Abstract
Mosquito-transmitted arthropod-borne viruses (arboviruses) such as dengue virus, chikungunya virus, and West Nile virus constitute a major public health burden and are increasing in severity and frequency worldwide. The microbiota associated with mosquitoes (comprised of viruses, bacteria, fungi and protozoa) can profoundly influence many host phenotypes including vector competence, which can either be enhanced or suppressed. Thus, the tripartite interactions between the mosquito vector, its microbiota and the pathogens they transmit offer novel possibilities to control arthropod-borne diseases.
Introduction
It is becoming increasingly apparent that organisms do not function in isolation. A complex consortium of microbes resides within a host, which influences the individual's phenotype. This holds true for mosquitoes that transmit medically important arboviruses such as dengue virus (DENV), chikungunya virus (CHIKV), and West Nile virus (WNV). Interactions within the mosquito holobiome (host and associated microbes) can profoundly impact many phenotypes, including vector competence for pathogens. Here, we highlight recent advances in our understanding of how the microbial members of the mosquito holobiome influence arbovirus transmission. We focus our attention on bacteria with extracellular phases predominantly found within the mosquito gut, rather than obligate intracellular bacteria of mosquitoes that have been extensively reviewed elsewhere [1–3].
Composition of the mosquito microbiome
Comprehending the diversity and dynamics of the microbial community is imperative in understanding the relationships within the mosquito holobiome. Studies exploiting high throughput sequencing have revealed that the bacterial microbiome of mosquito vectors has low diversity but is highly variable. Most studies thus far have focused on Anopheles mosquitoes [4•,5,6•,7••,8••,9], and while the number of studies examining the bacterial microbiomes from Aedes and Culex mosquitoes is growing [6•,8••,10], this is an area that needs further attention. Although not as comprehensive, culture dependent approaches have been used for characterization of the microbiome of important Aedes and Culex arboviral vectors [11,12]. Bacterial members that appear abundant across a range of mosquito species include aerobes and facultative anaerobes within the Gammaproteobacteria, Flavobacteria and Alphaproteobacteria [6•]. The variability within the mosquito microbial community appears to be influenced by environmental factors such as diet and host factors such as sex, species and developmental stage. While distinct differences are notable, there does appear to be some commonality, and in particular, Aedes and Anopheles vectors share common taxa including Pseudomonas, Asaia, Serratia and Enterobacter [6•,8••]. These bacteria are often located in the mosquito gut, meaning they are proximal to any ingested arboviruses, but they may also infect other tissues including the germline, salivary glands and malpighian tubules [13–15]. Intriguingly, the salivary glands of Anopheles culicifacies harbors a more diverse microbiota compared to the gut [15]. Whereas obligate intracellular symbionts such as Wolbachia are predominantly maternally transmitted, extracellular bacteria found in the gut likely have intracellular stages enabling their vertical transmission route (see [16] describing paternal transmission) and can also be horizontally acquired. In an elegant study, Coon et al. [8••] demonstrated that bacteria are transstadially transmitted in the important viral vector, Aedes aegypti.
While most studies have focused on bacteria, other organisms contribute to the species richness of the mosquito microbiome, including viruses, fungi and protozoans. Shotgun metagenomic sequencing offers a PCR-independent high throughput method to characterize these taxa and has been used to identify novel viruses within the Bunyaviridae and Rhabdoviridae in addition to many genera of fungi in the arboviral vector Culex pipiens [17]. Studies such as this fuel the growing appreciation that non-bacterial microbes contribute meaningfully to mosquito biology. To this end, insect-specific viruses have been discovered in several mosquito species [18–20], while culture dependent methods identified yeast species in Aedes and Anopheles mosquitoes [12,21]. Although the role of both insect-specific viruses and yeast in mosquito biology is unclear, there is evidence that these microbes can influence vector competence [22,23].
