Abstract
An emerging hypothesis postulates that internal noise is a key factor influencing perceptual abilities in autism spectrum disorder (ASD). Given fundamental and inescapable effects of noise on nearly all aspects of neural processing, this could be a critical abnormality with broad implications for perception, behavior, and cognition. However, this proposal has been challenged by both theoretical and empirical studies. A crucial question is whether and how internal noise limits perception in ASD, independently from other sources of perceptual inefficiency, such as the ability to filter out external noise. Here, we separately estimated internal noise and external noise filtering in ASD. In children and adolescents with and without ASD, we computationally modeled individuals’ visual orientation discrimination in the presence of varying levels of external noise. The results revealed increased internal noise and worse external noise filtering in individuals with ASD. For both factors, we also observed high inter-individual variability in ASD, with only the internal noise estimates significantly correlating with severity of ASD symptoms. We provide evidence for reduced perceptual efficiency in ASD that is due to both increased internal noise and worse external noise filtering, while highlighting internal noise as a possible contributing factor to variability in ASD symptoms.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and the presence of restricted and repetitive behaviors1. The diagnostic criteria include atypical reactivity to sensory stimuli, which can manifest as either hyper- or hypo-sensitivity. For example, individuals with ASD frequently report extreme sensitivity to lights, sounds, and textures (hyper-sensitivity) or demonstrate decreased response to salient stimuli (hypo-sensitivity). In fact, the two states may co-occur, fluctuating across time within an individual2. This variability, both within and across individuals, presents substantial challenges to understanding the neural underpinnings of sensory processing in ASD.
In the typically developing (TD) population, perceptual variability can be explained as being derived from both the physical randomness in the external environment3 and internal variability in the neural system4–7. Environmental noise, often referred to as external noise, arises from inherent variations in sensory input. For instance, in vision, fluctuations in the number of photons emitted by a light source can fundamentally limit our ability to detect dim light3. However, even when such fluctuations in external input are effectively held constant, perception and associated behavioral responses still exhibit significant variability8,9. Such variability is in part caused by internal noise that broadly exists throughout the nervous system, which together with external noise, limits the quality and efficiency of perceptual processing10 (but see McDonnell & Ward11 for a notable exception).
In individuals with ASD, there is growing evidence that increased internal noise might play an important role in their behavioral symptoms12–15. Specifically, it has been hypothesized that elevated internal noise in ASD may produce atypically large fluctuations in neural responses, leading to unreliable and less predictable representations of the environment16. The idea is supported by fMRI and EEG studies that showed increased trial-by-trial variability in blood-oxygen-level dependent (BOLD) and electrical responses to sensory stimuli in ASD17–19. Dinstein et al.19 found that trial-by-trial variability in BOLD responses within visual, auditory, and somatosensory cortices in adults with ASD was increased compared to neurotypical controls, with no significant group differences in the average BOLD amplitude. A possible role of internal noise in contributing to behaviors associated with ASD is suggested by a recent study20, where neurotypical individuals with more autistic traits exhibited more variable responses to identical stimuli over multiple repetitions.
However, whether internal noise is actually elevated in individuals with ASD remains an open question. We do not yet have a good understanding of causes and computational principles that might underlie increased neural variability in ASD21, and several theoretical accounts suggest an opposite possibility that internal noise may be reduced in ASD22,23. Notably, a number of studies challenge the noisy brain hypothesis in ASD by demonstrating typical levels of variability in evoked EEG24 and MEG25 responses to sensory stimulation, as well as in psychophysically estimated internal noise26,27. At the core of this controversy lies the issue of whether a domain-general account of ASD, such as the ones involving elevated internal noise and neural variability, can truly explain the complex phenotypes in individual with ASD24. This has been further complicated by the fact that the estimates of neural variability have been based on responses to task-irrelevant stimuli17–19,24,25, making it difficult to estimate the degree to which internal noise limits perceptual performance in ASD.
Furthermore, the effects of internal noise have often not been adequately separated from neural processes associated with external noise28. As discussed above, neural variability arises from many different sources10, including the variability in the external environment. External noise can be effectively filtered out by relying on appropriate perceptual ‘templates’ that weigh task-relevant sensory information9,29. Using templates that are not suitable for a given task increases the influence of task-irrelevant information and leads to inaccurate and noisy representations of the environment30. In fact, recent studies showed that individuals with ASD are more impacted by the presence of task-irrelevant stimulus noise27,31, suggesting heightened sensitivity to external noise in ASD.
Given these gaps in knowledge, we aimed to quantitatively estimate different sources of noise that limit perceptual processing in individuals with ASD using psychophysical and computational methods. Specifically, we employed a well-established equivalent noise paradigm32–36 in the context of a simple visual orientation discrimination task. This paradigm allows us to computationally estimate the input noise ‘equivalent’ to the total amount of internal noise that constrains perceptual ability. In this framework, perceptual sensitivity is measured at varying levels of added external noise. The assumptions are that A) there is a constant amount of internal noise in the system, and that B) perceptual performance at a given external noise level is influenced by whichever noise (i.e., internal or external) dominates. An analogy would be one’s ability to hear at a noisy party. In the absence of any additional noise, the baseline party noise—corresponding to internal noise—will determine the limits of what one can hear. Added external noise (e.g., caused by air conditioning)—corresponding to external noise—will have negligible effects as long as its level is lower than the baseline party noise. However, if the added noise is strong (e.g., a malfunctioning air conditioner), it will start affecting the ability of partygoers to hear each other. Here, the negative effects of added noise can be mitigated by an appropriate perceptual template that excludes irrelevant noise in the sensory input—a process described as external noise filtering in the framework used here. Taken together, by measuring perceptual sensitivity across varying levels of external noise, we can separately characterize the influence of both internal and external noises on perception (Fig. 1a). Performance was modeled using the Perceptual Template Model (PTM)36,37 to quantitatively estimate different sources of noise that constrain perception.
Figure 1.
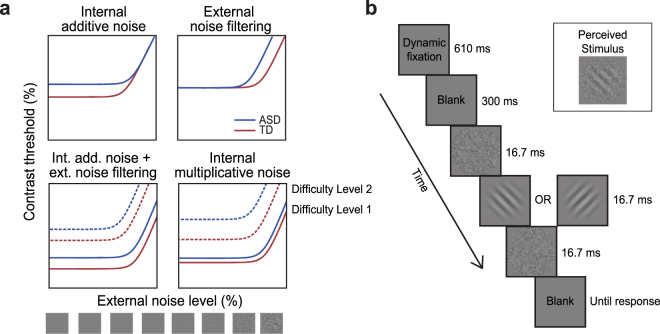
Predictions from PTM and experimental procedure. (a) Typical threshold-vs-noise (TvN) functions and predictions from the PTM. TvN functions have a characteristic shape where a flat segment is followed by a rising segment. At low external noise, internal noise dominates such that added external noise has little effect on perceptual performance. This produces the flat portion of the TvN curve. Once the external noise level begins to exceed that of internal noise, perceptual thresholds will be affected by external noise. Consequently, thresholds will increase because stronger signal is needed to overcome high levels of external noise. The PTM predicts distinct changes in TvN depending on the source of noise that is atypical in ASD (blue curves), relative to a TD baseline (red curves). Elevated internal additive noise will result in increased thresholds at the flat portion of the curve (top left). Worse external noise filtering, on the other hand, causes increased thresholds at the rising portion of the curve (top right). A combination of the two should yield increased thresholds across all levels of external noise levels (bottom left). A similar pattern is expected if internal multiplicative noise is elevated in ASD. However, the two can be distinguished by comparing the curves across at least two different difficulty levels. The ratio between thresholds at two difficulty levels will be different across groups in the case of elevated internal multiplicative noise in ASD (bottom right). (b) Stimuli, task, and timeline. Each trial began with a dynamic fixation point (see Methods). After a blank screen, the stimulus sequence started. Stimuli were oriented gratings temporally sandwiched by Gaussian pixel noise. This sequence yields merged perception of grating and noise (see insert). Participant’s task was to judge whether the grating was tilted left or right from vertical.
