Abstract
Background
Sub-Saharan Africa (SSA) has confronted decades of the HIV epidemic with substantial improvements in access to life-saving antiretroviral therapy (ART). Now, with improved survival, people living with HIV (PLWH) are at increased risk for non-communicable diseases (NCDs), including atherosclerotic cardiovascular disease (CVD). We assessed the existing literature regarding the association of CVD outcomes and HIV in SSA.
Methods
We used the PRISMA guidelines to perform a systematic review of the published literature regarding the association of CVD and HIV in SSA with a focus on CVD surrogate and clinical outcomes in PLWH.
Results
From January 2000 until March 2017, 31 articles were published regarding CVD outcomes among PLWH in SSA. Data from surrogate CVD outcomes (n = 13) suggest an increased risk of CVD events among PLWH in SSA. Although acute coronary syndrome is reported infrequently in SSA among PLWH, limited data from five studies suggest extensive thrombus and hypercoagulability as contributing factors. Additional studies suggest an increased risk of stroke among PLWH (n = 13); however, most data are from immunosuppressed ART-naïve PLWH and thus are potentially confounded by the possibility of central nervous system infections.
Conclusions
Given ongoing gaps in our current understanding of CVD and other NCDs in PLWH in SSA, it is imperative to ascertain the burden of CVD outcomes, and to examine strategies for intervention and best practices to enhance the health of this vulnerable population.
Electronic supplementary material
The online version of this article (10.1186/s12889-017-4940-1) contains supplementary material, which is available to authorized users.
Keywords: HIV, CVD, Atherosclerosis, Africa, Review
Background
With more than 26 million people living with human immunodeficiency virus (PLWH) in sub-Saharan Africa (SSA), the daunting immediacy of health needs has necessitated expanding infrastructure to provide care for HIV infection [1]. As the beneficial effects of antiretroviral therapy (ART) are increasingly apparent [2], attention has now shifted to expanding this growing healthcare infrastructure to also encompass chronic care for non-infectious, highly prevalent co-morbidities [3, 4].
Non-communicable diseases (NCDs), specifically cardiovascular disease (CVD), increasingly affect the general population in SSA [5–7]. The effects of urbanization and increased life expectancy have been linked to an increased prevalence of traditional CVD risk factors, including changes in diet and exercise patterns [8], although CVD mortality has decreased in SSA over the past few decades [9, 10]. Concerns regarding the potential impact of CVD in SSA have focused appropriate and necessary attention on its diagnosis, treatment, and prevention [11, 12], and disparities in CVD prevention and treatment across SSA have been highly publicized [12–14]. Interventions in prevention, screening, and treatment have been shown to be effective and cost-effective among the general population [15, 16].
PLWH are at increased risk for CVD [17, 18], and a synergistic intersection of these two distinct epidemics may emerge in SSA [11, 19]. As access to ART expands, more PLWH are living past 50 years of age [20, 21] and face an increased risk of CVD due to traditional CVD risk factors alone [22]. The additional impact of HIV infection with its associated inflammation and prothrombotic state may further increase CVD risk. Limited data are available regarding optimal methods for CVD risk factor screening, primary and secondary prevention, risk stratification, outcomes, and management in PLWH living in SSA [23, 24]. Now is the time to anticipate and consider this challenge in the face of rising multimorbidity due to NCDs and chronic infectious diseases [25].
To complement previously published reviews of CVD among PLWH in SSA [26–33], we provide a systematic review and qualitative summary of the existing primary literature regarding the association of CVD and HIV in SSA with a focused emphasis on CVD surrogate and clinical outcomes. We identify current gaps in knowledge, focusing on the need for additional outcomes data, implementation strategies, and best practices for risk factor modification and treatment.
Methods
Search strategy
This systematic review of CVD surrogate and clinical outcomes in PLWH in SSA was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1) [34]. We identified articles published from January 2000 to March 2017 through searches in PubMed that included Medical Subject Headings (MeSH) terms, “Africa” and “HIV,” as well as any of the following terms: “cardiovascular disease,” “cardiometabolic,” “metabolic syndrome,” “myocardial infarction,” “acute coronary syndrome,” “dyslipidemia,” “diabetes,” “dysglycemia,” “hypertension,” “surrogate marker,” “cIMT,” “pulse wave velocity,” “aortic augmentation index,” “ankle-brachial index,” “endothelial activation,” “radial tonometry,” “flow-mediated dilation,” or “stroke.” We included additional articles found in review of bibliographies or suggested by co-authors based on their relevance to the selected search terms. The full list of search terms is reported in Additional file 1.
