Abstract
Itk−/− mice exhibit defects in activation, development and function of CD4+ and CD8+ T cells and iNKT cells. These and other defects in these mice make it difficult to uncouple the developmental versus functional requirement of Itk signaling. Here we report an allele sensitive mutant of Itk (Itkas) whose catalytic activity can be selectively inhibited by analogs of the PP1 kinase inhibitor. We show that Itkas behaves like WT Itk in the absence of the inhibitor and can rescue the development of Itk−/− T cells in mice. Using this mutant, we show that Itk activity is required not only for Th2, Th17 and iNKT cell cytokine production, but also surprisingly, for Th1 cytokine production. This work has important implications for understanding the role of Itk signaling in development vs. function of iNKT cells, Th1, Th2 and Th17.
Keywords: Tec kinase, kinase inhibitor, allele sensitive, iNKT cell
Introduction
Interleukin-2 inducible T cell kinase (Itk) is involved in T cell receptor (TCR) signaling (for review see (1, 2)), and its absence results in select defects in T cell development, affecting naïve CD4+ and CD8+ T cells, iNKT cells and γδ T cells (2–7). Itk−/− mice preferentially develop a population of CD4+ and CD8+ T cells with an innate memory phenotype (8–11). The restricted expression of Itk along with the requirement for Itk signaling for T cell activation makes it an attractive target for therapeutic intervention in T cell mediated diseases. Indeed, Itk−/− mice exhibit reduced production of cytokines by iNKT cells (4, 12–14), reduced Th2 effector cytokines and IL-17A by T cells (2, 15, 16), and resistance to developing allergic asthma (17, 18).
While much has been uncovered regarding the function of Itk using Itk−/− mice, the developmental defects could affect subsequent T cell functions. However these differences are unclear. In addition, interpretations from cells lacking the expression of Itk are confounded by possible functional compensation by related Tec kinases (19), and the inability to temporally study the effects of loss of expression/function of Itk such as would happen with a small molecule inhibitor. Despite much interest, small molecule inhibitors of Itk to date either have poor pharmacodynamic properties or lack specificity for Itk. Here we take advantage of a regulatory feature in kinases, the presence of unique gatekeeper residues in the kinase domain, to develop an allele sensitive Itk (Itkas) that allows temporal inhibition of the kinase activity of Itk using a small molecule inhibitor (20). Gatekeeper residues are typically found within the hydrophobic ATP binding pocket of the kinase domain and hence can regulate catalytic activity of the kinase. Substitution of this gatekeeper residue in kinases, usually a bulky hydrophobic residue, with a smaller less hydrophobic residue can allow for bulkier analogs of PP1 kinase inhibitors to efficiently compete for this pocket with ATP thus preventing activation of the mutated kinase (21). The PP1 analogs include 1-NM-PP1, 3-MB-PP1 and 3-IB-PP1 (21).
A gatekeeper residue, F434, has been identified in Itk, which we alter to generate the allele sensitive Itk (Itkas) mutant. We also report the generation and characterization of Itk deficient mice carrying this Itkas. Using these mice, we have identified a previously unappreciated difference in sensitivity for Itk signaling in the proliferation of CD4+ versus CD8+ T cells, as well as show that the kinase activity of Itk is critical for the production of cytokines by not only Th2, Th17 and iNKT cells as predicted by the analysis of Itk−/− mice, but also of Th1 cells.
Results
Generation of transgenic mice expressing an allele sensitive Itk
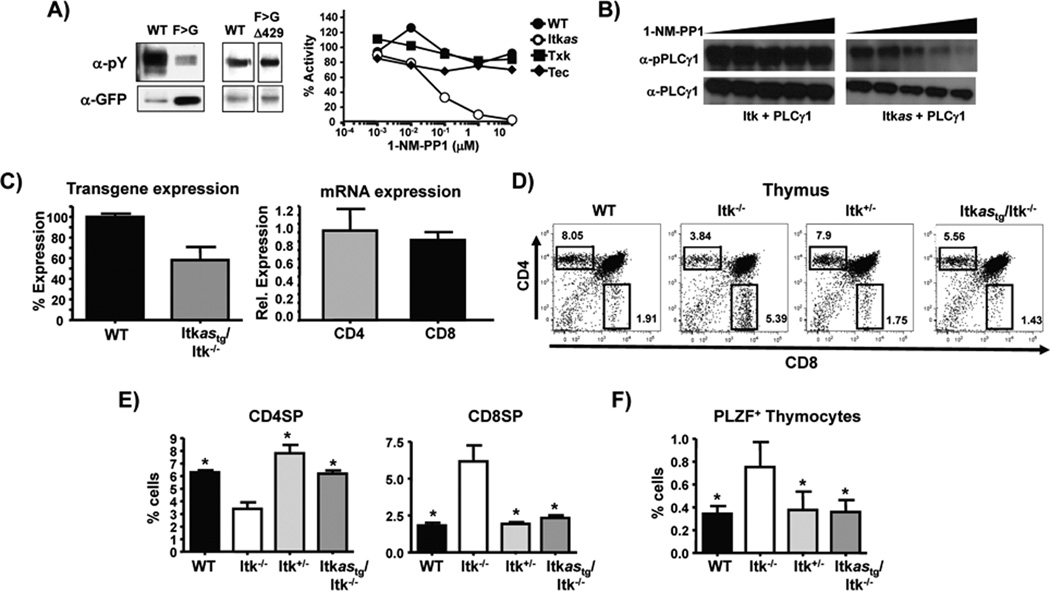
To generate an allele sensitive mutant of Itk, we substituted the gatekeeper residue, F434 with a glycine residue (Fig. 1), however, this resulted in the loss of greater than 95% of its kinase activity, suggesting that Itk behaves differentially from the majority of other tyrosine kinases in which this change has been tested (Fig. 2A)(22). Similar experiments with the kinase GRK2 also revealed intolerance to changes in its gatekeeper residue, with significant loss of activity, however compensating mutations were identified that partially rescued kinase activity (23). We therefore tested a range of potential compensating mutants of Itk, and found partial rescue of Itk kinase activity in the F434G/E395P, F434G/A429R, F434G/Δ429, F434G/N384G mutants. These mutants displayed varying sensitivity to inhibition by 1-NM-PP1 (A429R>Δ429>N384G>E395P). Based on stability of the variants, we chose the F434G/Δ429 (cellular IC50 for 1-NM-PP1 of ~50 nM) referred to as Itkas for the remainder of our studies (Fig. 1A). By contrast, 1-NM-PP1 did not appreciably inhibit the activity of the related Tec kinases expressed in T cells, Tec (as has been previously reported (24, 25), and Txk (Fig. 2A). Furthermore, 1-NM-PP1 inhibited the ability of Itkas, but not WT Itk, to phosphorylate PLCγ1 in co-transfected HEK 293T cells (Fig. 2B). We generated transgenic mice expressing the Itkas using the T cell specific human CD2 promoter/enhancer regions (26), and two founder lines that showed highest expression of the transgene were chosen for further breeding. Founders were backcrossed to Itk−/− mice to generate Tg(CD2-hItkas)Itk−/− mice, and offspring of both lines showed comparable expression of Itk (confirmed by immunoblot (Fig. 2C), with approximately 60% of WT expression), isolated CD4+ and CD8+ T cells from these mice did not exhibit any differences in Itkas mRNA levels (Fig. 2C). Given the comparable expression of Itkas, we do not distinguish between different founders in subsequent experiments.