The benefits of using equivalent noise paradigms to investigate internal noise in ASD have been previously highlighted12,14. In fact, two studies used a reduced version of this paradigm and found no significant evidence for elevated internal noise in ASD26,27. However, two important unanswered questions remain. First, given the extensive number of trials typically required for equivalent noise experiments, these previous studies estimated thresholds at only two noise levels (i.e., no-noise and high-noise). While the noise levels used are the two most diagnostic ones, such an approach is limited relative to the inferences that can be made by measuring a full threshold function over varying levels of external noise (Fig. 1a). Second, the previous studies used the equivalent noise approach in the context of direction or orientation integration across multiple stimuli. This is of relevance as visual integration relies on mechanisms distinct from those involved in processing of individual stimuli38. Moreover, internal noise for integration depends on the number of stimuli in the display39 and is shown to be even lower than that for processing a single item40. Thus, while these prior studies provide important insights on the visual integration abilities in ASD, it is not given that those results will generalize to other settings. Here, we aimed to estimate internal noise in the context of an ostensibly simpler stimulus and task. Specifically, we chose stimulus and task parameters to stay clear of known perceptual impairments in ASD. We used a coarse orientation discrimination task with larger stimulus size41,42 and low spatial frequency43,44. Our goal here was to minimize the influence of known atypicalities in ASD that are specific to processing of stimulus attributes per se. Additionally, to improve upon the methods previously used, we applied an advanced adaptive psychophysical technique to significantly increase the efficiency in data collection. The technique considerably reduced the time it takes to perform the experiment by 70%, which, together with hierarchical Bayesian model techniques, allowed us to behaviorally quantify the effects of noise on perception in ASD at both the group and individual levels.
Results
Perceptual Template Model and its predictions
We used an equivalent noise paradigm32–36 where perceptual thresholds are measured as a function of varying external noise levels added to the stimuli. This results in a characteristic threshold versus noise (TvN) function (Fig. 1a). To quantify the effects of noise on perception, data were fitted with the PTM35,36, a model where perceptual performance is limited by three different sources of inefficiency: external noise, internal additive noise, and internal multiplicative noise. Here, perceptual thresholds (c τ) are characterized by:
| 1 |
where the input (signal + external noise (N ext)) is filtered through a perceptual ‘template’ relevant to a given task (see Supplementary Material 1 for derivation). This results in signal enhancement by a gain factor β. The output of the filter is transformed through a nonlinear transducer function that amplifies the inputs to the γ th power, and various sources of internal noise—additive and multiplicative—are added. Internal additive noise (N add) remains constant at all signal levels (i.e., it is independent of signal). On the other hand, internal multiplicative noise (N mul) is proportional to the signal strength. Finally, a decision process determines the perceptual performance level (d′ ). N ext is manipulated by the experimenter along with the input signal.
Given the background detailed in the Introduction, we hypothesized that individuals with ASD have increased internal additive noise. This predicts worse perceptual thresholds at lower external noise levels (Fig. 1a, top left). Secondarily, we hypothesized worse external noise filtering in ASD27,31, which would impair performance at higher external noise levels (Fig. 1a, top right). A combined effect of higher internal additive noise and worse external noise filtering would be evident as increased thresholds in ASD at all external noise levels (Fig. 1a, bottom left). This expected pattern of results is similar to the effects of an increase in internal multiplicative noise. In the PTM, multiplicative noise is characterized similarly to contrast gain control mechanisms36,37,45. Increased multiplicative noise reduces the system’s response to stimulus contrast29, leading to worse performance across all external noise levels. However, because this effect is multiplicative, the expected result is a non-uniform shift in the TvN curve across different difficulty levels (Fig. 1a, bottom right; i.e., the ratio between the two difficulty levels will be different across groups)29,37,45. Therefore, to distinguish the effect of internal additive noise and external noise filtering from that of internal multiplicative noise, perceptual performance must be measured at multiple difficulty levels.
Increased internal additive noise and worse external noise filtering in ASD
Twenty-one children and adolescents with ASD and 20 age- and IQ-matched TD controls completed an orientation discrimination task, judging whether a foveally presented grating (3° radius; Fig. 1b) was tilted to the left (−45°) or right (45°) from the vertical. External noise was manipulated by presenting Gaussian pixel noise with the same size as the grating immediately before and after stimulus presentation. Note that because of brief frames (16.7 ms) this sequence results in perceptual merging of grating and noise frames. This is typically done in PTM experiments to allow finer adjustments of target stimulus contrast29,35,37. Contrast thresholds at each of eight fixed external noise levels (0–21%) were measured. Stimulus presentation was controlled using an advanced adaptive psychophysical technique46. As detailed in Methods, this allowed us to accurately estimate relevant model parameters in just 480 trials per participant.
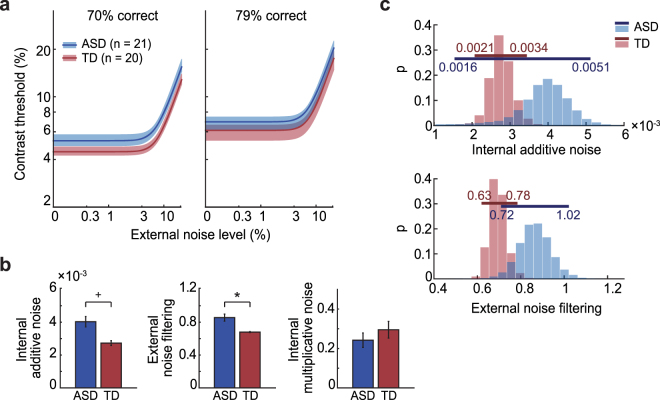
We first analyzed the data using the conventional PTM (Methods) which allows us to estimate the relative difference in noise-limiting factors between groups36. TvN curves estimated by the conventional PTM analysis revealed the characteristic non-linear patterns for both ASD and TD (Fig. 2). On average, individuals with ASD had a tendency to perform worse than TD across external noise levels. This pattern of results was best explained by a combined effect of a 70% increase in internal additive noise in ASD paired with 13% worse external noise filtering (r2 = 98.2%). This model was significantly different from a model that assumed no group differences in model parameters (F2, 26 = 9.41, p < 0.001), but was not statistically different from a full model that assumed all noise sources were different between the groups (F1, 25 = 0.139, p = 0.71). These results support the hypothesis that individuals with ASD have both elevated internal additive noise and worse ability to filter out external noise in the stimuli. We found no evidence for group differences in internal multiplicative noise.
