Abstract
The glial cell line–derived neurotrophic factor (GDNF) family of ligands (GFLs) comprising of GDNF, neurturin, artemin, and persephin plays an important role in the development and maintenance of the central and peripheral nervous system, renal morphogenesis, and spermatogenesis. Here we review our current understanding of GFL biology, and supported by recent progress in the area, we examine their emerging role in endocrine-related and other non–hormone-dependent solid neoplasms. The ability of GFLs to elicit actions that resemble those perturbed in an oncogenic phenotype, alongside mounting evidence of GFL involvement in tumor progression, presents novel opportunities for therapeutic intervention.
Introduction
The glial cell line–derived neurotrophic factor (GDNF) family of ligands (GFLs) is comprised of four structurally related factors: GDNF, neurturin (NRTN), artemin (ARTN), and persephin (PSPN) [1], [2], [3]. As their name suggests, these transforming growth factor beta–like growth factors have traditionally been implicated in the development and maintenance of central and peripheral neurons [2], [4] and consequently have generated therapeutic interest for combating neurodegenerative diseases such as Parkinson's disease [5]. Outside the nervous system, these factors mediate many other processes such as renal morphogenesis in the kidney [6], [7].
It is well known that tumors use growth factor pathways to progress an oncogenic phenotype and consequently escape the typical constraints of cellular growth. Growth factor pathways such as the epidermal growth factor (EGF) pathway [8], [9] and the vascular endothelial growth factor (VEGF) pathway [10], [11] have become the basis for development of anticancer therapeutics. Several lines of evidence suggests that the GFL/RET (rearranged during transfection) receptor tyrosine kinase signaling pathway may also present further potential targets for a multitude of cancers [12], [13].
The purpose of this review is to provide a renewed perspective of the emerging role of neurotrophic factors, specifically the GDNF family, in neoplasm.
GFL Signaling
Each member of the GDNF family is expressed as a pre-pro-precursor protein, which is proteolytically cleaved at a putative furin-like cleavage site (RAAR) by yet unidentified enzymes to generate an active form [14], [15]. GFLs act as biologically active homodimers that signal canonically through the transmembrane receptor RET. This is facilitated by each GFL binding to a preferred glycosyl phosphatidylinositol (GPI)–linked GDNF family receptor α (GFRα) co-receptor, e.g., GDNF predominantly binds to GFRα1, NRTN to GFRα2, ARTN to GFRα3, and PSPN to GFRα4. However, this ligand-specific binding to GFRα co-receptors at times is promiscuous with a GFL ligand capable of interacting and functionally signaling with one of other nonpreferred GFRα proteins. GDNF can also bind to GFRα2, NRTN to GFRα1, and ARTN to GFRα1 [16], [17]. In neuronal populations, transforming growth factor beta is responsible for recruiting GFRα1 to the plasma membrane to allow GDNF activation [18], [19], [20]. The stoichiometry of GFL:GFRα:RET binding interaction (as based on GDNF) is postulated to be one ligand homodimer to two GFRα molecules to two RET receptors, forming a heterohexameric complex [21]. RET homodimerization and subsequent autophosphorylation activate downstream signal transduction. Although not fully understood, all three components appear to be requisite for downstream signaling, as mice with homozygous deletions in either Ret or Gfra1 exhibit a similar phenotype to mutant Gdnf−/− mice, which die shortly after birth due to kidney defects and a lack of enteric innervation [3]. However, GFRα-independent signaling has also been observed. For example, heparan sulfate proteoglycan syndecan-3 can serve as a co-receptor to transduce GFL signal to the RET with the involvement of Src kinase activation [22].
The RET receptor tyrosine kinase has three functional regions: an extracellular domain containing four cadherin-like domains (CLD1 to 4) followed by a cysteine-rich domain (CRD), a hydrophobic transmembrane region and an intracellular dual tyrosine kinase domain (TK1 and TK2) [23]. RET possesses several glycosylation sites, resulting in a mature protein molecular weight of 170 kDa. RET is present in at least three isoforms, RET51, RET43, and RET9, which contain 51, 43, or 9 amino acids in their unique C-terminal tails, respectively [23]. Isoforms RET9 and RET51 have distinctive signaling properties. The internalization process of RET isoforms was studied using total internal reflection fluorescence microscopy. The RET51 was robustly internalized from the cell surface into endosomal compartments upon the activation by GDNF, while RET9 was considerably slow [24].
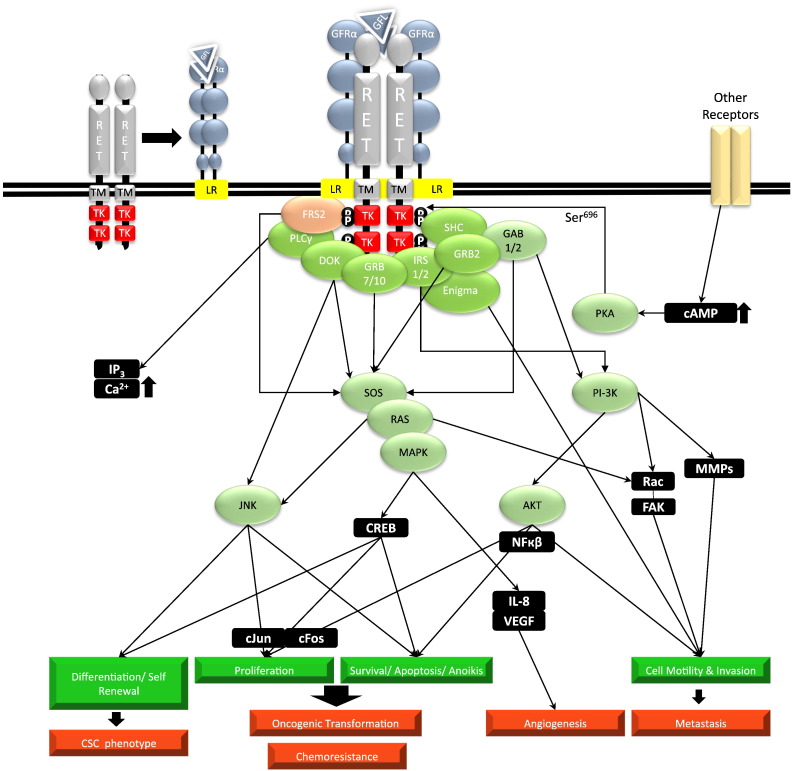
GFLs activate several signal transduction pathways, including phosphatodylinositol-3-kinase (PI3K)/protein kinase B (AKT), RAS/mitogen activated protein kinase (MAPK), phospholipase C gamma (PLCγ), and c-Jun N-terminal kinase (JNK) pathways. These pathways propagate the cellular effects of RET activation such as cell survival, proliferation, differentiation, migration, branching morphogenesis, chemotaxis, and potentially oncogenesis (Figure 1).
Figure 1.
Traditional RET-dependent signaling pathways of GFLs with physiological and oncogenic consequences. Upon binding of the GFL dimer to its respective GFRα pair, the complex induces RET dimerization and consequently tyrosine kinase domain (TK) autophosphorylation. A series of SH2 (Src Homology 2) domain adapter proteins (green) including FRS2 (fibroblast growth factor receptor substrate 2), PLCγ (phospholipase C gamma), DOK (downstream of kinase) 4/5, GRB (growth factor receptor-bound protein) 7/10, IRS 1/2 (insulin receptor substrate 1 or 2), GRB2 (growth receptor binding protein 2), SHC (Src homology 2 domain containing), and Enigma binds to their respective phosphorylated tyrosine residues, predominantly Tyr 905, Tyr1015, Tyr1062, or Tyr1096. The signaling cascade activates downstream effector molecules (orange and black), resulting in functional responses such as differentiation, self-renewal, proliferation, survival, apoptosis, and cell motility. When perturbed, these processes become the driving facets of oncogenesis. Arrows are not restricted to only direct interactions.
GFL, GDNF family ligand; TK, tyrosine kinase domain; TM, transmembrane domain; LR, lipid raft; P, phosphorylated tyrosine residue; GFRα, GDNF family receptor alpha; PI3K, phosphatidylinositol 3 kinase; AKT, protein kinase B; IP3, Inositol triphosphate; SOS, Son of Sevenless; MAPK, mitogen activate protein kinase; FAK, focal adhesion kinase; MMPs, matrix metalloproteinases; cAMP, cyclic adenosine mono phosphate; PKA, protein kinase A; JNK, c-Jun N-terminal Kinase; CREB, cAMP response element binding; NFB, nuclear factor-kappa beta; IL-8, interleukin 8; GAB1, GRB2 associated binding protein 1; CSC, cancer stem cell.
Four key tyrosine residues, Tyr905, Tyr1015, Tyr1062, and Tyr1096, are responsible for initiating the downstream phosphorylation cascade following RET autophosphorylation. Only the long isoform (RET51) possesses Tyr1062 and Tyr1096 [25]. Tyr905, Tyr1015, and Tyr1096 are binding sites for the adapter proteins GRB7/10, PLCγ, and GRB2, respectively. Additionally, Tyr1062 can bind to at least five additional families of docking proteins: Src homology 2 domain containing (SHC), fibroblast growth factor receptor substrate 2 (FRS2), insulin receptor substrate 1 or 2 (IRS1/2), the DOK (downstream of kinase) family of proteins (DOK1/4/5/6), and Enigma [3], [26], [27], [28]. Activation of RET via GPI-linked GFRα takes place predominantly within lipid rafts, while signaling outside lipid rafts is mediated by soluble GFRα bound to extracellular GFL [29]. In addition to tyrosine phosphorylation, RET phosphorylation can also occur at Ser696 in response to increased cAMP levels, resulting in protein kinase A (PKA) activation [30].
A recent development in GFL signaling has been the identification of an essential interaction of heparan-sulfate proteo-glycosaminoglycans (HSPGs), such as syndecans and glypicans, [31], [32] with GFLs and their receptors. An interaction between GDNF and HSPGs had always been suspected since the discovery that ex vivo kidney development failed under heparin-sulfate deprivation, in a similar fashion to GDNF or GFRα1 deletion [33], [34], [35], [36]. GDNF, NRTN, and ARTN, but not PSPN, have been shown to bind to syndecan-3 [22]. Likewise, ARTN is also known to bind heparin-sulfates [37]. GDNF promotes migration of cortical neurons via interaction with syndecan-3 [22]. It was demonstrated that the presence of heparin-sulfates is required for GDNF activation of RET tyrosine kinase activation in a MDCK cell line [31]. It was suggested that the low-affinity GDNF:GFRα1 interaction, identified by [21] in RET-deficient cells, is likely to be due to heparan-sulfate binding. It has been found that HSPGs have a role in facilitating RET activation, acting to increase the local GDNF concentration through low-affinity binding in the vicinity of the GFRα receptors [2], and providing a linking mechanism to activate Src kinases and subsequently Met during RET-independent signaling [38].
Unlike other receptor tyrosine kinases, binding of different GFLs to their cognate co-receptors GFRα does not appear to result in a differential activation of downstream signal transduction pathways. In fact, different GFLs actually induce coordinated phosphorylation of the same four key RET tyrosine residues (Tyr905, Tyr1015, Tyr1062, and Tyr1096) with similar kinetics and eliciting a similar signaling pathway profile [39]. The Tyr1062 plays a key role in RET signaling during development as revealed by mice with a silencing mutation at Tyr1062 displaying a similar phenotype as Ret−/− null mutants [40], [41]. The RET Tyr1062 phosphotyrosine serves as a docking site for multiple intracellular adaptor proteins, which are differentially used by different GFL-GFRα complexes to regulate alternative RET-stimulated cellular events [42]. Therefore, the binding promiscuity of Tyr1062 may prove to be an avenue of further research in this regard, with yet unidentified adaptor proteins serving as distinct modifiers of biological activity. Furthermore, there is a mounting body of evidence for RET-independent signaling mechanisms.
RET-Independent Signaling
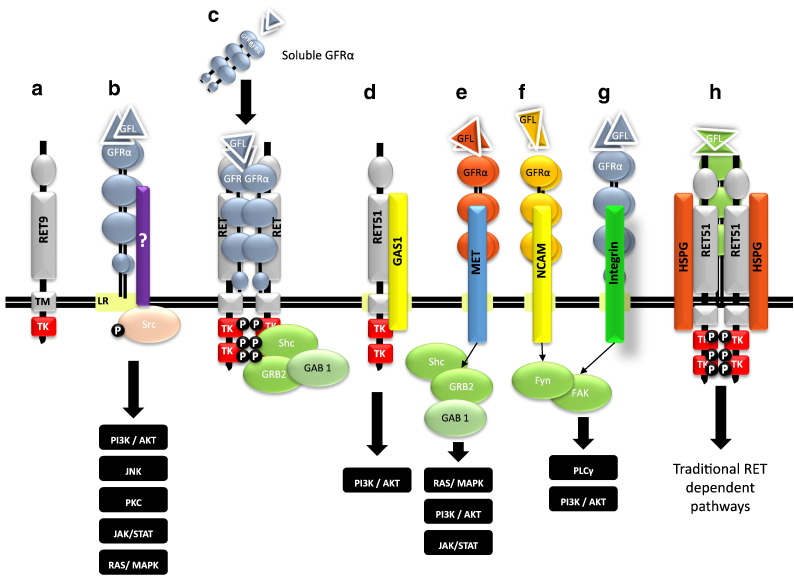
Recent questions have been raised as to how GFL molecules elicit discrete functions. Adaptor proteins, GFL-GFRα-RET internalization rates, and RET stimulation in trans (via soluble GFRα) can partially explain this; however, RET-independent signaling mechanisms hold more promise. It has been demonstrated that many tissues express GDNF and GFRα1, but not RET, suggesting the presence of RET-independent pathways [43]. In addition to the aforementioned HSPG-dependent signaling, other mechanisms exist through which GFL members can interact with other growth factor receptors such as neural cell adhesion molecules (NCAMs) or integrins [44] (Figure 2).
Figure 2.
Alternative signaling pathways of GFLs. In addition to the traditional GFL:GFRα:RET51 pathway, several other novel pathways have been discovered to modulate GFL signaling [from left to right]. (a) Alternative RET isoforms, e.g., RET9 (ret proto-oncogene isoforms c); (b) activated Src (v-src sarcoma viral oncogene homolog) signaling associated with lipid rafts (LR) by means of yet an unknown transmembrane protein; (c) soluble GFRα responsible for RET activation outside lipid rafts; (d) GAS1 (growth arrest-specific 1), a recent GFRα alternative receptor; (e) MET (met proto-oncogene); (f) NCAM (neural cell adhesion molecule); (g) integrins; and (h) HSPGs (heparan sulfate proteoglycans) are essential for RET activity.
TM, transmembrane domain; TK, tyrosine kinase domain; Fyn, p59fyn kinase; FAK, focal adhesion kinase; GAB1, GRB2 associated binding protein 1;GRB2, growth receptor binding protein 2; PI3K, phosphatidylinositol 3 kinase; AKT, protein kinase B; JAK, Janus kinase; STAT, signal transducer and activator of transcription; SHC, Src homology 2 domain containing.
In the absence of RET, GDNF is able to signal through lipid raft associated Src family kinases (SFKs). Despite the report of the co-immunoprecipitation of GFRα1 and Src [38], a direct interaction is not plausible due to their positions on opposite sides of the lipid bilayer [43]. Hence, the existence of a new transmembrane receptor linking Src and GDNF:GFRα1 has been postulated [2], [38]. A potential candidate is the Met tyrosine kinase receptor, as Met phosphorylation in cultured Ret-deficient epithelial and neuronal cells is dependent on SFKs [45]. Additionally, there is an overlap between GDNF:Ret and hepatocyte growth factor (HGF):Met signaling pathways, resulting in similar cellular phenotypic consequences [45]. This suggests an indirect GFRα1-Met association as Met does not immunoprecipitate with GFRα1 [45].