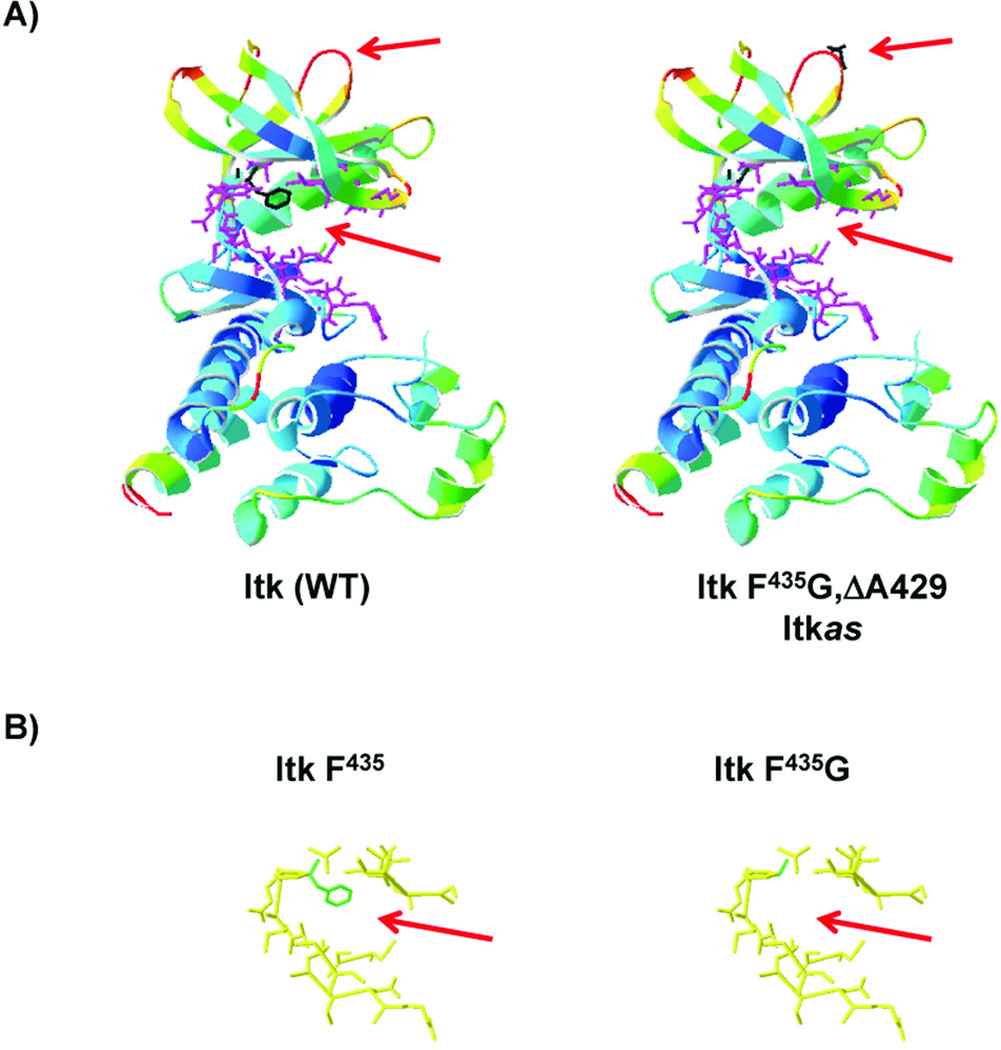
Figure 1. Location of gatekeeper and allele sensitive residues in kinase domain of Itk.
A) Ribbon structure of the kinase domain of Itk with F435 indicated in black pointing into the ATP binding pocket (left), and mutation of F435 to G435 and site of the Δ429 in the loop of the upper lobe of the kinase domain (right). B) Residues lining the binding pocket and location of F435 (green reside in left panel), and alteration of the binding pocket in the F435G mutant (right panel).
Figure 2. Generation of transgenic mice expressing an allele sensitive mutant of Itk.
A) Analysis of tyrosine phosphorylation of immunoprecipitated WT Itk, Itk F434G or Itk F434G/Δ429 (Itkas) following transfection into HEK293T cells (left panels). Effect of indicated concentrations of 1-NM-PP1 on tyrosine phosphorylation of WT Itk, Itkas or related kinases Tec and Txk or in transfected cells (right panel), representative of at-least 2 independent experiments. B) Effect of increasing concentrations of 1-NM-PP1 (1 nM to 10 µM) on tyrosine phosphorylation of PLCγ1 by WT Itk or Itkas. Total cell lysates probed with phospho- and total PLCγ1. Expression of Itk was confirmed, representative of at-least 2 independent experiments. C) Quantification of western analysis of Itk expression in thymocytes from WT or Tg(CD2-hItkas)Itk−/− mice (left panel). Quantification of Itkas mRNA in purified CD4+ and CD8+ T cells from Tg(CD2-hItkas)Itk−/− mice, expression level in CD4+ T cells set as 1 (right panel), Values are means ± SEMs of n=3. D) Thymocytes were analyzed for CD4 and CD8 SP cells by FACS and E) percentages determined by gating on TCRβ+CD4+CD8− (CD4SP) TCRβ+CD4−CD8+ (CD8SP) cells. F) Percentage of PLZF+ thymocytes. Values are means ± SEMs of n≥5, *p < .05 by unpaired student t test.
