Abstract
Mobile social media often feature the ability to “Like” content posted by others. The present study examined the effect of Likes on youths’ neural and behavioral responses to photographs. High school and college students (N = 61, ages 13–21) viewed theirs and others’ Instagram photographs while undergoing fMRI. Participants more often Liked photos that appeared to have received many (vs. few) Likes. Popular photos elicited greater activity in multiple brain regions, including the nucleus accumbens, a hub of the brain’s reward circuitry. Nucleus accumbens responsivity increased with age for high school but not college students. When viewing images depicting risk-taking (vs. non-risky photographs), high school students, but not college students, showed decreased activation of neural regions implicated in cognitive control.
Since the advent of early social networking sites, adolescents and young adults have been among the first and most enthusiastic users of social media. More recently, youth have flocked to social media designed for mobile devices, such as Instagram and Snapchat (Lenhart, 2015). Despite early concerns that adolescents might use the Internet to meet strangers, they primarily use social media to interact with existing friends (Reich, Subrahmanyam, & Espinoza, 2012). Furthermore, many offline social and emotional processes typical of adolescence are also enacted on social media, including peer influence (e.g., Cohen & Prinstein, 2006). Recently, we (reference omitted for blind review) investigated how the quantifiable nature of certain digital interactions might play into adolescent peer influence at the behavioral and neural level. In the present study, we expand upon these findings by examining possible developmental differences between adolescents and young adults.
While peers can influence one another to positive or negative behaviors, much of the extant literature has focused on peer influence in the context of risky behaviors, a pressing public health concern. The teen years are a time of heightened risk-taking relative to childhood (e.g., Steinberg, 2008). However, certain risky behaviors such as binge drinking actually peak in the college years (Esser, 2014), a period that has been characterized as a continuation of adolescence but also as a developmental stage of its own: emerging adulthood (Arnett, 2000). This increased risk-taking is likely the result of greater independence, as well as a shift for many young adults to living with peers and away from parents (e.g. Willoughby et al., 2013). Nonetheless, the neural systems hypothesized to underlie decision-making in adolescence do mature considerably during the late teens and early 20s. Executive functions improve throughout this period, likely as a result of pruning and myelination in the frontal and parietal lobes (Luciana, 2013; Paus, 2005).
The younger adolescent brain, which has not yet experienced this maturation, is also characterized by heightened sensitivity of areas involved in affect and reward processing. In particular, the extant literature has demonstrated that the nucleus accumbens (NAcc) shows greater responsivity to monetary reward during the second decade of life, peaking in mid-to-late adolescence (e.g. Braams et. al., 2015; Galvan et al., 2006). The increased sensitivity of the NAcc and other subcortical structures in adolescence is thought to be triggered by puberty, but the exact mechanisms are unknown (van Duijvenvoorde et al., in press). The NAcc is considered a hub of the brain’s reward circuitry: it is involved in the subjective experience of reward and pleasure (Berridge & Kringleback, 2013), including social rewards (e.g., Fareri and Delago, 2014; Izuma, Saito, & Sadato, 2008), and in motivating goal-directed behavior (Ikemoto & Pankepp, 1999). The NAcc is also involved in the implicit learning of culturally specific cues (Schaefer & Rotte, 2007), and has been implicated in behaviors common in social media environments (Meshi, Tamir, & Heekeren, 2015), such as sharing information (Tamir & Mitchell, 2012) and receiving positive feedback (e.g., Davey et al., 2010). Further, the level of NAcc response to positive social feedback has been linked to intensity of social media use (Meshi et al., 2013).
Indeed, social media easily afford the learning of social norms, as they involve simple, fast, quantifiable measures of peer endorsement (e.g., “Likes”). “Likes’ provide an opportunity for social proof (Cialdini, 2009), or the use of social comparison with peers to determine appropriate social behavior, but they are unique in that interactions that were previously qualitative are now primarily or exclusively quantitative. Previously, we dubbed Likes and similar features “Quantifiable Social Endorsement” (reference omitted for blind review) and demonstrated that the level of Quantifiable Social Endorsement on Instagram photos–that is, the popularity of photos posted online—affected both behavioral and neural responses to those images. Adolescents were more likely to “Like” photographs they believed to be popular, and neural responses differed as a function of popularity. When adolescents received many Likes (vs. few) on their own photographs, they showed significantly greater activation of the NAcc, lending confidence to the hypothesis that Likes motivate online behavior and continued use of social media. Is this motivation particularly high during adolescence? Given the trajectory of NAcc sensitivity through late adolescence, and given that resistance to peer influence is found to be higher in the college years than in the earlier teen years (e.g., Steinberg & Monahan, 2007), we tested if neural responses to social media increased throughout adolescence before tapering off, or perhaps decreasing in a cohort of young adults. We also investigated how responses to images of risk-taking behavior (e.g., alcohol and drug use) posted online might be different in older, more independent college students. Finally, we were eager to test if our original findings replicated in a new sample. As with our original study, we investigated the role of Likes at both the behavioral and neural level. This approach reflected our desire to understand 1) measurable behavioral outcomes of peer influence on social media, and 2) neural processes potentially underlying these effects. We thus had several overarching goals for the present study:
Replicate prior behavioral and neural findings in an older population that is nonetheless still experiencing social and brain development (i.e., college students). We hypothesized that, similar to high school students, college students would be more likely to “Like” popular than unpopular Instagram photos. We also hypothesized that college students’ neural responses would differ as a function of photo popularity, and in particular that receiving many (vs. few) Likes on one’s own photographs would elicit significant activation in the NAcc.
