen S.N., de Gorter D.J., Sier C.F., and Schipper I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop 35, 1271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer E.A., Gottardi R., Fedorchak M.V., and Little S.R. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Control Release 219, 129, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Tiwari G., Tiwari R., Sriwastawa B., Bhati L., Pandey S., Pandey P., and Bannerjee S.K. Drug delivery systems: an updated review. Int J Pharm Investig 2, 2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo T.N., Kasper F.K., and Mikos A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev 64, 1292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H., VandeVord P., Gong W., Wu B., Song Z., Matthew H., Wooley P., and Yang S. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res 26, 1147, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Endres S., Hiebl B., Hägele J., Beltzer C., Fuhrmann R., Jäger V., Almeida M., Costa E., Santos C., Traupe H., Jung E.M., Prantl L., Jung F., Wilke A., and Franke R.P. Angiogenesis and healing with non-shrinking, fast degradeable PLGA/CaP scaffolds in critical-sized defects in the rabbit femur with or without osteogenically induced mesenchymal stem cells. Clin Hemorheol Microcirc 48, 29, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kempen D.H., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A., Yaszemski M.J., and Dhert W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30, 2816, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Farokhi M., Mottaghitalab F., Ai J., and Shokrgozar M.A. Sustained release of platelet-derived growth factor and vascular endothelial growth factor from silk/calcium phosphate/PLGA based nanocomposite scaffold. Int J Pharm 454, 216, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E., and Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez A., Reyes R., Sanchez E., Rodriguez-Evora M., Delgado A., and Evora C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. J Biomed Mater Res A 100, 2382, 2012 [DOI] [PubMed] [Google Scholar]
- 18.McFadden T.M., Duffy G.P., Allen A.B., Stevens H.Y., Schwarzmaier S.M., Plesnila N., Murphy J.M., Barry F.P., Guldberg R.E., and O'Brien F.J. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater 9, 9303, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Tengood J.E., Kovach K.M., Vescovi P.E., Russell A.J., and Little S.R. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials 31, 7805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tengood J.E., Ridenour R., Brodsky R., Russell A.J., and Little S.R. Sequential delivery of basic fibroblast growth factor and platelet-derived growth factor for angiogenesis. Tissue Eng Part A 17, 1181, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo T., Zhang W., Shi B., Cheng X., and Zhang Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clin Oral Implants Res 23, 467, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.C., Kaigler D., Rice K.G., Krebsbach P.H., and Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20, 848, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kanczler J.M., and Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15, 100, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Bayer E.A., Fedorchak M., and Little S. The influence of PDGF and BMP presentation on tubule organization by HUVECs and human mesenchymal stem cells in co-culture. Tissue Eng Part A 22, 1296, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumta P., Sfeir C., and Roy A. Bone Substitute Compositions, Methods of Preparation and Clinical Applications. Pittsburgh: University of Pittsburgh, 2013. US Patent: US 8357364B2 [Google Scholar]
- 26.Roy A., Jhunjhunwala S., Bayer E., Fedorchak M., Little S.R., and Kumta P.N. Porous calcium phosphate-poly (lactic-co-glycolic) acid composite bone cement: a viable tunable drug delivery system. Mater Sci Eng C Mater Biol Appl 59, 92, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Bose S., and Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater 8, 1401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramay H.R., and Zhang M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 25, 5171, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ambard A.J., and Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont 15, 321, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Hutmacher D.W., Schantz J.T., Lam C.X., Tan K.C., and Lim T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med 1, 245, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Karageorgiou V., and Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Martino M.M., Tortelli F., Mochizuki M., Traub S., Ben-David D., Kuhn G.A., Müller R., Livne E., Eming S.A., and Hubbell J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 3, 100ra89, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Vila O.F., Martino M.M., Nebuloni L., Kuhn G., Pérez-Amodio S., Müller R., Hubbell J.A., Rubio N., and Blanco J. Bioluminescent and micro-computed tomography imaging of bone repair induced by fibrin-binding growth factors. Acta Biomater 10, 4377, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Balmert S.C., Zmolek A.C., Glowacki A.J., Knab T.D., Rothstein S.N., Wokpetah J.M., Fedorchak M.V., and Little S.R. Positive charge of “sticky” peptides and proteins impedes release from negatively charged PLGA matrices. J Mater Chem B Mater Biol Med 3, 4723, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K.Y., Peters M.C., and Mooney D.J. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release 87, 49, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Lee K.Y., and Mooney D.J. Alginate: properties and biomedical applications. Prog Polym Sci 37, 106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva E.A., and Mooney D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 31, 1235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino M.M., Brkic S., Bovo E., Burger M., Schaefer D.J., Wolff T., Gürke L., Briquez P.S., Larsson H.M., Gianni-Barrera R., Hubbell J.A., and Banfi A. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front Bioeng Biotechnol 3, 45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellich B., Borgogna M., Cok M., and Cersaro A. Release properties of hydrogels: water evaporation from alginate gel beads. Food Biophys 6, 259, 2011 [Google Scholar]
- 40.Mitchell A.C., Briquez P.S., Hubbell J.A., and Cochran J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater 30, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King W.J., and Krebsbach P.H. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv Drug Deliv Rev 64, 1239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ai-Aql Z.S., Alagl A.S., Graves D.T., Gerstenfeld L.C., and Einhorn T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 87, 107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino M.M., Briquez P.S., Maruyama K., and Hubbell J.A. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev 94, 41, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Salsac A., Zhang L., and Gherbezza J. Measurement of mechanical properties of alginate beads using ultrasound. Marseille: 19th French Congress of Mechanics, Aug. 24–28, 2009 [Google Scholar]
- 45.Xu H.H., and Simon C.G. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials 26, 1337, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wang P., Zhao L., Liu J., Weir M.D., Zhou X., and Xu H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res 2, 14017, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplan A.I., and Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res 29, 1795, 2011 [DOI] [PubMed] [Google Scholar]