Abstract
Orthopedic research into chronic discogenic back pain has commonly focused on aging- and degeneration-related changes in intervertebral disc structure, biomechanics, and biology. However, the primary spine-related reason for physician office visits is pain. The ambiguous nature of the human condition of discogenic low back pain motivates the use of animal models to better understand the pathophysiology. Discogenic back pain models must consider both emergent behavioral changes following pain induction and changes in the nervous system that mediate such behavior. Looking beyond the intervertebral disc, this review describes the different ways to classify pain in human patients and in animal models. We describe several behavioral assays that can be used in rodent models to augment disc degeneration measurements and characterize different types of pain. We review rodent models of discogenic pain that employed behavioral pain assays and highlight a need to better integrate neuroscience and orthopedic science methods to extend current understanding of the complex and multifactorial pathophysiology of discogenic back pain.
Keywords: intervertebral disc, discogenic pain, behavior, rodent model, nervous system
Introduction
Low back pain (LBP) is an extremely common musculoskeletal disorder and a leading cause of disability worldwide.1,2 It is defined as pain, muscle tension, or stiffness localized to the region of human body below the costal margin and above the inferior gluteal fold, either with or without leg pain.3–5 LBP affects more than 70–85% of the population at some time in their life.6 LBP can also induce psychological problems, including depression, anxiety, stressful responsibility, job dissatisfaction, and mental stress at work.6 Consequently, the U.S. economic costs for back and neck pain are approximately $200 billion, with healthcare spending estimated at $87.6 billion and additional costs attributed to lost economic productivity from missed work and lower wages.7–9
Anatomically, the source of LBP may arise from any innervated structures at the lumbar spine, including vertebrae, ligaments, muscles, fasciae, facet joints, and intervertebral discs (IVDs). Among these structures, the IVD is the most prevalent source of LBP,10 and degeneration of the IVD was shown to be highly associated with LBP.11–14 Pain originating from a damaged IVD is commonly referred to as discogenic pain, and in this review, we use the term to refer to nonspecific back pain associated with degenerated IVDs without larger structural defects (such as nucleus pulposus herniations).
Importantly, in both animal models and human patients, pain is a behavior. In order to perceive pain, there must be cortical activity.15 That is, while pain may arise from damage in the periphery, a brain is required in order to feel it. Thus, while in vitro and ex vivo models can help us better understand changes within a degenerated IVD or a single neuron, in vivo models are essential for studying higher-order behavior. Behavior is an emergent property that arises from complicated neural circuits and must not be inferred from lower-order changes but instead studied in its own right, for attempting to reduce it will limit our understanding.16
The IVD is the hydrated fibrocartilaginous soft tissue between vertebrae along the spine. Morphologically, the IVD can be divided into three major components: nucleus pulposus (NP), annulus fibrosus (AF), and cartilage endplate (CEP). The NP is a highly hydrated and proteoglycan-rich structure at the center of the IVD surrounded by the AF.17 The AF is an angle-ply and lamellar tissue.17 The CEP is a thin layer of hyaline cartilage at the superior and inferior margins of the IVD. The NP and AF establish the biomechanical properties of the IVD, including hydrostatic pressure to maintain IVD height and flexibility to allow spine motion, whereas the CEP mainly regulates the transportation of nutrients, cytokines, and waste products between the IVD and adjacent vertebrae.17 The structural, biochemical, and biomechanical properties of the IVD change with degeneration.17
The causes of IVD degeneration are complex and multifactorial.17 Mechanical loading, traumatic injury, inadequate nutrient supply, intradiscal inflammation, and aging are all major risk factors for IVD degeneration.17,18 Although risk factors for IVD degeneration have been widely studied, the relationship between IVD degeneration and nonspecific discogenic back pain is still not fully understood. Degeneration accumulates in human IVDs over many years and usually exhibits dehydration of the NP,19 disorganization of the AF lamellae,19 undistinguished NP–AF boundary,19 defects in the endplates,17 and a loss of IVD height.20 Degenerated IVDs may also develop annular fissures,17 which can lead to NP herniation and CEP injury. In the degenerated IVD, the balance between anabolism and catabolism is lost. There is significant downregulation of proteoglycans, water, and collagen content (except type I collagen in NP) and an increase in matrix-degrading enzymes and proinflammatory cytokines,17,21 such as tumor necrosis factor α (TNF-α),22 interleukin-1 β(IL-1β),22 and chemokine C-C motif 2.23 TNF-α may play a more important role in discogenic pain, while IL-1β may be more critical for the progression of disc degeneration.24,25 The structural defects and loss of proteoglycan have been considered to create a permissive environment for the ingrowth of nerves and vessels in this otherwise largely avascular and aneural structure of the IVD.26,27 Additionally, both vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) are increased in degenerated IVDs,28–30 further enabling neurovascular ingrowth. Ingrowth of capillaries and nerves from the peripheral AF to the inner AF or even NP are observed in painful IVD degeneration, which may be a result of matrix breakdown and structural failure.27,31
Here, we describe different pain classifications and how they are measured using behavioral assays in rodent models and review existing rodent models that employ some of these behavioral assays. We also discuss the importance of studying the nervous system when modeling pain and provide some future considerations when utilizing animal models for the study of discogenic pain.
Low back pain in the clinic
Chronic discogenic LBP can be difficult to diagnose and treat. Numerous imaging studies attempted to determine a definitive association between IVD degeneration and LBP. IVD degeneration is strongly associated with LBP, and degenerative disc disease is the most common diagnosis in back pain patients.6,13,14,32 However, IVD degeneration is not a sufficient diagnosis for pain development, as evidenced by large numbers of asymptomatic patients with abnormal findings on MRI or CT. Using MRI, IVD herniations are seen in 22–67% of asymptomatic adults and spinal stenosis in 21% of asymptomatic adults over 60, and CT evidence of spinal facet joint osteoarthritis was shown to have no correlation with LBP.33–36 In addition to structural defects, a 1990 MRI study by Boden et al. found IVD degeneration in approximately one-third of asymptomatic subjects.34 A 7-year follow-up further concluded that abnormal findings on MRI scans were not predictive of the development or duration of LBP.37 Thus, spine pathology can be observed in the absence of LBP and should not be used as a proxy for LBP in research.
While IVD degeneration may be found in asymptomatic patients, the severity of IVD degeneration as measured by MRI has been shown to correlate with the severity of LBP. Takatalo et al. found that lumbar IVD degeneration correlated with pain severity independent of other degenerative findings.38 Additionally, in asymptomatic individuals, increasing IVD degeneration score from MRI is predictive for developing future first-time LBP episodes.39 Therefore, while it is inappropriate to use IVD degeneration as a proxy for chronic LBP, it is likely a major contributing factor.
