Abstract
Background: Travelers’ diarrhea causes significant morbidity including some sequelae, lost travel time and opportunity cost to both travelers and countries receiving travelers. Effective prevention and treatment are needed to reduce these negative impacts.
Methods: This critical appraisal of the literature and expert consensus guideline development effort asked several key questions related to antibiotic and non-antibiotic prophylaxis and treatment, utility of available diagnostics, impact of multi-drug resistant (MDR) colonization associated with travel and travelers’ diarrhea, and how our understanding of the gastrointestinal microbiome should influence current practice and future research. Studies related to these key clinical areas were assessed for relevance and quality. Based on this critical appraisal, guidelines were developed and voted on using current standards for clinical guideline development methodology.
Results: New definitions for severity of travelers’ diarrhea were developed. A total of 20 graded recommendations on the topics of prophylaxis, diagnosis, therapy and follow-up were developed. In addition, three non-graded consensus-based statements were adopted.
Conclusions: Prevention and treatment of travelers’ diarrhea requires action at the provider, traveler and research community levels. Strong evidence supports the effectiveness of antimicrobial therapy in most cases of moderate to severe travelers’ diarrhea, while either increasing intake of fluids only or loperamide or bismuth subsalicylate may suffice for most cases of mild diarrhea. Further studies are needed to address knowledge gaps regarding optimal therapies, the individual, community and global health risks of MDR acquisition, manipulation of the microbiome in prevention and treatment and the utility of laboratory testing in returning travelers with persistent diarrhea.
Keywords: Clinical practice guideline, emporiatrics, evidence-based medicine
Introduction
Travelers’ diarrhea (TD) has always been, and continues to be, a problem globally.1 The overall impact of TD is substantial though it may be declining given the improvements of global health, sanitation and hygiene.2 Regardless, TD remains a frequent event and can result in many adverse consequences including lost time and opportunity, changes to itinerary, overseas medical encounters and hospitalization.1
Upon discovery that bacterial etiologies were a predominant cause, the debate surrounding appropriate management has been evolving and aims to balance safety and efficacy of antimicrobial therapy. Some of the first randomized controlled treatment trials demonstrating antibiotic efficacy superior to placebo were conducted in the early 1980s, with consensus and expert-based treatment guidelines developed shortly thereafter.3 Studies have also evaluated various antibiotic regimens in combination with loperamide, and randomized controlled trials (RCT) demonstrate improved efficacy compared to antibiotics alone when evaluating duration of post-treatment symptoms and clinical cure.4
While a number of guidelines are currently available which discuss recommendations around prevention, management and treatment of travelers’ diarrhea,5–11 new data are emerging which may have an impact on recommendations provided to travelers. These new issues include emerging data on both the acute and chronic health consequences of travelers’ diarrhea and treatment, and in particular the growing recognition of antibiotic resistance acquisition associated with TD and self‐treatment abroad, and the corresponding paramount concern of the impacts on individual and population health.12,13 Furthermore, advances in technology of diagnostics, the microbiome and novel therapeutics have brought new questions and opportunities to the field.14–20
This guideline aims to provide practical guidance to providers faced with common questions regarding recommendations on use of antibiotic and non-antibiotic therapies in the prevention and treatment of TD. We sought to apply a rigorous process to the review and assessment of evidence and to make guideline recommendations informed and supported by that evidence. Unfortunately, rigorous data needed to address important questions in the TD management space are often absent or insufficient. We therefore present a hybrid document. When sufficiently strong evidence from randomized clinical trials addressing a clinically important question is available, we have used this as a basis to develop our guideline recommendation statements. When evidence is absent or insufficient to provide evidence-based guidelines, we provide our best expert advice as consensus statements with the goal of helping providers of all training levels navigate important management questions.
Methods
The goal of this guideline project was to produce clinically relevant and useful recommendations on management of TD, to be used by a range of health care providers who provide pre- and post-travel consultation to travelers. Providers may use these guidelines to assist with treatment choices that optimize benefits and minimize harms and burdens associated with this common acute infection. This guideline also considers other important aspects of TD, as it relates to the ill returning traveler, the emerging concern of multi-drug resistant organism (MDRO) acquisition, and current limitations of evidence.
In 2011, the Institute of Medicine (IOM) released new guideline standards that required significantly more scientific rigor and high-quality evidence, as well as a series of processes in guideline development.21 A highly rigorous and transparent technical process for evaluating evidence and applying these evaluations to guideline development, known as GRADE (Grades of Recommendations, Assessment, Development and Evaluation), has also been described and continues to evolve.22 The panel supported by the International Society of Travel Medicine (ISTM) is committed to upholding the IOM standards in guideline development. However, it should be noted that the ISTM does not have a formal institutionalized process or dedicated resources for developing clinical practice guidelines. This project represents panel members’ best attempt to follow the principles of the GRADE framework and IOM standards, with limitations as described below. Furthermore, the panel also recognizes the need to present information that describes good practice and currently evolving and relevant data, where strong clinical evidence is either unavailable or not relevant to the question under consideration. In such clinical practice scenarios, we provide guidance in the form of ungraded consensus statements, accompanied by a summary of the available evidence. When there is insufficient evidence to make any recommendation, this has been noted in the text.
Composition and Selection of Panel Members
For this guideline, a Chair was appointed based on experience in the area of TD. The Chair had the authority, along with Guideline Organizing Committee (GOC), to nominate other panelists with experience in relevant areas of TD management. Conflicts of interest for the panel members were reviewed and it was determined that disclosure to the panel was necessary, but did not exclude them for participation in any of the voting. The panel was made up of 15 voting members, with support from 3 non-voting members.
Identifying and Reviewing the Evidence
Key Questions and Systematic Search: The GOC developed a list of key clinical questions based on their knowledge of practice gaps among practitioners managing cases of TD:
Should antibiotic prophylaxis be considered for some travelers, and if so, what traveler characteristics should prompt consideration of prophylaxis and with what agents?
What diagnostic modalities should be employed in travelers with diarrhea and/or persistent abdominal symptoms?
Given growing recognition of MDR colonization in returning travelers, how should travelers be directed with respect to 1) expectant management, 2) Over-the-counter (OTC) agents for symptom management, 3) antibiotic use, or 4) seeking care during travel?
In the context of MDR acquisition, how can providers clearly and effectively convey the risks and benefits of TD treatment, including acute and chronic consequences and community risks, when counseling on antibiotic use while traveling?
How does our knowledge (and potential manipulation) of the microbiome in the setting of TD influence practice recommendations or future research?
Questions were formulated as explicit or implicit PICO questions (population of interest, intervention, comparison and outcome), or related contextual questions which inform the PICO questions. To inform the evidence around these questions all panelists were provided with read-ahead materials including existing TD management guidelines and systematic reviews, key articles on diagnostics, prophylaxis, and treatment, data on the influence of travel, diarrhea, antibiotics, diet and the environment on changes in the gut microbiome, as well as recent data on MDR organism carriage.1,4,10,14,16,23–41 In addition, prior to the closed panel session where guidelines were developed, the ISTM sponsored a 2 day open session Summit Meeting where experts in the field gave presentations focused on the Key Clinical Questions identified.
A formal systematic review with data extraction tables detailing all relevant articles for each of the key questions was not performed. However, the panel did make use of the 2015 Committee to Advise on Tropical Medicine and Travel (CATMAT) Statement on Travellers' Diarrhea from the Public Health Agency of Canada, which had detailed summary tables on several of Key Clinical Questions, mostly pertaining to prophylaxis and treatment with antibiotics and non-antibiotic agents.10 The evidence used for other key clinical questions was based on the provided read-ahead documents, invited presentations, and subject matter expertize among panel members.
Drafting Recommendations
The guidelines panel discussion and deliberation was facilitated by an expert Chairperson in clinical practice and research in TD. The process for development of each recommendation for a given key clinical question followed the same algorithm: recommendation formulation, grading the quality of the evidence in terms of the confidence in the estimates of the efficacy and harms of the intervention, and grading the strength of the recommendation based on the balance of harms and benefits, and knowledge of the values and preferences of travelers. Each of these steps in the recommendation development and grading utilized the Delphi process including features of anonymity, iteration, controlled feedback and statistical group responses.42
Recommendation Formulation
The entire closed panel participated in the crafting of each of the recommendation statements. This process was achieved through the use of a facilitator (the Chair) who posed the key clinical question and solicited from each of the panelists ideas on a draft recommendation statement. Points from each of the panelists were written down on a flip charter and grouped accordingly. Similar suggestions were grouped together where appropriate. From this process one or more recommendation statements emerged. A statement would be collectively crafted and designed to explicitly or implicitly match the PICO format. When an apparent consensus was reached on the draft recommendation statement, the statement was put to an anonymous vote by means of a Likert-type question of strongly agree, weakly agree, weakly disagree, and strongly disagree (using web-based PollEverywhere.com platform). If there was 80% or greater agreement (combining votes for strongly agree and weakly agree), the draft recommendation was accepted and moved forward for grading of strength and quality of evidence. In recommendations where there was less than 80% agreement achieved, the draft recommendation was revisited by discussion, modified and vote repeated until at least 80% agreement was achieved. Draft recommendations that could not attain 80% agreement were abandoned.
Evidence Grading Process for Individual Recommendations
Upon consensus of each recommendation statement, the panel considered two dimensions on grading of the evidence. First, the balance of benefits to harms, risks or burdens, including the confidence in the estimate of effect (e.g. “net benefit rating”) was considered. For this assessment, recommendations were considered “strong” when desirable effects clearly outweighed the undesirable effects, or vice versa. In the latter case, there could be a strong negative recommendation (e.g., a strong recommendation not to use a specific intervention). Strong recommendations (Grade 1) would be phrased using persuasive language such as “we recommend.” However, when desirable and undesirable effects were more closely balanced and it was plausible that the direction or strength of a recommendation could be changed by patient values and preferences, additional research information, or other conditions specific to an individual case, it was graded as “weak”, and statements included phrasing such as “we suggest.” For a strong recommendation, where desirable effects were considered to clearly outweigh undesirable effects, we judge that most if not all informed service providers and their clients would choose the intervention. For a weak recommendation, the variability in provider and client preference, and tradeoffs between desirable and undesirable consequences are less clear, we judge that the majority of well-informed people would want the recommended course of action, but a minority (perhaps a large minority) would not.22
The second dimension involved rating of the quality of the entire body of evidence for each recommendation, in terms of confidence in the estimates of the effects. The higher the quality of the evidence, the more likely a strong recommendation is warranted. GRADE terminology was used for summarizing and grading the evidence as high, moderate, or low/very low quality. Ratings of the evidence are evaluated based on criteria of study design, imprecision, indirectness (relative to the recommendation statement elements), inconsistency or heterogeneity of results across studies, and risk of reporting or publication bias. In general, study designs such as RCTs start as high-quality evidence but are subject to downgrading based on these criteria. Observational studies start low but may be upgraded if they meet design standards and (1) there is a large magnitude of effect, (2) there is a statistically significant effect even with the presence of bias, or (3) there is a dose-response gradient. A grade (high, moderate, or low/very low) was assigned by the panel to for the quality of evidence supporting each recommendation. A high grade was evidenced from >1 properly RCT that met most or all of the criteria in terms of quality study design, precision, directness, consistency and minimized risk of publication bias. A moderate grade was considered appropriate based on evidence from >1 well-designed clinical trial, without randomization or from cohort or case-controlled analytic studies, from multiple time-series, or from dramatic results from uncontrolled experiments. A low/very low grade was relegated to evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees.
For the grading of both the strength of the recommendation and the quality of evidence ratings, a similar Delphi process was used to arrive at a consensus grading from the entire voting panel. For each recommendation, the facilitator would lead the discussion. Each participant was allowed to summarize his or her own opinions and thoughts. An anonymous voting method was used to record participants individual grading categories. If 80% or greater agreement was achieved, the consensus grade was accepted. If less than 80% agreement was obtained, the facilitator would lead a re-discussion of the recommendation and criteria supporting a particular grade, to solicit clarifications and comments from the panelists. Upon completion of the re-discussion, another anonymous vote was taken. This process continued iteratively until at least 80% agreement was attained. In situations where 80% agreement could not be attained, those recommendations with grades achieving 67 to 79% agreement were accepted, but reported with “additional remarks,” which included a description of the minority opinions and justifications. Recommendations achieving less than 67% agreement were not retained.
