Abstract
Social behaviours are frequently utilised for defence and stress avoidance in nature. Both Caenorhabditis elegans and Pristionchus pacificus nematodes display social behaviours including clumping and bordering, to avoid hyperoxic stress conditions. Additionally, both species show natural variation in social behaviours with “social” and “solitary” strains. While the single solitary C. elegans N2 strain has evolved under laboratory domestication due to a gain-of-function mutation in the neuropeptide receptor gene npr-1, P. pacificus solitary strains are commonplace and likely ancestral. P. pacificus therefore provides an opportunity to further our understanding of the mechanisms regulating these complex behaviours and how they evolved within an ecologically relevant system. Using CRISPR/Cas9 engineering, we show that Ppa-npr-1 has minimal influence on social behaviours, indicating independent evolutionary pathways compared to C. elegans. Furthermore, solitary P. pacificus strains show an unexpected locomotive response to hyperoxic conditions, suggesting a novel regulatory mechanism counteracting social behaviours. By utilising both forward and reverse genetic approaches we identified 10 genes of the intraflagellar transport machinery in ciliated neurons that are essential for this inhibition. Therefore, a novel cilia-mediated environmental input adds an additional level of complexity to the regulation of hyperoxia-induced social behaviours in P. pacificus, a mechanism unknown in C. elegans.
Introduction
Nematodes have a highly developed chemosensory system and are able to respond to a multitude of environmental cues. These cues can be associated with food, stress conditions and also other animals with whom they can have phoretic, necromenic or parasitic interactions1,2. Some of the behavioural responses to chemosensory cues are increasingly recognized to be associated with substantial natural variation, such as the intensely studied so-called “social feeding behaviours” clumping and bordering in Caenorhabditis elegans. Clumping is the aggregation of nematodes in feeding groups under laboratory conditions, which occurs in all wild isolates of C. elegans. Clumping correlates with bordering behaviour and indicates the preference of the worms for the border of the bacterial lawn over the centre. Both behaviours are found together in so-called “social strains”. In contrast, strains that do not exhibit clumping and bordering behaviours and instead display a solitary feeding behaviour have been called “solitary strains”; for example, the C. elegans laboratory reference strain N23,4.
The terms “social” and “solitary” provide a simple way to differentiate between clumping//bordering vs. non-clumping/bordering strains. However, these terms oversimplify the nature of these complex behaviours. It has been suggested that clumping and bordering behaviours are instigated to avoid the hyperoxic stress conditions induced by the 21% oxygen concentration [O2] present in the laboratory4, since both behaviours result in the nematodes being exposed to lower [O2]. Clumping lowers the [O2] due to increased consumption by the more tightly clustered nematodes5, while via bordering the nematodes are exposed to lower [O2] created by the boundary of the bacterial lawn being thicker than that of the centre and thus consuming more oxygen4. Therefore, “hyperoxia-avoidance behaviours” is a more accurate term for these behaviours. However, the original terms “social” and “solitary” will be utilised in this work for consistency with previous literature.
The solitary foraging behaviour of the N2 strain has evolved in the laboratory from ancestors adapted towards 21% [O2] avoidance in their natural habitats (soil, compost and rotten fruits)6,7. Specifically, this solitary foraging behaviour is the result of a gain-of-function mutation in the neuropeptide Y-like receptor encoded by the npr-1 gene, which creates a hyperactive neural circuit with pleiotropic effects on a wide variety of other life-history traits3,8–12. The two alleles of npr-1 in C. elegans differ at codon 215, with social strains encoding for the ancestral npr-1F (phenylalanine) allele and N2 encoding npr-1V (valine)3. The standard lab husbandry of C. elegans has strongly selected for the npr-1 215 V allele and the associated solitary feeding behaviour, which has been retained through clonal propagation during N2 maintenance7.
The nematode Pristionchus pacificus has been established as a model for evolutionary studies in comparison to C. elegans 13,14. In addition, the ecology and population genetics of P. pacificus have by now been well characterised and have contributed to its establishment as a model for integrative eco-evo-devo studies14. Further to this, many of its behaviours are now being dissected including its foraging behaviour as P. pacificus also shows natural variation in foraging behaviours similar to that observed in C. elegans. However, it is apparent that the origin of this natural variation is associated with differing evolutionary histories between the two species15. In contrast to C. elegans, solitary feeding behaviour is widespread among P. pacificus natural isolates (Fig. 1a) and the molecular phylogeny of P. pacificus strains suggests this pattern to be ancestral15. However, all strains of the phylogenetic clade B of P. pacificus display social behaviours under laboratory conditions15 (Fig. 1b). This clade is endemic to high-altitude locations (2100–2400 metres above mean sea level, hereafter m.a.s.l.) on La Réunion Island16 (Fig. 1c), characterized by an atmospheric oxygen partial pressure of around 16.4 kPa17. Since the social behaviours of clade B nematodes at 21% [O2] are suppressed when [O2] drops to 16% this may reflect a possible adaptation of this clade to high-altitude-associated hypobaric hypoxia found in the wild15 (Fig. 1d). Therefore, clade B strains may perform social behaviours to avoid hyperoxic conditions in the laboratory, but they behave also as solitary strains when the O2 levels are similar to those that they experience in the wild, suggesting that all P. pacificus strains behave solitary in natural conditions. These observations may be related to the necromenic association of P. pacificus and its connection to scarab beetle hosts, in which the arrested dauer stage of the nematode colonizes the insect and resumes development only after the death of the beetle by feeding on the proliferating microbes on the beetles’ carcass18. The need for the nematodes to search for a new host may have favoured the emergence of a mechanism that induces solitary foraging and ultimately favours dispersion.
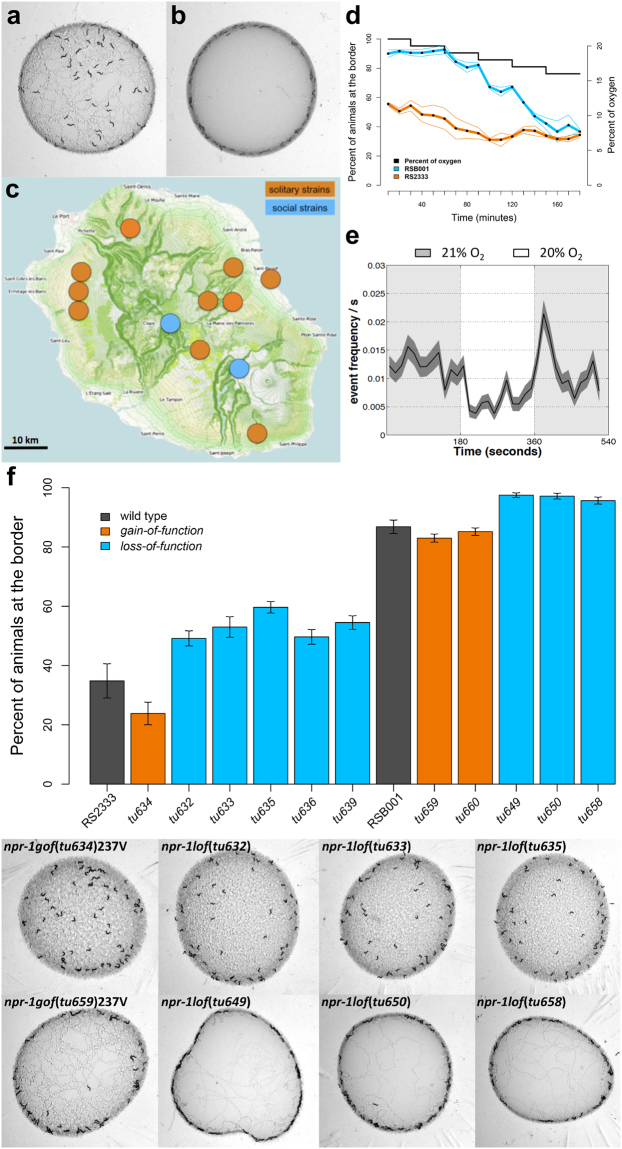
Figure 1.