Not only is IVD degeneration seen on imaging studies not indicative of LBP, but patients with discogenic back pain are also poorly indicated for surgery. In IVD degeneration, painful conditions are difficult to associate with specific anatomical and radiographic findings, in contrast to nucleus pulposus herniations or spinal stenosis. In 2007, a Medicare advisory committee concluded that the effectiveness of lumbar spinal fusion surgeries for treating chronic LBP was uncertain, owing to conflicting evidence and large variations in surgical technique.40 In a prospective Swedish cohort study, while fusion was found to be superior to nonsurgical treatment, only 63% of patients showed pain improvement after fusion surgery, and pain significantly increased from 1 to 2 years after surgery.41 Identifying which patients are likely to benefit from fusion surgery is difficult: a systematic review found that immobilization, provocative discography, and temporary external fixation were not useful in predicting which patients would benefit from fusion surgery.42 Given the difficulties in determining who will benefit from surgery, the American College of Physicians recently updated their LBP treatment guidelines, recommending noninvasive, nonpharmacologic treatments as the first line of therapy.43 However, nonsurgical treatments also have mixed results. TNF-α has been considered a promising target, as it is associated with painful IVD degeneration in rodents,44,45 and expression of the TNF-α receptor TNFR1 in the nucleus pulposus correlates with pain in human patients.46 Given the success of TNF-α inhibitors, such as infliximab, in treating pain in patients with rheumatoid arthritis, they have been considered as therapy for discogenic pain. Yet, a 2014 meta-analysis of TNF-α inhibitors as treatment for sciatica by Wang et al. found that they did not significantly improve LBP, leg pain, or rates of return to work at short-term, middle-term, or long-term follow-up.47 There is a significant clinical need to better understand how IVD degeneration may lead to chronic LBP and how to best classify this pain, so that we may better tailor our treatments to combat the progression from degenerative changes to chronic discogenic pain.
Types of pain
The International Association for the Study of Pain (IASP) defines pain as “unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”48 This broad definition can be further divided into different types of pain on the basis of mechanism, chronicity, spontaneity, and stimulus intensity required.
Neuropathic versus nociceptive pain
Pain can be classified on the basis of its underlying mechanism as either nociceptive or neuropathic. Nociceptive pain is the transmission of painful stimuli resulting from an injury to a non-neural tissue. Inflammation-related pain, which is observed in osteoarthritis or ankylosing spondylitis, is an example of nociceptive pain. Harmful stimuli, including specific neuropeptides, proinflammatory cytokines, and mechanical insult, stimulate peripheral nociceptive neurons, which then transmit the pain signal to the central nervous system.49 It is important to note that nociceptive pain is a protective mechanism to discourage the use of damaged tissues and allow for healing. Neuropathic pain, on the other hand, is pain resulting from injury to the nervous system itself. In this mode, a nerve is injured or impinged upon, resulting in pain. The damage can be focal, such as in the case of IVD-related radiculopathy, wherein the NP herniates and directly impinges the nerve root. Neuropathic pain can also be a diffuse systemic pathology, such as in painful diabetic neuropathy, wherein neurons are damaged throughout the body. However, it is important to note that these pain modalities are not exclusive, and LBP is likely to be a mixed pain with both nociceptive and neuropathic elements. Both nociceptive and neuropathic pain can arise from the same pathology, such as IVD degeneration, where innervating neurons are sensitized by intradiscal inflammation and degeneration-related stenosis directly impinges nerves. Distinguishing whether pain is nociceptive or neuropathic is important for determining treatment. Opioids are the standard of care for nociceptive pain50 but are not helpful for treating neuropathic pain.51 For patients with neuropathic pain syndromes, drugs that act on the neurotransmitter γ-aminobutyric acid (GABA) receptors, such as gabapentin and baclofen, are typically prescribed.52,53
Acute versus chronic pain
Pain is often classified on the basis of its duration as either acute, subacute, or chronic. Acute pain typically occurs in response to tissue trauma and is defined when pain onset and recovery occur within 1 month.54 Acute LBP is usually self-limiting, with 90% of patients recovering within 6 weeks.5 However, following acute injury, 2–7% of cases will progress to chronic pain.5 When this persistent pain lasts 4–12 weeks, it is classified as subacute, and pain that lasts longer than 12 weeks is classified as chronic pain. The American Pain Society defines chronic pain in two ways: (1) pain that extends beyond the period of healing (3–6 months), with levels of identified pathology that often are low and insufficient to explain the presence and/or extent of the pain, and (2) persistent pain that disrupts sleep and normal living, ceases to serve a protective function, and instead degrades health and functional capability.55 Chronic pain is not uncommon and is believed to affect 20–30% of the population.56,57
Evoked versus spontaneous pain
Pain may also be classified according to spontaneity as either spontaneous or evoked. Spontaneous pain, sometimes referred to as clinical pain, is seen in chronic pain disorders.58 It is not stimulus-dependent, and in animal studies spontaneous pain is described as voluntary behavior and can be measured by assessing the behaviors of animals in unrestrained condition 59. Unlike spontaneous pain, evoked pain is stimulus dependent and can be measured across different sensory modalities, including mechanical and thermal stimuli. As different sensory modalities utilize different neural pathways, correlation between pain sensitivity across modalities is variable and may be differentially modulated by interventions.58 Since evoked pain requires a provocative stimulus, it is sometimes referred to as experimental pain. Evoked pain measurements are often used to assess changes in pain thresholds in animal studies and to diagnose human pathology.59 For patients presenting with LBP, evoked pain tests, such as the straight leg raise, or provocative discography are commonly used to determine if the pain is discogenic in origin.60,61
Hyperalgesia versus allodynia
Pain can be classified clinically by the intensity of the stimulus required to produce a pain response as either hyperalgesia or allodynia. IASP has clear definitions for both hyperalgesia and allodynia. Allodynia is defined as pain in response to a stimulus that does not normally provoke pain in healthy subjects, whereas hyperalgesia is defined as increased pain from a stimulus that normally provokes pain in healthy subjects.48 While allodynia and hyperalgesia are clinical definitions, not mechanistic ones, they are believed to arise from changes in different types of peripheral nerve fibers. Hyperalgesia is thought to arise primarily from sensitization of Aδ- and C-fibers, which are peripheral nerve fibers responsible for normal pain sensation.62 Thus, the normal pain pathway is overactive and generates a greater response to already painful stimuli. In addition to sensitization of peripheral nerve endings, the heightened behavioral responses of hyperalgesia may also involve sensitization of the central nervous system.62 In allodynia, peripheral Aδ-fibers, which normally respond to nonpainful touch, are thought to undergo a phenotypic switch to become more similar to pain-sensing C-fibers.63 In addition, Aβ input to the superficial dorsal horn of the spinal cord (an important component of the pain pathway) increases in models of neuropathic pain, thus amplifying the pain signal input to this region, and further demonstrating the change in the role of Aβ-fibers in allodynia.64–66
Measurements of pain in rodent models
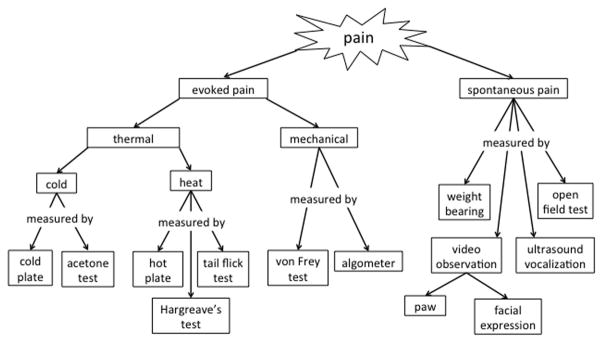
For human patients with LBP, the 10-point visual analog scale is commonly used to assess the severity of LBP. However, as rodents cannot communicate their pain status, we must employ alternative methods. Fortunately, there are multiple validated assays to determine whether an animal is experiencing pain (Fig. 1). This review focuses on the application of behavioral assays in rodent models, as these rodent behavioral pain assays have been well studied, are less technically challenging in rodents compared with other animal models, and have been shown to be sensitive to pain associated with IVD degeneration. While some behavior assays are tested directly on the spine to assess LBP, many of these behavioral assays are tested on the plantar surface of the hindpaw. The plantar surface of the rodent hindpaw is innervated primarily by the tibial nerve, which is composed of spinal nerve roots from L4 to S2.67 Thus, increased pain sensitivity that refers to the plantar surface of the hindpaw is considered a measure of LBP.
Figure 1.
Pain types and how they are measured in rodent models.