Itkas rescues T cell development in the absence of Itk
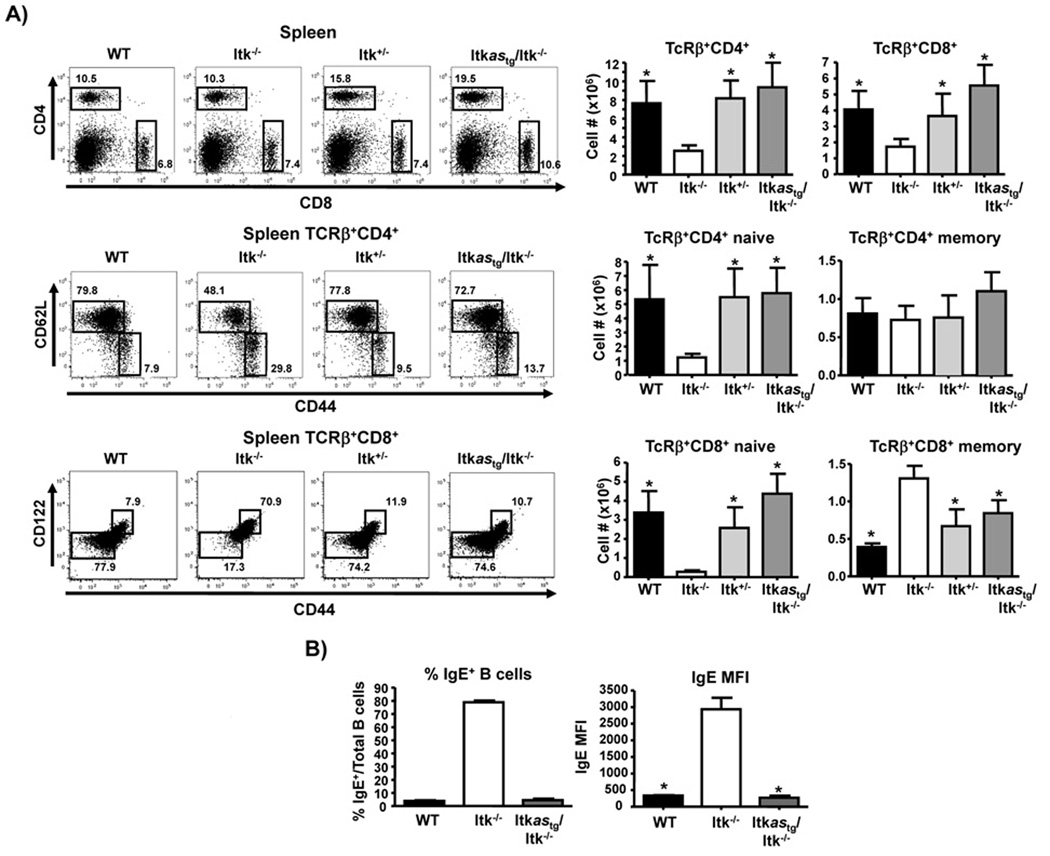
Analysis of the Itkas transgenic mice revealed that Itkas rescues T cell development in Itk−/− mice to the levels seen in WT or Itk+/− mice (percentage and number of both CD4SP and CD8SP cells, and ratio of CD4SP to CD8SP cells in thymus) (Fig. 2D, E). Furthermore, while Itk−/− mice exhibit increased proportion of PLZF+ thymocytes, this was normalized to WT levels by expression of the Itkas transgene (Fig. 2F). Thus the Itkas can function in the place of WT Itk in allowing T cell development to occur, and normalizes gross T cell developmental abnormalities seen in the absence of Itk. Analysis of peripheral T cells in the spleen revealed similar rescue of the percentage and number of peripheral CD4+ and CD8+ T cells, along with respective naïve and memory populations back to levels in WT and Itk+/− mice (Fig. 3). Along with the normalization of naïve and memory T cell populations, the Itkas transgene also rescued the elevated IgE observed in the absence of Itk (6, 18, 27). We have shown that elevated serum IgE in Itk−/− mice is reflected by the increased percentage of B cells that carry surface IgE (6, 27). We found that B cells from the Itkas mice carried similar levels of surface IgE to that seen in WT mice (Fig. 3B).
Figure 3. Itkas rescues peripheral CD4+ and CD8+ T cells and normalizes memory phenotype T cells.
A) Splenocytes were analyzed for CD4+ and CD8+ T cells (top), or gated on CD4+TCRβ+ or CD8+TCRβ+ (middle panels), percentages and numbers of CD4+TCRβ+CD44lowCD62Lhigh (naïve) and CD4+TCRβ+CD44highCD62Llow (memory), or CD8+TCRβ+CD44lowCD122− (naïve) and CD8+TCRβ+CD44highCD122− (memory) cells (bottom panels). Values are means ± SEMs of n≥5, *p < .05 by unpaired student t test. B) Splenic B cells were analyzed for percentage of cells IgE+ cells (percentage, left panel) or MFI (right panel). Values are means ± SEMs of n=4, *p < .05 by unpaired student t test.
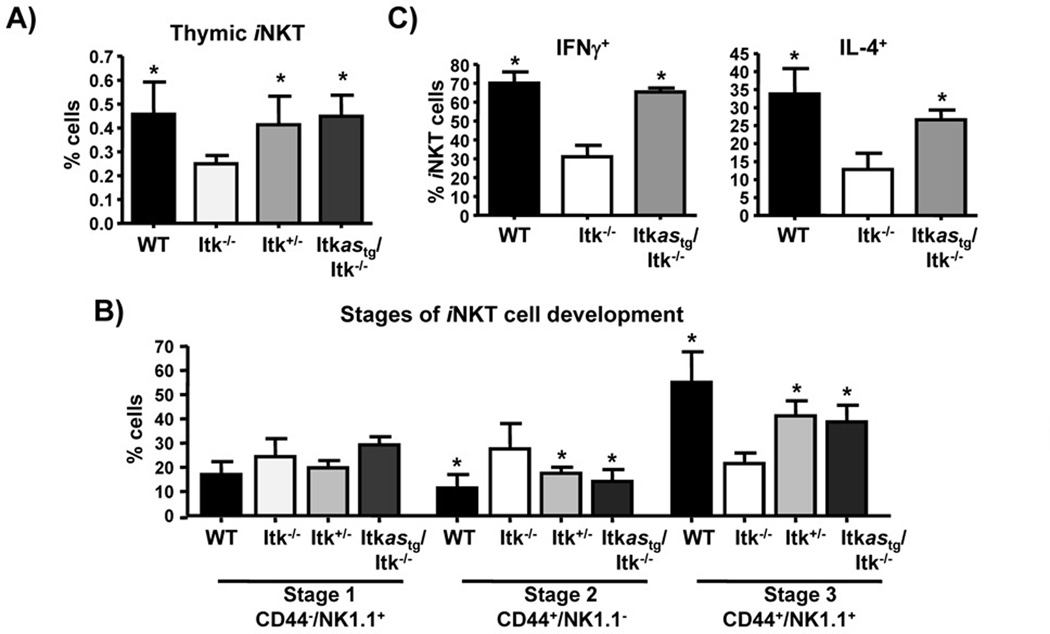
The expression of Itkas also rescued the development of iNKT cells in Itk−/− mice. Both the frequency, as well as the developmental stages in the thymus of Itkastg/Itk−/− mice, were comparable to levels in WT and Itk+/− mice (Fig. 4A, B). The total number iNKT cells were also comparable (WT= 3.70e5+/−1.86e5; Itk−/−=2.49e5+/−1.18e5; Itk+/−= 4.19e5+/−1.84e5; Itkastg/Itk−/−=3.12e5+/−2.19e5, not statistically significant). Furthermore, unlike Itk−/− iNKT cells, Itkas iNKT cells were functional, responding to in vivo stimulation by α-GalCer with rapid production of both IFNγ and IL4 (Fig. 4C). These results suggest that expression of the Itkas transgene completely rescues T and iNKT cell development.