Figure 2.
Results from the conventional PTM analysis. The two panels show thresholds (filled dots) at each external noise level for two difficulty levels. Overall, individuals with ASD (blue) performed worse than those with TD (red) across external noise levels. Error bars are ±SEM. Curves were obtained by fitting the conventional PTM (Equation (3) in Methods), which revealed a 70% increase in internal additive noise and 13% worse external noise filtering in ASD (p < 0.001; compared to the model that assumed no group differences).
Group differences in independently estimated contrast thresholds at each external noise level, however, did not reach significance (main effect of group; F1, 39 = 2.05, p = 0.16). This was expected. Given that our empirical approach was optimized for estimating relevant model parameters with as few individual trials as possible, independent threshold estimates were noisy (a more sensitive analysis of group differences in thresholds is presented in the next sub-section). We also calculated the Jeffreys, Zellener, and Siow (JZS) Bayes factor to quantify the evidence for the null hypothesis versus the alternative, using the prior on effect size following a Cauchy distribution with a scale factor of 0.707 as recommended by Rouder et al.47. By convention, Bayes factors ranging between 0.33 and 3 indicate data insensitivity while those below 0.33 or above 3 suggests that data supports the null or the alternative hypothesis, respectively47,48. The calculated Bayes factor was 0.7, suggesting that comparison of individual thresholds was not sensitive and that the alternative hypothesis cannot be fully excluded. Turning to the multiplicative noise, both groups had similar ratios of thresholds at two difficulty levels (ASD: mean = 0.53, SD = 0.09; TD: mean = 0.54, SD = 0.11; t39 = 0.34, p = 0.74). The JZS Bayes factor for the group difference in threshold ratio was 0.32. These findings are consistent with a lack of group differences in multiplicative noise.
Individual differences in internal noise and external noise filtering
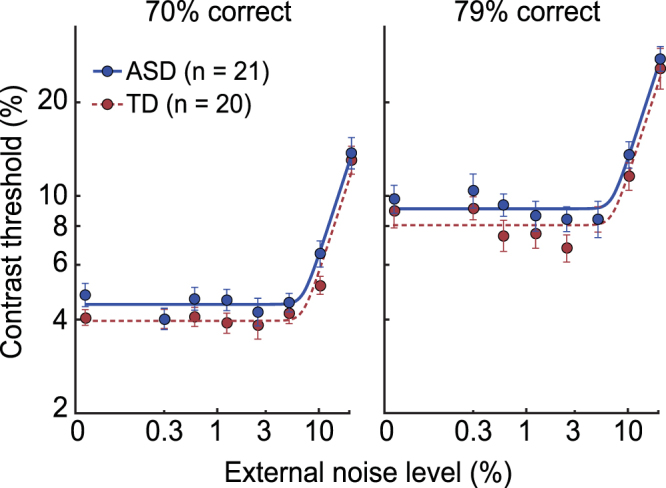
TvN curves and parameter estimates from the hierarchical Bayesian model exhibited a similar pattern to those estimated from conventional PTM (Fig. 3a,b). As this analysis removes some of the noise in threshold estimates by assuming that the variability between subjects in model parameters follows a population-level distribution49,50, we re-examined group differences in thresholds. Notably, individuals with ASD performed worse overall across external noise levels (F1,39 = 4.39, p = 0.04). There was no significant interaction between group and external noise level (F7, 273 = 0.42, p = 0.89), or between group and difficulty level (F1,39 = 0.5, p = 0.48). The estimated internal additive noise was 48% higher in ASD (population mean = 0.004) compared to TD (population mean = 0.0027). This group difference, however, was marginal (95% credible interval on difference = −0.0012, 0.0025; 91.3% of population posterior samples greater than zero). Here, the large increase in internal noise estimates was paired with greater individual variability in the ASD group (Fig. 3c, top). The 95% credible interval in the population posterior distribution for internal additive noise in ASD (0.0016, 0.0051) was 2.7 times broader than that in TD (0.0021, 0.0034). We will return to this individual variability when considering links with ASD symptoms. Turning to external noise filtering, we found that ASD was characterized by 25% worse external noise filtering abilities (population mean = 0.86) compared to TD (population mean = 0.69; 95% credible interval on difference = 0.003, 0.34; 97.6% of population posterior samples greater than zero). As with internal additive noise, we observed large individual variability (Fig. 3c, bottom), with the 95% credible interval in the population posterior distribution for external noise filtering in ASD (0.72, 1.02) being 2.0 times broader than in TD (0.63, 0.78). Finally, the two groups were not different in internal multiplicative noise (population mean for ASD = 0.11; for TD = 0.26; 95% credible intervals on difference = −0.48, 0.41; 42.8% of population posterior samples greater than zero).
Figure 3.
Results from hierarchical Bayesian analysis. (a) TvN functions estimated from the hierarchical Bayesian analysis show worse performance in ASD (blue) compared to TD (red), consistent with the conventional PTM analysis. Shaded regions are the 68% credible intervals obtained from the posterior distributions. (b) The average individual parameter estimates from the hierarchical Bayesian model. The estimated population means show 48% elevated internal additive noise, and 25% greater effect of external noise (i.e., worse external noise filtering) in ASD (+marginal significance using a 90% criterion on difference in population posterior samples; *significance using a 95% criterion). No significant group difference was found for internal multiplicative noise. Note that the absolute values of different types of noise cannot be meaningfully compared. Error bars are ±SEM. (c) Population posterior distributions estimated from the hierarchical Bayesian model for internal additive noise (top) and external noise filtering (bottom). Horizontal lines indicate 95% credible intervals. In both cases, the population posterior distributions for ASD (blue) are broader than TD (red), indicating greater inter-individual variability in ASD. In the case of internal additive noise, the 95% credible interval for ASD fully includes and extends beyond the credible interval for TD.
Internal additive noise is correlated with ASD symptoms
There was no significant correlation between internal additive noise and external noise filtering within ASD (r19 = 0.19, p = 0.42), suggesting, at least in part, independent contributions of these two sources of noise to perceptual inefficiencies in ASD. To test for links with behavioral ASD symptoms, we tested whether our noise estimates can predict individuals’ calibrated severity score derived from the Autism Diagnostic Observation Schedule (ADOS). Severity scores ranged from 4–9 on this dimensional measure of ASD severity, where scores of 4 and above are indicative of ASD (see Fig. 4 for simple correlations between symptom severity and each of the noise parameters). We then conducted a multiple linear regression analysis in the ASD group to understand the unique contributions of each noise type (estimated model parameters of internal additive noise and external noise filtering) on ADOS severity. Together, internal additive noise and external noise filtering accounted for 47% of the variance in ADOS severity score (R2 = 0.38, F2,18 = 5.56, p = 0.01). The results indicated that internal additive noise uniquely and significantly predicted the ADOS severity score in this combined model (β = 555.88, t18 = 2.98, p = 0.008). Specifically, higher internal additive noise was associated with increased severity of assessed ASD symptoms. On the other hand, external noise filtering did not significantly predict the ADOS severity score (β = 1.25, t18 = 0.91, p = 0.37). The results did not change when IQ was included as a predictor in the analysis. Finally, given the correlation between internal additive noise and ASD symptom severity, we repeated our analysis on the independently estimated Weibull thresholds by comparing the TD participants with those individuals with ASD who had more severe symptoms (median split using ADOS severity score, n = 11). This revealed a main effect of group (F1,29 = 4.78, p = 0.04) where this more severe ASD group performed significantly worse than the TD group. Taken together, these results suggest that the psychophysically estimated internal additive noise might be linked to the severity of core behavioral symptoms of ASD and support our hypothesis that, for basic visual processing, individuals with ASD have elevated internal noise.