The GDNF:GFRα1 complex is also able to bind to NCAM with high affinity to activate SFKs and FAK [44]. NCAM may also be responsible for facilitating the GFRα-Met interaction alluded to above. GDNF signaling through NCAM is known to occur independently of GFRα1 through direct interaction with the third immunoglobulin domain of NCAM. However, the introduction of GFRα1 results in a higher binding affinity to NCAM. In fact, GFRα1 can also signal through NCAM in the absence of GDNF [44], [46]. NCAM is expressed in many human cancers including mammary and small cell lung carcinoma, and NCAM expression in biliary carcinoma correlates with perineural invasion [47], [48], [49], [50], [51], [52]. In the physiological context, GDNF utilizes NCAM signaling pathways to promote axonal growth [44], and may play a role in regulating cell adhesion and synaptic plasticity in the CNS [53]. The effects of GDNF on survival and growth of midbrain dopaminergic neurons can be suppressed by inhibitory anti-NCAM antibodies [54]. GFRα1 has two alternative splicing isoforms, GFRα1a and GFRα1b, which differ by only five amino acids [55], [56]. GFRα1b, not GFRα1a, mediates GDNF-stimulated cell migration in C6 glioma cells through GDNF-GFRα1b-NCAM-RhoA signaling pathway, which is independent of RET [57].
The recently discovered growth arrest specific gene 1 (GAS1), a protein structurally similar to the GFRα family of receptors, has been shown to regulate RET signaling by binding and sequestering the receptor tyrosine kinase to lipid rafts in the plasma membrane in a similar fashion to the “classic” GFL receptors [58]. Like the GFRα molecules, GAS1 is a GPI-linked protein and constitutively localized in the lipid raft compartment of the plasma membrane. GAS1 is a protein with pleiotropic functions depending on its spatiotemporal expression in development. Such functions include growth inhibition, proliferation, and apoptosis [59]. GAS1 has also been shown to be co-expressed with RET in various tissues [60], [61]. As a co-receptor, GAS1 modulates RET signaling in a ligand- and GFRα1-independent fashion, specifically through the reduction of AKT phosphorylation, and without affecting MAPK activation [58].
CD133, also known as AC133 or prominin-1, is a transmembrane glycoprotein expressed on the surface of normal and cancer cells [62]. GDNF signaling can be regulated by CD133 in the absence of RET in neuroblastoma. It was shown that CD133 repressed neuroblastoma cell differentiation in part by downregulating RET transcription [63]. In CD133-expressing cells, GDNF-induced neurite outgrowth can be rescued by RET overexpression, whereas GDNF-induced expression of neuronal cell differentiation markers cannot be recovered by RET in CD133-expressing cells [63]. Therefore, GDNF can also function in a RET-independent manner in neuroblastoma cells.
The Physiological Role of GFLs
GDNF
GDNF was originally purified from a rat glial cell line (B49) [64] and is widely expressed throughout the rat and mouse central and peripheral nervous system, as well as the inner ear, corneal keratinocytes, olfactory epithelium, skin, submandibular gland, bone, seminiferous tubules, cochlea, oocytes, kidney, teeth, gastrointestinal tract, and carotid body [65], [66], [67]. GDNF expression is detected in mouse embryonic development during early stages of neurogenesis between E7.5 and E10.5 [68]. During organogenesis, GDNF is found primarily in the mesenchyme and its derived tissues, specifically in tissues where epithelial-mesenchymal interaction occurs, such as in the previously mentioned kidney, submandibular gland, and tooth [66], [67]. Similarly, in humans, GDNF is known to be expressed in the nervous system (central and peripheral), retina, kidney, lung [69], pituitary gland [70], skeletal muscle [71], testis, and mammary gland [72].
GDNF was first identified as a promoter of survival and morphological differentiation of rat embryonic midbrain dopaminergic neurons [14]. This observation had dramatic implications for the pursuit of a treatment for Parkinson's disease. But despite early success during human clinical trials [73], [74], treatment of patients with GDNF by direct brain infusion failed to improve the symptoms [75].
Recently, it has been reported that GDNF's protection of dopamine neurodegeneration is due to an inhibition of caspase-3 activation and suppression of endoplasmic reticulum stress–related genes [76]. As a survival factor for motor neurons, GDNF is significantly more potent (75-fold) than the neurotrophins brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and cholinergic differentiation factor–leukemia inhibitor factor (CDF-LIF); GDNF successfully rescued facial motor neurons from atrophy after deprivation of other survival factors following lesions [77]. Further evidence is provided from studies on Gdnf and Gfra1 knockout mice, which experience a substantial loss of motor neurons due to increased cell death [33], [35], [78], [79]. In these mice, enteric neurons, where GDNF stimulates migration, proliferation, differentiation, and survival of multipotent enteric precursor cells, fail to develop, and the mice die soon after birth [33], [34], [80], [81]. Interestingly, mice lacking other GFLs or co-receptors are viable and fertile [2]. GDNF was shown to induce regeneration of spinal motor neurons after injury [82]. It was observed that GDNF is expressed by skeletal muscle and is localized at the plasma membrane, predominantly near neuromuscular junctions, providing a source of GDNF for nearby motor neurons [83].
Reports of a functional role for GDNF outside the nervous system are accumulating. Most notable is a pivotal role that GDNF plays in renal morphogenesis [7]. GDNF signaling from mesenchymal cells around the ureteric bud tips is required for them to branch out. This happens through the RET receptor kinase and GFRα1, which then form the renal collecting duct system. It was conferred that the RET/Etv4 signaling promotes directed cell movements in the ureteric bud tips [84]. GDNF also plays a role in spermatogenesis [85], [86]. Firstly, GDNF is expressed by the nephrogenic mesenchyme in the developing kidney, located in immediate proximity to the RET-expressing tips of the ureteric bud. GFRα1 is localized to both the mesenchyme and ureteric bud. This mesenchymal-epithelial interaction induces the initial branching from the Wolffian duct, continuing into morphogenesis of ureteric bud [87]. While mesenchyme-derived GDNF expression is initially quite sparse across the Wolffian duct, upon branching, RET and GDNF become more concentrated around distal tips of the ureteric bud and periphery, respectively. GDNF is essential for kidney development as Gdnf knockout mice show defects in this respect and die soon after birth [34]. However, while GDNF is essential, it cannot promote development on its own [88]. It does appear that RET-independent pathways are utilized as RET-deficient mice exhibit metanephric development to some degree [80]. Moreover, the PI3K/PTEN axis was identified as being critical for chemotaxis and branching morphogenesis [89].
The enteric nervous system (ENS) develops following the migration of neural crest cells (NCC). GDNF is an essential trophic factor for the developing ENS, as knockout mice with deletions in Gdnf or Gfrα1 lack enteric neurons [35]. Ret knockout mice lack enteric neurons in the small and large intestines but do develop them in the esophagus [90], although the density of neurons in this region is significantly reduced [91]. Nedd4-related E3 ubiquitin ligase-2 (NEDL2) is pivotal in regulating ENS development and GDNF/Ret signaling. The NEDL2-deficient mice die within 2 weeks after birth and have a low body weight. These mice showed a progressive bowel motility defect [92]. GDNF is chemoattractive to NCC, thereby promoting their migration into the gastrointestinal tract [91], [93]. Additionally, GDNF supports the survival, proliferation, and differentiation of NCC [94], [95], [96].
GDNF is secreted by Sertoli cells, somatic cells of the seminiferous tubule, and ensures the self-renewal of spermatogonial stem cells (SSCs) and prevents terminal differentiation [85], [86], [97], predominantly acting in the perinatal period [98]. GDNF achieves this through the inhibition of Notch signaling, halting differentiation, and promoting the expansion of the stem cell pool [99], or via the Src family kinase/PI3K/Akt pathway that leads to the upregulation of both c- and N-Myc [100], [101], [102], [103], [104]. In addition to this crucial role in the self-renewal and maintenance of SSCs, GDNF may also play a role in the proliferation and differentiation of these cells into Apaired and Aaligned spermatogonia via the GDNF-ERK1/2-FOS pathway [105]. Evidence of a role for GDNF in SSC cell fate has been established in mouse Gdnf knockout models, which results in the depletion of the SSC population [106]. Conversely, overexpression of GDNF results in a larger, undifferentiated spermatogonia population, leading to the shutdown of spermatogenesis and generation of a nonmetastatic, seminoma-like tumor [106]. To complete this autocrine interaction, both RET and GFRα1 are located on undifferentiated spermatogonia and are routinely used as markers for spermatogonia isolation [86]. ARTN and NRTN also exhibit the capacity to regulate spermatogonial stem cell proliferation [107]. A study with double-mutant mice model has showed that the NOTCH signaling is activated in the Sertoli cells in vivo, and in vitro, the NOTCH ligand works through a ligand called JAG1 [108]. However, a negative feedback regulation was indicated in the testicular stem or progenitor cells. The activation of NOTCH signaling in Sertoli cells upregulates the transcriptional repressors HES1 and HEYL, which directly downregulate GDNF expression by binding to the Gdnf promoter [108], [109].
GDNF is also known to functionally interact with BMP4, a key inhibitory protein of embryonic stem cell differentiation, to enhance neuronal development during kidney development [110], [111]. Further evidence of a role for GDNF:RET signaling in stem cell differentiation and biology includes an involvement in the regulation of hematopoietic cell differentiation [112] and the co-localization of GDNF and GFRα1 with ABCG2 and p63, two known stem cell markers within the human corneal epithelium [113]. It was suggested that GDNF and GFRα1 may represent a phenotypic property that identifies a population of stem-like precursor cells [113]. GDNF also interacts with integrin β1 and was observed to be upregulated in mammospheres, neurospheres, and hematopoietic and embryonic stem cells [114]. GDNF is also capable of dimerizing with α6 integrin, an adhesion molecule required for cancer stem cell tumorigenicity [115]. Moreover, integrin β1 could form a complex with GFRαl, which is enhanced by GDNF [116]. Furthermore, cancer stem–like cells derived from medullary thyroid carcinoma (MTC) on the basis of CD133 positivity have demonstrated a dependence on RET for their self-renewal and differentiation [117]. Lastly, significant cross talk exists between the Notch and RET:GFRα1 signaling pathways during nephrogenesis [118].
GDNF also functions in oocyte development [119], [120], follicular proliferation in ovaries [121], regulation of hair growth [122], tooth innervation [123], corneal regeneration and wound healing [113], [124], [125], immune homeostasis and response, and psychoregulation including drug abuse [126]. A recent study observed reduced levels of GDNF and ARTN mRNA in the peripheral blood cells of patients with major depressive disorders compared to those same individuals in a remissive state [127]. Furthermore, GDNF may promote angiogenesis through increasing production of IL-8, a potent angiogenic factor, in SK-N-MC human primitive neuroectodermal tumor cells [128]. Functionally, GDNF is known to exhibit cross talk with the VEGF:VEGFR and NGF:TrkA pathways [129], [130]. GDNF is a key regulator of the endothelial cell network formation. GDNF, by itself and in the presence of adipose-derived stem cells, was associated with enhanced capillary network formation [131].
GDNF expression and activity are regulated by a complex network of factors and mechanisms ranging from epigenetic regulation in peripheral blood cells [132] to regulation by a host of small molecule drugs such as antidepressants [133], [134], [135], [136], glucocorticoids [137], [138], and follicle-stimulating hormone [139]. This extends to the neurotransmitters dopamine, serotonin, glutamate, and adenosine [140], [141]. Additionally, lipopolysaccharides and inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and TNF-β, regulate GDNF in C6 glioma cells [142], [143], [144], [145]. The regulation of GDNF expression in the nervous system has been extensively reviewed [146].
While the neuroprotective effects of estrogens may be mediated by neurotrophic factors [147], it appears that GDNF expression is not estrogen regulated in certain tissues. Instead, GDNF signaling can be significantly modulated by, or can participate in, cross talk with estrogen. The estrogen receptor (ER) modulator tamoxifen had only a limited effect on GDNF levels in C6 glioma cells [148], and Esr1 knockout mice demonstrated no alteration of GDNF expression within the murine midbrain [149]. Paradoxically, 17β-estradiol stimulates GDNF expression in developing mice hypothalamic neurons [150]. This effect, however, appears to manifest itself through nonclassical estrogen action since treatment with ER antagonist ICI182,780 (fulvestrant) does not alter GDNF expression. This nonclassical action is mediated by Ca2+ and cAMP/PKA signaling [151]. Conversely, in mammary carcinoma, RET and GFRα1 are highly regulated by estrogen, and significant cross talk exists between the pathways (see below) [152], [153], [154]. Genomic profiling of invasive melanoma found that GDNF was one of the genes that were highly amplified in invasive cell lines as compared to noninvasive ones, suggesting an important function in the melanoma metastasis [155].
Signaling pathway, genome-wide chromatin binding, and transcriptome analyses have demonstrated that SPIN1 has a direct impact on the expression of GDNF, which activates RET pathway [156]. It was shown that knockdown of SPIN1 in liposarcoma cells reduced GDNF expression, which was activated in human liposarcoma tissues as compared to normal tissues [156].
ARTN
ARTN, the most recently discovered member of the GFLs, promotes the survival of peripheral ganglia and dopaminergic neurons in vitro [157], [158]. Through alternative splicing, human ARTN mRNA possesses at least five functional transcript variants, which encode three pre-pro-ARTN isoforms forming identical mature proteins [15]. These splice variants appear to be differentially expressed in different human tissues, with some tissues only expressing nonfunctional variants, with the highest expression levels of total variants observed in nonneural tissues [15]. According to the National Center for Biotechnology Information UniGene database and as reported by our laboratory, human ARTN is expressed in a number of tissues, including esophagus, intestine, kidney, larynx, lung, pancreas, parathyroid, placenta, prostate, uterus, colon, trachea, cerebellum, adipose, cartilage, and stomach [159].
Artn and Gfra3 knockout mice are viable and fertile but exhibit ptosis (a characteristic drooping of the eyelids) due to the lack of sympathetic innervation to the superior tarsus muscle [160], [161]. In wild-type mice, GFRα3 is expressed in the ganglia of the peripheral nervous system at a higher level than GFRα1 or GFRα2. In particular, GFRα3-mediated signaling is required for the survival and migration of superior cervical ganglion neurons (SCG), as Gfra3−/− mice show gross postnatal SCG cell death and impaired rostral migration of SCG neurons to target organs [160], [162]. This impaired migration most likely contributes to the death of neurons due to a lack of target-derived growth factors such as nerve growth factor (NGF). Despite expression in other sympathetic ganglia such as the dorsal root ganglion, these ganglia appear to be normal in Gfra3−/− mice. Specifically, a study using low-density dissociated cultures demonstrated a role for ARTN in the embryonic generation and in the survival and growth of sympathetic neurons, in fact promoting survival to the same degree as NGF [160], [162]. ARTN also appears to have a transient effect on the survival of mature SCG neurons as they lose their survival dependence on NGF with age, and concomitant treatment of ARTN and NGF increases neurite growth to a greater extent than NGF alone [162]. The source of this endogenous ARTN, however, is unclear as an inhibitory anti-GFRα3 antibody did not have any effect on mature sympathetic neurons cultured in the absence of growth factors, indicating the lack of an autocrine loop [160], [162]. The parasympathetic ganglia normally innervate some of the same targets as sympathetic ganglia. Mice do not appear to be affected by Gfra3 deletion. Gdnf−/− mice have also been shown to have subtle defects in the SCG, suggesting some degree of cross talk between GDNF and GFRα3 [35]. Unlike Gdnf knockout mice, Artn/Gfra3 knockout mice have normal ENS development [160], [161], and ARTN does not induce the migration or neurite outgrowth of NCC [91]. ARTN complexed with GFRα3 is believed to have a normalizing effect on the pathophysiology of mechanisms of neuropathic pain [163]. In addition to GDNF and NRTN, ARTN was also studied for its effects in the gastrointestinal tract. While GDNF and NRTN promoted the migration of neurite outgrowth in the esophagus, ARTN had no effect [91], [164].
Immunohistochemistry studies in the normal human brainstem and hippocampus have revealed a distinct temporospatial expression pattern for each of the GFLs and GFRα subtypes at prenatal, perinatal, and adult ages [165], [166], [167], [168], [169]. ARTN and its cognate receptor GFRα3 consistently show a more restricted distribution compared to the other GFLs and associated receptor subtypes [167], [168]. In particular, ARTN/GFRα3 localize to the caudal spinal trigeminal nucleus, which corroborates with the findings of other studies that suggest a role for ARTN in nociception, touch, and thermal sensory perception [170], [171], [172].