Figure 4. Itkas rescues iNKT cell development.
A) Thymocytes from the indicated mice were analyzed for percent of iNKT cells by gating on CD1d tetramer+TCRβ+ cells. (B) Thymic iNKT-cells were analyzed for expression of CD44 and NK1.1 and divided into Stage 1, Stage 2, and Stage 3 according to CD44 and NK1.1 expression. Data are shown as mean ± SEM of n= 5 mice/group, representative of at-least two independent experiments. *p < 0.05 by unpaired Student t-test. (C) WT, Itk−/− and Itkastg/Itk−/− mice were injected with 2 µg of α-GalCer. Two hours later, spleens were isolated and analyzed for production of IFN-γ and IL-4 by iNKT cells using flow cytometry.
CD4+ and CD8+ T cell TCR-induced proliferation show differential sensitivity to inhibition of Itk
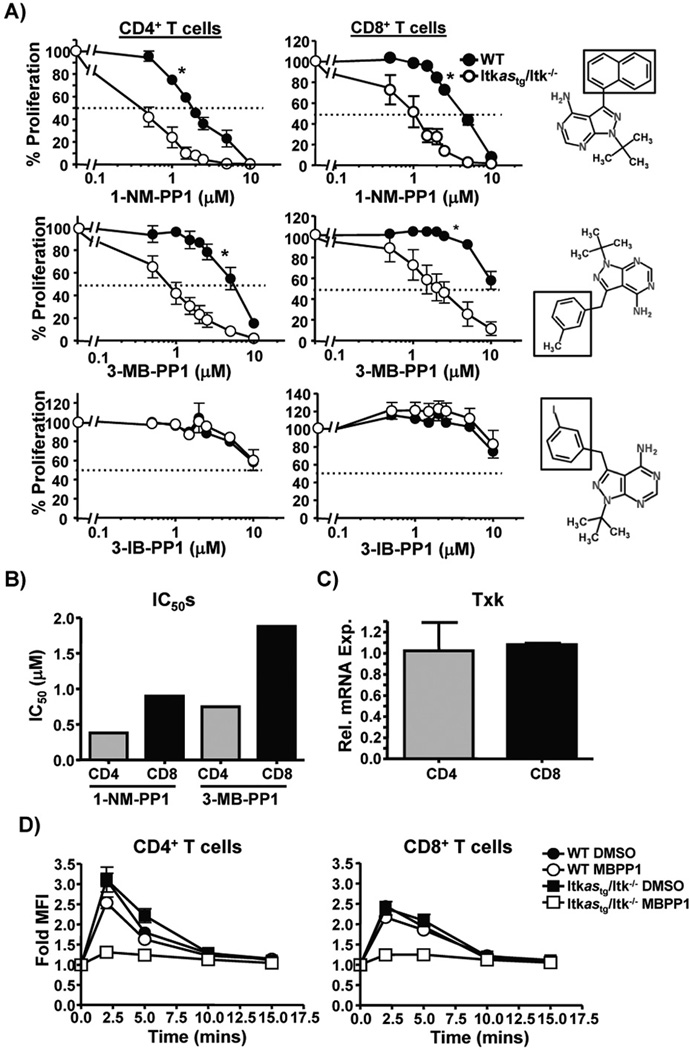
CD4+ and CD8+ T cells are known to have differences in the relationship between TCR signal strength and activation of downstream signaling (28), and we hypothesized that they would have differential sensitivity to signaling through Itk. We compared TcR induced proliferation of CD4 and CD8 T cells in vitro for sensitivity to inhibition of Itk kinase activity. We found that while α-CD3/CD28-induced the proliferation of both CD4+ and CD8+ T cells as expected, and that CD4+ T cells was significantly more sensitive to inhibition by both 1-NM-PP1 and the related analog 3-MB-PP1 compared to CD8+ T cells (Fig. 5A, B). Notably, the analog 3-IB-PP1 (29) had little effect on the proliferation of Itkas T cells (Fig. 5A), suggesting that our system is highly selective and discriminative in terms of inhibition with PP1 analogs. Note that there is no difference in expression of the Itkas in CD4+ and CD8+ T cells (Fig. 2C), and both CD4+ and CD8+ T cells express similar levels of both the transgene and the related Tec family kinase Txk, which can partially compensate for the absence of Itk ((19, 30), (Fig. 5C)). Analysis of TcR induced activation of ERK, a downstream target of Itk, revealed no difference in inhibition by 3-MB-PP1, suggesting that differential sensitivity to ERK activation does not explain the differential sensitivity between CD4+ and CD8+ T cells (Fig. 5D). However, analysis of the proliferative patterns of CD4+ and CD8+ T cells revealed that CD8+ T cells proliferated more, based on dilution of CFSE dye (Supplemental Fig. 1), suggesting that as a potential explanation for the differential effect of inhibiting Itk on proliferation.
Figure 5. TCR induced proliferation of CD4+ and CD8+ T cells show differential sensitivity to inhibition of Itk.