Examine between-group differences and age-related effects in the high school and college cohorts in neural regions implicated in reward and executive functions. First, we hypothesized that NAcc activation in response to social reward (i.e., receiving many “Likes” on one’s own photographs) would increase with age in our high school sample, just as NAcc response to monetary reward increases in adolescence (Braams et al., 2015), but that NAcc response would not continue to increase in a college cohort. Second, we previously reported that high school students showed significantly less activation of neural regions considered hubs of the Central Executive Network when viewing Risky images compared with neutral images (reference omitted for blind review), including parts of the dorsomedial prefrontal cortex, lateral prefrontal cortex, and posterior parietal cortex. Given the maturation of executive function in early adulthood, we did not expect that this decreased activation to risky photographs would occur in our college sample; in other words, we expected high school students to show significantly less activation in regions implicated in executive function than college students.
Explore individual differences in neural activity as a function of health-related risky behavior. Previous research has linked neural responses during a variety of fMRI paradigms to adolescents’ tendency to engage in real-world risky behaviors like drinking and smoking. We tested whether neural responses would similarly vary in response to photographs depicting risky behavior. Previous research on adolescent risk-taking has implicated a variety of regions including the NAcc (Galvan et al., 2007), ventromedial prefrontal cortex (vmPFC; van Leijenhorst et al., 2010) and posterior cingulate cortex (PCC; Saxbe et al., 2015). Given the distributed nature of previous findings, we used a bottom-up approach for this analysis.
Method
Participants
An adolescent sample of thirty-four high school students (Mage = 16.8, SDage = 1.4, 18 female) was recruited from the Los Angeles community through flyers and message board postings. A young adult sample of 27 university students (Mage = 19.9, SDage = 1.1, 17 female) was recruited through flyers posted on campus. Of these participants, two high-school and one college participant were excluded from fMRI data analysis due to scanner malfunction or excessive movement. Participants had not been diagnosed with any developmental, psychiatric, or neurological disorder. College participants were all enrolled in the same four-year university, where over 90% of students live on campus in their first year. Thus, our college sample was not only older on average than our high school sample, but had entered a qualitatively different developmental stage. Given the unique experiences of emerging adulthood (Arnett, 2000), as well as evidence suggesting that some neural changes may be attributable specifically to higher education (e.g., Bennett and Baird, 2006; Noble et al., 2014), we performed all fMRI analyses, including examining age-related trends, separately in our high school and college samples. All participants gave written consent (or, for individuals under 18, written assent and parental consent), and were fully debriefed and compensated monetarily following study procedures. All procedures were approved by the Institutional Review Board of the University of California, Los Angeles.
Procedure
Data were collected between July of 2013 and December of 2014. Before the MRI scan, participants were asked to submit photographs from their own accounts on the popular photo-sharing app Instagram. They were told that these photographs would be used to create an “internal social network,” and that each participant would see a feed of these images in the scanner, appearing as they would on Instagram. High school participants were told that other participants were fellow high school students from the same city. College participants were told that other participants were also students at their university. In order to establish the size of the “audience,” participants were instructed that approximately fifty other individuals had already participated in the study. In reality, participants did not see one another’s photographs in the scanner. Rather, they saw their own photographs, as well as a standardized set of photographs selected by the study team from publicly available images on Instagram. They were told that they could see how many “Likes” each photo had received from other participants. In reality, the number of Likes was manipulated by the study team, as described below.