Fig. 1.
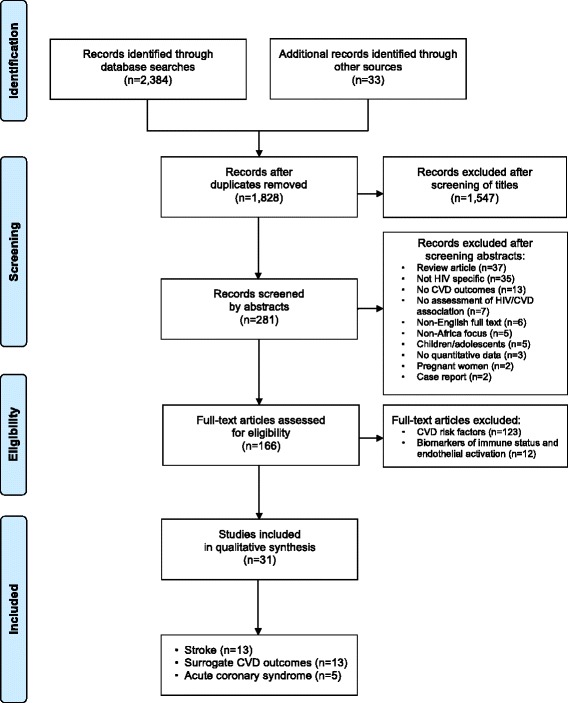
Flow diagram for the selection of studies. We followed PRISMA guidelines for screening articles in the systematic review. We identified articles via a systematic search of the PubMed database and derived additional records from reference lists of previously identified articles or co-author input. Articles were screened first by title, then by abstract, and finally on explicit inclusion/exclusion criteria. Full-text articles considered eligible for inclusion were categorized based on emphasis of CVD risk factors, biomarkers of immune status and endothelial activation, surrogate CVD outcomes, acute coronary syndrome, and stroke
Study selection
We selected relevant articles in a stepwise manner. Two co-authors (EPH and EBM) independently screened articles by title to ensure that the analysis included articles regarding PLWH, CVD, and sub-Saharan Africa. We next reviewed the selected abstracts, using the following inclusion criteria: study population without children or pregnant women; primary quantitative data; specific CVD risk factors or outcomes; assessment of an association between CVD and HIV; English written text. Last, we assessed the remaining full-text articles for eligibility and determined which studies would be included in qualitative synthesis.
Data extraction
Two co-authors (EPH and MM) extracted data for qualitative synthesis, including location, year of study, study design, sample size, population age (in years), and ART status, and summarized the main findings of eligible analyses. We did not perform quantitative synthesis because published study data included diverse populations and study designs, rarely offered effect sizes, and often included unmeasured confounders.
Results
Study selection
Full-text articles selected for eligibility were further separated into CVD risk factors (n = 123), biomarkers of immune status and endothelial activation (n = 12), and CVD outcomes (n = 31) (Fig. 2). Studies categorized as CVD risk factors or biomarkers of immune status and endothelial activation are reported in an additional reference list (see Additional file 2). The eligible 31 analyses evaluated the impact of HIV status on surrogate CVD outcomes (n = 13), acute coronary syndrome (ACS) (n = 5), and stroke (n = 13) for inclusion in qualitative synthesis and detailed data extraction.
Fig. 2.