Splenocytes from Tg(CD2-hItkas)Itk−/− mice were loaded with CFSE and stimulated with α-CD3/CD28 for 72 hrs in the presence of varying concentrations of (A) 1-NM-PP1 (Top), 3-MB-PP1 (middle) or 3-IB-BPP1 (bottom) and CFSE dilution of CD4+TCRβ+ and CD8+TCRβ+ cells determined. Percent inhibition of proliferation was calculated by normalizing to respective vehicle control. Values are means ± SEMs of n≥3, representative of at-least 3 independent experiments, *p < .05 by 2-way ANOVA. B) IC50 values for inhibition of proliferation of CD4+ and CD8+ T cells by 1-NM-PP1 and 3-MB-PP1 determined from the data shown in (A). C) Quantification of Txk mRNA in purified CD4+ and CD8+ T cells from Tg(CD2-hItkas)Itk−/− mice, expression level in CD4+ T cells set as 1, values are means ± SEMs of n=3. D) Splenocytes from Tg(CD2-hItkas)Itk−/− mice were stimulated with anti-CD3 and crosslinking antibody, and CD4 and CD8 T cells analyzed for phospho-ERK by flow cytometry. Fold change in MFI is plotted with non-stimulated cells set as 1. Values are means ± SEMs, of 2 experiments.
Itk kinase activity controls cytokine production by iNKT cells, Th1, Th2 and Th17 cells
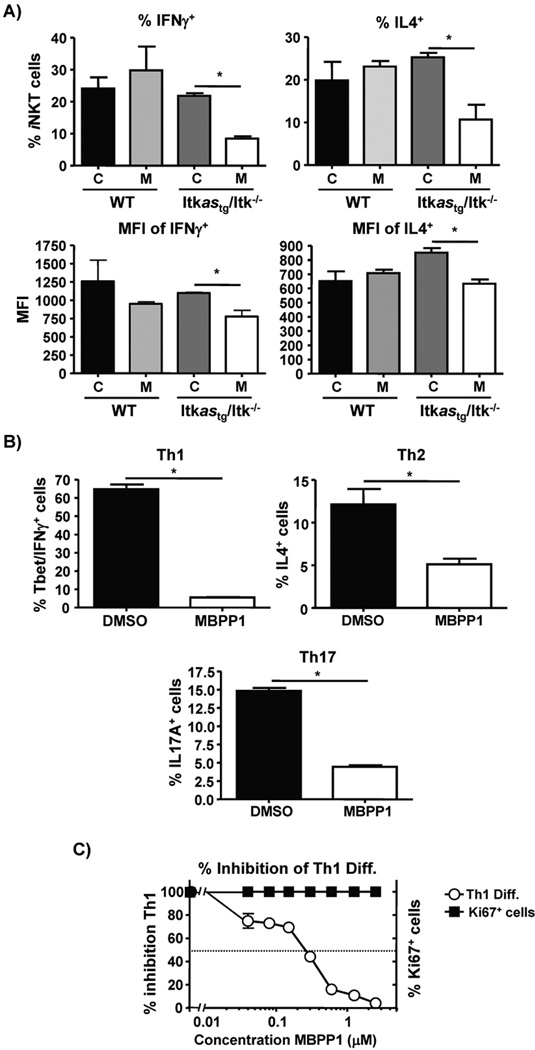
The Itkas transgene rescued development of iNKT cells, and the production of cytokines in vivo by these cells (Fig. 4). Although Itk−/− iNKT cells exhibit defects in the production of cytokines upon stimulation via the TcR with α-GalCer (4, 12–14), this could be secondary to alterations in development. To determine whether cytokine production by iNKT cells is sensitive to inhibition of Itk kinase activity, we examined cytokine production in mice injected with both α-GalCer and the allele sensitive kinase inhibitor 3-MB-PP1, or controls. Splenocytes from injected mice were isolated 2 hours post α-GalCer injection and cultured with BFA for 4 hours, followed by intracellular staining for iNKT cell cytokines as previously described (13). We found that production of both IFNγ and IL4 (as measured by % cytokine positive iNKT cells, Fig. 6A), as well as amount of cytokine/cell (as measured by MFI if the cytokine positive cells, Fig. 6B), was sensitive to inhibition in the Itkas expressing iNKT cells, but not WT iNKT. These results indicate that Itk activity is important for production of cytokines by iNKT cells. Itk has also been shown to be critical for the generation of Th2 and Th17 cells (16, 31). In line with this we find that inhibition of the kinase activity of the Itk resulted in a significant reduction in the ability of Th1, Th2 and Th17 cells to produce IFNγ, IL-4 or IL-17 (Fig. 6B). Since the effect on Th1 cells was unexpected based on the precious reports that Itk does not affect Th1 responses (2, 15, 16), we explored this further and found that inhibiting Itk during Th1 differentiation dose dependently inhibited Th1 differentiation, without significantly affecting proliferation (as measured by Ki67 expression) (Fig. 6C). Thus while defects in Th2 and Th17 cytokine production seen in Itk−/− Th cells are likely due to the absence of Itk kinase activity, the lack of effect of Itk deficiency on Th1 cytokine production we and others have previously differs when the kinase activity of Itk is specifically inhibited.
Figure 6. Itk catalytic activity is critical for iNKT cell, Th1, Th2 and Th17 cytokine production.
A) IFNγ and IL-4 production by iNKT cells from indicated mice 2 hours post injection with 2 µg of α-GalCer with vehicle or 3-MB-PP1. Percentage (top panels) and mean fluorescence intensity (bottom panels) of the cytokine. C= vehicle (DMSO), M= 3-MB-PP1. Values are means ± SEMs of n≥5, representative of at-least 2 independent experiments, *p < .05 by unpaired student t test. B) Naïve CD4+ T cells were differentiated into Th1 (left panel), Th2 (middle panel) or Th17 cells (right panel) in the presence of vehicle (DMSO) or 3-MB-PP1, then restimulated for analysis of cytokine production. B) Naïve CD4+ T cells were cultured under Th1 conditions with the indicated concentrations of 3-MB-PP1, then analyzed for expression of IFNγ and T-bet. Cells cultured in the presence of vehicle were set at 100% and differentiation compared to the indicated concentrations of compound. Values are means ± SEMs of n≥5, representative of at-least 3 independent experiments, *p < .05 by unpaired student t test.
Discussion
In this report, we show that an allele sensitive mutant of Itk whose catalytic activity can be selectively inhibited by analogs of the PP1 kinase inhibitor. Our results show that this Itkas behaves similar to the WT Itk in the absence of the inhibitor, rescuing T and iNKT cells development in the absence of Itk. Furthermore, we used this allele to show that Itk activity is required for Th1, Th2, Th17 and iNKT cell cytokine production in vitro. This novel murine model will allow more subtle analysis of the function of Itk in T and iNKT cell development and function.