In the scanner, participants viewed each photograph for 3s, with the number of Likes ostensibly provided by peers displayed underneath (Figure 1). Participants saw three categories of images. “Risky” photo depicted alcohol and partying behaviors, smoking paraphernalia, rude gestures, or other adolescents (male and female) wearing provocative or “skimpy” clothing. “Neutral” photo depicted typical images found on adolescent social media profiles (e.g., pictures of people, food, and possessions; Hu, Manikonda, & Kambhampati, 2014). Neutral and Risky images did not differ in the overall proportion featuring people versus objects only (X2= 0.002, p = .999). Each participant also saw a selection of images he or she had submitted from his/her own Instagram account. These images were selected to minimize risky content; therefore, they were comparable to the neutral photographs ostensibly submitted by peers. Across participants, all neutral and risky images were assigned both a “popular” value and an “unpopular” value. Two versions of the imaging paradigm were created: in Version One, half of the photographs in each category (Risky, Neutral) were displayed with a “popular” value of 23–45 Likes and half were displayed with an “unpopular” value of 0–22 Likes. In Version Two, the values were reversed. Similarly, half of each participant’s own photographs were assigned many Likes (23–45), and the other half assigned few Likes (0–22). Participants were asked to view the photographs and decide whether to Like each image. Participants could select either “Like” or “Next” by pressing buttons on a handheld button box.
Figure 1. Example of a photograph presented during the Instagram experiment.
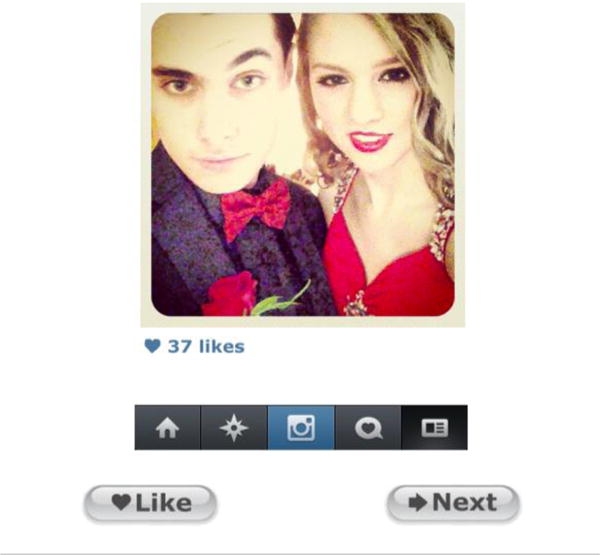
Participants viewed a series of photographs while in the MRI scanner, depicted in a simplified version of the Instagram user interface (as of 2014). Under each photograph was a blue heart, as well as the number of “Likes” ostensibly provided by peers. The Instagram menu bar appeared below the Likes. Beneath the Instagram display, participants saw two buttons, prompting them to choose “Like” to Like an image or “Next” to move on without Liking the image.
Following the MRI scan, participants completed the Revised Cognitive Appraisal of Risky Events (CARE-R; Katz, Fromme, & D’amico, 2000). This questionnaire consists of two sections. “Risks and Benefits” assesses participants’ appraisal of the risks and benefits associated with risky drinking, drug use, and sexual behavior. “Past Experiences” assesses the frequency with which participants engaged in these behaviors in the past six months.
Data Analysis
Behavioral data were analyzed in Stata (v14.1) using a mixed-effects logistic regression (Stata’s ‘melogit’ function). Button-press choice for each trial (i.e. selecting “Like” or “Next” on each image) was modeled as the binary outcome variable and participant was modeled as a random effect, to determine if participants’ likelihood to “Like” images was predicted by three categorical variables –the popularity of the image (Popular, Unpopular), the image content (Neutral, Risky, Participant’s Own), the sample (High School, College) –and all possible interactions.
To test our a priori hypothesis that viewing popular photographs would elicit greater activation in the bilateral NAcc than unpopular photographs, and to compare NAcc findings between samples and across conditions, we used a region of interest (ROI) approach (see Supplementary Methods for more details about the analytic pipeline, including the ROI analysis). We also tested whether NAcc response to receiving social approval (Popular > Unpopular for participant’s own photographs) was correlated with age in our two samples, and compared these correlation coefficients using a Fischer’s r-to-z transformation. In addition to our ROI analyses, we used a bottom-up approach to investigate effects in other brain regions exhibiting significant task-related activity. Specifically, we modeled contrasts examining the effect of popularity (Popular > Unpopular and the reverse) for the three types of photograph, and contrasts comparing all Neutral photographs to all Risky photographs (see Supplementary Methods).