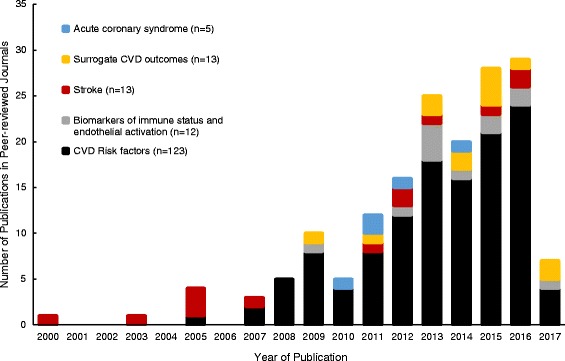
Publication year for studies on HIV and CVD risk factors and outcomes in sub-Saharan Africa. Full-text articles were stratified by year of publication and description of CVD risk factors, biomarkers of immune status and endothelial activation, surrogate CVD outcomes, acute coronary syndrome, or stroke. Of the 166 studies assessed in this review, the majority (n = 102) were published between 2013 and 2016. Only 3 months of 2017 publications were included
CVD risk factors
Traditional CVD risk factors are prevalent in SSA among the general population and include hypertension (6-22%) [35–37], dyslipidemia (5–70%) [38], diabetes (1-12%) [35, 39], and smoking (males 15%, females 0.6%) [40, 41]. Despite these high prevalence estimates, almost 67% of diabetics and 50% of hypertensives are thought to be unaware of their status [14, 42]. Tobacco use varies widely in different regions and is likely under-reported, particularly among women [40].
Among PLWH in the US and Europe, traditional CVD risk factors are highly prevalent, even when accounting for age [43]. Hypertension is associated with CVD risk in PLWH [44], and an important interplay also occurs between lipid indices, HIV, chronic inflammation, and antiretroviral medications [45–47]. PLWH are at risk for impaired glucose tolerance (IGT) and for diabetes [48]. Smoking is at least twice as prevalent in PLWH in the US [49] and has a greater impact on overall mortality than HIV itself in the setting of available ART [50].
Considerably less is known about the determinants and impact of CVD risk factors among PLWH in SSA. The association of hypertension with higher CD4 counts and older age suggests that hypertension may be a substantial problem among PLWH in SSA as they age on ART [22, 51–54]. Wide ranges in prevalence of IGT (16-24%) [55–58] and diabetes (1-18%) [58–64] are reported in PLWH in SSA. Total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) all rise following ART initiation, yet a relatively smaller increase in HDL results in a pro-atherosclerosis lipid profile [65–71]. Smoking is a public health concern in SSA, yet the role of HIV with regards to smoking rates in this setting remains unclear. Some cohorts demonstrate an increased prevalence of smoking compared with the general public in the region (23% vs 16%) [72, 73], while others show decreased smoking rates among PLWH (48% vs 31%) [74] or no difference [69, 75–78].
Chronic inflammation plays a role in the development of atherosclerotic CVD among PLWH, and evidence is mounting for the contribution of viremia and immunosuppression towards the premature development of CVD [79, 80]. Although several SSA studies demonstrated immune activation among both ART-naïve and –experienced PLWH [78, 81–84], a South African study focused on older PLWH (≥50 years) demonstrated a reduction in inflammatory markers (i.e., CRP, IL-1, IL-6, and TNF-α) after ART-initiation [85]. Evaluating the role of immune activation and cardiovascular risk in a cohort of PLWH on ART in southwestern Uganda, lower absolute levels of sCD14 and IL-6, markers of monocyte activation and generalized inflammation, were found to be significantly associated with lower future carotid intima media thickness (cIMT), a marker of preclinical atherosclerosis, after adjusting for traditional CVD risk factors [86]. Several studies in SSA have also consistently demonstrated that PLWH experience excess endothelial activation [78, 87], which persists even after the introduction of ART [82]. Given the potential impact of chronic inflammation on PLWH in SSA and possible concomitant mediators of inflammation from other infections endemic to the region [88], further research into the pathogenesis of atherosclerotic CVD among PLWH in SSA will be valuable and may offer additional insights into molecular markers, predictive tests, and treatment options.
Patterns of screening and diagnosis of CVD risk factors in SSA may differ by HIV status. PLWH are less likely to be asked about CVD risk factors at routine clinical visits compared with HIV-uninfected groups [89]. However, when CVD risk factors are actively assessed, PLWH on ART may be more likely to be diagnosed with hypertension or diabetes than patients with unknown HIV status. In a South African study using pharmacy records, for instance, hypertension was the most common second diagnosis among PLWH on ART, making it more common than tuberculosis (TB) [25].
