Abstract
The concept of the ovarian continuum can be understood as a process that occurs during a woman’s lifetime and begins during intrauterine life with fertilization. Women start their reproductive years with approximately five hundred thousand follicles containing oocytes, of which only around five hundred will be released during ovulation. Ovulation has been recognized as an event linked with reproduction; however, recent evidence supports the role of ovulation as a sign of health. The use of biomarkers that help women recognize ovulation enables them to identify their health status. This knowledge helps medical healthcare providers in the prevention, diagnosis, and treatment of different pathologies related with endocrine disorders, gynecological abnormalities, autoimmune, genetic, and neoplastic diseases, as well as pregnancy-related issues. The knowledge of the ovarian continuum and the use of biomarkers to recognize ovulation should be considered a powerful tool for women and medical professionals.
Summary
The ovarian continuum is a process that occurs during a woman’s lifetime. It begins during intrauterine life with fertilization and ends with menopause. This process can be greatly affected by different conditions such as changes in hormonal levels and illnesses. Therefore, understanding and promoting the knowledge and use of biomarkers of ovulation in women is a key aspect to consider when evaluating their health status. The knowledge and education about the ovarian continuum should be taken into account as a powerful tool for women and medical professionals.
Keywords: Ovarian continuum, Women’s health, Ovulation, Biomarkers
Introduction
The concept of the ovarian continuum can be understood as a process that occurs during a woman’s lifetime and starts during intrauterine life (Brown 2011). This continuum begins with fertilization. Two months later the primordial germ cells leave the embryo to avoid differentiation and migrate to the yolk sac, where they remain for four weeks. After this time, they are found in the gonadal ridge where they are surrounded by somatic cells. In this way, ovarian primordial follicles start to organize (Motta, Makabe, and Nottola 1997), and around seven million primordial follicles are formed in the ovaries, but only one to two million remain at birth (Block 1952). The rest of the primordial follicles degenerate via an apoptotic process called follicular atresia. Later on during her reproductive life, a woman will ovulate around five hundred oocytes (Lunenfeld and Insler 1993).
Often, healthcare providers have focused on regularizing bleeding patterns, without paying attention to ovulation in reproductive age women. The fact that women have biomarkers that enable them to recognize ovulation and hence which stage of the ovarian continuum they are in, allows them to evaluate their own health. Accordingly, normal ovulatory activity during reproductive years can be considered a sign of health, because it implies adequate endocrine and gonadal function. Women in conditions such as breastfeeding or pregnancy will also be able to identify their anovulatory state as part of the continuum. Periods of transition from anovulation to regular ovulation, such as those found during puberty and perimenopause, can also be identified as a physiological part of the continuum by women using their biomarkers.
The first sign of an underlying health problem a woman may experience is usually an abnormality in ovulation followed by irregular cycles or amenorrhea. Indeed, when pregnancy, lactation, or menopause are not the causes, persistent irregularities in the ovulatory cycle can be associated with lifestyle, stress, and endocrine, gynecological, autoimmune, nutritional, genetic, and iatrogenic disorders (Vigil et al. 2006) (fig. 1). Importantly, while regular menstrual cycles are generally considered a sufficient indicator of ovulation, they can also be anovulatory (Malcolm and Cumming 2003). Therefore, it is not the presence of regular menstruation but the presence of regular ovulation, which helps in monitoring women’s health.
Figure 1.
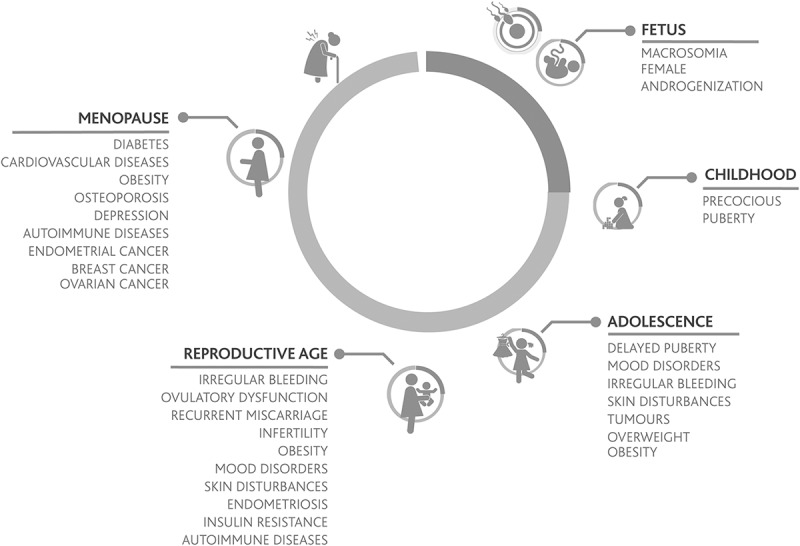
Physiological and pathological conditions affecting ovulation. The lower portion of the figure illustrates conditions that drive toward an anovulatory state while the upper part of the figure illustrates conditions that drive toward an ovulatory state.
Care of Women in Various Stages of Life
During childhood, follicles are continuously developing until the antral stage, at which they become gonadotrophin dependent. Due to the low gonadotrophin levels present during this period of life many follicles undergo atresia (Skinner 2005). Considering the ovarian continuum, a healthy child is in an anovulatory state, although having a considerable pool of follicles in the ovaries (Peters, Byskov, and Grinsted 1978). Interestingly, during puberty, when gonadotrophin levels start to rise, primordial follicles that have developed to the antral stage will continue their development until one of them reaches the pre-ovulatory stage and ovulation occurs. Adrenarche, which generally occurs between the ages of 8 and 10, triggers pubertal development and its accompanying hormonal changes, which culminate with the first ovulation that usually leads to menarche, the first menses. Menarche occurs on average between ages 12 and 13. In general, menses is considered to be the visible sign of the onset of ovarian cyclicity. Menarche indicates that the first ovulation has probably occurred or is about to occur, in an event that marks the beginning of the gonadotrophin-dependent period of ovarian cyclical activity characteristic of reproductive years. During the first two years after menarche, occasional anovulatory cycles can occur. Once the reproductive system fully matures, typically women exhibit regular monthly ovulations characterized by 24- to 36-day cycles, 27 plus or minus 1 day being the most frequent length (Fraser et al. 2007).