The approach that we have taken here with Itk has been shown by others to be useful in the analysis of the kinase activity of Itk, Lck, ZAP70 and most recently Csk in T cell function (24, 32–34). In the work with Lck, ZAP70 and Csk, it was found that a simple alteration of the gatekeeper residue allowed sensitivity to 1-NM-PP1 and/or 3-MB-PP1, similar to what Shokat and colleagues had originally reported for the Src kinases (20, 21). However, in our work, and that of Andreotti et al on Itk (32), who also recently reported a similar Itk allele sensitive mutant, alteration of the gatekeeper residue resulted in a complete loss of kinase activity, and we and Andreotti et al have had to generate compensating changes to rescue this activity. In Andreotti et al’s work, changing Leucine 432 to Isoleucine on an F434A gatekeeper mutant background resulted in ~74% rescue of kinase activity, likely by restoring the regulatory spine in the kinase domain (32). Our Δ429R mutant on the F434G background is in a similar location and likely also restores the regulatory spine in the kinase domain, although this remains to be determined. Nevertheless, these results suggest that Itk behaves differently from the other kinases with regards to the regulation of its kinase activity, and tolerance for changes in the ATP binding pocket.
We do note however, that Weiss and colleagues did report that the Zap70 allele sensitive mutant they generated exhibited reduced kinase activity, but this was sufficient for further experiments and rescue of T cell development and function (24). We also tested related the 1-NM-PP1 analog 3-IB-PP1, which differs from 3-MB-PP1 by the substitution of an Iodo-group instead of a methyl group at the 3 position of the benzyl ring (3-methylbenzyl vs. 3-Iodobenzyl, see Fig. 5 for structures, (21)). The fact that 3-IB-PP1 did not affect cells expressing the Itkas and 3-MB-PP1 and 1-NM-PP1 do, suggests that the Itkas ATP binding pocket can discriminate between these substitutions, and allows the use of a closely related compound for subsequent inhibitor studies.
The loss of Itk results in altered T cell development, fewer CD4+ and CD8+ T cells and a significant increase in the proportion of innate memory CD4+ and CD8+ T cells (8–11, 35). Itk signaling also has different outcomes dependent on the cell type (3). The allele sensitive system we report here has allowed us to isolate an apparent differential requirement for Itk kinase activity during TCR induced proliferation of CD4+ and CD8+ T cells. However, while proliferation of naïve CD4+ T cells may be more sensitive than CD8+ T cells to deletion of the adaptor protein Gads (36), a part of the signaling complex along with the Slp-76/Itk complex, which is recruited to LAT during TCR signaling and upstream of the ERK pathway (37, 38), our analysis of the sensitivity to ERK activation revealed no difference between CD4+ and CD8+ T cells. Our data suggest that this apparent difference may be due to the observation that CD8+ T cells proliferate more in vitro in response to anti-CD3/CD28 stimulation compared to CD4+ T cells.
Using transgenic mice carrying a kinase inactive mutant of Itk, we have previously shown that development of iNKT cells is partially independent of kinase activity (14). Furthermore, we and others have shown that like WT iNKT cells, Itk−/− iNKT cells carry preformed mRNA for both IFNγ and IL-4, yet show defects in production of these cytokines upon stimulation with α-GalCer (3, 4, 12, 14). Our data here showing that Itk kinase activity is required for iNKT production of IFNγ and IL-4 are in support of view that kinase activity is required for the production of these cytokines in iNKT cells. While iNKT cells carry preformed mRNA for these cytokines and can rapidly translate these messages for rapid cytokine production, shown to be dependent on the p38 pathway ((39, 40)), production of these cytokines is also dependent on transcriptional events (41). Itk is not required for the production of the preformed mRNA for IFNγ or IL-4 in iNKT cells (3, 4, 12, 14), but its activity is required for the combination of translation and transcriptional events required for efficient production of these cytokines. Given that the absence of Itk, or the kinase domain, does still allow for some cytokine production in iNKT cells (3, 4, 12, 14), it will be interesting to determine whether translation and/or transcriptional regulation of cytokine production in iNKT cells exhibit differential sensitivity, particularly since Itk regulates the p38 pathway, involved in rapid translation of cytokine message in iNKT cells (40), as well as the NFAT transcription factor, involved in transcriptional regulation of these cytokines (41).
We and others have previously shown that the absence of Itk affect the differentiation and/or cytokine production of Th2 and Th17 cells, while Th1 differentiation and function was not affected (16, 30, 31, 42, 43). However, our current studies show that Itk kinase activity is required for the differentiation and cytokine production of Th1, Th2 and Th17 cells, indicating that for Th2 and Th17 cells, the absence of Itk phenotypically mirrors the inhibition of its kinase activity, while for Th1 cells this is not the case. The difference in the Th1 response between Itk−/− T cells and inhibiting the kinase activity of Itk is likely due to the fact that Itk−/− T cells exhibit elevated expression of the T-bet transcription factor and are more likely to differentiate to the Th1 lineage upon activation (31, 43). Using T cells from these Itk allele sensitive mice, we have also recently reported that kinase activity suppresses the development of T regulatory cells (44). Thus our data suggest that Itk inhibitors may also be useful for suppressing Th1, Th2 and Th17, while enhancing T regulatory cells and their suppressive responses.
This Itkas mouse model will be very useful in unraveling how Itk signaling fine-tunes the differentiation and effector function of the different T helper cell subsets as well as its role in the generation of effector and memory CD8+ T cells. It would be interesting to determine whether the level of sensitivity to Itk inhibition is different among the different T helper cell subsets and between effector and memory CD8+ T cells, as well as in mouse models of T cell mediated disease.
Materials and Methods
Mice
All mice were on the C57Bl/6 background. Tg(CD2-hItkas) mice were generated by cloning the cDNA of human Itkas into a transgenic expression cassette driven by the CD2 promoter and CD2 enhancer as described (8). Founder lines were backcrossed to Itk−/− mice to generate the Tg(CD2-hItkas)Itk−/− mice. These, along with Itk+/− and WT mice were housed in specific pathogen free environment and used between 6- to 12- weeks of age. All experiments were performed in accordance with the IACUC at Cornell University.
Transfection, western blotting and analysis of kinase activity
HEK 293T cells were transfected as previously described (45), with plasmids encoding GFP-tagged human WT Itk or mutants, HA-tagged Txk or Flag-tagged Tec. In some cases cells were transfected with GFP-tagged WT Itk or F434G/Δ429 mutant with or without PLCγ1 plasmid as indicated. 48 hrs after transfection, cells were treated with 1-NM-PP1 or vehicle for 1 hr prior to harvest and analysis with antibodies against HA (clone HA-7, Sigma). Kinase activity in cells was determined by analysis of phosphotyrosine content of Itk or the substrate PLCγ1, which correlates with kinase activity of Itk (46). IC50 values were determined by treating similar transfectants with varying concentrations of 1-NM-PP1 and analysis of phosphotyrosine content in Itk.
