Abstract
During transcription initiation, RNA polymerase (RNAP) binds to promoter DNA, unwinds promoter DNA to form an RNAP-promoter open complex (RPo) containing a single-stranded ‘transcription bubble,’ and selects a transcription start site (TSS). TSS selection occurs at different positions within the promoter region, depending on promoter sequence and initiating-substrate concentration. Variability in TSS selection has been proposed to involve DNA ‘scrunching’ and ‘anti-scrunching,’ the hallmarks of which are: (i) forward and reverse movement of the RNAP leading edge, but not trailing edge, relative to DNA, and (ii) expansion and contraction of the transcription bubble. Here, using in vitro and in vivo protein-DNA photocrosslinking and single-molecule nanomanipulation, we show bacterial TSS selection exhibits both hallmarks of scrunching and anti-scrunching, and we define energetics of scrunching and anti-scrunching. The results establish the mechanism of TSS selection by bacterial RNAP and suggest a general mechanism for TSS selection by bacterial, archaeal, and eukaryotic RNAP.
Research organism: E. coli
eLife digest
Genes store the information needed to build and repair cells. This information is written in a chemical code within the structure of DNA molecules. To make use of the information, cells copy sections of a gene into a DNA-like molecule called RNA. An enzyme called RNA polymerase makes RNA molecules from DNA templates in a process called transcription. RNA polymerase can only make RNA by attaching to DNA and separating the two strands of the DNA double helix. This creates a short region of single-stranded DNA known as a “transcription bubble”.
RNA polymerase can start transcription at different distances from the sites where it initially attaches to DNA, depending on the DNA sequence and the cell’s environment. It had not been known how RNA polymerase selects different transcription start sites in different cases. One hypothesis had been that differences in the size of the transcription bubble – the amount of unwound single-stranded DNA – could be responsible for differences in transcription start sites. For example, RNA polymerase could increase the size of the bubble through a process called “DNA scrunching”, in which RNA polymerase pulls in and unwinds extra DNA from further along the gene.
Yu, Winkelman et al. looked for indicators of DNA scrunching to see whether it contributes to the selection of transcription start sites. By mapping the positions of the two edges of RNA polymerase relative to DNA, they saw that RNA polymerase pulls in extra DNA when selecting a transcription start site further from its initial attachment site. Next, by measuring the amount of DNA unwinding, they saw that RNA polymerase unwinds extra DNA when it selects a transcription start site further from its initial attachment site. This was the case for both RNA polymerase in a test tube and RNA polymerase in living bacterial cells. The results showed that DNA scrunching accounts for known patterns of selection of transcription start sites.
The findings hint at a common theory for the selection of transcription start sites across all life by DNA scrunching. Understanding these basic principles of biology reveals more about how cells work and how cells adapt to changing conditions. The experimental methods developed for mapping the positions of proteins on DNA and for measuring DNA unwinding will help scientists to learn more about other aspects of how DNA is stored, copied, read, and controlled.
Introduction
During transcription initiation, RNA polymerase (RNAP) and one or more transcription initiation factor bind to promoter DNA through sequence-specific interactions with core promoter elements, unwind a turn of promoter DNA to form an RNAP-promoter open complex (RPo) containing an unwound ‘transcription bubble,’ and select a transcription start site (TSS). The distance between core promoter elements and the TSS can vary. TSS selection is a multi-factor process, in which the outcome reflects the contributions of promoter sequence and reaction conditions. TSS selection by bacterial RNAP and the bacterial transcription initiation factor σ involves four promoter-sequence determinants: (i) distance relative to the promoter −10 element (preference for TSS selection at the position 7 bp downstream of the promoter −10 element; Aoyama and Takanami, 1985; Sørensen et al., 1993; Jeong and Kang, 1994; Liu and Turnbough, 1994; Walker and Osuna, 2002; Lewis and Adhya, 2004; Vvedenskaya et al., 2015; Winkelman et al., 2016a; Winkelman et al., 2016b); (ii) identities of the template-strand nucleotide at the TSS and the template-strand nucleotide immediately upstream of the TSS (strong preference for a template-strand pyrimidine at the TSS and preference for a template-strand purine immediately upstream of the TSS; Aoyama and Takanami, 1985; Sørensen et al., 1993; Jeong and Kang, 1994; Liu and Turnbough, 1994; Walker and Osuna, 2002; Lewis and Adhya, 2004; Vvedenskaya et al., 2015; Winkelman et al., 2016a; Winkelman et al., 2016b); (iii) the promoter ‘core recognition element,’ a segment of nontemplate-strand sequence spanning the TSS that interacts sequence-specifically with RNAP (preference for nontemplate-strand G immediately downstream of the TSS; Vvedenskaya et al., 2016), and (iv) the promoter ‘discriminator element,’ a nontemplate-strand sequence immediately downstream of the promoter −10 element that interacts sequence-specifically with σ (preference for TSS selection at upstream positions for purine-rich discriminator sequences, and preference for TSS selection at downstream positions for pyrimidine-rich discriminator sequences; Winkelman et al., 2016a, 2016b). In addition to these four promoter-sequence determinants, the concentrations of initiating NTPs (Sørensen et al., 1993; Liu and Turnbough, 1994; Walker and Osuna, 2002; Vvedenskaya et al., 2015; Wilson et al., 1992; Qi and Turnbough, 1995; Tu and Turnbough, 1997; Walker et al., 2004; Turnbough, 2008; Turnbough and Switzer, 2008) and DNA topology (Vvedenskaya et al., 2015) also influence TSS selection.
It has been hypothesized that variability in the distance between core promoter elements and the TSS is accommodated by DNA ‘scrunching’ and ‘anti-scrunching,’ the defining hallmarks of which are: (i) forward and reverse movements of the RNAP leading edge, but not the RNAP trailing edge, relative to DNA, and (ii) expansion and contraction of the transcription bubble (Vvedenskaya et al., 2015; Winkelman et al., 2016a, 2016b; Vvedenskaya et al., 2016; Robb et al., 2013). In previous work, we showed that TSS selection exhibits the first hallmark of scrunching in vitro (Winkelman et al., 2016a). Here, we show that TSS selection also exhibits the first hallmark of scrunching in vivo, show that TSS selection exhibits the second hallmark of scrunching and anti-scrunching, and define the energetics of scrunching and anti-scrunching.
Results and discussion
TSS selection exhibits first hallmark of scrunching--movements of RNAP leading edge but not RNAP trailing edge--both in vitro and in vivo
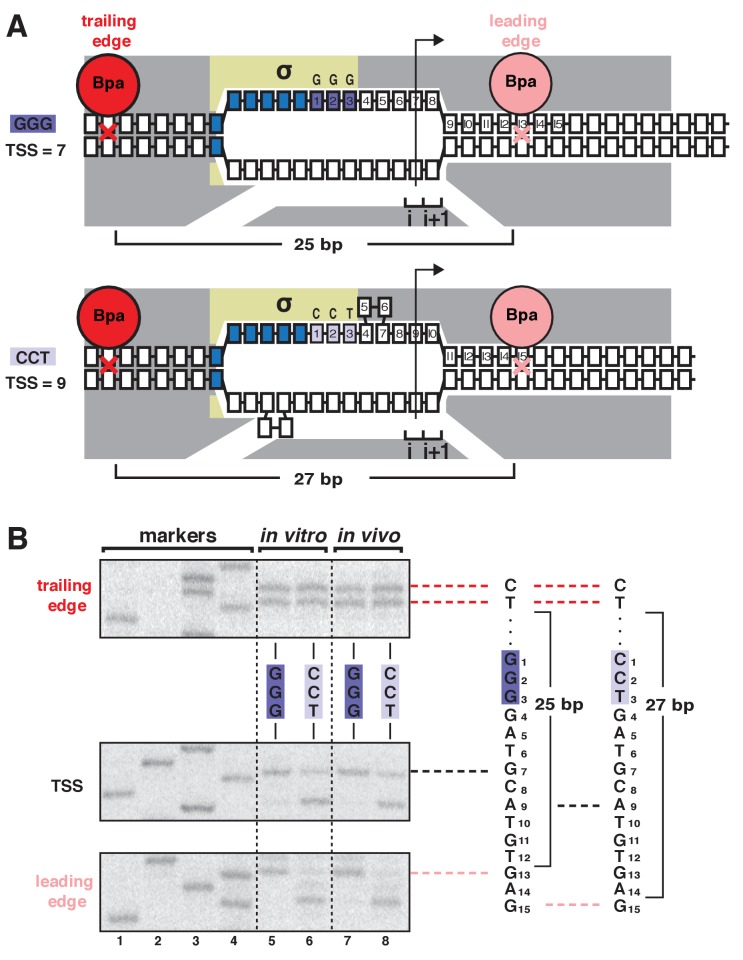
In our prior work, we demonstrated that bacterial TSS selection in vitro exhibits the first hallmark of scrunching by defining, simultaneously, the TSS, the RNAP leading-edge position, and RNAP trailing-edge position for transcription complexes formed on a library of 106 promoter sequences (Winkelman et al., 2016a). We used RNA-seq to define the TSS, and we used unnatural-amino-acid-mutagenesis, incorporating the photoactivatable amino acid p-benzoyl-L-phenylalanine (Bpa), and protein-DNA photocrosslinking to define RNAP-leading-edge and trailing-edge positions (Winkelman et al., 2016a). The results showed that the discriminator element (Haugen et al., 2006; Feklistov et al., 2006) influences TSS selection and does so through effects on sequence-specific σ-DNA interaction that select between two alternative paths of the DNA nontemplate strand (Winkelman et al., 2016a). The results further showed that, as the TSS changes for different discriminator sequences, the RNAP-leading-edge position changes, but the RNAP-trailing-edge position does not change (Winkelman et al., 2016a). For example, replacing a GGG discriminator by a CCT discriminator causes a 2 bp downstream change in TSS (from the position 7 bp downstream of the −10 element to the position 9 bp downstream of the −10 element, due to differences in sequence-specific σ-DNA interaction that result in different paths of the DNA nontemplate strand), causes a 2 bp downstream change in RNAP leading-edge position, but does not cause a change in RNAP trailing-edge position (Figure 1A).
Figure 1. TSS selection exhibits first hallmark of scrunching--movement of RNAP leading edge but not RNAP trailing edge--both in vitro and in vivo.
(A) RNAP leading-edge and trailing-edge positions at promoters having GGG discriminator (TSS 7 bp downstream of −10 element; top) and CCT discriminator (TSS 9 bp downstream of −10 element; bottom). Changes in TSS selection result from changes in discriminator-sequence-dependent DNA scrunching. Gray, RNAP; yellow, σ; blue, −10-element nucleotides; dark purple, GGG-discriminator nucleotides; light purple, CCT-discriminator nucleotides; i and i + 1, NTP binding sites; arrow, TSS; boxes, DNA nucleotides (nontemplate-strand nucleotides above template-strand nucleotides; nucleotides downstream of −10 element numbered); red, trailing-edge Bpa and nucleotide crosslinked to Bpa; pink, leading-edge Bpa and nucleotide crosslinked to Bpa. Scrunching is indicated by bulged-out nucleotides. Distance between leading-edge and trailing-edge crosslinks is indicated below RNAP. (B) RNAP trailing-edge crosslinking (top), TSS (middle), and RNAP leading-edge crosslinking (bottom) for promoters having GGG discriminator and CCT discriminator, in vitro (lanes 5–6) and in vivo (lanes 7–8). Horizontal dashed lines relate bands on gel (left) to nucleotide sequences (right).