At approximately four years prior to the final menstrual period, the functional capacity of the ovary diminishes, and women enter into the perimenopausal period characterized by changes such as hot flashes, sleep disturbances, mood symptoms, and vaginal dryness. An increase of FSH during the first days of the follicular phase can be observed, together with a decrease of inhibin B and an increase of activin A. The decline in oocyte number that occurs with age, together with the increase in activin (Lobo 2014), causes an accelerated follicular depletion that leads to menopause. In this period, due to gonadotrophin stimulation, increased estrogen levels are produced by the ovaries, which cause endometrial growth that can be associated with heavy bleeding and irregular cycles (Harlow et al. 2012).
Endocrine Regulation of the Ovulatory Cycle
The ovulatory cycle is regulated by positive and negative feedback mechanisms. Steroid sex hormones produced by the ovary regulate the secretion pattern of kisspeptin, gonadotrophin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), which in turn, modify the release of ovarian hormones (Hawkins and Matzuk 2008).
At the beginning of each cycle, an increase in FSH levels causes follicular recruitment and development, with subsequent elevation of estradiol levels (Miro and Aspinall 2005). Increasing estradiol levels secreted by maturing follicles produce endometrial proliferation, a change in the size of the cervical os, and an increase in the amount of cervical mucus with modifications in its rheological and physicochemical properties. After follicular recruitment, during the follicular phase of the cycle, estradiol together with inhibin exert a negative feedback mechanism upon the hypothalamic-hypophyseal-gonadal (HHG) axis that causes a decrease in FSH levels (Laven and Fauser 2004). During this period, estradiol also inhibits kisspeptin expression in the arcuate nucleus of the hypothalamus. Later on, when a follicle becomes the dominant follicle, it produces increasingly high levels of estradiol, which result in a stimulation of kisspeptinergic neurons in the anteroventral periventricular nucleus of the hypothalamus, thus changing the negative feedback mechanism to a positive one. Kisspeptin induces GnRH secretion and the following pre-ovulatory LH peak, which initiates follicular luteinization that leads to the formation of the corpus luteum (Cortés et al. 2015). After the initiation of the midcycle gonadotrophin surge, a pre-ovulatory rise in progesterone occurs. This early progesterone rise produced in the pre-ovulatory follicle is critical for ovulation and development of a functional corpus luteum. LH and progesterone trigger follicular-wall degradation by proteases, prostaglandins, and other compounds (Stouffer 2003), resulting in release of the oocyte which normally survives 12 to 24 hours (Ferin, Van Vught, and Wardlaw 1984). After ovulation, LH contributes to the development of the corpus luteum (Misao et al. 1998), which continues to produce progesterone and estrogen during the luteal phase (Blackwell et al. 2003). Progesterone modifies the endometrial lining from proliferative to secretory type and changes the cervical mucus from estrogenic to progestational. About 8 days later, in the absence of fertilization, the corpus luteum begins to regress and lasts for approximately 11 to 17 days (Blackwell et al. 2013), with an average lifespan of 14 days. As a consequence, estrogen and progesterone concentrations return to the levels observed in the early follicular phase about two weeks after the initial formation of the corpus luteum (Miro and Aspinall 2005). This eliminates the suppression exerted on the HHG axis and causes the beginning of a new cycle (table 1).
Table 1.
Main hormonal events during the ovulatory cycle, ordered chronologically
| Hormonal event | |
| 1 | An increase in FSH levels leads to recruitment and development of ovarian follicles. |
| 2 | Selected follicles produce rising estradiol levels. |
| 3 | Estradiol together with inhibin exerts a negative feedback upon the HHG axis, thus decreasing FSH levels. Estradiol also causes a negative feedback upon the kisspeptinergic neurons. |
| 4 | One of the selected ovarian follicles becomes dominant. Increasing high levels of estradiol change the negative feedback upon the hypothalamus and hypophysis to a positive one, which causes the midcycle gonadotrophin surge. |
| 5 | LH surge is initiated, which causes follicular luteinization and an initial progesterone rise. Progesterone maintains the LH peak and is necessary for follicular rupture and adequate ovulation. |
| 6 | Ovulation |
| 7 | LH and progesterone release pattern help in the formation and support of the corpus luteum. The corpus luteum secretes progesterone and estrogens. |
| 8 | If fertilization does not occur, the corpus luteum will start to regress after 6 days and will last for 11 to 17 days. |
| 9 | Estradiol and progesterone concentrations drop, which eliminates the negative feedback exerted upon the HHG axis. |
| 10 | A new cycle begins. |
Cervical Mucus as a Biomarker of Ovulation
There are several biomarkers that can help a woman to identify ovulation (table 2). The most used are changes in the cervical mucus, basal body temperature, and modifications of the cervical os.
Table 2.