Influence on vector competence
The bacterial microbiome is a potent modulator of mosquito vector competence. While several studies have investigated the effect of the bacterial microbiome on Plasmodium infection in Anopheles mosquitoes [24,25], it is also evident the microbiome modulates arboviral vector competence. Most studies employ antibiotic treatment or reinfection of cultured microbes to perturb the microbiome. While antibiotic treated mosquitoes are far from aseptic [7••], this dysbiosis is sufficient to influence viral pathogen dynamics. For example, DENV serotype 2 (DENV-2) titer decreased when A. aegypti were supplemented with a cocktail of antibiotics [26]. Similarly, when bacterial isolates were administered to mosquitoes, DENV-2 titers within A. aegypti were reduced. A Chromobacterium isolated from field caught A. aegypti colonized the midgut upon reinfection when fed to mosquitoes in a sugar meal and significantly reduced DENV-2 replication [27•]. Likewise, bacteria in the genus Proteus and Paenibacillus also inhibited DENV-2 density when administered to mosquitoes in the blood meal [28]. Proteus also inhibited DENV-2 titer when administered in a sugar meal [28]. In contrast to these findings, some microbes increase arboviruses in mosquitoes [29–31]. A Serratia odorifera isolate has been shown to increase both DENV and CHIKV replication [30,31]. Similarly, elegant work has shown that the resident microbiota are essential for O'nyong nyong virus (ONNV) to infect Anopheles [32••]. It was further demonstrated that infection of ONNV was rescued in antibiotic treated mosquitoes when supplementing the blood meal with a live culture of midgut bacteria, but not a heat killed culture [32••]. Using a comparative approach, the midgut bacterial composition was found to be different in three A. aegypti lines that vary in their susceptibility to DENV [33], although antibiotic treatment of these strains did not effect DENV vector competence [34]. These reinfection studies demonstrate the utility of culture-based approaches for dissecting the influence of microbes on arboviruses in mosquitoes and the mechanisms behind such traits.
Microbiota influence on vectorial capacity
In addition to direct and indirect effects on vector competence, the microbiota can also alter other mosquito traits that can influence vectorial capacity. These include various physiological traits like nutrition, reproduction and development. For example, antibiotic treatment of larvae results in aborted development, however this effect can be rescued by bacterial supplementation [8••,35]. In another case, bacteria isolated from the midgut of A. aegypti have been shown to influence blood digestion and egg development [36]. Parameters such as mosquito survival, development time, and reproductive capacity can have large influences on the population-level vectorial capacity for arboviruses that are equal or greater than the direct effects on vector competence.
Influence of virus infection on the microbiome
While it is evident that the microbiome affects arboviruses, there is evidence that the reciprocal interaction also occurs. In the Asian tiger mosquito Aedes albopictus, CHIKV infection increases the abundance of bacteria in the family Enterobacteriaceae and reduces Wolbachia and Blattabacterium [37]. Whether bacterial titers are responding directly to CHIKV or changing in response to other stimuli remains to be determined. For example, CHIKV is known to suppress the Toll pathway [38], and virus-mediated immune modulation inhibits the overall bacterial abundance in A. aegypti [28]. Alternatively, bacteria in the Enterobacteriaceae may be expanding in response to the reduction of Wolbachia and Blattabacterium.
Microbial interactions
While we recognize that important interactions occur between microbiota and arboviruses within mosquitoes, less is known regarding the interplay between the other members of the mosquito microbiome. However, some intriguing results are beginning to illuminate this field. This is important given the impact of the microbiome on many aspects of mosquito biology. The acetic acid bacterium Asaia interferes with Wolbachia transmission in Anopheles mosquitoes [7••], and recent work suggests this antagonism extends into Culex and Aedes species [39]. Culture-based experiments have also found inhibition between bacterial isolates from mosquitoes [27•,40], although this interplay needs to be confirmed in vivo. Contrary to these inhibitory interactions, a positive correlation was seen between Asaia and Acinetobacter in the midgut of A. albopictus [41].