Natural variation of social behaviours in P. pacificus independent of Ppa-npr-1. (a) Clumping/bordering assay for the P. pacificus reference strain RS2333, which shows a solitary behaviour under laboratory conditions, characterized by low levels of both clumping and bordering behaviours. (b) Clumping/bordering assay for the P. pacificus RSB001 strain, isolated at 2327 m.a.s.l. on La Réunion Island, which shows strong clumping and bordering behaviours under laboratory conditions. (c) Map of La Réunion Island showing the locations from which P. pacificus nematode strains have been isolated. Social strains, belonging to the phylogenetic lineage B, have been isolated from only two locations above 2000 m.a.s.l. (modified from OpenStreetMap® under the Open Database License (ODbL), © OpenStreetMap contributors (https://www.openstreetmap.org/copyright)). (d) Regulation of bordering behaviour by oxygen levels: comparison of the dynamic changes in bordering behaviour between RSB001 and RS2333 strains during oxygen shifts from 21% to 16%, with a 1% decrement occurring every 30 min (modified from Moreno et al.15). Black dots represent the mean and thin lines represent the standard error of the mean (SEM). (e) Ω-turn rate response of RS2333 to 21% −>20% −>21% oxygen shifts on a lawn of Escherichia coli OP5015. The black line represents the mean Ω-turn and the grey area, the SEM. (f) Bordering behaviour of RS2333, RSB001 and the Ppa-npr-1 mutants generated using CRISPR/Cas9 system (Suppl. Table S1). In all bar-plots arrows represent the standard error of the mean (SEM). For statistical analysis see Suppl. Table S2.
Previous QTL analyses indicated that the genetic architecture of social behaviours in P. pacificus is more complex than in C. elegans, with at least three major QTLs involved, none of which are associated with the P. pacificus npr-1 locus15. Additionally, both the solitary RS2333 and the social RSB001 strains encode the npr-1F allele at codon 237, corresponding to the npr-1F allele at codon 215 present in social C. elegans strains15. These observations suggested that npr-1 is not a major contributor to social behaviours in P. pacificus, while a smaller contribution of this gene cannot be ruled out. Here, we disentangled the contribution of npr-1 to the regulation of the social behaviours in P. pacificus by producing both gain-of-function (gof) and loss-of-function (lof) mutations using the CRISPR/Cas9 system recently established for P. pacificus 19.
In addition to clumping and bordering, nematode locomotive behaviours are likewise influenced by environmental oxygen. Hyperoxic stress conditions increase the rate of reorientation movements (omega (Ω) turns) while crawling. The variation in the rate of Ω-turns in response to small shifts from 20% to 21% in [O2] correlates with the social behaviours in C. elegans. For instance, the social C. elegans strain CB4856 increases the rate of Ω-turns when [O2] shifts from 20% to 21%, whereas the solitary N2 strain does not modify its Ω-turn rate in response to these shifts7,20. This variation reflects a different neuronal sensitivity to [O2], dependent on the globin encoding gbl-5 gene. The glb-5 N2 variant has also arisen under laboratory conditions and has been spread during N2 maintenance on agar plates7.
On the contrary, P. pacificus RS2333 shows a surprising and unexpected locomotive response to [O2] shifts from 20% to 21%, indicating that nematodes of this strain perceive 21% [O2] as hyperoxic stress but do not avoid it by performing social behaviours15 (Fig. 1e). Here we show that a similar locomotive response is also observed among solitary strains from La Réunion Island, indicating that this behaviour is not specific to RS2333. It has therefore not been acquired due to any adaptation to laboratory conditions and is a common feature found among solitary P. pacificus strains. These findings suggest that solitary strains in P. pacificus have acquired a regulatory mechanism that inhibits hyperoxia-avoidance by social feeding. We tested this hypothesis by performing a mutagenesis screen on RS2333, through which we isolated 15 social mutants. By means of whole-genome sequencing and complementation tests we identified four genes encoding intraflagellar transport (IFT) proteins, all of which are involved in the structure and function of neuronal sensory cilia. We subsequently produced mutants in six additional cilia-related IFT genes using the CRIPSR/Cas9 system. The social behaviours displayed by these mutants are regulated by oxygen, similar to the clade B strain and wild type C. elegans strains. Our results indicate that P. pacificus solitary strains have acquired a novel regulatory mechanism, dependent on environmental sensing through sensory cilia, which prevents social behaviours in response to hyperoxic conditions. Ultimately, these results reveal an additional level of complexity in the regulation of the hyperoxia-induced social behaviours in P. pacificus in comparison with C. elegans.
Results
Ppa-npr-1 does not play a major role in natural variation of bordering behaviour
Our previous study on the genetic architecture of social behaviours in P. pacificus did not provide any evidence for a major contribution of Ppa-npr-1 to these behaviours although a smaller contribution cannot be ruled out15. To investigate the role of Ppa-npr-1 in the regulation of the social behaviours in P. pacificus further, we generated a set of gof and lof mutants in both, the RS2333 and RSB001 strains, using the CRISPR/Cas9 system (Suppl. Table 1 and Suppl. Fig. S1). sgRNAs were designed to target the first and the sixth exons of the gene to create lof mutations (Suppl. Fig. S1a,b,e). In addition, the gof mutation that is found in C. elegans N2 was induced in both P. pacificus strains by co-injection of the sgRNA for exon 6 with a repair template oligonucleotide that recapitulates the change of phenylalanine to valine (Suppl. Table 1 and Suppl. Fig. S1c,d). The social behaviours of these mutants were evaluated by the clumping and bordering assay previously established for P. pacificus 15. Since both behaviours are correlated in P. pacificus, we focused on bordering behaviour as an indicator of the social/solitary character of the strains and mutants along this study.
In the solitary P. pacificus RS2333 strain, the gof mutation Ppa-npr-1(tu634)RS2333 reduced bordering behaviour even further, although the comparison with the wild type phenotype was not statistically significant (Fig. 1f and Supp. Table S2). More strikingly, a similar gof mutation produced in the social RSB001 background Ppa-npr-1(tu659)RSB001 did not induce any observable change in social behaviour (Fig. 1f and Suppl. Table S2). These results indicate that this amino acid substitution either does not induce a solitary phenotype in P. pacificus, or has only a minor effect on bordering behaviour.
If Ppa-NPR-1 was responsible for the solitary behaviour of P. pacificus RS2333, Ppa-npr-1 lof mutants produced in this strain should show social behaviour under laboratory conditions. Although we observed a slight increase in the levels of bordering behaviour in five lof alleles of RS2333, these levels were still much lower than those typical of social strains (Fig. 1f and Suppl. Table S2). Similarly, an increase in bordering behaviour was observed in three Ppa-npr-1 lof alleles generated in the RSB001 background (Fig. 1f and Suppl. Table S2). These results are consistent with Ppa-npr-1 playing only a minor role in the modulation of bordering behaviour in P. pacificus. Importantly, these findings indicate that Ppa-npr-1 is not responsible for the natural variation observed among wild isolates.