The committee did not consider the cost of individual treatment or prophylaxis regimens in formulating recommendations. It is recognized that such consideration is important and depending on different provider, patient and/or payer perspectives, preferential recommendation choices should be made. Finally, as described above, some recommendations were not graded by this process. In such situations, the panel developed an ungraded consensus-based statement. Consensus in this instance was determined through open discussion and debate among the panel, and a draft recommendation put to a consensus vote required 80% or greater consensus for the recommendation to be made.
The Chair, co-Chairs and select panelists drafted the initial manuscript with recommendations combined with the corresponding grades and summary of the evidence. Panelists were asked to comment and suggest modifications and wording refinements. A final consensus document was reviewed and approved by all panelists.
Review by External Reviewers
When the final manuscript was completed and endorsed by the GOC, the manuscript underwent peer review process by the Editorial Board of the Journal of Travel Medicine to consider content, methods, and adherence to process. Reviewers were self-nominated and vetted through the Editor-in-Chief, were not involved in the CPG development process, and included infectious diseases and travel medicine experts.
Mild (acute): diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Moderate (acute): diarrhea that is distressing or interferes with planned activities.
Severe (acute): diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools) is considered severe.
Persistent: diarrhea lasting ≥ 2 weeks.
Recommendations (Table 1)
Table 1.
Summary of recommendations and statements
| Travelers’ diarrhea definitions |
| Mild (acute): diarrhea that is tolerable, is not distressing, and does not interfere with planned activities. |
| Moderate (acute): diarrhea that is distressing or interferes with planned activities. |
| Severe (acute): diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools) is considered severe. |
| Persistent: diarrhea lasting ≥2 weeks. |
| Prophylaxis |
| 1. Antimicrobial prophylaxis should not be used routinely in travelers (Strong recommendation, low/very low level of evidence). |
| 2. Antimicrobial prophylaxis should be considered for travelers at high risk of health-related complications of travelers’ diarrhea (Strong recommendation, low/very low level of evidence). |
| 3. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent travelers’ diarrhea (Strong recommendation, high level of evidence). |
| 4. When antibiotic prophylaxis is indicated, rifaximin is recommended (Strong recommendation, moderate level of evidence). |
| 5. Fluoroquinolones are not recommended for prophylaxis of travelers’ diarrhea (Strong recommendation, low/very low level of evidence). |
| Therapy of mild travelers’ diarrhea |
| 6. Antibiotic treatment is not recommended in patients with mild travelers’ diarrhea (Strong recommendation, moderate level of evidence). |
| 7. Loperamide or BSS may be considered in the treatment of mild travelers’ diarrhea (Strong recommendation, moderate level of evidence). |
| Therapy of moderate travelers’ diarrhea |
| 8. Antibiotics may be used to treat cases of moderate travelers’ diarrhea (Weak recommendation, moderate level of evidence). |
| 9. Fluoroquinolones may be used to treat moderate travelers’ diarrhea (Strong recommendation, moderate level of evidence. Qualifying Remarks: Emergence of resistance to this class of drug though without strong evidence of clinical failure outside of SE Asia, combined with the potential for adverse dysbiotic (reduction in diversity of intestinal microbiota) and musculoskeletal consequences, contribute important uncertainties to the risk-benefit assessment and underlie a non-unanimous GRADE of this recommendation). |
| 10. Azithromycin may be used to treat moderate travelers’ diarrhea (Strong recommendation, high level of evidence). |
| 11. Rifaximin may be used to treat moderate travelers’ diarrhea (Weak recommendation, moderate level of evidence). Qualifying Remarks: Caution should be exercised in provision of rifaximin as empirical therapy of moderate diarrhea in regions or itineraries in which high risk of invasive pathogens are anticipated. |
| 12. Loperamide may be used as adjunctive therapy for moderate to severe travelers’ diarrhea (Strong recommendation, high level of evidence). |
| 13. Loperamide may be considered for use as monotherapy in moderate travelers’ diarrhea (Strong recommendation, high level of evidence). |
| Therapy in severe travelers’ diarrhea |
| 14. Antibiotics should be used to treat severe travelers’ diarrhea (Strong recommendation, high level of evidence). |
| 15. Azithromycin is preferred to treat severe travelers’ diarrhea (Strong recommendation, moderate level of evidence). |
| 16. Fluoroquinolones may be used to treat severe, nondysenteric travelers’ diarrhea (Weak recommendation, moderate level of evidence). |
| 17. Rifaximin may be used to treat severe, nondysenteric travelers’ diarrhea (Weak recommendation, moderate level of evidence). |
| 18. Single-dose antibiotic regimens may be used to treat moderate or severe travelers’ diarrhea (Strong recommendation, high level of evidence). |
| Follow-up and diagnostic testing |
| 19. Microbiologic testing is recommended in returning travelers with severe or persistent symptoms or in those who fail empiric therapy (Strong recommendation, low/very low level of evidence). |
| 20. Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or non-molecular tests have failed to establish a diagnosis (ungraded). Qualifying Remarks: No studies have been published as of yet which show that using these tests improves patient outcomes. |
| Additional consensus statements (ungraded) |
| 21. There is insufficient evidence to recommend the use of commercially available prebiotics or probiotics to prevent or treat travelers’ diarrhea. |
| 22. Studies are needed on changes in the gut microbiome in travelers with and without diarrhea to clarify the benefits and harms of current and novel preventive, diagnostic, and therapeutic approaches. |
| 23. There is an incrementally increasing association between travel, travelers’ diarrhea, and antibiotic use with the acquisition of multidrug-resistant bacteria. Pretravel counseling should include information about this risk, balanced against the benefits of antibiotic use. |
Travelers’ Diarrhea Definitions
Comment on Definitions
A classification of TD using functional impact for defining severity is advised rather than the traditional frequency-based algorithm that has been utilized.11 If passing numerous stools represents a functional impairment, it will be judged as more severe than an illness where fewer stools are passed. Previous definitions have been variably classified functionally as mild, moderate or severe or based on number of unformed stools passed in 24 hours (e.g. 1-2 stools mild, 3-5 stools moderate and ≥ 6-9 stools for severe). Passage of small number of stools with fever and severe cramps may be more disabling than passage of six watery diarrheal stools without cramps or pain. We support an approach that matches the therapeutic intervention with the severity of illness, in terms of both safety and effectiveness. Therefore, we recommend definitions based on functional impact. Prior to travel, the definitions of diarrhea should be discussed with travelers so they understand when to begin self-treatment and what treatment modalities should be utilized. It should be discussed that TD is the sudden onset of abnormally loose or liquid, frequent stools such that the individual’s assessment is that the illness is tolerable (mild), distressing (moderate) or incapacitating (severe). It is recognized that this is a departure from conventional definitions and will bring challenges to the interpretation and design of future trials related to TD which have relied on stool frequency-based outcomes; however this classification system will likely lead to more tailored therapy for the individual. Further research on defining valid patient-reported and non-frequency based outcomes in the setting of TD is needed.
The definition of dysentery should be discussed with future travelers and is defined as passage of stools that contain gross blood admixed with stool in the commode and is often accompanied by more severe constitutional symptoms including fever. It should be emphasized that normal appearing stools in the commode with streaks of blood on the toilet paper may well represent bleeding haemorrhoids and not dysentery. As most travelers are unlikely to carry a thermometer to take their temperatures, providers should discuss symptomatology associated with fever and how fever may alter the assessment of disease severity. The traveler should be instructed that if their diarrhea lasts longer than 14 days it is considered persistent diarrhea, and may be associated with a higher frequency of certain bacterial and protozoal pathogens therefore may justifying evaluation (see specific recommendations).43 Functional bowel disease (FBD) may occur after bouts of travelers’ diarrhea and other enteric infections.44 Many of these former travelers with functional gastrointestinal complaints will meet Rome III or IV criteria for irritable bowel syndrome (IBS).45 While there are no recommendations on diagnosis or treatment of IBS or functional gastrointestinal disease after travel, we feel it is important to recognize this travel associated phenomenon, which is discussed in relation to a number of the included recommendations in this clinical practice guideline. We offer the following definitions to consider post-infectious FBD and IBS in the returning traveler.
Post-infectious irritable bowel syndrome (PI-IBS) and Functional Bowel Disease (PI-FBD): PI-IBS or PI-FBD are diagnosed by Rome III or Rome IV criteria after a bout of TD in a traveler without pre-travel gastrointestinal disease where the evaluation for microbial etiologies and underlying GI disease is negative.
Prophylaxis
1. Antimicrobial prophylaxis should not be used routinely in travelers (Strong recommendation, low/very low level of evidence).
2. Antimicrobial prophylaxis should be considered for travelers at high risk of health-related complications of travelers’ diarrhea (Strong recommendation, low/very low level of evidence).
3. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent travelers’ diarrhea (Strong recommendation, high level of evidence).
4. When antibiotic prophylaxis is indicated, rifaximin is recommended (Strong recommendation, moderate level of evidence).
5. Fluoroquinolones are not recommended for prophylaxis of travelers’ diarrhea (Strong recommendation, low/very low level of evidence).
Summary of the Evidence
There is strong evidence from multiple randomized controlled trials (RCT) that, depending on compound and destination, antimicrobials demonstrate prevention of classically defined TD in about 58 to 88% of travelers.1,26 By contrast, studies demonstrating the benefit of antimicrobials in primary or secondary prevention of long-term health consequences that have been strongly linked to TD36 such as irritable bowel syndrome, reactive arthritis, and Guillain-Barré syndrome are absent. Moreover, this cannot be readily inferred from efficacy data against clinical TD, as subclinical pathogen contact may suffice to trigger the presumed patho-etiology involved in post-TD chronic disease morbidity.36 In theory, however, lowering pathogen burden and limiting mucosal damage through the prophylactic use of antimicrobials may be anticipated to prevent long-term morbidity in a fraction of travelers thus justifying their use in secondary prevention. Indeed, studies in non-traveler populations have shown that more severe and longer illness durations are associated with increased risk of post-infectious IBS,46–49 supporting the notion that prevention of TD disease (and/or severity and duration) could mitigate the post-TD chronic health consequences given the similarities of infection types. Similarly, the rationale of preventing TD-related complications in chronically ill travelers by prophylactic antimicrobials has not been subject to rigorous research, but is supported by clinical reasoning on the vulnerability of certain patient groups to dehydration and bacteremia.
Against this background, and based on opinion and clinical expertize, the expert panel gives a strong recommendation in favor of considering antimicrobials for the prevention of TD in travelers at high risk of health-related complications. This recommendation applies to individuals with a desire to travel and a history of clinically significant long-term morbidity following an enteric infection (e.g. reactive arthritis) or serious chronic illness that predisposes for TD-related complications.
At the same time, and also based on opinion and expertize, the expert panel gives a strong recommendation against the routine use of antimicrobial prophylaxis for the prevention of TD in travelers’ sub-groups other than those outlined above. Antimicrobial resistance has been recognized as a major threat to health globally and is known to be fostered by antimicrobial overuse.50 On an individual level, colonization with resistant bacteria is discussed as potentially harmful consequence of antimicrobial intake. One cohort study in travelers showed that the use of antimicrobials at destinations with high-prevalence of multi-drug resistant organisms (MDROs) in the environment doubled the risk of intestinal colonization with these bacteria upon return.25 Together with research from population based surveillance that found past travel to India to be a risk factor for infection with MDROs in outpatients,51 these findings point towards a potentially increased risk of infection with MDROs in travelers using antibiotics as chemoprophylaxis. Further research is needed to clarify the clinical significance of MDRO colonization in returning travelers who use antimicrobial chemoprophylaxis.
Other traveler populations which may benefit from antimicrobials for the prevention of TD but are not explicitly included in the current panel recommendation have been described. These include individuals who because of occupation or itinerary (e.g. athlete in competition, professional musician, politician, etc.) cannot afford to become sick with TD10 or may not have access to adequate medical treatment of complicated TD. Given the rapid effectiveness of antibiotics with or without loperamide in combination and the increasing threat of antimicrobial resistance, the rationale supporting the aforementioned sub-group as populations who might benefit from antimicrobial chemoprophylaxis may not be as strong anymore. Therefore, the panel recommends that, when assessing travelers of this sub-group, travel medicine practitioners take clear public health responsibility by conducting an individual risk benefit analysis of antimicrobial chemoprophylaxis vs self-treatment with self-treatment of moderate to severe TD representing the standard approach, and chemoprophylaxis the rare exception.
