Abstract
Objective
To investigate current status of diagnosis and treatment of bladder cancer in China.
Methods
A database was generated by Chinese Bladder Cancer Consortium (CBCC). From January 2007 to December 2012, 14,260 cases from 44 CBCC centers were included. Data of diagnosis, treatment and pathology were collected.
Results
The average age was 63.5 year-old and most patients were male (84.3%). The most common histologic types were urothelial carcinoma (91.4%), adenocarcinoma (1.8%), and squamous carcinoma (1.9%). According to 1973 and 2004 WHO grading system, 42.0%, 41.0%, and 17.0% of patients were grade 1, 2, and 3, and 16.0%, 48.7%, and 35.3% of patients were papillary urothelial neoplasms of low malignant potential, low, and high grade, respectively. Non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) were 25.2% and 74.1%, respectively (0.8% not clear). Carcinoma in situ was only 2.4%. Most patients were diagnosed by white-light cystoscopy with biopsy (74.3%). Fluorescence and narrow band imaging cystoscopy had additional detection rate of 1.0% and 4.0%, respectively. Diagnostic transurethral resection (TUR) provided detection rate of 16.9%. Most NMIBCs were treated with TUR (89.2%). After initial TUR, 2.6% accepted second TUR, and 45.7%, 69.9%, and 58.7% accepted immediate, induced, and maintenance chemotherapy instillation, respectively. Most MIBCs were treated with radical cystectomy (RC, 59.7%). Laparoscopic RCs were 35.1%, while open RC 63.4%. Extended and standard pelvic lymph node dissection were 7% and 66%, respectively. Three most common urinary diversions were orthotopic neobladder (44%), ileal conduit (31%), and ureterocutaneostomy (23%). Only 2.3% of patients accepted neo-adjuvant chemotherapy and only 18% of T3 and T4 patients accepted adjuvant chemotherapy.
Conclusion
Disease characteristics are similar to international reports, while differences of diagnosis and treatment exist. This study can provide evidences for revisions of the guideline on bladder cancer in China.
Keywords: Bladder cancer, Diagnosis, Treatment
1. Introduction
Bladder cancer (BCa) is the most common urologic malignancy in China. During the past few years, the incidence and mortality rates have increased gradually [1], [2]. However, most data referred for guideline of Chinese BCa are not originally from China [3]. In fact, discriminations of diagnosis and treatment in practice for BCa may exist between China and Western countries. To gain unique data of BCa in China, Chinese Bladder Cancer Consortium (CBCC) conducted a cross-sectional investigation in 44 membership centers and generated a database of BCa. From January 2007 to December 2012, clinical data of 14,260 BCa cases were retrospectively collected. The present study was to investigate the current status of diagnosis and treatment for Chinese BCa by analyzing the database and to give evidences for Chinese guideline.
2. Patients and methods
2.1. Patients
The CBCC includes 44 academic centers from China. This study was approved by Institutional Review Boards in all CBCC centers. An electronic CBCC database was generated for data transfer and collection. Before data analysis, initial verifications were performed for identifying data consistencies and integrity. Communication with CBCC centers permitted resolution of identified anomalies. In all, 14,260 in-patient cases diagnosed as BCa from January 1, 2007 to December 31, 2012 were included.
2.2. Clinical features
The clinical features evaluated included age, gender, body mass index (BMI), prior history of bladder cancer, ways of diagnosis and treatment (transurethral resection [TUR], radical cystectomy [RC], partial cystectomy [PC] and others), perioperative information, histology, pathological T stage and grade, instillation therapy, neoadjuvant chemotherapy, adjuvant chemotherapy, and follow-up. All pathological specimens were reviewed by individual institutional pathologists using the American Joint Committee on Cancer TNM staging system relevant to time of diagnosis.
2.3. Statistical analysis
All statistical computations were performed using Stata 12/SE (StataCorp LP, TX, USA). Continuous and categorical data were summarized as mean and rate, respectively. Survival data were analyzed by Kaplan–Meier methods and compared by Log-rank test. All tests were considered statistically significant at p < 0.05.