Figure 4.
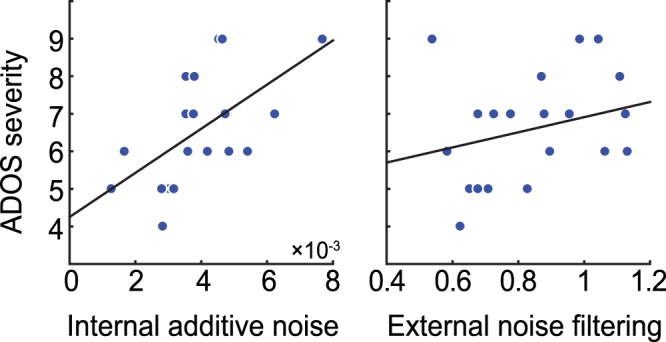
Relationship between the model parameters and ADOS calibrated severity score within ASD. Correlation analyses indicated that symptom severity was related to internal additive noise (left panel; r19 = 0.59, p = 0.005) and not to external noise filtering (right panel; r19 = 0.28, p = 0.22; see main text for results from multiple regression analysis which include both noise estimates in the model).
Discussion
The present study utilized psychophysical and computational methods to elucidate the sources of noise that influence perceptual performance in individuals with ASD. We found evidence for elevated internal noise and poor external noise filtering in ASD. Moreover, the estimated internal noise was correlated with ASD symptom severity, suggesting that individual variability in internal noise may be related to ASD symptomatology. Although the observed perceptual effect was relatively small (6.91% average increase in thresholds in ASD), evidence for higher internal noise in ASD is consistently observed across our model analyses. These findings support current theories that argue for an important role of increased internal noise in atypical sensory abilities in ASD, and suggest external noise filtering as another possible source of perceptual variability in ASD.
Our findings that individuals with ASD have elevated internal noise at the group level is consistent with previous studies that showed increased trial-by-trial variability in BOLD and EEG responses to sensory stimuli in ASD17–19 (but see Butler et al.24 and Coskun et al.25). While trial-by-trial variability in neural responses in the sensory areas is likely driven by a number of factors, noise levels in the nervous system are a key determinant10. Internal noise is also an important factor in perceptual variability5,8. Individuals with ASD report more varying perceptual judgments to repeated presentations of a sensory stimulus51. Such perceptual and neural fluctuations may have a substantial effect on the reliability of sensory representations, which in turn may cause greater difficulties for individuals with ASD when interacting with the surrounding environment. We also found significantly worsened external noise filtering in ASD. This may reflect abnormalities in the ability to extract task-relevant structures from visual inputs. In the typical population, there exist individual differences in the use of noise-excluding ‘templates’, which are believed to reflect variations in the ability to extract task-relevant information52 and to integrate the outputs of lower-level sensory channels into decision45—an ability that can be improved through perceptual learning29,53.
The present study extends the existing literature on internal noise in ASD in several important ways. Unlike recent studies that were based on brain responses to task-irrelevant stimuli17–19,24, our estimates of internal noise were functionally relevant to the task at hand (i.e., specifically associated with the limitations in visual perception in ASD). This is notable because of known atypicalities in task-dependent changes in neural processing in ASD54. However, this also raises a question of generalizability of our findings to other task settings. By definition, estimates of internal noise index the total amount of noise that is equivalent to the observer’s intrinsic noise. This intrinsic noise could be derived from sources specific to processing stimulus attributes required by a given task55. For example, internal noise estimates can be substantially elevated within an individual if the task/stimulus involves a known impairment56. On the other hand, a portion of estimated internal noise should also reflect the baseline noise that can have limiting effects on a wide range of processes within a system. In this study, we utilized a coarse orientation discrimination task that relies on the measurement of contrast thresholds with low spatial frequency stimuli whose processing is largely intact in ASD43,44. This makes it less likely that our internal noise estimates are elevated due to selective impairments associated with our specific task and stimuli, and suggests that perception may be limited by the same noise in other settings.
Our study presents discrepant findings from previous studies that used similar equivalent noise paradigms and did not find atypical levels of internal noise in ASD26,27. There are several possible reasons for this difference. First, as discussed in the Introduction, these studies used an impoverished version of the equivalent noise paradigm, relying on only two noise levels. While the selected noise levels were the most diagnostic ones, this approach precludes the measurement of full TvN curves and, consequently, application of arguably more sensitive model analyses used in the present study. Second, the nature of the task, namely an explicit need for stimulus integration in prior studies, may play a role in the observed discrepancy. While the ecological importance of visual integration processes motivates its study in context of ASD, visual integration also involves distinct mechanisms which are known to have differential impacts on the estimated internal noise40. In addition, a third factor that should be considered is the variability within individuals with ASD themselves21, which is frequently observed in ASD42,57–62. In fact, enhanced orientation sensitivity in ASD has been reported60, which would predict lower internal noise in ASD based on our assumptions in the model. This is in contrast with the previous account of internal noise in ASD (e.g., stochastic resonance) which argues that elevated internal noise, as a global trait of ASD, can explain both enhanced and diminished perceptual sensitivity in this population12. Instead, our findings reveal large inter-individual variability in both internal additive noise and external noise filtering among ASD. While such variability in ASD results emphasizes the need for replication of our findings, this also raises an important possibility that subgroups may exist within the ASD population that are characterized by different levels of internal noise. Our observation that internal noise estimates were positively related to severity of ASD symptoms, as well as a previous report of the link between internal noise and autistic traits20, further supports this idea.
An alternative explanation of our findings may involve deficits in attentional deployment in ASD. In TD, spatial attention can reduce the effects of internal additive noise, without affecting external noise filtering37. While only partially consistent with our results, this still raises a possibility that increased internal additive noise in ASD may result from impairments in spatial attention. However, previous reaction time studies report mixed findings on spatial attention deficits in ASD (for a review: Ames and Fletcher-Watson63). Moreover, the studies that directly investigated the effects of spatial attention on visual performance in ASD found either no significant deficits64,65 or, in one case, a sharper window for spatial attention in ASD66. Additionally, if our results were caused by group differences in global attention or task motivation, we would expect similar effects on internal noise and external noise filtering estimates—an expectation inconsistent with a lack of significant correlation between the two noise factors. Therefore, although we cannot completely rule out effects of attention, it is unlikely that attentional differences would cause the specific pattern of results observed in this study.