Mode of action
Experimental evidence suggests there are several mechanisms by which bacteria affect arboviruses in mosquitoes (Figure 1). These fall into the categories of immunity [26,32••], production of metabolites [27•], resource competition [42] and regulating miRNAs [43,44], however it is likely that further mechanisms will be uncovered in the future.
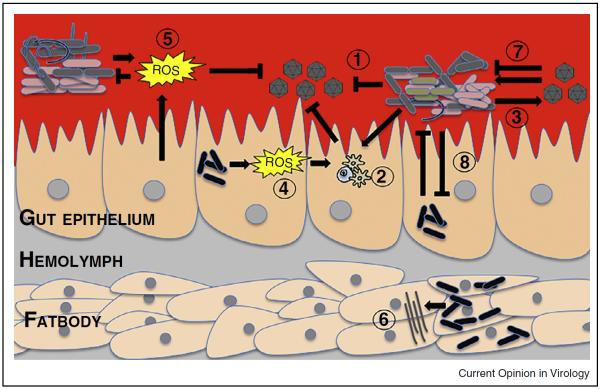
Figure 1.
Schematic illustrating the tripartite interactions between the mosquito host, the microbiome and arboviruses. Members of the microbiome can directly impede viruses (1), or can stimulate basal immunity leading to virus suppression (2). Conversely, some bacterial species can enhance viruses (3). Intracellular bacteria such as Wolbachia can also stimulate immunity by production of reactive oxygen species (ROS) (4). ROS can also be generated by the mosquito host and members of the microbiome and suppresses bacteria and pathogens (5). Intracellular bacteria can also manipulate host miRNA expression (6). Arboviruses can both suppress and enhance members of the microbiome (7) while bacterial interactions also influence the microbiome composition (8).
Host immunity
There is a complex interplay between the resident microbiota and the insect immune system. Insights from Drosophila indicate the microbiome primes and matures host immunity while the immune system keeps microbial levels under tight control [45]. While less is known about the role of bidirectional cross talk in mosquito immune homeostasis, it is clear that immune pathways that ward off invading arboviruses also influence the microbiota. For example, overlap exists within the JAK-STAT pathway which is elicited by bacteria and fungi, but is also effective against DENV and WNV [46,47]. Similarly, the Toll pathway is activated by gram-positive bacteria and has anti-viral properties [26], while the IMD pathway has been shown to influence Serratia and ONNV densities in An. gambiae [32••,48•]. Another important class of immune molecules, which also have complex interactions with pathogens and the microbiota, are reactive oxygen species (ROS). ROS has anti-pathogenic properties, regulates the microbiota density, and can activate the Toll pathway [49•,50]. RNAi is another major antiviral defense of mosquitoes [51], however the interplay between this pathway and the microbiota is yet to be determined. These complex interactions between host immunity, the microbiome and invading pathogens has led to the `holo-immunome' concept [52], which emphasizes the role of the microbial community's (and pathogenic microbes) influence on the immune status of an organism.
Metabolites
Bacteria produce secondary metabolites with anti-viral properties. Several bacterial isolates from the A. albopictus midgut produce bioactive compounds that inhibit La Crosse virus [53]. Additionally, a Chromobacterium isolated from field A. aegypti mosquitoes possesses anti-dengue and anti-Plasmodium activity [27•]. These compounds are promising candidates for anti-viral drugs. However, further work is required to examine the biological relevance of these metabolites in vivo and their effect on arbovirus dissemination into the mosquito gut.