3. Results
3.1. Patient and disease characteristics
Data from 14,260 patients were available at the time of final analysis. Patient and disease characteristics were presented in Table 1. The average age of patients was 63.5 years old. Most patients were male (84.3%) and had primary onset (84.4%). The majority patients had histology of urothelial carcinoma (91.4%). Adenocarcinoma, squamous carcinoma and other carcinomas had the rate of 1.8%, 1.9% and 4.9%, respectively. Most patients were graded by both 1973 and 2004 WHO grading systems. A total of 8673 patients were graded by the 1973 WHO grading system. Among them, 42.0%, 41.0%, and 17.0% of patients were grade 1, 2, and 3, respectively. A total of 10,893 patients were graded by 2004 WHO grading system. Among them, 16.0%, 48.7%, and 35.3% of patients had papillary urothelial neoplasm of low malignant potential, low grade, and high grade, respectively. Most patients were diagnosed as non-muscle invasive bladder cancer (NMIBC, 74.1%), including Ta (32.7%), primary carcinoma in situ (CIS, 0.8%), and T1 (40.6%). The other 25.2% of patients had muscle invasive bladder cancer (MIBC). Total morbidity rate of CIS was 2.4%, including concurrent CIS (1.6%) and primary CIS (0.8%).
Table 1.
Patient and disease characteristics.
| Number of patients | Proportion (%) | |
|---|---|---|
| Total | 14,260 | – |
| Age | 63.5 ± 13.2a | – |
| Sex | ||
| Male | 12,022 | 84.3 |
| Female | 2238 | 15.7 |
| BMI | 23.1 ± 3.2a | – |
| Onset | ||
| Primary | 12,037 | 84.4 |
| Recurrent | 2223 | 15.6 |
| Histology | ||
| Urothelial carcinoma | 13,037 | 91.4 |
| Squamous carcinoma | 274 | 1.9 |
| Adenocarcinoma | 261 | 1.8 |
| Others | 688 | 4.9 |
| Grade, 1973 WHO | 8673 | |
| Grade 1 | 3627 | 42.0 |
| Grade 2 | 3546 | 41.0 |
| Grand 3 | 1466 | 17.0 |
| Grade, 2004 WHO | 10,893 | |
| PUNLMP | 1732 | 16.0 |
| Low-grade | 5276 | 48.7 |
| High-grade | 3831 | 35.3 |
| T classification | ||
| Ta | 4667 | 32.7 |
| Primary CIS | 108 | 0.8 |
| T1 | 5786 | 40.6 |
| T2 or higher | 3591 | 25.2 |
| Unclear | 108 | 0.8 |
| Concurrent CIS | 223 | 1.6 |
BMI, body mass index; PUNLMP, papillary urothelial neoplasm of low malignant potential; CIS, carcinoma in situ.
Mean ± SD.
3.2. Current status of diagnosis
The majority patients were diagnosed by white-light cystoscopy with biopsy (74.3%). Application of fluorescence and NBI cystoscopy had additional detection rates of 1.0% and 4.0%, respectively. Only 16.9% of BCa were detected by diagnostic TUR. The other 3.8% of patients were recurrent cases. Ultrasonography was also a basic diagnostic technique with the positive rate of 95.5%. Computed tomography and magnetic resonance techniques were used for staging with the positive rate of 96.0% and 93.8%, respectively. The applications of urinary cytology and FISH were low (11.9% and 1.2%, respectively). However, FISH could provide the non-invasive detection rate up to 56.9%.