Expression of ARTN, GFRα3, and RET was detected in early murine embryo cultures [173]. They established that endogenous ARTN promoted embryonic development by increasing trophectoderm cells, decreasing blastocyst cell apoptosis, and ultimately increasing the percentage of early embryos or zygotes progressing into mature blastocysts [173]. Conversely, depletion of ARTN suppressed embryo development. Similarly ARTN and RET, but not GFRα3, were detected in early pregnancy oviducts, suggesting that paracrine ARTN from the oviduct ampulla epithelium also facilitates preimplanted embryo development. GDNF has also been reported to exhibit a similar function [174].
ARTN also facilitates the development and maintenance of vascular system innervation. The vascular and nervous systems have a very close relationship, such that vascular-derived neurotrophic factors have long been postulated to regulate vascular innervations [175]. The vascular system largely exerts its effects on the neuronal system by signaling to receptors located on postganglionic sympathetic neurons to direct the innervation of the vasculature [175]. In mice and rats, ARTN is secreted by arterial smooth muscle cells and acts as a chemoattractant to developing axonal processes from SCG at the final stages of innervation [161], [176]. Likewise, ARTN expression is present within smooth muscle cells of blood vessels of normal human breast tissue and mammary carcinoma [159]. Vascular-derived ARTN is also a determinant of neurite outgrowth in adult rats [176]. It is alone in this regard, as GDNF- and NRTN-deficient mice do not exhibit abnormal sympathetic nervous systems, and PSPN does not act on the peripheral nervous system [35], [177], [178]. ARTN-, RET-, and GFRα3-deficient mice have grossly abnormal development of the sympathetic nervous system [161], [179].
PSPN and NRTN
PSPN has several unspliced (nonfunctional) and spliced (functional) transcripts. The unspliced forms are detected in most human tissues, while the spliced transcripts are only detectable at very low levels in the human adrenal gland, cerebellum, spinal cord, and testis [180]. PSPN specifically binds GFRα4, of which there are several splice variants, one of which is a non–GPI-linked soluble form [180], [181]. In newborn and adult mice, the expression pattern of these splice variants is restricted to the thyroid, adrenal medulla, and the pituitary intermediate lobe, with RET co-expression occurring only in the thyroid C-cells and adrenal chromaffin cells [182], [183]. In the adult human, expression of GFRα4 is restricted to the thyroid gland, while RET was found to be widely expressed [180]. PSPN knockdown in oral squamous cell carcinoma cells significantly reduced cell proliferation, and overexpression of PSPN was closely related to tumoral size [184].
NRTN is particularly important in the development, maintenance, and function of the parasympathetic system, both centrally [168], [185] and peripherally [186], [187], [188], [189], [190], [191], [192]. Mice homozygous for NRTN or GFRα2 (the co-receptor for NRTN) deletion show defects in parasympathetic ganglia [177], [178]. Additionally, a stem/progenitor cell niche was identified within the pituitary (predominantly localized at the marginal zone between the intermediate lobe and adenopituitary) that is characterized by the expression of GFRα2, Prop-1 (Prophet of Pit-1), and several stem/progenitor cell markers. It has been suggested that these nonendocrine cells are responsible for maintaining postnatal pituitary homeostasis and expansion as they exhibit the ability to differentiate into hormone-producing cells or neuron-like cells [193].
GFLs in Cancers
As shown in Table 1, GFLs have been implicated in a variety of cancers. In this section, the roles of individual GFL signaling in those neoplasms will be discussed.
Table 1.
GFL Signaling Involved in Cancers
| Cancer Types | GFL Signaling Involved | Other Signaling Pathways Implicated |
|---|---|---|
| Neuroendocrine tumors | RET mutations [195], [196], [197]; RET [200], [201]; GDNF [70]; RET/GFRα4 [180]; PSPN (?) [198] | p53/Pit-1 [200], [201] |
| Pancreatic cancer | GDNF and RET/GFRα1 [203], [204], [205], [206], [207] | PI3K/AKT and NFκB [213], [214]; integrin β1 and MMP-9 [218], [219], [221], [222] |
| ARTN and RET/GFRα3 [208], [209], [210], [211], [216], [223] | MMP-2 and E-cadherin [211] | |
| NRTN and GFRα2 [225] | ||
| Glioma | GDNF and RET [227], [228]; GFRα1 [227], [229] | MAPK and JNK [227], [228]; GAS1 [232], [233]; PCDNA and Ki-67 [235] |
| NRTN and GFRα1/2 [231], [234] | ||
| Colorectal cancer | GDNF [237]; RET/GFRα1 [236] | integrin β1[236]; VEGF-VEGFR1, p38, PI3K/AKT, and HIF1α [237] |
| NRTN [238] | ||
| Breast cancer | RET [255]; GDNF [249], [250]; RET/GFRα1 [72] | TNFα and IL-1 β [72]; ER and PR [241]; HER2 [250], [252]; p44/42 (ERK1/2)/mTOR and JNK [152], [154]; SRC [262]; PAX2 [263], [264]. |
| ARTN [153]; GFRα1 and GFRα3 [265] | ER [153]; HER2 [159]; TWIST1 [256],[257]; BCL-2 [258]; VEGF-A [259] | |
| Endometrial cancer | ARTN and RET/GFRα3 [266] | AKT and CD24 [267] |
| Lung cancer | ARTN and RET/GFRα3 [268] | BCL-2 [268] |
| Ovarian and testicular cancers | GDNF/GFRα1 [120], [273], [274], [275], [276] | NCAM/FYN [273]; ERK1/2 and AKT [275] |
| Melanoma | GDNF and RET/GFRα1 [278], [279], [280], [281], [282] | ERK1/2, c-JUN, MMP-9, c-Kit and p38 [278], [279], [280] |
| Oral cancer and salivary adenoid cystic carcinoma | GDNF [283] | NF-κB, MMP-9 and integrin β1 [283] |
| PSPN and RET [184] | ERK and CDKs [184] | |
| Prostate cancer | GDNF, RET/GFRα1 [284], [285] | ERK and AKT [284] |
| Liver cancer | ARTN [286] | AKT/HIF1α [286] |
| GDNF [131] | ||
| Bone cancer | GDNF [287], [288] | ERK [287], [288] |
| Gastric cancer | GFRα1 methylation [289]; GFRα3 methylation [290] |
RET Germline Mutations and Neuroendocrine Tumors
Germline activating mutations of the RET proto-oncogene, which is highly expressed by several cell lines of the neural crest lineage [194], cause several forms of neuroendocrine cancer including multiple endocrine neoplasia type 2A and 2B, familial medullary thyroid carcinoma, papillary thyroid carcinoma, pheochromocytomas, and neuroblastomas [195], [196], [197]. However, not all cases of MEN2 have mutated RET. In addition, GFRα4 has also been proposed as a modifying factor for this set of diseases, as it is only detected in MTC, and no other thyroid tumors such as follicular thyroid adenomas, follicular thyroid carcinomas, or papillary thyroid carcinomas [180]. Whether PSPN functions as the GFRα4/RET ligand in MTC is unclear as its expression pattern is limited. MTC cells secrete calcitonin, which is used as a marker of tumor burden. Exogenously applied PSPN appears to regulate the expression of calcitonin in MTC, a process mediated by the oncogenic forms of RET [198]. Alternatively, one particular mutation in GFRα4 results in the formation of a truncated, soluble form of the receptor, capable of activating RET independent of its ligand PSPN [199]. However, use of calcitonin as a biomarker in MTC may need to be reassessed in light of the evidence that treatment with a RET inhibitor results in a dose-dependent reduction of calcitonin levels, while tumor volumes remain the same [198].
In normal anterior and neoplastic pituitaries, GDNF and RET are predominantly expressed in almost all somatotrophs but absent in most of the other cell types (corticotrophs and gonadotrophs) in normal pituitaries. Furthermore, GDNF was expressed in all growth hormone–secreting pituitary adenomas and absent in almost all other types of pituitary tumors [70]. These findings suggest a link between the GDNF and growth hormone signaling pathways, although the physiological significance has not yet been ascertained [70]. One role may be the regulation of cell populations because RET was shown to control the somatotroph population numbers through the p53 apoptotic pathway [200]. This is mediated by RET activating Pit-1, a potent transcription factor that also binds and activates the human growth hormone promoter [200], [201]. However, upon the application of GDNF, Pit-1 expression and apoptosis are suppressed [200].
In contrast, Hirschsprung disease is a congenital malformation associated with aganglionosis of the gastrointestinal tract resulting from inactivating mutations in RET [25]. These individuals tend to lack certain parts of the enteric nervous system [197]. Polymorphisms in the GFL genes do not appear to be a modifying factor in Hirschsprung disease phenotypes [202]. Beyond these neuroendocrine disorders, GFLs have also been implicated in a series of other cancers of epithelial origin particularly pancreatic, testicular, bile duct, colon, glioma, mammary, endometrium, ovarian, and lung.
Pancreatic Cancer
GDNF is upregulated following acute induction of pancreatitis in mice [203]. Pancreatic cancer tissues and pancreatic cancer cell lines express GDNF and the RET and GFRα1 receptors [204]. GDNF promotes pancreatic cancer cell proliferation and invasion through the GFRα-1/RET receptor complex in an autocrine/paracrine manner [205], [206]. Endoneurial macrophages are shown to secrete high levels of GDNF, which activates RET and promotes perineural invasion of pancreatic cancer [207]. ARTN may be involved in chronic pancreatitis, a precursor disease to pancreatic cancer characterized by progressive and irreparable damage to the organ, severe fibrosis, and intense abdominal pain leading to functional insufficiency [208], [209]. ARTN is closely related to a normalizing effect of neural pain. In chronic pancreatitis where there is increased pain, ARTN and its co-receptor GFRα3 are upregulated [210]. ARTN, through GFRα3, was found to promote pancreatic cancer cell motility and invasiveness in MIA PaCa-2 cell lines [211].
Both GDNF and ARTN are strongly expressed in the endocrine cells and intrapancreatic nerves of normal pancreatic tissue, as well as in pancreatic cancer tissue using immunohistochemical methods [212]. Intrapancreatic neural invasion is correlated to GDNF expression and is facilitated through the PI3K/AKT and nuclear factor kappa B (NFκB) pathways [213], [214]. GDNF and RET both correlate with survival rate and clinical parameters in pancreatic cancer [215], [216], [217]. GDNF enhances the expression of integrins in the pancreas, specifically the β1 subunit [218], [219], which combined with GDNF-induced upregulation of matrix metalloproteinase-9 (MMP-9) production and activation facilitates metastasis [212], [218], [219], [220], [221], [222]. Another group found that both ARTN and its receptor complex GFRα3/RET were both increased in expression in cases of pancreatic cancer, with strong expression in both primary cancer cells, in liver metastases, and in surrounding tissues (the strongest of which was in hypertrophic nerves and arterial walls) [216], [223]. Most notably, ARTN appeared to increase cell migration and invasion in pancreatic cancer cell lines in a similar manner to GDNF but did not affect proliferation [223]. While ARTN did not affect the expression either of MMP-2 or of MMP-9 mRNA in their study [223], another study showed that ARTN treatment resulted in an increase of MMP-2 and a decrease of E-cadherin expression [211]. Moreover, MMP-9 was found to mediate GDNF-stimulated invasion of pancreatic cancer cells [221], [222]. Therefore, the signal transduction pathways involved in ARTN-mediated migration/invasion need to be investigated further. It is possible that the invasion of pancreatic cancer cells into nerves results in injury and inflammation, which in previous research have been shown to result in the upregulation of GFLs in Schwann cells, in an effort to regenerate nerves; GFLs then act in a paracrine/autocrine fashion to perpetuate further intravasation by pancreatic cancer cells [210]. Additionally, certain RET polymorphisms can increase the effect of GDNF-induced pancreatic cell invasion [224].
Pancreatic cancer tissue and pancreatic cancer cells expressed increased amounts of NRTN. NRTN promoted invasiveness and silencing of NRTN reduced proliferation and invasion of pancreatic cancer cells [225]. RET was also upregulated in pancreatic adenocarcinoma, and GDNF depletion in perineurial macrophages, or inhibition of RET with shRNA or a small-molecule inhibitor, reduced perineurial invasion in a mouse model [226].
Glioma
GDNF expression is abundant in the highly invasive C6 rat glioma cell line but is significantly lower in the noninvasive Hs683 human glioma cell line, suggesting that an autocrine/paracrine mechanism stimulates glioma cell migration [227]. GDNF induces glioma cell migration via MAPK and JNK pathways [227], [228]. RET and GFRα1 are also reported to be highly expressed in glioma cells [227], [229]. GDNF appears to confer chemoresistance while promoting mitogenesis in glioblastoma cell lines [230]. It was reported that GDNF additionally permitted neuroblastoma cells to proliferate in the presence of a range of cytotoxic chemotherapeutic agents [231]. GAS1 was shown to inhibit the growth of gliomas by blocking the GDNF-RET signaling pathway [232], [233].
There is a contentious role for NRTN and GDNF in neuroblastomas. It was found that NRTN and GDNF potentiated retinoic acid–induced differentiation in neuroblastoma [234]. This effect was much more pronounced in nonaggressive tumors compared to aggressive ones. Conversely, GDNF and NRTN were found to induce faster growth of neuroblastoma cell lines, conferring protection against chemotherapeutic drug treatment [231]. GDNF has also been shown to stimulate proliferation of glioma cells by upregulating expression of cyclins PCDNA and Ki-67 [235].
Colorectal Cancer
Colorectal cancer cells express the RET/GFRα1 receptor complex for GDNF, and their β1 integrin expression is also significantly enhanced by GDNF, and consequently, the enhancement and associated increase in adhesion and invasive abilities in response to GDNF were inhibited by blocking the GDNF receptor or the integrin β1 subunit [236]. GDNF is also shown to enhance the migration of colon cancer cells by increasing VEGF-VEGFR interaction, which is mainly regulated by the p38, PI3K/Akt, and HIF1α signaling pathways [237]. In a case of intestinal ganglion neuromatosis associated with colon adenocarcinoma, GDNF and NRTN were found to be highly expressed in the adenocarcinoma cells, while co-receptors GFRα1 and RET were expressed in surrounding ganglion and glial cells [238].
Methylation may affect GDNF signaling in colorectal cancer. GDNF locus in colonic mucosa of ulcerative colitis patients was found highly methylated and the level of methylation was significantly higher in active inflamed mucosa than in quiescent mucosa [207]. GDNF gene is among the 15 genes that have been differentially methylated in colorectal cancer in comparison to adjacent normal mucosas [239].
RET has been shown to be an oncogene in many cancers, but a recent study has shown that RET is a potential tumor suppressor gene in colorectal cancer [240]. RET locus was methylated in 27% of colon adenomas and in 63% of colorectal cancers, resulting in a decrease in RET expression, whereas the restoration of RET expression in colorectal cancer cell lines caused apoptosis [240].
Breast Cancer
Increased expression of GFRα1 and RET transcripts is observed in mammary carcinoma compared to normal breast tissue [72]. GFRα1 mRNA expression was detected in 59.4% of the tumor samples, and was associated with ER receptor expression and lymphovascular invasion/lymph node metastasis at diagnosis. GFRα1 mRNA expression was inversely correlated with p53, EGFR, basal markers, and basal-like tumors but positively correlated with luminal-type tumors [72]. There are many other studies in the cancer microarray database Oncomine that have identified a significant correlation of GFRα1 and RET receptors with both estrogen and progesterone receptor (PR) positive status [241]. Elevated GFRα1 levels in mammary carcinoma have been reported in several studies [242], [243], [244]. Of the 212 tumor samples surveyed, RET mRNA expression was expressed in 29.7% of the tumors [245]. Of those, only 18.1% co-expressed RET and GFRα1 compared with 41.9% of the tumors being GFRα1 positive/ RET negative. This suggests that, in mammary carcinoma, GDNF (or other GFL acting through GFRα1) predominantly acts via a RET-independent signaling mechanism.
We have observed similar correlations for GDNF in human normal mammary tissue and mammary carcinoma in an analysis of data extracted from the Oncomine database [159]. However, we did not observe a repeatable correlation between GDNF expression and grade, which had been previously demonstrated by other groups [246], [247], [248]. Although additional studies have detected GDNF upregulation in mammary carcinoma [249], [250] and GDNF is significantly linked to luminal/apocrine but not basal tumors [251], there is still a lack of evidence that GDNF expression is significantly correlation with disease and outcome. However, it is clear that GDNF correlates consistently with HER2 positive status [250], [252] but not ER status [159], [250], [253].