Resource competition
Both arboviruses and bacteria scavenge for resources in mosquitoes. Cholesterol and lipids are common molecules required by these microbes. In Drosophila, Spiroplasma utilizes lipids and vitamins from the host for replication [54], while Wolbachia sequesters cholesterol in mosquitoes [55]. Studies on the interplay between the gut microbiota and cholesterol in insects are limited, but evidence from a murine model system suggests microbes regulate cholesterol homeostasis [56]. DENV is known to perturb host lipid levels to facilitate replication, while cholesterol is essential for flavivirus replication [57,58]. Supplementation of exogenous cholesterol ablated the viral protection effect of Wolbachia in flies suggesting there is competition between these microbes for this molecule [42].
miRNA
The endosymbiotic bacterium Wolbachia modulates miRNA expression in A. aegypti [43]. Wolbachia has also been found to express miRNAs that manipulate mosquito gene expression [44]. Host derived miRNA can influence viral dynamics in A. albopictus [59]. However, it is unknown if gut bacteria influence mosquito miRNA expression, but insights from mammalian models suggest the microbiota has the capacity to modulate host miRNA levels [60].
Microbes for applied vector control
By far the most developed strategy for microbial control of arboviruses is the use of the intracellular bacterium Wolbachia (comprehensively reviewed [1–3]). However, other microbes of mosquitoes also have great potential for applied control strategies. These include not only harnessing their innate anti-viral abilities, but also to engineer microbes to interfere with pathogens; essentially using microbes as a delivery platform for the production of anti-viral molecules that either target the virus, mosquito pathways essential for virus replication, or to induce mosquito pathways antagonistic to arboviruses. This process, termed paratransgenesis, is being investigated in mosquitoes mainly for control of malaria parasites, but this application has the potential to be used for arbovirus control. In addition to bacteria, fungi and viruses are also promising candidates for use in paratransgenic control [61,62]. Excitingly, bacteria have been used to generate and transfer dsRNA to manipulate mosquito gene expression, offering new prospects for paratransgenesis strategies to reduce arboviruses [63•,64•]. The ability to deliver RNAi into field mosquito populations would be highly desirable and enable the development of a myriad of control approaches. The findings that close relatives of genetically tractable model bacteria are found in field mosquitoes [6•,7••,8••], that these model bacteria appear to be beneficial to the mosquito host [8••], and translocate into numerous mosquito tissues [65] will further enhance research in this field.
Future prospects
Studies perturbing the mosquito microbiome have elucidated its role in arboviral transmission. The next challenge in this area is to determine the biological relevance of these manipulations, and how this relates to natural variation of the mosquito microbiome in the field. Studies in an Anopheles–Plasmodium system suggest that the variability in the microbiome in field mosquitoes influences vector competence [9]. While we are beginning to characterize the bacterial microbiome in mosquitoes, we have a poor understanding of the viral and fungal communities, and this needs to be urgently addressed. Further to this, the role of bacteriophage in shaping the gut microbiome of mosquitoes or other insects is virtually unknown. This is an area of increasing interest in the vertebrate community [66], and insight from these systems may provide stimulus for studies in mosquitoes. Elucidating the core microbiome from transient microbes within mosquitoes and determining their influence upon the host, and whether bacterial members have functional redundancy are further challenges. While a mechanistic understanding of these tripartite interactions is desirable, the development of high throughput in vitro assays capable of assessing host–microbe–pathogen interactions will undoubtedly be useful in this regard [67,68]. Alarmingly, recent work suggests it is possible that the medicinal use of antibiotics and other products is also perturbing the microbiota within mosquitoes [69••,70]. Mosquitoes that fed upon humans treated with antibiotics were shown to have an altered microbiome compared to mosquitoes imbibing a blood meal from humans not using antibiotics [69••]. Similarly, the expulsion of antibiotics and other personal products from humans into the environment was shown in laboratory experiments to modify the larval microbiome of mosquitoes [70]. These findings suggest anthropogenic alteration of the environment by antibiotics and pharmaceutical products could manipulate the microbiota of mosquitoes, thus have wide-reaching implications for mosquito biology and pathogen transmission. This area of research warrants further investigation.
Conclusions
The influence of the microbiome on host biology is a burgeoning area of research. For mosquitoes that transmit arboviruses, this question is of great and timely importance, not only to increase our basic understanding of host–microbe interactions but also for its relevance for vector control. Given the increase in arboviral disease, novel microbial control approaches offer promising strategies to combat these pathogens.