Analysis of T cell proliferation and T helper cell cytokine production
To determine proliferation of T cells, splenocytes were loaded with CFSE (Molecular Probes), washed and stimulated with 1µg/ml anti-CD3ε (BD Biosciences) and 3 µg/ml anti-CD28 (BD Biosciences).
For Th polarizing conditions, naïve CD4+ T cells were isolated using the naïve CD4+ T cell isolation kit (Miltenyi Biotec) and antigen-presenting cells (APCs) were isolated by depletion of CD3+ T cells from spleens of C57Bl/6 mice using biotin-anti-CD3 (eBioscience) and Miltenyi anti-biotin microbead kit (Miltenyi Biotec). T cells were cultured at a ratio of 1:5 with mitomycin C treated antigen-presenting cells (APCs) in complete RPMI 1640. Th1 polarizing conditions included 1 µg/mL anti-CD3 (BD Biosciences), 3 µg/ml anti-CD28 (BD Biosciences), and 40 ng/mL IL4 (Peprotech). Th2 polarizing conditions included 1 µg/mL anti-CD3 (BD Biosciences) and 3 µg/ml anti-CD28 (BD Biosciences), 40 ng/mL IL12 (Peprotech) and 10 µg/mL of anti-IFNγ and anti-IL12 (Biolegend). Th17 polarizing conditions included 1 µg/mL anti-CD3 and 1 µg/mL anti-CD28 (BD Biosciences), 20 ng/mL of IL6 (Peprotech), 5 ng/ml TGFβ and 10 µg/mL anti-IFNγ and anti-IL12 (Biolegend). Related analogs that inhibit allele sensitive kinases 1-NM-PP1, 3-MB-PP1 and 3-I-PP1 (21, 29) were used at the indicated concentrations.
α-GalCer stimulation
Mice were treated (i.p.) with vehicle (DMSO) or 3-MB-PP1 2 hours prior to i.p. injection with 2 µg α-GalCer or vehicle. Splenocytes were isolated 2 hours post α-GalCer injection and cultured with BFA for 4 hours, followed by intracellular staining for iNKT cell cytokines as previously described (13).
Analysis of iNKT cell development
Thymus and spleens were collected and T cells analyzed by staining for the indicated markers. iNKT cells were characterized using Allophycocyanin-Cy7-TCRβ were from BD Biosciences (San Jose, CA), V500-CD44, Allophycocyanin-NK1.1 BioLegend (San Diego, CA), and PE-PBS57 loaded CD1d tetramer from the National Institute of Allergy and Infectious Diseases Tetramer Facility. IgE positive B cells were determined by staining with Alexa Fluor 647-B220 and PE-IgE from eBioscience (San Diego, CA).
T cell stimulation and pERK staining
Freshly isolated splenocytes were pre-incubated with 5µg/ml hamster anti-CD3ε (eBioscience) and 10 µg/ml hamster anti-CD28 (eBioscience) at 2 × 107 cells/ml, on ice for 30 mins, followed by cross-linking with 20 µg/ml goat anti-hamster IgG (855387, MP Biomedicals) for the indicated time points. Stimulation was terminated by adding pre-chilled 4 volume 5% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Cells were then fixed, permeabilized, then stained with PE-CF594-CD8α (BD Biosciences), PE-Cy7-CD4 (BioLegend) and Alexa Fluor 488- Phospho-p44/42 (Erk1/2) (Cell Signaling, E10). All flow cytometry data were acquired on LSRII (BD Biosciences), and analyzed in FlowJo (Tree Star, Ashland, OR).
Reverse transcription/ Quantitative PCR
cDNA was synthesized using You Prime First-Strand beads kit (GE Healthcare) on RNA that was extracted using RNAeasy kit (Invitrogen). qPCR was performed using 7500 Fast Real-Time PCR instrument (Applied Biosystems). Ct values was normalized to respective GAPDH values and analyzed using the 2−ΔΔCT method as described in the figure legend.
Statistical analysis
Un-paired two-tailed Student’s t test was performed using GraphPad Prism v5.00 (GraphPad, San Diego, CA), with p < 0.05 considered statistically significant.
Supplementary Material
Acknowledgements
We thank Amie Wood for animal care. This work was supported by grants from the National Institutes of Health (AI51626 & AI065566) to AA.
Abbreviations Used
- α-GalCer
Alpha-Galactosyl Ceramide
- BFA
Brefeldin A
- CFSE
Carboxyfluorescein succinimidyl ester
- 3-IB-PP1
3-iodobenzyl-PP1
- 1-NM-PP1
1-naphthylmethyl-PP1
- 3-MB-PP1
3-methylbenzyl-PP1.
Footnotes
Conflict of Interest Disclosure
The authors have no conflicting financial interests.