To examine the relation between task-related neural activity and CARE-R scores, we performed a second bottom-up fMRI analysis with composite scores on the CARE-R modeled at the group level. This analysis involved 57 participants, because one high school participant did not complete the CARE-R. Because age and scores on the CARE-R were correlated (r = .32, p = .02), we included age as a control variable. More details about participant demographics, the fMRI paradigm, data acquisition, and data analysis are available in the Supplementary Methods.
Results
Goal 1a: Replication of Behavioral Findings
We previously reported that high school participants were more likely to select “Like” for popular images and “Next” for unpopular images than expected by chance, as determined by a binomial test. This finding was significant for all three categories of photographs (reference omitted for blind review). For the present inquiry, we utilized a statistical model that additionally allowed us to 1) model within-subject variability for each participant, and 2) report the likelihood of a participant Liking an image given its popularity and type. The full model was significant (χ2 = 1437.20, p < .0001). High school and college students did not differ in their overall tendency to Like images (z = 1.34, p = .18), and the interaction between popularity and cohort was not significant (z = 1.04, p = 0.30), which suggests that our cohorts did not significantly differ in their tendency to Like popular vs. unpopular images; we therefore report behavioral results for the two cohorts combined. Participants were significantly more likely to Like popular images than unpopular images (z = 7.28, p < .001). While the effect of popularity was significantly larger for participants’ own images than for either neutral images (z = 5.03, p < .001) or risky images (z = 3.86, p < .001), the effect of popularity was significant for all three types of photograph (all ps < .01). In other words, for each photograph type, participants more frequently “Liked” popular than unpopular photographs. Table 1 presents the probability of participants Liking a photograph, given its popularity and type; Table S1 presents all main and interactive effects, including effects not related to our hypotheses (e.g. main effect of photo type).
Table 1.
Likelihood to Like Photographs Based on Popularity and Type
| Image Type | Popular Likelihood (SE) |
Unpopular Likelihood (SE) |
|---|---|---|
| Participant’s Own | .88 (.01) | .74 (.02) |
| Neutral | .49 (.02) | .43 (.02) |
| Risky | .26 (.02) | .21 (.02) |
Goal 1b: Replication of fMRI Results for Popularity Effect
We previously reported that high school students showed significantly greater activation in the left and right NAcc when viewing their own photographs that had received many Likes compared to few (citation omitted for blind review). Using the same ROI, we replicated this finding in our college sample, in the left NAcc (t(25) = 2.95; p = .007) and right NAcc (t (25)= 3.43 p = .002). Furthermore, high school and college students did not significantly differ in activation in left or right NAcc for this comparison (Left, t(56) = .39, p = .70; Right, t(56) = .251, p = .80). Similarly, the cohorts did not differ in NAcc response when viewing popular (compared to unpopular) risky (Left, t(56) = 1.91, p = .06; Right, t(56) = .96, p = .34), or neutral (Left, t(56) = .97, p = .34; Right, t(56) = .14, p = .89) images. In the college cohort, viewing popular risky images was associated with significantly greater activation than unpopular risky images in the left NAcc (t(25) = 2.78, p = .01) but this same comparison in the right NAcc did not reach significance (t (25)= 1.50, p = .146). Viewing popular neutral images (compared to unpopular neutral images) was not associated with significant activation in the NAcc in either hemisphere (LH: t(25) = 0.54, p = .596; RH: t(25) = 1.27, p = .216).
Supplementary Figure 1 depicts results of the bottom-up analysis comparing popular to unpopular photos for the three photo categories (Neutral, Risky, Participant’s Own) in our college sample. When viewing photographs with many likes (popular) compared with few likes (unpopular), college students demonstrated significantly greater activation in several brain regions. The regions differed by photo type, but included areas implicated in social cognition (e.g., precuneus, medial prefrontal cortex), reward (NAcc, caudate, orbitofrontal cortex), and visual attention (occipital cortex). College students showed no areas of significant activation when viewing photographs of any type for the opposite contrast (unpopular > popular). When directly comparing our college and high school samples in our bottom-up analysis, we only found a single contrast in which the two cohorts differed significantly in brain responses to the effects of popularity. Specifically, when viewing Neutral images with many likes (popular) > few likes (unpopular), high school students showed significantly greater activation than college students in one region of visual cortex (MNI coordinates of max voxel, x =6, y=−72, z=16; Max Z = 3.40, 526 voxels).
Goal 2a: Age Differences in NAcc Responsivity to Social Media
Figure 2 presents the results of the correlational analysis relating age to NAcc response when viewing one’s own photographs with many Likes compared to few Likes. As hypothesized, bilateral NAcc responsivity to the many likes (popular) > few likes (unpopular) contrast increased with age in our high school sample (Left NAcc: r = .47, p = .006; Right NAcc: : r = .38, p = .03) but not in our college sample (Left NAcc: r = −.07, p = .72; Right NAcc: r = .05, p = .82). The correlation coefficients for college and high school students were significantly different in the left NAcc (Z = 2.1, p = .04), though not the right NAcc (Z = 1.27, p = .20).