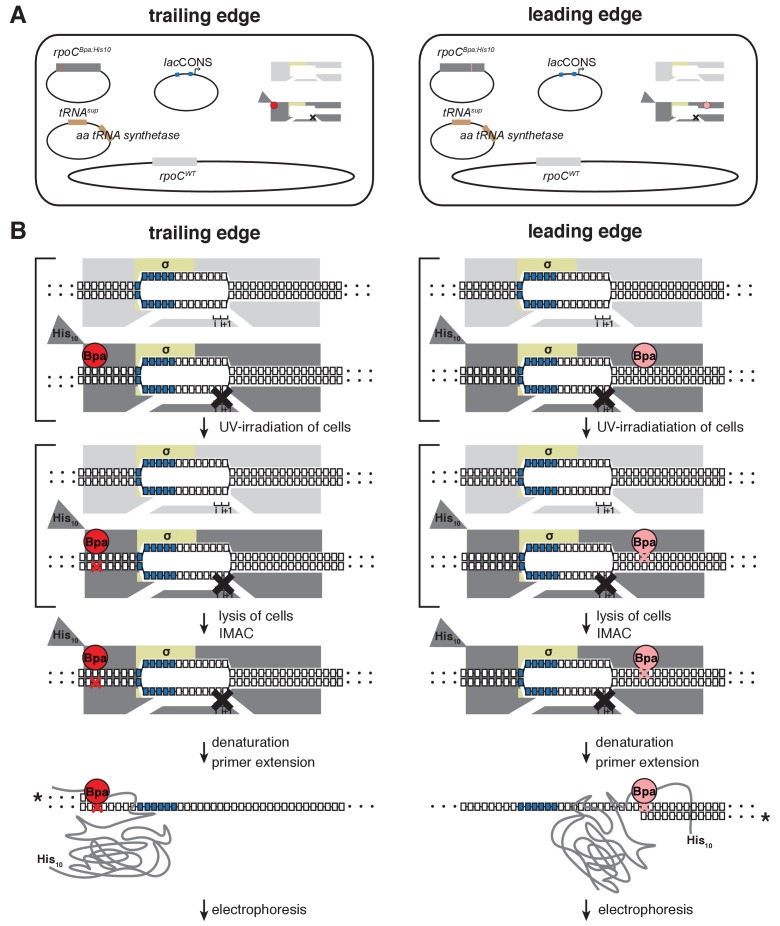
Figure 1—figure supplement 1. Unnatural-amino-acid mutagenesis and protein-DNA photocrosslinking in vivo.
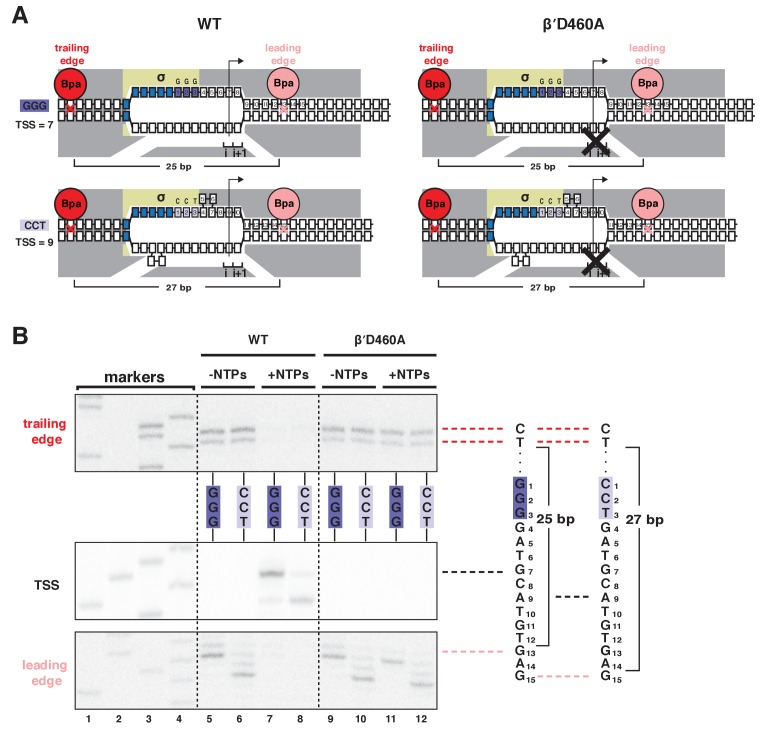
Figure 1—figure supplement 2. Mutationally inactivated RNAP derivative traps RPo in presence of NTPs, enabling protein-DNA photocrosslinking of RPo in presence of NTPs.
Here, to determine whether bacterial TSS selection in vivo also exhibits the first hallmark of scrunching, we adapted the above unnatural-amino-acid-mutagenesis and protein-DNA-photocrosslinking procedures to define RNAP leading-edge and trailing-edge positions in TSS selection in living cells (Figure 1, Figure 1—figure supplements 1–2). We developed approaches to assemble, trap, and UV-irradiate RPo formed by a Bpa-labeled RNAP derivative in living cells, to extract crosslinked material from cells, and and to map crosslinks at single-nucleotide resolution (Figure 1—figure supplement 1). In order to assemble, trap, and UV-irradiate RPo in living cells, despite the presence of high concentrations of initiating substrates that rapidly convert RPo into transcribing complexes, we used a mutationally inactivated RNAP derivative, β’D460A, that lacks a residue required for binding of the RNAP-active-center catalytic metal ion and initiating substrates (Zaychikov et al., 1996) (Figure 1—figure supplements 1–2). Control experiments confirm that, in vitro, in both the absence and presence of initiating substrates, the mutationally inactivated RNAP derivative remains trapped in RPo, exhibiting the same pattern of leading-edge and trailing-edge crosslinks as for wild-type RNAP in the absence of initiating substrates (Figure 1—figure supplement 2). In order to introduce Bpa at the leading-edge and trailing-edge of RPo in living cells, we co-produced, in Escherichia coli, a Bpa-labeled, decahistidine-tagged, mutationally inactivated RNAP derivative in the presence of unlabeled, untagged, wild-type RNAP, using a three-plasmid system comprising (i) a plasmid carrying a gene for RNAP β’ subunit that contained a nonsense codon at the site for incorporation of Bpa, the βʹD460A mutation, and a decahistidine coding sequence; (ii) a plasmid carrying genes for an engineered Bpa-specific nonsense-suppressor tRNA and an engineered Bpa-specific aminoacyl-tRNA synthase (Chin et al., 2002); and (iii) a plasmid containing a promoter of interest (Figure 1—figure supplement 1A). (Using this merodiploid system, with both a plasmid-borne mutant gene for βʹ subunit and a chromosomal wild-type gene for βʹ subunit, enabled analysis of the mutationally inactivated RNAP derivative without loss of viability.) In order to perform RNAP-DNA crosslinking and to map resulting RNAP-DNA crosslinks, we then grew cells in medium containing Bpa, UV-irradiated cells, lysed cells, purified crosslinked material using immobilized metal-ion-affinity chromatography targeting the decahistidine tag on the Bpa-labeled, decahistidine-tagged, mutationally inactivated RNAP derivative, and mapped crosslinks using primer extension (Figure 1—figure supplement 1B). The results showed an exact correspondence of crosslinking patterns in vitro and in vivo (Figure 1B, ‘in vitro’ vs. ‘in vivo’ lanes). The RNAP leading edge crosslinked 2 bp further downstream on CCT than on GGG, whereas the RNAP trailing edge crosslinked at the same positions on CCT and GGG (Figure 1B). We conclude that TSS selection in vivo shows the first hallmark of scrunching.
TSS selection exhibits second hallmark of scrunching--changes in size of transcription bubble
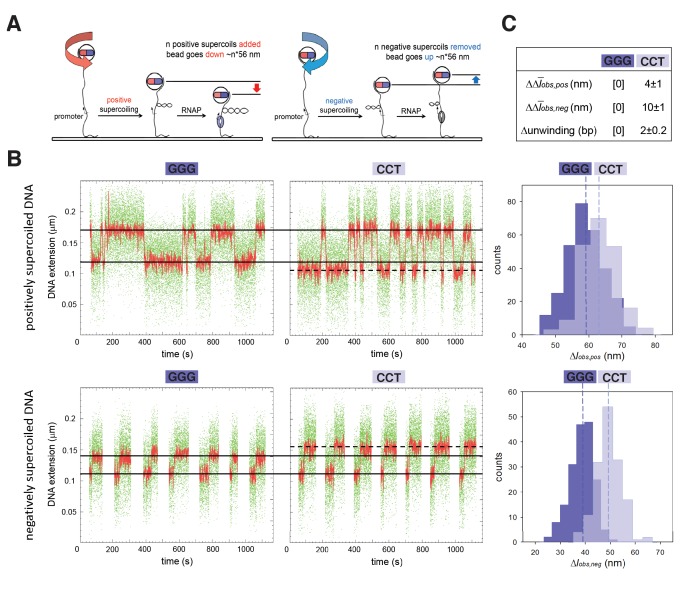
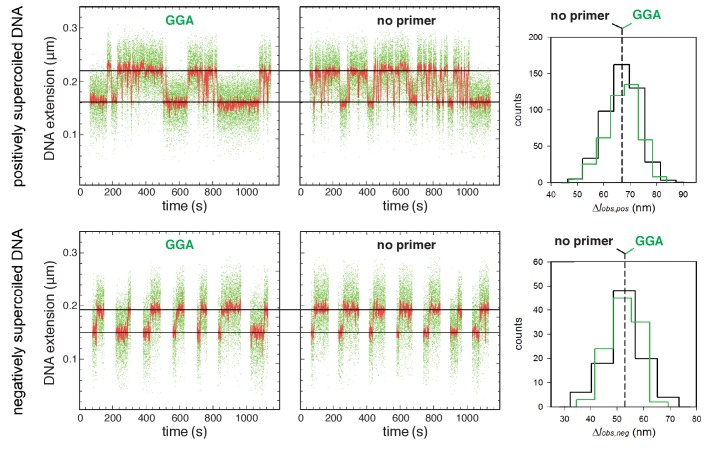
The results in Figure 1 establish that TSS selection in vitro and in vivo exhibits the first hallmark of scrunching. However, definitive demonstration that TSS selection involves scrunching also requires demonstration of the second hallmark of scrunching: that is, changes in transcription-bubble size. To determine whether bacterial TSS selection exhibits the second hallmark of scrunching we used a magnetic-tweezers single-molecule DNA-nanomanipulation assay that enables detection of RNAP-dependent DNA unwinding with near-single-base-pair spatial resolution and sub-second temporal resolution (Revyakin et al., 2004, 2005, 2006) to assess whether TSS selection correlates with transcription-bubble size for GGG and CCT promoters (Figure 2). The results indicate that transition amplitudes for RNAP-dependent DNA unwinding upon formation of RPo with CCT are larger than those for formation of RPo with GGG, on both positively and negatively supercoiled templates (Figure 2B, left and center). Transition-amplitude histograms confirm that transition amplitudes with CCT are larger than with GGG, on both positively and negatively supercoiled templates (Figure 2B, right). By combining the results with positively and negatively supercoiled templates to deconvolve effects of RNAP-dependent DNA unwinding and RNAP-dependent compaction (Revyakin et al., 2004, 2005, 2006), we find a 2 bp difference in RNAP-dependent DNA unwinding for CCT vs. GGG (Figure 2C), corresponding exactly to the 2 bp difference in TSS selection (Figure 1B). We conclude that TSS selection shows the second hallmark of scrunching.
Figure 2. TSS selection exhibits second hallmark of scrunching--change in size of transcription bubble.