Our findings are relevant to the growing literature on Bayesian perceptual priors2 and predictive coding in ASD67,68. Bayesian priors are helpful in attenuating the effects of noise69, and play an important role in constraining sensory noise throughout development70. In this context, atypically strong effects of noise in ASD may, in part, reflect diminished reliance on priors31,71. Supporting this idea, several studies report reduced adaptation in ASD across multiple domains72–74, suggesting another example of perceptual inefficiency in this population. Such an account is also consistent with the weaker divisive normalization hypothesis in ASD, which can lead to reduced influence of contextual information75. On the other hand, unreliability in sensory representations due to noisier processing may also prevent individuals from forming reliable priors. In a predictive coding framework, noisier perceptual processing can be explained by individuals’ tendencies to more heavily weigh prediction errors caused by irrelevant information68,76. Indeed, a recent empirical finding suggests that individuals with ASD have an increased tendency to attribute aberrant events to changes in the environment77. This can increase unpredictability in perception, and might be further related to the desire for sameness frequently observed in ASD68,78. Thus, future research should consider how the influence of noise in each individual relates to the use of top-down information such as prior and prediction, as well as their possible interactions across development.
In conclusion, we report evidence for elevated internal noise and poor external noise filtering in ASD. Our results further reveal large inter-individual variability in estimated levels of internal noise and external noise filtering, the former of which is related to severity of ASD symptoms. Evidently, visual processing in ASD may be less efficient, limited by multiple sources of noise. These results are pertinent to existing theories on atypical sensory processing in ASD, providing important insights about the constraints of reliable perceptual representations that could have substantial effects on individuals’ everyday lives. Future studies should investigate how our findings relate to neurophysiological measures of noise and generalize to other sensory modalities.
Methods
Participants
Participants were 21 children and adolescents (ages 9–17 years) with ASD (19 male) and 20 TD controls (18 male). This sample size was determined to be the larger of (a) previous studies that investigated perceptual efficiencies across groups using the PTM56,79 and (b) the typical sample size in studies that examined various aspects of sensory processing in ASD (~20 participants in a group)80–84. We assessed IQ using abbreviated versions of the Wechsler Intelligence Scale for Children, 4th Edition85 or the Wechsler Adult Intelligence Scale, 4th Edition86. Groups were matched on both age (ASD: mean age = 13.49, SD = 2.08; TD: mean age = 13.47, SD = 2.31; t39 = 0.02, p = 0.98) and full scale IQ (ASD: mean FSIQ = 110.14, SD = 14.14; TD: mean FSIQ = 113.70, SD = 15.10; t39 = 0.78, p = 0.44). ASD diagnosis was confirmed or ruled out with a combination of the ADOS87, and either the Autism Diagnostic Interview–Revised (ADI-R)88 with parents of participants with ASD or the Social Communication Questionnaire (SCQ)89 with parents of TD participants. The ADOS and ADI-R were administered by a research-reliable examiner and a licensed clinical psychologist made final diagnostic decisions. From the ADOS, we derived the calibrated severity score90,91 (range: 4–9) to use in our correlation analyses. Thirteen of the participants with ASD were taking medications at the time of the study, which included stimulants and other psychoactive medications (e.g., guanfacine, fluoxetine). There were no effects of medication on any of our experimental measures within ASD (all p’s > 0.4), and no effects of medication on correlations between our measures and symptom severity. However, given the limited power we have to investigate medicated versus un-medicated participants, we cannot completely rule out effects of medication. All parents gave written informed consent, and participants gave assent. All were paid for participation. Procedures were conducted according to a protocol approved by the University’s Research Subjects Review Board.
Stimuli, task, and experimental procedure
Stimuli were created using MATLAB and Psychophysics Toolbox92. They were shown on a linearized monitor (24-inch Sony GDM-FW900 CRT; 1024 × 640 resolution; 120 Hz). Viewing distance was 77 cm with each pixel subtending 0.036°, and a chin rest was used. An experimenter was present in the room throughout to encourage on-task behavior.
Participants performed a coarse orientation discrimination task where they judged the tilt of the stimulus (±45° from the vertical; Fig. 1b). The stimulus was a sinewave grating (1 cycle/°) presented at the center of the display in a two-dimensional raised cosine envelope (radius = 3°). The stimulus was temporally sandwiched by two independent external noise samples (Fig. 1b). The grey-level pixel values for external noise (each subtending 0.14° × 0.14°) were sampled from a Gaussian distribution with a mean of 0 and SD that varied over trials, such that its root mean square (RMS) contrast defined the external noise level (0, 0.3, 0.61, 1.24, 2.51, 5.1, 10.3, or 21%). A trial began with a dynamic fixation sequence presented at the center of the screen62. Participants indicated their response by pressing the left or right arrow key. Auditory feedback followed correct responses.
Psychophysical procedure
Stimulus contrast was adjusted on every trial to estimate the signal strength required to reliably perceive stimulus orientation for a given external noise level. To do this, we used an advanced adaptive psychophysical method, Functional Adaptive Sequential Testing (FAST toolbox; Vul et al., 2010), to efficiently sample stimulus contrast around participants’ contrast thresholds. FAST optimally and adaptively selected a stimulus contrast that is most informative for estimating the model parameters. We implemented two interleaved FAST structures that independently estimated psychophysical functions at two different difficulty levels (d′) using Equation (1). For each of the two FAST structures, d′ was set at 1.089 and 1.634, corresponding to 70.71% and 79.37% difficulty levels, respectively. Unlike conventional approaches that first aim to estimate thresholds at each external noise level, FAST substantially increases data collection efficiency by directly constraining the shape of the full TvN function using all data points46. Compared to conventional methods that require ~1500 trials29, this technique allowed us to test our hypotheses in a more efficient manner, requiring only 480 trials total for each participant (~25 minutes). We settled on this design after extensive piloting, first in adults and then in children. For this, we conducted longer experiments and estimated the number of trials at which estimates of the PTM parameters asymptoted. Similar techniques have been shown to be effective for testing visual performance in TD children93.
Note that the data analyses were performed independent from the FAST procedure. This was to minimize possible effects of erroneous responses in ASD, which could result in biased threshold estimations if estimated directly using these types of adaptive techniques12,14. Specifically, we analyzed the data in two complementing ways as described below. First, we estimated model parameters by fitting the conventional PTM, with an aim to test for group differences in internal additive noise, internal multiplicative noise, and external noise filtering29,37. Second, we directly estimated model parameters from single trial data using hierarchical Bayesian model fitting. This method estimated model parameters for each individual participant, providing a better index of individual differences94. This further allowed us to test for links between behavioral ASD symptoms and noise-related perceptual limitations.
Conventional PTM analysis
To analyze the data using a conventional PTM fitting method, we first estimated psychophysical thresholds for each participant at each of the external noise levels. We pooled the data from the two FAST structures to estimate thresholds, resulting in 60 trials for each external noise level. This yielded independent, albeit not very precise, threshold estimates at each noise level. Thresholds were estimated by fitting a Weibull function at each external noise level independently:
| 2 |
where P denotes percent correct, c is stimulus contrast, α is threshold at 75% performance level and η is the slope. To estimate the two free parameters of the Weibull function (η and α), we used a Bayesian model fitting method that implements a Markov Chain Monte Carlo (MCMC) technique. Specifically, we sampled the posterior distributions of the parameters using JAGS software (http://mcmc-jags.sourceforge.net/). We assumed a broad uniform prior on each parameter with a range that includes all practically possible values. Maximum a posteriori (the mode of the posterior) were used as the best estimates of the model parameters. We discarded the first 15,000 samples as a burn-in period, and thinned the samples to reduce correlations by only selecting every 200 samples. A total of 10 chains were run in parallel, resulting in 1,000 posterior samples from each chain. Thresholds at 70.71% and 79.37% were then computed from the estimated Weibull functions.