Acknowledgements
This work has been supported by a University of Texas Rising Star Award and Brown Foundation grant to GLH and by NIH/NIAID grants R21AI111175, R21AI088311, R56AI116636 and R01AI067371 to JLR.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, Bossin HC, Moretti R, Baton LA, Hughes GL, et al. Harnessing mosquito–Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Hughes GL, Rasgon JL. Transinfection: a method to investigate Wolbachia–host interactions and control arthropod-borne disease. Insect Mol Biol. 2014;23:141–151. doi: 10.1111/imb.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rainey SM, Shah P, Kohl A, Dietrich I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol. 2014;95:517–530. doi: 10.1099/vir.0.057422-0. [DOI] [PubMed] [Google Scholar]
- 4•.Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes the microbiome of A. gambaie mosquitoes across various life stages and diets.
- 5.Gimonneau G, Tchioffo MT, Abate L, Boissière A, Awono-Ambene PH, Nsango SE, Christen R, Morlais I. Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect Genet Evol. 2014;28:715–724. doi: 10.1016/j.meegid.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 6•.Osei Poku J, Mbogo CM, Palmer WJ, Jiggins FM. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol Ecol. 2012;21:5138–5150. doi: 10.1111/j.1365-294X.2012.05759.x. [DOI] [PubMed] [Google Scholar]; This article characterizes the gut microbiome of several field caught mosquito species.
- 7••.Hughes GL, Dodson BL, Johnson RM, Murdock CC, Tsujimoto H, Suzuki Y, Patt AA, Cui L, Nossa CW, Barry RM, et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc Natl Acad Sci USA. 2014;111:12498–12503. doi: 10.1073/pnas.1408888111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that Asaia inhibits Wolbachia transmission in Anopheles mosquitoes and provides a possible explanation for the lack of Wolbachia in many Anopheles species.
- 8••.Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, a novel method was developed to create axenic larvae that were subsequently used in experiments that suggest bacteria are transmitted transstadially. It was also found that Aedes and Anopheles mosquitoes have a similar microbiome.
- 9.Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minard G, Tran FH, Dubost A, Tran-Van V, Mavingui P, Moro CV. Pyrosequencing 16S rRNA genes of bacteria associated with wild tiger mosquito Aedes albopictus: a pilot study. Front Cell Infect Microbiol. 2014;4:59. doi: 10.3389/fcimb.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pidiyar VJ, Jangid K, Patole MS, Shouche YS. Studies on cultured and uncultured microbiota of wild Culex quinquefasciatus mosquito midgut based on 16S ribosomal RNA gene analysis. Am J Trop Med Hyg. 2004;70:597–603. [PubMed] [Google Scholar]
- 12.Gusmão DS, Santos AV, Marini DC, Bacci M, Berbert-Molina MA, Lemos FJA. Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop. 2010;115:275–281. doi: 10.1016/j.actatropica.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci USA. 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavshin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Zarenejad F, Terenius O. Malpighian tubules are important determinants of Pseudomonas transstadial transmission and longtime persistence in Anopheles stephensi. Parasites Vectors. 2015;8:36. doi: 10.1186/s13071-015-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Sharma S, Maurya RK, De Das T, Thomas T, Lata S, Singh N, Pandey KC, Valecha N, Dixit R. Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Parasites Vectors. 2014;7:235. doi: 10.1186/1756-3305-7-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damiani C, Ricci I, Crotti E, Rossi P, Rizzi A, Scuppa P, Esposito F, Bandi C, Daffonchio D, Favia G. Paternal transmission of symbiotic bacteria in malaria vectors. Curr Biol. 2008;18:R1087–R1088. doi: 10.1016/j.cub.