There is strong evidence to recommend the option of using bismuth subsalicylate (BSS) for prophylaxis of TD in travelers.1,52,53 There have been three sound RCTs using BSS vs placebo in travelers where BSS was shown to have a strong protective effect (>60%).54–56 BSS has been studied using either 2.1 g/day or 4.2 g/day in 4 divided doses (with meals and at bedtime), and has been studied in both the liquid and tablet forms. A lower dose of 1.05 g/day has also been shown to be preventive, though it is unclear whether it is as effective as the higher doses. There are no data on usage of BSS beyond 4 weeks and no data regarding differences in the liquid form vs the tablet form. It is unknown what effect BSS may have on the microbiome or the acquisition of MDROs. BSS is well tolerated; adverse effects are mild in adults and consist of black tongue, black stools, tinnitus, potential for constipation, and potential interference with other medications.56,57 Those who are allergic to aspirin, who are pregnant, who have chronic renal insufficiency, and theoretically those who have severe enteric disease where there may be bismuth absorption should not take BSS. The panel is aware of the fact that BSS is not available in many countries.
There is strong evidence to opt for rifaximin in those travelers that require antibiotic prophylaxis. Five RCTs conducted between 2005 and 2013 demonstrated that 200 to 1100 mg of prophylactic rifaximin per day divided into one to three doses confer strong protection against TD.58–62 Most enteric pathogens causing TD are susceptible to rifaximin63–65 with the exception of Campylobacter spp. which are resistant.63 This may explain rifaxmin’s only moderate protective effectiveness in South and Southeast Asia where Campylobacteriosis is more common.58 As a non-absorbable antibiotic, rifaximin has an extremely favorable safety profile. This has been confirmed in trials in traveler58–62 and non-traveler populations.66–68 The impact of rifaximin prophylaxis on MDRO acquisition has not been studied. While the number of RCTs to date (N = 5) supporting the recommendation would rate a high level of evidence, the panel downgraded the strength of evidence for this recommendation due to concerns of unpublished data from at least one other RCT with rifaximin and to a lesser extent concern with efficacy in regions where invasive pathogens are more common.
Four RCTs conducted from 1986 to 1994 demonstrated that prophylactic fluoroquinolones (FQ) confer strong protection against classic TD.69–72 Since then, resistance of enteric pathogens to FQs has been emerging,65,73,74 reaching 70-80% in Campylobacter spp. from Nepal and Thailand73,74 and 65% in ETEC and EAEC from India.65 Hence, the current efficacy of FQs in preventing TD is unknown, and may be lower than in these earlier trials. There is also increasing evidence from non-traveler populations that, in situations where FQs are not essential, the potential for harm to the peripheral and central nervous system, tendons, muscles and joints may outweigh the benefit of their use. This has led to a recent change of the safety labeling for FQs by the U.S. Food and Drug administration.75 Together with the known association of FQs with C. difficile-associated diarrhea,76 these potential harms underline the concern that FQs should be used with more caution in the TD setting. Therefore, based on expert opinion, the expert panel does not recommend FQs for the prophylaxis of TD.
Therapy of Mild Travelers’ Diarrhea
6. Antibiotic treatment is not recommended in patients with mild travelers’ diarrhea (Strong recommendation, moderate level of evidence).
7. Loperamide or BSS may be considered in the treatment of mild travelers’ diarrhea (Strong recommendation, moderate level of evidence).
Summary of the Evidence
Increasing concerns on MDRO and the implications on individual, community and global health, requires the conservation of antibiotics used in the self-treatment of TD. We recommend an approach to provide antibiotics for judicious self-treatment to travelers at risk for TD and counsel them on use according to the functional impact of symptoms on their travel-related activities.
The rates of mild illness may vary widely depending on such factors as the geographic area, type of trip, age of traveler, and previous exposure; however, a sizeable proportion of illnesses will fall into the mild category defined as causing little or no interference in normal daily activities.77–79 For mild diarrhea, the traveler is encouraged to use supportive measures such as rehydration and non-antibiotic, anti-motility drugs such as loperamide.
The loperamide starting dose is 2 tablets (4 mg), followed by with uncontrolled diarrhea an additional 2 mg after each additional loose or liquid stool with a total dose of up to 16 mg per day. Travelers should be counseled that it takes 1 to 2 hours for loperamide to reach its therapeutic effect, so additional dosing should be spaced accordingly so as to avoid rebound constipation. It is important to counsel the traveler that if diarrhea worsens or is accompanied by moderate-severe or invasive symptoms (e.g. fever, moderate to severe abdominal pain or bloody diarrhea), then antibiotics should be used.
While dated, the evidence supporting loperamide in treatment of mild TD is strong, and loperamide has an FDA labeled indication for treatment of mild TD. In a first 1983-84 study comparing loperamide to bismuth subsalicylate (no placebo), loperamide resulted in fewer unformed stools being passed when compared to the bismuth subsalicylate treatment group during the first 48 hours of therapy, though nearly a quarter of volunteers opted in for antibiotic rescue therapy.80 Subsequently, in a RCT of 227 US adults with TD in Mexico, a single high dose of sulfamethoxazole and trimethoprim (1600/320 mg) or 3 days of therapy with loperamide hydrochloride (4-mg loading dose, then 2 mg orally after each loose stool) or sulfamethoxazole-trimethoprim (800/160 mg orally twice daily), or the combination of both was evaluated.81 While subjects treated with the combination had the shortest average duration of diarrhea compared with the placebo group (1 vs 59 hours), loperamide alone was significantly effective only when treatment failures (13% vs 2% for combination therapy) were treated with antibiotics. Finally, loperamide was compared to the non-absorbable antibiotic, rifaximin, and to the combination of rifaximin plus loperamide for the treatment of TD in a large study of travelers to Mexico.82 Subjects met a standard definition of TD to be included in the study and there was no stratification of results according to illness severity. The mean number of diarrheal stools in the entire illness was 6.7 for loperamide alone compared to 6.2 for rifaximin and 4.0 for the combination. The time to last unformed stool was 69 hours for loperamide alone compared to 33 hours for rifaximin and 27 hours for rifaximin plus loperamide. None of the subjects treated with loperamide required treatment with antibiotics. Finally, a number of observational studies also support treatment of mild diarrhea with antimotility agents.77,83
No RCTs have been conducted to compare loperamide with azithromycin or fluoroquinolones (alone or in combination). Evidence in support of the efficacy of loperamide is stronger than that for BSS. Other OTC products, such as activated charcoal or dimenhydrinate, are not recommended. The panel recognizes that there are other antisecretory agents such as racecadotril, crofelemer and zaldaride which may have a role in travelers’ diarrhea management. Crofelemer84,85 and zaldaride19,20 have had limited study in the setting of travelers’ diarrhea, while racecadotril has not been evaluated in this relevant setting. While crofelemer and racecadotril are available in many regions of the world, the lack of a labeled indication for use in a travelers’ diarrhea setting, and no apparent advantage compared to loperamide or BSS, prevented the panel from considering recommendations regarding these novel anti-secretory agents. We recognize, however, that there may be a potential use for these agents and encourage future evaluation alone and in combination with antibiotics for moderate and severe travelers’ diarrhea.
Therapy of Moderate Travelers’ Diarrhea
Summary of the Evidence
8. Antibiotics may be used to treat moderate travelers’ diarrhea (Weak recommendation, moderate level of evidence).
9. Fluoroquinolones may be used to treat moderate travelers’ diarrhea (Strong recommendation, moderate level of evidence. Qualifying Remarks: Emergence of resistance to this class of drug though without strong evidence of clinical failure outside of SE Asia, combined with the potential for adverse dysbiotic (reduction in diversity of intestinal microbiota) and musculoskeletal consequences, contribute important uncertainties to the risk-benefit assessment and underlie a non-unanimous GRADE of this recommendation).
10. Azithromycin may be used to treat moderate travelers’ diarrhea (Strong recommendation, high level of evidence).
11. Rifaximin may be used to treat moderate travelers’ diarrhea (Weak recommendation, moderate level of evidence). Qualifying Remarks: Caution should be exercised in provision of rifaximin as empirical therapy of moderate diarrhea in regions or itineraries in which high risk of invasive pathogens are anticipated.
12. Loperamide may be used as adjunctive therapy for moderate to severe travelers’ diarrhea (Strong recommendation, high level of evidence).
13. Loperamide may be considered for use as monotherapy in moderate travelers’ diarrhea (Strong recommendation, high level of evidence).
There is no question that antimicrobial agents have been demonstrated to be effective in shortening the illness to about one and a half days,10,52,86 and when combined with loperamide, to less than one half day.52,87 Even if there were more side effects as compared to placebo, antimicrobial agents are well tolerated overall,86 and a movement towards single dose regimens likely improves the safety profile. In addition, the reduction in the duration of illness associated with impact on travel with timely and effective self-treatment will often enable the traveler to continue the journey as planned. This results not only in stress reduction, but also may have an economic impact as well. For example, rebooking flights — sometimes for an entire family — may be costly and there also may be lost business opportunities.88 It may be extremely frustrating and ultimately can also have financial consequences if a major excursion must be cancelled because of TD, or if individual travelers are unable to fully participate in activities for which they may have expended considerable energy and resources. Furthermore, lack of availability of empiric therapy may result in the traveler seeking care from locally, providers which may result in inappropriate, ineffective and even dangerous care acquired abroad.89,90
On the other hand, antibiotic treatment is not without potential adverse consequences. While adverse events for the commonly used antibiotics are rare, they do occur. Most recently, the FDA released a statement on avoidance of FQ use for non-complicated bacterial infections due to emerging and accumulating concerns of peripheral neuropathy, central nervous system, cardiac, dermatologic effects and hypersensitivity reactions.91 Although the FQ warning was targeted for non-complicated upper respiratory tract and urinary infections, and is likely dose and duration dependent, a lesser concern in treatment of moderate TD is apparent. Particularly in the older traveler, such musculoskeletal and cardiotoxic risks need to be balanced with the adverse consequences of TD in this population who may also be more susceptible to significant dehydration. In addition, the absolute risk of these rare complications associated with a single dose regimen is uncertain, but are likely much lower.
Of growing concern is the demonstration that antibiotic use in the context of travel (more than travel alone and untreated TD alone) is associated with increased rates of acquisition of ESBL-PE.25,92 These resistant pathogens are usually not associated with symptoms in the otherwise healthy traveler, carriage is usually transient in duration, and the impact on the spread of MDRO related infection in the community and globally is uncertain.23 Nevertheless, their potential importance warrants consideration in balancing the benefit of acute illness treatment against both individual and population health consequences. Furthermore, there are increasing concerns about alteration of the individual’s microbiota with antibiotic use.16 Use of antibiotics may also be associated with subsequent C. difficile infection,93 though this has not been commonly reported in travellers. There are also concerns on negative effects of antibiotics use (e.g. prolonged carriage) on enteric infection caused by non-typhoidal Salmonella.94 Here, as well, single dose antibiotic regimens are likely to minimize the risk of such consequences; however, data to support an unqualified recommendation regarding the advantage of single dose regimens preventing with respect to these adverse outcomes are lacking.
One potential value of early and effective treatment of TD relates to its potential of mitigating the well-described chronic health consequences of TD, including IBS. The rationale behind this theoretical benefit relates to the results of a number of epidemiological studies, which have identified increased risk of PI-IBS following longer duration and more severe acute enteric infections.46–49,77,95 Also an animal model demonstrates that co-administration of an antibiotic (rifaximin) in a rat model mitigates the risk of a PI-IBS disease phenotype.96 So far, there have been no studies assessing whether any use and particularly early use of effective antibiotics against TD reduces or increases the risk of sequelae, such research is sorely needed. Thus, taking into consideration all of the real and theoretical risks and benefits of therapy for moderate TD, we recommend that antibiotics may be used for self-treatment for moderate TD. Counselling and traveler preferences should also be taken into consideration.
With respect to antibiotic choice in moderate TD, we have made a number of class- and regimen-specific recommendations (Table 2). In nine RCTs, FQs have proven effective against moderate TD.10,52 Widespread resistance has developed mainly in Southeast and South Asia, particularly against Campylobacter spp.; thus, these agents are to be avoided in empiric treatment of moderate or severe TD arising from travel to these areas.1 Observational data also show increasing rates of resistance elsewhere,97 though studies documenting clinical failures outside of SE Asia are not available. These findings, in addition to some panellists’ concerns about the tendinopathy and dysbiotic effects of this drug classclass resulted in a non-unanimous GRADE of this recommendation and the associated qualifying remarks.
Table 2.