It has been demonstrated that in MCF-7 cells (RET+/GFRα1+), treatment with exogenous GDNF resulted in increased S-phase entry, enhanced cell survival as measured by cell attachment after serum starvation, and increased cell scattering [72]. GDNF treatment also resulted in the loss of cortical actin organization and formation of actin stress fibers [72]. In a wild-type MCF-7 xenograft mouse model, the authors also detected strong GDNF-expressing, infiltrating fibroblasts around the tumor and a low level of GDNF expression by the tumor itself, with localization of GDNF on the invasive margin of the tumor [72]. Untreated MCF-7 cells showed very little endogenous GDNF mRNA, but treatment with inflammatory cytokines TNFα and IL-1 β resulted in a substantial increase [72]. Together, these results suggest that mammary carcinoma cells not only secrete GDNF in response to inducing cytokines, but also respond to paracrine- and autocrine-derived GDNF sources. Additionally, MCF-7 cells have been found to have robust expression of both GFRα1 and RET proteins [72], [159], [254].
RET gene has previously been reported to contain an estrogen response element located in its promoter region [255]. Both RET and GFRα1 mRNA expressions were strongly increased following estradiol treatment with similar kinetics to that of early response genes such as the trefoil family factor TFF1 and c-Myc. It appears that, rather than altering GDNF levels, estrogens contribute to an enhanced level of GDNF signaling, albeit in a cell line–dependent manner [152]. In fact, GDNF stimulates anchorage-independent proliferation through RET, an effect mediated by p44/42 (ERK1/2) MAPK and JNK [152].
ARTN expression is regulated by estrogen. Overexpression of ARTN is associated with resistance to antiestrogen drugs like tamoxifen in patients with ER-positive mammary carcinoma, and inhibition of ARTN restores the tamoxifen sensitivity [153]. ARTN is also expressed in ER-negative breast cancers. ARTN synergizes with TWIST1 to promote metastasis and poor survival outcome in patients with ER-negative tumors [256]. TWIST1 is a transcription factor. High TWIST1 expression is associated with breast cancer invasion and metastasis [257]. ARTN has also been reported to stimulate radio- and chemoresistance by promoting TWIST1-BCL-2–dependent cancer stem cell–like behavior in mammary carcinoma cells [258] and to promote de novo angiogenesis in ER-negative mammary carcinoma through activation of TWIST1-VEGF-A signaling [259]. Enhanced GDNF/RET signaling in ER positive breast cancers promotes resistance to aromatase inhibitors in postmenopausal patients with ER breast cancers [260].
The RET kinase inhibitor NVP-AST487, superior to the aromatase inhibitor letrozole, inhibited the GDNF-induced motility and tumor spheroid growth in ER-positive breast cancer cells in vitro and demonstrated similar efficacy in impairing tumor growth in vivo [261].
GDNF was able to neutralize trastuzumab-induced apoptosis in HER2+ breast cancer cells in vitro and induce in vivo growth in xenograft tumors [262]. Interestingly, the SRC kinase inhibitor saracatinib effectively blocked GDNF-stimulated growth of trastuzumab-sensitive cells but did not inhibit GDNF-promoted growth of trastuzumab-resistant cells, indicating that SRC mediates GDNF prosurvival functions by bridging RET-HER2 cross talk in trastuzumab-responsive breast cancer tumors, whereas GDNF is also linked to trastuzumab resistance by acting independently from SRC in trastuzumab-resistant tumors [262].
Therefore, targeting the GDNF- and ARTN-induced RET signaling may be an effective way to overcome endocrine resistance in ER-positive breast cancers [153], [154], [261] as well as trastuzumab resistance in HER2-positive breast cancers [262].
A previous study demonstrated that PAX2 (paired box 2 gene), a transcriptional activator, coordinated GDNF expression by binding to the 5′UTR of exon 1 within the gene, driving kidney development [263]. This is interesting not only because GDNF may be under similar transcriptional control within the breast but also because PAX2 has been implicated in mediation of tamoxifen resistance through an interaction with ER and the HER2 promoter [264].
Unlike GDNF, ARTN appears to play more of an autocrine role within mammary carcinomas. ARTN protein expression was detected in 65% of human mammary carcinoma samples and correlated to HER2 positivity and higher tumor stage, and increased ARTN expression is linked to decreased overall survival of stage III and HER2-negative patients [159].
Forced expression of ARTN in MCF-7 mammary carcinoma cells increased cell survival, promoted anchorage-independent growth and enhanced cell migration and invasion, and promoted a more aggressive cellular morphology with formation of proliferative and disorganized colonies in a three-dimensional basement membrane culture model [159]. Like GDNF [152], ARTN had little effect on anchorage-dependent proliferation under serum-replete conditions but displayed a stimulatory effect under serum-depleted conditions [159].
ARTN significantly increased the expression of the antiapoptotic BCL-2 protein and several genes involved in invasion and metastasis, such as SERPINE1, MMP1, and PLAU [159]. Forced expression of ARTN significantly promoted tumor formation and tumor progression in vivo, with a greater than two-fold increase in size after 6 weeks compared to control tumors [159]. Additionally, ARTN is correlated with ER-positive status in mammary carcinoma, and its expression is correlated with poorer distant metastasis-free survival [153]. ARTN both increases ER transcriptional activity and function, as well as mediates resistance to antiestrogen therapies. Resistance appears to be driven by ARTN-induced BCL-2 expression [153]. Concordantly, targeting of the RET receptor sensitizes mammary carcinoma cells to antiestrogen therapies, and recurrent invasive tumors are twice as likely to exhibit RET positivity following adjuvant tamoxifen treatment [154]. GDNF stimulation of RET was also shown to increase ERα phosphorylation and estrogen-independent activation of ERα transcriptional activity by a mammalian target of rapamycin (mTOR)–dependent mechanism [154]. We have demonstrated that ARTN co-receptors GFRα1 and GFRα3, but not Syndecan-3, are significantly upregulated in breast cancer and that their expression is significantly associated with survival outcome of breast cancer patients, especially in ER-negative or HER2-negative mammary carcinoma [265].
It has been demonstrated that ARTN can induce epithelial to epithelial-to-mesenchymal transition (EMT) in ER-negative mammary carcinoma cells through the upregulation of TWIST1 [256]. In vivo, this resulted in enhanced local invasion and distant metastasis. Interestingly, low expression of both ARTN and TWIST1 was sufficient to predict 100% relapse-free and overall survival in patients with ER-negative breast cancer [256]. On the other hand, high expression of both ARTN and TWIST1 was correlated with a poor survival.
Endometrial Cancer
ARTN exhibits similar oncogenic effects in endometrial carcinoma to mammary carcinoma [266]. Expression of ARTN was significantly associated with higher-grade tumors and myometrial invasion. ARTN stimulated the in vitro proliferation and cell survival of RL95-2 and AN3 cells, regulating such key genes as CDC25A, CDK2, Bcl2, p53, Bad, Bax, and TERT. Furthermore, anchorage-independent growth was enhanced, while ARTN overexpression also induced a mesenchymal phenotype, which was correlated to deregulation of vimentin, Met, MMP1, MMP9, PLAUR, SERPINE1, and SERPINEB5. Mechanistically, AKT phosphorylation was indicated as pivotal to the survival and invasive response demonstrated by ARTN. In vivo, xenografts yielded almost two-fold larger tumors that were poorly differentiated and significantly more invasive, infiltrating surrounding supporting tissues. Enhanced proliferation and survival were also evident in vivo. We have also demonstrated that ARTN confers endometrial carcinoma chemoresistance through transcriptional activation of CD24 [267]. Specifically, ARTN alleviates the G2/M arrest initiated by doxorubicin and the apoptosis induced via microtubule disruption by paclitaxel treatment. Additionally, ARTN inhibition by functional antibody or small-interference RNA (siRNA) delivery enhanced the efficacy of the respective chemotherapeutic drug treatments [267]. Forced expression of ARTN in endometrial carcinoma cells was shown to decrease sensitivity to chemodrugs doxorubicin and paclitaxel, and ARTN-stimulated resistance can be abrogated through inhibition of CD24 expression [266].
Lung Cancer
ARTN has also been implicated in non–small cell lung carcinoma (NSCLC) [268]. ARTN, RET, and GFRα3 have been demonstrated to be unregulated in primary neoplasms relative to their normal counterparts, while high ARTN expression also correlated with lymph node metastasis and increasing grade of NSCLC, according to an examination of the Oncomine database [268]. Transcriptional activation of BCL-2 by ARTN enhances in vitro survival in monolayer and anchorage-independent conditions. Furthermore, ARTN significantly enhanced invasive capability of H1299 and H1975 NSCLC cell lines. The former, when injected into immunodeficient mice, yielded 63% larger tumors at day 30 compared to control. Tumors exhibited increased S-phase entry and reduced apoptotic levels. Conversely, these oncogenic effects abated following siRNA or functional antibody inhibition. GDNF and GFRα1 on the other hand lacked any significant correlation between normal and cancerous lung tissue [268]. GFRα2 in the majority of cases exhibited a downregulation in the neoplastic setting (squamous cell carcinoma and adenocarcinoma) [268]. In terms of RET mutations causing SCLC, several novel somatic RET mutations were reported in squamous small cell lung carcinoma cell lines and tumor samples [269]. However, another study was unable to detect the same RET (or GDNF) mutations in 54 SCLC cell lines [270]. Moreover, there was no consistent pattern for RET, GDNF, or GFRα1 expression in 21 SCLC cell lines assayed [270]. Therefore, RET mutations may not be an important step in the tumorigenesis of SCLC. Nevertheless, RET fusion genes occur at a rate of approximately 1% (84/6899) in NSCLC patients, and female patients (or those less than 60 years old of age) usually have higher frequencies than male patients (or those aged 60 years and older), particularly in patients from Asian [271]. For example, RET fusion genes occur at 1.9% (3 of 156) of NSCLC patients in Koreans [272].
Ovarian and Testicular Cancers
GDNF and GFRα1 are localized to follicles at various stages of development, being actively secreted by oocytes and acting in an autocrine/paracrine fashion [120]. GDNF has also been implicated in ovarian tumorigenesis, being influenced by androgens [273], [274]. Interestingly, the expression of GDNF in the ovary is distinct to that in the testes, where it is the somatic Sertoli cells which express GDNF. Testicular tumors develop regularly in older GDNF-overexpressing mice [275], and GDNF promotes invasive behavior in testicular seminoma cells [276]. Because spermatocytic seminoma cells share many phenotypic markers with SSCs, whose self-renewal is triggered by GDNF, it is possible that GDNF contributes to spermatocytic seminoma by promoting the formation of SSCs [277].
Melanoma
Activated RET signaling is found to be correlated with the development of malignant melanoma in a mouse model [278], [279], [280]. Several human melanoma cell lines express RET and GFRα1, and GDNF stimulation significantly enhances the proliferation of human melanoma cells [281], [282]. The expression level of intrinsic Ret, Gdnf, and Gfrα1 transcripts in malignant melanomas from RET-transgenic mice was significantly upregulated compared with those in benign melanocytic tumors, and GDNF treatment activated RET via phosphorylated Tyr905 in human malignant melanoma cells [282]. Therefore, GDNF-mediated RET kinase activation is associated with the pathogenesis of malignant melanoma.
Oral cancer and salivary adenoid cystic carcinoma
PSPN mRNA and protein were significantly upregulated in oral squamous cell carcinoma (OSCC)–derived cells compared with human normal oral keratinocytes. Pspn knockdown significantly decreased cell proliferation and reduced receptor tyrosine kinase signaling and cell cycle arrest at the G1 phase. Primary OSCCs have significantly higher PSPN protein expression than normal counterparts, and overexpression of PSPN is closely related to tumoral size, suggesting that PSPN is a regulator of OSCC progression and may be a diagnostic marker for OSCC [184].
GDNF protein was strongly expressed in salivary adenoid cystic carcinoma and adjacent nerve fibers and positively correlated to the expression of NF-κB, MMP-9, and integrin β1 [283]. This observation suggests that GDNF may increase the matrix degrading and cell adhesion in the process of perineural invasion of salivary adenoid cystic carcinoma.
Prostate Cancer
A recent study found that RET was expressed in all prostate cancer cell lines tested, but GFRα1 was only expressed in 22Rv1 cells; as a result, 22Rv1 cells responded to exogenous GDNF, while all cell lines responded to combined GDNF and GFRα1 treatment [284]. RET knockdown inhibits tumor growth in vivo, and mechanically, RET activates ERK or AKT signaling to promote transformation-associated phenotypes, including perineural invasion via activation of p70S6 kinase [284]. Immunohistochemical analysis of tumor tissues revealed that GDNF expression was significantly stronger in higher-stage prostate tumors [285].
Our group has unpublished evidence examining the role of ARTN in prostate carcinoma. Immunohistochemistry indicated a significant increase in the expression of ARTN in 50 cases of human prostate carcinoma in comparison to 23 cases of human benign prostate hyperplasia. ARTN does not appear to regulate cell proliferation or survival, as ARTN overexpression, knockdown by siRNA, and inhibitory antibody treatment had negligible effects (unpublished observations). This was also the case for chemotherapeutic resistance to doxorubicin and paclitaxel. However, ARTN overexpression increased the metastatic potential of cells, registering significant changes in monolayer and 3D Matrigel culture cell morphology, lamellipodia formation, cell migration and invasion, and characteristic deregulation of mRNA species involved in EMT (unpublished observations). ARTN also promoted anchorage-independent growth, an effect that is inexplicably not abrogated by ARTN inhibitory antibody or chemodrugs.
Liver Cancer
ARTN has been shown to be a hypoxia-responsive factor essential for hypoxia-induced expansion of cancer stem cells in hepatocellular carcinoma (HCC). In addition, increased ARTN expression was associated with larger tumor size and worse clinical outcome of HCC patients. Similar to the role of ATRN in breast cancer, forced ARTN expression reduced apoptosis; increased proliferation; and enhanced EMT and motility, tumorsphere formation, and the tumor-initiating capacity of HCC cells. ARTN was also shown to dramatically increased xenograft tumor size and metastasis in vivo [286]. GDNF is secreted by adipose-derived stem cells and HCC and contributes to pathological neovascularization [131]. Therefore, targeting ARTN and GDNF signaling may be an effective approach to treating HCC.
Bone Cancer
Metastasis of any cancers to the bone causes the bone cancer pain in cancer patients. GDNF was shown to be involved in bone cancer pain in an animal model. Lentivirus-mediated GDNF RNAi significantly attenuated mechanical and thermal hyperalgesia and downregulated the ratio of pERK/ERK, where its activation is crucial for pain signaling [287], [288]. Therefore, it is possible to target GDNF signaling as a therapeutic treatment for bone cancer pain.
Gastric Cancer
Genome-wide DNA methylation profiling of metastatic and nonmetastatic gastric carcinomas and their surgical margins has identified that GFRA1 gene is 1 of the 15 genes that were significantly differentially methylated in gastric carcinoma compared with the surgical margins, and methylation changes of GFRA1, together with SRF and ZNF382, may be potential biomarkers for gastric carcinoma metastasis prediction [289]. Similarly, GFRA3 promoter region was shown to be markedly hypermethylated in almost all gastric tumors [290].
Concluding Remarks
The GDNF family represents a group of four structurally related ligands that have traditionally taken up developmental roles within the neuronal system. However, more recently, they have been ascribed additional developmental and maintenance functions within extraneuronal tissues, including the kidney, testis, eyes, ovary, hematopoietic system, vascular system, pituitary, and endometrium. Owing to their ability to regulate numerous physiological actions in these tissues such as cell survival, motility, and proliferation, their deregulation has always been suspected to yield oncogenic effects. Central to this has been their indirect implication in neuroendocrine tumors, and from the aforementioned studies, this has been recently expanded to include cancers of epithelial origin in endocrine-responsive tissues—most notably breast, endometrial, and pancreatic cancers. Here we have reviewed GFL biology and discussed the evidence and rationale behind whether components of the GFL signaling complex, particularly the ligands themselves, present novel opportunities for therapeutic intervention in cancer.