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Chandler JA, Liu RM, Bennett SN. RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front Microbiol. 2015;6:185. doi: 10.3389/fmicb.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasar F, Palacios G, Gorchakov RV, Guzman H, Da Rosa APT, Savji N, Popov VL, Sherman MB, Lipkin WI, Tesh RB, et al. Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proc Natl Acad Sci USA. 2012;109:14622–14627. doi: 10.1073/pnas.1204787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blitvich B, Firth A. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses. 2015;7:1927–1959. doi: 10.3390/v7041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolling BG, Vasilakis N, Guzman H, Widen SG, Wood TG, Popov VL, Thangamani S, Tesh RB. Insect-specific viruses detected in laboratory mosquito colonies and their potential implications for experiments evaluating arbovirus vector competence. Am J Epidemiol. 2015;92:422–428. doi: 10.4269/ajtmh.14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricci I, Mosca M, Valzano M, Damiani C, Scuppa P, Rossi P, Crotti E, Cappelli A, Ulissi U, Capone A, et al. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): perspectives on vector-borne diseases symbiotic control. Antonie Van Leeuwenhoek. 2010;99:443–450. doi: 10.1007/s10482-010-9532-3. http://dx.doi.org/10.1007/s10482-010-9532-3. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Morton JC, Ramirez JL, Souza-Neto JA, Dimopoulos G. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem Mol Biol. 2012;42:126–132. doi: 10.1016/j.ibmb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenney JL, Solberg OD, Langevin SA, Brault AC. Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J Gen Virol. 2014;95:2796–2808. doi: 10.1099/vir.0.068031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennison NJ, Jupatanakul N, Dimopoulos G. The mosquito microbiota influences vector competence for human pathogens. Curr Opin Insect Sci. 2014;3:6–13. doi: 10.1016/j.cois.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul-Ghani R, Al-Mekhlafi AM, Alabsi MS. Microbial control of malaria: biological warfare against the parasite and its vector. Acta Trop. 2012;121:71–84. doi: 10.1016/j.actatropica.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S, Tripathi A, Mlambo G, Dimopoulos G. Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog. 2014;10:e1004398. doi: 10.1371/journal.ppat.1004398. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that a Chromobacterium isolate from field mosquitoes has anti-dengue and anti-Plasmodium properties.
- 28.Ramirez JL, Souza-Neto J, Torres Cosme R, Rovira J, Ortiz A, Pascale JM, Dimopoulos G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl Trop Dis. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis. 2014;8:e2965. doi: 10.1371/journal.pntd.0002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apte-Deshpande A, Paingankar M, Gokhale MD, Deobagkar DN. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE. 2012;7:e40401. doi: 10.1371/journal.pone.0040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apte-Deshpande AD, Paingankar MS, Gokhale MD, Deobagkar DN. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J Med Res. 2014;139:762–768. [PMC free article] [PubMed] [Google Scholar]
- 32••.Carissimo G, Pondeville E, McFarlane M, Dietrich I, Mitri C, Bischoff E, Antoniewski C, Bourgouin C, Failloux A-B, Kohl A, et al. Antiviral immunity of Anopheles gambiaeis highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc Natl Acad Sci USA. 2015;112:E176–E185. doi: 10.1073/pnas.1412984112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Anopheles mosquitoes were shown to have compartmentalized immune responses to O'nyong nyong virus and the microbiome was demonstrated to be essential for virus infection.