Physiological changes experienced by women during the follicular and luteal phases of the menstrual cycle
| Follicular phase | Luteal phase | ||||
|---|---|---|---|---|---|
| Signs | Early | Mid | Late | Early | Late |
| Menstruation | Includes ovulation | ||||
| Cervical mucus | None or estrogenic | None or estrogenic | Abundant estrogenic | Progestative | Progestative |
| Basal body temperature | Low | Low | Low | High | High |
| Position of cervix in vaginal canal | Low | Low | High | Low | Low |
| Consistency of cervix | Firm | Firm | Soft | Firm | Firm |
| Opening of cervical canal | Partially open | Closed | Open | Closed | Closed |
| Vaginal sensation | Variable | Variable | Wet | Dry | Dry |
| Pain | Lower abdomen | More intense, lower abdomen | |||
| Skin | Cleaner, healthier | Greasier | |||
| Fluid retention | Hands, feet, abdomen | Hands, feet, abdomen | |||
| Tenderness | Higher in breasts | Higher in breasts | |||
| Mood | Easily irritable | ||||
Source: Vigil (2004).
Increasing estrogen levels produced during follicular selection result in a noticeable rise in the secretion of estrogenic cervical mucus. In this phase, the mucus is aqueous, transparent, fluid, and crystalline, and tends to form characteristic geometric patterns (Menárguez, Pastor, and Odeblad 2003). Estrogenic mucus is made up of 98–99 percent water (Vigil, Croxatto, and Cortés 2014), and its ultrastructure shows a network with channels and pores that increase in size as ovulation nears (Chretien and Dubois 1991).
Progesterone has the opposite effect of estradiol, inhibiting the production and changing the characteristics of the mucus to those of G mucus (e.g., lower water content, which decreases to 90%). G mucus is opaque and less fluid and loses its ability to crystallize into characteristic patterns (Vigil, Blackwell, and Cortés 2012). The ultrastructure of G mucus shows a dense network with small diameter pores (Vigil et al. 2009).
It has been demonstrated that recognizing mucus patterns can help women to identify the different stages of the ovarian continuum (Billings et al. 1972) and in this way be able to recognize changes in the ovulatory pattern and detect a number of gynecological disorders.
Ovulation Monitoring Devices and Apps
Devices and apps are available to assist women in monitoring the menstrual cycle (Duane et al. 2016). Several popular apps are based on the rhythm method and predict ovulation, the fertile window, and the next menstruation. However, this prediction is inaccurate as it does not consider the variability between different women and within the same woman. The apps that are more accurate—since they consider personal variability—are those based on biomarkers such as cervical mucus symptoms charting and basal body temperature.
In the near future, apps connected with point-of-care devices will be available. The most direct indicators of ovulation are estrogen, LH, and progesterone. The development of apps and/or point-of-care devices that consider these hormones will be helpful for medical providers and women around the world (Brown, Blackwell, and Cooke 2017), as they are highly accurate and precise indicators of ovulation and the fertility window.
Ovulatory Dysfunction and Underlying Health Disorders
The most frequent causes of menstrual irregularities associated with ovulatory dysfunctions are hormonal abnormalities. These can be hypothalamic, pituitary, thyroid, adrenal, ovarian, and metabolic disorders.
Hypothalamic disorders are characterized by a change in the normal pattern of secretion of GnRH, delaying the increase of FSH levels above threshold. Hypothalamic disorders can be caused by excessive exercise, nutritional imbalances, stress, or psychiatric disorders, such as anorexia (Unuane et al. 2011). Nutritional deficits and/or low body fat result in low levels of leptin, a hormone that is secreted by adipocytes and promotes the secretion of kisspeptin. Low kisspeptin levels affect GnRH release and therefore ovulation (Clarke, Dhillo, and Jayasena 2015). In addition, an increase in adipose tissue may result in an increase in leptin levels that generates leptin resistance (Sahu 2002) thus affecting kisspeptin release (Elias et al. 1999). Hypothalamic disorders may also be the result of hypercortisolemia. Increased cortisol levels block both the secretion of GnRH and the action of gonadotrophins. Therefore, these disorders may result in hypoestrogenic cycles, anovulation, and amenorrhea (Saketos, Sharma, and Santoro 1993).
Prolactinomas are the most common pituitary tumors and are generally associated with hyperprolactinemia. Stress (Johansson, Karonen, and Laakso 1983) and/or the use of antidepressant drugs (Mondal et al. 2013) may also cause an increase in prolactin production. High prolactin levels inhibit GnRH by negative modulation of kisspeptinergic neurons (Araujo-Lopes et al. 2014) and by activation of dopaminergic neurons in the hypothalamus (Koike et al. 1991). High circulating prolactin levels also activate adrenal androgen secretion (Higuchi et al. 1984) and decrease androgen aromatization in the ovary (Krasnow, Hickey, and Richards 1990), causing higher androgen and lower estrogen levels. Women with hyperprolactinemia present symptoms that include menstrual irregularities (sometimes amenorrhea), short luteal phases, decreased libido, dyspareunia, and galactorrhea (Barron 2004). In our experience, these women frequently present with allergies, warts, and a higher tendency to suffer from infections (Vigil, del Río et al. 2007). Elevated prolactin levels are observed in several autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Hashimoto’s thyroiditis, and multiple sclerosis. Also, prolactin impairs the negative selection of autoreactive B lymphocytes and enhances the proliferative response to antigens and mitogens, and the production of autoantibodies. Therefore, this hormone has an immuno-stimulatory effect, promoting autoimmunity (Orbach and Shoenfeld 2007). In this way, ovulatory dysfunctions can be an early signal of more serious underlying health issues.