Evoked pain tests
Evoked pain tests are those in which an experimenter must expose an animal to a painful stimulus. Such stimuli may be processed across various sensory modalities: in this section, we will examine evoked pain tests used to measure mechanical pain and thermal pain (both cold and heat).
Mechanical allodynia is most commonly measured using the von Frey assay. In this assay, rodents are placed in wire mesh–floored cages, allowed to acclimate, and then tested with calibrated microfilaments. These filaments are calibrated such that they will buckle when the appropriate amount of mechanical force has been transmitted. Typically, von Frey filaments are applied in ascending force to the plantar surface of the hindpaw with sufficient strength to cause buckling of the filament, although they can be used at other locations, such as the tail, lower back, or face. The most common application of the von Frey assay is using the up–down method described by Chaplan et al. Hindpaws are probed a prescribed number of times, and a positive response is defined as brisk withdrawal of the probed foot. Once a positive response is seen, the previous filament is applied. If positive, the lower filament is determined to be the 50% paw-withdrawal threshold. If negative, the next ascending filament is applied, and if that next filament provokes a positive response, the original filament is considered to be the 50% withdrawal threshold. If the next ascending filament is negative, further ascending filaments are applied until a response is provoked.68,69
Mechanical hyperalgesia, sometimes referred to as pressure hyperalgesia, can be measured using an algometer.70,71 An algometer is an applied force gauge that can be applied to a localized region, such as the hind paw or posterior lumbar spine.72 The applied force is gradually increased until an audible vocalization is elicited to determine the pressure-pain threshold.55,56 The algometer is a useful assay in rodent models, as it can be similarly applied in human LBP patients to measure mechanical hyperalgesia in the musculature of the lower back.57
Thermal pain sensitivity can be measured as either a sensitivity to heat or to cold, as the two stimulus modalities activate different populations of neurons.73,74 Tests for sensitivity to heat-provoked pain have been performed for over half a century.75 One of the first tests for heat hyperalgesia was the hot-plate test. In this assay, developed in the 1940s, a mouse or rat is placed on a hot plate in order to evoke a behavioral response to heat-induced pain, and the latency to the first behavior is measured.76 In mice, these behaviors include hindpaw licking, brisk hindpaw withdrawal, and jumping; in rats, hindpaw licking or brisk hindpaw withdrawal may be seen in the presence of pain.75 The hot plate is typically set between 50 ºC and 55 ºC for both mice and rats.77 Importantly, this temperature range is well above the 42–43 ºC threshold for the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor, responsible for the sensation of noxious heat.78 As a result, naive animals will still experience pain at this temperature, but will have increased latency to respond, compared with animals in pain. However, the development of dynamic hot plates, in which the temperature can be steadily ramped up from a nonpainful temperature, enables the assessment of heat allodynia owing to its initially reduced stimulus intensity.75
While the hot-plate test can test heat hyperalgesia in the paws, the tail-flick test is used to test the same sensitivity in the tail. Developed around the same time as the hot-plate test, in this assay, a rat’s tail is exposed to heat either via immersing it in hot water or by a radiant infrared beam, and the latency until the animal flicks its tail is measured.79,80 This test is typically performed on rats, as it can be difficult to perform on mice.81 While the tail-flick test may be helpful in assessing heat hyperalgesia in the tail, immersing the tail in water may have other effects, and one should not equate water immersion and radiant-heat tail-flick tests, as the surface area exposed to the stimulus may vary significantly.80
More recently, a radiant heat test known as the Hargreaves’ test was developed. Named after the Hargreaves et al. paper in which it was first described, this test uses radiant heat to stimulate rodent hindpaws, and latency to response is measured.82 Because an infrared beam is used, the experimenter can independently evaluate the left and right paws, allowing internal controls for lateralized pain models, such a sciatic nerve injury.81 The Hargreaves’ test enables more specific targeting of the heat stimulus but takes longer to test and requires more elaborate experimental equipment than the hot-plate test.
Following the understanding that heat pain and cold pain are not processed by the same populations of neurons, tests for cold-provoked pain have become more popular. Two of the most common tests for cold hyperalgesia are the cold-plate test and the acetone test. In the cold-plate test, a mouse or rat is placed on a cold plate, and stereotyped pain behaviors are measured similarly to the hot-plate test 83,84. However, in the cold-plate test, both latency to first pain behavior and total number of pain behaviors during a prescribed time frame are often measured, as there is considerable behavioral variability in cold nociception.81 Cold plates are usually set between 5 ºC and −5ºC.85 This is well below the firing threshold for transient receptor potential cation channel subfamily M member 8 (TRPM8), the receptor responsible for responding to both menthol and noxious cold.86 The threshold for TRPM8 is 20 ºC, but noxious cold is not felt until below 15 ºC.86 This may explain why the cold-plate test is more technically challenging than the hot-plate test, as the sensation of cold pain appears to exist on more of a gradient than heat pain. While static cold plates measure cold hyperalgesia, dynamic cold plates in which the temperature ramps down from a non-painful temperature can be used to evaluate cold allodynia.75
Cold hyperalgesia can also be measured using the acetone test. In this test, a drop of liquid acetone is touched to the hindpaw of a mouse or rat, where it quickly spreads. The evaporation of the acetone causes a cold sensation, and the latency to hindpaw licking or withdrawal and number of these behaviors are measured.87,88 The acetone test has an advantage over the cold-plate test because it can be applied to a single hindpaw, allowing for the use of internal controls. However, acetone evaporation is considered to be not only a measure of thermal hyperalgesia but also a measure of sensitivity to chemical-induced pain.89 This lack of specificity, as well as the inability to measure the reaction to a specific temperature, makes this assay variable.
Spontaneous pain tests
Spontaneous pain tests do not require an experimenter to expose an animal to a nociceptive stimulus, and instead rely on observing voluntary behaviors. Spontaneous pain can be difficult to evaluate in rodents, because, as prey animals, they hide signs of injury or pain.90 However, a variety of measurements have been developed.
One such measurement is the analysis of weight bearing following injury. Unrestrained animals are placed on a sensor plate, and distribution of weight on each paw is assessed. This test can be performed statically or dynamically and is sometimes part of gait analysis.59 Importantly, analysis of weight bearing is only relevant in unilateral injury models, for weight bearing can be compared between injured and uninjured sides, and changes in weight bearing are unlikely in symmetrical injuries.