- 33.Charan SS, Pawar KD, Severson DW, Patole MS, Shouche YS. Comparative analysis of midgut bacterial communities of Aedes aegypti mosquito strains varying in vector competence to dengue virus. Parasitol Res. 2013;112:2627–2637. doi: 10.1007/s00436-013-3428-x. [DOI] [PubMed] [Google Scholar]
- 34.Hill CL, Sharma A, Shouche Y, Severson DW. Dynamics of midgut microflora and dengue virus impact on life history traits in Aedes aegypti. Acta Trop. 2014;140:151–157. doi: 10.1016/j.actatropica.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chouaia B, Rossi P, Epis S, Mosca M, Ricci I, Damiani C, Ulissi U, Crotti E, Daffonchio D, Bandi C, et al. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiol. 2012;12:S2. doi: 10.1186/1471-2180-12-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaio A, de O, Gusmão DS, Santos AV, Berbert-Molina MA, Pimenta PFP, Lemos FJA. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.) Parasites Vectors. 2011;4:105. doi: 10.1186/1756-3305-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zouache K, Michelland RJ, Failloux A-B, Grundmann GL, Mavingui P. Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Mol Ecol. 2012;21:2297–2309. doi: 10.1111/j.1365-294X.2012.05526.x. [DOI] [PubMed] [Google Scholar]
- 38.McFarlane M, Arias-Goeta C, Martin E, O'Hara Z, Lulla A, Mousson L, Rainey SM, Misbah S, Schnettler E, Donald CL, et al. Characterization of Aedes aegypti innate-immune pathways that limit chikungunya virus replication. PLoS Negl Trop Dis. 2014;8:e2994. doi: 10.1371/journal.pntd.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi P, Ricci I, Cappelli A, Damiani C, Ulissi U, Mancini MV, Valzano M, Capone A, Epis S, Crotti E, et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasites Vectors. 2015;8:278. doi: 10.1186/s13071-015-0888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terenius O, Lindh JM, Eriksson-Gonzales K, Bussière L, Laugen AT, Bergquist H, Titanji K, Faye I. Midgut bacterial dynamics in Aedes aegypti. FEMS Microbiol Ecol. 2012;80:556–565. doi: 10.1111/j.1574-6941.2012.01317.x. [DOI] [PubMed] [Google Scholar]
- 41.Minard G, Tran FH, Raharimalala FN, Hellard E, Ravelonandro P, Mavingui P, Valiente Moro C. Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol Ecol. 2013;83:63–73. doi: 10.1111/j.1574-6941.2012.01455.x. [DOI] [PubMed] [Google Scholar]
- 42.Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, McGraw EA. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussain M, Frentiu FD, Moreira LA, O'Neill SL, Asgari S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:9250–9255. doi: 10.1073/pnas.1105469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayoral JG, Hussain M, Joubert DA, Iturbe-Ormaetxe I, O'Neill SL, Asgari S. Wolbachia small noncoding RNAs and their role in cross-kingdom communications. Proc Natl Acad Sci USA. 2014;111:18721–18726. doi: 10.1073/pnas.1420131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol. 2013;11:615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- 46.Paradkar PN, Duchemin J-B, Voysey R, Walker PJ. Dicer-2-dependent activation of Culex vago occurs via the TRAF-Rel2 signaling pathway. PLoS Negl Trop Dis. 2014;8:e2823. doi: 10.1371/journal.pntd.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Stathopoulos S, Neafsey DE, Lawniczak MKN, Muskavitch MAT, Christophides GK. Genetic dissection of Anopheles gambiae gut epithelial responses to Serratia marcescens. PLoS Pathog. 2014;10:e1003897. doi: 10.1371/journal.ppat.1003897. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that host genetics influence susceptibility to orally acquired microbes.
- 49•.Oliveira JHM, Gonçalves RLS, Lara FA, Dias FA, Gandara ACP, Menna-Barreto RFS, Edwards MC, Laurindo FRM, Silva-Neto MAC, Sorgine MHF, et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011;7:e1001320. doi: 10.1371/journal.ppat.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, reductions in reactive oxygen species after a blood meal were demonstrated to enable bacteria to proliferate in the mosquito midgut.