PLWH may also be more likely to have multiple CVD risk factors, thus increasing their overall risk of CVD outcomes. Among patients on ART for more than 34 months in Cameroon, 61% had one CVD risk factor, and 18% had two or more CVD risk factors [90]. Among virologically suppressed PLWH ages 40-50 in Botswana, the American Heart Association (AHA)/American College of Cardiology (ACC) risk equation demonstrated elevated risk of CVD outcomes, which correlated with cIMT measurements [91]. However, only 3.6% of patients who were actively screened for CVD risk factors in a South African study were found to have more than a 10% risk of a CVD event in the next 10 years, using a risk stratification tool from the World Health Organization and International Society of Hypertension [63]. Strategies to estimate CVD risk among PLWH have not been formally assessed in SSA, and accurate risk prediction algorithms will be important to guide preventive care.
CVD outcomes
Although data are increasingly available regarding CVD risk factors among PLWH in SSA, data on CVD outcomes remain scarce (Fig. 2).
Surrogate CVD outcomes
Assessment of surrogate CVD outcomes (e.g., cIMT, pulse wave velocity (PWV), aortic augmentation index (AI), ankle-brachial index (ABI), flow-mediated dilation (FMD), radial tonometry) has demonstrated the potential for an increased risk of CVD events among PLWH in SSA (Table 1). Although the surrogate outcomes studied are diverse and reflect discrete vascular functions, the majority of studies showed increased atherosclerosis among PLWH, either versus controls or in relation to HIV disease duration or treatment status. Although some studies demonstrate no evidence for increased atherosclerosis among PLWH [78, 92], most studies show early subclinical atherosclerosis by surrogate CVD outcomes [81, 93–98]. A large Ugandan study demonstrated subclinical atherosclerosis via cIMT in 18% of PLWH attending clinic with higher risk among ART-experienced or older patients with elevated body mass index (BMI) or LDL [95]. A second study in Uganda diagnosed arterial stiffness by ABI in 33% (19%) of male (female) PLWH on ART for 7 years, which was twice the prevalence compared to age- and sex-matched HIV-uninfected controls after adjustment for traditional CVD risk factors [97]. A cross-sectional study from South Africa demonstrated that 12% of PLWH (median age 41 years, 69% female) had subclinical atherosclerosis by cIMT that was associated with traditional but not HIV-related factors [99]. Studies comparing surrogate CVD outcomes in PLWH versus comparator HIV-uninfected groups will further elucidate the role that HIV could play in the development of atherosclerosis. The long-term implications of these altered vascular indices remain unknown among PLWH in SSA and warrant further investigation.
Table 1.
Published studies on surrogate CVD outcomes in PLWH in sub-Saharan Africa
| Study (location, dates) |
Study Design | N = | Age (years)a | ART-status | Findings |
|---|---|---|---|---|---|
| Fourie et al. [93] (SA, 2005) |
Case-control | PLWH: 300 HIV-: 300 |
44 ± 8 44 ± 8 |
100% ART-naïve | • Untreated HIV associated with higher biomarkers of endothelial injury |
| Lazar et al. [92] (Rwanda, 2005) |
Prospective cohort | PLWH: 276 HIV-: 67 |
35 ± 7 41 ± 10 |
59% ART-naïve 41% on ART |
• HIV not associated with increased arterial wave reflection |
| Botha et al. [132] (SA, 2005-10) |
Prospective cohort | PLWH: 137 | no ART: 47.6 ± 1.9 on ART: 47.8 ± 1.6 |
52% ART-naïve 48% on ART |
• ART exposure associated with higher pulse pressure |
| Fourie et al. [78] (SA, 2005-10) |
Prospective cohort | PLWH: 144 HIV-: 165 |
no ART: 48 ± 1 on ART: 49 ± 1 50 ± 1 |
54% ART-naïve 46% on ART |
• >5y HIV infection associated with increased biomarkers of endothelial activation |
| Ngatchou et al. [94] (Cameroon, 2009-10) |
Cross-sectional | PLWH: 108 HIV-: 96 |
39 ± 11 41 ± 12 |
100% ART-naïve | • HIV associated with aortic stiffness as assessed by radial tonometry |