Another test for spontaneous pain is the open-field test. This test was first developed as a measure of anxiety-like behavior or “emotionality” and involves placing the rat or mouse in a plexiglass square and measuring exploratory behavior.91 Briefly, the rodent is allowed to move freely about the space, and the time spent in each region of the box is quantified. Typically, the square is virtually divided into 16 equally sized square regions so that there is a clear center region. The number of central squares visited, the time spent in the central squares, and overall locomotion can be quantified. Both the number of central squares visited and the time spent in the central squares are markers of exploratory behavior, which is reduced in rodents exhibiting anxiety-like behavior.92 While this test is traditionally used to assess anxiety-like behavior, it has also been adapted as a non-reflexive pain assay. As animals experiencing chronic pain may also exhibit anxiety-like behaviors, both the traditional measurements of the open field test and additional measures of rearing behaviors can be used to evaluate pain behavior without stimulation.92,93
Spontaneous pain in rodents can also be measured by evaluating ultrasound vocalizations in a sound-free environment. Rodents are capable of producing both audible and ultrasound vocalizations, so ultrasound vocalizations may be measured when audible vocalizations are not present.94 Adult rats emit different types of ultrasound vocalizations depending on their environment and affective state: 22-kHz vocalizations are produced in anticipation of aversive stimuli, and 50-kHz vocalizations are produced when the rat has a positive affective state.95 Thus, when measuring ultrasound vocalizations, it is important to note not only the presence or absence of such vocalizations but also their frequency. Using ultrasound vocalization measurements to assess pain is somewhat controversial. Technically, it can be difficult to maintain a sufficiently sound-free environment, making measurements unreliable. Additionally, it is unclear whether rodents will reliably produce ultrasound vocalizations when in pain. In a study of neuropathic pain in mice, ultrasound vocalizations were increased in mice with neuropathic pain and reduced when they were given analgesic drugs.96 However, in a separate study of acute pain in mice, 65% produced no vocalization, and in those that produced ultrasound vocalizations, audible vocalizations were also produced, rendering the ultrasound measurements redundant.97
Lastly, spontaneous pain can be measured via video observation. Such observation is divided into observations of facial expressions and observations of paw behaviors. For evaluation of facial expressions, both a rat and a mouse grimace scale exist. The rat grimace scale assess rats across four action units: orbit tightening, nose/cheek flattening, ear changes, and whisker changes. An automated software may be used to photograph rat facial expressions, and then expressions are manually scored using the rat grimace scale. This scale has been shown to reliably and accurately quantify spontaneous pain across a variety of pain models.98 Similar to the rat grimace scale, the mouse grimace scale assesses orbital tightening, nose bulge, cheek bulge, ear position, and whisker change.99
In addition to facial expressions, paw behaviors may also be observed. For this assay, rodents are placed on a room-temperature plate enclosed in a plastic box or under a plastic dome, and the number of hindpaw lifts not related to locomotion are recorded.87 However, unprovoked paw lifts may not reliably measure spontaneous pain, as they are only seen in some pain models. Spontaneous paw lifting is seen in the spared nerve injury model and a modified spinal nerve ligation model (in which the L5 spinal nerve is ligated and axotomized and the L4 nerve is loosely ligated), but does not occur in the traditional spinal nerve ligation model.100,101
Existing models of painful IVD degeneration
In vivo animal models of IVD degeneration exist in a wide variety of species, including rodents, rabbits, ovine, canine, and primates.102 Mechanical and structural methods have been used to induce IVD degeneration experimentally, and some species develop IVD degeneration with age and have been extensively reviewed previously.102–104 However, given the practical limitations of housing large animal species, such as sheep and cattle, and the ethical considerations of the use of others, such as canines and nonhuman primates, small animals have often been the model organism of choice for IVD degeneration research. The use of rodent models is further supported by the existence of validated practical assays for probing pain.20
The relationship of IVD degeneration to pain is frequently cited as a motivation for investigating IVD degeneration models, yet few studies have directly measured pain in a non-herniation lumbar IVD degeneration model (Table 1). An increase in spontaneous behaviors associated with pain was found up to 3 weeks after performing a facetectomy, puncturing the L4/5 IVD, and inducing NP leakage in a rat model, suggesting increased pain.105 However, the facetectomy alters the whole motion segment biomechanics so may not be a true mimic of isolated IVD degeneration. A significant increase in pain behavior—as measured by algometer, von Frey assay, and gait analysis—was found after L4/5 and L5/6 IVD injury and nucleotomy using a 0.5-mm diameter microdrill in a rat model.72 A transient increase in intradiscal TNF-α, IL-1β, IL-6, and substance P at the gene level was reported but returned to preoperative levels within 1 month. A significantly impaired gait was found after L5/6 IVD puncture using a 24-gauge needle in another rat model, but rats returned to normal gait 4 weeks after injury.106 Another group found an increase in mechanical and heat-induced pain behavior after facetectomy and posterior L4/5 puncture but not anterior puncture without facetectomy.107
Table 1.
Existing models of IVD degeneration
| Model type | Species/age | Sex | Intervention | Experiment duration | Biochemical and structural assays | Behavior assays | Reference |
|---|---|---|---|---|---|---|---|
| Structural | Rat/not specified | Female | L4/5 L facetectomy + L4/5 posterolateral puncture + induced nuclear leakage | 21 days | N/A | Spontaneous: Stereotypical behavior instances | Olmarker et al.90 |
| Rat/adult | Not specified | L4/5, 5/6 anterior puncture + nucleotomy | 7 weeks (behavioral) 9 weeks (molecular) |
X-ray Histology qPCR (DRG) |
Mechanical: von Frey (hindpaw) Algometer (lumbar) |
Kim et al.56 | |
| Rat/8 weeks | Male | L5/6 anterior 10× puncture | 4 weeks | IHC (DRG) | Spontaneous: Gait analysis | Miyagi et al.91 | |
| Rat/not specified | Male | L4/5 anterior puncture OR L4/5 L facetectomy + L4/5 posterior puncture |
6 weeks | MRI Histology IHC (NP) IHC (DRG) qPCR (NP) Western blot (NP) |
Mechanical: von Frey (hindpaw) Heat: Hot plate (hindpaw) |
Li et al.92 | |
| Structural + inflammatory | Rat/not specified | Male | L5/6 anterior puncture + 10μL CFA | 8 weeks | Histology IHC (spinal cord, IVD) qPCR (DRG) |
Spontaneous: Weight loading Mechanical: von Frey (hindpaw, lumbar) Cold: Tail flick Heat: Withdrawal (tail) |
Lee et al.93 |
| Rat/4 months | Male | L3/4, 4/5, 5/6 anterior puncture + 0.25ng TNF-α or NGF/VEGF injection | 6 weeks | X-ray Histology IHC (IVD, DRG) |
Mechanical: von Frey | Lai et al.94 | |
| Genetic | Mouse/3 months and 9 months | Male | SPARC−/− | N/A | N/A |
Mechanical: von Frey (hindpaw, lumbar) Cold: Acetone test (hindpaw, back) Withdrawal (tail) Heat: Withdrawal (hindpaw, tail) |
Millecamps et al.96 |
NOTE: DRG, dorsal root ganglia; IHC, immunohistochemistry
Other models have used an inflammatory bolus in addition to an IVD puncture injury. A puncture with an injection of an adjuvant adds an additional intradiscal inflammatory component. A significantly decreased pain threshold—as measured by vocal responses to microfilaments applied to the spinous process—was found up to 7 weeks after puncturing the L5/6 IVD ventrolaterally with a 26-gauge needle and injection of 10 μL complete Freund’s adjuvant (CFA) with an anterior approach in a rat.108 Our group has developed a model of painful IVD degeneration wherein an anterior IVD puncture with TNF-α injection induces a decreased mechanical hindpaw withdrawal threshold.44 We believe the use of TNF-α is more physiologically relevant than CFA, as TNF-α has been extensively implicated in IVD degeneration and may better lend itself to understanding the underlying pathophysiology of IVD degeneration–related pain in an animal model.
Any intentional anatomical alterations to induce spinal injury or instability should parallel the human condition. The IVD is one component of a three-point spinal joint; the other two are the posterior facet joints. Although the facetectomy is a convenient mode to access the IVD and allow for puncture, this fundamentally changes the biomechanics of the spinal joint. Even in a well-controlled study, a facetectomy mimics a situation unlike what is seen in the human patient. The human degenerated IVD is still part of a three-point joint, so models of the condition should aim to preserve the interrelated anatomy. The anterior IVD puncture, which is done via a ventral approach through the abdomen, both preserves the complex spinal joint anatomy and creates a degeneration-inducing injury and is therefore a more physiologically appropriate model of the human condition.