3.3. Current status of treatment
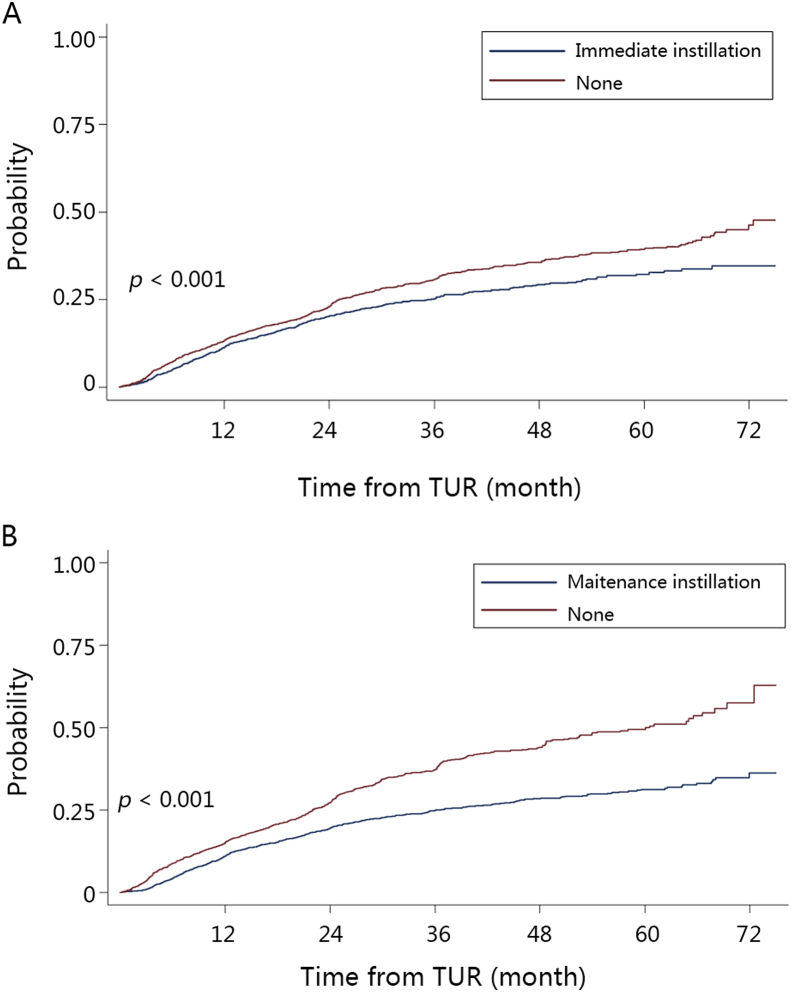
Treatment methods for NMIBC and MIBC were detailed in Table 2. Most NMIBC patients were treated with TUR (89.2%). Other NMIBCs were treated with RC (5.9%), PC (3.1%), and laser therapy or without operations (1.8%). After the initial TUR, second TUR was performed in only 2.6% of NMIBC patients. At the time of second TUR, the recurrence rate at primary site was only 15%. Postoperative adjuvant therapy included immediate (45.7%), induced (69.9%), and maintenance (58.7%) intravesical instillations. A total of 9038 patients had intermediate or high risk NMIBCs. Among them, 66.0% of patients had maintenance intravesical instillations. There were still 16.7% of patients without any intravesical instillation after TUR. All instillation regimens were based on chemotherapy drugs without Bacillus Calmette-Guérin (BCG). The three most common medicines were pirarubicin (51.4%), epirarubicin (20.6%), and mitomycin (7.0%). Survival analysis showed that 5-year cumulative recurrence rate was significantly lower in patients with immediate instillation (Fig. 1A, log-rank p < 0.001). Similarly, survival analysis showed that 5-year cumulative recurrence rate was significantly lower in patients with maintenance instillations in intermediate or high risk patients (Fig. 1B, log-rank p < 0.001).
Table 2.
Treatment for NMIBCs and MIBCs.
| NMIBC |
MIBC |
|||
|---|---|---|---|---|
| n | % | n | % | |
| TUR | 9422 | 89.2 | 547 | 15.2 |
| RC | 623 | 5.9 | 2304 | 64.1 |
| PC | 330 | 3.1 | 427 | 11.9 |
| Others | 186 | 1.8 | 313 | 8.8 |
| Totala | 10,561 | 3591 | ||
NMIBC, non-muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer; TUR, transurethral resection of bladder tumor; RC, radical cystectomy; PC, partial cystectomy.
Patients with unclear treatments: 108 cases.
Figure 1.
Kaplan–Meier plots of cumulative recurrence comparing (A) immediate instillation with none; (B) maintenance instillation with none.
Most MIBC patients were treated with RC (64.1%, Table 2). Other MIBCs were treated with TUR (15.2%), PC (11.9%) and reservative non-surgical therapy (8.8%). Laparoscopic RC was performed with the rate of 35.1%, while open RC with the rate of 63.4%. In recent years, robotic RC began to be applied (1.5%). RC with pelvic lymph node dissection (PLND) was performed in 73% of patients, including 7% extended and 66% standard PLND. The three most common urinary diversions were orthotopic neobladder (44%), ileal conduit (31%), and ureterocutaneostomy (23%). Only 2.31% of patients were treated with neo-adjuvant chemotherapy. Among 738 pT3–T4 patients, only 18% of patients were treated with adjuvant chemotherapy post RC.