Acute diarrhea antibiotic treatment recommendations
| Antibiotica | Dose | Treatment duration |
|---|---|---|
| Azithromycinc, d | 1000 mg by mouth or | Single or 1-day dividedb |
| 500 mg by mouth | 3 day course | |
| Levofloxacin | 500 mg by mouth | Single doseb or 3 day course |
| Ciprofloxacin | 750 mg by mouth or | Single doseb |
| 500 mg by mouth | 3 day course | |
| Ofloxacin | 400 mg by mouth | Single doseb or 3 day course |
| Rifaximine | 200 mg by mouth three times daily | 3 days |
Antibiotic regimens may be combined with loperamide, 4 mg first dose, then 2 mg dose after each loose stool, not to exceed 16 mg in a 24 hour period.
If symptoms are not resolved after 24 hours, continue daily dosing for up to 3 days.
Use empirically as first line in Southeast Asia and India to cover fluoroquinolone resistant Campylobacter or in other geographical areas if Campylobacter or resistant ETEC are suspected.
Preferred regimen for dysentery or febrile diarrhea.
Do not use if clinical suspicion for Campylobacter, Salmonella, Shigella or other causes of invasive diarrhea.
Azithromycin has been evaluated in several RCTs, often in comparison to FQs for therapy of moderate TD. Studies support that there are no significant differences in efficacy between FQs and azithromycin.10 So far there is only limited resistance to common TD pathogens, but there are concerns in Nepal,74 and increasing concentrations of drug needed to inhibit ETEC and EAEC, which have not yet been shown to translate into clinical failure.97 Tolerability to azithromycin is good, but nausea and vomiting are more frequently reported compared to FQs, particularly when 1,000 mg is ingested as a single dose.10,98 While there were no increased risks for mortality or cardiovascular events associated with azithromycin therapy compared to placebo,99,100 sustained ventricular tachycardia has been observed in patients with prolonged QTc. This represents a potential risk for certain patient populations who might have pre-existing conditions or are taking concomitant medications.101
In two RCTs rifaximin has been found to be a more effective agent, as compared to placebo, in treating TD associated with non-invasive pathogens, and in another two RCTs, it has been shown to be non-inferior to ciprofloxacin.10 So far there are only limited data on resistance,10 but in contrast to FQs and azithromycin, there is no indication that MICs increase among recovered enteric pathogen isolates.65 The safety profile is excellent with this poorly absorbed agent. So far there is a lack of documentation as to whether rifaximin therapy is associated with acquisition of ESBL-PE, but it has been demonstrated to alter the microbiome, possibly in a beneficial way.102,103 Therefore, rifaximin is recommended as an acceptable alternative for treatment of moderate TD, however should be used with caution in areas where invasive pathogens are likely to be encountered.
Since loperamide is the fastest acting therapeutic against TD, the rationale of combination therapy with antibiotics is to add symptomatic relief with curative treatment. Five studies have shown that the combination of an antibiotic plus loperamide increases the rate of short term cure, whereas in one study utilizing a FQ in SE Asia where high rates of resistance were known to occur, no benefit was seen.4,10,52 There is no evidence that the combined therapy results in increased rates of adverse events,82 but a secondary analysis of a single observational study found that combining an antibiotic (of unknown duration and class) with loperamide was associated with a higher rate of ESBL-PE recovery from stool samples as compared to the use of either agent independently.104 Studies evaluating the dysbiotic and ESBL-PE colonization effects in combined single dose regimens are lacking.
Loperamide may be used as monotherapy for moderate TD.105 There is unsubstantiated concern of increasing TD patients’ exposure to pathogens when motility is slowed. However, numerous studies have demonstrated the effectiveness of loperamide against TD, among them three travel–related RCTs comparing loperamide to placebo in settings generally limited to 3 days.10 It is very well tolerated, except for constipation, particularly in females, and if there is a lack of adherence to prescribed dosing instructions.106 In most countries loperamide is contraindicated in children below the age of 2 years. In children with acute diarrhea between 2 and 11 years of age, loperamide was beneficial without causing severe adverse events.107 Although there have been few published cases of significant adverse effects related to empiric use in travelers’ despite very common use of this agent,108 continued use of loperamide alone, or in combination with antibiotics, in the face of worsening of symptoms or development of dysentery is cautioned.
Therapy in Severe Travelers’ Diarrhea
14. Antibiotics should be used to treat severe travelers’ diarrhea (Strong recommendation, high level of evidence).
15. Azithromycin is preferred to treat severe travelers’ diarrhea (Strong recommendation, moderate level of evidence).
16. Fluoroquinolones may be used to treat severe, nondysenteric travelers’ diarrhea (Weak recommendation, moderate level of evidence).
17. Rifaximin may be used to treat severe, nondysenteric travelers’ diarrhea (Weak recommendation, moderate level of evidence).
18. Single-dose antibiotic regimens may be used to treat moderate or severe travelers’ diarrhea (Strong recommendation, high level of evidence).
Summary of the Evidence
The decision to treat TD with non-specific anti-diarrheal medications and/or an antimicrobial agent is based on assessment of the severity of illness and the effects it will have on the traveler’s activities and plans. The classification “severe travelers’ diarrhea” includes both severe non-dysenteric watery diarrhea leading to incapacitation and/or inability to carry out planned activities, as well as dysentery. It is important to consider the syndromes separately since their antibiotic management may differ as detailed in the following summary of evidence.
Antibiotics have been demonstrated to reduce symptom duration in TD from an average of 50-93 to 16–30 hours.86 Antibiotic therapy has also been demonstrated to be efficacious in moderate to severe acute bacterial diarrhea in community-based studies in industrialized countries, and particularly in infections due to Shigella spp. and (early on in the treatment of) campylobacteriosis (< 72 hours).109–116 An important caveat when translating the RCT evidence to practice guidance is that trials include subjects with TD across a range of severity and occasionally exclude individuals with more severe illness such as dysentery or those in need of hospitalization.
Key considerations in the selection of an empiric antibiotic include the likelihood of treatment efficacy and rapidity of response, regional patterns of probable target pathogens and their antimicrobial resistance, safety and tolerance profile of the antibiotic, simplicity of treatment regimen (and hence patient adherence) and cost. Equivalent efficacy for treatment of watery noninvasive diarrhea has been demonstrated between FQs (3-day and single dose), azithromycin (3-day and single dose), and rifaximin (three times daily for 3 days).117–119 The efficacy of single dose regimens for both FQs and azithromycin supports their acceptability (Strong recommendation, high level of evidence).118,120,121
Azithromycin should be considered the first-line agent in cases of dysentery (Strong recommendation, high level of evidence) as well as acute watery diarrhea with greater than mild fever given the increased likelihood of FQ-resistant Campylobacter, and other bacterial causes such as Shigella spp., enteroinvasive E. coli, Aeromonas spp., Plesiomonas spp., and Yersinia enterocolitica.118,122–124 Azithromycin (single 1-gm dose or 500 mg daily for 3 days) has been shown to be superior to levofloxacin (500 mg daily for 3 days) in achieving clinical cure in Thailand in a setting with extremely high rates (exceeding 90%) of FQ-resistant Campylobacter spp.118 Other randomized trials have demonstrated equivalent efficacy between azithromycin and FQs; however, these studies were conducted in areas with TD cases caused primarily by diarrheagenic E. coli, or in an earlier time period in Thailand when C. jejuni FQ-resistance rates were significantly lower. In those settings, ciprofloxacin treatment failures requiring rescue therapy did occur (5%) but overall clinical efficacy in post treatment duration was comparable.120,122 FQ-resistant travel-associated and domestic Campylobacter cases in industrialized countries have been increasingly reported and are not restricted to countries such as Thailand, thus leading to the strong recommendation, high level of evidence recommendation for azithromycin as first-line dysentery therapy irrespective of geographic region.125–127 Further support for this is provided by the emergence of nalidixic acid and FQ resistance in Shigella spp. and Salmonella spp. from India and in a variety of enteric pathogens in sub-Saharan Africa.128–131 Azithromycin has also demonstrated effective and comparable cure rates with shigellosis, another common agent causing dysentery.113,132
Azithromycin is generally well tolerated with minimal side effects, usually dose-related gastrointestinal complaints.133 Incident or worsening nausea or vomiting are exacerbated by the primary gastrointestinal infection,134 and are more common than in the treatment of non-gastrointestinal infections, with rates of 3% and <1%, respectively.135–138 Comparable efficacy may be achieved with potentially lower side effect rates, by splitting the single 1-gm dose over the first day; however, this remains to be proven.
FQs retain efficacy in much of the developing world with the previous caveats regarding resistance in Campylobacter spp. and now other enteric pathogens. The recommendation is based mainly on concerns about reduced benefit due to the likelihood of FQ-resistant Campylobacter spp., or Shigella spp. being the cause of dysentery as well as significant risk concerns. FQs are generally well tolerated, with most adverse effects being mild and transient. Gastrointestinal (nausea, vomiting, or diarrhea) and central nervous system (headache, dizziness, or insomnia) side effects are shared by all quinolones.139 In addition, the FQ class has several concerns regarding adverse effects including the potential for Achilles tendon rupture (“black box warning” issued by US FDA), an increased risk for C. difficile infection, and rarely the prolongation of the QT interval that may lead to fatal dysrhythmias.140–142 Consideration should be given to the potential for drug interactions in a given patient. Recent evidence is accumulating for acquisition of travel-associated gut dysbiosis as well as MDR bacteria during international travel.16,25,143 The possible potentiating effects of antimicrobials, particularly FQs, should be factored into the decision to use these agents for treatment of TD.
Rifaximin, a nonabsorbable antibiotic, has demonstrated comparable efficacy to FQs in non-invasive TD caused by diarrheagenic E. coli.119,144,145 These trials have often excluded patients with more severe illness, which typically accounts for a minority of TD cases. Rifaximin is less effective for the treatment of invasive pathogens, with failure to achieve wellness in up to 50% of the treated subjects.144 As invasive pathogens such as Campylobacter, Salmonella and Shigella spp. may account for approximately 10-20% of TD cases, rifaximin cannot be recommended for areas where these invasive pathogens are common and it is not appropriate for treatment of dysentery, irrespective of illness severity. With the above caveats, rifaximin has the best safety profile compared to other first-line antibiotics (Weak recommendation, moderate level of evidence).
Follow-up and Diagnostic Testing
19. Microbiologic testing is recommended in returning travelers with severe or persistent symptoms or in those who fail empiric therapy (Strong recommendation, low/very low level of evidence).
20. Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or non-molecular tests have failed to establish a diagnosis (ungraded). Qualifying Remarks: No studies have been published as of yet which show that using these tests improves patient outcomes.
Summary of the Evidence
Detection and identification of specific etiologic agents may be helpful for the management and treatment of patients with TD; however, studies demonstrating the benefit with considerable cost and clinical practicability challenges are lacking. Therefore, the recommendations made here are based largely on expert opinion and clinical judgement. Specifically, it is recommended that microbiologic diagnosis be attempted in returning travelers with severe or persistent (chronic) symptoms and if there is bloody diarrhea or mucus present in the stools. In such cases, knowing an etiology may assist in directing pathogen-specific treatment, or withholding antibiotics in the case of a viral etiology being identified. No clinical trials or well-designed observational studies have been performed to assess the cost-benefit of a test and treatment strategy in such situations, so clinician discretion should be used.
While the decision to test is qualified, selection of which diagnostic test to be performed is also complicated. Due to the heterogeneity of etiologies, (i.e. bacteria, viruses and parasites), current microbiological diagnosis requires multiple strategies. Selective and differential culture media remain the reference method to isolate bacterial enteropathogens but significant limitations such as low sensitivity and a prolonged turn-around-time for results may reduce the meaningful impact on a patient’s management.146 For detection of parasites, microscopic examination is carried out to detect ova and parasites in stools either directly from fresh concentrated stools or after staining. However, this method lacks sensitivity, is time consuming, and requires highly trained personnel for detection and interpretation. Specific tests for parasite antigen detection, such as Giardia lamblia, Cryptosporidium spp., or Entamoeba histolytica have also been developed. However, antigen detection tests can only detect a few specific parasites and therefore cannot completely replace microscopic examination which detects a wider variety of pathogens. Antigen detection tests have also been used to detect some viruses causing gastroenteritis such as rotavirus and adenovirus; these tests are usually quite specific and their sensitivity is variable.