(A) Magnetic-tweezers single-molecule DNA nanomanipulation (Revyakin et al., 2004; Revyakin et al., 2005; Revyakin et al., 2006). End-to-end extension (l) of a mechanically stretched, positively supercoiled (left), or negatively supercoiled (right), DNA molecule is monitored. Unwinding of n turns of DNA by RNAP results in compensatory gain of n positive supercoils or loss of n negative supercoils, and movement of the bead by n*56 nm. (B) Single-molecule time traces and transition-amplitude histograms for RPo at promoters having GGG discriminator or CCT discriminator. Upper subpanel, positively supercoiled DNA; lower subpanel, negatively supercoiled DNA. Green points, raw data (30 frames/s); red points, averaged data (1 s window); horizontal black lines, wound and unwound states of GGG promoter; dashed horizontal black line, unwound state of CCT promoter; vertical dashed lines, means; Δlobs,pos, transition amplitude with positively supercoiled DNA; Δlobs,neg, transition amplitude with negatively supercoiled DNA; Δobs,pos, mean Δlobs,pos; Δobs,neg, mean Δlobs,neg. (C) Differences in Δobs,pos, Δobs,neg, and DNA unwinding between GGG-discriminator promoter and CCT-discriminator promoter (means ± SEM).
TSS selection downstream and upstream of modal TSS involves scrunching and anti-scrunching, respectively: forward and reverse movements of RNAP leading edge
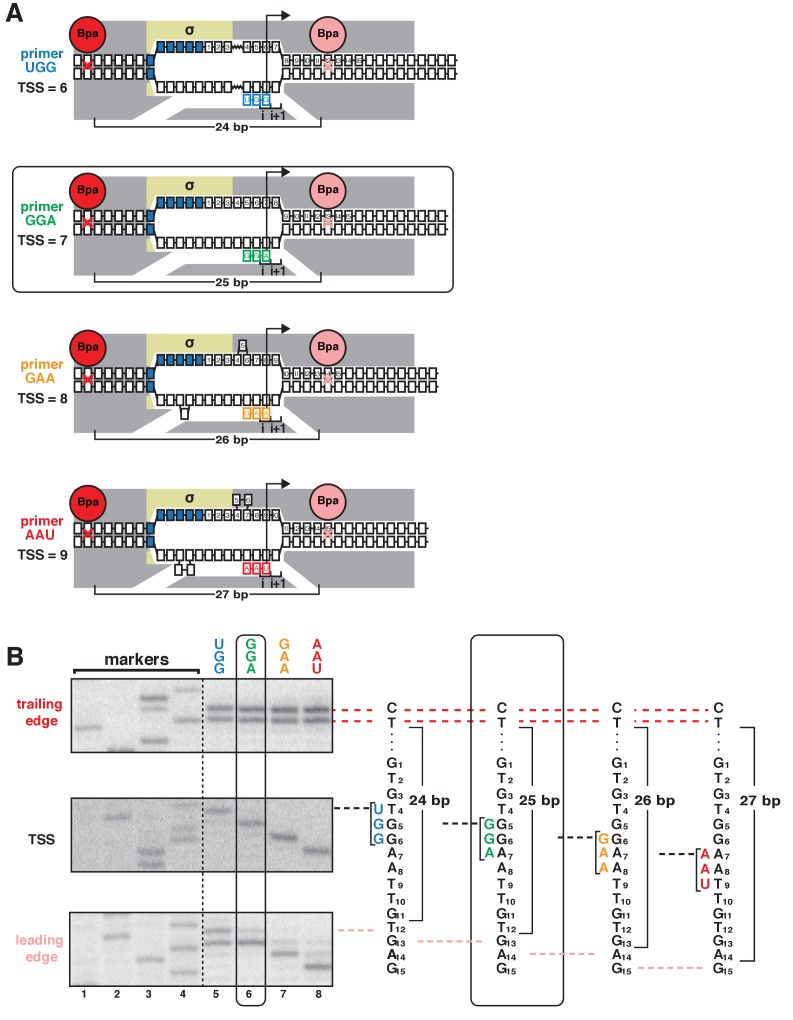
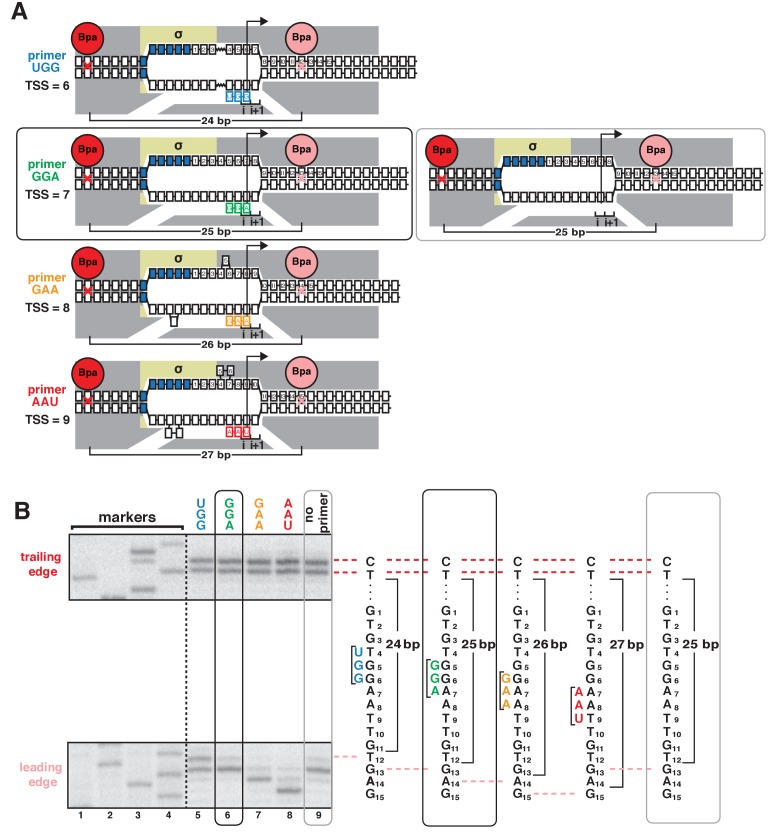
According to the hypothesis that TSS selection involves scrunching or anti-scrunching, TSS selection at the most frequently observed, modal TSS position (7 bp downstream of −10 element for majority of discriminator sequences, including GGG) involves neither scrunching nor anti-scrunching, TSS selection downstream of the modal position involves scrunching (transcription-bubble expansion), and TSS selection upstream of the modal position involves anti-scrunching (Vvedenskaya et al., 2015; Winkelman et al., 2016a, 2016b; Vvedenskaya et al., 2016; Robb et al., 2013). The results in Figures 1–2 apply to the modal TSS position and a TSS position 2 bp downstream of the modal TSS position. To generalize and extend the results to a range of different TSS positions, including a position upstream of the modal TSS position expected to involve anti-scrunching, we exploited the ability of oligoribonucleotide primers (‘nanoRNAs’; Goldman et al., 2011) to program TSS selection (Figure 3A, Figure 3—figure supplement 1). We analyzed a consensus bacterial promoter, lacCONS, and used four ribotrinucleotide primers, UGG, GGA, GAA, and AAU, to program TSS selection at positions 6, 7, 8, and 9 bp downstream of the −10 element (Figure 3A). Experiments analogous to those in Figure 1 show a one-for-one, bp-for-bp correlation between primer-programmed changes in TSS and changes in RNAP-leading-edge position. The leading-edge crosslink positions with the four primers differed in single-nucleotide increments, but the trailing-edge crosslink positions were the same (Figure 3B). With the primer GGA, which programs TSS selection at the modal position (7 bp downstream of −10 element for this discriminator sequence), the leading-edge crosslinks were exactly as in experiments with no primer (Figure 3—figure supplement 1). With primers GAA and AAU, which program TSS selection 1 and 2 bp downstream (positions 8 and 9), leading-edge crosslinks were 1 and 2 bp downstream of crosslinks with GGA (Figure 3B). With primer UGG, which programs TSS selection 1 bp upstream (position 6), leading-edge crosslinks were 1 bp upstream of crosslinks with GGA (Figure 3B). The results show that successive single-base-pair changes in TSS selection are matched by successive single-base-pair changes in RNAP leading-edge position. We conclude that the first hallmark of scrunching is observed for a full range of TSS positions, including, importantly, a position upstream of the modal TSS expected to involve anti-scrunching.
Figure 3. TSS selection downstream and upstream of the modal TSS involves, respectively, forward and reverse movements of RNAP leading edge.
(A) Ribotrinucleotide primers program TSS selection at positions 6, 7, 8, and 9 bp downstream of −10 element (UGG, GGA, GAA, and AAU). Cyan, green, orange, and red denote primers UGG, GGA, GAA, and AAU, respectively. Rectangle with rounded corners highlights case of primer GGA, which programs TSS selection at same position as in absence of primer (7 bp downstream of −10 element). Other colors as in Figure 1A. (B) Use of protein-DNA photocrosslinking to define RNAP leading-edge and trailing-edge positions in vitro. RNAP trailing-edge crosslinking (top), TSS (middle), and RNAP leading-edge crosslinking (bottom) with primers UGG, GGA, GAA, and AAU (lanes 5–8). Horizontal dashed lines relate bands on gel (left) to nucleotide sequences (right).
Figure 3—figure supplement 1. Protein-DNA photocrosslinking in primer-programmed TSS selection: primer GGA yields same pattern of RNAP leading-edge and trailing-edge crosslinking as in absence of primer.