A lone genetic model of IVD degeneration has assayed associated pain behaviors. A genetically modified Sparc−/− (secreted protein, acidic, and rich in cysteine) mouse model also has been found to yield spontaneous IVD degeneration with age109 with evidence of IVD degeneration–related pain.110 A drawback of this model, however, is degeneration occurs in all IVDs, which is dissimilar to humans, and it is not clear that the effects of SPARC−/− are restricted to the IVDs.
IVD degeneration and the nervous system
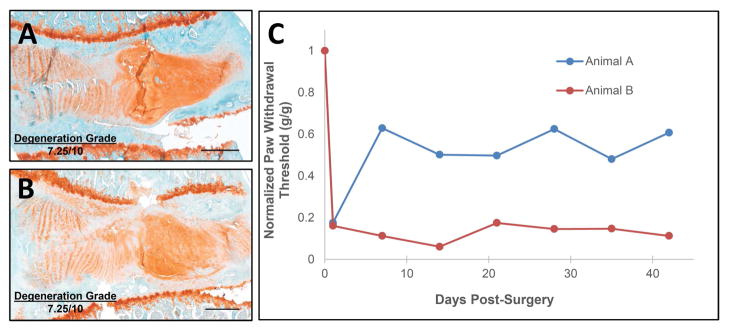
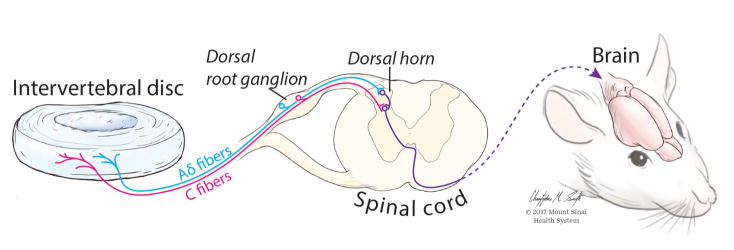
As illustrated by Boden, IVD degeneration is not sufficient for pain development.34 Similar findings are observed in preclinical models. For example, two animals with equally degenerated IVDs111,112 can have vastly different pain behaviors (Fig. 2). In this example, IVD degeneration was induced using anterior puncture and injection as described previously.20 Both animals had a reduced mechanical paw-withdrawal threshold immediately following injury, illustrating that IVD injury and degeneration can induce painful conditions. However, the paw-withdrawal threshold of one rat slightly recovered 2 weeks postsurgery, while a low threshold was maintained in the other rat throughout the 6-week experiment. Accordingly, these two rats serve as an example of why analyzing the IVD in isolation is insufficient for identifying the structural and molecular bases of IVD degeneration–related pain: these rats may have differences in their nervous systems that underlie the observed differences in their pain behavior. Discogenic pain signals are transmitted from the IVD and adjacent structures via peripheral afferent nerve fibers whose cell bodies lie in the dorsal root ganglia and synapse with projection neurons in the dorsal horn of the spinal cord to multiple brain regions that comprise the “pain matrix” (Fig. 3). The specific molecular basis of painful IVD degeneration in the context of the peripheral nervous system is an important area of investigation that has been reviewed elegantly elsewhere113–115 and is beyond the scope of this review. Understanding the neural pathway of painful IVD degeneration and regulation will inform tissue targets for both analysis and intervention.
Figure 2.
IVD degeneration–related pain has greater variance than IVD degeneration. IVD degeneration and pain do not have a linear relationship, as demonstrated by two animals with similarly degenerated IVDs having vastly different mechanical paw-withdrawal thresholds. Both animals exhibited reduced mechanical paw withdrawal thresholds during the acute postoperative period, but animal A recovered to a greater extent than Animal B. (A and B) Mid-sagittal sections of rat lumbar IVDs stained with safranin-O/light green exhibited equal degeneration grades as determined by a semiquantitative histological grading scale of a total grade (0–10, least to most degenerated) of AF integrity, AF/NP border definition, NP cellularity, NP matrix condensation, and CEP regularity made by two graders at two time points. (C) Mechanical paw-withdrawal thresholds of corresponding rats normalized to presurgery values exhibited variability. Pain behavior evaluated using the von Frey assay. Scale bars = 250 μm.
Figure 3.
Pain pathway from the intervertebral disc to the brain. Following IVD degeneration, pain may be evoked by a variety of possible mechanisms, such as nerve root irritation, neurovascular ingrowth, sensitized peripheral nerves, and/or abnormal concentrated stresses. Peripheral Aδ and C fibers transmit pain signals from the IVD and adjacent structures to the central nervous system. The cell bodies of these afferent peripheral nerves form the dorsal root ganglia. Within the spinal cord, Aδ and C fibers synapse with ascending neurons in the dorsal horn, which carry the pain signal to the brain. Thus, modulation of the pain pathway can occur at multiple hubs in the pain pathway: at the site of injury, in the dorsal root ganglion, in the dorsal horn of the spinal cord, or within the brain.
Peripheral nerve endings at the IVD
Neural innervation into the IVD has long been hypothesized to play an important role in discogenic pain. In the healthy IVD, nerves innervate the outer AF.116 In degenerated IVDs, nerve innervation is much more extensive, and these fibers are predominantly nociceptive Aδ and C-fibers.113,117,118 Importantly, nerve endings in the inner AF and NP were found more often in painful IVDs than non-painful IVDs,31 suggesting that the extent of neural ingrowth is important for pain development. Nerve growth into the IVD may lead to pain through neuropathic mechanical (i.e., direct nerve impingement) or nociceptive sensitization (inflammation-related) mechanisms, or more likely a combination of the both.
Dorsal root ganglia
Sensory signals transmitted from the periphery to the spinal cord are carried by a single neuron, and the cell bodies of these neurons form the dorsal root ganglia (DRG).114 As such, any changes in gene and protein expression in the nerves innervating the IVD that follow repeated stimulation and peripheral sensitization are expected to be found within the DRG, and the DRG has rightly been a popular target for investigating neural involvement with IVD degeneration. Previous rodent discogenic pain studies have shown that injury-induced degeneration and pain are associated with upregulation of intradiscal proinflammatory cytokines and pain-related neuropeptides in the DRG (Table 1). A series of experiments by Ohtori et al. using retrograde and anterograde tracers demonstrated that L5/6 IVD in a rat, which corresponds to the human L4/5 IVD, is innervated by T13–L2 DRGs.119,120 As such, changes in the nervous system are likely to be found in DRGs several levels cranial to the IVD of interest rather than the IVD-adjacent DRG.