In the last decade multi-pathogen molecular-based clinical assays which can detect a variety of relevant bacteria, viruses and parasites have been developed which provide the potential for quick identification and potential impact on treatment decisions.11 The molecular multiplex most studied is xTAG® Gastrointestinal Pathogen Panel (GPP) (Luminex Molecular Diagnostics, Toronto, Canada). This panel detects 15 pathogens, including nine bacteria, three parasites and three viruses, and has been evaluated for the diagnosis of diarrhea in different populations, including travelers. In a study performed to evaluate the utility of this panel to diagnose TD, it was found that the GPP test might be especially sensitive for the detection of Shigella, ETEC and Giardia. However, this panel does not include EAEC and Cyclospora cayetanensis, two microorganisms responsible for TD.31 Among other FDA approved multiplex molecular tests on the market is the FilmArray GI panel (BioFire Diagnostics, LLC a bioMérieux Company, Salt Lake City, UT), which allows the simultaneous detection of 22 enteropathogens and has the advantage of being an integrated system requiring only five minutes of sample handling and providing results in 1 hour.15,147
While these assays have high sensitivity and specificity, the clinical outcomes advantage and financial impact of these molecular panels has not yet been fully evaluated, and there are some concerns that molecular testing may, in some cases, detect colonization rather than infection. Interpretation therefore may be difficult and implementation will depend on the economics of each hospital or clinic system, and perhaps cost-effectiveness will vary depending on the type of patients tested (pediatric, hospital or community diarrhea, TD, etc.). Furthermore, the use of molecular diagnostic methods does not allow for the resistance characterization of etiologies which may be important for directing care. Thus, the clinician may be in a position of ordering both culture-based and molecular tests to be able to fully evaluate the patient’s illness and identify the best therapy. Further studies are needed to evaluate the utility of these assays in a clinical setting of the returning traveler with diarrhea. Until such studies are conducted, an evidence-based recommendation for their use cannot be made.
Additional Consensus Statements
21. There is insufficient evidence to recommend the use of commercially available prebiotics or probiotics to prevent or treat travelers’ diarrhea (ungraded).
22. Studies are needed on changes in the gut microbiome in travelers with and without diarrhea to clarify the benefits and harms of current and novel preventive, diagnostic, and therapeutic approaches (ungraded).
23. There is an incrementally increasing association between travel, travelers’ diarrhea, and antibiotic use with the acquisition of multidrug-resistant bacteria. Pre-travel counseling should include information about this risk, balanced against the benefits of antibiotic use (ungraded).
Summary of Evidence
The use of probiotics and prebiotics to prevent or treat acute diarrheal infection is an appealing concept because of their ease of use and relative safety. The concept of host resistance to enteric infection, also commonly referred to as colonization resistance, describes the microbiota's capability to prevent and limit pathogen colonization and growth. Over the past few decades our understanding of the varied colonization resistance mechanisms has expanded to understand key direct and indirect mechanisms.148,149
While studies assessing probiotic/prebiotic effectiveness in prevention of acute diarrheal infection in adults have been conducted in the traveler setting,41,150–158 these suffer from variability in setting, causes of acute diarrhea, probiotic strains, short follow up and lack of person-time analysis. There are also large variations in the dosage of probiotics, frequency of administration and formulations used. Further variation is seen with regard to timing and administration of these preparations relative to a number of factors including travel populations and locations, and concurrent treatment with antimicrobials.150 Although two meta-analyses suggest a marginal benefit of probiotics in prevention of TD, both studies also suggest there is insufficient evidence for extrapolation to global recommendations for their use.41,150
With regards to a potential treatment indication, a 2010 Cochrane Collaboration systematic review on probiotics and treatment of intestinal infection identified 63 randomized and quasi-randomized controlled trials comparing specific probiotic agent(s) vs placebo or no-treatment for acute diarrhea of presumed infectious etiology.40 However, only 6 of these trials were among adults, none of which were in a TD setting though pathogen-attributable infections described were somewhat comparable to common TD etiologies and thus worth consideration.159–164 While one product, Enterococcus LAB SF68, demonstrated efficacy when examining clinical improvement at 4 days, the theoretical safety concerns raised about this product limits any further recommendation.165 (Note: Probiotic use for the treatment of antibiotic associated diarrhea has been recommended).166 For probiotics and prebiotics the challenge has been to understand the right formulation or combination for the right condition or individual. In summary, in considering the totality of evidence, the panel reached a consensus that the data supporting the use of probiotics and prebiotics for either a prevention or treatment indication in TD is not adequate to make a graded recommendation. More research is needed, however a more thorough knowledge of the host microbiome and mechanisms of action might be necessary before appropriate trials can be designed using specific agents for prevention and treatment of TD.
For the last three decades, RCTs have consistently and clearly demonstrated that antibiotics significantly shorten the duration and alleviate the morbidity of TD which if left untreated can last 3 days or more and significantly disrupt travel plans. Treatment with an effective antibiotic can shorten the average duration to slightly longer than 1 day, and to less than one half day if combined with loperamide. However, recently the concern over antibiotic induced colonization with MDRO has called into question the role of antibiotics for what is often is a self-limiting, albeit often disruptive experience. While the impact to the average traveler of this colonization appears limited it has been identified as an important fraction of MDRO urinary tract infections in community and hospital-based settings.167,168 Carriage appears to be transient, but has been described to persist in approximately 10% at a year post-travel and be transmitted to close household contacts.12 The challenge that we now face as providers is to balance the clear benefits of individual treatment with the impact of colonization on the given individual, and in the larger context of risk to close contacts, the community to which the traveler returns, and the global spread of resistance. The elderly traveler or the traveler with a history of recurrent urinary tract infections present a unique challenge as these individuals may be at higher risk of individual health consequences from ESBL-PE colonization acquisition, but also, in case of the elderly or otherwise vulnerable traveler, the consequences of delayed or untreated illness must be measured. At a minimum travelers should be made aware of this risk, and should they become ill following travel, they ought to convey their travel exposure history to their treating providers. While the role of travelers’ serving as mobility agents of infectious disease and resistance cannot be ignored, the ecology of ESBL-PE infections is complex and includes important environmental, dietary, migration and local nosocomial transmission dynamics.30 The expert panel recognizes that the indiscriminant and overuse of antibiotics in the clinical and livestock settings in the developing world as a major source of resistance globally, and the burden of these infection in the developed world is non-travel associated. ESBL-PE infections are clearly an important health threat and addressing this complex problem will likely take a multi-faceted approach. We are then left with considering what equipoise exists in the balance of treatment vs no treatment in the traveler setting. More evidence is needed. However, in the interim we recommend discussion with the individual about the multi-level (individual, community, global) risks of travel, TD, and the role of antibiotic treatment take place.
Conclusions
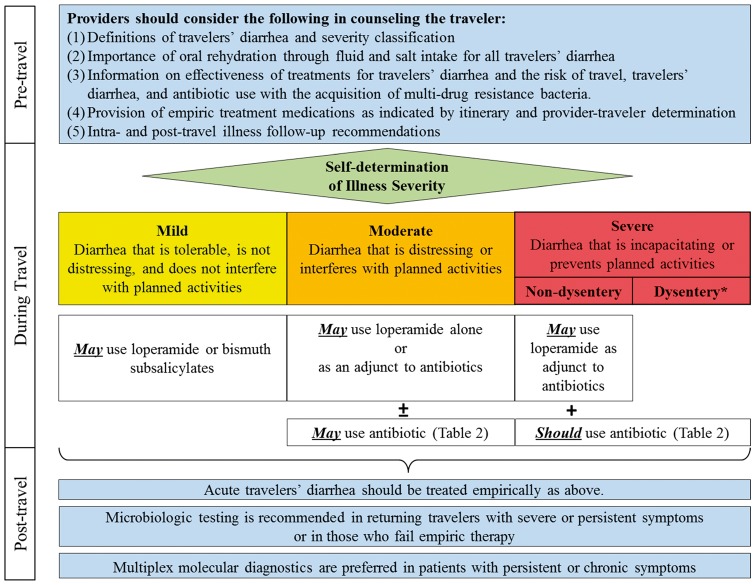
The practice of travel medicine is a unique clinical scenario where we expect the traveler to be the diagnostician, practitioner, and patient when it comes to management of TD. The translation, conveyance and provision of effective confidence in the traveler to follow complicated guidelines with various alternatives and caveats are an obvious challenge in the already compact interaction in which discussion of other topics such as vaccines, prevention measures of malaria, and other pre-travel counseling occurs. Therefore, we attempted to simplify the recommendations (see Figure 1) into “take home points” which can guide the provider as well as be discussed with the traveler:
Figure 1.
Travelers’ diarrhea management algorithm Footnote: *All Dysentery is considered severe
Most if not all travelers should be provided with loperamide and an antibiotic for self-treatment.
If the traveler is going to SE Asia, azithromycin should be prescribed. A FQ, azithromycin or rifaximin can be used in all other regions. (Note: if rifaximin given as first-line agent, azithromycin should also be given in case of dysentery or febrile diarrhea).
The traveler should be informed that if the illness does not impact his or her travel, he or she should keep up on fluids and consider taking loperamide.
If the travelers’ illness is having some impact but is tolerable, they should be instructed to start loperamide and consider taking a single dose antibiotic for expeditious resolution of symptoms, while understanding the benefits and risk of antibiotics for moderate disease.
If the travelers’ illness is keeping them in bed or confined to their room, they should be informed to start antibiotics and add loperamide if expeditious relief is desired.
The traveler should be counseled that if symptoms do not begin to improve within 24 – 36 hours despite self-treatment, it may be necessary to seek medical attention.
Prophylaxis should be considered only in high risk groups (due to underlying health conditions or performance critical occupation/itinerary) with rifaximin being the first choice and bismuth subsalicylate as second option (with a reduced threshold for use).
If rifaximin is used as prophylaxis, azithromycin should be provided for standby break-through therapy.
A rational approach of minimizing antibiotic exposure through use of single dose regimens and selection of a first line antibiotic agent with less evidence of microbiome disruption (rifaximin) and resistance colonization may be advised (and needs immediate further study). Additionally, as travel itself and untreated TD also increase the individual risk of ESBL-PE colonization, non-antibiotic chemoprophylactic strategies, including use of bismuth subsalicylate, may decrease both the acute and post-travel risk concerns. We also recognize that there are differences in the construct, strength and grading of recommendations of this guideline when compared to the recent American College of Gastroenterology guideline and CATMAT statement on the topic of TD treatment, diagnosis and prevention.10,11 We attribute these differences to factors including the accrual of new information since the time of these publications, different methodologies of development, and a dissimilar constitution of experts involved in guideline development and formulation. The expert members involved in this guideline were representative of a broad diversity of country of practice, clinical specialty, and scientific discipline. Finally, making such calculations and decisions can be a challenge for even the most astute traveler and added to the anxiety provoked by the onset of that first abdominal cramp and disturbing urgency while traveling in sometimes austere and inconvenient settings. As such, better and effective educational methods and tools are needed to help travelers’ prepare for and make the best decisions for each individual.
Acknowledgements
We are entirely grateful to leadership and staff from the ISTM for their support and assistance in the conduct of the workshop, coordination of attendance, and other activities. Special thanks go to Diane Nickolson, Jodi Metzgar, Peter Leggat and Annelies Wilder-Smith. In addition, we appreciate the support of Clifford McDonald, Doug Esposito and Ronnie Henry from the Centers of Disease Control and Prevention (CDC) who also supported this effort through development of meeting agenda and focus, expert panel participation, reviewing and managing conflict of interest disclosures, and recording minutes.
Funding: Supported by an unrestricted grantfrom the International Society of Travel Medicine Foundation including a contribution from the Rudin Foundation.
Conflict of interest: The ISTM Foundation strives to produce clinical guidelines that reflect the best available evidence supplemented with the judgment of expert clinicians. Significant efforts are taken to minimize the potential for conflicts of interest to influence guideline content. Funding of guideline production by medical or pharmaceutical entities is prohibited, full disclosure is obtained and evaluated for all guideline contributors, and recusal is used to manage identified relationships. Individual conflict of interest statements for each of the panel voting members are summarized.