| Ngatchou et al. [133] (Cameroon, 2009-10) |
Cross-sectional | PLWH: 238 | no ART: 39 ± 11 on ART: 41 ± 12 |
45% ART-naïve 55% on ART |
• ART exposure associated with increased pulse pressure and augmentation index among ART-experienced patients compared to untreated PLWH |
| Ssinabulya et al. [95] (Uganda, 2012) |
Cross-sectional | PLWH: 245 | 37 (31-37) | 59% ART-naïve 41% on ART |
• HIV associated with 18% risk of pre-clinical carotid atherosclerosis on ultrasound imaging |
| Awotedu et al. [96] (SA, 2012-13) |
Cross-sectional | PLWH: 106 HIV-: 63 |
no ART: 36 ± 11 on ART: 40 ± 10 36 ± 11 |
51% ART-naïve 49% on ART |
• HIV associated with increased aortic pulse wave velocity • Highest increase among ART-experienced |
| Schoffelen et al. [99] (SA, 2013) |
Cross-sectional | PLWH: 904 | 41 (35-48) | 13% ART-naïve 87% on ART |
• cIMT associated with traditional CVD risk factors, not HIV-specific factors |
| Siedner et al. [97] (Uganda, 2013-14) |
Cross-sectional | PLWH: 105 HIV-: 100 |
49 (45-51) 50 (46-54) |
100% on ART | • HIV associated with twice the risk of arterial stiffness as assessed by calculating ankle-brachial index |
| Feinstein et al. [98] (Uganda, not stated) |
Cross-sectional | PLWH: 105 HIV-: 100 |
49 ± 6 52 ± 9 |
100% on ART | • HIV not associated with carotid intima media thickness |
| Gleason et al. [81] (Ethiopia, not stated) |
Cross-sectional | PLWH: 281 HIV-: 36 |
no ART: 38 (32-45) EFV: 38 (32-45) LPV/r: 39 (35-44) NVP: 37 (32-42) 39 (29-45) |
18% ART-naïve 82% on ART |
• Use of EFV & LPV/r, nut not NVP, is associated with elevated pulse wave velocity, normalized cIMT, and abnormal FMD |
| Mosepele et al. [91] (Botswana, not stated) |
Cross-sectional | PLWH: 208 | 39 (5) | 25% ART-naïve 75% on ART |
• Atherosclerotic CVD risk score and cIMT measurement similarly identify high CVD risk |
amean ± SD or median (IQR)
PLWH people living with HIV, SA South Africa, ART antiretroviral therapy, EFV efavirenz, LPV/r lopinavir/r, NVP nevirapine, cIMT carotid intima-media thickness, FMD flow-mediated dilation
Acute coronary syndrome
Acute coronary syndrome (ACS) is reported relatively infrequently in SSA [11, 27]. Events may be underreported due to “silent” myocardial infarctions, limited access to diagnostic tests, and lack of patient and healthcare provider awareness. However, a recent study shows that more than 5% of cardiac hospitalizations are attributable to ACS in South Africa [100]. With increasing life expectancy and a growing burden of CVD risk factors, the incidence of ACS and subsequent morbidities may rise in SSA.
Specifically among PLWH living in SSA, ACS is not reported frequently, and no studies have investigated ACS incidence in PLWH relative to control patients [24, 27]. In a large cohort study in Soweto, South Africa following 5328 new cases of heart disease, ACS was described in only 3% of the 518 cases of cardiac hospitalization among PLWH (although HIV testing was performed only when “clinically indicated”) (Table 2) [24]. The presentation of ACS was further examined in a separate study from the same population in which all patients with ACS were tested for HIV. In comparison to HIV-uninfected controls, 30 HIV-infected cases were younger and more likely to have a history of smoking but less likely to have traditional CVD risk factors such as diabetes, hypertension, and dyslipidemia [101]. Angiographic features among the HIV-infected cases revealed acute thrombus with a low burden of underlying atherosclerotic plaque, consistent with US studies showing fewer involved vessels and a greater relative burden of inflammatory plaque [102]. A follow-up study of this same study population demonstrated a greater prevalence of coagulopathy among the PLWH who presented with myocardial infarction, specifically elevated protein C levels [103]; anti-phospholipid antibodies were not found to be associated with ACS among PLWH [104].
Table 2.