Dorsal horn of the spinal cord
The peripheral primary afferent fibers synapse in the dorsal horn of the spinal cord with both small interneurons and projection neurons that travel to the brain.121 Of the 10 lamina of the spinal cord, lamina I and II are most important for pain, as this is where Aδ and C fibers primarily synapse,121 as well as where Aβ fibers may synapse in allodynia states.64–66 Just as repeated stimulation of peripheral nerves by painful stimuli may cause gene and protein expression changes in the DRG, similar changes farther down the pain pathway would be expected to be seen in the dorsal horn of the spinal cord, either in the projection neurons, interneurons, or both. Lee et al. found increased calcitonin gene–related peptide in the dorsal horn following IVD injury and degeneration in a rodent model (Table 1). Importantly, the dorsal horn is a key site of pain modulation.121–126 The small interneurons of the dorsal horn may inhibit or reduce pain signaling locally127 or in response to top-down modulation from higher brain structures.123 In top-down modulation, efferent signaling, predominantly from the rostral ventromedial medulla in the brain stem to the dorsal horn of the spinal cord, is able to modulate pain signals such that less pain is perceived, independent of intensity of signal from the periphery.121,122 This descending system is believed to play a role in the placebo effect.126
Brain
From the dorsal horn of the spinal cord, projection neurons target multiple brain regions, primarily in the brain stem and thalamus.121 More specifically, brain stem regions where dorsal horn neurons synapse include the caudal ventrolateral medulla,128,129 the nucleus of the solitary tract,130 the parabrachial area,129,131 and the periaqueductal gray matter (PAG).126,132 Importantly, the PAG is involved in the psychological modulation of pain, as it plays a role in the top-down modulation of pain in the dorsal horn and is implicated in depression.126,133,134 Thalamic nuclei implicated in pain include the ventral posterolateral and posteromedial nuclei,135,136 the posterior group,135 the ventral posterior parvicellular nuclei,135 and the posterior triangular nucleus.135,137 From these initial projections, pain is processed in the brain across several brain regions, which are sometimes referred to as the pain matrix. In addition to the thalamic and brain stem nuclei, the pain matrix includes components of both the limbic system and the cortex. Limbic structures involved in pain include the hippocampus124,138–140 and the amygdala.126,134 At the cortex, the pain matrix includes the prefrontal cortex,124,141 insular cortex,124,134,142 somatosensory cortex,124,134 and the anterior and posterior cingulate cortices.124,134,143 As pain processing occurs across a network of brain areas, changes in the brain from chronic pain may be widespread. Luchtmann et al. investigated the morphometric changes in the brain in patients suffering from chronic LBP and IVD herniation using MRI and found that patients with herniated IVD exhibited significant changes in both gray and white matter volumes throughout the brain, predominantly in regions of the pain matrix.133,134
Important considerations for future studies
A strong and growing body of research is investigating the etiology and potential therapeutic interventions for IVD degeneration in the setting of LBP. However, the complex mechanisms by which IVD injury and degeneration may lead to pain remain poorly understood. Given the importance in associating pain with IVD injury and degeneration, we propose several considerations for study design to ensure that animal models of discogenic pain are applicable to the human condition and to advance our understanding of the mechanism of such pain.
The obvious goal of animal models of IVD degeneration is to closely mimic the human condition of chronic discogenic pain. This raises the question of what is truly considered chronic pain in both human patients and animal models. The current literature presents two different definitions. The first is that chronic pain involves a change in cognitive and emotional cortical areas.125,134 Chronic pain cannot be completely explained by identifiable somatic pathology and involves structural brain changes.144 Thus, it is likely that the transition from acute to chronic pain reflects a change from a protective response due to tissue damage to a pathologic change within the nervous system. Using this definition, an experiment would need to establish that an induced IVD injury resulted in long-term brain changes. A more practical means of ensuring a chronic pain model is by considering the duration of an experiment. If the experiment is too short, the pathology may reflect an acute IVD injury rather than chronic degeneration, especially in an injury-induced model. While chronic pain in humans is defined as pain lasting greater than 3 months, in rodents, it is thought that 2–8 weeks is appropriate to establish chronicity, depending on the model.125,145 Specifically, in the spared nerve injury model, hyperalgesia and allodynia are first seen about 2 weeks after injury,146 but mood-related symptoms of chronic pain may not be evident until around 8 weeks.145 As such, experiments investigating pain in a rodent IVD injury model should last for at least 2 weeks, but ideally longer, in order to assess a more complete picture of the chronic pain state. Alternatively, chronic pain may be achieved when pain assays reach a sustained maximum value, with models adjusted accordingly.
Additionally, both male and female animals should be used in future studies. The National Institutes of Health (NIH) has strongly recommended using both sexes in preclinical research studies in order to identify and evaluate any sex-dependent differences.147 An additional benefit of this is the ability to evaluate the effects of sex in the absence of confounding gender effects that may be seen in human populations. Assessing sex as a biological variable in the study of discogenic pain is particularly important, as differences are seen between males and females in both spine pathology and mechanisms of pain. Spine impairment is more common in women (70.3 per 1000 population) than in men (57.3 per 1000 population).6 Women typically exhibit lower pain thresholds across multiple modalities.139 However, this may be a product of physiological factors (due to sex) or psychosocial factors (due to gender/culture) or an interaction of both.148 In addition, at the cellular level, males and females may use different immune cells and molecular pathways in the perception of pain.149,150 Investigating sex effects in IVD degeneration models may elucidate pathophysiological differences and inform sex-specific therapeutic strategies.
Considering the complexity of the pain pathway and the nervous system’s essential role in pain pathogenesis, animal models of discogenic pain must evaluate both pain behaviors and cellular and molecular changes within the nervous system. In order to determine whether an animal is in pain, we must employ behavioral assays. Further, these assays can be used to more precisely define the type of pain the animal is experiencing and to suggest which neural pathways may be involved. Once pain in an animal model of IVD degeneration has been more clearly defined, the knowledge gained from behavioral measures may be used to inform investigations of cellular and molecular changes within hubs in the pain pathway found in both the peripheral and central nervous system. Examining such changes in the nervous system at a more granular level may enable both better understanding of mechanisms of discogenic pain and treatment precision.
Summary
IVD degeneration is highly associated with LBP, but the complex relationships between the two are unclear, as IVD injury and degeneration does not always result in LBP. Thus, animal models are necessary to probe the underlying pathophysiology of discogenic pain. The majority of animal models on IVD degeneration provide precise anatomical, biomechanical, biochemical, and radiographic measures to quantify the extent of IVD degeneration. A notably small number of animal model studies on IVD degeneration have directly assayed pain, despite the existence of a variety of different pain behavioral assays exist to measure pain, each assessing different pain modalities. Furthermore, long-term behavioral change likely implies adaptations of the central nervous system, for this is the network from which behavior arises. In addition to incorporating diverse behavioral pain assays into studies of discogenic pain, careful consideration must be made to design models that reflect the chronicity and mechanisms of the human clinical condition. Additionally, models should utilize both male and female animals to account for possible sex differences in LBP. Beyond the IVD, future studies should consider the behavioral changes and the nervous system changes that lead to them for a more complete picture of the ways in which IVD injury and degeneration can lead to discogenic pain.
Acknowledgments
This work was funded by the National Institutes of Health with grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR064157) and from the Mount Sinai Center for Molecular Integrative Neuroresilience and the National Center for Complementary and Integrative Health (P50AT008661). All authors substantially contributed to conception, literature review, analysis, writing and revising of this manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Murray CJL, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Tulder M, Koes B, Bombardier C. Low back pain. Best Pract Res Clin Rheumatol. 2002;16:761–775. doi: 10.1053/berh.2002.0267. [DOI] [PubMed] [Google Scholar]
- 4.van Tulder M, Koes B. Low back pain (chronic) Clin Evid. 2006:1634–1653. [PubMed] [Google Scholar]
- 5.van Tulder M, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(Suppl 2):S169–91. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson GB. Epidemiological features of chronic low-back pain. The Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 7.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Dieleman JL, et al. US Spending on Personal Health Care and Public Health, 1996–2013. JAMA. 2016;316:2627–2646. doi: 10.1001/jama.2016.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178–188. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- 11.Luoma K, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 12.Luoma K, Vehmas T, Kerttula L, Grönblad M, Rinne E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. 2016;25:2873–2881. doi: 10.1007/s00586-016-4715-x. [DOI] [PubMed] [Google Scholar]
- 13.Livshits G, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samartzis D, et al. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93:662–670. doi: 10.2106/JBJS.I.01568. [DOI] [PubMed] [Google Scholar]
- 15.Saab CY. Chronic Pain and Brain Abnormalities. Academic Press; 2013. [Google Scholar]
- 16.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience needs behavior: correcting a reductionist bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 18.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai A, et al. Assessment of functional and behavioral changes sensitive to painful disc degeneration. J Orthop Res. 2015;33:755–764. doi: 10.1002/jor.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vo NV, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34:1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–16. doi: 10.22203/ecm.v030a08. discussion 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruber HE, et al. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Exp Mol Pathol. 2015;98:102–105. doi: 10.1016/j.yexmp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita M, et al. Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine. 2008;33:1836–1842. doi: 10.1097/BRS.0b013e31817bab2a. [DOI] [PubMed] [Google Scholar]
- 25.Rajan NE, et al. Toll-Like Receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine. 2013;38:1343–1351. doi: 10.1097/BRS.0b013e31826b71f4. [DOI] [PubMed] [Google Scholar]
- 26.Stefanakis M, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine. 2012;37:1883–1891. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 27.Purmessur D, Cornejo MC, Cho SK, Hecht AC, Iatridis JC. Notochordal cell-derived therapeutic strategies for discogenic back pain. Global Spine J. 2013;3:201–218. doi: 10.1055/s-0033-1350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohba T, et al. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27:229–235. doi: 10.1002/jor.20727. [DOI] [PubMed] [Google Scholar]
- 29.Haro H, Kato T, Komori H, Osada M, Shinomiya K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J Orthop Res. 2002;20:409–415. doi: 10.1016/S0736-0266(01)00150-4. [DOI] [PubMed] [Google Scholar]
- 30.Abe Y, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 31.Freemont AJ, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. The Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 32.BMUS: The Burden of Musculoskeletal Diseases in the United States | Prevalence, Societal and Economic Cost. at < http://www.boneandjointburden.org/>.