The group averages of the independently estimated thresholds were then used to fit the conventional PTM. To characterize the group difference, the PTM introduces three coefficient indices (A m(group), A a(group),A f(group)) to Equation (1), each to be multiplied by N mul, N add, and N ext:
| 3 |
The coefficient indices for TD are fixed to be A m(TD) = A a(TD) = A e(TD) = 1. To estimate group differences in how the three types of noise influenced performance, A m(ASD), A a(ASD), and A e(ASD) are varied. That is, these coefficients for ASD describe the relative differences in the effects of noise between the groups. Specifically, if the estimated A m(ASD), A a(ASD) and A e(ASD) are greater than 1, it suggests that the amount of corresponding types of noise in ASD is higher than TD. The three coefficient indices for ASD could potentially vary or be fixed at 1, resulting in eight candidate models to compare. For example, the null model, assuming no group differences, had only four free parameters (N mul, N add, β and γ), whereas the full model, assuming group differences in all three types of noise, had seven free parameters (A m(ASD) and A a(ASD), and A e(ASD), in addition to the four in the null model). The remaining six candidate models had different combinations of the three types of noise, resulting in five or six free parameters. Each of the candidate models was fitted to the average group thresholds estimated from the above-described method using a least-squares procedure.
For model comparison, goodness-of-fit for each candidate model was evaluated using r 2:
| 4 |
where Σ and mean were computed over all difficulty levels, external noise levels, and groups. The best-fitting model was identified using the F-test for nested models:
| 5 |
where df 1 = k full − k reduced and df 2 = N data − k full. k full is the number of free parameters in the full model, and k reduced is the number of free parameters in reduced models. N data is the number of predicted data points. The model with the fewest number of free parameters that was not significantly different from the full model was determined to be the best-fitting model. A significance level of α < 0.05 was used.
Hierarchical Bayesian model analysis
To test for links between ASD symptoms and individual variability in the noise estimates, we fitted the PTM for each participant using a hierarchical Bayesian modeling technique. By assuming that each participant is drawn from a population distribution (ASD or TD), this method increases statistical power49,50. Importantly, for our purposes, it allows estimation of model parameters for each participant within a population, providing a better understanding of individual differences94. Furthermore, using this approach, we can estimate model parameters directly from single trial data points (instead of relying on the independently estimated thresholds at each external noise level).
We used a variation of PTM that minimized the number of free parameters and allowed us to estimate the effects of all three types of noise for each participant. Specifically, we assumed that an individual’s response (response ijk), whether correct or incorrect, for a given trial is drawn from a Bernoulli distribution:
where i is difficulty level, j is external noise level, and k is trial number. The probability of making a correct response (θ ijk) is given by:
| 6 |
where is stimulus contrast, η is slope, and
| 7 |
The parameter q is the difficulty level and thus is either 0.7071 or 0.7937. Following the PTM, the perceptual threshold, , is defined by:
| 8 |
Equation (8) is identical to Equation (1) except that a coefficient for external noise (w ext) is added to characterize the influence of external noise on the individual’s perception (i.e., extent of external noise filtering; see Supplementary Material 1 for derivations). For each participant, we estimated three parameters N mul, N add, and w ext that were the most relevant for our hypotheses, in addition to the slope of the psychometric function (η). To do so, we simplified the model by assuming β = 1.25 for all participants, and further set γ = 2. We note that setting these two parameters to different values did not change the results (Supplementary Material 1 for details). The fixed values we used are within a reasonable range reported in previous studies29,36,37,45,56. This also simplified the model by eliminating strong correlations among model parameters (see Supplementary Material 2, Supplementary Figs. 1 and 2 for additional details). In other words, we ensured that all model parameters are identifiable and sufficiently constrained based on the available data.
Similar to the Weibull fitting procedure, an MCMC technique was used to sample from the posterior distributions and estimate the free parameters. Here, we assumed hierarchical priors on each of the model parameters. That is, each model parameter that characterizes an individual participant’s data was assumed to be drawn from an independent Gaussian population distribution with a mean and SD that characterized each group. The priors for these population means and SDs were set to broad uniform distributions. Maximum a posteriori were used as the best parameter estimates for both individual and population parameters (using the mean did not change the results). To test the group difference, 95% credible intervals on the population posterior samples were used95. When the credible interval for the group difference (ASD-TD) does not include zero, the null hypothesis that there is no group difference is rejected. We used 15,000 iterations for burn-in, and only selected every 200 samples for thinning. Ten chains were run in parallel, each of which sampled 2,000 posterior samples (see Supplementary Material 3 and Supplementary Fig. 3 for sample convergence and autocorrelation). To test the link between the noise-related perceptual limitations and ASD symptoms, we used the estimated individual model parameters to correlate with the calibrated severity score derived from the ADOS. A significance level of α < 0.05 was used for correlation analyses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We would like to thank the children and families that participated in this study. The study was supported by an Autism Science Foundation Pre-doctoral Fellowship 16–006 (to W.P.), R01 EY019295 and T32 EY007125 (to D.T.), R01 DC009439 (to L.B.), a University of Rochester PumpPrimer II award (to D.T. and L.B.), a NASRAD Independent Investigator Grant (to D.T.), and Schmitt Program on Integrative Brain Research (to L.B. and D.T.). The Center for Integrated Research Computing at the University of Rochester provided computing resources.
Author Contributions
All authors designed research; W.P., and K.S. performed research and analyzed data; R.Z. and W.P. developed software and analysis; W.P. wrote the first draft and all authors edited the paper. L.B. and D.T. provided supervision.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Woon Ju Park and Kimberly B. Schauder contributed equally to this work.
Loisa Bennetto and Duje Tadin jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17676-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.APA. Diagnostic and statistical manual of mental disorders, (DSM-5). (American Psychiatric Publishing, 2013).
- 2.Pellicano E, Burr DC. When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends Cogn Sci. 2012;16:504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Bialek W. Physical limits to sensation and perception. Annu Rev Biophys Biophys Chem. 1987;16:455–478. doi: 10.1146/annurev.bb.16.060187.002323. [DOI] [PubMed] [Google Scholar]
- 4.Deneve S, Latham PE, Pouget A. Efficient computation and cue integration with noisy population codes. Nat Neurosci. 2001;4:826–831. doi: 10.1038/90541. [DOI] [PubMed] [Google Scholar]
- 5.Shadlen MN, Britten KH, Newsome W, Movshon J. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stocker AA, Simoncelli EP. Noise characteristics and prior expectations in human visual speed perception. Nat Neurosci. 2006;9:578–585. doi: 10.1038/nn1669. [DOI] [PubMed] [Google Scholar]
- 7.Rolls, E. T. & Deco, G. The noisy brain: Stochastic dynamics as a principle of brain function. (Oxford University Press, 2010).