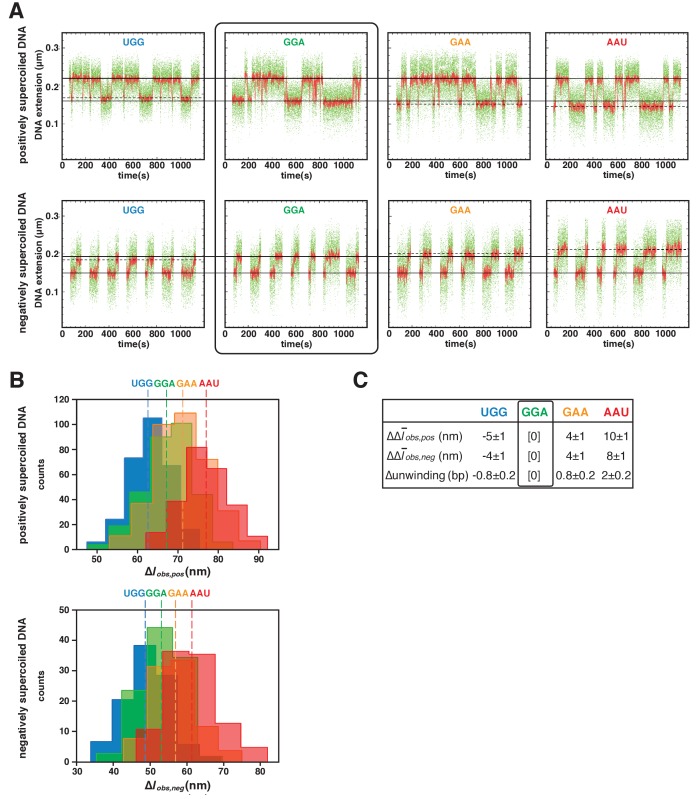
TSS selection downstream and upstream of modal TSS involves scrunching and anti-scrunching, respectively: increases and decreases in RNAP-dependent DNA unwinding
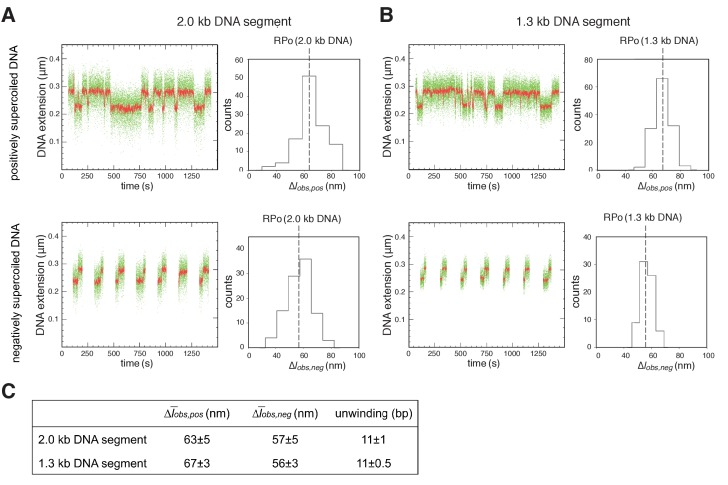
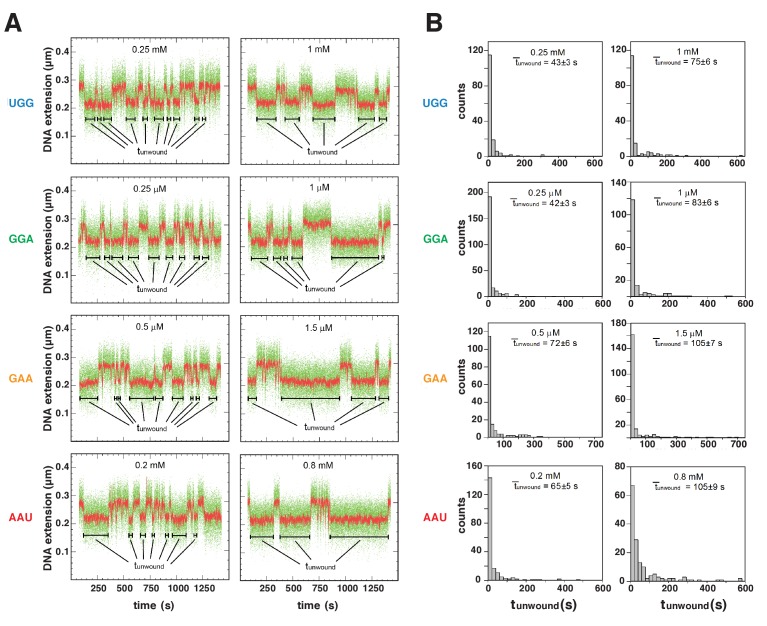
We next used magnetic-tweezers single-molecule DNA-nanomanipulation to analyze primer-programmed TSS selection. To enable single-base-pair resolution, we reduced the DNA-tether length from 2.0 kb to 1.3 kb, thereby reducing noise due to compliance (Figure 4—figure supplement 1; see Revyakin et al., 2005). The resulting transition amplitudes, transition-amplitude histograms, and RNAP-dependent DNA unwinding values for TSS selection with saturating concentrations of the four primers show a one-for-one, base-pair-for-base-pair correlation between primer-programmed changes in TSS and changes in RNAP-dependent DNA unwinding (Figure 4). With primer GGA, which programs TSS selection at the modal position (7 bp downstream of the −10 element for this discriminator sequence), DNA unwinding was exactly as in experiments with no primer (Figure 4—figure supplement 2). With primers GAA and AAU, which program TSS selection 1 and 2 bp further downstream (positions 8 and 9), DNA unwinding was ~1 and ~2 bp greater than with GGA (Figure 4). With primer UGG, which programs TSS selection 1 bp upstream, DNA unwinding was ~1 bp less than that in experiments with GGA (Figure 4). The results show that successive single-base-pair changes in TSS selection are matched by successive single-base-pair changes in DNA unwinding for a full range of TSS positions including, importantly, a position upstream of the modal TSS expected to involve anti-scrunching. Taken together, the results of protein-DNA photocrosslinking (Figure 3, Figure 3—figure supplement 1) and DNA-nanomanipulation (Figure 4, Figure 4—figure supplement 2) demonstrate, definitively, the scrunching/anti-scrunching hypothesis for TSS selection.
Figure 4. TSS selection downstream and upstream of the modal TSS involves, respectively: increases and decreases in RNAP-dependent DNA unwinding.
(A) Use of single-molecule DNA nanomanipulation to define RNAP-dependent DNA unwinding. Single-molecule time traces with primers UGG, GGA, GAA, and AAU (positively supercoiled DNA in upper panel; negatively supercoiled DNA in lower panel). Rectangle with rounded corners highlights case of primer GGA, which programs TSS selection at position 7. Colors as in Figure 2B. (B) Transition-amplitude histograms (positively supercoiled DNA in upper panel; negatively supercoiled DNA in lower panel). (C) Differences in Δobs,pos, Δobs,neg, and DNA unwinding (bottom panel) with primers UGG, GGA, GAA, and AAU (means ± SEM).
Figure 4—figure supplement 1. Single-molecule DNA-nanomanipulation: shorter DNA fragment enables detection of RNAP-dependent DNA unwinding with single-base-pair resolution.
Figure 4—figure supplement 2. Single-molecule DNA-nanomanipulation: analysis of primer-programmed TSS selection with primer GGA.
Energetic costs of scrunching and anti-scrunching
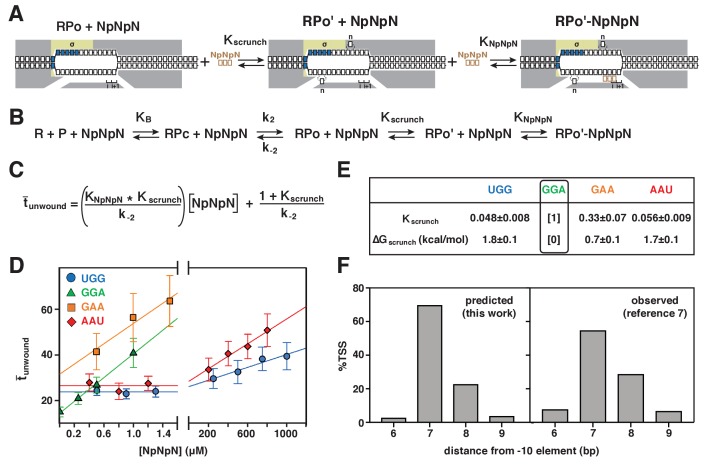
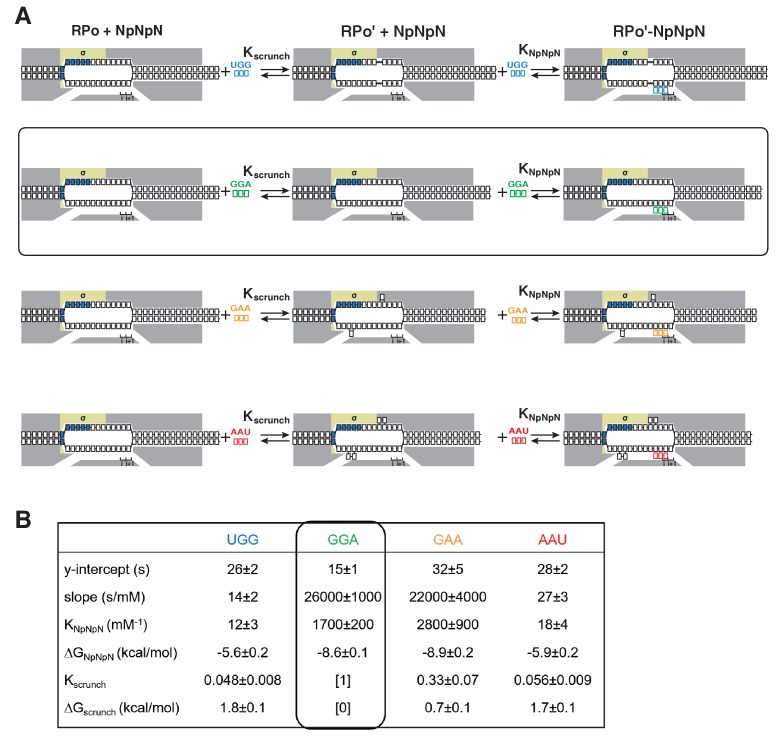
To quantify the energetic costs of scrunching and anti-scrunching, we measured primer-concentration dependences of lifetimes of unwound states (Figures 5–6, Figure 6—figure supplement 1). For each primer, increasing the primer concentration increases the lifetime of the unwound state (tunwound), as expected for coupled equilibria of promoter unwinding, promoter scrunching, and primer binding (Figures 5–6). The results in Figure 6D show that the slopes of plots of mean tunwound (unwound) vs. primer concentration differ for different primers. Fitting the results to the equation describing the coupled equilibria (Figure 6C) yields values of KNpNpN, ΔGNpNpN, Kscrunch, and ΔGscrunch for the four primers (Figure 6E, Figure 6—figure supplement 1). The results indicate that scrunching by 1 bp requires 0.7 kcal/mol, scrunching by 2 bp requires 1.7 kcal/mol, and anti-scrunching by 1 bp requires 1.8 kcal/mol (Figure 6E, Figure 6—figure supplement 1).
Figure 5. Energetic costs of scrunching and anti-scrunching: primer-concentration dependences of unwound-state lifetimes with primers UGG, GGA, GAA, and AAU.
(A) Single-molecule time traces at low (left) and high (right) primer concentrations. Black bars, lifetimes of unwound states (tunwound). Colors as in Figure 2B. (B) tunwound distributions and mean tunwound (unwound). unwound increases with increasing primer concentrations.
Figure 6. Energetic costs of scrunching and anti-scrunching: calculation of energetic costs and relationship between energetic costs and range and relative utilization of TSS positions.
(A) Coupled equilibria for promoter scrunching or anti-scrunching (Kscrunch) and primer binding (KNpNpN). (B) Equations for promoter binding (KB), promoter unwinding (k2/k-2), promoter scrunching or anti-scrunching (Kscrunch), and primer binding (KNpNpN). (C) Relationship between unwound, Kscrunch, KNpNpN, and primer concentration. (D) Dependences of mean lifetimes of unwound states (unwound) on primer concentration for primers UGG, GGA, GAA, and AAU (means ± SEM). (E) Values of Kscrunch and ΔGscrunch calculated by fitting data in (D) to equation in (C) using k-2 = 1/unwound in absence of primer (Revyakin et al., 2004) (means ± SEM). Colors as in Figure 3. (F) TSS distributions predicted by Boltzmann-distribution probabilities for ΔGscrunch values in (E) (left) and observed in analysis of comprehensive library of TSS-region sequences in Vvedenskaya et al., 2015 (right).
Figure 6—figure supplement 1. Single-molecule DNA-nanomanipulation: calculation of Kscrunch and ΔGscrunch in primer-programmed TSS selection with primers UGG, GGA, GAA, and AAU.