Figure 2. NAcc response to social reward and its relation to age.
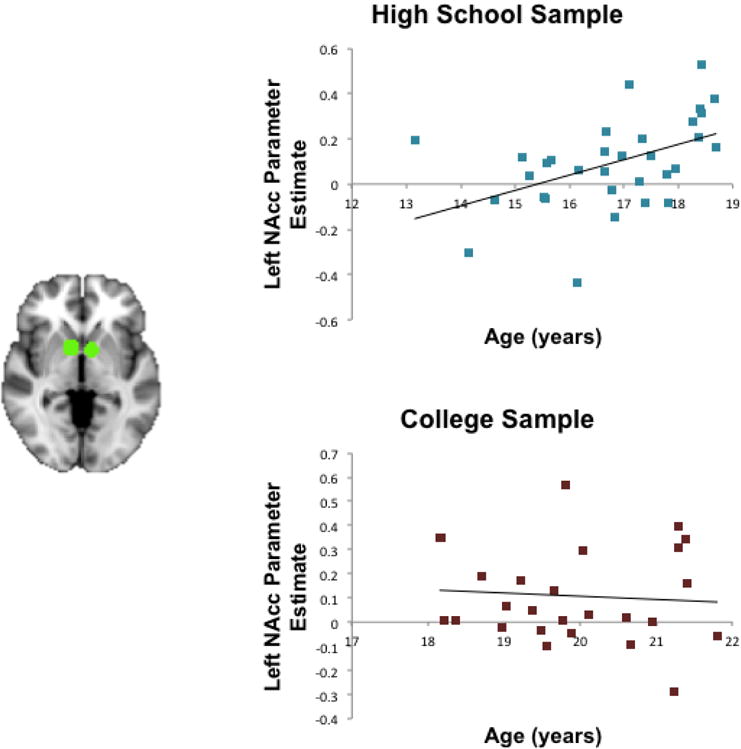
For participants in the high school cohort, NAcc response to the Popular (Many Likes) > Unpopular (Few Likes) contrast increased linearly with age (Left NAcc: r = .47, p = .006). However, for participants in the college cohort, NAcc response was not associated with age (Left NAcc: r = -.07, p = .72). Responses differed significantly for the high school and college samples in the Left NAcc, depicted in the figure. Parameter estimates are reported in percent signal change, determined using an isolated 3-second event with a double-gamma HRF. The NAcc region of interest was selected from an independent sample of young adults completing a Monetary Incentive Delay task (Tamir & Mitchell, 2013).
Goal 2: Cohort Differences in Neural Responses to Risky vs. Neutral Photographs
High-school and college participants differed significantly in their neural responses to Risky (compared to Neutral) photographs. We previously reported that when high-school students viewed risky images (vs. neutral images), they demonstrated significantly less activation in the dorsomedial prefrontal cortex (dmPFC), lateral parietal cortices, and bilateral prefrontal cortices, as well as a portion of the visual cortex. Notably, while the college student sample showed a similar decrease in activation in visual and right parietal cortices, we found no significant decreases in frontal areas (Figure 3). Indeed, significant differences between high-school and college students were observed in dmPFC (MNI coordinates of max voxel, x = 14, y = 54, z = 24; Max Z = 3.60, 706 voxels) and left dorsolateral prefrontal cortex (MNI coordinates of maximum voxel, x = −44, y = 20, z = 38; Max Z = 3.77, 393 voxels.
Figure 3. Differences in decreased activation for comparison of risky and neutral photographs.

When viewing Instagram photographs depicting risk-taking activities, compared to photographs depicting non-risky, neutral activities, only high-school students showed a significant decrease in activity in frontal regions including the dorsomedial prefrontal cortex (dmPFC) and the dorsolateral prefrontal cortex (dlPFC). The high school and college samples differed significantly in the dmPFC and left dlPFC, depicted in green. All images thresholded at Z > 2.3, with cluster correction to maintain p < .05.