Taken together, the literature suggests that GFLs have a very complex and tissue-dependent role in both homeostatic physiology and oncogenesis. Indicative of this is the multitude of heterogeneous phenotypes and mechanisms that cell populations can exhibit with respect to combinations of GFLs and GFRαs: both membrane-bound and -soluble forms, RET, nontraditional receptors (HSPG, NCAM, integrins, Met), GFL paracrine versus autocrine actions, and GFL extracellular concentration and release. This inherent complexity ensures that challenges remain and highlights the importance of further research that links in vitro biology with clinical significance.
All GFLs have a pronounced role in early development and postnatal repair. Oncogenesis sees their reactivation and/or deregulation of homeostatic levels. With respect to GDNF, it appears that it functions primarily in a paracrine nature whether it is from the surrounding microenvironment—more specifically, support or stromal cells (adipose, fibroblasts, immune, epithelial ,and muscle)—or the neuronal architecture supporting the tissue. Endogenously sourced GDNF seems to act in a supporting role as opposed to being a key driver of nonneuronal function. The lack of clinical and primary tumor correlation is indicative of this. Conversely, ARTN exhibits a predominantly autocrine role within tumors of various origins. Currently, there is a lack of sufficient data to attribute an oncogenic role to NRTN and PSPN.
Disclosure of Potential Conflicts of Interest
D. X. L. and P. E. L. are inventors on PCT application PCT/NZ2008/00152, US provisional application 61/234902, China ZL200880104674.9, EPO 2164870, and US 14/482512. P. E. L. is an inventor on US provisional application 61/252513 and US 2015/0079106A1. The other authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgements
This work was supported by the Breast Cancer Foundation of New Zealand and the Breast Cancer Cure of New Zealand. G. C. F. is supported by a scholarship from the Genesis Oncology Trust and the University of Auckland. T. W. S. Y. is supported by a scholarship from the University of Auckland.
References
- 1.Airaksinen MS, Holm L, Hätinen T. Evolution of the GDNF family ligands and receptors. Brain Behav Evol. 2006;68(3):181–190. doi: 10.1159/000094087. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13(5):313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- 4.Enomoto H. Regulation of neural development by glial cell line–derived neurotrophic factor family ligands. Anat Sci Int. 2005;80(1):42–52. doi: 10.1111/j.1447-073x.2005.00099.x. [DOI] [PubMed] [Google Scholar]
- 5.Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- 6.Costantini F. GDNF/Ret signaling and renal branching morphogenesis: from mesenchymal signals to epithelial cell behaviors. Organogenesis. 2010;6(4):252–262. doi: 10.4161/org.6.4.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain S. The many faces of RET dysfunction in kidney. Organogenesis. 2009;5(4):177–190. doi: 10.4161/org.5.4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troiani T, Martinelli E, Capasso A, Morgillo F, Orditura M, De Vita F, Ciardiello F. Targeting EGFR in pancreatic cancer treatment. Curr Drug Targets. 2012;13(6):802–810. doi: 10.2174/138945012800564158. [DOI] [PubMed] [Google Scholar]
- 9.Howe LR, Brown PH. Targeting the HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res (Phila) 2011;4(8):1149–1157. doi: 10.1158/1940-6207.CAPR-11-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61(2):47–56. doi: 10.2302/kjm.61.47. [DOI] [PubMed] [Google Scholar]
- 11.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72(8):1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mologni L. Development of RET kinase inhibitors for targeted cancer therapy. Curr Med Chem. 2011;18(2):162–175. doi: 10.2174/092986711794088308. [DOI] [PubMed] [Google Scholar]
- 13.Wells SA, Jr., Santoro M. Targeting the RET pathway in thyroid cancer. Clin Cancer Res. 2009;15(23):7119–7123. doi: 10.1158/1078-0432.CCR-08-2742. [DOI] [PubMed] [Google Scholar]
- 14.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line–derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 15.Masure S, Geerts H, Cik M, Hoefnagel E, Van Den Kieboom G, Tuytelaars A, Harris S, Lesage AS, Leysen JE, Van Der Helm L. Enovin, a member of the glial cell-line–derived neurotrophic factor (GDNF) family with growth promoting activity on neuronal cells. Existence and tissue-specific expression of different splice variants. Eur J Biochem. 1999;266(3):892–902. doi: 10.1046/j.1432-1327.1999.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EM, Jr., Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18(5):793–802. doi: 10.1016/s0896-6273(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 17.Trupp M, Raynoschek C, Belluardo N, Ibanez CF. Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol Cell Neurosci. 1998;11(1–2):47–63. doi: 10.1006/mcne.1998.0667. [DOI] [PubMed] [Google Scholar]
- 18.Peterziel H, Unsicker K, Krieglstein K. TGFbeta induces GDNF responsiveness in neurons by recruitment of GFRalpha1 to the plasma membrane. J Cell Biol. 2002;159(1):157–167. doi: 10.1083/jcb.200203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schober A, Hertel R, Arumae U, Farkas L, Jaszai J, Krieglstein K, Saarma M, Unsicker K. Glial cell line–derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor-beta as cofactor in vivo. J Neurosci. 1999;19(6):2008–2015. doi: 10.1523/JNEUROSCI.19-06-02008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieglstein K, Henheik P, Farkas L, Jaszai J, Galter D, Krohn K, Unsicker K. Glial cell line–derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci. 1998;18(23):9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85(7):1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 22.Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhaes AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol. 2011;192(1):153–169. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4–5):441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Crupi MJ, Yoganathan P, Bone LN, Lian E, Fetz A, Antonescu CN, Mulligan LM. Distinct temporal regulation of RET isoform internalization: roles of clathrin and AP2. Traffic. 2015;16(11):1155–1173. doi: 10.1111/tra.12315. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12(4):361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9(2):213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 27.Kurotsuchi A, Murakumo Y, Jijiwa M, Kurokawa K, Itoh Y, Kodama Y, Kato T, Enomoto A, Asai N, Terasaki H. Analysis of DOK-6 function in downstream signaling of RET in human neuroblastoma cells. Cancer Sci. 2010;101(5):1147–1155. doi: 10.1111/j.1349-7006.2010.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida M, Enomoto A, Fukuda T, Kurokawa K, Maeda K, Kodama Y, Asai N, Hasegawa T, Shimono Y, Jijiwa M. Dok-4 regulates GDNF-dependent neurite outgrowth through downstream activation of Rap1 and mitogen-activated protein kinase. J Cell Sci. 2006;119(Pt 15):3067–3077. doi: 10.1242/jcs.03043. [DOI] [PubMed] [Google Scholar]
- 29.Worley DS, Pisano JM, Choi ED, Walus L, Hession CA, Cate RL, Sanicola M, Birren SJ. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development. 2000;127(20):4383–4393. doi: 10.1242/dev.127.20.4383. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda T, Kiuchi K, Takahashi M. Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem. 2002;277(21):19114–19121. doi: 10.1074/jbc.M200643200. [DOI] [PubMed] [Google Scholar]
- 31.Barnett MW, Fisher CE, Perona-Wright G, Davies JA. Signalling by glial cell line–derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci. 2002;115(Pt 23):4495–4503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka M, Xiao H, Kiuchi K. Heparin facilitates glial cell line–derived neurotrophic factor signal transduction. Neuroreport. 2002;13(15):1913–1916. doi: 10.1097/00001756-200210280-00016. [DOI] [PubMed] [Google Scholar]
- 33.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 34.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 36.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans. 2006;34(Pt 3):458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 37.Silvian L, Jin P, Carmillo P, Boriack-Sjodin PA, Pelletier C, Rushe M, Gong B, Sah D, Pepinsky B, Rossomando A. Artemin crystal structure reveals insights into heparan sulfate binding. Biochemistry. 2006;45(22):6801–6812. doi: 10.1021/bi060035x. [DOI] [PubMed] [Google Scholar]
- 38.Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumae U, Saarma M. GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett. 1999;463(1–2):63–66. doi: 10.1016/s0014-5793(99)01590-2. [DOI] [PubMed] [Google Scholar]
- 39.Coulpier M, Anders J, Ibanez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem. 2002;277(3):1991–1999. doi: 10.1074/jbc.M107992200. [DOI] [PubMed] [Google Scholar]
- 40.Jain S, Knoten A, Hoshi M, Wang H, Vohra B, Heuckeroth RO, Milbrandt J. Organotypic specificity of key RET adaptor-docking sites in the pathogenesis of neurocristopathies and renal malformations in mice. J Clin Invest. 2010;120(3):778–790. doi: 10.1172/JCI41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M. A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol. 2004;24(18):8026–8036. doi: 10.1128/MCB.24.18.8026-8036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustin JA, Yang M, Johnson EM, Jr., Milbrandt J. Deciphering adaptor specificity in GFL-dependent RET-mediated proliferation and neurite outgrowth. J Neurochem. 2007;102(4):1184–1194. doi: 10.1111/j.1471-4159.2007.04624.x. [DOI] [PubMed] [Google Scholar]
- 43.Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line–derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274(30):20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 44.Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113(7):867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- 45.Popsueva A, Poteryaev D, Arighi E, Meng X, Angers-Loustau A, Kaplan D, Saarma M, Sariola H. GDNF promotes tubulogenesis of GFRalpha1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase. J Cell Biol. 2003;161(1):119–129. doi: 10.1083/jcb.200212174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjostrand D, Carlsson J, Paratcha G, Persson B, Ibanez CF. Disruption of the GDNF binding site in NCAM dissociates ligand binding and homophilic cell adhesion. J Biol Chem. 2007;282(17):12734–12740. doi: 10.1074/jbc.M701588200. [DOI] [PubMed] [Google Scholar]
- 47.Seki H, Tanaka J, Sato Y, Kato Y, Umezawa A, Koyama K. Neural cell adhesion molecule (NCAM) and perineural invasion in bile duct cancer. J Surg Oncol. 1993;53(2):78–83. doi: 10.1002/jso.2930530205. [DOI] [PubMed] [Google Scholar]
- 48.Zecchini S, Cavallaro U. Neural cell adhesion molecule in cancer: expression and mechanisms. Adv Exp Med Biol. 2010;663:319–333. doi: 10.1007/978-1-4419-1170-4_20. [DOI] [PubMed] [Google Scholar]
- 49.Wai Wong C, Dye DE, Coombe DR. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol. 2012;2012:340296. doi: 10.1155/2012/340296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen M, Berthold F. Targeting the neural cell adhesion molecule in cancer. Cancer Lett. 2007;258(1):9–21. doi: 10.1016/j.canlet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Tajima S, Maeda I, Kanemaki Y, Nakajima Y, Tatsunami S, Fukuda M, Takagi M. Evaluation of CD56 and CD57 immunostainings for discrimination between endocrine ductal carcinoma in situ and intraductal papilloma. Pathol Int. 2010;60(6):459–465. doi: 10.1111/j.1440-1827.2010.02544.x. [DOI] [PubMed] [Google Scholar]
- 52.Zoltowska A, Stepinski J, Lewko B, Serkies K, Zamorska B, Roszkiewicz A, Izycka-Swieszewska E, Kruszewski WJ. Neural cell adhesion molecule in breast, colon and lung carcinomas. Arch Immunol Ther Exp (Warsz) 2001;49(2):171–174. [PubMed] [Google Scholar]
- 53.Sjostrand D, Ibanez CF. Insights into GFRalpha1 regulation of neural cell adhesion molecule (NCAM) function from structure-function analysis of the NCAM/GFRalpha1 receptor complex. J Biol Chem. 2008;283(20):13792–13798. doi: 10.1074/jbc.M800283200. [DOI] [PubMed] [Google Scholar]
- 54.Chao CC, Ma YL, Chu KY, Lee EH. Integrin alphav and NCAM mediate the effects of GDNF on DA neuron survival, outgrowth, DA turnover and motor activity in rats. Neurobiol Aging. 2003;24(1):105–116. doi: 10.1016/s0197-4580(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 55.Dey BK, Wong YW, Too HP. Cloning of a novel murine isoform of the glial cell line–derived neurotrophic factor receptor. Neuroreport. 1998;9(1):37–42. doi: 10.1097/00001756-199801050-00008. [DOI] [PubMed] [Google Scholar]
- 56.Shefelbine SE, Khorana S, Schultz PN, Huang E, Thobe N, Hu ZJ, Fox GM, Jing S, Cote GJ, Gagel RF. Mutational analysis of the GDNF/RET-GDNFR alpha signaling complex in a kindred with vesicoureteral reflux. Hum Genet. 1998;102(4):474–478. doi: 10.1007/s004390050724. [DOI] [PubMed] [Google Scholar]
- 57.Wan G, Too HP. A specific isoform of glial cell line–derived neurotrophic factor family receptor alpha 1 regulates RhoA expression and glioma cell migration. J Neurochem. 2010;115(3):759–770. doi: 10.1111/j.1471-4159.2010.06975.x. [DOI] [PubMed] [Google Scholar]
- 58.Cabrera JR, Sanchez-Pulido L, Rojas AM, Valencia A, Manes S, Naranjo JR, Mellstrom B. Gas1 is related to the glial cell–derived neurotrophic factor family receptors alpha and regulates Ret signaling. J Biol Chem. 2006;281(20):14330–14339. doi: 10.1074/jbc.M509572200. [DOI] [PubMed] [Google Scholar]
- 59.Schueler-Furman O, Glick E, Segovia J, Linial M. Is GAS1 a co-receptor for the GDNF family of ligands? Trends Pharmacol Sci. 2006;27(2):72–77. doi: 10.1016/j.tips.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Lee CS, May NR, Fan C-M. Transdifferentiation of the ventral retinal pigmented epithelium to neural retina in the growth arrest specific gene 1 mutant. Dev Biol. 2001;236(1):17–29. doi: 10.1006/dbio.2001.0280. [DOI] [PubMed] [Google Scholar]
- 61.Lee KK, Leung AK, Tang MK, Cai DQ, Schneider C, Brancolini C, Chow PH. Functions of the growth arrest specific 1 gene in the development of the mouse embryo. Dev Biol. 2001;234(1):188–203. doi: 10.1006/dbio.2001.0249. [DOI] [PubMed] [Google Scholar]
- 62.Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, Kunz-Schughart LA. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol. 2013;229(3):355–378. doi: 10.1002/path.4086. [DOI] [PubMed] [Google Scholar]
- 63.Takenobu H, Shimozato O, Nakamura T, Ochiai H, Yamaguchi Y, Ohira M, Nakagawara A, Kamijo T. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30(1):97–105. doi: 10.1038/onc.2010.383. [DOI] [PubMed] [Google Scholar]
- 64.Lin LF, Zhang TJ, Collins F, Armes LG. Purification and initial characterization of rat B49 glial cell line–derived neurotrophic factor. J Neurochem. 1994;63(2):758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- 65.Stover T, Gong TL, Cho Y, Altschuler RA, Lomax MI. Expression of the GDNF family members and their receptors in the mature rat cochlea. Brain Res Mol Brain Res. 2000;76(1):25–35. doi: 10.1016/s0169-328x(99)00328-9. [DOI] [PubMed] [Google Scholar]
- 66.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158(2):504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- 67.Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286(2):191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- 68.Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A. Embryonic expression of glial cell-line–derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech Dev. 1996;54(1):95–105. doi: 10.1016/0925-4773(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 69.Fromont-Hankard G, Philippe-Chomette P, Delezoide AL, Nessmann C, Aigrain Y, Peuchmaur M. Glial cell–derived neurotrophic factor expression in normal human lung and congenital cystic adenomatoid malformation. Arch Pathol Lab Med. 2002;126(4):432–436. doi: 10.5858/2002-126-0432-GCDNFE. [DOI] [PubMed] [Google Scholar]
- 70.Japon MA, Urbano AG, Saez C, Segura DI, Cerro AL, Dieguez C, Alvarez CV. Glial-derived neurotropic factor and RET gene expression in normal human anterior pituitary cell types and in pituitary tumors. J Clin Endocrinol Metab. 2002;87(4):1879–1884. doi: 10.1210/jcem.87.4.8383. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki H, Hase A, Miyata Y, Arahata K, Akazawa C. Prominent expression of glial cell line–derived neurotrophic factor in human skeletal muscle. J Comp Neurol. 1998;402(3):303–312. [PubMed] [Google Scholar]