Hyperoxic stress avoidance is not a result of laboratory domestication in P. pacificus
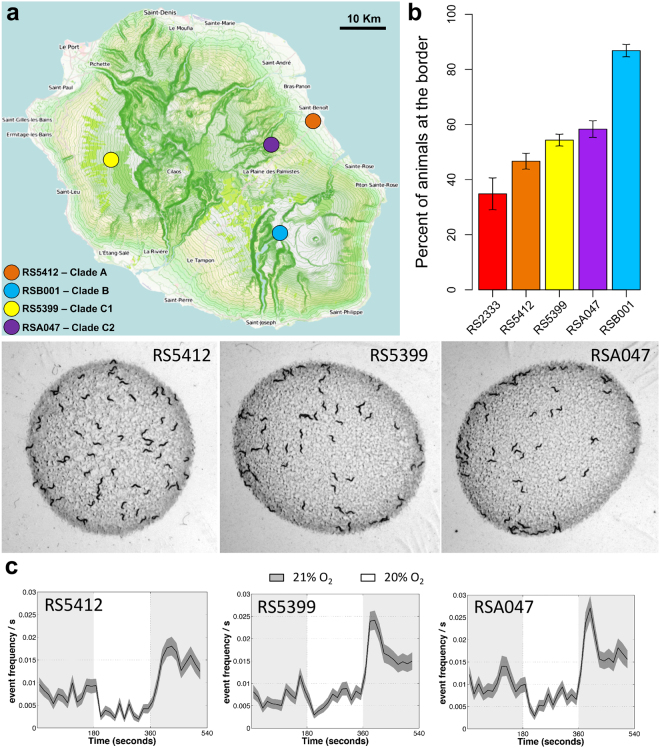
Our previous study revealed an unexpected locomotive response to [O2] shifts from 20% to 21% in RS233315. We therefore, tested whether a similar response is conserved among other solitary strains of P. pacificus, focusing on three selected strains isolated from different locations on La Réunion Island, which belong to different phylogenetic clades15,16 (Fig. 2a,b). Similarly to RS2333, these strains also modulate their omega-turn rate in response to [O2] (Fig. 2c and Suppl. Table S3). These findings indicate that the locomotive response to hyperoxic stress conditions as seen in P. pacificus RS2333 has not been a result of domestication in the approximately 3,000 generations this strain is cultured under laboratory conditions, but rather represents a general feature of solitary P. pacificus strains under natural conditions.
Figure 2.
Omega-turn locomotive response in solitary P. pacificus strains. (a) Locations on La Réunion Island from which the selected solitary strains have been isolated (modified from OpenStreetMap® under the Open Database License (ODbL), © OpenStreetMap contributors (https://www.openstreetmap.org/copyright)). (b) Bordering behaviour of selected P. pacificus solitary strains. (c) Ω-turn rate response to 21% −>20% −>21% oxygen shifts on a lawn of E. coli OP50 in selected P. pacificus solitary strains. In all graphs, the black line represents the mean Ω-turn and the grey area, the S.E.M. For statistical analysis see Suppl. Table S3.
Multiple genes control the inhibition of social phenotype in RS2333
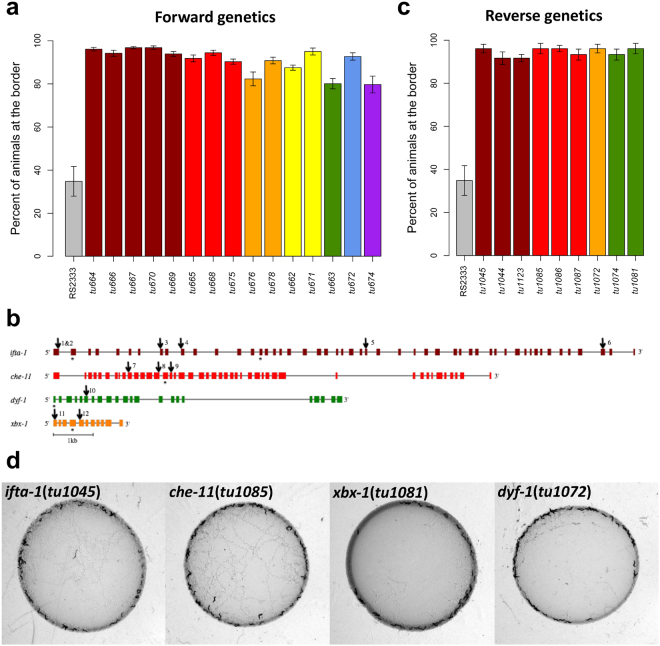
To explore the potential existence of alternative regulatory mechanisms in P. pacificus solitary strains, which block the social behaviours even under hyperoxic conditions, we performed a mutagenesis screen in the RS2333 background searching for social mutants. From a screen of approximately 2,250 gametes we isolated 15 mutants with social behaviours, indicating a mutation frequency of approximately 1 in 150 gametes for this phenotype. Mutant lines were characterised according to the strength of the social phenotype and complementation tests were performed by pairwise crosses, which allowed the identification of seven complementation groups (Fig. 3a and Suppl. Table S4). Thus, social behaviours can be readily induced by mutations in many complementation groups, although its occurrence in nature is restricted to wild isolates from a single phylogenetic lineage on La Réunion Island.
Figure 3.
Social behaviours induced by mutations in the Ppa-ifta-1, Ppa-che-11, Ppa-dyf-1 and Ppa-xbx-1 genes. (a) Bordering behaviour of RS2333 and the 15 social mutants isolated in forward genetic screens by EMS mutagenesis. Colours indicate complementation groups. For statistical analysis see Suppl. Table S4 and for more information about the mutant alleles see Suppl. Table S5. (b) Gene structure of the Ppa-ifta-1, Ppa-che-11, Ppa-dyf-1 and Ppa-xbx-1 genes. Colour boxes indicate exons and solid lines indicate introns. Arrows indicate genetic lesions generated by EMS mutagenesis in mutant alleles (see Suppl. Table S5). Asterisks indicate sgRNA positions. (c) Bordering behaviour of RS2333 and mutant alleles of Ppa-ifta-1, Ppa-dyf-1, Ppa-che-11 and Ppa-xbx-1 produced using CRISPR/Cas9 system (Suppl. Table S6). Colours correspond with genes in Fig. 2b and complementation groups in Fig. 2a. In all bar-plots arrows represent the standard error of the mean (SEM). For statistical analysis see Suppl. Table S7. (D) Clumping/bordering assay for representatives of Ppa-ifta-1, Ppa-dyf-1, Ppa-che-11 and Ppa-xbx-1 mutant alleles.
Mutations in genes encoding intraflagellar transport proteins cause social behaviours
To identify causative mutations, we sequenced the genomes of all mutant lines using an Illumina platform. We used bioinformatic procedures to search for genes sharing SNPs in coding regions according to the complementation groups previously established. This methodology allowed us to identify candidate genes in three complementation groups: i) tu664, tu666, tu667, tu669 and tu670 shared mutations in the coding region of the gene contig6-snap.265; ii) tu665, tu668 and tu675 shared mutations in coding region of contig31-snap.23; and iii) tu676 and tu678 share mutations in coding region of contig56-snap.118 (Suppl. Table S5). However, we could not find a common mutation in the mutant alleles of the fourth complementation group (tu662 and tu671), probably due to assembly errors in our reference genome.
Based on combination of automated and manual orthology prediction methods, we found that Contig6-snap.265 corresponds to Ppa-ifta-1, which encodes for an evolutionary conserved protein required for retrograde intraflagellar transport (IFT) along ciliary axonemes21. IFT refers to the movement of protein complexes called IFT-trains along the axonemes that constitute the cilia of all eukaryotic cells, an essential process for the proper assembly of the cilia and its function in motility, sensing and signalling22–24. In C. elegans IFTA-1 is associated with the IFT-A sub-complex, which consists of at least six proteins including CHE-1121. Indeed, the second gene we identified, contig31-snap.23, is the P. pacificus ortholog of che-11. In C. elegans, CHE-11 is required for the proper incorporation of IFTA-1 into the IFT machinery assembly21. Finally, the third identified gene, contig56-snap.118, corresponds to Ppa-xbx-1, which encodes for the light intermediate dynein chain equally required for retrograde IFT in C. elegans 25.