- 33.Jensen MC, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 34.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 35.Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661–666. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- 36.Kalichman L, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33:2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borenstein DG, et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am. 2001;83-A:1306–1311. doi: 10.2106/00004623-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Takatalo J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine. 2011;36:2180–2189. doi: 10.1097/BRS.0b013e3182077122. [DOI] [PubMed] [Google Scholar]
- 39.Samartzis D, Karppinen J, Luk K, Cheung K. Baseline MRI Characteristics in Asymptomatic Subjects as Predictors for Future First-Time LBP Episode. Global Spine J. 2012;2 s-0032-1319911-s-0032-1319911. [Google Scholar]
- 40.Schafer J, O’Connor D, Feinglass S, Salive M. Medicare Evidence Development and Coverage Advisory Committee Meeting on lumbar fusion surgery for treatment of chronic back pain from degenerative disc disease. Spine. 2007;32:2403–2404. doi: 10.1097/BRS.0b013e3181573841. [DOI] [PubMed] [Google Scholar]
- 41.Fritzell P, Hägg O, Wessberg P, Nordwall A Swedish Lumbar Spine Study Group. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521–32. doi: 10.1097/00007632-200112010-00002. discussion 2532. [DOI] [PubMed] [Google Scholar]
- 42.Willems P. Decision making in surgical treatment of chronic low back pain: the performance of prognostic tests to select patients for lumbar spinal fusion. Acta Orthop Suppl. 2013;84:1–35. doi: 10.3109/17453674.2012.753565. [DOI] [PubMed] [Google Scholar]
- 43.Qaseem A, Wilt TJ, McLean RM, Forciea MA Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the american college of physicians. Ann Intern Med. 2017 doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 44.Lai A, et al. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016;16:420–431. doi: 10.1016/j.spinee.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine. 2003;28:1635–41. doi: 10.1097/01.BRS.0000083162.35476.FF. discussion 1642. [DOI] [PubMed] [Google Scholar]
- 46.Andrade P, et al. Tumor necrosis factor-α levels correlate with postoperative pain severity in lumbar disc hernia patients: opposite clinical effects between tumor necrosis factor receptor 1 and 2. Pain. 2011;152:2645–2652. doi: 10.1016/j.pain.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Wang YF, et al. Clinical significance of tumor necrosis factor-α inhibitors in the treatment of sciatica: a systematic review and meta-analysis. PLoS ONE. 2014;9:e103147. doi: 10.1371/journal.pone.0103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.IASP Taxonomy - IASP. at < http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576>.
- 49.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120:3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholson B. Responsible prescribing of opioids for the management of chronic pain. Drugs. 2003;63:17–32. doi: 10.2165/00003495-200363010-00002. [DOI] [PubMed] [Google Scholar]
- 51.Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 52.Zuniga RE, Schlicht CR, Abram SE. Intrathecal Baclofen Is Analgesic in Patients with Chronic Pain. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2000 doi: 10.1097/00000542-200003000-00037. at < http://anesthesiology.pubs.asahq.org/article.aspx?articleid=1945736>. [DOI] [PubMed]
- 53.Serpell MG Neuropathic pain study group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557–566. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 54.Pengel LHM, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enduring Materials | APS. at < http://americanpainsociety.org/education/enduring-materials>.
- 56.Reid KJ, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27:449–462. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- 57.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Gracely RH, Grant MAB, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17:593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 59.Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur J Neurosci. 2014;39:1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- 60.Edgar MA, Park WM. Induced pain patterns on passive straight-leg raising in lower lumbar disc protrusion. A prospective clinical, myelographic and operative study in fifty patients. J Bone Joint Surg Br. 1974;56-B:658–667. doi: 10.1302/0301-620X.56B4.658. [DOI] [PubMed] [Google Scholar]
- 61.Carragee EJ, Alamin TF, Miller J, Grafe M. Provocative discography in volunteer subjects with mild persistent low back pain. Spine J. 2002;2:25–34. doi: 10.1016/s1529-9430(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 62.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 63.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 64.Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Abeta afferents. J Neurosci. 2000;20:1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okamoto M, et al. Functional reorganization of sensory pathways in the rat spinal dorsal horn following peripheral nerve injury. J Physiol (Lond) 2001;532:241–250. doi: 10.1111/j.1469-7793.2001.0241g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol (Lond) 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kambiz S, et al. Innervation mapping of the hind paw of the rat using Evans Blue extravasation, Optical Surface Mapping and CASAM. J Neurosci Methods. 2014;229:15–27. doi: 10.1016/j.jneumeth.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 69.Kartha S, Zeeman ME, Baig HA, Guarino BB, Winkelstein BA. Upregulation of BDNF and NGF in cervical intervertebral discs exposed to painful whole-body vibration. Spine. 2014;39:1542–1548. doi: 10.1097/BRS.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Culp WJ, Ochoa J, Cline M, Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Brain. 1989;112:1317–1331. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- 71.Kinser AM, Sands WA, Stone MH. Reliability and validity of a pressure algometer. J Strength Cond Res. 2009;23:312–314. doi: 10.1519/jsc.0b013e31818f051c. [DOI] [PubMed] [Google Scholar]
- 72.Kim JS, et al. The rat intervertebral disk degeneration pain model: relationships between biological and structural alterations and pain. Arthritis Res Ther. 2011;13:R165. doi: 10.1186/ar3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu WW, Mazor O, Wilson RI. Thermosensory processing in the Drosophila brain. Nature. 2015;519:353–357. doi: 10.1038/nature14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frank DD, Jouandet GC, Kearney PJ, Macpherson LJ, Gallio M. Temperature representation in the Drosophila brain. Nature. 2015;519:358–361. doi: 10.1038/nature14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009;10:767–773. doi: 10.1016/j.jpain.2009.01.325. [DOI] [PubMed] [Google Scholar]
- 76.WOOLFE G, MACDONALD AD. THE EVALUATION OF THE ANALGESIC ACTION OF PETHIDINE HYDROCHLORIDE (DEMEROL) | Journal of Pharmacology and Experimental Therapeutics. Journal of Pharmacology and Experimental Therapeutics. 1944;80:300–307. [Google Scholar]
- 77.O’Callaghan JP, Holtzman SG. Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. J Pharmacol Exp Ther. 1975;192:497–505. [PubMed] [Google Scholar]
- 78.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 79.AMOUR FE, D’SMITH DL. A METHOD FOR DETERMINING LOSS OF PAIN SENSATION | Journal of Pharmacology and Experimental Therapeutics. Journal of Pharmacology and Experimental Therapeutics. 1941;72:74–79. [Google Scholar]
- 80.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 81.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 82.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 83.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 84.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 85.Tsagareli MG, et al. Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci Lett. 2010;473:233–236. doi: 10.1016/j.neulet.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKemy DD. In: TRP ion channel function in sensory transduction and cellular signaling cascades. Liedtke WB, Heller S, editors. CRC Press/Taylor & Francis; 2007. [PubMed] [Google Scholar]