Nicholas Beeching receives partial support from the National Institute of Health Research (NIHR) Health Protection Unit in Gastrointestinal Infections, Farr Institute, University of Liverpool, in partnership with PHE and the Universities of Oxford and of East Anglia. Bradley Connor has received consultancy fees/honorarium/support for travel or accommodation from PaxVax, Romark, Clasado and Valneva. Dr. Connor has also participated in the development and drafting of guidelines for the American College of Gastroenterology (2016), and the US Department of Defense (2016). Herbert DuPont has received consultancy fees/honorarium/support for travel or accommodation from Salix, Merck, Cubist, and Seres Health. Dr. DuPont also has active grants from Takeda, Seres Health, Rebiiotix, and the Texas Department of State Health Services, participated in the development and drafting of guidelines for the American College of Gastroenterology (2016), and the US Department of Defense (2016), as well as published multiple articles and given lectures on treatment and prevention of travelers’ diarrhea. Charles Ericsson has received meals from various pharmaceutical companies, and has published research which supports self-treatment and a relatively liberal approach to prevention. Phyllis Kozarsky is the Medical Editor of CDC Health Information for International Travel which publishes guidance for travelers’ diarrhea. Michael Libman led development of the recent Canadian Committee to Advise on Tropical Medicine and Travel statement on Travelers’ Diarrhea treatment and prevention. Mark Riddle is a U.S. military service members and this work was prepared as part of official duties. Dr. Riddle has received support for travel and accommodations to attend various meetings to give lectures on travelers’ diarrhea. Dr. Riddle is principal investigator on a Cooperative Research and Development Agreement between Salix and the Naval Medical Research Center to study efficacy of rifaximin in prevention of travelers’ diarrhea in deployed military populations (Salix provided in-kind drug and placebo only). Dr. Riddle has participated in the development and drafting of guidelines for the American College of Gastroenterology (2016), and the US Department of Defense (2016) on the topic of travelers’ diarrhea management. Robert Steffen has received consultancy fees and travel support from Clasado and Takeda relating to pharmaceutical agents and vaccines for travelers’ diarrhea indications not yet marketed, and received honorarium from Dr Falk Pharma as coordinating investigator in India and Latin America in a trial with a new therapeutic agent against TD. David Tribble is a U.S. federal employee and this work was prepared as part of official duties. David Tribble has been actively involved in the development of guidelines for the Department of Defense on clinical management of acute infectious diarrhea during military deployment and is principal investigator on a Cooperative Research and Development Agreement between Salix and the Uniformed Services University of the Health Sciences to study efficacy of rifaximin in prevention of travelers’ diarrhea in deployed military populations (Salix provided in-kind drug and placebo only). Jordi Vila has published on the evaluation of culture-independent methods in diagnosis of travelers’ diarrhea. Philipp Zanger has published on a randomized controlled trial on the effectiveness of rifaximin in prevention of diarhea in individuals traveling to south and southeast Asia.
Douglas Esposito, Davidson Hamer, Ronald Henry and David Taylor have no conflicts of interest to disclose.
The views expressed are those of the authors and do not reflect those of their respective institutions, organizations or governments.
References
- 1. Steffen R, Hill DR, DuPont HL.. Traveler's diarrhea: a clinical review. JAMA 2015; 313:71–80. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Barber RM, Foreman KJ. et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet 2015; 386:2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travelers' diarrhea. National Institutes of Health Consensus Development Conference Statement. Natl Inst Health Consens Dev Conf Consens Statement 1985; 5:1–7. [PubMed] [Google Scholar]
- 4. Riddle MS, Arnold S, Tribble DR.. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in traveler's diarrhea: a systematic review and meta-analysis. Clin Infect Dis 2008; 47:1007–14. [DOI] [PubMed] [Google Scholar]
- 5. Zaidi D, Wine E.. An update on travelers' diarrhea. Curr Opin Gastroenterol 2015; 31:7–13. [DOI] [PubMed] [Google Scholar]
- 6. Costa M, Oberholzer-Riss M, Hatz C. et al. Pre-travel health advice guidelines for humanitarian workers: a systematic review. Travel Med Infect Dis 2015; 13:449–65. [DOI] [PubMed] [Google Scholar]
- 7. Hagmann SH, Leshem E, Fischer PR. et al. Preparing children for international travel: need for training and pediatric-focused research. J Travel Med 2014; 21:377–83. [DOI] [PubMed] [Google Scholar]
- 8. Dupont HL. Traveling internationally: avoiding and treating travelers' diarrhea. Clin Gastroenterol Hepatol 2010; 8:490–3; quiz e71. [DOI] [PubMed] [Google Scholar]
- 9. Advice for travelers. Treat Guidel Med Lett 2009; 7:83–94. quiz 2 p following 94. [PubMed] [Google Scholar]
- 10. Libman M. Statement on Travellers' Diarrhea. An Advisory Committee Statement (ACS). Committee to Advise on Tropical Medicine and Travel (CATMAT). In: (CATMAT) CtAoTMaT, ed. http://www.phac-aspc.gc.ca/tmp-pmv/catmat-ccmtmv/diarrhea-diarrhee-eng.php: Public Health Agency of Canada, 2015. [DOI] [PMC free article] [PubMed]
- 11. Riddle MS, DuPont HL, Connor BA.. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016; 111:602–22. [DOI] [PubMed] [Google Scholar]
- 12. Arcilla MS, van Hattem JM, Haverkate MR. et al. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 2017; 17:78–85. [DOI] [PubMed] [Google Scholar]
- 13. Armand-Lefevre L, Ruppe E, Andremont A.. ESBL-producing Enterobacteriaceae in travellers: doctors beware. Lancet Infect Dis 2017; 17:8–9. [DOI] [PubMed] [Google Scholar]
- 14. Lalani T, Tisdale MD, Maguire JD. et al. Detection of enteropathogens associated with travelers' diarrhea using a multiplex Luminex-based assay performed on stool samples smeared on Whatman FTA Elute cards. Diagn Microbiol Infect Dis 2015; 83:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spina A, Kerr KG, Cormican M. et al. Spectrum of enteropathogens detected by the FilmArray GI Panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect 2015; 21:719–28. [DOI] [PubMed] [Google Scholar]
- 16. Youmans BP, Ajami NJ, Jiang ZD. et al. Characterization of the human gut microbiome during travelers' diarrhea. Gut Microbes 2015; 6:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teixeira AG, Stephens L, Divers TJ. et al. Effect of crofelemer extract on severity and consistency of experimentally induced enterotoxigenic Escherichia coli diarrhea in newborn Holstein calves. J Dairy Sci 2015; 98:8035–43. [DOI] [PubMed] [Google Scholar]
- 18. Mehta S, Khandelwal PD, Jain VK, Sihag M.. A comparative study of racecadotril and single dose octreotide as an anti-secretory agent in acute infective diarrhea. J Assoc Physicians India 2012; 60:12–5. [PubMed] [Google Scholar]
- 19. Silberschmidt G, Schick MT, Steffen R. et al. Treatment of travellers' diarrhea: zaldaride compared with loperamide and placebo. Eur J Gastroenterol Hepatol 1995; 7:871–5. [PubMed] [Google Scholar]
- 20. Okhuysen PC, DuPont HL, Ericsson CD. et al. Zaldaride maleate (a new calmodulin antagonist) versus loperamide in the treatment of traveler's diarrhea: randomized, placebo-controlled trial. Clin Infect Dis 1995; 21:341–4. [DOI] [PubMed] [Google Scholar]
- 21. Institute of M, Committee on Standards for Developing Trustworthy Clinical Practice G, Graham R.Clinical Practice Guidelines we can Trust. Washington, D.C: National Academies Press, 2011. [Google Scholar]
- 22. Guyatt GH, Oxman AD, Vist GE. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruppe E, Armand-Lefevre L, Estellat C. et al. High rate of acquisition but short duration of carriage of multidrug-resistant enterobacteriaceae after travel to the tropics. Clin Infect Dis 2015; 61:593–600. [DOI] [PubMed] [Google Scholar]
- 24. Lubbert C, Straube L, Stein C. et al. Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol 2015; 305:148–56. [DOI] [PubMed] [Google Scholar]
- 25. Kantele A, Laaveri T, Mero S. et al. Antimicrobials increase travelers' risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis 2015; 60:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alajbegovic S, Sanders JW, Atherly DE, Riddle MS.. Effectiveness of rifaximin and fluoroquinolones in preventing travelers' diarrhea (TD): a systematic review and meta-analysis. Syst Rev 2012; 1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DeBruyn G, Hahn S, Borwick A.. Antibiotic treatment for travelers' diarrhea. Cochrane Database Syst Rev 2000; 3:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reuland EA, Sonder GJ, Stolte I. et al. Travel to Asia and traveller's diarrhea with antibiotic treatment are independent risk factors for acquiring ciprofloxacin-resistant and extended spectrum beta-lactamase-producing Enterobacteriaceae-a prospective cohort study. Clin Microbiol Infect 2016; 22:731.e1–7. [DOI] [PubMed] [Google Scholar]
- 29. Mathai D, Kumar VA, Paul B. et al. Fecal carriage rates of extended-spectrum beta-lactamase-producing Escherichia coli among antibiotic naive healthy human volunteers. Microb Drug Resist 2015; 21:59–64. [DOI] [PubMed] [Google Scholar]
- 30. Woerther PL, Burdet C, Chachaty E, Andremont A.. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26:744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zboromyrska Y, Hurtado JC, Salvador P. et al. Aetiology of traveller's diarrhea: evaluation of a multiplex PCR tool to detect different enteropathogens. Clin Microbiol Infect 2014; 20:O753–9. [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Kabir F, Manneh J. et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhea: a multicentre study. Lancet Infect Dis 2014; 14:716–24. [DOI] [PubMed] [Google Scholar]
- 33. Laaveri T, Pakkanen SH, Antikainen J. et al. High number of diarrheal co-infections in travellers to Benin, West Africa. BMC Infect Dis 2014; 14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beckmann C, Heininger U, Marti H, Hirsch HH.. Gastrointestinal pathogens detected by multiplex nucleic acid amplification testing in stools of pediatric patients and patients returning from the tropics. Infection 2014; 42:961–70. [DOI] [PubMed] [Google Scholar]
- 35. Mutsch M, Pitzurra R, Hatz C, Steffen R.. Post-infectious sequelae of travelers' diarrhea: irritable bowel syndrome. J Travel Med 2014; 21:141–3. [DOI] [PubMed] [Google Scholar]