Next, we used a reverse genetic approach by CRISPR/Cas9 engineering to obtain additional alleles in Ppa-ifta-1, Ppa-che-11 and Ppa-xbx-1 (Fig. 3b, Suppl. Table S6 and Suppl. Fig. S2). In total, we generated seven mutant lines in the RS2333 background, all of which showed very strong social behaviours (Fig. 3c,d and Suppl. Table S7). Finally, we performed complementation tests between the original mutants isolated in the mutagenesis screen and the CRIPSR/Cas9-generated mutants. The F1 progeny derived from these crosses failed to rescue the original solitary phenotype, confirming that the mutations in these genes are responsible for the social behaviour. In summary, we found that the three genes encoding IFT proteins Ppa-ifta-1, Ppa-che-11 and Ppa-xbx-1, result in strong social behavioural phenotypes, suggesting a role for sensory cilia in the regulatory mechanisms that prevent social phenotypes in P. pacificus RS2333.
A single allele of Ppa-dyf-1 shows similar social behavioural phenotypes
Next, we searched among the list of candidate mutations in the three remaining mutant alleles tu663, tu772 and tu674, looking for other candidate genes involved in IFT. Indeed, for tu663 we found a mutation in the gene contig10-snap.384 (Suppl. Table S5), the P. pacificus ortholog of dyf-1. dyf-1 encodes an evolutionary conserved protein required for docking the kinesin OSM-3 onto anterograde IFT transport particles26. To test whether the mutation in Ppa-dyf-1 is the causative mutation of the social phenotype of tu663, we generated two additional alleles using the CRISPR/Cas9 system in RS2333, tu1074 and tu1081 (Fig. 3b, Suppl. Table S6 and Fig. S2), which also showed very strong social behaviours (Fig. 3c,d and Suppl. Table S7). In addition, complementation tests between these alleles and tu663 confirmed that the mutation in Ppa-dyf-1 is responsible for the social phenotype of tu663. Taken together, our forward genetic screen for mutants with social phenotypes in the solitary RS2333 background has resulted in the isolation of 15 mutants in seven complementation groups, four of which encode for IFT proteins.
Mutations in genes encoding other IFT proteins also cause social phenotypes in P. pacificus
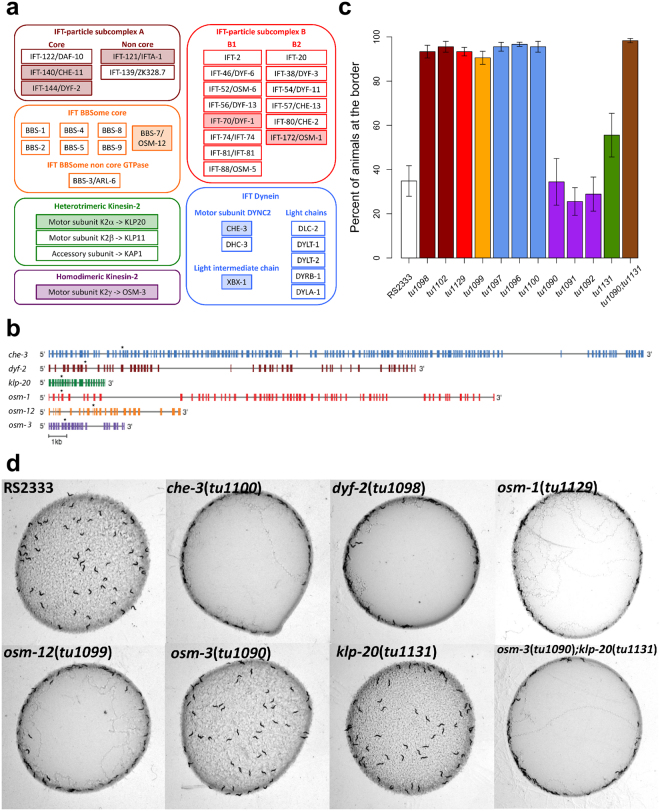
IFT requires the coordinated action of six multi-protein sub-complexes: the IFT-sub-complex A and B, the BBSome, the homodimeric and heterotrimeric kinesin motors and the dynein motors22,23. To test whether other IFT proteins are also involved in the inhibition of social behaviours in RS2333, we identified the P. pacificus orthologs of all IFT related genes (see methods) (Fig. 4a). Next, we employed a candidate gene approach by selecting one representative of each of the six multi-protein sub-complexes to produce knock-out mutants using the CRISPR/Cas9 system: dyf-2 and osm-1 from the IFT-A and IFT-B sub-complexes, osm-12 from the BBSome, the heavy chain che-3 component of the IFT dynein motor, the klp-20 component from the heterotrimeric kinesin-II motor and the homodimeric OSM-3-kinesin motor (Fig. 4b and Suppl. Fig. S3).
Figure 4.
Social behaviours induced by mutations in other genes encoding intraflagellar transport proteins. (a) Orthologs of IFT-related genes in P. pacificus. Colour-filled boxes indicate genes for which mutant alleles have been produced using CRISPR/Cas9 system in this study. (b) Gene structure of the Ppa-che-3, Ppa-dyf-2, Ppa-osm-1, Ppa-osm-3, Ppa-osm-12 and Ppa-klp-20 genes. Colour boxes indicate exons and solid lines indicate introns. Asterisks indicate sgRNA positions. (c) Bordering behaviour of RS2333, mutant alleles of Ppa-che-3, Ppa-dyf-2, Ppa-osm-1, Ppa-osm-3, Ppa-osm-12 and Ppa-klp-20 produced using CRISPR/Cas9 system (Suppl. Table S8), plus Ppa-osm-3(tu1090); Ppa-klp-20(tu1131) double mutant. Colours correspond to genes in Fig. 4b and functional groups in Fig. 4a. In all bar-plots arrows represent the standard error of the mean (SEM). For statistical analysis see Suppl. Table S9. (d) Clumping/bordering assay for representatives of Ppa-che-3, Ppa-dyf-2, Ppa-osm-1, Ppa-osm-3, Ppa-osm-12 and Ppa-klp-20 mutant alleles and the Ppa-osm-3(tu1090); Ppa-klp-20(tu1131) double mutant.
We were able to isolate between one and three alleles per targeted gene (Suppl. Table S8). Mutants in Ppa-dyf-2, Ppa-osm-1, Ppa-osm-12 and Ppa-che-3 showed social phenotypes (Fig. 4c,d and Suppl. Table S9), indicating that proteins of the IFT-A, IFT-B, the BBSome and the IFT dynein motor sub-complexes are indeed involved in the inhibition of social feeding in P. pacificus RS2333. These findings also indicate that our original mutagenesis screen was far from saturation.
In contrast to the CRISPR/Cas9-induced mutants in Ppa-dyf-2, Ppa-osm-1, Ppa-osm-12 and Ppa-che-3, mutants of each kinesin motor holoenzymes remained solitary. Specifically, all three alleles in Ppa-osm-3 and the single Ppa-klp-20(tu1131) mutant showed a normal solitary phenotype (Fig. 4c,d and Suppl. Table S9). Two possible scenarios might explain these findings; either, kinesin motors are not involved in the inhibition of the social behaviours, or alternatively, there might be functional redundancy between the heterotrimeric kinesin-II and the homodimeric OSM-3-kinesin motors as previously described for C. elegans 27. To distinguish between these scenarios, we generated a kinesin motor double mutant and indeed, Ppa-osm-3(tu1090); Ppa-klp-20(tu1131) double mutant animals showed strong social behaviours (Fig. 4c,d and Suppl. Table S9). These findings suggest that kinesin motors are involved in the inhibition of social behaviours in the wild type RS2333 strains and that the kinesin motors function redundantly in P. pacificus.