Thyroid hormones influence ovulation by acting upon folliculogenesis and steroidogenesis at the ovarian level, and by affecting sex hormone-binding globulin (SHBG) and GnRH secretion. Women with thyroid disorders can suffer from menstrual abnormalities, such as hypomenorrhea, hypermenorrhea, menorrhagia, polymenorrhea, intermenstrual bleeding, oligomenorrhea, or amenorrhea (Krassas et al. 1994, 1999). Menorrhagia is a common symptom of hypothyroidism due to reduced levels of SHBG, which increase free estradiol that promotes endometrial growth. Moreover, the higher levels of thyrotropin-releasing hormone (TRH), present in primary hypothyroidism, stimulate the secretion of prolactin and dopamine that inhibits GnRH, causing an ovulatory dysfunction. In contrast, SHBG levels rise in patients with hyperthyroidism, which decreases free estradiol levels. Ovarian and hypophyseal hormones may also be increased, resulting in ovulatory dysfunction (Poppe, Velkeniers and Glinoer 2007).
Adrenal and/or ovarian disorders are frequently associated with ovulatory dysfunction. Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women (Amer 2009). These patients have hyperandrogenemia and may exhibit acne, hirsutism, alopecia, increased body weight, and mood changes. But the most common symptom perceived by these patients is the presence of irregular menstrual cycles and an atypical pattern of cervical mucus, associated with ovulatory dysfunction. Obesity, insulin resistance, and consequent hyperinsulinemia are highly prevalent co-morbidities of PCOS and can impair ovulation (Pauli et al. 2011). Elevated insulin levels are present in about half of these patients and are correlated to body mass index (BMI), but not all PCOS patients are insulin resistant (Vigil, Contreras et al. 2007). High insulin levels further increase androgen production by stimulating ovarian theca cells to produce more androgens which lead to premature follicular atresia and even anovulation (Diamanti-Kandarakis 2006). Increased testosterone production inhibits the granulosa cell aromatase, thus decreasing estradiol production. High levels of testosterone and insulin will decrease SHBG, increasing the free estradiol fraction. This, together with an increase in peripheral production of estrogens by adipose tissue may inhibit the kisspeptinergic system decreasing GnRH and gonadotrophins. PCOS is also associated to an increased risk of type 2 diabetes, metabolic syndrome (Ranasinha et al. 2015), cardiovascular disease, and endometrial, ovarian, and/or breast cancer (Fauser et al. 2012).
Congenital adrenal hyperplasia (CAH) is a family of disorders caused by mutations in genes that encode for enzymes involved in one of the various steps of adrenal steroid synthesis. In some of these disorders, the synthesis of cortisol is impaired, stimulating the adenohypophysis to secrete high levels of adrenocorticotrophic hormone (ACTH), which in turn causes hyperandrogenemia due to adrenal overstimulation (Merke and Bornstein 2005). This leads to gonadal dysfunction, precocious puberty, delay of menarche, menstrual disorders, anovulation, and infertility (New 2004). Another adrenal disorder, Addison’s disease or premature adrenal insufficiency, is characterized by a deficiency of cortisol, aldosterone, and adrenal androgen hormonal precursors, and in some women is associated with premature ovarian failure (Erichsen et al. 2009, 2010).
Premature ovarian senescence affects about 10 percent of all women, being primary occult ovarian insufficiency the most common condition found in this group of patients. The three principal causes are autoimmune, genetic, and iatrogenic diseases (Gleicher et al. 2013). These women have low estrogen and androgen production. Early deficiency of estrogen will be identified by a dry mucus pattern. Estrogen and/or androgen replacement in these women will improve mood, and mitigate the risk of cardiovascular disease (Rocca et al. 2012), osteoporosis (Svejme et al. 2012), and other complications (Shuster et al. 2010).
Vitamin D has also been associated with a proper ovarian function, stimulating steroidogenesis, and follicular development. Hypovitaminosis D will increase androgen and decrease estrogen levels by a decrease in the aromatase activity of granulosa cells (Irani and Merhi 2014). Obese patients will present low vitamin D levels as a consequence of its sequestering by the adipose tissue (Lerchbaum and Obermayer-Pietsch 2012).
Women presenting with genetic conditions such as Turner’s syndrome will also present an ovulatory dysfunction. This condition is usually diagnosed early in life because of poor growth and development; however, in some cases, it will remain undiagnosed until adolescence. Amenorrhea associated with high FSH levels and a hypoestrogenic state will be present. Karyotyping is mandatory to confirm the diagnosis and to rule out a Y component as in cases of mosaicism (Nader 2000).
Gynecological disorders that include anatomical abnormalities, neoplasia, and inflammatory diseases may also cause abnormal uterine bleeding (AUB). In women with AUB, leukemia and abnormalities in blood clotting factors must be ruled out. Iatrogenic causes, such as hormonal contraceptives, anabolics, and selective estrogen receptor modulators (SERMs) could cause AUB. After contraceptives discontinuation, cycles are variable in length, likely because the HHG axis is normalizing itself after it has been suppressed during contraceptive use, and the quality of cervical mucus is diminished for at least the first six menstrual cycles (Nassaralla et al. 2011). Traumatic events in the pelvic area, pregnancy-related disorders, such as spontaneous abortion, and ectopic pregnancies must also always be ruled out in cases of AUB.
Ovulation as a Marker of Endocrine Homeostasis and Health Status
Women who learn how to read their biomarkers will be able to recognize if ovulation is occurring. Monitoring her ovulation can allow a woman to be prepared for the onset of her next period. Indeed, many women cannot accurately predict the onset of menstruation and do not know how long their cycles are (Jukic et al. 2008). Women should learn to identify the duration and flow of menstruation, cervical-mucus quality, ovulation day, and duration of follicular and luteal phases (Vigil et al. 2006). A normal menstrual ovulatory cycle is one that has a length between 24 and 36 days, and a luteal phase length between 11 and 17 days calculated from the estrogen peak, measured by its glucuronides in urine, to the day before the ensuing bleed (Blackwell et al. 2013). Abnormal cycles are short cycles (less than 24 days), long cycles (more than 36 days), or normal length cycles with a short luteal phase, or its absence (anovulation). If a woman identifies three or more abnormal cycles in a year or two consecutive abnormal cycles, she should consult a specialist, and a hormonal profile should be done.