Published studies on acute coronary syndrome in PLWH in sub-Saharan Africa
| Study (location, dates) |
Study Design | N = | Age (years)a | ART-status | Findings |
|---|---|---|---|---|---|
| Becker et al. [101] (SA, 2004-08) |
Prospective case-control | ACS + PLWH: 30 ACS + HIV-: 30 |
43 ± 7 54 ± 13 | 100% ART-naïve | • Traditional risk factors more prevalent in HIV-, except for smoking • PLWH more likely to have single vessel disease and greater thrombus burden • PLWH more likely to have MACE and need TLR at follow-up |
| Becker et al. [103] (SA, 2004-08) |
Same study population as above | • PLWH with ACS more likely to have lower protein C and higher Factor VIII, Anti-cardiolipin IgG and Anti-prothrombin IgG | |||
| Becker et al. [104] (SA, 2004-08) |
Prospective case-control | ACS-PLWH: 30 ACS + PLWH: 30 ACS + HIV-: 30 |
41 ± 8 43 ± 7 54 ± 13 |
100% ART-naïve | • PLWH are more likely to have anti-phospholipid antibodies but this is not associated with ACS |
| Sliwa et al. [24] (SA, 2006-08) |
Cohort | PLWH: 518 | 39 ± 13 | 46% ART-naïve 54% on ART |
• 170 (32.8%) were new HIV diagnoses • 14 (2.7%) were admitted with ACS and 18 (3.5%) with cerebrovascular disease |
| Redman et al. [105] (SA, 2008-11) |
Prospective cohort of vascular surgery patients | PLWH: 73 HIV-: 152 |
41 ± 10 56 ± 13 |
68% ART-naïve 23% on ART |
• Lower RCRI score among PLWH • No difference in 30 day outcomes (13% vs 15%), even though PLWH were younger |
amean ± SD or median (IQR)
PLWH people living with HIV, SA South Africa, ACS acute coronary syndrome, ART antiretroviral therapy, MACE major adverse cardiovascular events, TLR target lesion revascularization, RCRI Revised Cardiac Risk Index
PLWH may be at higher risk for coronary artery disease than suggested by traditional CVD risk factors alone, given data on post-operative cardiovascular morbidity and mortality. In a South African cohort of 255 patients undergoing vascular surgery, 32% were HIV-infected of whom 23% were on ART [105]. When compared to vascular surgery patients confirmed to be HIV-uninfected, PLWH were equally as likely to have myocardial infarction as measured by troponin elevations on post-operative day three or to experience 30-day mortality. PLWH were less likely to have known traditional CVD risk factors and were less likely to be on cardiovascular medications; it is not known if the prevalence of traditional CVD risk factors was lower among PLWH or if they were less likely to have been previously screened for these diseases.
Stroke
Stroke is a substantial problem in the general population in SSA and is associated with high rates of mortality, morbidity, and post-stroke disability [13, 106–108]. An association between HIV infection and stroke has been described in high-income countries [109–111]; data emerging from SSA also suggest a high prevalence of stroke among PLWH. Relative to ACS, data regarding stroke among PLWH in SSA are more extensive, which may be due to the more readily recognizable symptoms of stroke that are likely to result in presentation to medical attention, as well as the high prevalence of hypertension in SSA. Three distinct study designs have been used to investigate an association of stroke and HIV in SSA: 1) comparisons of PLWH and HIV-uninfected patients who present with stroke; 2) comparisons of stroke patients with population controls, evaluating for HIV status in both groups 3) assessment of cohorts of PLWH, some of whom have had stroke (Table 3).
Table 3.