- 8.Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature. 2005;437:412–416. doi: 10.1038/nature03961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold JM, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178. doi: 10.1038/46027. [DOI] [PubMed] [Google Scholar]
- 10.Faisal AA, Selen LPJ, Wolpert D. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonnell MD, Ward LM. The benefits of noise in neural systems: Bridging theory and experiment. Nat Rev Neurosci. 2011;12:415–426. doi: 10.1038/nrn3061. [DOI] [PubMed] [Google Scholar]
- 12.Simmons DR, et al. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JLR, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183X.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dakin SC, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Baron-Cohen S, Belmonte MK. Autism: A window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- 16.Dinstein I, Heeger DJ, Behrmann M. Neural variability: Friend or foe? Trends Cogn Sci. 2015;19:322–328. doi: 10.1016/j.tics.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Milne E. Increased intra-participant variability in children with autistic spectrum disorders: Evidence from single-trial analysis of evoked EEG. Front Psychol. 2011;2:1–12. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haigh SM, Heeger DJ, Dinstein I, Minshew NJ, Behrmann M. Cortical variability in the sensory-evoked response in autism. J Autism Dev Disord. 2015;45:1176–1190. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinstein I, et al. Unreliable evoked responses in autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilidaite G, Yu M, Baker DH. Internal noise estimates correlate with autistic traits. Autism Res. 2017;10:1384–1391. doi: 10.1002/aur.1781. [DOI] [PubMed] [Google Scholar]
- 21.Heeger DJ, Behrmann M, Dinstein I. Vision as a beachhead. Biol Psychiatry. 2016;81:832–837. doi: 10.1016/j.biopsych.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Brock J. Alternative Bayesian accounts of autistic perception: Comment on Pellicano and Burr. Trends Cogn Sci. 2012;16:573–574. doi: 10.1016/j.tics.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Davis G, Plaisted-Grant K. Low endogenous neural noise in autism. Autism. 2014;19:351–362. doi: 10.1177/1362361314552198. [DOI] [PubMed] [Google Scholar]
- 24.Butler, J. S., Molholm, S., Andrade, G. N. & Foxe, J. J. An examination of the neural unreliability thesis of autism. Cereb Cortex 185–200 (2017). [DOI] [PMC free article] [PubMed]
- 25.Coskun MA, et al. Increased response variability in autistic brains? Behav Integr Clin Neurosci. 2009;20:1543–1548. doi: 10.1097/WNR.0b013e32833246b5. [DOI] [PubMed] [Google Scholar]
- 26.Manning C, Tibber MS, Dakin SC. Visual integration of direction and orientation information in autistic children. Autism Dev Lang Impair. 2017;2:1–16. doi: 10.1177/2396941517694626. [DOI] [Google Scholar]
- 27.Manning C, Tibber MS, Charman T, Dakin SC, Pellicano E. Enhanced integration of motion information in children with autism. J Neurosci. 2015;35:6979–6986. doi: 10.1523/JNEUROSCI.4645-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Marin FJ, Padilla-Medina JA. A psychophysical test of the visual pathway of children with autism. J Autism Dev Disord. 2008;38:1270–1277. doi: 10.1007/s10803-007-0507-9. [DOI] [PubMed] [Google Scholar]
- 29.Lu Z-L, Dosher BA. Perceptual learning retunes the perceptual template in foveal orientation identification. J Vis. 2004;4:44–56. doi: 10.1167/4.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Beck JM, Ma WJ, Pitkow X, Latham PE, Pouget A. Not noisy, just wrong: The role of suboptimal inference in behavioral variability. Neuron. 2012;74:30–39. doi: 10.1016/j.neuron.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaidel A, Goin-Kochel RP, Angelaki DE. Self-motion perception in autism is compromised by visual noise but integrated optimally across multiple senses. Proc Natl Acad Sci. 2015;112:6461–6466. doi: 10.1073/pnas.1506582112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess AE, Wagner RF, Jennings RJ, Barlow HB. Efficiency of human visual signal detection. Science (80-) 1981;214:93–94. doi: 10.1126/science.7280685. [DOI] [PubMed] [Google Scholar]
- 33.Pelli DG, Farell B. Why use noise? J Opt Soc Am A Opt Image Sci Vis. 1999;16:647–653. doi: 10.1364/JOSAA.16.000647. [DOI] [PubMed] [Google Scholar]
- 34.Barlow HB. Retinal noise and absolute threshold. J Opt Soc Am. 1956;46:634–639. doi: 10.1364/JOSA.46.000634. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z-L, Dosher BA. Characterizing human perceptual inefficiencies with equivalent internal noise. J Opt Soc Am A Opt Image Sci Vis. 1999;16:764–778. doi: 10.1364/JOSAA.16.000764. [DOI] [PubMed] [Google Scholar]
- 36.Lu Z-L, Dosher BA. Characterizing observers using external noise and observer models: assessing internal representations with external noise. Psychol Rev. 2008;115:44–82. doi: 10.1037/0033-295X.115.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Lu Z-L, Dosher BA. External noise distinguishes attention mechanisms. Vision Res. 1998;38:1183–1198. doi: 10.1016/S0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 38.Cohen MA, Dennett DC, Kanwisher N. What is the bandwidth of perceptual experience? Trends Cogn Sci. 2016;20:324–335. doi: 10.1016/j.tics.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dakin SC, Mareschal I, Bex PJ. Local and global limitations on direction integration assessed using equivalent noise analysis. Vision Res. 2005;45:3027–3049. doi: 10.1016/j.visres.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Im HY, Halberda J. The effects of sampling and internal noise on the representation of ensemble average size. Attention, Perception, Psychophys. 2013;75:278–86. doi: 10.3758/s13414-012-0399-4. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzkopf DS, Anderson EJ, de Haas B, White SJ, Rees G. Larger extrastriate population receptive fields in autism spectrum disorders. J Neurosci. 2014;34:2713–2724. doi: 10.1523/JNEUROSCI.4416-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauder, K. B., Park, W. J., Tadin, D. & Bennetto, L. Larger receptive field size as a mechanism underlying atypical motion perception in autism spectrum disorder. Clin Psychol Sci 1–16 10.1177/2167702617707733 (2017). [DOI] [PMC free article] [PubMed]
- 43.Jemel B, Mimeault D, Saint-Amour D, Hosein A, Mottron L. VEP contrast sensitivity responses reveal reduced functional segregation of mid and high filters of visual channels in Autism. J Vis. 2010;10:1–13. doi: 10.1167/10.6.13. [DOI] [PubMed] [Google Scholar]
- 44.Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biol Psychiatry. 2009;65:22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Dosher BA, Lu Z-L. Mechanisms of perceptual learning. Vision Res. 1999;39:3197–3221. doi: 10.1016/S0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 46.Vul E, Bergsma J, MacLeod DI. Functional Adaptive Sequential Testing. Seeing Perceiving. 2010;23:483–515. doi: 10.1163/187847510X532694. [DOI] [PubMed] [Google Scholar]
- 47.Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- 48.Dienes Z. Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5:1–17. doi: 10.3389/fpsyg.2014.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morey RD. A Bayesian hierarchical model for the measurement of working memory capacity. J Math Psychol. 2011;55:8–24. doi: 10.1016/j.jmp.2010.08.008. [DOI] [Google Scholar]
- 50.Rouder JN, Sun D, Speckman PL, Lu J, Zhou D. A hierarchical Bayesian statistical framework for response time distributions. Psychometrika. 2003;68:589–606. doi: 10.1007/BF02295614. [DOI] [Google Scholar]