IFT mutants in P. pacificus are defective for chemosensation and dye-filling of amphid neurons
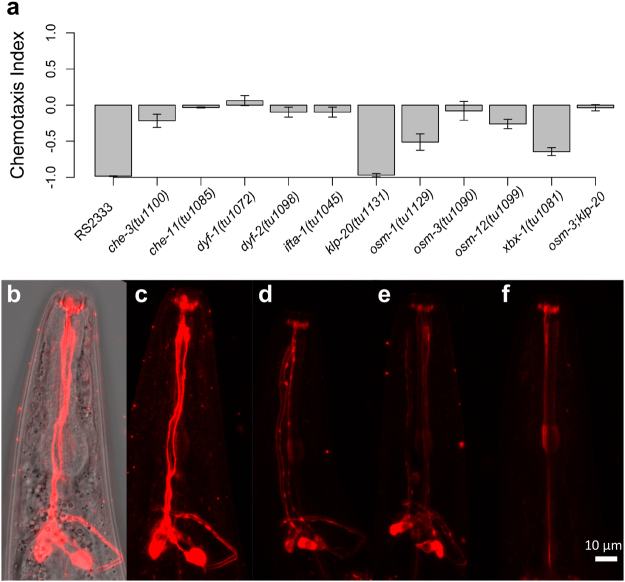
In C. elegans IFT mutants were characterized by performing dye-filling assays for the staining of ciliated neurons, chemotaxis and osmotic avoidance behaviours28. To study whether IFT mutants in P. pacificus show abnormal chemotaxis behaviour we assayed repulsion from 1-Octanol, a chemical known to induce strong avoidance in P. pacificus wild type animals29 (Fig. 5a and Suppl. Table S10). Indeed, the chemotaxis index was close to 0 for most of the IFT mutants, indicating that they are unable to sense 1-Octanol. However, Ppa-osm-1(tu1129) and Ppa-xbx-1(tu1081) mutants showed only a reduction in the avoidance behaviour, while Ppa-klp-20(tu1131) mutants appeared wild type. The difference in the repulsion behaviour between Ppa-osm-3(tu1090) and Ppa-klp-20(tu1131) mutants could indicate that the receptor triggering this behaviour is located at the distal segment of the cilia, since in C. elegans only the homodimeric OSM-3-kinesin motor carries the IFT particles to the distal cilia tip27.
Figure 5.
Chemosensation and dye-filling defects in P. pacificus IFT mutants. (a) 1-Octanol avoidance behaviour of RS2333 and the IFT-related mutant alleles generated in this study. For statistical analysis see Suppl. Table S10. (b) Overlay of bright field and fluorescent images showing dye-filling staining of amphid neurons in a RS2333 adult individual. (c) Dye-filling staining of amphid neurons in a RS2333 adult. (d) Dye-filling staining of amphid neurons in an osm-3(tu1090) adult. (e) Dye-filling staining of amphid neurons in a Ppa-klp-20(tu1131) adult. (f) Dye-filling defective staining of amphid neurons in an Ppa-osm-3(tu1090); Ppa-klp-20(tu1131) double mutant.
Absence of aversion by 1-Octanol in IFT mutants in addition to the previously described social phenotype supports the conserved function of the studied IFT proteins in ciliogenesis. To detect crude morphological changes in ciliated neurons in IFT mutants, we used a dye-filling technique, whereby nematodes were incubated with the lipophilic dye DiI, which enters amphids and other sensory neurons if they are fully formed and open to the outside30. In the wild-type RS2333 strain, we observed intense staining of the neurons corresponding to amphid neurons in C. elegans also known to be stained by DiI (Fig. 5b,c). Strikingly, staining was absent in most of the mutants examined. The only exceptions were Ppa-osm-3(tu1090) and Ppa-klp-20(tu1131) (Fig. 5d,e), however, the double mutant was also dye-filling defective, similar to all of the other IFT mutants (Fig. 5f). The absence of dye-filling is consistent with the expectation of abnormal morphogenesis of ciliated neurons regulated by IFT genes. This conclusion is further supported by strong correlation of the dye-filling pattern and the social phenotypes of the mutant strains.
P. pacificus IFT mutants modulate clumping and bordering and locomotive behaviours in response to environmental oxygen levels
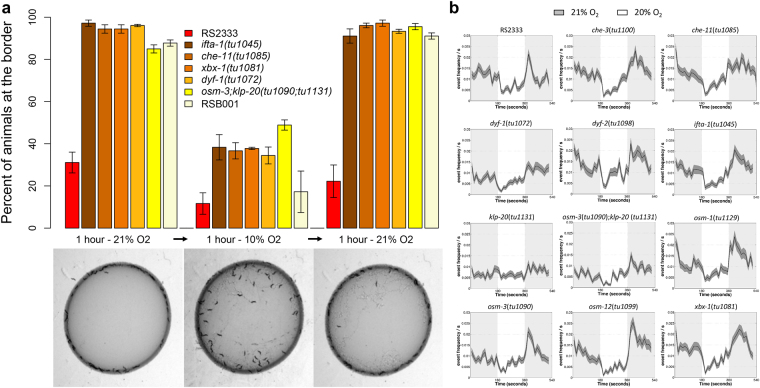
Finally, we tested whether the social behaviours of IFT mutants were still regulated by [O2] using an aerotaxis chamber (see methods). In all mutant lines tested, the social behaviour showed the following dynamics: 10% [O2] induced a strong inhibition of bordering from 90–100% to 35–40%, while the increase to 21% [O2] induced bordering close to 100% (Fig. 6a, Suppl. Table S11a,b). These findings indicate that the social behaviour observed in the IFT mutants are established with the intent to avoid hyperoxic conditions, since a significant decrease in the [O2] produced a strong reduction in bordering. Since these mutant lines are most probably defective in sensory-cilia related processes, our experiments suggest that environmental inputs sensed through cilia are required in the solitary P. pacificus RS2333 strain to counteract hyperoxic stress conditions, resulting in the inhibition of clumping and bordering. In addition, the bordering behaviour of RS2333 fluctuated from 20–30% at 21% [O2] to 12% at 10% [O2]. This reduction is equivalent in magnitude to what we observed in the IFT mutants, which suggests that [O2] affects both wild-type and IFT mutant strains equivalently (Fig. 6a and Suppl. Table S11a,b), likely indicating parallel regulatory inputs consisting of the low [O2] and cilia-mediated mechanism contributing to the inhibition of social behaviours in RS2333.
Figure 6.
Hyperoxia avoidance behaviours in P. pacificus IFT mutants. (a) Regulation of bordering behaviour by oxygen levels in RS2333, RSB001 and representatives of Ppa-ifta-1, Ppa-che-11, Ppa-dyf-1 and Ppa-xbx-1 mutants. [O2] shifts every hour from 21% to 10% to 21%. Three replicates were performed for each strain/mutant. Bar-plots arrows represent the standard error of the mean (SEM). For statistical analysis see Suppl. Table S11. An example of the influence of oxygen in the dynamic of clumping/bordering behaviours for tu1072 is shown under the bar-plot. (b) Ω-turn rate response to 21% −>20% −>21% [O2] shifts on a lawn of E. coli OP50 of RS2333 and all cilia-related mutants generated using CRISPR/Cas9 system in this study. In all graphs, the black line represents the mean Ω-turn and the grey area, the S.E.M. For statistical analysis see Suppl. Table S12.