The first sign of an underlying health problem is often an abnormality in ovulation followed by irregular cycles. It has also been shown that varying cycle lengths, short cycle lengths, and long cycle lengths are associated with decreased fecundity, and that menstrual cycle patterns may predict whether a pregnancy will survive (Kolstad et al. 1999). However, menstrual cycles with a normal length are not an indicator of proper ovarian function, because these women can also present anovulatory cycles (Prior et al. 2015). Therefore, it is regular ovulation and not regular menstruation which evidences a good health state.
Monitoring the ovulatory cycle should begin in puberty and adolescence. Special attention must be given to precocious or delayed puberty, as they are linked to endocrine abnormalities (Stanhope and Brook 1986). Because the conditions that alter ovulation during adolescence will only worsen if a correct diagnosis is not made, identifying ovulatory abnormalities can allow for early treatment of underlying health problems (Popat et al. 2008). Importantly, it has been shown that perimenarcheal girls from diverse ethnic and socioeconomic groups are able to learn how to recognize their cervical mucus patterns and to use this information to distinguish ovulatory from anovulatory cycles (Klaus and Martin 1989). It has been demonstrated that the menstrual cycle pattern during the first years after menarche is a better predictor for ovulatory dysfunction in adulthood than androgen or LH levels (van Hooff et al. 2004).
Concluding Remarks
Understanding the ovarian continuum and recognizing abnormalities within it allows women and healthcare providers to identify, diagnose, prevent, and treat different pathologies that may present during a woman’s life (fig. 2). After menarche, during reproductive years, ovulation can be considered and used as a sign of health.
Figure 2.
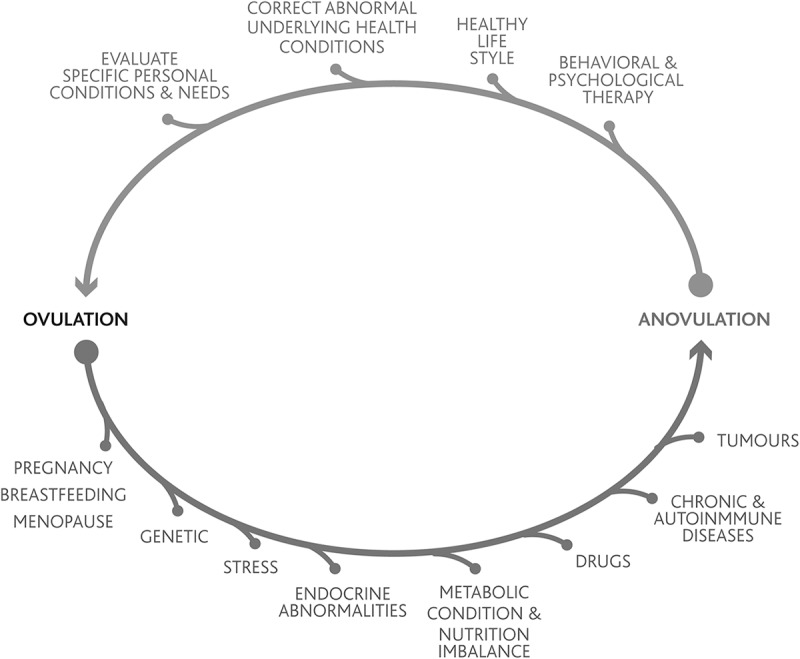
Conditions that can be identified and/or prevented during a woman’s lifetime when understanding the ovarian continuum and ovulation as a sign of health.
Endocrine disorders are predominantly associated with irregular menstrual cycles and are the most common cause of ovulatory dysfunction (Vigil et al. 1993). Normal menstrual patterns do not guarantee that ovulation is occurring since it has been shown that normal length cycles may be anovulatory. For this reason, the use of biomarkers that help women to identify ovulation is essential. This is knowledge that every woman and every healthcare provider should have.
It is also worth noting that ovulatory dysfunction in women usually has its origin early in her life; and, if not diagnosed and treated, it normally worsens with adulthood. Educating younger girls in schools, from puberty on, about identifying when they are ovulating will be of great value to them throughout their life (Vigil et al. 2008). Tracking ovulation biomarkers is a reliable method for managing health and fertility as well as identifying abnormal patterns that may point to disorders needing treatment.
Interestingly, Carl Djerassi, a pioneer in the development of contraceptives, wrote that, “Eventually, many a woman in our affluent society may conclude that the determination of when and whether she is ovulating should be a routine item of personal health information to which she is entitled as a matter of course” (Djerassi 1990).
Biographies
Pilar Vigil, M.D., Ph.D., FACOG, is associate professor at the Pontificia Universidad Católica de Chile, Santiago, and medical director of the Reproductive Health Research Institute (RHRI), Santiago, Chile. In addition, Dr. Vigil is president of Teen STAR International.
Carolina Lyon, N.T., is a nurse practitioner and midwife and researcher at RHRI.
Betsi Flores is a biochemist and worked as a researcher at RHRI.
Hernán Rioseco, M.D., is a physician and researcher at RHRI.
Felipe Serrano, M.Sc., is a biologist and research director at RHRI.