- 72.Esseghir S, Todd SK, Hunt T, Poulsom R, Plaza-Menacho I, Reis-Filho JS, Isacke CM. A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor up-regulation in breast cancer. Cancer Res. 2007;67(24):11732–11741. doi: 10.1158/0008-5472.CAN-07-2343. [DOI] [PubMed] [Google Scholar]
- 73.Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 74.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line–derived neurotrophic factor. J Neurosurg. 2005;102(2):216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 75.Check E. Second chance. Nat Med. 2007;13(7):770–771. doi: 10.1038/nm0707-770. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Peng C, Li L, Ming M, Yang D, Le W. Glial cell–derived neurotrophic factor protects against proteasome inhibition-induced dopamine neuron degeneration by suppression of endoplasmic reticulum stress and caspase-3 activation. J Gerontol A Biol Sci Med Sci. 2007;62(9):943–950. doi: 10.1093/gerona/62.9.943. [doi:62/9/943 pii] [DOI] [PubMed] [Google Scholar]
- 77.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 78.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21(1):53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20(13):4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 81.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr., Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21(2):317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 82.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki H, Hase A, Kim BY, Miyata Y, Nonaka I, Arahata K, Akazawa C. Up-regulation of glial cell line–derived neurotrophic factor (GDNF) expression in regenerating muscle fibers in neuromuscular diseases. Neurosci Lett. 1998;257(3):165–167. doi: 10.1016/s0304-3940(98)00817-9. [DOI] [PubMed] [Google Scholar]
- 84.Riccio P, Cebrian C, Zong H, Hippenmeyer S, Costantini F. Ret and Etv4 promote directed movements of progenitor cells during renal branching morphogenesis. PLoS Biol. 2016;14(2):e1002382. doi: 10.1371/journal.pbio.1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288(1–2):95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28(2):117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 88.Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V. Glial-cell-line–derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124(20):4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- 89.Kim D, Dressler GR. PTEN modulates GDNF/RET mediated chemotaxis and branching morphogenesis in the developing kidney. Dev Biol. 2007;307(2):290–299. doi: 10.1016/j.ydbio.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 91.Yan H, Bergner AJ, Enomoto H, Milbrandt J, Newgreen DF, Young HM. Neural cells in the esophagus respond to glial cell line–derived neurotrophic factor and neurturin, and are RET-dependent. Dev Biol. 2004;272(1):118–133. doi: 10.1016/j.ydbio.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 92.Wei R, Qiu X, Wang S, Li Y, Wang Y, Lu K, Fu Y, Xing G, He F, Zhang L. NEDL2 is an essential regulator of enteric neural development and GDNF/Ret signaling. Cell Signal. 2015;27(3):578–586. doi: 10.1016/j.cellsig.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 93.Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129(22):5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 94.Focke PJ, Swetlik AR, Schilz JL, Epstein ML. GDNF and insulin cooperate to enhance the proliferation and differentiation of enteric crest-derived cells. J Neurobiol. 2003;55(2):151–164. doi: 10.1002/neu.10204. [DOI] [PubMed] [Google Scholar]
- 95.Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200(1):116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- 96.Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126(12):2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- 97.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101(47):16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dadoune JP. New insights into male gametogenesis: what about the spermatogonial stem cell niche? Folia Histochem Cytobiol. 2007;45(3):141–147. [PubMed] [Google Scholar]
- 99.Braydich-Stolle L, Nolan C, Dym M, Hofmann MC. Role of glial cell line–derived neurotrophic factor in germ-line stem cell fate. Ann N Y Acad Sci. 2005;1061:94–99. doi: 10.1196/annals.1336.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol. 2007;304(1):34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134(10):1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 102.Oatley JM, Avarbock MR, Brinster RL. Glial cell line–derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knoepfler PS. Why myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2(1):18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Laurenti E, Varnum-Finney B, Wilson A, Ferrero I, Blanco-Bose WE, Ehninger A, Knoepfler PS, Cheng PF, MacDonald HR, Eisenman RN. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3(6):611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lucas BE, Fields C, Joshi N, Hofmann MC. Mono-(2-ethylhexyl)-phthalate (MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology. 2012;299(1):10–19. doi: 10.1016/j.tox.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 107.Hamra FK, Chapman KM, Nguyen D, Garbers DL. Identification of neuregulin as a factor required for formation of aligned spermatogonia. J Biol Chem. 2007;282(1):721–730. doi: 10.1074/jbc.M608398200. [DOI] [PubMed] [Google Scholar]
- 108.Garcia TX, Parekh P, Gandhi P, Sinha K, Hofmann MC. The NOTCH Ligand JAG1 regulates GDNF expression in Sertoli cells. Stem Cells Dev. 2017;26(8):585–598. doi: 10.1089/scd.2016.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia TX. Regulation of germ line stem cell homeostasis.pdf. Anim Reprod. 2015;12(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- 110.Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134(13):2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- 111.Chalazonitis A, D'Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3–dependent subset. J Neurosci. 2004;24(17):4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakayama S, Iida K, Tsuzuki T, Iwashita T, Murakami H, Asai N, Iwata Y, Ichihara M, Ito S, Kawai K. Implication of expression of GDNF/Ret signalling components in differentiation of bone marrow haemopoietic cells. Br J Haematol. 1999;105(1):50–57. [PubMed] [Google Scholar]
- 113.Qi H, Li DQ, Bian F, Chuang EY, Jones DB, Pflugfelder SC. Expression of glial cell–derived neurotrophic factor and its receptor in the stem-cell-containing human limbal epithelium. Br J Ophthalmol. 2008;92(9):1269–1274. doi: 10.1136/bjo.2007.132431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl. 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, Purushotham AD. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer. 2008;122(2):298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 116.Cao JP, Yu JK, Li C, Sun Y, Yuan HH, Wang HJ, Gao DS. Integrin beta1 is involved in the signaling of glial cell line–derived neurotrophic factor. J Comp Neurol. 2008;509(2):203–210. doi: 10.1002/cne.21739. [DOI] [PubMed] [Google Scholar]
- 117.Zhu W, Hai T, Ye L, Cote GJ. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab. 2010;95(1):439–444. doi: 10.1210/jc.2009-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuure S, Sainio K, Vuolteenaho R, Ilves M, Wartiovaara K, Immonen T, Kvist J, Vainio S, Sariola H. Crosstalk between Jagged1 and GDNF/Ret/GFRalpha1 signalling regulates ureteric budding and branching. Mech Dev. 2005;122(6):765–780. doi: 10.1016/j.mod.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 119.Linher K, Wu D, Li J. Glial cell line–derived neurotrophic factor: an intraovarian factor that enhances oocyte developmental competence in vitro. Endocrinology. 2007;148(9):4292–4301. doi: 10.1210/en.2007-0021. [DOI] [PubMed] [Google Scholar]
- 120.Dole G, Nilsson EE, Skinner MK. Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis. Reproduction. 2008;135(5):671–682. doi: 10.1530/REP-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Streiter S, Fisch B, Sabbah B, Ao A, Abir R. The importance of neuronal growth factors in the ovary. Mol Hum Reprod. 2016;22(1):3–17. doi: 10.1093/molehr/gav057. [DOI] [PubMed] [Google Scholar]
- 122.Botchkareva NV, Botchkarev VA, Welker P, Airaksinen M, Roth W, Suvanto P, Muller-Rover S, Hadshiew IM, Peters C, Paus R. New roles for glial cell line–derived neurotrophic factor and neurturin: involvement in hair cycle control. Am J Pathol. 2000;156(3):1041–1053. doi: 10.1016/S0002-9440(10)64972-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luukko K, Suvanto P, Saarma M, Thesleff I. Expression of GDNF and its receptors in developing tooth is developmentally regulated and suggests multiple roles in innervation and organogenesis. Dev Dyn. 1997;210(4):463–471. doi: 10.1002/(SICI)1097-0177(199712)210:4<463::AID-AJA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 124.You L, Ebner S, Kruse FE. Glial cell–derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;42(11):2496–2504. [PubMed] [Google Scholar]
- 125.You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41(3):692–702. [PubMed] [Google Scholar]
- 126.Messer CJ, Eisch AJ, Carlezon WA, Jr., Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26(1):247–257. doi: 10.1016/s0896-6273(00)81154-x. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Otsuki K, Uchida S, Watanuki T, Wakabayashi Y, Fujimoto M, Matsubara T, Funato H, Watanabe Y. Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res. 2008;42(14):1145–1153. doi: 10.1016/j.jpsychires.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 128.Iwahashi N, Murakami H, Nimura Y, Takahashi M. Activation of RET tyrosine kinase regulates interleukin-8 production by multiple signaling pathways. Biochem Biophys Res Commun. 2002;294(3):642–649. doi: 10.1016/S0006-291X(02)00528-4. [DOI] [PubMed] [Google Scholar]
- 129.Tsui-Pierchala BA, Milbrandt J, Johnson EM., Jr. NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron. 2002;33(2):261–273. doi: 10.1016/s0896-6273(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 130.Tufro A, Teichman J, Banu N, Villegas G. Crosstalk between VEGF-A/VEGFR2 and GDNF/RET signaling pathways. Biochem Biophys Res Commun. 2007;358(2):410–416. doi: 10.1016/j.bbrc.2007.04.146. [DOI] [PubMed] [Google Scholar]
- 131.Zhong Z, Gu H, Peng J, Wang W, Johnstone BH, March KL, Farlow MR, Du Y. GDNF secreted from adipose-derived stem cells stimulates VEGF-independent angiogenesis. Oncotarget. 2016;7(24):36829–36841. doi: 10.18632/oncotarget.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Widschwendter M, Apostolidou S, Raum E, Rothenbacher D, Fiegl H, Menon U, Stegmaier C, Jacobs IJ, Brenner H. Epigenotyping in peripheral blood cell DNA and breast cancer risk: a proof of principle study. PLoS One. 2008;3(7):e2656. doi: 10.1371/journal.pone.0002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hisaoka K, Nishida A, Koda T, Miyata M, Zensho H, Morinobu S, Ohta M, Yamawaki S. Antidepressant drug treatments induce glial cell line–derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J Neurochem. 2001;79(1):25–34. doi: 10.1046/j.1471-4159.2001.00531.x. [DOI] [PubMed] [Google Scholar]
- 134.Shao Z, Dyck LE, Wang H, Li XM. Antipsychotic drugs cause glial cell line–derived neurotrophic factor secretion from C6 glioma cells. J Psychiatry Neurosci. 2006;31(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- 135.Koyama Y, Egawa H, Osakada M, Baba A, Matsuda T. Increase by FK960, a novel cognitive enhancer, in glial cell line–derived neurotrophic factor production in cultured rat astrocytes. Biochem Pharmacol. 2004;68(2):275–282. doi: 10.1016/j.bcp.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 136.Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695–714. doi: 10.2165/00023210-200216100-00004. [doi:161004 [pii]] [DOI] [PubMed] [Google Scholar]
- 137.Capes-Davis A, Andrew SD, Hyland VJ, Twigg S, Learoyd DL, Dwight T, Marsh DJ, Robinson BG. Glucocorticoids differentially inhibit expression of the RET proto-oncogene. Gene Expr. 1999;8(5–6):311–326. [PMC free article] [PubMed] [Google Scholar]
- 138.Nakashima S, Matsuyama Y, Yu Y, Kiuchi K, Ishiguro N. Suppression of GDNF production by MPSS treatment following spinal cord injury in the rat. Neuroreport. 2004;15(15):2337–2340. doi: 10.1097/00001756-200410250-00007. [doi:00001756-200410250-00007 [pii]] [DOI] [PubMed] [Google Scholar]
- 139.Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113(1):29–39. doi: 10.1016/s0925-4773(02)00004-7. [doi:S0925477302000047 [pii]] [DOI] [PubMed] [Google Scholar]
- 140.Yamagata K, Hakata K, Maeda A, Mochizuki C, Matsufuji H, Chino M, Yamori Y. Adenosine induces expression of glial cell line–derived neurotrophic factor (GDNF) in primary rat astrocytes. Neurosci Res. 2007;59(4):467–474. doi: 10.1016/j.neures.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 141.Tsuchioka M, Takebayashi M, Hisaoka K, Maeda N, Nakata Y. Serotonin (5-HT) induces glial cell line–derived neurotrophic factor (GDNF) mRNA expression via the transactivation of fibroblast growth factor receptor 2 (FGFR2) in rat C6 glioma cells. J Neurochem. 2008;106(1):244–257. doi: 10.1111/j.1471-4159.2008.05357.x. [DOI] [PubMed] [Google Scholar]
- 142.Woodbury D, Schaar DG, Ramakrishnan L, Black IB. Novel structure of the human GDNF gene. Brain Res. 1998;803(1–2):95–104. doi: 10.1016/s0006-8993(98)00627-1. [doi:S0006-8993(98)00627-1 [pii]] [DOI] [PubMed] [Google Scholar]
- 143.Tanaka M, Ito S, Kiuchi K. Novel alternative promoters of mouse glial cell line–derived neurotrophic factor gene. Biochim Biophys Acta. 2000;1494(1–2):63–74. doi: 10.1016/s0167-4781(00)00218-9. [doi:S0167-4781(00)00218-9 [pii]] [DOI] [PubMed] [Google Scholar]
- 144.Verity AN, Wyatt TL, Lee W, Hajos B, Baecker PA, Eglen RM, Johnson RM. Differential regulation of glial cell line–derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J Neurosci Res. 1999;55(2):187–197. doi: 10.1002/(SICI)1097-4547(19990115)55:2<187::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 145.Tanabe K, Nishimura K, Dohi S, Kozawa O. Mechanisms of interleukin-1beta-induced GDNF release from rat glioma cells. Brain Res. 2009;1274:11–20. doi: 10.1016/j.brainres.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 146.Saavedra A, Baltazar G, Duarte EP. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol. 2008;86(3):186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 147.Morale MC, Serra PA, L'Episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS. Estrogen, neuroinflammation and neuroprotection in Parkinson's disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138(3):869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 148.Kim YJ, Lee CJ, Lee U, Yoo YM. Tamoxifen-induced cell death and expression of neurotrophic factors in cultured C6 glioma cells. J Neurooncol. 2005;71(2):121–125. doi: 10.1007/s11060-004-0984-z. [DOI] [PubMed] [Google Scholar]
- 149.Kuppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-alpha knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33(6):832–838. doi: 10.1016/j.psyneuen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 150.Ivanova T, Karolczak M, Beyer C. Estradiol stimulates GDNF expression in developing hypothalamic neurons. Endocrinology. 2002;143(8):3175–3178. doi: 10.1210/endo.143.8.8794. [DOI] [PubMed] [Google Scholar]
- 151.Platania P, Seminara G, Aronica E, Troost D, Vincenza Catania M, Angela Sortino M. 17beta-estradiol rescues spinal motoneurons from AMPA-induced toxicity: a role for glial cells. Neurobiol Dis. 2005;20(2):461–470. doi: 10.1016/j.nbd.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 152.Boulay A, Breuleux M, Stephan C, Fux C, Brisken C, Fiche M, Wartmann M, Stumm M, Lane HA, Hynes NE. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008;68(10):3743–3751. doi: 10.1158/0008-5472.CAN-07-5100. [DOI] [PubMed] [Google Scholar]
- 153.Kang J, Qian PX, Pandey V, Perry JK, Miller LD, Liu ET, Zhu T, Liu DX, Lobie PE. Artemin is estrogen regulated and mediates antiestrogen resistance in mammary carcinoma. Oncogene. 2010;29(22):3228–3240. doi: 10.1038/onc.2010.71. [DOI] [PubMed] [Google Scholar]