- 51.Haigh SM, Minshew NJ, Heeger DJ, Dinstein I, Behrmann M. Over-responsiveness and greater variability in roughness perception in autism. Autism Res. 2016;9:393–402. doi: 10.1002/aur.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon JA. Noise reveals visual mechanisms of detection and discrimination. J Vis. 2002;2:105–120. doi: 10.1167/2.1.7. [DOI] [PubMed] [Google Scholar]
- 53.Gold JM, Sekuler AB, Bennett PJ. Characterizing perceptual learning with external noise. Cogn Sci. 2004;28:167–207. doi: 10.1207/s15516709cog2802_3. [DOI] [Google Scholar]
- 54.You X, et al. Atypical modulation of distant functional connectivity by cognitive state in children with Autism Spectrum Disorders. Front Hum Neurosci. 2013;7:1–13. doi: 10.3389/fnhum.2013.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldwin AS, Baker DH, Hess RF. What do contrast threshold equivalent noise studies actually measure? Noise vs. nonlinearity in different masking paradigms. PLoS One. 2016;11:1–25. doi: 10.1371/journal.pone.0150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavanaugh MR, et al. Visual recovery in cortical blindness is limited by high internal noise. J Vis. 2015;15:1–18. doi: 10.1167/15.10.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milne E, et al. Visual motion coherence thresholds in people with autism. J Child Psychol Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- 58.Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–1053. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Tsermentseli S, O’Brien J, Spencer JV. Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. J Autism Dev Disord. 2008;38:1201–1210. doi: 10.1007/s10803-007-0500-3. [DOI] [PubMed] [Google Scholar]
- 60.Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- 61.Meilleur AAS, Berthiaume C, Bertone A, Mottron L. Autism-specific covariation in perceptual performances: ‘g’ or ‘p’ Factor? PLoS One. 2014;9:1–13. doi: 10.1371/journal.pone.0103781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foss-Feig J, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J Neurosci. 2013;33:8243–8249. doi: 10.1523/JNEUROSCI.1608-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ames C, Fletcher-Watson S. A review of methods in the study of attention in autism. Dev Rev. 2010;30:52–73. doi: 10.1016/j.dr.2009.12.003. [DOI] [Google Scholar]
- 64.Grubb MA, et al. Endogenous spatial attention: evidence for intact functioning in adults with autism. Autism Res. 2013;6:108–118. doi: 10.1002/aur.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grubb MA, et al. Exogenous spatial attention: Evidence for intact functioning in adults with autism spectrum disorder. J Vis. 2013;13:1–13. doi: 10.1167/13.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Tunnel vision: Sharper gradient of spatial attention in autism. J Neurosci. 2013;33:6776–6781. doi: 10.1523/JNEUROSCI.5120-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Boxtel JJA, Lu H. A predictive coding perspective on autism spectrum disorders. Front Psychol. 2013;4:1–3. doi: 10.3389/fpsyg.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van de Cruys S, et al. Precise minds in uncertain worlds: Predictive coding in autism. Psychol Rev. 2014;121:649–75. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- 69.Knill DC, Pouget A. The Bayesian brain: The role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27:712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Sciutti A, Burr DC, Saracco A, Sandini G, Gori M. Development of context dependency in human space perception. Exp Brain Res. 2014;232:3965–3976. doi: 10.1007/s00221-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 71.Karaminis T, et al. Central tendency effects in time interval reproduction in autism. Sci Rep. 2016;6:1–13. doi: 10.1038/srep28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turi M, et al. Children with autism spectrum disorder show reduced adaptation to number. Proc Natl Acad Sci USA. 2015;112:7868–72. doi: 10.1073/pnas.1504099112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pellicano E, Jeffery L, Burr DC, Rhodes G. Abnormal adaptive face-coding mechanisms in children with autism spectrum disorder. Curr Biol. 2007;17:1508–1512. doi: 10.1016/j.cub.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 74.Turi M, Karaminis T, Pellicano E, Burr D. No rapid audiovisual recalibration in adults on the autism spectrum. Sci Rep. 2016;6:21756. doi: 10.1038/srep21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenberg A, Patterson JS, Angelaki DE. A computational perspective on autism. Proc Natl Acad Sci. 2015;112:9158–9165. doi: 10.1073/pnas.1510583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van de Cruys, S., Van der Hallen, R. & Wagemans, J. Disentangling signal and noise in autism spectrum disorder. Brain Cogn (2016). [DOI] [PubMed]
- 77.Lawson RP, Mathys C, Rees G. Adults with autism overestimate the volatility of the sensory environment. Nat Neurosci. 2017;20:1293–1299. doi: 10.1038/nn.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL. Autism as a disorder of prediction. Proc Natl Acad Sci. 2014;111:15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bejjanki VR, et al. Action video game play facilitates the development of better perceptual templates. Proc Natl Acad Sci. 2014;111:16961–16966. doi: 10.1073/pnas.1417056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chowdhury R, et al. Auditory Pitch Perception in Autism Spectrum Disorder Is Associated With Nonverbal Abilities. Perception. 2017;46:1298–1320. doi: 10.1177/0301006617718715. [DOI] [PubMed] [Google Scholar]
- 81.Jao Keehn RJ, et al. Impaired downregulation of visual cortex during auditory processing is associated with autism symptomatology in children and adolescents with autism spectrum disorder. Autism Res. 2017;10:130–143. doi: 10.1002/aur.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freyberg J, Robertson CE, Baron-Cohen S. Typical magnitude and spatial extent of crowding in autism. J Vis. 2016;16:1–10. doi: 10.1167/16.5.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown AC, Crewther DP. Autistic children show a aurprising relationship between global visual perception, non-verbal intelligence and visual parvocellular function, not seen in typically developing children. Front Hum Neurosci. 2017;11:1–9. doi: 10.3389/fnhum.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zachi EC, et al. Color vision losses in autism spectrum disorders. Front Psychol. 2017;8:1–11. doi: 10.3389/fpsyg.2017.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wechsler, D. Wechsler Intelligence Scale for Children. (Pearson, 2003).
- 86.Wechsler. WAIS-IV: Wechsler Adult Intelligence Scale. (Pearson, 2008).
- 87.Lord, C., Rutter, M., DiLavore, P. C. & Risi, S. Autism diagnostic observation schedule-WPS edition. (Western Psychological Services, 1999).
- 88.Rutter, M., Le Couteur, A. & Lord, C. Autism Diagnostic Interview, Revised. (Western Psychological Services, 2003).
- 89.Rutter, M., Bailey, A. & Lord, C. The social communication questionnaire: Manual. (Western Psychological Services, 2003).
- 90.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hus V, Lord C. The autism diagnostic observation schedule, module 4: Revised algorithm and standardized severity scores. J Autism Dev Disord. 2014;44:1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- 93.Bogfjellmo L-G, Bex PJ, Falkenberg HK. The development of global motion discrimination in school aged children. J Vis. 2014;14:1–12. doi: 10.1167/14.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Navarro DJ, Griffiths TL, Steyvers M, Lee MD. Modeling individual differences using Dirichlet processes. J Math Psychol. 2006;50:101–122. doi: 10.1016/j.jmp.2005.11.006. [DOI] [Google Scholar]
- 95.Kruschke JK. Bayesian estimation supersedes the t test. J Exp Psychol Gen. 2012;142:573–603. doi: 10.1037/a0029146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.