References
- Amer S. 2009. Polycystic ovarian syndrome: Diagnosis and management of related infertility. Obstetrics, Gynaecology and Reproductive Medicine 19: 263–70. [Google Scholar]
- Araujo-Lopes R., Crampton J.R., Aquino N.S., Miranda R.M., Kokay I.C., Reis A.M., Franci C.R., Grattan D.R., and Szawka R.E.. 2014. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 155: 1010–20. [DOI] [PubMed] [Google Scholar]
- Barron M.L. 2004. Proactive management of menstrual cycle abnormalities in young women. Journal of Perinatal and Neonatal Nursing 18: 81–92. [DOI] [PubMed] [Google Scholar]
- Billings E.L., Brown J.B., Billings J.J., and Burger H.G.. 1972. Symptoms and hormonal changes accompanying ovulation. Lancet 1: 282–84. [DOI] [PubMed] [Google Scholar]
- Blackwell L.F., J.B. Brown P. Vigil B. Gross S. Sufi, and d’Arcangues C.. 2003. Hormonal monitoring of ovarian activity using the Ovarian Monitor, part I. Validation of home and laboratory results obtained during ovulatory cycles by comparison with radioimmunoassay. Steroids 68: 465–76. [DOI] [PubMed] [Google Scholar]
- Blackwell L.F., P. Vigil D. Cooke G., d’Arcangues C., and Brown J.B.. 2013. Monitoring of ovarian activity by daily measurement of urinary excretion rates of oestrone glucuronide and pregnanediol glucuronide using the Ovarian Monitor, Part III: Variability of normal menstrual cycle profiles. Human Reproduction 28: 3306–15. [DOI] [PubMed] [Google Scholar]
- Block E. 1952. Quantitative morphological investigations of the follicular system in women; Variations at different ages. Acta Anatomica (Basel) 14: 108–23. [DOI] [PubMed] [Google Scholar]
- Brown J.B. 2011. Types of ovarian activity in women and their significance: The continuum (a reinterpretation of early findings). Human Reproduction Update 17: 141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Blackwell L., and Cooke D.. 2017. Online fertility monitoring: Some of the issues. International Journal of Open Information Technologies 5: 85–91. [Google Scholar]
- Chretien F.C., and Dubois R.. 1991. Effect of nomegestrol acetate on spinability, ferning and mesh dimension of midcycle cervical mucus. Contraception 43: 55–65. [DOI] [PubMed] [Google Scholar]
- Clarke H., Dhillo W.S., and Jayasena C.N.. 2015. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinology & Metabolism (Seoul) 30: 124–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés M.E., Carrera B., Rioseco H., del Rio J.P., and Vigil P.. 2015. The role of Kisspeptin in the onset of puberty and in the ovulatory mechanism: A mini-review. Journal of Pediatric Adolescent Gynecology 28: 286–91. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. 2006. Insulin resistance in PCOS. Endocrine 30: 13–17. [DOI] [PubMed] [Google Scholar]
- Djerassi C. 1990. Fertility awareness: Jet-age rhythm method? Science 248: 1061–62. [DOI] [PubMed] [Google Scholar]
- Duane M., Contreras A., Jensen E.T., and White A.. 2016. The performance of fertility awareness-based method apps marketed to avoid pregnancy. Journal of the American Board of Family Medicine 29: 508–11. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Aschkenasi C., Lee C., Kelly J., Ahima R.S., Bjorbaek C., Flier J.S., Saper C.B., and Elmquist J.K.. 1999. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23: 775–86. [DOI] [PubMed] [Google Scholar]
- Erichsen M.M., Husebye E.S., Michelsen T.M., Dahl A.A., and Lovas K.. 2010. Sexuality and fertility in women with Addison’s disease. Journal of Clinical Endocrinology & Metabolism 95: 4354–60. [DOI] [PubMed] [Google Scholar]
- Erichsen M.M., Lovas K., Skinningsrud B., Wolff A.B., Undlien D.E., Svartberg J., Fougner K.J., et al. 2009. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: Observations from a Norwegian registry. Journal of Clinical Endocrinology & Metabolism 94: 4882–90. [DOI] [PubMed] [Google Scholar]
- Fauser B.C., Tarlatzis B.C., Rebar R.W., Legro R.S., Balen A.H., Lobo R., Carmina E., et al. 2012. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility and Sterility 97: 28–38 e25. [DOI] [PubMed] [Google Scholar]
- Ferin M., Van Vugt D., and Wardlaw S.. 1984. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Progress in Hormone Research 40: 441–85. [DOI] [PubMed] [Google Scholar]
- Fraser I.S., Critchley H.O., Munro M.G., and Broder M.. 2007. Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding? Human Reproduction 22: 635–43. [DOI] [PubMed] [Google Scholar]
- Gleicher N., Kim A., Weghofer A., Kushnir V.A., Shohat-Tal A., Lazzaroni E., Lee H.J., and Barad D.H.. 2013. Hypoandrogenism in association with diminished functional ovarian reserve. Human Reproduction 28: 1084–91. [DOI] [PubMed] [Google Scholar]
- Harlow S.D., Gass M., Hall J.E., Lobo R., Maki P., Rebar R.W., Sherman S., Sluss P.M., de Villiers T.J., and Group Straw Collaborative. 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 19: 387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins S.M., and Matzuk M.M.. 2008. The menstrual cycle: Basic biology. Annals of the New York Academy of Science 1135: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Nawata H., Maki T., Higashizima M., Kato K., and Ibayashi H.. 1984. Prolactin has a direct effect on adrenal androgen secretion. Journal of Clinical Endocrinology & Metabolism 59: 714–18. [DOI] [PubMed] [Google Scholar]