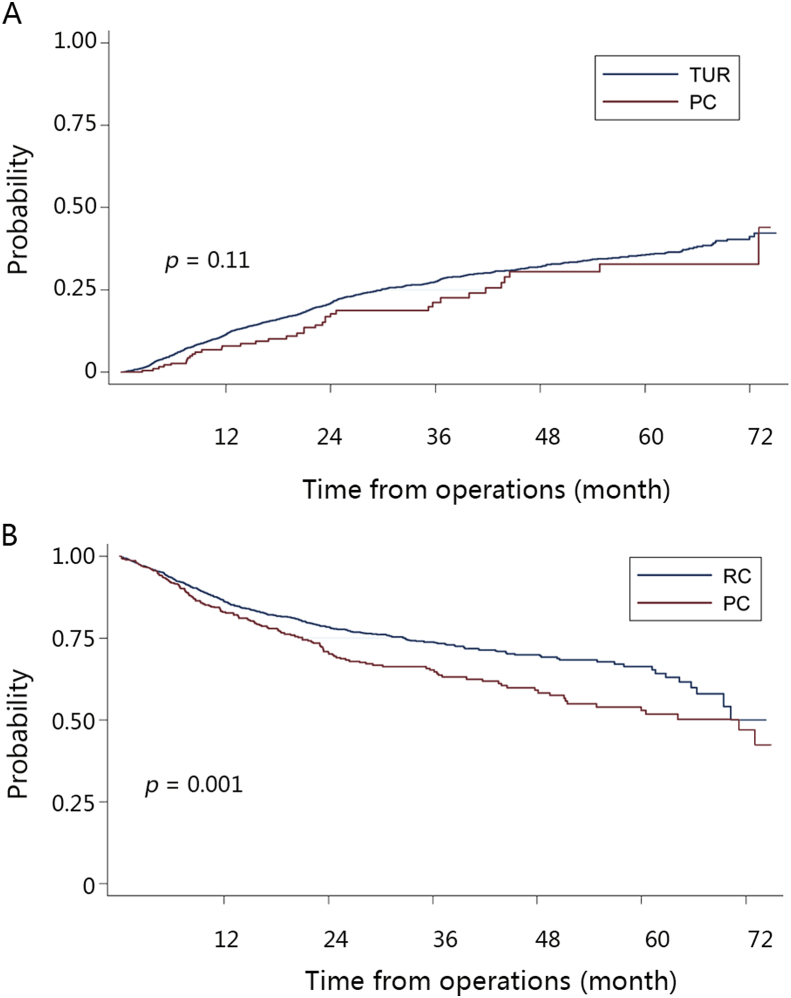
In all, 777 patients were treated with PC (5.4%), including 427 NMIBCs and 350 MIBCs. The predicted 5-year cumulative recurrence rate in vesicle was comparable in TUR and PC groups in NMIBC patients (Fig. 2A, log-rank p = 0.11). However, recurrence-free survival rate was significantly lower in PC compared with RC group in MIBC patients (Fig. 2B, log-rank p = 0.001).
Figure 2.
Prognosis of partial cystectomy. Kaplan–Meier plots of (A) cumulative intravesicle recurrence comparing partial cystectomy (PC) and transurethral resection (TUR) in non-muscle invasive bladder cancers (NMIBCs); (B) tumor-free survival comparing PC and radical cystectomy (RC) in muscle invasive bladder cancers (MIBCs).
4. Discussion
To our knowledge, this is the largest multicenter cross-sectional study to evaluate the status of diagnosis and treatment of BCa in China. Furthermore, a multicenter database for Chinese BCa is generated and unique data for Chinese BCa are obtained in the first time. This study revealed the status of diagnosis and treatment of BCa had some difference between China and Western countries. Completely referred to clinical evidence from Western countries was not enough to make the diagnosis and treatment of BCa best in China. The present results could provide evidence for clinical practice of Chinese BCa.