- 154.Plaza-Menacho I, Morandi A, Robertson D, Pancholi S, Drury S, Dowsett M, Martin LA, Isacke CM. Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene. 2010;29(33):4648–4657. doi: 10.1038/onc.2010.209. [DOI] [PubMed] [Google Scholar]
- 155.Koroknai V, Ecsedi S, Vizkeleti L, Kiss T, Szasz I, Lukacs A, Papp O, Adany R, Balazs M. Genomic profiling of invasive melanoma cell lines by array comparative genomic hybridization. Melanoma Res. 2016;26(2):100–107. [Google Scholar]
- 156.Franz H, Greschik H, Willmann D, Ozretic L, Jilg CA, Wardelmann E, Jung M, Buettner R, Schule R. The histone code reader SPIN1 controls RET signaling in liposarcoma. Oncotarget. 2015;6(7):4773–4789. doi: 10.18632/oncotarget.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr., Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21(6):1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 158.Zihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR. The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull. 2005;68(1–2):42–53. doi: 10.1016/j.brainresbull.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 159.Kang J, Perry JK, Pandey V, Fielder GC, Mei B, Qian PX, Wu ZS, Zhu T, Liu DX, Lobie PE. Artemin is oncogenic for human mammary carcinoma cells. Oncogene. 2009;28(19):2034–2045. doi: 10.1038/onc.2009.66. [DOI] [PubMed] [Google Scholar]
- 160.Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, Matsuda Y, Fujii H, Saijoh Y, Hamada H. GFR[alpha]3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron. 1999;23(4):725–736. doi: 10.1016/s0896-6273(01)80031-3. [DOI] [PubMed] [Google Scholar]
- 161.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth RO, Johnson EM, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35(2):267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 162.Andres R, Forgie A, Wyatt S, Chen Q, de Sauvage FJ, Davies AM. Multiple effects of artemin on sympathetic neurone generation, survival and growth. Development. 2001;128(19):3685–3695. doi: 10.1242/dev.128.19.3685. [DOI] [PubMed] [Google Scholar]
- 163.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9(11):1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- 164.Yan H, Newgreen DF, Young HM. Developmental changes in neurite outgrowth responses of dorsal root and sympathetic ganglia to GDNF, neurturin, and artemin. Dev Dyn. 2003;227(3):395–401. doi: 10.1002/dvdy.10294. [DOI] [PubMed] [Google Scholar]
- 165.Del Fiacco M, Quartu M, Serra MP, Follesa P, Lai ML, Bachis A. Topographical localization of glial cell line–derived neurotrophic factor in the human brain stem: an immunohistochemical study of prenatal, neonatal and adult brains. J Chem Neuroanat. 2002;23(1):29–48. doi: 10.1016/s0891-0618(01)00139-9. [DOI] [PubMed] [Google Scholar]
- 166.Pina Serra M, Quartu M, Ambu R, Follesa P, Del Fiacco M. Immunohistochemical localization of GDNF in the human hippocampal formation from prenatal life to adulthood. Brain Res. 2002;928(1–2):138–146. doi: 10.1016/s0006-8993(01)03377-7. [DOI] [PubMed] [Google Scholar]
- 167.Quartu M, Serra MP, Boi M, Ferretti MT, Lai ML, Del Fiacco M. Tissue distribution of Ret, GFRalpha-1, GFRalpha-2 and GFRalpha-3 receptors in the human brainstem at fetal, neonatal and adult age. Brain Res. 2007;1173:36–52. doi: 10.1016/j.brainres.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 168.Quartu M, Serra MP, Boi M, Sestu N, Lai ML, Del Fiacco M. Tissue distribution of neurturin, persephin and artemin in the human brainstem at fetal, neonatal and adult age. Brain Res. 2007;1143:102–115. doi: 10.1016/j.brainres.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 169.Quartu M, Serra MP, Manca A, Mascia F, Follesa P, Del Fiacco M. Neurturin, persephin, and artemin in the human pre- and full-term newborn and adult hippocampus and fascia dentata. Brain Res. 2005;1041(2):157–166. doi: 10.1016/j.brainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 170.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26(33):8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orozco OE, Walus L, Sah DWY, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13(11):2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 172.Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line–derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33(30):12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li J, Klein C, Liang C, Rauch R, Kawamura K, Hsueh AJ. Autocrine regulation of early embryonic development by the artemin-GFRA3 (GDNF family receptor-alpha 3) signaling system in mice. FEBS Lett. 2009;583(15):2479–2485. doi: 10.1016/j.febslet.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 174.Kawamura K, Ye Y, Kawamura N, Jing L, Groenen P, Gelpke MS, Rauch R, Hsueh AJ, Tanaka T. Completion of Meiosis I of preovulatory oocytes and facilitation of preimplantation embryo development by glial cell line–derived neurotrophic factor. Dev Biol. 2008;315(1):189–202. doi: 10.1016/j.ydbio.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 175.Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. Fourth ed. McGraw-Hill; 2000. [Google Scholar]
- 176.Damon DH, Teriele JA, Marko SB. Vascular-derived artemin: a determinant of vascular sympathetic innervation? Am J Physiol Heart Circ Physiol. 2007;293(1):H266–273. doi: 10.1152/ajpheart.00859.2006. [DOI] [PubMed] [Google Scholar]
- 177.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM., Jr. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22(2):253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 178.Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR[alpha]2, a functional neurturin receptor. Neuron. 1999;22(2):243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 179.Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr., Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128(20):3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- 180.Lindahl M, Poteryaev D, Yu L, Arumae U, Timmusk T, Bongarzone I, Aiello A, Pierotti MA, Airaksinen MS, Saarma M. Human glial cell line–derived neurotrophic factor receptor alpha 4 is the receptor for persephin and is predominantly expressed in normal and malignant thyroid medullary cells. J Biol Chem. 2001;276(12):9344–9351. doi: 10.1074/jbc.M008279200. [DOI] [PubMed] [Google Scholar]
- 181.Vanhorne JB, Andrew SD, Harrison KJ, Taylor SA, Thomas B, McDonald TJ, Ainsworth PJ, Mulligan LM. A model for GFR alpha 4 function and a potential modifying role in multiple endocrine neoplasia 2. Oncogene. 2005;24(6):1091–1097. doi: 10.1038/sj.onc.1207826. [DOI] [PubMed] [Google Scholar]
- 182.Lindahl M, Timmusk T, Rossi J, Saarma M, Airaksinen MS. Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci. 2000;15(6):522–533. doi: 10.1006/mcne.2000.0845. [DOI] [PubMed] [Google Scholar]
- 183.Lindfors PH, Lindahl M, Rossi J, Saarma M, Airaksinen MS. Ablation of persephin receptor glial cell line–derived neurotrophic factor family receptor {alpha}4 impairs thyroid calcitonin production in young mice. Endocrinology. 2006;147(5):2237–2244. doi: 10.1210/en.2005-1620. [DOI] [PubMed] [Google Scholar]
- 184.Baba T, Sakamoto Y, Kasamatsu A, Minakawa Y, Yokota S, Higo M, Yokoe H, Ogawara K, Shiiba M, Tanzawa H. Persephin: a potential key component in human oral cancer progression through the RET receptor tyrosine kinase-mitogen-activated protein kinase signaling pathway. Mol Carcinog. 2015;54(8):608–617. doi: 10.1002/mc.22127. [DOI] [PubMed] [Google Scholar]
- 185.Peterziel H, Paech T, Strelau J, Unsicker K, Krieglstein K. Specificity in the crosstalk of TGFbeta/GDNF family members is determined by distinct GFR alpha receptors. J Neurochem. 2007;103(6):2491–2504. doi: 10.1111/j.1471-4159.2007.04962.x. [DOI] [PubMed] [Google Scholar]
- 186.Lahteenmaki M, Kupari J, Airaksinen MS. Increased apoptosis of parasympathetic but not enteric neurons in mice lacking GFRalpha2. Dev Biol. 2007;305(1):325–332. doi: 10.1016/j.ydbio.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 187.Laurikainen A, Hiltunen JO, Vanhatalo S, Klinge E, Saarma M. Glial cell line–derived neurotrophic factor is expressed in penis of adult rat and retrogradely transported in penile parasympathetic and sensory nerves. Cell Tissue Res. 2000;302(3):321–329. doi: 10.1007/s004410000273. [DOI] [PubMed] [Google Scholar]
- 188.Kato R, Wolfe D, Coyle CH, Wechuck JB, Tyagi P, Tsukamoto T, Nelson JB, Glorioso JC, Chancellor MB, Yoshimura N. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 2009;16(1):26–33. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nangle MR, Keast JR. Loss of nitrergic neurotransmission to mouse corpus cavernosum in the absence of neurturin is accompanied by increased response to acetylcholine. Br J Pharmacol. 2006;148(4):423–433. doi: 10.1038/sj.bjp.0706760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stewart AL, Anderson RB, Kobayashi K, Young HM. Effects of NGF, NT-3 and GDNF family members on neurite outgrowth and migration from pelvic ganglia from embryonic and newborn mice. BMC Dev Biol. 2008;8:73. doi: 10.1186/1471-213X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311(2):175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- 192.Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 2008;507(2):1169–1183. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- 193.Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS One. 2009;4(3):e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakamura T, Ishizaka Y, Nagao M, Hara M, Ishikawa T. Expression of the RET proto-oncogene product in human normal and neoplastic tissues of neural crest origin. J Pathol. 1994;172(3):255–260. doi: 10.1002/path.1711720305. [DOI] [PubMed] [Google Scholar]
- 195.Dahia PL, Toledo SP, Mulligan LM, Maher ER, Grossman AB, Eng C. Mutation analysis of glial cell line–derived neurotrophic factor (GDNF), a ligand for the RET/GDNF receptor alpha complex, in sporadic phaeochromocytomas. Cancer Res. 1997;57(2):310–313. [PubMed] [Google Scholar]
- 196.Marsh DJ, Zheng Z, Arnold A, Andrew SD, Learoyd D, Frilling A, Komminoth P, Neumann HP, Ponder BA, Rollins BJ. Mutation analysis of glial cell line–derived neurotrophic factor, a ligand for an RET/coreceptor complex, in multiple endocrine neoplasia type 2 and sporadic neuroendocrine tumors. J Clin Endocrinol Metab. 1997;82(9):3025–3028. doi: 10.1210/jcem.82.9.4197. [DOI] [PubMed] [Google Scholar]
- 197.Plaza-Menacho I, Burzynski GM, de Groot JW, Eggen BJ, Hofstra RM. Current concepts in RET-related genetics, signaling and therapeutics. Trends Genet. 2006;22(11):627–636. doi: 10.1016/j.tig.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 198.Akeno-Stuart N, Croyle M, Knauf JA, Malaguarnera R, Vitagliano D, Santoro M, Stephan C, Grosios K, Wartmann M, Cozens R. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res. 2007;67(14):6956–6964. doi: 10.1158/0008-5472.CAN-06-4605. [DOI] [PubMed] [Google Scholar]
- 199.Yang J, Runeberg-Roos P, Leppanen VM, Saarma M. The mouse soluble GFRa4 receptor activates RET independently of its ligand persephin. Oncogene. 2007;26(26):3892–3898. doi: 10.1038/sj.onc.1210161. [DOI] [PubMed] [Google Scholar]
- 200.Canibano C, Rodriguez NL, Saez C, Tovar S, Garcia-Lavandeira M, Borrello MG, Vidal A, Costantini F, Japon M, Dieguez C. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J. 2007;26(8):2015–2028. doi: 10.1038/sj.emboj.7601636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shewchuk BM, Ho Y, Liebhaber SA, Cooke NE. A single base difference between Pit-1 binding sites at the hGH promoter and locus control region specifies distinct Pit-1 conformations and functions. Mol Cell Biol. 2006;26(17):6535–6546. doi: 10.1128/MCB.00267-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fernandez RM, Ruiz-Ferrer M, Lopez-Alonso M, Antinolo G, Borrego S. Polymorphisms in the genes encoding the 4 RET ligands, GDNF, NTN, ARTN, PSPN, and susceptibility to Hirschsprung disease. J Pediatr Surg. 2008;43(11):2042–2047. doi: 10.1016/j.jpedsurg.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 203.Toma H, Winston JH, Micci M-A, Li H, Hellmich HL, Parsricha PJ. Characterization of the neurotrophic response to acute pancreatitis. Pancreas. 2002;25(1):31–38. doi: 10.1097/00006676-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 204.Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line–derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826(1):112–120. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 205.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Muller MW, Giese NA, Friess H, Schafer KH. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374(3):442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 206.Liu H, Ma Q, Li J. High glucose promotes cell proliferation and enhances GDNF and RET expression in pancreatic cancer cells. Mol Cell Biochem. 2011;347(1–2):95–101. doi: 10.1007/s11010-010-0617-0. [DOI] [PubMed] [Google Scholar]
- 207.Cavel O, Shomron O, Shabtay A, Vital J, Trejo-Leider L, Weizman N, Krelin Y, Fong Y, Wong RJ, Amit M. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72(22):5733–5743. doi: 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- 208.Klöppel G, Detlefsen S, Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch. 2004;445(1):1–8. doi: 10.1007/s00428-004-1021-5. [DOI] [PubMed] [Google Scholar]
- 209.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332(22):1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 210.Ceyhan GO, Bergmann F, Kadihasanoglu M, Erkan M, Park W, Hinz U, Giese T, Muller MW, Buchler MW, Giese NA. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56(4):534–544. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meng LX, Chi YH, Wang XX, Ding ZJ, Fei LC, Zhang H, Mou L, Cui W, Xue YJ. Neurotrophic artemin promotes motility and invasiveness of MIA PaCa-2 pancreatic cancer cells. Asian Pac J Cancer Prev. 2012;13(5):1793–1797. doi: 10.7314/apjcp.2012.13.5.1793. [DOI] [PubMed] [Google Scholar]
- 212.Ito Y, Okada Y, Sato M, Sawai H, Funahashi H, Murase T, Hayakawa T, Manabe T. Expression of glial cell line–derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery. 2005;138(4):788–794. doi: 10.1016/j.surg.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 213.Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, Giehl K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line–derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64(15):5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- 214.Takahashi H, Funahashi H, Sawai H, Sakamoto M, Matsuo Y, Yamamoto M, Okada Y, Hayakawa T, Manabe T. Glial cell line–derived neurotrophic factor enhances nuclear factor-kappaB activity and invasive potential in human pancreatic cancer cells. Pancreas. 2004;29(1):22–27. doi: 10.1097/00006676-200407000-00051. [DOI] [PubMed] [Google Scholar]
- 215.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, Carlson DL, Shah JP, Fong Y, Wong RJ. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3:97. doi: 10.3389/fphys.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, Zhou M, Han S, Zhang Q. The relationship between overexpression of glial cell–derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36(4):656–664. doi: 10.1177/147323000803600406. [DOI] [PubMed] [Google Scholar]
- 218.Funahashi H, Takeyama H, Sawai H, Furuta A, Sato M, Okada Y, Hayakawa T, Tanaka M, Manabe T. Alteration of integrin expression by glial cell line–derived neurotrophic factor (GDNF) in human pancreatic cancer cells. Pancreas. 2003;27(2):190–196. doi: 10.1097/00006676-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 219.Funahashi H, Okada Y, Sawai H, Takahashi H, Matsuo Y, Takeyama H, Manabe T. The role of glial cell line–derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J Surg Oncol. 2005;91(1):77–83. doi: 10.1002/jso.20277. [DOI] [PubMed] [Google Scholar]
- 220.Kikuchi K. Expression of GDNF (glial cell line–derived neurotrophic factor) and Ret in normal human and cancerous pancreatic tissues. Hokkaido Igaku Zasshi. 2004;79(5):585–595. [PubMed] [Google Scholar]
- 221.Okada Y, Eibl G, Duffy JP, Reber HA, Hines OJ. Glial cell–derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery. 2003;134(2):293–299. doi: 10.1067/msy.2003.239. [DOI] [PubMed] [Google Scholar]