- 87.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 88.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 89.van der Wal S, et al. Behavior of neuropathic pain in mice following chronic constriction injury comparing silk and catgut ligatures. Springerplus. 2015;4:225. doi: 10.1186/s40064-015-1009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arras M. Improvement of pain therapy in laboratory mice. ALTEX. 2007;24(Spec No):6–8. [PubMed] [Google Scholar]
- 91.Denenberg VH. Open-field behavior in the rat: what does it mean? Ann N Y Acad Sci. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- 92.Parent AJ, et al. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res. 2012;229:160–167. doi: 10.1016/j.bbr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho H, et al. Voluntary movements as a possible non-reflexive pain assay. Mol Pain. 2013;9:25. doi: 10.1186/1744-8069-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts LH. The rodent ultrasound production mechanism. Ultrasonics. 1975;13:83–88. doi: 10.1016/0041-624x(75)90052-9. [DOI] [PubMed] [Google Scholar]
- 95.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 96.Kurejova M, et al. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain. 2010;6:18. doi: 10.1186/1744-8069-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams WO, Riskin DK, Mott AKM. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci. 2008;47:8–10. [PMC free article] [PubMed] [Google Scholar]
- 98.Sotocinal SG, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langford DJ, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 100.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee KS, Huang YH, Yen CT. Periaqueductal gray stimulation suppresses spontaneous pain behavior in rats. Neurosci Lett. 2012;514:42–45. doi: 10.1016/j.neulet.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 102.Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J. 2005;5:267S–279S. doi: 10.1016/j.spinee.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 103.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 104.Alini M, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olmarker K. Puncture of a lumbar intervertebral disc induces changes in spontaneous pain behavior: an experimental study in rats. Spine. 2008;33:850–855. doi: 10.1097/BRS.0b013e31816b46ca. [DOI] [PubMed] [Google Scholar]
- 106.Miyagi M, et al. Assessment of pain behavior in a rat model of intervertebral disc injury using the CatWalk gait analysis system. Spine. 2013;38:1459–1465. doi: 10.1097/BRS.0b013e318299536a. [DOI] [PubMed] [Google Scholar]
- 107.Li Z, et al. Both expression of cytokines and posterior annulus fibrosus rupture are essential for pain behavior changes induced by degenerative intervertebral disc: An experimental study in rats. J Orthop Res. 2014;32:262–272. doi: 10.1002/jor.22494. [DOI] [PubMed] [Google Scholar]
- 108.Lee M, et al. Complete Freund’s adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth Analg. 2009;109:1287–1296. doi: 10.1213/ane.0b013e3181b31f39. [DOI] [PubMed] [Google Scholar]
- 109.Gruber HE, et al. Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J Histochem Cytochem. 2005;53:1131–1138. doi: 10.1369/jhc.5A6687.2005. [DOI] [PubMed] [Google Scholar]
- 110.Millecamps M, Tajerian M, Sage EH, Stone LS. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine. 2011;36:95–102. doi: 10.1097/BRS.0b013e3181cd9d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masuda K, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 112.Rutges JPHJ, et al. A validated new histological classification for intervertebral disc degeneration. Osteoarthr Cartil. 2013;21:2039–2047. doi: 10.1016/j.joca.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 113.Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135–1139. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- 114.García-Cosamalón J, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15:1347–1355. doi: 10.1016/j.spinee.2013.07.490. [DOI] [PubMed] [Google Scholar]
- 116.Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication) Acta Anat (Basel) 1959;38:96–113. [PubMed] [Google Scholar]
- 117.Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine. 1997;22:2342–9. doi: 10.1097/00007632-199710150-00005. discussion 2349. [DOI] [PubMed] [Google Scholar]
- 118.Cavanaugh JM, Kallakuri S, Ozaktay AC. Innervation of the rabbit lumbar intervertebral disc and posterior longitudinal ligament. Spine. 1995;20:2080–2085. doi: 10.1097/00007632-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Ohtori S, et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295–2299. doi: 10.1097/00007632-199911150-00002. [DOI] [PubMed] [Google Scholar]
- 120.Ohtori S, et al. Neurones in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. A study using diI, an anterograde neurotracer. J Bone Joint Surg Br. 2001;83:1191–1194. doi: 10.1302/0301-620x.83b8.11012. [DOI] [PubMed] [Google Scholar]
- 121.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antal M, Petkó M, Polgár E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 123.Kato G, et al. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats. J Neurosci. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 125.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Polgár E, et al. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 128.Lima D, Albino-Teixeira A, Tavares I. The caudal medullary ventrolateral reticular formation in nociceptive-cardiovascular integration. An experimental study in the rat. Exp Physiol. 2002;87:267–274. doi: 10.1113/eph8702354. [DOI] [PubMed] [Google Scholar]
- 129.Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 130.Boscan P, Pickering AE, Paton JFR. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002;87:259–266. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- 131.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 132.Ikeda H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 133.Descalzi G, et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal. 2017;10 doi: 10.1126/scisignal.aaj1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- 136.Kupers RC, Gybels JM. Electrical stimulation of the ventroposterolateral thalamic nucleus (VPL) reduces mechanical allodynia in a rat model of neuropathic pain. Neurosci Lett. 1993;150:95–98. doi: 10.1016/0304-3940(93)90116-3. [DOI] [PubMed] [Google Scholar]
- 137.Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Covey WC, Ignatowski TA, Knight PR, Spengler RN. Brain-derived TNFalpha: involvement in neuroplastic changes implicated in the conscious perception of persistent pain. Brain Res. 2000;859:113–122. doi: 10.1016/s0006-8993(00)01965-x. [DOI] [PubMed] [Google Scholar]
- 139.Ignatowski TA, Sud R, Reynolds JL, Knight PR, Spengler RN. The dissipation of neuropathic pain paradoxically involves the presence of tumor necrosis factor-alpha (TNF) Neuropharmacology. 2005;48:448–460. doi: 10.1016/j.neuropharm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Ignatowski TA, et al. Brain-derived TNFalpha mediates neuropathic pain. Brain Res. 1999;841:70–77. doi: 10.1016/s0006-8993(99)01782-5. [DOI] [PubMed] [Google Scholar]
- 141.Apkarian AV, et al. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett. 2006;407:176–181. doi: 10.1016/j.neulet.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blom SM, Pfister JP, Santello M, Senn W, Nevian T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci. 2014;34:5754–5764. doi: 10.1523/JNEUROSCI.3667-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 145.Terzi D, et al. RGS9-2 modulates sensory and mood related symptoms of neuropathic pain. Neurobiol Learn Mem. 2014;115:43–48. doi: 10.1016/j.nlm.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 147.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melchior M, Poisbeau P, Gaumond I, Marchand S. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience. 2016;338:63–80. doi: 10.1016/j.neuroscience.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 149.Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mogil JS, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]