Screening for BCa in high risk populations is not a routine in China. White-light cystoscopy and biopsy was still the most common diagnosis technique for Chinese BCa. Narrow-band imaging and fluorescence cystoscopy could provide additional detection rates of BCa and apt to detect minimal lesions and CIS. They can be popularized and applied widely in China. Studies have reported that the detection rates of BCa are 73%–96%, 4%–31%, and 8%–35% in white-light, narrow-bind imaging, and fluorescence cystoscopy, respectively [4], [5], [6], which are similar to the results in China. Recent studies have also presented that diagnostic TUR can be directly performed without cystoscopy in the condition of mass found in bladder by iconography [7]. In NMIBC patients, it can simultaneously obtain the histology and pathology and resect the tumor. In MIBC patients, it can also provide evidence for next-step treatments and prognosis. However, diagnostic TUR is not widely applied and needs popularizations in China. Ultrasonography is mostly selected for review or patients with hematuria and is widely applied with a high positive rate in China. However, abdominal ultrasonography has disadvantages for BCa less than 5 mm [8]. European Association of Urology suggests urinary cytology for patients with high risk factors. However, a meta-analysis reports that the sensitivity of cytology is low. In low-grade tumors, the sensitivity is only 4%–31% [9]. In recent years, urinary molecular markers of BCa in urine have been widely used for a new non-invasive technique for detection of BCa in the world. The most common markers were BTAstat, BTAtrak, NMP22, and FISH, etc. [10]. However, no ideal marker exists which can be replaced for cystoscopy in terms of diagnosis, treatment, follow-up, and prognosis [3]. In China, the application of cytology and markers (mainly FISH) were low. The detection rate of FISH in BCa was relatively high (56.9%). With some commercial FISH kits, it can also be popularized and applied widely in China.
Urothelial carcinoma was the most common histological subtype (91.4%). The other subtypes were squamous carcinoma, glandular carcinoma, small cell carcinomas, etc. In Western countries, the proportions of urothelial, squamous, and glandular carcinomas are more than 90%, 3%–5% and less than 2%, respectively [11], [12], which are similar to proportions in China. The rate of CIS was low in China (only 2.6%), which was far less than in Western countries (24.4%) [13]. Low rate of CIS may be the result of low incidence rate of CIS in China or low diagnostic level. The real reason is still uncertain, which needs further research. Application of new diagnostic techniques such as narrow-band imaging and fluorescence cystoscopy may have values for detection of CIS. Nowadays, tumors were graded using both the 1973 and the 2004 WHO classifications in China. The use of the 2004 WHO classification had a trend to increase, while the use of the 1973 WHO classification had a trend to decrease. For unifying diagnosis of tumors and reflecting risk potential more better, guideline of BCa in China recommends the use of the 2004 WHO classification [3].
NMIBC were mainly treated with TUR. There may be a significant risk of leaving residual tumor in the bladder after the initial TUR. Residual disease is observed in up to 36% of patients [14]. Therefore, a second TUR is recommended to be performed if the initial resection is incomplete or when a high-grade, non-muscle-invasive tumour or a T1 tumour is detected at the initial TUR. However, the rate of TUR was only 2.6% in China, which was far less than the rate of 25.8%–62.4% in Western countries [15], [16]. In the meanwhile, the rate of primary recurrence was only 15% at the time of second TUR in China, which was also far less than the rate of 36%–38.9% in Western countries [17], [14]. The reasons of differences above may be: more complete resection of bladder tumor by the initial resection which lower the primary recurrence; surgeons were unwilling to perform second TUR for decreasing the economic burden and avoiding unnecessary medical dispute. Thereafter, the inclusion criteria of second TUR should be more restricted in China. Indication of second TUR in Western countries should not be completely followed. Furthermore, clinical researches of higher level are needed for proving the applicability of second TUR in China. After TUR, the proportion of immediate intravesical instillation of chemotherapy was low. Survival analysis showed that 5-year cumulative recurrence rate was lower in patients with immediate instillation therapy than without. The result indicated that immediate instillation of postoperative chemotherapy should be considered immediately after TUR in all NMIBCs without overt or suspected intra- or extraperitoneal perforation or bleeding that requires bladder irrigation. Survival analysis also showed that 5-year cumulative recurrence rate was lower in intermediate and high risk patients with maintenance instillation therapy than without, which indicated the value of maintenance instillation in these patients. In this study, all instillation regimens were based on chemotherapy and no BCG was used. By far, there exists no obvious clinical evidence apt to the best regimen or drug [3]. Most regimens or drugs selected for instillations still rely on the experience of surgeons. After BCG is on the market in China, more research will be conducted for evaluating the efficacy of BCG in Chinese BCa.