Goal 3: Correlation Between fMRI Results and Risky Decision-Making
Composite scores on the CARE-R ranged from 1.1 –3.2 (M = 1.8) in our high school sample and 0.9 to 3.7 (M = 2.1) in our college sample, with higher scores indicating more past experiences with alcohol, drugs, and sexual risk-taking, as well as ratings of these activities as more beneficial and less risky. Frequency and appraisal scores were highly correlated (r(55) = .73, p < .001). Frequency scores for college students were higher than those of high school students (t (55) = 2.1, p = .04) but appraisal scores were not significantly different (t(55) = 1.2, p = .24). In our bottom-up correlational analyses with the CARE-R measures, no significant differences were observed between the college and high-school groups; however, some significant correlations emerged for only one cohort. Composite CARE-R scores were not significantly related to brain activity when participants viewed their own photographs with Many vs. Few Likes or others’ Neutral photos with Many vs. Few Likes. However, when viewing Risky images with Many > Few Likes, high-school students with higher composite CARE-R scores showed greater activation in a region of the occipital cortex (MNI coordinates of max voxel, x = −20, y = −74, z = 4; Max Z = 3.45, 467 voxels; Figure S2, top row). When comparing all Risky photos to all Neutral photos for all participants, those with higher CARE-R scores again showed significantly greater activation in visual areas (MNI coordinates of max voxel, x = −6, y = −96, z = 0; Max Z = 3.78, 598 voxels), as well as the precuneus/ PCC (MNI coordinates of max voxel, x = 8, y = −54, z = 12; Max Z = 3.14, 321 voxels; Figure S2, middle row). For college students only, higher CARE-R scores were additionally associated with greater activation in the mPFC (MNI coordinates of max voxel, x = −8, y = 66, z = 4; Max Z = 3.70, 672 voxels) and superior lateral occipital cortex (MNI coordinates of max voxel, x = −28, y = −76, z = 50; Max Z = 3.45, 304 voxels; Figure S2, bottom row). We extracted parameter estimates for each of these regions and correlated them to appraisal scores and frequency scores separately on the CARE-R. In all cases, correlations with parameter estimates were higher for appraisal scores than frequency scores, but the correlation coefficients were not significantly different (p > .05 for all).
Discussion
The first goal of the present study was to extend our prior findings from our sample of high school social media users (reference omitted for blind review) in a new, college-aged sample. As hypothesized, participants in both cohorts were more likely to Like photographs when they were popular; this effect was especially strong for participants’ own photographs. College students, like adolescents, showed significantly greater activation in the NAcc when viewing their own photographs that had received many Likes, as compared to few, and showed significantly greater activity in multiple brain regions when viewing popular photographs; however, they showed no areas of greater activity when viewing unpopular photographs compared to popular. Indeed, when comparing popular and unpopular photographs, high school and college participants differed significantly in only one brain region, for a single comparison: When viewing neutral images that were popular (vs. unpopular), high school students demonstrated significantly greater activity than college students in visual areas.
In addition to corroborating to our original findings, the observed agreement across the two samples for both behavioral results and NAcc activation suggests that Quantifiable Social Endorsement (QSE) plays a significant role in influencing how young adults perceive and respond to information on social media; in other words, QSE is a mechanism of peer influence, and a potential means by which individuals learn about their social environment, in a wider age-range than previously shown. These findings make intuitive sense given the continuing importance of the peer context in emerging adulthood (Arnett, 2000) and the popularity of mobile social media among college students. While the present inquiry used an Instagram-like interface, “Likes” are a feature of many social media, including Facebook and Twitter; thus, we expect that our findings would generalize to other digital platforms. Quantifiable Social Endorsement is not unique to mobile media use; indeed, Likes existed before smartphones were widely used by adolescents. However, all major social media tools are widely used on mobile phones, and Likes play an especially prominent role on Instagram, an tool initially designed for and primarily used on smartphones.
While the present study focused specifically on peer influence, it is possible that non-peers (e.g., older adults, parents, etc.) might have a similar effect, in line with work suggesting overlapping mechanisms between peer and parental influence (Welborn et al., 2016, though see also Telzer et al., 2015, for an discussion of differential responses to parents and peers). Of note, we artificially assigned Likes in order to implement our experimental manipulation and to avoid possible image-specific confounds; however, this meant that participants saw the same average number of Likes on their photos regardless of their own popularity. Given that visual attention is moderated by adolescents’ status (e.g., Lansu, Cilessen, & Karremans, 2014), future research should consider how users’ own popularity influences their behavioral and neural responses to popular and unpopular images.