- Irani M., and Merhi Z.. 2014. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertility & Sterility 102: 460–68e3. [DOI] [PubMed] [Google Scholar]
- Johansson G.G., Karonen S.L., and Laakso M.L.. 1983. Reversal of an elevated plasma level of prolactin during prolonged psychological stress. Acta Physiologica Scandinavica 119: 463–64. [DOI] [PubMed] [Google Scholar]
- Jukic A.M., Weinberg C.R., Wilcox A.J., McConnaughey D.R., Hornsby P., and Baird D.D.. 2008. Accuracy of reporting of menstrual cycle length. American Journal of Epidemiology 167: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus H., and Martin J.L.. 1989. Recognition of ovulatory/anovulatory cycle pattern in adolescents by mucus self-detection. Journal of Adolescent Health Care 10: 93–96. [DOI] [PubMed] [Google Scholar]
- Koike K., Miyake A., Aono T., Sakumoto T., Ohmichi M., Yamaguchi M., and Tanizawa O.. 1991. Effect of prolactin on the secretion of hypothalamic GnRH and pituitary gonadotropins. Hormone Research 35, Suppl 1: 5–12. [DOI] [PubMed] [Google Scholar]
- Kolstad H.A., Bonde J.P., Hjollund N.H., Jensen T.K., Henriksen T.B., Ernst E., Giwercman A., Skakkebaek N.E., and Olsen J.. 1999. Menstrual cycle pattern and fertility: A prospective follow-up study of pregnancy and early embryonal loss in 295 couples who were planning their first pregnancy. Fertility & Sterility 71: 490–96. [DOI] [PubMed] [Google Scholar]
- Krasnow J.S., Hickey G.J., and Richards J.S.. 1990. Regulation of aromatase mRNA and estradiol biosynthesis in rat ovarian granulosa and luteal cells by prolactin. Molecular Endocrinology 4: 13–21. [DOI] [PubMed] [Google Scholar]
- Krassas G.E., Pontikides N., Kaltsas T., Papadopoulou P., and Batrinos M.. 1994. Menstrual disturbances in thyrotoxicosis. Clinical Endocrinology (Oxf) 40: 641–44. [DOI] [PubMed] [Google Scholar]
- Krassas G.E., Pontikides N., Kaltsas T., Papadopoulou P., Paunkovic J., Paunkovic N., and Duntas L.H.. 1999. Disturbances of menstruation in hypothyroidism. Clinical Endocrinology (Oxf) 50: 655–59. [DOI] [PubMed] [Google Scholar]
- Laven J.S., and Fauser B.C.. 2004. Inhibins and adult ovarian function. Molecular and Cellular Endocrinology 225: 37–44. [DOI] [PubMed] [Google Scholar]
- Lerchbaum E., and Obermayer-Pietsch B.. 2012. Vitamin D and fertility: A systematic review. European Journal of Endocrinology 166: 765–78. [DOI] [PubMed] [Google Scholar]
- Lobo R. 2014. Menopause and aging In Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 7th ed., 325–356. Philadelphia: Elsevier/Saunders. [Google Scholar]
- Lunenfeld B., and Insler V.. 1993. Follicular development and its control. Gynecology and Endocrinology 7: 285–91. [DOI] [PubMed] [Google Scholar]
- Malcolm C.E., and Cumming D.C.. 2003. Does anovulation exist in eumenorrheic women? Obstetrics and Gynecology 102: 317–18. [DOI] [PubMed] [Google Scholar]
- Menárguez M., Pastor L.M., and Odeblad E.. 2003. Morphological characterization of different human cervical mucus types using light and scanning electron microscopy. Human Reproduction 18: 1782–89. [DOI] [PubMed] [Google Scholar]
- Merke D.P., and Bornstein S.R.. 2005. Congenital adrenal hyperplasia. Lancet 365: 2125–36. [DOI] [PubMed] [Google Scholar]
- Miro F., and Aspinall L.J.. 2005. The onset of the initial rise in follicle-stimulating hormone during the human menstrual cycle. Human Reproduction 20: 96–100. [DOI] [PubMed] [Google Scholar]
- Misao R., Nakanishi Y., Iwagaki S., Fujimoto J., and Tamaya T.. 1998. Expression of progesterone receptor isoforms in corpora lutea of human subjects: Correlation with serum oestrogen and progesterone concentrations. Molecular Human Reproduction 4: 1045–52. [DOI] [PubMed] [Google Scholar]
- Mondal S., Saha I., Das S., Ganguly A., Das D., and Tripathi S.K.. 2013. A new logical insight and putative mechanism behind fluoxetine-induced amenorrhea, hyperprolactinemia and galactorrhea in a case series. Therapeutic Advances in Psychopharmacology 3: 322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta P.M., Makabe S., and Nottola S.A.. 1997. The ultrastructure of human reproduction. I. The natural history of the female germ cell: Origin, migration and differentiation inside the developing ovary. Human Reproduction Update 3: 281–95. [DOI] [PubMed] [Google Scholar]
- Nader, S 2000. Case Studies in Reproductive Endocrinology. London: Arnold. [Google Scholar]
- Nassaralla C.L., Stanford J.B., Daly K.D., Schneider M., Schliep K.C., and Fehring R.J.. 2011. Characteristics of the menstrual cycle after discontinuation of oral contraceptives. Journal of Womens Health (Larchmt) 20: 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New M.I. 2004. An update of congenital adrenal hyperplasia. Annals of the New York Academy of Science 1038: 14–43. [DOI] [PubMed] [Google Scholar]
- Orbach H., and Shoenfeld Y.. 2007. Hyperprolactinemia and autoimmune diseases. Autoimmunity Reviews 6: 537–42. [DOI] [PubMed] [Google Scholar]