Muscle invasive bladder cancers were mainly treated with radical cystectomy. Open RC is still the standard operation for MIBC and the most common RC in China. In recent years, laparoscopic RC has been improved with high feasibility and safety. In China, more than 30% of RC were performed under the assistance of laparoscopy. Robotic assisted RC was started to be performed with its low postoperative complications and accurate manipulation. However, Da Vinci system is too expensive, which limits its popularization. Most researchers suggest PLND simultaneously with RC. Without clinical evidences of high level, the exact area of PLND is still uncertain. Although extending the area of PLND could dissect more lymph nodes, longer operation time and more postoperative complications may influence the final prognosis [18]. It may be an important reason of low proportion of extended PLND in China. The three most common types of urinary diversions in the world are orthotopic neobladder, ileal conduit, and ureterocutaneostomy. A review reports that the proportions of orthotopic neobladder, ileal conduit, and ureterocutaneostomy are 38%, 42.2%, and 10.4%, respectively [19]. In China, orthotopic neobladder was used more frequently than in Western countries. Because orthotopic neobladder can maintain the quality of life and self-image, it is adopted as a main diversion by more and more large centers. However, the proportion of ureterocutaneostomy was still high. Patients with ureterocutaneostomy have high risk of stricturing on skin level and ascending urinary tract infection. The use of ureterocutaneostomy should be reduced in China, except for patients with short predicted life time, metastasis, or intolerance to complicated operations.
Partial cystectomy is considered for NMIBC patients not appropriate to TUR or MIBC with conservation therapy in Western countries. A study from the USA reports the rate of PC is only 2.8% [20]. The 5-year local recurrence rate and tumor-free survival rate for PC were 38%–78% and ∼50%, respectively [21], [22]. In China, the proportion of PC was high (5.4%). Survival analysis showed that PC could provide prognosis no better than TUR for NMIBC patients. However, PC could result in more complications, which make the recovery slower. Another survival analysis showed worse prognosis in PC compared with RC for MIBC patients. Therefore, the use of PC should be reduced for the treatment of BCa in China.
5. Conclusion
The present study concludes that the demographic characteristics of BCa in China are similar to Western countries, while some differences in diagnosis and treatment of BCa exist. Diagnosis is mainly based on cystoscopy with biopsy. Diagnostic TUR, fluorescence cystoscopy, NBI, and FISH should be applied more widely in China. NMIBC is mainly operated by TUR and too many partial cystectomies were performed. MIBC is mainly operated by RC with PLND, but the operations of PC and RC without PLND still exist. Orthotopic neobladders and ileal conduits are the most two common urinary diversions, while the proportion of ureterocutaneostomy is high. By investigating the current status of diagnosis and treatment of Chinese BCa, this study could provide evidences for revisions of the guideline of BCa in China.
Conflicts of interest
The author declares no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Urological Association and SMMU.