- 36. Connor BA, Riddle MS.. Post-infectious sequelae of travelers' diarrhea. J Travel Med 2013; 20:303–12. [DOI] [PubMed] [Google Scholar]
- 37. Verdu EF, Riddle MS.. Chronic gastrointestinal consequences of acute infectious diarrhea: evolving concepts in epidemiology and pathogenesis. Am J Gastroenterol 2012; 107:981–9. [DOI] [PubMed] [Google Scholar]
- 38. Lim PL, Han P, Chen LH. et al. Expatriates ill after travel: results from the Geosentinel Surveillance Network. BMC Infect Dis 2012; 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jalanka J, Salonen A, Fuentes S, de Vos WM.. Microbial signatures in post-infectious irritable bowel syndrome–toward patient stratification for improved diagnostics and treatment. Gut Microbes 2015; 6:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allen SJ, Martinez EG, Gregorio GV, Dans LF.. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst Rev 2010; Cd003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis 2007; 5:97–105. [DOI] [PubMed] [Google Scholar]
- 42. Jones J, Hunter D.. Consensus methods for medical and health services research. BMJ 1995; 311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DuPont HL. Persistent diarrhea: a clinical review. JAMA 2016; 315:2712–23. [DOI] [PubMed] [Google Scholar]
- 44. Nair P, Okhuysen PC, Jiang ZD. et al. Persistent abdominal symptoms in us adults after short-term stay in Mexico. J Travel Med 2014; 21:153–8. [DOI] [PubMed] [Google Scholar]
- 45. Connor BA. Sequelae of traveler's diarrhea: focus on postinfectious irritable bowel syndrome. Clin Infect Dis 2005; 41(Suppl 8):S577–86. [DOI] [PubMed] [Google Scholar]
- 46. Thabane M, Simunovic M, Akhtar-Danesh N, Marshall JK.. Development and validation of a risk score for post-infectious irritable bowel syndrome. Am J Gastroenterol 2009; 104:2267–74. [DOI] [PubMed] [Google Scholar]
- 47. Porter CK, Gloor K, Cash BD, Riddle MS.. Risk of functional gastrointestinal disorders in U.S. military following self-reported diarrhea and vomiting during deployment. Dig Dis Sci 2011; 56:3262–9. [DOI] [PubMed] [Google Scholar]
- 48. Wang LH, Fang XC, Pan GZ.. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004; 53:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neal KR, Hebden J, Spiller R.. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 1997; 314:779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. WHO. Antimicrobial resistance. Fact sheet N°194. WHO; http://www.wpro.who.int/mediacentre/releases/2014/AMR_factsheet_FINAL.pdf. [Google Scholar]
- 51. Laupland KB, Church DL, Vidakovich J. et al. Community-onset extended-spectrum beta-lactamase (ESBL) producing Escherichia coli: importance of international travel. J Infect 2008; 57:441–8. [DOI] [PubMed] [Google Scholar]
- 52. DuPont HL, Ericsson CD, Farthing MJ. et al. Expert review of the evidence base for self-therapy of travelers' diarrhea. J Travel Med 2009; 16:161–71. [DOI] [PubMed] [Google Scholar]
- 53. DuPont HL, Ericsson CD, Johnson PC, de la Cabada FJ.. Use of bismuth subsalicylate for the prevention of travelers' diarrhea. Rev Infect Dis 1990; 12(Suppl 1):S64–7. [DOI] [PubMed] [Google Scholar]
- 54. DuPont HL, Sullivan P, Evans DG. et al. Prevention of traveler's diarrhea (emporiatric enteritis). Prophylactic administration of subsalicylate bismuth). JAMA 1980; 243:237–41. [PubMed] [Google Scholar]
- 55. Steffen R, DuPont HL, Heusser R. et al. Prevention of traveler's diarrhea by the tablet form of bismuth subsalicylate. Antimicrob Agents Chemother 1986; 29:625–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DuPont HL, Ericsson CD, Johnson PC. et al. Prevention of travelers' diarrhea by the tablet formulation of bismuth subsalicylate. JAMA 1987; 257:1347–50. [PubMed] [Google Scholar]
- 57. DuPont HL. Therapy for and prevention of traveler's diarrhea. Clin Infect Dis 2007; 45(Suppl 1):S78–84. [DOI] [PubMed] [Google Scholar]
- 58. Zanger P, Nurjadi D, Gabor J. et al. Effectiveness of rifaximin in prevention of diarrhea in individuals travelling to south and southeast Asia: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis 2013; 13:946–54. [DOI] [PubMed] [Google Scholar]
- 59. Flores J, Dupont HL, Jiang ZD. et al. A randomized, double-blind, pilot study of rifaximin 550 mg versus placebo in the prevention of travelers' diarrhea in Mexico during the dry season. J Travel Med 2011; 18:333–6. [DOI] [PubMed] [Google Scholar]
- 60. Martinez-Sandoval F, Ericsson CD, Jiang ZD. et al. Prevention of travelers' diarrhea with rifaximin in US travelers to Mexico. J Travel Med 2010; 17:111–7. [DOI] [PubMed] [Google Scholar]
- 61. Armstrong AW, Ulukan S, Weiner M. et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of rifaximin for the prevention of travelers' diarrhea in US military personnel deployed to Incirlik Air Base, Incirlik, Turkey. J Travel Med 2010; 17:392–4. [DOI] [PubMed] [Google Scholar]
- 62. DuPont HL, Jiang ZD, Okhuysen PC. et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers' diarrhea. Ann Intern Med 2005; 142:805–12. [DOI] [PubMed] [Google Scholar]
- 63. Sierra JM, Ruiz J, Navia MM. et al. In vitro activity of rifaximin against enteropathogens producing traveler's diarrhea. Antimicrob Agents Chemother 2001; 45:643–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruiz J, Mensa L, O'callaghan C. et al. In vitro antimicrobial activity of rifaximin against enteropathogens causing traveler's diarrhea. Diagn Microbiol Infect Dis 2007; 59:473–5. [DOI] [PubMed] [Google Scholar]
- 65. Mendez Arancibia E, Pitart C, Ruiz J. et al. Evolution of antimicrobial resistance in enteroaggregative Escherichia coli and enterotoxigenic Escherichia coli causing traveller's diarrhea. J Antimicrob Chemother 2009; 64:343–7. [DOI] [PubMed] [Google Scholar]
- 66. Menees SB, Maneerattannaporn M, Kim HM, Chey WD.. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 2012; 107:28–35; quiz 36. [DOI] [PubMed] [Google Scholar]
- 67. Lawrence KR, Klee JA.. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy 2008; 28:1019–32. [DOI] [PubMed] [Google Scholar]
- 68. Mullen KD, Sanyal AJ, Bass NM. et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014; 12:1390–7 e2. [DOI] [PubMed] [Google Scholar]
- 69. Johnson PC, Ericsson CD, Morgan DR. et al. Lack of emergence of resistant fecal flora during successful prophylaxis of traveler's diarrhea with norfloxacin. Antimicrob Agents Chemother 1986; 30:671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wistrom J, Norrby SR, Burman LG. et al. Norfloxacin versus placebo for prophylaxis against travellers' diarrhea. J Antimicrob Chemother 1987; 20:563–74. [DOI] [PubMed] [Google Scholar]
- 71. Scott DA, Haberberger RL, Thornton SA, Hyams KC.. Norfloxacin for the prophylaxis of travelers' diarrhea in U.S. military personnel. Am J Trop Med Hyg 1990; 42:160–4. [DOI] [PubMed] [Google Scholar]
- 72. Heck JE, Staneck JL, Cohen MB. et al. Prevention of travelers' diarrhea: ciprofloxacin versus trimethoprim/sulfamethoxazole in adult volunteers working in Latin America and the Caribbean. J Travel Med 1994; 1:136–42. [DOI] [PubMed] [Google Scholar]
- 73. Hoge CW, Gambel JM, Srijan A. et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis 1998; 26:341–5. [DOI] [PubMed] [Google Scholar]
- 74. Pandey P, Bodhidatta L, Lewis M. et al. Travelers' diarrhea in Nepal: an update on the pathogens and antibiotic resistance. J Travel Med 2011; 18:102–8. [DOI] [PubMed] [Google Scholar]
- 75. U.S. Food and Drug Administration. FDA Drug Safety Communication. FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. 2016.
- 76. Pepin J, Saheb N, Coulombe MA. et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis 2005; 41:1254–60. [DOI] [PubMed] [Google Scholar]
- 77. Lalani T, Maguire JD, Grant EM. et al. Epidemiology and self-treatment of travelers' diarrhea in a large, prospective cohort of department of defense beneficiaries. J Travel Med 2015; 22:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hill DR. Occurrence and Self-treatment of Diarrhea in a large cohort of Americans traveling to developing countries. Am J Tropical Med Hygiene 2003; 62:585–9. [DOI] [PubMed] [Google Scholar]
- 79. Soonawala D, Vlot JA, Visser LG.. Inconvenience due to travelers' diarrhea: a prospective follow-up study. BMC Infect Dis 2011; 11:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johnson PC, Ericsson C, DuPont HL, Morgan DR, Bitsura JM, Wood LV.. Comparison of loperamide with bismuth subsalicylate for the treament of acute travelers' diarrhea. J Am Med Assoc 1986; 255:757–60. [PubMed] [Google Scholar]
- 81. Ericsson CD, Dupont H, Mathewson JJ, West MS, Johnson PC, Bitsura JA.. Treatment of travelers' diarrhea with sulfamethoxazole and trimethoprim and loperamide. J Am Med Assoc 1990; 263:257–61. [PubMed] [Google Scholar]
- 82. DuPont HL, Jiang Z-D, Belkind-Gerson J. et al. Treatment of travelers' diarrhea: randomized trial comparing rifaximin, rifaximin plus loperamide, and loperamide alone. Clin Gastroenterol Hepatol 2007; 5:451–6. [DOI] [PubMed] [Google Scholar]
- 83. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg 2000; 62:585–9. [DOI] [PubMed] [Google Scholar]
- 84. Cottreau J, Tucker A, Crutchley R, Garey KW.. Crofelemer for the treatment of secretory diarrhea. Expert Rev Gastroenterol Hepatol 2012; 6:17–23. [DOI] [PubMed] [Google Scholar]
- 85. DiCesare D, DuPont HL, Mathewson JJ. et al. A double blind, randomized, placebo-controlled study of SP-303 (Provir) in the symptomatic treatment of acute diarrhea among travelers to Jamaica and Mexico. Am J Gastroenterol 2002; 97:2585–8. [DOI] [PubMed] [Google Scholar]
- 86. De Bruyn G, Hahn S, Borwick A.. Antibiotic treatment for travellers' diarrhea. Cochrane Database Syst Rev 2000; Cd002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ericsson CD, DuPont HL, Okhuysen PC. et al. Loperamide plus azithromycin more effectively treats travelers' diarrhea in Mexico than azithromycin alone. J Travel Med 2007; 14:312–9. [DOI] [PubMed] [Google Scholar]
- 88. Wang M, Szucs TD, Steffen R.. Economic aspects of travelers' diarrhea. J Travel Med 2008; 15:110–8. [DOI] [PubMed] [Google Scholar]
- 89. Nayyar GM, Attaran A, Clark JP. et al. Responding to the pandemic of falsified medicines. Am J Trop Med Hyg 2015; 92:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wyss MN, Steffen R, Dhupdale NY. et al. Management of travelers' diarrhea by local physicians in tropical and subtropical countries–a questionnaire survey. J Travel Med 2009; 16:186–90. [DOI] [PubMed] [Google Scholar]
- 91. FDA. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Safety Announcement. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf: US Food and Drug Administration, 2016.