- 50.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–E32. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blair CD. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011;6:265–277. doi: 10.2217/fmb.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dheilly NM. Holobiont–holobiont interactions: redefining host–parasite interactions. PLoS Pathog. 2014;10:e1004093. doi: 10.1371/journal.ppat.1004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyce JD, Nogueira JR, Bales AA, Pittman KE, Anderson JR. Interactions between La Crosse virus and bacteria isolated from the digestive tract of Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2011;48:389–394. doi: 10.1603/me09268. [DOI] [PubMed] [Google Scholar]
- 54.Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. Insect endosymbiont proliferation is limited by lipid availability. Elife. 2014;3:e02964. doi: 10.7554/eLife.02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caragata EP, Rancès E, O'Neill SL, McGraw EA. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol. 2013;67:205–218. doi: 10.1007/s00248-013-0339-4. http://dx.doi.org/10.1007/s00248-013-0339-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhong C-Y, Sun W-W, Ma Y, Zhu H, Yang P, Wei H, Zeng B-H, Zhang Q, Liu Y, Li W-X, et al. Microbiota prevents cholesterol loss from the body by regulating host gene expression in mice. Sci Rep. 2015;5:10512. doi: 10.1038/srep10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C-J, Lin H-R, Liao C-L, Lin Y-L. Cholesterol effectively blocks entry of flavivirus. J Virol. 2008;82:6470–6480. doi: 10.1128/JVI.00117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan H, Zhou Y, Liu Y, Deng Y, Chen X. miR-252 of the Asian tiger mosquito Aedes albopictus regulates dengue virus replication by suppressing the expression of the dengue virus envelope protein. J Med Virol. 2014;86:1428–1436. doi: 10.1002/jmv.23815. [DOI] [PubMed] [Google Scholar]
- 60.Masotti A. Interplays between gut microbiota and gene expression regulation by miRNAs. Front Cell Infect Microbiol. 2012;2:137. doi: 10.3389/fcimb.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang W, Vega-Rodriguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St Leger RJ. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331:1074–1077. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki Y, Niu G, Hughes GL, Rasgon JL. A viral over-expression system for the major malaria mosquito Anopheles gambiae. Sci Rep. 2014;4:5127. doi: 10.1038/srep05127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Whyard S, Erdelyan CN, Partridge AL, Singh AD, Beebe NW, Capina R. Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasites Vectors. 2015;8:181–220. doi: 10.1186/s13071-015-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that orally administered RNAi can induce gene silencing in mosquitoes.
- 64•.Taracena ML, Oliveira PL, Almendares O, Umaña C, Lowenberger C, Dotson EM, Paiva-Silva GO, Pennington PM. Genetically modifying the insect gut microbiota to control Chagas disease vectors through systemic RNAi. PLoS Negl Trop Dis. 2015;9:e0003358. doi: 10.1371/journal.pntd.0003358. [DOI] [PMC free article] [PubMed] [Google Scholar]; Escherichia coli were used to delivery RNAi to the insect gut to manipulate the vector.
- 65.Chaxhin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Raeisi A, Zarenejad F. Escherichia coli expressing a green fluorescent protein (GFP) in Anopheles stephensi: a preliminary model for paratransgenesis. Symbiosis. 2013;60:17–24. [Google Scholar]
- 66.Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat Rev Microbiol. 2012;10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes GL, Pike AD, Xue P, Rasgon JL. Invasion of Wolbachia into Anopheles and other insect germlines in an ex vivo organ culture system. PLoS ONE. 2012;7:e36277. doi: 10.1371/journal.pone.0036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pyles RB, Vincent KL, Baum MM, Elsom B, Miller AL, Maxwell C, Eaves-Pyles TD, Li G, Popov VL, Nusbaum RJ, et al. Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS ONE. 2014;9:e93419. doi: 10.1371/journal.pone.0093419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Gendrin M, Rodgers FH, Yerbanga RS, Ouedraogo JB, Basáñez M-G, Cohuet A, Christophides GK. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6:5921. doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence is provided that antibiotic use in humans is manipulating the mosquito microbiome, which has implications for pathogen transmission.
- 70.Pennington MJ, Rivas NG, Prager SM, Walton WE, Trumble JT. Pharmaceuticals and personal care products alter the holobiome and development of a medically important mosquito. Environ Pollut. 2015;203:199–207. doi: 10.1016/j.envpol.2015.04.004. [DOI] [PubMed] [Google Scholar]