The results provide the first experimental determination of the energetic costs of scrunching and anti-scrunching in any context. We hypothesize that energetic costs on the same scale, ~0.7–1.8 kcal/mol per scrunched bp, also apply in the structurally and mechanistically related scrunching that occurs during initial transcription by RNAP (Revyakin et al., 2006; Kapanidis et al., 2006). We note that, according to this hypothesis, the scrunching by ~10 bp that occurs during initial transcription (Revyakin et al., 2006) results in an increase in the state energy of the transcription initiation complex by a total of ~7–18 kcal/mol (~10 x ~0.7–1.8 kcal/mol). This is an increase in state energy potentially sufficient to yield a ‘stressed intermediate’ (Revyakin et al., 2006; Straney and Crothers, 1987) having scrunching-dependent ‘stress’ comparable to the free energies of RNAP-promoter and RNAP-initiation-factor interactions that anchor RNAP at a promoter (~7–9 kcal/mol for sequence-specific component of RNAP-promoter interaction and ~13 kcal/mol for RNAP-initiation-factor interaction; 24) and therefore is an increase in state energy potentially sufficient to pay energetic costs of breaking RNAP-promoter and RNAP-initiation-factor interactions in the transition from transcription initiation to transcription elongation.
Energetic costs of scrunching and anti-scrunching in TSS selection explain range and relative utilization of TSS positions
The ΔGscrunch values for bacterial TSS selection obtained in this work account for the range of TSS positions and the relative utilization of different TSS positions in bacterial transcription initiation. The ΔGscrunch values for TSS selection at positions 6, 7, 8, and 9 of a promoter where the modal TSS is position 7 (0–1.8 kcal/mol) all are less than or comparable to 3kBT (~2 kcal/mol), where kB is the Bolztmann constant and T is temperature in °K, indicating that TSS selection at these positions requires no energy beyond energy available in the thermal bath. Indeed, the probabilities of TSS selection at positions 6, 7, 8, and 9 as observed in a comprehensive analysis of TSS-region sequences (8%, 55%, 29%, 7%; Vvedenskaya et al., 2015) can be predicted from the Boltzmann-distribution probabilities for the ΔGscrunch values for TSS selection at these positions (3%, 70%, 23%, 4%; Figure 6F). The finding that values of ΔGscrunch for scrunching and anti-scrunching in TSS selection are ~1 kcal mol−1 bp−1 and ~2 kcal mol−1 bp−1, respectively, implies that TSS selection at positions >2 bp downstream or >1 bp upstream of the modal position would exceed the energy fluctuations available to 99% of molecules at 20–37°C, and therefore explains the observation that TSS selection >2 bp downstream or >1 bp upstream of the modal position occurs rarely (Vvedenskaya et al., 2015).
Unified mechanism of TSS selection by multisubunit RNAP
TSS selection by archaeal RNAP, eukaryotic RNAP I, eukaryotic RNAP II from most species, and eukaryotic RNAP III involves the same range of TSS positions as TSS selection by bacterial RNAP (positions ± 2 bp from the modal TSS; Learned and Tjian, 1982; Samuels et al., 1984; Thomm and Wich, 1988; Reiter et al., 1990; Fruscoloni et al., 1995; Zecherle et al., 1996). We propose that TSS selection by all of these enzymes is mediated by scrunching and anti-scrunching driven by energy available in the thermal bath. In contrast, TSS selection by S. cerevisiae RNAP II involves a range of TSS positions of 10 s to 100 s of bp (long-range TSS scanning; Giardina and Lis, 1993; Kuehner and Brow, 2006). We propose that TSS scanning by S. cerevisiae RNAP II also is mediated by scrunching and anti-scrunching, but, in this case, involves not only energy from the thermal bath, but also energy from the ATPase activity of RNAP II transcription factor TFIIH (Sainsbury et al., 2015). This proposal could account for the ATP-dependent, TFIIH-dependent cycles of DNA compaction and de-compaction of 10 s to 100 s of bp observed in single-molecule optical-tweezer analyses of TSS scanning by S. cerevisiae RNAP II (Fazal et al., 2015).
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| strain, strain background(E. coli) | BL21(DE3) | New England BioLabs | Cat# C2527I | |
| strain, strain background(E. coli) | NiCo21(DE3) | New England BioLabs | Cat# C2529H | |
| strain, strain background (T. thermophilus) | HB8 | ATCC | Cat# ATCC 27634 | |
| recombinant DNA reagent | pUC18 | Thermo-Fisher | ||
| recombinant DNA reagent | pUC18-T20C2-lacCONS-CCT (plasmid) | this paper | progenitors: PCR of T. thermophilus HB8, pUC18 | |
| recombinant DNA reagent | pUC18-T20C2-lacCONS-CCT (plasmid) | this paper | progenitors: PCR of T. thermophilus HB8, pUC18 | |
| recombinant DNA reagent | pUC18-T20C2-lacCONS-CCT (plasmid) | this paper | progenitors: PCR of T. thermophilus HB8, pUC18 | |
| recombinant DNA reagent | pVS10 (plasmid) | PMID: 12511572 | ||
| recombinant DNA reagent | pIA900 (plasmid) | PMID: 25665556 | ||
| recombinant DNA reagent | pIA900-beta'R1148Bpa (plasmid) | PMID: 26257284 | ||
| recombinant DNA reagent | pIA900-beta'T48Bp | PMID: 26257284 | ||
| recombinant DNA reagent | pIA900-beta'R1148Bpa;beta'D460A | this paper | progenitor: pIA900 | |
| recombinant DNA reagent | pIA900-beta'T48Bpa;beta'D460A | this paper | progenitor: pIA900 | |
| recombinant DNA reagent | pEVOL-pBpF (plasmid) | PMID: 12154230; Addgene | cat# 31190 | |
| sequence-based reagent | UGG (oligoribonucleotide) | Trilink Biotechnologies | ||
| sequence-based reagent | GGA (oligoribonucleotide) | Trilink Biotechnologies | ||
| sequence-based reagent | GAA (oligoribonucleotide) | Trilink Biotechnologies | ||
| sequence-based reagent | AAU (oligoribonucleotide) | Trilink Biotechnologies | ||
| sequence-based reagent | JW 30 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | JW 61 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | JW 62 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | JW 85 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | LY10 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | LY11 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | S128a (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | S1219 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | S1220 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | RPOC820 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | RPOC3140 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | SbfRPOC50 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | Taq_rpoC_F (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | Taq_rpoC_R (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| sequence-based reagent | XbaRPOC4050 (oligodeoxyribonucleotide) | Integrated DNA Technologies | ||
| peptide, recombinant protein (E.coli) | RNAP polymerase core enzyme | PMID: 12511572 | ||
| peptide, recombinant protein (E.coli) | sigma70 | PMID: 9157885 | ||
| peptide, recombinant protein (E.coli) | RNAP core enzyme derivative beta'R1148Bpa | PMID: 26257284 | ||
| peptide, recombinantprotein (E.coli) | RNAP core enzyme derivative beta'T48Bpa | PMID: 26257284 | ||
| peptide, recombinantprotein (E.coli) | RNAP core enzyme derivative beta'R1148Bpa;D460A | this work | ||
| peptide, recombinant protein (E.coli) | RNAP core enzyme derivative beta'R1148Bpa;D460A | this work | ||
| peptide, recombinant protein | Dam methyltransferase | New England BioLabs | Cat# M0222S | |
| peptide, recombinantprotein | restriction endonuclease SbfI-HF | New England BioLabs | Cat# R3642S | |
| peptide, recombinantprotein | restriction endonuclease XbaI | New England BioLabs | Cat# R0145S | |
| peptide, recombinant protein | T4 polynucleotide kinase | New England BioLabs | Cat# M0201S | |
| antibody | anti-digoxigenin antibody | Sigma-Aldrich | Cat# 11333089001 | |
| commercial assay or kit | Dynabeads MyOne Streptavidin C1 | Thermo-Fisher | Cat# 650.01 | |
| commercial assay or kit | MagneHis protein purification system | Promega | Cat# V8500 | |
| commercial assay or kit | TRI reagent | Molecular Research Center | Cat# TR118 | |
| chemical compound,drug | carbenicillin | Gold Biotechnology | cat# c-103-25 | |
| chemical compound,drug | chloramphenicol | Gold Biotechnology | cat# c-105-25 | |
| chemical compound,drug | spectinomycin | Gold Biotechnology | cat# 22189-32-8 | |
| chemical compound,drug | streptomycin | Omnipur | cat# 3810-74-0 | |
| software, algorithm | SigmaPlot | Systat Software | ||
| software, algorithm | xvin | PMID: 16156080 |
Proteins
Wild-type E. coli RNAP core enzyme used in transcription experiments was prepared from E. coli strain NiCo21(DE3) (New England BioLabs) transformed with plasmid pIA900 (Svetlov and Artsimovitch, 2015) as described (Winkelman et al., 2015). Wild-type RNAP for single-molecule DNA-nanomanipulation experiments was prepared from E. coli strain BL21(DE3) (New England Biolabs) transformed with plasmid pVS10 (Artsimovitch et al., 2003) as described (Artsimovitch et al., 2003).
Bpa-containing RNAP core-enzyme derivatives for in vitro protein-DNA photocrosslinking (βʹR1148Bpa for analysis of RNAP leading-edge positions; βʹT48Bpa for analysis of RNAP trailing-edge positions) were prepared from E. coli strain NiCo21(DE3) (New England BioLabs) co-transformed with plasmid pEVOL-pBpF (Chin et al., 2002; Addgene) and plasmid pIA900-βʹR1148Bpa (Winkelman et al., 2015) or pIA900-βʹT48Bpa (Winkelman et al., 2015), as in Winkelman et al. (2015).
Bpa-containing, mutationally inactivated, RNAP core-enzyme derivatives for in vitro and in vivo protein-DNA photocrosslinking (βʹR1148Bpa;βʹD460A for analysis of RNAP leading-edge positions; βʹT48Bpa;βʹD460A for analysis of RNAP trailing-edge positions) were prepared from E. coli strain NiCo21(DE3) (New England BioLabs) co-transformed with plasmid pEVOL-pBpF (Chin et al., 2002; Addgene) and plasmid pIA900-βʹR1148Bpa;βʹD460A or pIA900-βʹT48Bpa;βʹD460A [constructed from pIA900-βʹR1148-Bpa (Winkelman et al., 2015) and pIA900-βʹT48-Bpa (Winkelman et al., 2015) by use of site-directed mutagenesis with primer ‘JW30’, as in Winkelman et al. (2015)].
σ70 was prepared from E. coli strain BL21(DE3) (New England Biolabs) transformed with plasmid pσ70-His (Marr and Roberts, 1997) as described (Marr and Roberts, 1997). To form RNAP holoenzyme, 1 μM RNAP core enzyme and 5 μM σ70 in 10 mM Tris-Cl, pH 8.0, 100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol were incubated 30 min at 25°C.
Oligonucleotides
Oligodeoxyribonucleotides (desalted) were purchased from IDT (sequences in Supplementary file 1). Oligoribonucleotides (HPLC-purified) were purchased from Trilink Biotechnologies.