Furthermore, we tested the ability of the mutants to respond to small shifts from 20% to 21% [O2] by increasing their rate of Ω-turns. We observed that all IFT mutants were responsive to [O2] as predicted (Fig. 6b and Suppl. Table S12), although the rate of Ω-turns varies among them. Specifically, for the Ppa-dyf-1(tu1072), Ppa-klp-20(tu1131) and the Ppa-osm-3; Ppa-klp-20 double mutants the difference in the Ω-turn rate was lower in comparison to wild type (Fig. 6b and Suppl. Table S12). These findings indicate that both the klp-20 and dyf-1 genes may play a role in the regulation of the Ω-turn rate in response to shifts in [O2]. However, the reduction of the locomotive response in these mutants seems not to affect the regulation of its social behaviour by [O2]. Indeed, the social behavior of the Ppa-osm-3; Ppa-klp-20 double mutant was strongly suppressed by hypoxia (Fig. 6a, Suppl. Table S11a,b), in spite of its weak Ω-turn response to [O2] shifts.
Discussion
Social behaviour in animals is frequently employed for stress avoidance and also as a means of defence. Examples of social cooperation to build shelters are found among both vertebrates and invertebrates, e.g. beehives, rodent burrows and termite nests31. In addition, living in groups offers passive or active protection from predators as passive collective defence involves surrounding themselves with others32, while active involves adopting radial defensive formation, which has been observed among insects33 and vertebrates34. Aggregation is likewise employed to gain protection from environmental stress, especially in thermoregulation, which has been observed among mammals, reptiles and insects35–37.
Among nematodes, aggregation is addressed to avoid another kind of stress: hyperoxia. Wild type isolates of C. elegans perform a clumping behaviour under laboratory conditions in order to escape from the atmospheric 21% [O2]3,5. In addition, they aggregate at the border of the bacterial lawn, which is thicker and consumes more O2 than the centre, to further reduce the [O2] to which they are exposed4. This hyperoxia-avoidance behaviour is probably related to the adaptation of C. elegans to the hypoxic natural habitats where it is usually found, i.e. compost heaps and rotten fruits6. In these environments avoidance of 21% [O2] would be beneficial in order to escape desiccation by surface exposure, while additionally enabling their accumulation on bacterial food sources38. On the contrary, most of the P. pacificus nematode wild isolates show a solitary foraging behaviour under laboratory conditions, which indicates that unlike C. elegans, solitary foraging in P. pacificus is ancestral, and it does not constitute a lab-derived artefact related to domestication15.
Surprisingly P. pacificus solitary strains show a strong Ω-turn response to shifts from 20% to 21% in [O2], which indicates that these strains are able to perceive 21% [O2] as hyperoxic stress, but nonetheless, they do not try to escape the hyperoxic stress conditions by performing clumping and bordering. In this work, we have demonstrated the existence of a novel regulatory mechanism in the solitary strain RS2333, which blocks the social behaviours even under hyperoxic stress conditions. By means of both forward and reverse mutagenesis, we have produced social mutants from RS2333, which are defective in IFT genes, essential for the proper assembly of the cilia and its function in sensing and signalling24. Therefore, these experiments provide strong evidence for the existence of a regulatory pathway triggered by an environmental signal, which must be recognised by ciliated neurons and in turn leads to the inhibition of the social behaviours (Fig. 7a). On the contrary, in IFT defective mutants this regulatory pathway acting though the cilia is impeded, and as a consequence, the inhibition of social behaviours is abrogated allowing the nematodes to escape hyperoxic stress conditions by means of clumping and bordering (Fig. 7b).
Figure 7.
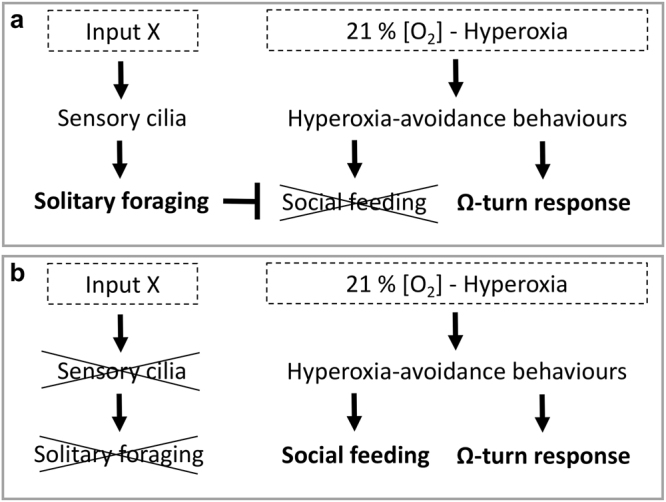
Model for the regulation of hyperoxia-avoidance behaviours (social feeding and Ω-turn rate) by oxygen and the input integrated through sensory-cilia. (a) In wild type solitary strains, hyperoxic conditions increase the Ω-turn rate while crawling. However, the hyperoxia-avoidance by social behaviour is suppressed by the input X, which is recognised by sensory cilia. (b) In cilia-defective mutants the input X does not inhibit hyperoxia-avoidance by social behaviour in response to hyperoxic conditions.
Our current hypothesis to explain the evolution of this mechanism proposes that it arose as a consequence of the ecological association of P. pacificus with beetle hosts. While in C. elegans social feeding may be advantageous in the wild in order to remain in contact with patches of bacterial food sources, the need for P. pacificus nematodes developing on the carcass of the previous beetle host to find a new host may have favoured the emergence of a mechanism that induces solitary foraging and ultimately favours dispersion. However, the nature of the environmental cue that triggers the solitary foraging behaviour remains unknown and it will be explored in future studies. Our observation of a strong correlation between social behaviours and dye-filling staining in amphid neurons in the IFT defective P. pacificus mutants, indicates that this putative environmental trigger is probably sensed by the amphid neurons. Future work will take advantage of the well-known synaptic connectivity of P. pacificus 39 to analyse the role of the amphid neurons in this inhibitory mechanism by neural ablation or induced apoptosis by reporter system methodologies.
In C. elegans, IFT defective mutants show abnormal chemotaxis behaviours as well as morphological defects in cilia28. Yet, to our knowledge, social behaviours have not been reported in C. elegans IFT defective mutants, indicating that the inhibitory mechanism of the social behaviours suggested for P. pacificus is absent in C. elegans. However, it has been reported that noxious stimuli from bacterial food sources sensed through the ASH and ADL amphid neurons induce aggregation in C. elegans npr-1(null) mutants. This mechanism requires the TRP-related transduction channels ocr-2 and osm-9, as well as odr-4 and odr-8, which localise sensory chemoreceptors to cilia40. Specifically, the solitary behaviour of npr-1; ocr-2 and npr-1; odr-4 double mutants was suppressed in triple mutants including osm-3, indicating that inputs from neurons that express osm-3 inhibit aggregation40. This inhibitory mechanism is fundamentally different from the one described here for P. pacificus since the latter constitutes the main mechanism suppressing clumping and bordering in natural strains while the former was evident only when osm-3 plus ocr-2 or odr-4 were knocked-out on a npr-1(null) background, whereas osm-3 single mutants show no social phenotype.
In conclusion, our study reveals a new additional level of complexity in the regulation of the hyperoxia-induced social behaviours in P. pacificus in comparison with C. elegans. In addition, it demonstrates the need to work on animal models with a well-known ecology in order to disentangle biological questions related to evolution and adaptation in the wild.
Methods
Strains
Three P. pacificus strains were used in this study: the reference strain PS312 (belonging to the phylogenetic lineage A and isolated from Pasadena, CA (USA) in 198813), the RS2333 strain (a laboratory derivative of the original strain PS312) and the RSB001 strain (belonging to the phylogenetic lineage B and isolated from a location at 2327 m.a.s.l. on La Réunion Island15,16). Strains were maintained at 20 °C using standard methods41.