References
- 1.Zhang S.W., Ma J.H., Li M., Na Y.Q., Chen W.Q. Incidence trends of bladder cancer in cities and counties in China. Chin J Urol. 2009;10:673–676. [Google Scholar]
- 2.Han S.J., Zhang S.W., Chen W.Q., Li C.L. Analysis of the status quo and trends: mortality in patients with bladder cancer in China. J Mod Urol. 2013;18:228–232. [Google Scholar]
- 3.Huang J. Guidelines of diagnosis and treatment on bladder cancer. In: Na Y.Q., Ye Z.Q., Sun Y.H., Sun G., editors. Guidelines on urology in China. 2014 ed. People's Medical Publishing House; Beijing: 2014. pp. 20–60. [Google Scholar]
- 4.Kamat A.M., Hegarty P.K., Gee J.R., Clark P.E., Svatek R.S., Hegarty N. ICUD-EAU International Consultation on Bladder Cancer 2012: screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 5.Kausch I., Sommerauer M., Montorsi F., Stenzl A., Jacqmin D., Jichlinski P. Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010;57:595–606. doi: 10.1016/j.eururo.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 6.He Y., Zha J., Wang Y., Liu W., Yang X., Yu P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res. 2013;73:629–639. doi: 10.1158/0008-5472.CAN-12-2704. [DOI] [PubMed] [Google Scholar]
- 7.Brausi M., Collette L., Kurth K., van der Meijden A.P., Oosterlinck W., Witjes J.A. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 8.Datta S.N., Allen G.M., Evans R., Vaughton K.C., Lucas M.G. Urinary tract ultrasonography in the evaluation of haematuria – a report of over 1,000 cases. Ann R Coll Surg Engl. 2002;84:203–205. [PMC free article] [PubMed] [Google Scholar]
- 9.Lotan Y., Roehrborn C.G. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118. doi: 10.1016/s0090-4295(02)02136-2. [DOI] [PubMed] [Google Scholar]
- 10.Tilki D., Burger M., Dalbagni G., Grossman H.B., Hakenberg O.W., Palou J. Urine markers for detection and surveillance of non-muscle-invasive bladder cancer. Eur Urol. 2011;60:484–492. doi: 10.1016/j.eururo.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Fleshner N.E., Herr H.W., Stewart A.K., Murphy G.P., Mettlin C., Menck H.R. The national cancer data base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–1513. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Kantor A.F., Hartge P., Hoover R.N., Fraumeni J.F., Jr. Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer Res. 1988;48:3853–3855. [PubMed] [Google Scholar]
- 13.Gontero P., Sylvester R., Pisano F., Joniau S., Vander Eeckt K., Serretta V. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with bacillus Calmette-Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol. 2014;16:00614–00619. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Divrik R.T., Yildirim U., Zorlu F., Ozen H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol. 2006;175:1641–1644. doi: 10.1016/S0022-5347(05)01002-5. [DOI] [PubMed] [Google Scholar]
- 15.Holmang S. High-grade non-muscle-invasive bladder cancer: is re-resection necessary in all patients before intravesical bacillus Calmette-Guerin treatment? Scand J Urol. 2013;47:363–369. doi: 10.3109/21681805.2013.769461. [DOI] [PubMed] [Google Scholar]
- 16.Angulo J.C., Palou J., Garcia-Tello A., de Fata F.R., Rodriguez O., Villavicencio H. Second transurethral resection and prognosis of high-grade non-muscle invasive bladder cancer in patients not receiving bacillus Calmette-Guerin. Actas Urol Esp. 2014;38:164–171. doi: 10.1016/j.acuro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Lazica D.A., Roth S., Brandt A.S., Bottcher S., Mathers M.J., Ubrig B. Second transurethral resection after Ta high-grade bladder tumor: a 4.5-year period at a single university center. Urol Int. 2014;92:131–135. doi: 10.1159/000353089. [DOI] [PubMed] [Google Scholar]
- 18.Linder B.J., Frank I., Cheville J.C., Tollefson M.K., Thompson R.H., Tarrell R.F. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013;63:839–845. doi: 10.1016/j.eururo.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Hautmann R.E., Abol-Enein H., Davidsson T., Gudjonsson S., Hautmann S.H., Holm H.V. ICUD-EAU International Consultation on Bladder Cancer 2012: urinary diversion. Eur Urol. 2013;63:67–80. doi: 10.1016/j.eururo.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Kassouf W., Swanson D., Kamat A.M., Leibovici D., Siefker-Radtke A., Munsell M.F. Partial cystectomy for muscle invasive urothelial carcinoma of the bladder: a contemporary review of the M. D. Anderson Cancer Center experience. J Urol. 2006;175:2058–2062. doi: 10.1016/S0022-5347(06)00322-3. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney P., Kursh E.D., Resnick M.I. Partial cystectomy. Urol Clin North Am. 1992;19:701–711. [PubMed] [Google Scholar]
- 22.Ma B., Li H., Zhang C., Yang K., Qiao B., Zhang Z. Lymphovascular invasion, ureteral reimplantation and prior history of urothelial carcinoma are associated with poor prognosis after partial cystectomy for muscle-invasive bladder cancer with negative pelvic lymph nodes. Eur J Surg Oncol. 2013;39:1150–1156. doi: 10.1016/j.ejso.2013.04.006. [DOI] [PubMed] [Google Scholar]