Determination of TSS position in vitro
Experiments in Figure 1B and Figure 1—figure supplement 2B were performed using reaction mixtures (60 μl) containing 20 nM RNAP holoenzyme derivative, 4 nM plasmid pCDF-CP-lacCONS-GGG or pCDF-CP-lacCONS-CCT, carrying derivatives of the lacCONS promoter (Mukhopadhyay et al., 2001) having a GGG or CCT discriminator [prepared by inserting a synthetic 248 bp DNA fragment (5'- GAAGCCCTGCATTAGGGGTACCCTAGAGCCTGACCGGCATTATAGCCCCAGCGGCGGATCCCTGCGGGTCGACAAGCTTGAATAGCCATCCCAATCGAACAGGCCTGCTGGTAATCGCAGGCCTTTTTATTTGGATGGAGCTCTGAGAGTCTTCGGTGTATGGGTTTTGCGGTGGAAACACAGAAAAAAGCCCGCACCTGACAGTGCGGGCTTTTTTTTTCGACCAAAGGGACGACCGGGTCGTTGGT- 3') between positions 3601 and 460 of pCDF-1b (EMD-Millipore), yielding plasmid pCDF-CP, followed by ligating a 200 bp BglI-digested DNA fragment carrying the lacCONS promoter with GGG or CCT discriminator (5'-GTTCAGAGTTCTACAGTCCGACGATCGCGGATGCTTGACAGAGTGAGCGCAACGCAATAACAGTCATCTAGATAGAACTTTAGGCACCCCAGGCTTGACACTTTATGCTTCGGCTCGTATAATGGGGATGCATGTGAGCGGATAACAATGCGGTTAGGCTTAGAGCGCTTAGTCGATGCTGGAATTCTCGGGTGCCAAGG−3' or 5'-GTTCAGAGTTCTACAGTCCGACGATCGCGGATGCTTGACAGAGTGAGCGCAACGCAATAACAGTCATCTAGATAGAACTTTAGGCACCCCAGGCTTGACACTTTATGCTTCGGCTCGTATAATCCTGATGCATGTGAGCGGATAACAATGCGGTTAGGCTTAGAGCGCTTAGTCGATGCTGGAATTCTCGGGTGCCAAGG−3'; −35 and −10 elements underlined; discriminator in bold) with BglI-digested plasmid pCDF-CP], 0 or 1 mM ATP, 0 or 1 mM CTP, 0 or 1 mM GTP, and 0 or 1 mM UTP in 60 µl 10 mM Tris-Cl, pH 8.0, 70 mM NaCl, 10 mM MgCl2, and 0.1 mg/ml bovine serum albumin. After 20 min at 37°C, reactions were terminated by addition of 100 µl 10 mM EDTA pH 8.0 and 1 mg/ml glycogen. Samples were extracted with acid phenol:chloroform (Sambrook and Russell, 2001) (Ambion), and RNA products were recovered by ethanol precipitation (Sambrook and Russell, 2001) and re-suspended in 6.5 µl water. The RNA products were analyzed by primer extension to define TSS positions. Primer-extension was performed by combining 6.5 µl RNA products in water, 1 µl 1 µM 32P-5'-end-labeled primer ‘s128a’ [Supplementary file 1; 200 Bq/fmol; prepared using [γ32P]-ATP (PerkinElmer) and T4 polynucleotide kinase (New England Biolabs); procedures as in Sambrook and Russell, 2001], and 1 µl 10x avian myelobastosis virus (AMV) reverse transcriptase buffer (New England BioLabs) heating 10 min at 90°C, cooling to 40°C at 0.1 °C/s, and incubating 15 min at 40°C; adding 0.5 μl 10 mM dNTP mix (2.5 mM dATP, 2.5 mM dGTP, 2.5 mM, dCTP, and 2.5 mM dTTP; New England Biolabs) and 1 μl 10 U/μl AMV reverse transcriptase (New England BioLabs); and incubating 1 hr at 50°C. Primer-extension reactions were terminated by heating 20 min at 85°C; 10 μl 1x TBE (Sambrook and Russell, 2001), 8 M urea, 0.025% xylene cyanol, and 0.025% bromophenol blue was added; and samples were analyzed by electrophoresis on 8 M urea, 1X TBE polyacrylamide gels UreaGel System; National Diagnostics) (procedures as in Sambrook and Russell, 2001), followed by storage-phosphor imaging (Typhoon 9400 variable-mode imager; GE Life Science). TSS positions were determined by comparison to products of a DNA-nucleotide sequencing reaction obtained using a PCR-generated DNA fragment containing positions −129 to +71 of the lacCONS-GGG promoter and primer ‘s128a’ (Thermo Sequenase Cycle Sequencing Kit; Affymetrix; methods as per manufacturer). Experiments in Figure 3B, were performed analogously, but using a 1.3 kb DNA fragment carrying positions −687 to +644 of the lacCONS promoter (Mukhopadhyay et al., 2001) prepared by PCR amplification of plasmid pUC18-T20C2-lacCONS [prepared by replacing the SbfI-XbaI segment of plasmid pUC18 (Thermo Scientific) with a 2.0 kb SbfI-XbaI DNA fragment obtained by PCR amplification of Thermus aquaticus rpoC gene with primers Taq_rpoC_F and Taq_rpoC_R (Supplementary file 1) and digestion with XbaI and SbfI-HF (New England BioLabs), yielding plasmid pUC18-T20C2, followed by inserting a synthetic 117 bp DNA fragment carrying the lacCONS promoter (5'-CGGATGCTTGACAGAGTGAGCGCAACGCAATAACAGTCATCTAGATAGAACTTTAGGCACCCCAGGCTTGACACTTTATGCTTCGGCTCGTATAATGTGTGGAATTGTGAGCGGATA-3'; −35 and −10 elements underlined; discriminator in bold) into the KpnI site of plasmid pUC18-T20C2] with primers ‘LY10’ and ‘LY11’ (Supplementary file 1), and performing experiments in the presence of 0 or 1 mM UGG, GGA, GAA, or AAU.
Determination of TSS position in vivo
E. coli strain NiCo21(DE3) (New England BioLabs) transformed with plasmid pCDF-CP-lacCONS-GGG or pCDF-CP-lacCONS-CCT was plated on LB agar (Sambrook and Russell, 2001) containing 50 μg/ml spectinomycin and 50 μg/ml streptomycin, single colonies were inoculated into 25 ml LB broth (Sambrook and Russell, 2001) containing 50 µg/ml spectinomycin and 50 µg/ml streptomycin in 125 ml Bellco flasks, and cultures were shaken (220 rpm) at 37°C. When cell densities reached OD600 = 0.6, 2 ml aliquots were centrifuged 2 min at 4°C at 23,000xg, and resulting cell pellets were frozen at −80°C. Cell pellets were thawed in 1 ml TRI Reagent (Molecular Research Center) at 25°C for 5 min, completely re-suspended by pipetting up and down, incubated 10 min at 70°C, and centrifuged 2 min at 25°C at 23,000 x g. Supernatants were transferred to fresh 1.7 ml microfuge tubes, 200 µl chloroform (Ambion) was added, vortexed, and samples were centrifuged 1 min at 25°C at 23,000 x g. Aqueous phases were transferred to a fresh tube and nucleic acids were extracted with acid phenol:chloroform (Sambrook and Russell, 2001). Nucleic acids were recovered by ethanol precipitation (Sambrook and Russell, 2001), and re-suspended in 20 μl 10 mM Tris-Cl, pH 8.0. Primer extension was performed as described in the preceding section.
Determination of RNAP leading-edge and trailing-edge positions in vitro: protein-DNA photocrosslinking in vitro
Experiments in Figure 1B were performed using reaction mixtures (10 μl) containing 50 nM Bpa-containing RNAP holoenzyme derivative βʹR1148Bpa (for analysis of RNAP leading-edge positions) or βʹT48Bpa (for analysis of RNAP trailing-edge positions) and 4 nM plasmid pCDF-CP-lacCONS-GGG or plasmid pCDF-CP-lacCONS-CCT in 10 mM Tris-Cl, pH 8.0, 70 mM NaCl, 10 mM MgCl2, and 0.1 mg/ml bovine serum albumin. Reaction mixtures were incubated 5 min at 37°C, UV-irradiated 5 min at 25°C in a Rayonet RPR-100 photochemical reactor equipped with 16 × 350 nm tubes (Southern New England Ultraviolet), and resulting protein-DNA crosslinks were mapped using primer extension. Primer-extension reactions (12.5 µl) were performed by combining 2 µl aliquot of crosslinking reaction, 1 µl 1 µM 32P-5'-end-labeled primer ‘s128a’ (for analysis of leading-edge positions) or primer ‘JW85’ (for analysis of trailing-edge positions) [Supplementary file 1; 200 Bq/fmol; prepared using [γ32P]-ATP (PerkinElmer) and T4 polynucleotide kinase (New England Biolabs); procedures as in Sambrook and Russell, 2001, 1 μl 10X dNTPs (2.5 mM dATP, 2.5 mM dCTP, 2.5 mM dGTP, 2.5 mM TTP, 0.5 μl 5 U/μl Taq DNA polymerase (New England BioLabs), 5 μl 5 M betaine, 0.625 μl 100% dimethyl sulfoxide, and 1.25 µl 10x Taq DNA polymerase buffer (New England BioLabs); and cycling 16–40 times through 30 s at 95°C, 30 s at 53°C, and 30 s at 72°C. Primer-extension reactions were terminated, and primer-extension products were analyzed as in the preceding section. Experiments in Figure 1—figure supplement 2B were performed analogously, but using Bpa-containing, mutationally inactivated, RNAP derivatives βʹR1148Bpa; β'D460A (for analysis of RNAP leading-edge positions) and βʹT48Bpa; β'D460A (for analysis of RNAP trailing-edge positions)
Experiments in Figure 3B and Figure 3—figure supplement 1B were performed analogously, but using reaction mixtures also containing 0 or 1 mM of ribotrinucleotide primers UGG, GGA, GAA, or AAU, and using 32P-5'-end-labeled primers ‘JW62’ and ‘JW61’ (Supplementary file 1) in primer-extension reactions.
Determination of RNAP leading-edge and trailing-edge positions in vivo: protein-DNA photocrosslinking in vivo
Experiments in Figure 1B were performed using a three-plasmid merodiploid system that enabled production of a Bpa-containing, mutationally inactivated, decahistidine-tagged RNAP holoenzyme derivative in vivo and enabled trapping of RPo consisting of the Bpa-containing, mutationally inactivated, decahistidine-tagged RNAP holoenzyme derivative and a lacCONS promoter with GGG or CCT discriminator in vivo, and UV-irradiation of cells (Figure 1—figure supplement 1).