Behavioural Assays
The assay for quantification of bordering and clumping behaviours in C. elegans 3 was modified for P. pacificus as previously indicated15. The regulation of bordering behaviour by oxygen was analysed by performing the above assay in a custom-fabricated Plexiglas chamber20 as previously indicated15 with three replicates completed per strain/mutant. Nematodes where exposed to shifting [O2] levels from 21% to 10% and back to 21% after one hour intervals. Oxygen-evoked turning responses were monitored as described previously15,20,42,43 with ten replicates completed per strain/mutant. Oxygen concentration was changed every three min. Ω-turn rate values were calculated in 15 sec windows. The means of Ω-turns during the 90 sec before and after the oxygen shift were calculated for each replicate. The chemotaxis assay to quantify 1-Octanol avoidance behaviour was modified from Hong et al.44 as follows: For each strain/mutant, nematode cultures were synchronized by transferring ten gravid hermaphrodites onto 6-cm NGM plates seeded with Escherichia coli OP50. Ten plates were prepared per strain/mutant, which were then incubated at 20 °C for five days. Nematodes were then washed with M9 buffer and filtered with a 5 µm nylon membrane filter (Merck Millipore, Billerica, MA USA) to remove the bacteria. The animals were loaded onto 8.5-cm NGM plates between the test compound (1 µl of 100% 1-Octanol (Sigma-Aldrich Co., St. Louis, MO USA)) and the control compound (1 µl of 100% Ethanol (Merck Millipore)), located at opposed ends of the plates. Plates were incubated at 20 °C for 12 hours. Animals within 2 cm radius-circle around each odour source were counted. Three replicates for each strain/mutant were carried out and the differences in chemotaxis index between the reference strain RS2333 and each mutant allele were calculated as in Hong et al.44 by means of the two-sample equal variance Student’s t test.
Mutant screen
We screened for bordering/clumping mutant strains using Ethyl methanesulfonate (EMS) mutagenesis41 in P. pacificus RS2333. We screened approximately 2,250 gametes (4,500 homozygous F2 lines) in three mutagenic screens over a six-month period. The screens were performed by placing single F2s in a 50 µl OP50 lawn. Then the bordering behaviour was screened on the F3 animals. Candidate mutants were confirmed by proper bordering/clumping assays as described before.
CRISPR/Cas9 mutagenesis
P. pacificus strains PS312, RS2333 and RSB001 were used for generating mutants using CRISPR/Cas9 system45 following the protocol of Witte et al.19. For most of the genes, sgRNAs were designed to target sequence regions conserved between P. pacificus and C. elegans, as identified in amino acid sequence alignments produced using Mafft version 746. sgRNAs were synthesized by ToolGen Inc. (Seoul, Korea) and Integrated DNA Technologies Inc. (Coralville, Iowa USA). We used Cas9 protein produced by ToolGen Inc. and New England BioLabs Inc. (Ipswich, MA USA). Injection Master Mix was prepared following the manufacturer instructions from Alt-RTM CRISPR-Cas9 System User guide from Integrated DNA Technologies Inc. Injections were performed on a Zeiss Axiovert microscope (Zeiss, Germany) coupled to an Eppendorf TransferMan micromanipualtor and Eppendorf FemtoJet injector (Eppendorf AG., Hamburg, Germany). Injected mothers were kept individually on NGM plates for 16 hours. Around 100 F1 progeny were transferred onto NGM plates before they became adults (one individual per plate). Once they have laid eggs, F1 individuals were lysed and assayed for the presence of a molecular lesion around the sgRNA target site. For npr-1 and ifta-1 genes this was performed by PCR and subsequent Sanger sequencing. For the rest of the genes, high resolution melting was performed using LightCycler 480 High Resolution Melting Master (Roche Diagnostics Ltd., Burgess Hill, England) and the identified mutant candidates were confirmed by Sanger sequencing. Primers for detecting gene lesions are listed in Suppl. Table S13.
Genetics
To analyse the dominant/recessive character of social feeding behaviours of the mutant alleles, we performed the bordering/clumping assays on heterozygotes made by crossing males of each mutant strain, with hermaphrodites of the dumpy (pdl-1) mutant version of RS2333. After backcrossed with the RS2333 pdl-1 mutant, mutant strains showing both social and dumpy phenotypes were isolated for further complementation tests, which were performed by crossing males of each mutant strain, with hermaphrodites of each backcrossed mutant strain.
Whole genome re-sequencing of mutant strains
Genomic DNA was prepared for all mutant strains following the protocol outlined in Rödelsperger et al.47. DNA was extracted from pooled individuals of each isogenic line using the GenEluteTM Mammalian Genomic DNA Miniprep Kits (Sigma-Aldrich Co., St. Louis, MO USA) and genomic DNAs were quantified with the Qubit® dsDNA BR Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA USA). Genomic libraries were generated using the TruSeq Nano DNA Library Prep Kit from Illumina (Illumina Inc., California, United States). DNA was sheared to 350 bp using the Covaris S2 System (Covaris Ltd., Woodingdean Brighton, United Kingdom) and end repair, adenylation, and adaptor ligation were performed following the kit protocol. After PCR amplification, libraries were validated on an Agilent Bioanalyzer DNA 1,000 chip (Agilent Technologies GmbH, Waldbronn, Germany) and pooled before sequencing on an Illumina HiSeq. 3000 platform.
Identification of mutant alleles from whole-genome sequencing data
Raw Illumina reads were aligned to the P. pacificus genome (version Hybrid1) and candidate variants were called, filtered, and classified as described in Rae et al.48. This yielded between 400–600 substitutions per mutant line, for which roughly 10% were located in coding regions and were predicted to affect the gene product (non-synonymous, nonsense, or splice-site mutations). To identify complementation groups, we intersected the lists of affected genes between different mutant samples.
Orthologs Gene Finding
P. pacificus orthologs for 31 out of 39 cilia-related C. elegans genes were obtained from automated orthology predictions by Baskaran et al.49. The orthologs for the remaining eight genes were identified by manual BLAST analysis against different P. pacificus databases (genome, gene annotations, and trancriptomes) on www.pristionchus.org.
Dye-filling
The dye-filling protocol was adapted from previously described methods50. Nematodes were transferred into 1.5 ml centrifuge tubes and prewashed 3 times with M9 medium. Washed animals were stained with Vybrant DiI Cell-Labelling Solution (Thermo Fisher Scientific Inc.) diluted 100-fold in 250 µl of M9 medium. After incubation in a rocking wheel for 3 h, nematodes were washed 3 times with M9 medium to remove excess dye. Adult individuals were picked to agar pads containing 0.3% w/v NaN3 and imaged using a Leica TCS SP8 confocal microscope. Identical acquisition settings were used for all images. Average intensity Z-projections and overlay of bright field and fluorescent images were created using FIJI software51. Intensity ranges displayed in the fluorescent images in Fig. 4 are identical.
Statistical analyses
Statistical analyses were performed in the computing environment R ver. 3.1.3 (R Core Team, 2015). Replicates of clumping/bordering assays, aerotaxis assays and chemotaxis assays were used to calculate means and standard errors (S.E.M.). Two-sample equal variance Student’s t test, with Bonferroni corrections for multiple hypothesis testing, was used to confirm significant differences in bordering averages between wild type and mutant strains, Ω-turn rate averages before and after oxygen shifts, and chemotaxis index between wild type and mutant strains.
Electronic supplementary material
Acknowledgements
We thank the Max Planck Society for funding the research.