- 92. Barreto Miranda I, Ignatius R, Pfuller R. et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med 2016; 23:tav024. [DOI] [PubMed] [Google Scholar]
- 93. Neuberger A, Saadi T, Shetern A, Schwartz E.. Clostridium difficile Infection in travelers–a neglected pathogen? J Travel Med 2013; 20:37–43. [DOI] [PubMed] [Google Scholar]
- 94. Onwuezobe IA, Oshun PO, Odigwe CC.. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev 2012; 11:Cd001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schwille-Kiuntke J, Mazurak N, Enck P.. Systematic review with meta-analysis: post-infectious irritable bowel syndrome after travellers' diarrhea. Aliment Pharmacol Ther 2015; 41:1029–37. [DOI] [PubMed] [Google Scholar]
- 96. Pimentel M, Morales W, Jee SR. et al. Antibiotic prophylaxis prevents the development of a post-infectious phenotype in a new rat model of post-infectious IBS. Dig Dis Sci 2011; 56:1962–6. [DOI] [PubMed] [Google Scholar]
- 97. Ouyang-Latimer J, Jafri S, VanTassel A. et al. In vitro antimicrobial susceptibility of bacterial enteropathogens isolated from international travelers to Mexico, Guatemala, and India from 2006 to 2008. Antimicrob Agents Chemother 2011; 55:874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sanders JW, Frenck RW, Putnam SD. et al. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler's diarrhea in United States military personnel in Turkey. Clin Infect Dis 2007; 45:294–301. [DOI] [PubMed] [Google Scholar]
- 99. Almalki ZS, Guo JJ.. Cardiovascular events and safety outcomes associated with azithromycin therapy: a meta-analysis of randomized controlled trials. Am Health Drug Benefits 2014; 7:318–28. [PMC free article] [PubMed] [Google Scholar]
- 100. Khosropour CM, Capizzi JD, Schafer SD. et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med 2014; 370:1961–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sears SP, Getz TW, Austin CO. et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes 2016; 3:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Acosta A, Camilleri M, Shin A. et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol 2016; 7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ponziani FR, Scaldaferri F, Petito V. et al. The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin. Dig Dis 2016; 34:269–78. [DOI] [PubMed] [Google Scholar]
- 104. Kantele A, Mero S, Kirveskari J, Laaveri T.. Increased risk for ESBL-producing bacteria from co-administration of loperamide and antimicrobial drugs for travelers' diarrhea. Emerg Infect Dis 2016; 22:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Epple HJ, Fromm M, Riecken EO, Schulzke JD.. Antisecretory effect of loperamide in colon epithelial cells by inhibition of basolateral K+ conductance. Scand J Gastroenterol 2001; 36:731–7. [DOI] [PubMed] [Google Scholar]
- 106. Meuris B. Observational study of travelers' diarrhea. J Travel Med 1995; 2:11–5. [DOI] [PubMed] [Google Scholar]
- 107. Kaplan MA, Prior MJ, McKonly KI. et al. A multicenter randomized controlled trial of a liquid loperamide product versus placebo in the treatment of acute diarrhea in children. Clin Pediatr (Phila) 1999; 38:579–91. [DOI] [PubMed] [Google Scholar]
- 108. Caumes E, Menegaux F, Hoang C. et al. From travelers' diarrhea to abdominal surgery: report of three cases. J Travel Med 2004; 11:117–9. [DOI] [PubMed] [Google Scholar]
- 109. Dryden MS, Gabb RJ, Wright SK.. Empirical treatment of severe acute community-acquired gastroenteritis with ciprofloxacin. Clin Infect Dis 1996; 22:1019–25. [DOI] [PubMed] [Google Scholar]
- 110. Oldfield EC 3rd, Bourgeois AL, Omar AK, Pazzaglia GL.. Empirical treatment of Shigella dysentery with trimethoprim: five-day course vs. single dose. Am J Trop Med Hyg 1987; 37:616–23. [DOI] [PubMed] [Google Scholar]
- 111. Gotuzzo E, Oberhelman RA, Maguina C. et al. Comparison of single-dose treatment with norfloxacin and standard 5-day treatment with trimethoprim-sulfamethoxazole for acute shigellosis in adults. Antimicrob Agents Chemother 1989; 33:1101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bennish ML, Salam MA, Khan WA, Khan AM.. Treatment of shigellosis: III. Comparison of one- or two-dose ciprofloxacin with standard 5-day therapy. A randomized, blinded trial. Ann Intern Med 1992; 117:727–34. [DOI] [PubMed] [Google Scholar]
- 113. Khan WA, Seas C, Dhar U. et al. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med 1997; 126:697–703. [DOI] [PubMed] [Google Scholar]
- 114. Murphy GS, Bodhidatta L, Echeverria P. et al. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann Intern Med 1993; 118:582–6. [DOI] [PubMed] [Google Scholar]
- 115. Salazar-Lindo E, Sack RB, Chea-Woo E. et al. Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. J Pediatr 1986; 109:355–60. [DOI] [PubMed] [Google Scholar]
- 116. Ternhag A, Asikainen T, Giesecke J, Ekdahl K.. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007; 44:696–700. [DOI] [PubMed] [Google Scholar]
- 117. Ericsson CD, DuPont HL, Mathewson JJ.. Single dose ofloxacin plus loperamide compared with single dose or three days of ofloxacin in the treatment of traveler's diarrhea. J Travel Med 1997; 4:3–7. [DOI] [PubMed] [Google Scholar]
- 118. Tribble DR, Sanders JW, Pang LW. et al. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis 2007; 44:338–46. [DOI] [PubMed] [Google Scholar]
- 119. DuPont HL, Jiang ZD, Ericsson CD. et al. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis 2001; 33:1807–15. [DOI] [PubMed] [Google Scholar]
- 120. Adachi JA, Ericsson CD, Jiang ZD. et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis 2003; 37:1165–71. [DOI] [PubMed] [Google Scholar]
- 121. Salam I, Katelaris P, Leigh-Smith S, Farthing MJ.. Randomised trial of single-dose ciprofloxacin for travellers' diarrhea. Lancet 1994; 344:1537–9. [DOI] [PubMed] [Google Scholar]
- 122. Kuschner RA, Trofa AF, Thomas RJ. et al. Use of azithromycin for the treatment of Campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis 1995; 21:536–41. [DOI] [PubMed] [Google Scholar]
- 123. Al-Abri SS, Beeching NJ, Nye FJ.. Traveller's diarrhea. Lancet Infect Dis 2005; 5:349–60. [DOI] [PubMed] [Google Scholar]
- 124. Barrett J, Brown M.. Travellers' diarrhea. BMJ 2016; 353:i1937. [DOI] [PubMed] [Google Scholar]
- 125. Hakanen A, Jousimies-Somer H, Siitonen A. et al. Fluoroquinolone resistance in Campylobacter jejuni isolates in travelers returning to Finland: association of ciprofloxacin resistance to travel destination. Emerg Infect Dis 2003; 9:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pollett S, Rocha C, Zerpa R. et al. Campylobacter antimicrobial resistance in Peru: a ten-year observational study. BMC Infect Dis 2012; 12:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stockdale AJ, Beeching NJ, Anson J, Beadsworth MB.. Emergence of extensive fluoroquinolone resistance in Campylobacter gastroenteritis in Liverpool, UK. J Infect 2016; 72:398–400. [DOI] [PubMed] [Google Scholar]
- 128. Mensa L, Marco F, Vila J. et al. Quinolone resistance among Shigella spp. isolated from travellers returning from India. Clin Microbiol Infect 2008; 14:279–81. [DOI] [PubMed] [Google Scholar]
- 129. Pons MJ, Gomes C, Martinez-Puchol S. et al. Antimicrobial resistance in Shigella spp. causing traveller's diarrhea (1995-2010): a retrospective analysis. Travel Med Infect Dis 2013; 11:315–9. [DOI] [PubMed] [Google Scholar]
- 130. Al-Mashhadani M, Hewson R, Vivancos R. et al. Foreign travel and decreased ciprofloxacin susceptibility in Salmonella enterica infections. Emerg Infect Dis 2011; 17:123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chattaway MA, Aboderin AO, Fashae K. et al. Fluoroquinolone-resistant enteric bacteria in sub-saharan africa: clones, implications and research needs. Front Microbiol 2016; 7:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shanks GD, Smoak BL, Aleman GM. et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis 1999; 29:942–3. [DOI] [PubMed] [Google Scholar]
- 133. Hopkins S. Clinical toleration and safety of azithromycin. Am J Med 1991; 91:40S–5S. [DOI] [PubMed] [Google Scholar]
- 134. Tribble D, Sanders J, Pang L. et al. Travelers’ Diarrhea in Thailand: randomized, double-blind trial comparing azithromycin-based regimens (single dose and 3-day) versus levofloxacin (3-Day). Clin Infect Dis 2007; 44:338–46. [DOI] [PubMed] [Google Scholar]
- 135. Treadway G, Pontani D, Reisman A.. The safety of azithromycin in the treatment of adults with community-acquired respiratory tract infections. Int J Antimicrob Agents 2002; 19:189–94. [DOI] [PubMed] [Google Scholar]
- 136. Lister PJ, Balechandran T, Ridgway GL, Robinson AJ.. Comparison of azithromycin and doxycycline in the treatment of non-gonococcal urethritis in men. J Antimicrob Chemother 1993; 31(Suppl E):185–92. [DOI] [PubMed] [Google Scholar]
- 137. Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother 1993; 31(Suppl E):193–8. [DOI] [PubMed] [Google Scholar]
- 138. Zuckerman JM, Qamar F, Bono BR.. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am 2009; 23:997–1026. ix-x. [DOI] [PubMed] [Google Scholar]
- 139. Owens RC Jr., Ambrose PG.. Antimicrobial safety: focus on fluoroquinolones. Clin Infect Dis 2005; 41(Suppl 2):S144–57. [DOI] [PubMed] [Google Scholar]
- 140. Kaleagasioglu F, Olcay E.. Fluoroquinolone-induced tendinopathy: etiology and preventive measures. Tohoku J Exp Med 2012; 226:251–8. [DOI] [PubMed] [Google Scholar]
- 141. Stahlmann R, Lode HM.. Risks associated with the therapeutic use of fluoroquinolones. Expert Opin Drug Saf 2013; 12:497–505. [DOI] [PubMed] [Google Scholar]
- 142. Abo-Salem E, Fowler JC, Attari M. et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther 2014; 32:19–25. [DOI] [PubMed] [Google Scholar]
- 143. Riddle MS, Connor BA.. The traveling microbiome. Curr Infect Dis Rep 2016; 18:29. [DOI] [PubMed] [Google Scholar]
- 144. Taylor DN, Bourgeois AL, Ericsson CD. et al. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am J Trop Med Hyg 2006; 74:1060. [PubMed] [Google Scholar]
- 145. Steffen R, Sack DA, Riopel L. et al. Therapy of travelers' diarrhea with rifaximin on various continents. Am J Gastroenterol 2003; 98:1073–8. [DOI] [PubMed] [Google Scholar]
- 146. Bennett WE Jr, Tarr PI.. Enteric infections and diagnostic testing. Curr Opin Gastroenterol 2009; 25:1–7. [DOI] [PubMed] [Google Scholar]
- 147. Khare R, Espy MJ, Cebelinski E. et al. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J Clin Microbiol 2014; 52:3667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kamada N, Chen GY, Inohara N, Nunez G.. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013; 14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Leslie JL, Young VB.. The rest of the story: the microbiome and gastrointestinal infections. Curr Opin Microbiol 2015; 23:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sazawal S, Hiremath G, Dhingra U. et al. Efficacy of probiotics in prevention of acute diarrhea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis 2006; 6:374–82. [DOI] [PubMed] [Google Scholar]
- 151. Kollaritsch H. H, Wiedermann G.. Prevention of traveler’s diarrhea: a double-blind randomized trial with Saccharomyces cerevisiae Hansen CBS 5926. Travel Medicine: Springer Science + Business Media, 1989:328–332. [Google Scholar]
- 152. Kollaritsch H, Holst H, Grobara P, Wiedermann G.. Prevention of Travelers' diarrhea with Saccharomyces Boulardii: results of a placebo controlled double-blind study. Fortschr Med 1993; 111:152–6. [PubMed] [Google Scholar]
- 153. Oksanen PJ, Salminen S, Saxelin M. et al. Prevention of travellers' diarrhea by Lactobacillus GG. Ann Med 1990; 22:53–6. [DOI] [PubMed] [Google Scholar]
- 154. Hilton E, Kolakowski P, Singer C, Smith M.. Efficacy of lactobacillus GG as a diarrheal preventive in travelers. J Travel Med 1997; 4:41–3. [DOI] [PubMed] [Google Scholar]
- 155. De Dios Pozo-Olano J, Warram JJ, Gomez R, Cazavos M.. Effect of lactobacilli preparation on travelers' diarrhea. Gastroenterology 1978; 74:829–30. [PubMed] [Google Scholar]
- 156. Katelaris PH, Salam I, Farthing MJ.. Lactobacilli to prevent traveler's diarrhea? N Engl J Med 1995; 333:1360–1. [DOI] [PubMed] [Google Scholar]
- 157. Drakoularakou A, Tzortzis G, Rastall RA, Gibson GR.. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers' diarrhea. Eur J Clin Nutr 2010; 64:146–52. [DOI] [PubMed] [Google Scholar]
- 158. Virk A, Mandrekar J, Berbari EF. et al. A randomized, double blind, placebo-controlled trial of an oral synbiotic (AKSB) for prevention of travelers' diarrhea. J Travel Med 2013; 20:88–94. [DOI] [PubMed] [Google Scholar]
- 159. Bruno F, Frigerio G.. A new therapeutic alternative for the treatment of enteritis–controlled double-blind tests with the strain SF 68. Schweiz Rundsch Med Prax 1981; 70:1717–20. [PubMed] [Google Scholar]
- 160. Bruno F, Nastasi A, Bruno M.. Double-blind controlled study of the effect of the lactogenic enterococcus SF68 strain on various enterocolitis associated manifestations and on salmonella infections. Clin Ter 1983; 105:203–7. [PubMed] [Google Scholar]
- 161. Buydens P, Debeuckelaere S.. Efficacy of SF 68 in the treatment of acute diarrhea. A placebo-controlled trial. Scand J Gastroenterol 1996; 31:887–91. [DOI] [PubMed] [Google Scholar]
- 162. Wunderlich PF, Braun L, Fumagalli I. et al. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhea and in the treatment of acute diarrhea. J Int Med Res 1989; 17:333–8. [DOI] [PubMed] [Google Scholar]
- 163. Mitra AK, Rabbani GH.. A double-blind, controlled trial of bioflorin (Streptococcus faecium SF68) in adults with acute diarrhea due to Vibrio cholerae and enterotoxigenic Escherichia coli. Gastroenterology 1990; 99:1149–52. [DOI] [PubMed] [Google Scholar]
- 164. Höchter W, Chase D, Hagenhoff G.. Saccharomyces boulardii in the treatment of acute adult diarrhea. [Saccharomyces boulardii bei acuter Erwachsenendiarrhoea]. Münchener Medizinische Wochenschrift 1990; 132:188–92. [Google Scholar]
- 165. Lund B, Edlund C.. Probiotic Enterococcus faecium strain is a possible recipient of the vanA gene cluster. Clin Infect Dis 2001; 32:1384–5. [DOI] [PubMed] [Google Scholar]
- 166. Hempel S, Newberry SJ, Maher AR. et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 2012; 307:1959–69. [DOI] [PubMed] [Google Scholar]
- 167. Epelboin L, Robert J, Tsyrina-Kouyoumdjian E. et al. High rate of multidrug-resistant gram-negative bacilli carriage and infection in hospitalized returning travelers: a cross-sectional cohort study. J Travel Med 2015; 22:292–9. [DOI] [PubMed] [Google Scholar]
- 168. Soraas A, Sundsfjord A, Sandven I. et al. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae–a case-control study in a low prevalence country. PLoS One 2013; 8:e69581. [DOI] [PMC free article] [PubMed] [Google Scholar]