E. coli strain NiCo21(DE3) (New England BioLabs) transformed sequentially with (i) plasmid pCDF-CP-lacCONS-GGG or plasmid pCDF-CP-lacCONS-CCT, (ii) plasmid pIA900-βʹT48Bpa; βʹD460A or plasmid pIA900-βʹR1148Bpa; βʹD460A, and (iii) plasmid pEVOL-pBpF (Chin et al., 2002; Addgene) was plated to yield a confluent lawn on LB agar (Sambrook and Russell, 2001) containing 100 μg/ml carbenicillin, 50 μg/ml spectinomycin, 50 μg/ml streptomycin, and 25 μg/ml chloramphenicol; cells were scraped from the plate and used to inoculate 250 ml LB broth (as described above) containing 1 mM Bpa (Bachem), 100 μg/ml carbenicillin, 50 μg/ml spectinomycin, 50 μg/ml streptomycin, and 25 μg/ml chloramphenicol in a 1000 ml flask (Bellco) to yield OD600 = 0.3; the culture was shaken (220 rpm) 1 hr at 37°C in the dark, isopropyl-β-D-thiogalactoside was added to 1 mM; and the culture was further shaken (220 rpm) 3 hr at 37°C in the dark. Aliquots (7 ml) were transferred to 13 mm x 100 mm borosilicate glass test tubes (VWR), UV-irradiated 20 min at 25°C in a Rayonet RPR-100 photochemical reactor equipped with 16 × 350 nm tubes (Southern New England Ultraviolet), harvested by centrifuging 15 min at 4°C at 3000xg, and cell pellets were frozen at −20°C. Cell pellets were thawed 30 min at 4°C, re-suspended in 40 ml 50 mM Na2HPO4 pH 8.0, 1.4 M NaCl, 20 mM imidazole, 14 mM β-mercaptoethanol, 0.1% Tween20, and 5% ethanol containing 2 mg egg white lysozyme. Cells were lysed by sonication 5 min at 4°C., cell lysates were centrifuged 40 min at 4°C at 23,000xg, and supernatants were added to 1 ml Ni-NTA-agarose (Qiagen, Germantown, MD) in 1 ml 50 mM Na2HPO4, pH 8.0, 1.4 M NaCl, 20 mM imidazole, 0.1% Tween-20, 5 mM β-mercaptoethanol, and 5% ethanol, and incubated 30 min at 4°C with gentle rocking. The Ni-NTA-agarose was loaded into a 15 ml polyprep column (BioRad), the resulting column was washed with 10 ml of 50 mM Na2HPO4, pH 8.0, 300 mM NaCl, 20 mM imidazole, 0.1% Tween-20, 5 mM β-mercaptoethanol, and 5% ethanol and eluted with 3 ml of the same buffer containing 300 mM imidazole. The eluate was concentrated to 0.2 ml using an 1000 MWCO Amicon Ultra-4 centrifugal filter (EMD Millipore); the buffer was exchanged to 0.2 ml 20 mM Tris-Cl, pH 8.0, 200 mM KCl, 20 mM MgCl2, 0.2 mM EDTA, and 1 mM DTT using the 1000 MWCO Amicon Ultra-4 centrifugal filter (EMD Millipore); 0.2 ml glycerol was added; and the sample was stored at −20°C. Protein-DNA crosslinks were mapped by denaturation followed by primer extension. Denaturation was performed by combining 25 μl crosslinked RNAP-DNA, 25 μl water, 15 μl 5 M NaCl, and 6 μl 100 µg/ml heparin; heating 5 min at 95°C; cooling on ice. Denatured crosslinked RNAP-DNA was purified by adding 20 µl MagneHis Ni-particles (Promega) equilibrated and suspended in 10 mM Tris-Cl, pH 8.0, 1.2 M NaCl, 10 mM MgCl2, 10 μg/ml heparin, and 0.1 mg/ml bovine serum albumin; washing once with 50 µl 10 mM Tris-Cl, pH 8.0, 1.2 M NaCl, 10 mM MgCl2, 10 μg/ml heparin, and 0.1 mg/ml bovine serum albumin; washed twice with 50 µl 1x Taq DNA polymerase buffer (New England BioLabs); and resuspended in 10 µl 1x Taq DNA polymerase buffer. Primer extension was performed using 2 μl aliquots of purified denatured crosslinked RNAP-DNA, using procedures essentially as described above for experiments in Figure 1B.
Determination of RNAP-dependent DNA unwinding by single-molecule DNA-nanomanipulation: DNA constructs
2.0 kb DNA fragments carrying single centrally located lacCONS-GGG, lacCONS-CCT, or lacCONS promoters were prepared by digesting plasmid pUC18-T20C2-lacCONS-GGG or plasmid pUC18-T20C2-lacCONS-CCT [prepared by inserting a synthetic 80 bp DNA fragment carrying a derivative of the lacCONS promoter (Mukhopadhyay et al., 2001) having a GGG or CCT discriminator (5'-CATCTAGATCACATTTTAGGCACCCCAGGCTTGACACTTTATGCTTCGGCTCGTATAATGGGGATGCATGTGAGCGGATA-3' or 5'-CATCTAGATCACATTTTAGGCACCCCAGGCTTGACACTTTATGCTTCGGCTCGTATAATCCTGATGCATGTGAGCGGATA −3'; −35 and −10 elements underlined; discriminator in bold) into the KpnI site of plasmid pUC18-T20C2] or plasmid pUC18-T20C2-lacCONS with XbaI and SbfI-HF (New England BioLabs), followed by agarose gel electrophoresis.
1.3 kb DNA fragments carrying a single centrally located lacCONS promoter were prepared by PCR amplification of plasmid pUC18-T20C2-lacCONS, using primers ‘LY10’ and ‘LY11’ (Supplementary file 1), followed by treatment with Dam methyltransferase (New England BioLabs), digestion with XbaI and SbfI-HF (New England BioLabs), and agarose gel electrophoresis.
DNA constructs for magnetic-tweezers single-molecule DNA-nanomanipulation were prepared from the above 2.0 kb and 1.3 kb DNA fragments by ligating, at the XbaI end, a 1.0 kb DNA fragment bearing multiple biotin residues on both strands [prepared by PCR amplification of plasmid pARTaqRPOC-lacCONS using primers ‘XbaRPOC4050’ and ‘RPOC3140’ Supplementary file 1) and conditions as described (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
Determination of RNAP-dependent DNA unwinding by single-molecule DNA-nanomanipulation: data collection
Experiments were performed essentially as described (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
Experiments in Figure 2 (experiments addressing TSS selection for promoters with GGG or CCT discriminator sequence), were performed using standard reactions containing mechanically extended, torsionally constrained, 2.0 kb DNA molecule carrying GGG or CCT promoter (extension force = 0.3 pN; superhelical density = 0.021 for experiments with positively supercoiled DNA; superhelical density = −0.021 for experiments with negatively supercoiled DNA) and RNAP holoenzyme (10 nM for experiments with positively supercoiled DNA; 0.5 nM for experiments with negatively supercoiled DNA) in 25 mM Na-HEPES, pH 7.9, 75 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 0.1% Tween-20, 0.1 mg/ml bovine serum albumin) at 30°C. Data from each of three single DNA molecules were pooled [differences in plectoneme size (at superhelical density ± 0.021) and Δobs ≤ 5%].
Experiments in Figure 4—figure supplement 1 (experiments demonstrating that reduction in DNA-fragment length from 2.0 to 1.3 kb enables single-bp resolution) were performed using standard reactions containing mechanically extended, torsionally constrained, 2.0 kb or 1.3 kb DNA molecule carrying lacCONS promoter (extension force = 0.3 pN; initial superhelical density = 0.021 or 0.024 for experiments with 2.0 kb or 1.3 kb positively supercoiled DNA; superhelical density = −0.021 or −0.024 for experiments with 2.0 kb or 1.3 kb negatively supercoiled DNA) and RNAP holoenzyme (10 nM for experiments with positively supercoiled DNA; 0.5 nM for experiments with negatively supercoiled DNA) in the buffer of the preceding paragraph at 30°C. For each DNA-fragment length, data were collected on one single DNA molecule.
Experiments in Figure 4 and Figure 4—figure supplement 2 (experiments addressing primer-programmed TSS selection with primers UGG, GGA, GAA, AAU) were performed using standard reactions containing mechanically extended, torsionally constrained 1.3 kb DNA molecule carrying lacCONS promoter (extension force = 0.3 pN; initial superhelical density = 0.024 for experiments with positively supercoiled DNA; superhelical density = −0.024 for experiments with negatively supercoiled DNA) and RNAP holoenzyme (10 nM for experiments with positively supercoiled DNA; 0.5 nM for experiments with negatively supercoiled DNA) in the buffer of the preceding paragraph at 30°C. Primers UGG, GGA, GAA, and AAU were present at 0 or 1 mM, 0 or 1 µM, 0 or 2.5 µM, and 0 or 1 mM, respectively. For experiments with positively supercoiled DNA, data from each of seven single DNA molecules were normalized based on Δobs,pos in absence of primer and pooled; for experiments with negatively supercoiled DNA, data from each of two single DNA molecules were normalized based on Δobs,neg in absence of primer and pooled.
Experiments in Figures 5–6 and Figure 6—figure supplement 1 (experiments addressing primer-concentration dependences of tunwound in primer-programmed TSS selection) were performed using standard reactions containing mechanically extended, torsionally constrained, 2.0 kb DNA molecule carrying lacCONS promoter (extension force = 0.3 pN; initial superhelical density = 0.021) and RNAP holoenzyme (10 nM) in the buffer of the preceding paragraph at 30°C. Each titration consisted of recordings in absence of primer followed by recordings in presence of primer at increasing concentrations. (0, 0.50, 0.90, 1.3, 250, 500, 750, and 1000 μM for UGG; 0, 0.25, 0.50, and 1.0 µM for GGA; 0, 0.50, 1.0, and 1.5 µM for GAA; 0, 0.40, 0.80, 1.2, 200, 400, 600, and 800 μM for AAU). For each titration, data were collected on one single DNA molecule.
For experiments with negatively supercoiled DNA, for which unwound >> 1 h (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
Determination of RNAP-dependent DNA unwinding by single-molecule DNA-nanomanipulation: data reduction for determination of DNA unwinding
Raw time traces were analyzed to yield DNA extension (l) as described (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
Changes in l attributable to DNA unwinding (Δlu) and changes in l attributable to DNA compaction (Δlc) were calculated as: Δlu = (Δlobs,neg + Δlobs,pos)/2, and Δlc = (Δlobs,pos - Δlobs,neg)/2, where Δlobs,pos and Δlobs,neg are observed changes in l in experiments with positively supercoiled DNA and negatively supercoiled DNA, as described (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
Determination of RNAP-dependent DNA unwinding by single-molecule DNA-nanomanipulation: data reduction for determination of energetics of scrunching and anti-scrunching
Lifetimes of unwound states (tunwound) were extracted from single-molecule traces as described (Revyakin et al., 2004, 2005, 2006, Revyakin et al., 2003
For experiments in absence of primer (Qi and Turnbough, 1995; Figure 6—figure supplement 1):
where R, P, RPc, and RPo denote RNAP holoenzyme, promoter, RNAP-promoter closed complex, and RNAP-promoter open complex; and
For experiments in presence of primer GGA, which programs TSS selection at modal position and therefore does not require scrunching or anti-scrunching for TSS selection (Figure 6—figure supplement 1):
where NpNpN denotes primer; and
For experiments in presence of primer UGG, or GAA, or AAU, which program TSS selection at positions different form modal TSS, and therefore require scrunching or anti-scrunching for TSS selection (Figures 4–6 and Figure 6—figure supplement 1):
where RPo' denotes a scrunched or anti-scrunched RPo; and
Kscrunch, KNpNpN, ΔGNpNpN, and ΔGscrunch were obtained by fitting slopes and y-intercepts of linear-regression fits of plots of unwound vs. primer concentration (Figure 6D and Figure 6—figure supplement 1) to the equation of Figure 6C, stipulating Kscrunch = 1 and ΔGscrunch = 0 for primer GGA, which programs TSS selection at modal position and therefore does not require scrunching or anti-scrunching for TSS selection.
Quantitation and statistical analysis
Data in Figure 2 are means ± SEM of at least 70 technical replicates of each of three biological replicates (three single DNA molecules) for positively supercoiled DNA and at least 50 technical replicates of each of three biological replicates (three single DNA molecules) for negatively supercoiled DNA.
Data in Figure 4—figure supplement 1A–B are means ± SEM of at least 100 technical replicates for a single DNA molecule (positively supercoiled DNA) or at least 70 technical replicates for a single DNA molecule (negatively supercoiled DNA).
Data in Figure 4—figure supplement 1C are means ± SEM of randomly selected subsets of n = 30. Similar results were obtained for ten different randomly selected subsets of n = 30.
Data in Figure 4B–C and Figure 4—figure supplement 2 are means ± SEM of at least 40 technical replicates for each of seven biological replicates (seven single DNA molecules) for positively supercoiled DNA and at least 50 technical replicates for each of two biological replicates (two single DNA molecules) for negatively supercoiled DNA.
Data in Figures 5–6 and Figure 6—figure supplement 1 are means ± SEM of at least 150 technical replicates for one single DNA molecule for each of the four primers.
Acknowledgements
Work was supported by NIH grants GM041376 (RHE), GM118059 (BEN), and a European Science Foundation EURYI grant (TRS). We thank Seth Goldman for help with construction of pCDF-CP.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Bryce E Nickels, Email: bnickels@waksman.rutgers.edu.