References
- 1.Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol. 2010;2:a002287. doi: 10.1101/cshperspect.a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Rodriguez J, Kraus Z, Schwartzberg P. Tec family kinases in T lymphocytes: Cross-regulation of cytokine production and T cell fates. FEBS Lett. 2011;278:1980–1989. doi: 10.1111/j.1742-4658.2011.08072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi Q, Kannan AK, August A. Tec family kinases: Itk signaling and the development of NKT alphabeta and gammadelta T cells. FEBS J. 2011;278:1970–1979. doi: 10.1111/j.1742-4658.2011.08074.x. [DOI] [PubMed] [Google Scholar]
- 4.Felices M, Berg L. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 5.Felices M, Yin C, Kosaka Y, Kang J, Berg L. Tec kinase Itk in gammadelta T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, August A. Enhanced development of CD4+ {gamma}{delta} T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. Epub 2009 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia M, Qi Q, Jin Y, Wiest D, August A, Xiong N. Differential Roles of IL-2-Inducible T Cell Kinase-Mediated TCR Signals in Tissue-Specific Localization and Maintenance of Skin Intraepithelial T Cells. J Immunol. 2010;184:6807–6814. doi: 10.4049/jimmunol.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broussard C, Fleischecker C, Horai R, Chetana M, Venegas A, Sharp L, Hedrick S, Fowlkes B, Schwartzberg P. Altered development of CD8+ T cell lineages in mice deficient for the tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Atherly L, Lucas J, Felices M, Yin C, Reiner S, Berg L. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Au-Yeung B, Fowell D. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. J Immunol. 2007;179:111–119. doi: 10.4049/jimmunol.179.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Qi Q, Huang W, Bai Y, Balmus G, Weiss RS, August A. A unique role for ITK in survival of invariant NKT cells associated with the p53-dependent pathway in mice. J Immunol. 2012;188:3611–3619. doi: 10.4049/jimmunol.1102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Q, Xia M, Bai Y, Yu S, Cantorna M, August A. Interleukin-2-inducible T cell kinase (Itk) network edge dependence for the maturation of iNKT cell. J Biol Chem. 2011;286:138–146. doi: 10.1074/jbc.M110.148205. Epub 2010 Oct 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.August A, Ragin MJ. Regulation of T-cell responses and disease by tec kinase Itk. Int Rev Immunol. 2012;31:155–165. doi: 10.3109/08830185.2012.668981. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Rodriguez J, Sahu N, Handon R, Davidson T, Anderson S, Kirby M, August A, Schwartzberg P. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara T, Mueller C, Sahu N, Ben-Jebria A, August A. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. J Allergy Clin Immunol. 2006;117:780–786. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- 18.Mueller C, August A. Attenuation of Immunological Symptoms of Allergic Asthma in Mice Lacking the Tyrosine Kinase ITK. J Immunol. 2003;170:5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 19.Sahu N, Venegas A, Jankovic D, Mitzner W, Gomez-Rodriguez J, Cannons J, Sommers C, Love P, Sher A, Schwartzberg P, August A. Selective expression rather than specific function of Txk and Itk regulate Th1 and Th2 responses. J Immunol. 2008;181:6125–6131. doi: 10.4049/jimmunol.181.9.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop A, Shah K, Liu Y, Witucki L, Kung C, Shokat K. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 21.Shokat K, Velleca M. Novel chemical genetic approaches to the discovery of signal transduction inhibitors. Drug Discov Today. 2002;7:872–879. doi: 10.1016/s1359-6446(02)02391-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Kenski D, Paulson J, Bonshtien A, Sessa G, Cross J, Templeton D, Shokat K. A second-site suppressor strategy for chemical genetic analysis of diverse protein kinases. Nat Methods. 2005;2:435–441. doi: 10.1038/nmeth764. [DOI] [PubMed] [Google Scholar]
- 23.Kenski DM, Zhang C, von Zastrow M, Shokat KM. Chemical genetic engineering of G protein-coupled receptor kinase 2. J Biol Chem. 2005;280:35051–35061. doi: 10.1074/jbc.M507594200. [DOI] [PubMed] [Google Scholar]
- 24.Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. 2008;283:15419–15430. doi: 10.1074/jbc.M709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- 27.Iyer A, August A. The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses. J Immunol. 2008;180:7869–7877. doi: 10.4049/jimmunol.180.12.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan A, Huang W, Huang F, August A. Signal transduction via the T cell antigen receptor in naive and effector/memory T cells. The international journal of biochemistry & cell biology. 2012;44:2129–2134. doi: 10.1016/j.biocel.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregan J, Zhang C, Rumpf C, Cipak L, Li Z, Uluocak P, Nasmyth K, Shokat KM. Construction of conditional analog-sensitive kinase alleles in the fission yeast Schizosaccharomyces pombe. Nat Protoc. 2007;2:2996–3000. doi: 10.1038/nprot.2007.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 31.Kannan A, Sahu N, Mohanan SSM, August A. Itk modulates allergic airway inflammation by suppressing IFNγ in naïve CD4+ T-cells. J. Allergy Clin. Immunol. 2013;132:811–820. e811–e815. doi: 10.1016/j.jaci.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph RE, Andreotti AH. Controlling the activity of the Tec kinase Itk by mutation of the phenylalanine gatekeeper residue. Biochemistry. 2011;50:221–229. doi: 10.1021/bi101379m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15:186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denzel A, Hare KJ, Zhang C, Shokat K, Jenkinson EJ, Anderson G, Hayday A. Cutting edge: a chemical genetic system for the analysis of kinases regulating T cell development. J Immunol. 2003;171:519–523. doi: 10.4049/jimmunol.171.2.519. [DOI] [PubMed] [Google Scholar]
- 35.Schaeffer E, Broussard C, Debnath J, Anderson S, McVicar D, Schwartzberg P. Tec Family Kinases Modulate Thresholds for Thymocyte Development and Selection. J. Exp. Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yankee TM, Yun TJ, Draves KE, Ganesh K, Bevan MJ, Murali-Krishna K, Clark EA. The Gads (GrpL) adaptor protein regulates T cell homeostasis. J Immunol. 2004;173:1711–1720. doi: 10.4049/jimmunol.173.3.1711. [DOI] [PubMed] [Google Scholar]
- 37.Bunnell S, Diehn M, Yaffe M, Findell P, Cantley L, Berg L. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J Biol Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 38.Law CL, Ewings MK, Chaudhary PM, Solow SA, Yun TJ, Marshall AJ, Hood L, Clark EA. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J Exp Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaleekar VK, Sabio G, Aktan I, Chant A, Howe IW, Thornton TM, Benoit PJ, Davis RJ, Rincon M, Boyson JE. Translational control of NKT cell cytokine production by p38 MAPK. J Immunol. 2011;186:4140–4146. doi: 10.4049/jimmunol.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atherly L, Brehm M, Welsh R, Berg L. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 2006;176:1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Kusam S, Munugalavadla V, Kapur R, Brutkiewicz R, Dent A. Regulation of Th2 cytokine expression in NKT cells: unconventional use of Stat6, GATA-3, and NFAT2. J Immunol. 2006;176:880–888. doi: 10.4049/jimmunol.176.2.880. [DOI] [PubMed] [Google Scholar]
- 42.Schaeffer E, Yap G, Lewis C, Czar M, McVicar D, Cheever A, Sher A, Schwartzberg P. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol. 2001;2:1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 43.Miller A, Wilcox H, Lai Z, Berg L. Signaling through Itk Promotes T Helper 2 Differentiation via Negative Regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Huang W, Jeong A-R, Kannan A, Huang L, August A. IL-2 inducible T cell kinase (ITK) tunes Treg development and is required for suppressive function. J Immunol. 2014;193:2267–2272. doi: 10.4049/jimmunol.1400968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi Q, August A. The Tec family kinase Itk exists as a folded monomer in vivo. J Biol Chem. 2009;284:29882–29892. doi: 10.1074/jbc.M109.003129. Epub 22009 Aug 29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.