- 222.Okada Y, Takeyama H, Sato M, Morikawa M, Sobue K, Asai K, Tada T, Kato T, Manabe T. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret proto-oncogene with reference to glial-cell-line–derived neurotrophic factor (GDNF) Int J Cancer. 1999;81(1):67–73. doi: 10.1002/(sici)1097-0215(19990331)81:1<67::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 223.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Buchler MW, Friess H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244(2):274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sawai H, Okada Y, Kazanjian K, Kim J, Hasan S, Hines OJ, Reber HA, Hoon DS, Eibl G. The G691S RET polymorphism increases glial cell line–derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res. 2005;65(24):11536–11544. doi: 10.1158/0008-5472.CAN-05-2843. [DOI] [PubMed] [Google Scholar]
- 225.Wang K, Demir IE, D'Haese JG, Tieftrunk E, Kujundzic K, Schorn S, Xing B, Kehl T, Friess H, Ceyhan GO. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2014;35(1):103–113. doi: 10.1093/carcin/bgt312. [DOI] [PubMed] [Google Scholar]
- 226.Amit M, Na'ara S, Leider-Trejo L, Binenbaum Y, Kulish N, Fridman E, Shabtai-Orbach A, Wong RJ, Gil Z. Upregulation of RET induces perineurial invasion of pancreatic adenocarcinoma. Oncogene. 2017;36(23):3232–3239. doi: 10.1038/onc.2016.483. [DOI] [PubMed] [Google Scholar]
- 227.Song H, Moon A. Glial cell–derived neurotrophic factor (GDNF) promotes low-grade Hs683 glioma cell migration through JNK, ERK-1/2 and p38 MAPK signaling pathways. Neurosci Res. 2006;56(1):29–38. doi: 10.1016/j.neures.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 228.Lu DY, Leung YM, Cheung CW, Chen YR, Wong KL. Glial cell line–derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem Pharmacol. 2010;80(8):1201–1209. doi: 10.1016/j.bcp.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 229.Wiesenhofer B, Stockhammer G, Kostron H, Maier H, Hinterhuber H, Humpel C. Glial cell line–derived neurotrophic factor (GDNF) and its receptor (GFR-alpha 1) are strongly expressed in human gliomas. Acta Neuropathol (Berl) 2000;99(2):131–137. doi: 10.1007/pl00007416. [DOI] [PubMed] [Google Scholar]
- 230.Ng WH, Wan GQ, Peng ZN, Too HP. Glial cell-line derived neurotrophic factor (GDNF) family of ligands confer chemoresistance in a ligand-specific fashion in malignant gliomas. J Clin Neurosci. 2009;16(3):427–436. doi: 10.1016/j.jocn.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 231.Hansford LM, Marshall GM. Glial cell line–derived neurotrophic factor (GDNF) family ligands reduce the sensitivity of neuroblastoma cells to pharmacologically induced cell death, growth arrest and differentiation. Neurosci Lett. 2005;389(2):77–82. doi: 10.1016/j.neulet.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 232.Jimenez A, Lopez-Ornelas A, Estudillo E, Gonzalez-Mariscal L, Gonzalez RO, Segovia J. A soluble form of GAS1 inhibits tumor growth and angiogenesis in a triple negative breast cancer model. Exp Cell Res. 2014;327(2):307–317. doi: 10.1016/j.yexcr.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 233.Dominguez-Monzon G, Benitez JA, Vergara P, Lorenzana R, Segovia J. Gas1 inhibits cell proliferation and induces apoptosis of human primary gliomas in the absence of Shh. Int J Dev Neurosci. 2009;27(4):305–313. doi: 10.1016/j.ijdevneu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 234.Hishiki T, Nimura Y, Isogai E, Kondo K, Ichimiya S, Nakamura Y, Ozaki T, Sakiyama S, Hirose M, Seki N. Glial cell line–derived neurotrophic factor/neurturin-induced differentiation and its enhancement by retinoic acid in primary human neuroblastomas expressing c-Ret, GFR alpha-1, and GFR alpha-2. Cancer Res. 1998;58(10):2158–2165. [PubMed] [Google Scholar]
- 235.Qu DW, Liu Y, Wang L, Xiong Y, Zhang CL, Gao DS. Glial cell line–derived neurotrophic factor promotes proliferation of neuroglioma cells by up-regulation of cyclins PCNA and Ki-67. Eur Rev Med Pharmacol Sci. 2015;19(11):2070–2075. [PubMed] [Google Scholar]
- 236.Furuta A, Funahashi H, Sawai H, Sato M, Okada Y, Takeyama H, Manabe T. The relationship between GDNF and integrins in human colorectal cancer cell activity. Hepatogastroenterology. 2007;54(77):1398–1402. [PubMed] [Google Scholar]
- 237.Huang SM, Chen TS, Chiu CM, Chang LK, Liao KF, Tan HM, Yeh WL, Chang GR, Wang MY, Lu DY. GDNF increases cell motility in human colon cancer through VEGF-VEGFR1 interaction. Endocr Relat Cancer. 2014;21(1):73–84. doi: 10.1530/ERC-13-0351. [DOI] [PubMed] [Google Scholar]
- 238.Qiao S, Iwashita T, Ichihara M, Murakumo Y, Yamaguchi A, Isogai M, Sakata K, Takahashi M. Increased expression of glial cell line–derived neurotrophic factor and neurturin in a case of colon adenocarcinoma associated with diffuse ganglioneuromatosis. Clin Neuropathol. 2009;28(2):105–112. doi: 10.5414/npp28105. [DOI] [PubMed] [Google Scholar]
- 239.Sambuudash O, Kim HS, Cho MY. Lack of aberrant methylation in an adjacent area of left-sided colorectal cancer. Yonsei Med J. 2017;58(4):749–755. doi: 10.3349/ymj.2017.58.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Luo Y, Tsuchiya KD, Il Park D, Fausel R, Kanngurn S, Welcsh P, Dzieciatkowski S, Wang J, Grady WM. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene. 2013;32(16):2037–2047. doi: 10.1038/onc.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 243.Radvanyi L, Singh-Sandhu D, Gallichan S, Lovitt C, Pedyczak A, Mallo G, Gish K, Kwok K, Hanna W, Zubovits J. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2005;102(31):11005–11010. doi: 10.1073/pnas.0500904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100(6):3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Esseghir S, Kennedy A, Seedhar P, Nerurkar A, Poulsom R, Reis-Filho JS, Isacke CM. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin Cancer Res. 2007;13(11):3164–3173. doi: 10.1158/1078-0432.CCR-07-0224. [DOI] [PubMed] [Google Scholar]
- 246.Ginestier C, Cervera N, Finetti P, Esteyries S, Esterni B, Adelaide J, Xerri L, Viens P, Jacquemier J, Charafe-Jauffret E. Prognosis and gene expression profiling of 20q13-amplified breast cancers. Clin Cancer Res. 2006;12(15):4533–4544. doi: 10.1158/1078-0432.CCR-05-2339. [DOI] [PubMed] [Google Scholar]
- 247.Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66(21):10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 248.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102(38):13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 250.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 251.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24(29):4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 252.Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, Muir B, Mohapatra G, Salunga R, Tuggle JT. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5(6):607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 253.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98(4):262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 254.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3(6):e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Banerjee A, Wu ZS, Qian P, Kang J, Pandey V, Liu DX, Zhu T, Lobie PE. ARTEMIN synergizes with TWIST1 to promote metastasis and poor survival outcome in patients with ER negative mammary carcinoma. Breast Cancer Res. 2011;13(6):R112. doi: 10.1186/bcr3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xu Y, Qin L, Sun T, Wu H, He T, Yang Z, Mo Q, Liao L, Xu J. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene. 2017;36(8):1157–1166. doi: 10.1038/onc.2016.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Banerjee A, Qian P, Wu ZS, Ren X, Steiner M, Bougen NM, Liu S, Liu DX, Zhu T, Lobie PE. Artemin stimulates radio- and chemo-resistance by promoting TWIST1-BCL-2-dependent cancer stem cell-like behavior in mammary carcinoma cells. J Biol Chem. 2012;287(51):42502–42515. doi: 10.1074/jbc.M112.365163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Banerjee A, Wu ZS, Qian PX, Kang J, Liu DX, Zhu T, Lobie PE. ARTEMIN promotes de novo angiogenesis in ER negative mammary carcinoma through activation of TWIST1-VEGF-A signalling. PLoS One. 2012;7(11):e50098. doi: 10.1371/journal.pone.0050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Morandi A, Martin LA, Gao Q, Pancholi S, Mackay A, Robertson D, Zvelebil M, Dowsett M, Plaza-Menacho I, Isacke CM. GDNF-RET signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res. 2013;73(12):3783–3795. doi: 10.1158/0008-5472.CAN-12-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Andreucci E, Francica P, Fearns A, Martin LA, Chiarugi P, Isacke CM, Morandi A. Targeting the receptor tyrosine kinase RET in combination with aromatase inhibitors in ER positive breast cancer xenografts. Oncotarget. 2016;7(49):80543–80553. doi: 10.18632/oncotarget.11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gardaneh M, Shojaei S, Kaviani A, Behnam B. GDNF induces RET-SRC-HER2-dependent growth in trastuzumab-sensitive but SRC-independent growth in resistant breast tumor cells. Breast Cancer Res Treat. 2017;162(2):231–241. doi: 10.1007/s10549-016-4078-3. [DOI] [PubMed] [Google Scholar]
- 263.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128(23):4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- 264.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456(7222):663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wu ZS, Pandey V, Wu WY, Ye S, Zhu T, Lobie PE. Prognostic significance of the expression of GFRalpha1, GFRalpha3 and syndecan-3, proteins binding ARTEMIN, in mammary carcinoma. BMC Cancer. 2013;13:34. doi: 10.1186/1471-2407-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pandey V, Qian PX, Kang J, Perry JK, Mitchell MD, Yin Z, Wu ZS, Liu DX, Zhu T, Lobie PE. Artemin stimulates oncogenicity and invasiveness of human endometrial carcinoma cells. Endocrinology. 2010;151(3):909–920. doi: 10.1210/en.2009-0979. [DOI] [PubMed] [Google Scholar]
- 267.Pandey V, Jung Y, Kang J, Steiner M, Qian PX, Banerjee A, Mitchell MD, Wu ZS, Zhu T, Liu DX. Artemin reduces sensitivity to doxorubicin and paclitaxel in endometrial carcinoma cells through specific regulation of CD24. Transl Oncol. 2010;3(4):218–229. doi: 10.1593/tlo.09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tang JZ, Kong XJ, Kang J, Fielder GC, Steiner M, Perry JK, Wu ZS, Yin Z, Zhu T, Liu DX. Artemin-stimulated progression of human non–small cell lung carcinoma is mediated by BCL2. Mol Cancer Ther. 2010;9(6):1697–1708. doi: 10.1158/1535-7163.MCT-09-1077. [DOI] [PubMed] [Google Scholar]
- 269.Futami H, Egawa S, Tsukada T, Maruyama K, Bandoh S, Noguchi M, Yamaguchi K. A novel somatic point mutation of the RET Proto-oncogene in tumor tissues of small cell lung cancer patients. Jpn J Cancer Res. 1995;86(12):1127–1130. doi: 10.1111/j.1349-7006.1995.tb03304.x. [doi:h0910505096852513 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mulligan LM, Timmer T, Ivanchuk SM, Campling BG, Young LC, Rabbitts PH, Sundaresan V, Hofstra RM, Eng C. Investigation of the genes for RET and its ligand complex, GDNF/GFR alpha-I, in small cell lung carcinoma. Genes Chromosomes Cancer. 1998;21(4):326–332. [PubMed] [Google Scholar]
- 271.Lin C, Wang S, Xie W, Chang J, Gan Y. The RET fusion gene and its correlation with demographic and clinicopathological features of non–small cell lung cancer: a meta-analysis. Cancer Biol Ther. 2015;16(7):1019–1028. doi: 10.1080/15384047.2015.1046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yoo SS, Jin G, Jung HJ, Hong MJ, Choi JE, Jeon HS, Lee SY, Lim JO, Park JY. RET fusion genes in Korean non–small cell lung cancer. J Korean Med Sci. 2013;28(10):1555–1558. doi: 10.3346/jkms.2013.28.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Aravindakshan J, Chen XL, Sairam MR. Age-dependent bimodal GDNF regulation during ovarian tumorigenesis in follitropin receptor mutant mice. Biochem Biophys Res Commun. 2006;351(2):507–513. doi: 10.1016/j.bbrc.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 274.Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006;66(3):1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 275.Meng X, de Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line–derived neurotrophic factor in mouse testis. Cancer Res. 2001;61(8):3267–3271. [PubMed] [Google Scholar]
- 276.Ferranti F, Muciaccia B, Ricci G, Dovere L, Canipari R, Magliocca F, Stefanini M, Catizone A, Vicini E. Glial cell line–derived neurotrophic factor promotes invasive behaviour in testicular seminoma cells. Int J Androl. 2012;35(5):758–768. doi: 10.1111/j.1365-2605.2012.01267.x. [DOI] [PubMed] [Google Scholar]
- 277.Waheeb R, Hofmann MC. Human spermatogonial stem cells: a possible origin for spermatocytic seminoma. Int J Androl. 2011;34(4 Pt 2):e296–305. doi: 10.1111/j.1365-2605.2011.01199.x. [discussion e305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17(14):1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 279.Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Hossain K, Tamakoshi A, Kunisada T, Kambayashi Y, Ogino K, Suzuki H. c-Kit-targeting immunotherapy for hereditary melanoma in a mouse model. Cancer Res. 2004;64(3):801–806. doi: 10.1158/0008-5472.can-03-2532. [DOI] [PubMed] [Google Scholar]
- 280.Kato M, Ohgami N, Kawamoto Y, Tsuzuki T, Hossain K, Yanagishita T, Ohshima Y, Tsuboi H, Yamanoshita O, Matsumoto Y. Protective effect of hyperpigmented skin on UV-mediated cutaneous cancer development. J Invest Dermatol. 2007;127(5):1244–1249. doi: 10.1038/sj.jid.5700659. [DOI] [PubMed] [Google Scholar]
- 281.Narita N, Tanemura A, Murali R, Scolyer RA, Huang S, Arigami T, Yanagita S, Chong KK, Thompson JF, Morton DL. Functional RET G691S polymorphism in cutaneous malignant melanoma. Oncogene. 2009;28(34):3058–3068. doi: 10.1038/onc.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ohshima Y, Yajima I, Takeda K, Iida M, Kumasaka M, Matsumoto Y, Kato M. c-RET molecule in malignant melanoma from oncogenic RET-carrying transgenic mice and human cell lines. PLoS One. 2010;5(4):e10279. doi: 10.1371/journal.pone.0010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zheng SC, Zhang YR, Luo SY, Zhang LP. The effect of GDNF on matrix-degrading and cell-adhesion during perineural invasion of salivary adenoid cystic carcinoma. Shanghai Kou Qiang Yi Xue. 2016;25(2):212–216. [PubMed] [Google Scholar]
- 284.Ban K, Feng S, Shao L, Ittmann M. RET Signaling in Prostate Cancer. Clin Cancer Res. 2017;23(16):4885–4896. doi: 10.1158/1078-0432.CCR-17-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Baspinar S, Bircan S, Ciris M, Karahan N, Bozkurt KK. Expression of NGF, GDNF and MMP-9 in prostate carcinoma. Pathol Res Pract. 2017;213(5):483–489. doi: 10.1016/j.prp.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 286.Zhang M, Zhang W, Wu Z, Liu S, Sun L, Zhong Y, Zhang X, Kong X, Qian P, Zhang H. Artemin is hypoxia responsive and promotes oncogenicity and increased tumor initiating capacity in hepatocellular carcinoma. Oncotarget. 2016;7(3):3267–3282. doi: 10.18632/oncotarget.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Meng FF, Xu Y, Dan QQ, Wei L, Deng YJ, Liu J, He M, Liu W, Xia QJ, Zhou FH. Intrathecal injection of lentivirus-mediated glial cell line–derived neurotrophic factor RNA interference relieves bone cancer-induced pain in rats. Cancer Sci. 2015;106(4):430–437. doi: 10.1111/cas.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ding Z, Xu W, Zhang J, Zou W, Guo Q, Huang C, Liu C, Zhong T, Zhang JM, Song Z. Normalizing GDNF expression in the spinal cord alleviates cutaneous hyperalgesia but not ongoing pain in a rat model of bone cancer pain. Int J Cancer. 2017;140(2):411–422. doi: 10.1002/ijc.30438. [DOI] [PubMed] [Google Scholar]
- 289.Liu Z, Zhang J, Gao Y, Pei L, Zhou J, Gu L, Zhang L, Zhu B, Hattori N, Ji J. Large-scale characterization of DNA methylation changes in human gastric carcinomas with and without metastasis. Clin Cancer Res. 2014;20(17):4598–4612. doi: 10.1158/1078-0432.CCR-13-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Eftang LL, Klajic J, Kristensen VN, Tost J, Esbensen QY, Blom GP, Bukholm IR, Bukholm G. GFRA3 promoter methylation may be associated with decreased postoperative survival in k. BMC Cancer. 2016;16:225. doi: 10.1186/s12885-016-2247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]