Author Contributions
E.M.: Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article. B.S.: Acquisition of data, Analysis and interpretation of data, Drafting or revising the article. H.W.: Acquisition of data. C.R.: Analysis and interpretation of data, Drafting or revising the article. J.W.L.: Acquisition of data, Drafting or revising the article. R.J.S.: Conception and design, Drafting or revising the article.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18019-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bargmann, C. I. Chemosensation in C. elegans. WormBook (2006). [DOI] [PMC free article] [PubMed]
- 2.McGaughran A, Morgan K, Sommer RJ. Natural variation in chemosensation: lessons from an island nematode. Ecol. Evol. 2013;3:5209–24. doi: 10.1002/ece3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–89. doi: 10.1016/S0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 4.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–22. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 5.Rogers C, Persson A, Cheung B, de Bono M. Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol. 2006;16:649–59. doi: 10.1016/j.cub.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Frézaland L, Félix MA. The natural history of model organisms: C. elegans outside the Petri dish. eLife. 2015;4:e05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath PT, et al. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–99. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers C, et al. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat. Neurosci. 2003;6:1178–85. doi: 10.1038/nn1140. [DOI] [PubMed] [Google Scholar]
- 9.Weber KP, et al. Whole genome sequencing highlights genetic changes associated with laboratory domestication of C. elegans. PLoS ONE. 2010;5:e13922. doi: 10.1371/journal.pone.0013922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behavior in C. elegans. Nature. 2009;458:1171–75. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen EC, Bloom JS, Gerke JP, Kruglyak L. A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genet. 2014;10:e1004156. doi: 10.1371/journal.pgen.1004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterken MG, Snoek LB, Kammenga JE, Andersen EC. The laboratory domestication of Caenorhabditis elegans. Trends Genet. 2015;31:224–31. doi: 10.1016/j.tig.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer RJ, Carta LK, Kim S-Y, Sternberg PW. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda: Neodiplogastridae) Nematology. 1996;19:511–22. [Google Scholar]
- 14.Sommer RJ, McGaughran A. The nematode Pristionchus pacificus as a model system for integrative studies in evolutionary biology. Mol. Ecol. 2013;22:2380–93. doi: 10.1111/mec.12286. [DOI] [PubMed] [Google Scholar]
- 15.Moreno E, McGaughran A, Rödelsperger C, Zimmer M, Sommer RJ. Oxygen-induced social behaviours in Pristionchus pacficus have a distinct evolutionary history and genetic regulation from Caenorhabditis elegans. Proc. R. Soc. B. 2016;283:20152263. doi: 10.1098/rspb.2015.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan K, et al. Multi-locus analysis of Pristionchus pacificus on La Réunion Island reveals an evolutionary history shaped by multiple introductions, constrained dispersal events, and rare out-crossing. Mol. Ecol. 2012;21:250–66. doi: 10.1111/j.1365-294X.2011.05382.x. [DOI] [PubMed] [Google Scholar]
- 17.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA. 2007;104:8655–60. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragsdale, E. J., Kanzaki, N. & Herrmann, M. Taxonomy and natural history: the genus Pristionchus. In Pristionchus Pacificus—A Nematode Model For Comparative And Evolutionary Biology (ed. R. J. Sommer), pp. 77–120. Leiden, The Netherlands: Brill (2015).
- 19.Witte H, et al. Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Dev. Genes Evol. 2015;225:55–62. doi: 10.1007/s00427-014-0486-8. [DOI] [PubMed] [Google Scholar]
- 20.Zimmer M, et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–79. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blacque OE, et al. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol. Biol. Cell. 2006;17:5053–62. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taschner M, Lorentzen E. The intraflagellar transport machinery. Cold Spring Harb. Perspect. Biol. 2016;8:a028092. doi: 10.1101/cshperspect.a028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevo, B., Scholey, J. M. & Peterman, E. J. G. Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery. FEBS J. (2017). [DOI] [PMC free article] [PubMed]
- 24.Sengupta P. Cilia and sensory signaling: The journey from “animalcules” to human disease. PLoS Biol. 2017;15:e2002240. doi: 10.1371/journal.pbio.2002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer JC, Haycraft CJ, Thomas J, Yoder BK, Swoboda P. XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Mol. Biol. Cell. 2003;14:2057–70. doi: 10.1091/mbc.E02-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–87. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 27.Snow JJ, et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 28.Inglis, P. N., Ou, G., Leroux, M. R. & Scholey, J. M. The sensory cilia of Caenorhabditis elegans. WormBook (2007). [DOI] [PMC free article] [PubMed]
- 29.Cinkornpumin JK, et al. A host beetle pheromone regulates development and behavior in the nematode Pristionchus pacificus. eLife. 2014;3:e03229. doi: 10.7554/eLife.03229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 31.Choe, J. C. & Crespi, B. J. The Evolution Of Social Behavior In Insects And Arachnids. Cambridge University Press (1997).
- 32.Hamilton WD. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- 33.Dury, G. J., Bede, J. C. & Windsor, D. M. Circular defence of immature insects: definition and occurrences of cycloalexy revisited. Psyche Article ID 642908 (2014).
- 34.Miller FG, Gunn A. Behavioral responses of musk ox to simulation of cargo slinging by helicopter, Northwest Territories. Can. field-nat. 1980;94:52–60. [Google Scholar]
- 35.Gilbert C, et al. One for all and all for one: the energetic benefits of huddling in endotherms. Biol. Rev. Camb. Philos. Soc. 2010;85:545–69. doi: 10.1111/j.1469-185X.2009.00115.x. [DOI] [PubMed] [Google Scholar]
- 36.Brischoux F, Bonnet X, Shine R. Kleptothermy: an additional category of thermoregulation, and a possible example in sea kraits (Laticauda laticaudata, Serpentes) Biol. Lett. 2009;5:729–31. doi: 10.1098/rsbl.2009.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport, J. Environmental Stress And Behavioural Adaptation. Croom Helm (1985).
- 38.Persson A, et al. Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature. 2009;458:1030–33. doi: 10.1038/nature07820. [DOI] [PubMed] [Google Scholar]
- 39.Bumbarger DJ, Riebesell M, Rödelsperger C, Sommer RJ. System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell. 2013;152:109–19. doi: 10.1016/j.cell.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 40.de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pires-daSilva, A. Pristionchus pacificus protocols. The C. elegans Research Community, WormBook (2013). [DOI] [PMC free article] [PubMed]
- 42.Chalasani SH, et al. Dissecting a circuit for olfactory behavior in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 43.Ramot D, Johnson BE, Berry TL, Jr., Carnell L, Goodman MB. Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS ONE. 2008;3:e2208. doi: 10.1371/journal.pone.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong RL, Witte H, Sommer RJ. Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL-4. Proc. Natl. Acad. Sci. USA. 2008;105:7779–84. doi: 10.1073/pnas.0708406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–80. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rödelsperger C, et al. Characterization of genetic diversity in the nematode Pristionchus pacificus from population-scale resequencing data. Genetics. 2014;196:1153–65. doi: 10.1534/genetics.113.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rae R, Witte H, Rödelsperger C, Sommer RJ. The importance of being regular: Caenorhabditis elegans and Pristionchus pacificus defecation mutants are hypersusceptible to bacterial pathogens. Int. J. Parasitol. 2012;42:747–53. doi: 10.1016/j.ijpara.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Baskaran P, et al. Ancient gene duplications have shaped developmental stage-specific expression in Pristionchus pacificus. BMC Evol. Biol. 2015;15:185. doi: 10.1186/s12862-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong Y, Bürglin TR. Conditions for dye-filling of sensory neurons in Caenorhabditis elegans. J. Neurosci. Methods. 2010;188:58–61. doi: 10.1016/j.jneumeth.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.