Our second and third study goals aimed to characterize age differences as well as individual differences in brain responses to social media. As hypothesized, we found that increased age was associated with greater NAcc response to having one’s own content Liked by peers in the high school but not college sample. This finding is consistent with recent longitudinal work in adolescents demonstrating that NAcc sensitivity to rewarding stimuli increases in adolescence and peaks around age 16–17 (Braams et al., 2015). Our results indicate that social reward may progress along a similar trajectory as monetary reward. They are also consistent with trends concerning the early adoption of social media tools. Throughout the history of social media, older adolescents have been among the first to flock to new media, and they tend to use the tools most frequently, compared to older adults and younger teens (Lenhart et al., 2007; Madden et al., 2013). While adolescents generally are early adopters of new media, the tendency for older teens to be even more voracious users than younger teens may reflect not only greater independence from parents, but also increased motivation to seek approval online.
In addition to age differences in the strength of NAcc responsivity to social reward, we found that high school and college students demonstrated significantly different brain responses to Risky vs. Neutral images. Unlike high school students, college students did not show a decrease in activity in regions of the dmPFC and lPFC that overlap with the Central Executive Network (CEN; Sherman et al., 2014). The CEN is frequently activated during tasks involving executive function, including response inhibition and cognitive control, and metrics of CEN connectivity have been found to relate executive function and IQ in youth and adults (e.g., Li & Tian, 2014; Seeley et al., 2007). In other words, high-school but not college students showed decreased activity in frontal cognitive control regions when viewing images of risky behaviors. This difference could reflect continued maturation of the frontal cortex into early adulthood (Luciana, 2013; Paus, 2005). Our findings are consistent with the Dual Systems theory of adolescent risk-taking (Shulman et al., 2016), which posits that in adolescence, frontal control regions are insufficient to inhibit responses to affective, and often risky stimuli.
It should be noted that even though college students did not demonstrate decreased activation of regions implicated in cognitive control while viewing risky photographs, they did report higher overall risk-taking. This heightened risk-taking is not surprising: it is reasonable to assume that factors in their social environment (e.g., living away from home, prevalence of friends’ risky behaviors) can largely explain the difference in our high-school and college students’ risk-taking behaviors (for further discussion of these factors, see Willoughby et al., 2013). However, our findings highlight the importance of considering the relation between neural and behavioral responses within the larger context of the sociocultural environment, particularly in instances where two distinct developmental cohorts are being compared.
We found that individual differences in neural activation in response to risky photographs were related to differences in participants’ risky behaviors and risk appraisal. Specifically, increased scores on our risk-taking measure were related to increased activity in the precuneus/PCC and (for college students) mPFC. Our results are in concert with Saxbe and colleagues’ (2015) findings that, during a task in which adolescents rated peers’ emotions from video, activity in the precuneus, PCC, and mPFC was correlated with adolescents’ reported risk-taking and their affiliation with risky peers. The precuneus/PCC and mPFC have been associated with social cognition (e.g., Mars et al., 2012; Zaki & Ochsner, 2009) and self-reference (Northoff et al., 2006). Perhaps for individuals who engage in greater risk-taking in real life, or who tend to appraise dangerous behaviors more positively, photographs depicting those behaviors feel more relevant to their own selves and social activities (though this is only one of many possible interpretations; for example, the mPFC is also implicated in valuation and memory; e.g., Euston, Gruber, & McNaughton, 2012). These reported findings of individual differences are only a first step; it will be important for future research to further examine the relation between neural responses in the social brain and real-world risk-taking behavior, in the health domain and more generally. Furthermore, longitudinal research will be necessary to determine whether neural responses to risky images posted online have predictive power. For researchers broadly interested in neural predictors of health-risk behaviors, we suggest that a social media paradigm be considered in addition to more classic risk-taking paradigms, because of the high ecological validity.
While risky behaviors like smoking and drinking do not occur on social media, social media tools offer an opportunity for adolescents and young adults to socialize one another to norms relating to these activities. With the increasing popularity and availability of mobile social media, youth are more able to document and post risky behaviors in the moment. Youth not only see images depicting risk-taking behavior online; they also learn how their peers feel about these behaviors. As we have shown, peer endorsement significantly affects their perception of these photos and subsequent behavior on social media.