- Pauli J.M., Raja-Khan N., Wu X., and Legro R.S.. 2011. Current perspectives of insulin resistance and polycystic ovary syndrome. Diabetic Medicine 28: 1445–54. [DOI] [PubMed] [Google Scholar]
- Peters H., Byskov A.G., and Grinsted J.. 1978. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clinics in Endocrinology and Metabolism 7: 469–85. [DOI] [PubMed] [Google Scholar]
- Popat V.B., Prodanov T., Calis K.A., and Nelson L.M.. 2008. The menstrual cycle: A biological marker of general health in adolescents. Annals of the New York Academy of Science 1135: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe K., Velkeniers B., and Glinoer D.. 2007. Thyroid disease and female reproduction. Clinical Endocrinology (Oxf) 66: 309–21. [DOI] [PubMed] [Google Scholar]
- Prior J.C., Naess M., Langhammer A., and Forsmo S.. 2015. Ovulation prevalence in women with spontaneous normal-length menstrual cycles - A population-based cohort from HUNT3, Norway. PLoS One 10: e0134473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinha S., Joham A.E., Norman R.J., Shaw J.E., Zoungas S., Boyle J., Moran L., and Teede H.J.. 2015. The association between Polycystic Ovary Syndrome (PCOS) and metabolic syndrome: A statistical modelling approach. Clinical Endocrinology (Oxf) 83: 879–87. [DOI] [PubMed] [Google Scholar]
- Rocca W.A., Grossardt B.R., Miller V.M., Shuster L.T., and Brown R.D. Jr.. 2012. Premature menopause or early menopause and risk of ischemic stroke. Menopause 19: 272–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A. 2002. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. Journal of Neuroendocrinology 14: 796–804. [DOI] [PubMed] [Google Scholar]
- Saketos M., Sharma N., and Santoro N.F.. 1993. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biology of Reproduction 49: 1270–1276. [DOI] [PubMed] [Google Scholar]
- Shuster L.T., Rhodes D.J., Gostout B.S., Grossardt B. R., and W.A. Rocca. 2010. Premature menopause or early menopause: long-term health consequences. Maturitas 65: 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.K. 2005. Regulation of primordial follicle assembly and development. Human Reproduction Update 11: 461–71. [DOI] [PubMed] [Google Scholar]
- Stanhope R., and Brook C.G.. 1986. Clinical diagnosis of disorders of puberty. British Journal of Hospital Medicine 35: 57–58. [PubMed] [Google Scholar]
- Stouffer R.L. 2003. Progesterone as a mediator of gonadotrophin action in the corpus luteum: Beyond steroidogenesis. Human Reproduction Update 9: 99–117. [DOI] [PubMed] [Google Scholar]
- Svejme O., Ahlborg H.G., Nilsson J.A., and Karlsson M.K.. 2012. Early menopause and risk of osteoporosis, fracture and mortality: A 34-year prospective observational study in 390 women. BJOG: An International Journal of Obstetrics and Gynaecology 119: 810–16. [DOI] [PubMed] [Google Scholar]
- Unuane D., Tournaye H., Velkeniers B., and Poppe K.. 2011. Endocrine disorders & female infertility. Best Practice & Research: Clinical Endocrinology & Metabolism 25: 861–73. [DOI] [PubMed] [Google Scholar]
- van Hooff M.H., Voorhorst F.J., Kaptein M.B., Hirasing R.A., Koppenaal C., and Schoemaker J.. 2004. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Human Reproduction 19: 383–92. [DOI] [PubMed] [Google Scholar]
- Vigil P. 2004. La fertilidad de la pareja humana. 3. ed, Lecciones Santiago, Chile: Ediciones Universidad Católica de Chile. [Google Scholar]
- Vigil P., Blackwell L., and Cortes M.E.. 2012. The Importance of Fertility Awareness in the Assessment of a Woman’s Health. Linacre Quarterly 4: 426–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil P., Ceric F., Cortes M.E., and Klaus H.. 2006. Usefulness of monitoring fertility from menarche. Journal of Pediatric Adolescent Gynecology 19: 173–79. [DOI] [PubMed] [Google Scholar]
- Vigil P., Contreras P., Alvarado J.L., Godoy A., Salgado A.M., and Cortes M.E.. 2007. Evidence of subpopulations with different levels of insulin resistance in women with polycystic ovary syndrome. Human Reproduction 22: 2974–80. [DOI] [PubMed] [Google Scholar]
- Vigil P., Cortés M.E., Zuniga A., Riquelme J., and Ceric F.. 2009. Scanning electron and light microscopy study of the cervical mucus in women with polycystic ovary syndrome. Journal of Electron Microscopy (Tokyo) 58: 21–27. [DOI] [PubMed] [Google Scholar]
- Vigil P., Croxatto H., and Cortés M.E.. 2014. Ciclo menstrual In Ginecologia. Edited by Sanchez Alfredo Perez, 4 ed. Santiago: Mediterraneo. [Google Scholar]
- Vigil P., del Río M., Socías M., González A., and Honeyman J.. 2007. Cellular immunity alterations in hyperprolactinemia. Acta Cytologica 51: 2. [Google Scholar]
- Vigil P., Orellana R., del Río M., and Cortés M.. 2008. Educación en afectividad y sexualidad para adolescentes: Resultados de la implementación del programa teen STAR. Ars Médica 17: 19. [Google Scholar]
- Vigil P., Rodríguez-Rigau L., Palacios X., Kauak S., and Morales P.. 1993. Diagnosis of menstrual disorders in adolescence In Reproductive Medicine. Edited by Frajese G., Steinberger E., and Rodríguez-Rigau L.J., 149–54. New York: Raven Press. [Google Scholar]