Richard H Ebright, Email: ebright@waksman.rutgers.edu.
Taekjip Ha, Johns Hopkins University School of Medicine, United States.
Funding Information
This paper was supported by the following grants:
National Institutes of Health GM041376 to Richard H Ebright.
National Institutes of Health GM118059 to Bryce E Nickels.
European Science Foundation EURYI to Terence Strick.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—review and editing.
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—review and editing.
Resources, Investigation.
Conceptualization, Funding acquisition, Methodology, Writing—review and editing.
Conceptualization, Resources, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Conceptualization, Resources, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Additional files
References
- Aoyama T, Takanami M. Essential structure of E. coli promoter II. Effect of the sequences around the RNA start point on promoter function. Nucleic Acids Research. 1985;13:4085–4096. doi: 10.1093/nar/13.11.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovitch I, Svetlov V, Murakami KS, Landick R. Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. Journal of Biological Chemistry. 2003;278:12344–12355. doi: 10.1074/jbc.M211214200. [DOI] [PubMed] [Google Scholar]
- Bustamante C, Marko JF, Siggia ED, Smith S. Entropic elasticity of lambda-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- Chin JW, Martin AB, King DS, Wang L, Schultz PG. Addition of a photocrosslinking amino acid to the genetic code of Escherichiacoli. PNAS. 2002;99:11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal FM, Meng CA, Murakami K, Kornberg RD, Block SM. Real-time observation of the initiation of RNA polymerase II transcription. Nature. 2015;525:274–277. doi: 10.1038/nature14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feklistov A, Barinova N, Sevostyanova A, Heyduk E, Bass I, Vvedenskaya I, Kuznedelov K, Merkiene E, Stavrovskaya E, Klimasauskas S, Nikiforov V, Heyduk T, Severinov K, Kulbachinskiy A. A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Molecular Cell. 2006;23:97–107. doi: 10.1016/j.molcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Franklin RE, Gosling RG. Molecular configuration in sodium thymonucleate. Nature. 1953;171:740–741. doi: 10.1038/171740a0. [DOI] [PubMed] [Google Scholar]
- Fruscoloni P, Zamboni M, Panetta G, De Paolis A, Tocchini-Valentini GP. Mutational analysis of the transcription start site of the yeast tRNA(Leu3) gene. Nucleic Acids Research. 1995;23:2914–2918. doi: 10.1093/nar/23.15.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina C, Lis JT. DNA melting on yeast RNA polymerase II promoters. Science. 1993;261:759–762. doi: 10.1126/science.8342041. [DOI] [PubMed] [Google Scholar]
- Goldman SR, Sharp JS, Vvedenskaya IO, Livny J, Dove SL, Nickels BE. NanoRNAs prime transcription initiation in vivo. Molecular Cell. 2011;42:817–825. doi: 10.1016/j.molcel.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jeong W, Kang C. Start site selection at lacUV5 promoter affected by the sequence context around the initiation sites. Nucleic Acids Research. 1994;22:4667–4672. doi: 10.1093/nar/22.22.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanidis AN, Margeat E, Ho SO, Kortkhonjia E, Weiss S, Ebright RH. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314:1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner JN, Brow DA. Quantitative analysis of in vivo initiator selection by yeast RNA polymerase II supports a scanning model. Journal of Biological Chemistry. 2006;281:14119–14128. doi: 10.1074/jbc.M601937200. [DOI] [PubMed] [Google Scholar]
- Learned RM, Tjian R. In vitro transcription of human ribosomal RNA genes by RNA polymerase I. Journal of Molecular and Applied Genetics. 1982;1:575–584. [PubMed] [Google Scholar]
- Lewis DE, Adhya S. Axiom of determining transcription start points by RNA polymerase in Escherichia coli. Molecular Microbiology. 2004;54:692–701. doi: 10.1111/j.1365-2958.2004.04318.x. [DOI] [PubMed] [Google Scholar]
- Lionnet T, Allemand JF, Revyakin A, Strick TR, Saleh OA, Bensimon D, Croquette V. Magnetic trap construction. Cold Spring Harbor Protocols. 2012;2012:pdb.prot067496. doi: 10.1101/pdb.prot067496. [DOI] [PubMed] [Google Scholar]
- Liu J, Turnbough CL. Effects of transcriptional start site sequence and position on nucleotide-sensitive selection of alternative start sites at the pyrC promoter in Escherichia coli. Journal of Bacteriology. 1994;176:2938–2945. doi: 10.1128/jb.176.10.2938-2945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Roberts JW. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science. 1997;276:1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay J, Kapanidis AN, Mekler V, Kortkhonjia E, Ebright YW, Ebright RH. Translocation of sigma(70) with RNA polymerase during transcription: fluorescence resonance energy transfer assay for movement relative to DNA. Cell. 2001;106:453–463. doi: 10.1016/S0092-8674(01)00464-0. [DOI] [PubMed] [Google Scholar]
- Qi F, Turnbough CL. Regulation of codBA operon expression in Escherichia coli by UTP-dependent reiterative transcription and UTP-sensitive transcriptional start site switching. Journal of Molecular Biology. 1995;254:552–565. doi: 10.1006/jmbi.1995.0638. [DOI] [PubMed] [Google Scholar]
- Reiter WD, Hüdepohl U, Zillig W. Mutational analysis of an archaebacterial promoter: essential role of a TATA box for transcription efficiency and start-site selection in vitro. PNAS. 1990;87:9509–9513. doi: 10.1073/pnas.87.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revyakin A, Allemand JF, Croquette V, Ebright RH, Strick TR. Single-molecule DNA nanomanipulation: detection of promoter-unwinding events by RNA polymerase. Methods in Enzymology. 2003;370:577–598. doi: 10.1016/S0076-6879(03)70049-4. [DOI] [PubMed] [Google Scholar]
- Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: detection by single-molecule DNA nanomanipulation. PNAS. 2004;101:4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revyakin A, Ebright RH, Strick TR. Single-molecule DNA nanomanipulation: improved resolution through use of shorter DNA fragments. Nature Methods. 2005;2:127–138. doi: 10.1038/nmeth0205-127. [DOI] [PubMed] [Google Scholar]
- Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314:1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb NC, Cordes T, Hwang LC, Gryte K, Duchi D, Craggs TD, Santoso Y, Weiss S, Ebright RH, Kapanidis AN. The transcription bubble of the RNA polymerase-promoter open complex exhibits conformational heterogeneity and millisecond-scale dynamics: implications for transcription start-site selection. Journal of Molecular Biology. 2013;425:875–885. doi: 10.1016/j.jmb.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen KI, Baker KE, Kelln RA, Neuhard J. Nucleotide pool-sensitive selection of the transcriptional start site in vivo at the Salmonella typhimurium pyrC and pyrD promoters. Journal of Bacteriology. 1993;175:4137–4144. doi: 10.1128/jb.175.13.4137-4144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury S, Bernecky C, Cramer P. Structural basis of transcription initiation by RNA polymerase II. Nature Reviews Molecular Cell Biology. 2015;16:129–143. doi: 10.1038/nrm3952. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor. NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- Samuels M, Fire A, Sharp PA. Dinucleotide priming of transcription mediated by RNA polymerase II. The Journal of Biological Chemistry. 1984;259:2517–2525. [PubMed] [Google Scholar]
- Straney DC, Crothers DM. A stressed intermediate in the formation of stably initiated RNA chains at the Escherichia coli lac UV5 promoter. Journal of Molecular Biology. 1987;193:267–278. doi: 10.1016/0022-2836(87)90218-X. [DOI] [PubMed] [Google Scholar]
- Svetlov V, Artsimovitch I. Purification of bacterial RNA polymerase: tools and protocols. Methods in Molecular Biology. 2015;1276:13–29. doi: 10.1007/978-1-4939-2392-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomm M, Wich G. An archaebacterial promoter element for stable RNA genes with homology to the TATA box of higher eukaryotes. Nucleic Acids Research. 1988;16:151–163. doi: 10.1093/nar/16.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu AH, Turnbough CL. Regulation of upp expression in Escherichia coli by UTP-sensitive selection of transcriptional start sites coupled with UTP-dependent reiterative transcription. Journal of Bacteriology. 1997;179:6665–6673. doi: 10.1128/jb.179.21.6665-6673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough CL, Switzer RL. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiology and Molecular Biology Reviews. 2008;72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough CL. Regulation of bacterial gene expression by the NTP substrates of transcription initiation. Molecular Microbiology. 2008;69:10–14. doi: 10.1111/j.1365-2958.2008.06272.x. [DOI] [PubMed] [Google Scholar]
- Vvedenskaya IO, Vahedian-Movahed H, Zhang Y, Taylor DM, Ebright RH, Nickels BE. Interactions between RNA polymerase and the core recognition element are a determinant of transcription start site selection. PNAS. 2016;113:E2899–E2905. doi: 10.1073/pnas.1603271113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vvedenskaya IO, Zhang Y, Goldman SR, Valenti A, Visone V, Taylor DM, Ebright RH, Nickels BE. Massively systematic transcript end readout, "master": transcription start site selection, transcriptional slippage, and transcript yields. Molecular Cell. 2015;60:953–965. doi: 10.1016/j.molcel.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Mallik P, Pratt TS, Osuna R. The Escherichia coli Fis promoter is regulated by changes in the levels of its transcription initiation nucleotide CTP. Journal of Biological Chemistry. 2004;279:50818–50828. doi: 10.1074/jbc.M406285200. [DOI] [PubMed] [Google Scholar]
- Walker KA, Osuna R. Factors affecting start site selection at the Escherichia coli fis promoter. Journal of Bacteriology. 2002;184:4783–4791. doi: 10.1128/JB.184.17.4783-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC. Helical repeat of DNA in solution. PNAS. 1979;76:200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Archer CD, Liu JK, Turnbough CL. Translational control of pyrC expression mediated by nucleotide-sensitive selection of transcriptional start sites in Escherichia coli. Journal of Bacteriology. 1992;174:514–524. doi: 10.1128/jb.174.2.514-524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JT, Chandrangsu P, Ross W, Gourse RL. Open complex scrunching before nucleotide addition accounts for the unusual transcription start site of E. coli ribosomal RNA promoters. PNAS. 2016b;113:E1787–E1795. doi: 10.1073/pnas.1522159113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JT, Vvedenskaya IO, Zhang Y, Zhang Y, Bird JG, Taylor DM, Gourse RL, Ebright RH, Nickels BE. Multiplexed protein-DNA cross-linking: scrunching in transcription start site selection. Science. 2016a;351:1090–1093. doi: 10.1126/science.aad6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JT, Winkelman BT, Boyce J, Maloney MF, Chen AY, Ross W, Gourse RL. Crosslink mapping at amino acid-base resolution reveals the path of scrunched dna in initial transcribing complexes. Molecular Cell. 2015;59:768–780. doi: 10.1016/j.molcel.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaychikov E, Martin E, Denissova L, Kozlov M, Markovtsov V, Kashlev M, Heumann H, Nikiforov V, Goldfarb A, Mustaev A. Mapping of catalytic residues in the RNA polymerase active center. Science. 1996;273:107–109. doi: 10.1126/science.273.5271.107. [DOI] [PubMed] [Google Scholar]
- Zecherle GN, Whelen S, Hall BD. Purines are required at the 5' ends of newly initiated RNAs for optimal RNA polymerase III gene expression. Molecular and Cellular Biology. 1996;16:5801–5810. doi: 10.1128/MCB.16.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]