Supplementary Material
Acknowledgments
This research was supported in part by Grants C06-RR012169 and C06-RR015431 from the National Center for Research Resources, by Grant S10-OD011939 from the Office of the Director of the National Institutes of Health (NIH), by a National Institute on Drug Abuse National Research Service Award F31-DA038578- 01A1 (to L. E. Sherman), and by the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson- Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55:469. [PubMed] [Google Scholar]
- Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: A voxel‐based morphometry study. Human Brain Mapping. 2006;27:766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current Opinion in Neurobiology. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. The Journal of Neuroscience. 2015;35:7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galván A, Somerville LH. Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini RB. Influence: Science and practice. Boston, MA: Pearson Education; 2009. [Google Scholar]
- Cohen GL, Prinstein MJ. Peer contagion of aggression and health risk behavior among adolescent males: An experimental investigation of effects on public conduct and private attitudes. Child Development. 2006;77:967–983. doi: 10.1111/j.1467-8624.2006.00913.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2010;31(4):660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MB. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Preventing Chronic Disease. 2014;11 doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR. The importance of social rewards and social networks in the human brain. The Neuroscientist. 2014;20:387–402. doi: 10.1177/1073858414521869. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss K, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk‐taking and the adolescent brain: who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hu Y, Manikonda L, Kambhampati S. What We Instagram: A First Analysis of Instagram Photo Content and User Types. Proceedings of the 8th International Conference on Weblogs and Social Media 2014 Jun; [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Katz E, Fromme K, D’Amico E. Effects of outcome expectancies and personality on young adults’ illicit drug use, heavy drinking, and risky sexual behavior. Cognitive Therapy and Research. 2000;24:1–22. [Google Scholar]
- Lansu TA, Cillessen AH, Karremans JC. Adolescents’ Selective Visual Attention for High-Status Peers: The Role of Perceiver Status and Gender. Child Development. 2014;85:421–428. doi: 10.1111/cdev.12139. [DOI] [PubMed] [Google Scholar]
- Lee MR, Chassin L, Villalta IK. Maturing out of alcohol involvement: Transitions in latent drinking statuses from late adolescence to adulthood. Development and Psychopathology. 2013;25:1137–1153. doi: 10.1017/S0954579413000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart A, Madden M, Smith A, Macgill A. Teens and social media. Pew Internet & American Life Project 2007 Dec 19; [Google Scholar]
- Lenhart A. Teens, Social Media & Technology Overview 2015. Pew Internet and American Life Project. 2015 Available at: http://www.pewinternet.org/2007/12/19/teens-and-social-media/ Accessed on March 10 2016.
- Li C, Tian L. Association between resting-state coactivation in the parieto-frontal network and intelligence during late childhood and adolescence. American Journal of Neuroradiology. 2014;35(6):1150–1156. doi: 10.3174/ajnr.A3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Development and Psychopathology. 2013;25:1325–1345. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M, Lenhart A, Duggan M, Cortesi S, Gasser U. Teens and Technology, 2013. 2013 Retrieved from http://www.pewinternet.org/Reports/2013/Teens-and-Tech/Summary-of-Findings.aspx.
- Mars RB, Neubert F, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain”. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Morawetz C, Heekeren HR. Nucleus accumbens response to gains in reputation for the self relative to gains for others predicts social media use. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Tamir DI, Heekeren HR. The emerging neuroscience of social media. Trends in Cognitive Sciences. 2015;19:771–782. doi: 10.1016/j.tics.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science. 2013;16:653–664. doi: 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, De Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perrin A. Social media usage: 2005–2015. Pew Internet and American Life Project. 2015 Available at: http://www.pewinternet.org/2015/10/08/social-networking-usage-2005-2015/ Accessed on March 10, 2015.
- Reich SM, Subrahmanyam K, Espinoza G. Friending, IMing, and hanging out face-to-face: Overlap in adolescents’ online and offline social networks. Developmental Psychology. 2012;48:356–368. doi: 10.1037/a0026980. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Del Piero L, Immordino-Yang MH, Kaplan J, Margolin G. Neural correlates of adolescents’ viewing of parents’ and peers’ emotions: Associations with risk-taking behavior and risky peer affiliations. Social Neuroscience. 2015;10(6):592–604. doi: 10.1080/17470919.2015.1022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Rotte M. Favorite brands as cultural objects modulate reward circuit. Neuroreport. 2007;18:141–145. doi: 10.1097/WNR.0b013e328010ac84. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental Cognitive Neuroscience. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2015.12.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Monahan KC. Age differences in resistance to peer influence. Developmental Psychology. 2007;43:1531–1543. doi: 10.1037/0012-1649.43.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, Qu Y. Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Peters S, Braams BR, Crone EA. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience & Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2016.06.037. (in press) [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Welborn BL, Lieberman MD, Goldenberg D, Fuligni AJ, Galván A, Telzer EH. Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience. 2016;11:100–109. doi: 10.1093/scan/nsv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby T, Good M, Adachi PJ, Hamza C, Tavernier R. Examining the link between adolescent brain development and risk taking from a social–developmental perspective. Brain and Cognition. 2013;83:315–323. doi: 10.1016/j.bandc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. Values, empathy, and fairness across social barriers. New York Academy of Sciences; New York, NY: 2009. The need for a cognitive neuroscience of naturalistic social cognition; pp. 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