Published studies on stroke in PLWH in sub-Saharan Africa
| Study (location, dates) |
Study Design | N = | Age (years)a | ART-status | Findings |
|---|---|---|---|---|---|
| Study population: stroke patients | |||||
| Patel et al. [116] (SA, 1987-2002) |
Retrospective case-controlb | PLWH: 56 HIV-: 154 |
15-44 | 100% ART-naïve | • No significant differences between PLWH and HIV- patients with stroke regarding cardiac etiologies or angiography |
| Hoffmann et al. [117] (SA, 1992-98) |
Prospective case-controlb | PLWH: 22 HIV-: 23 |
29.1 (20-42) 31.0 (19-44) |
100% ART-naïve | • PLWH with fewer traditional CVD risk factors • 20 of 22 (91%) PLWH with unknown etiology for stroke • All PLWH were diagnosed with HIV at admission for stroke |
| Mochan et al. [114] (SA, 1999-2000) |
Case series of PLWH and stroke | PLWH: 35 | 32.1 (20-61) | 100% ART-naïve | • 94% ischemic; 6% hemorrhagic • LP showed infection in 10 of 33 PLWH • 17 PLWH had coagulopathy |
| Tipping et al. [113] (SA, 2000-06) |
Prospective cohort of stroke patients | PLWH: 67 HIV-: 1020 (all subjects) PLWH: 61 HIV-: 205 (<46y) |
33.4 (19-76) 64 (17-96) |
12% < 6 months ART 88% ART-naïve |
• 96% ischemic; 4% hemorrhagic • ID etiologies predominant among PLWH (37% vs 8%) • 20% of PLWH had HIV vasculopathy • Fewer traditional risk factors among PLWH <46 years |
| Kumwenda et al. [112] (Malawi, 2001-02) |
Prospective case-control | PLWH: 47 HIV-: 51 |
37.5 ± 13.1 58.6 ± 16.8 |
100% ART-naïve | • 11 (23%) of PLWH diagnosed with infectious etiologies of stroke |
| Heikinheimo et al. [115] (Malawi, 2008-09) |
Cohort of 1st time stroke | PLWH: 50 HIV-: 84 |
39.8 ± 12.4 61.9 ± 14.0 |
22% on ART | • No difference in outcomes between PLWH and HIV- • PLWH were younger with fewer CVD risk factors |
| Owolabi et al. [37] (Nigeria, 2008-10) |
Prospective cohort of stroke patients (18-40 years) | PLWH: 6 HIV-: 65 |
31.9 ± 6 | Not stated | • 8.5% were PLWH but not all patients were tested • HIV is 5th most common risk factor |
| Study population: PLWH | |||||
| Longo-Mbenza et al. [134] (DRC, 2004-08) | Cross-sectional | PLWH: 116 | 45.3 ± 8.5 (men) 42.5 ± 11.2 (women) |
100% of stroke patients on ART | • 17 (15%) PLWH had stroke • 94% ischemic; 6% ICH • Uncertain ID workup • Fewer traditional risk factors among stroke patients, but lower CD4 and more WHO Stage 3-4 |
| Divala et al. [135] (Malawi, 2014) |
Cross-sectional | PLWH: 952 | 43.0 ± 10.2 | 4.1% ART-naïve 95.9% on ART |
• Self-reported past stroke: 4.3% |
| Study population: stroke (cases) and non-stroke from community (controls) | |||||
| Walker et al. [118] (Tanzania, 2003-06) |
Case-controlb | Stroke: 201 (PLWH: 25) Controls: 398 (PLWH: 15) |
61.7 (15.0) and 68.8 (14.8)c
61.4 (13.1) and 69.4 (14.6)c |
100% ART-naïve | • Stroke associated with traditional risk factors • HIV independently associated with stroke (aOR, 5.61) |
| Benjamin et al. [136] (Malawi, 2011-12) |
Case-controld | Stroke: 222 (PLWH: 69) Controls: 503 (PLWH: 95) |
60 (42-70) 57 (42-67) |
17% ART-naïve 7% ART <6 mo 6% ART ≥6 mo 9% ART-naïve 1% ART <6 mo 8% ART ≥6 mo |
• 78% ischemic; 22% hemorrhagic • Stroke associated with HIV (aOR, 3.28), especially if ART started in past 6 months (aOR, 15.6) • No effect modification for HIV and HTN • Diabetes and smoking were independently associated |
| Asiki et al. [137] (Uganda, not stated) |
Retrospective case-controlb | Stroke: 31 (PLWH: 5) Controls: 135 (PLWH: 8) |
59.0 (13.7) 60.2 (13.7) |
62% of PLWH are ART-naïve | • Increased risk of stroke among HIV+ (16% vs 6%) • Potentially biased sample given patients included only if they had frozen specimen available for varicella IgG testing |
| Mochan et al. [138] (SA, not stated) |
Case-controle | Stroke: 33 (PLWH: 33) Controls: 66 (PLWH: 33) |
32.1 (20-61) | Not stated | • Protein S deficiency associated with HIV, not stroke |
amean ± SD or median (IQR)
bmatched by age/sex
ctwo study regions (Dar-es-Salaam and Hai)
dmatched by age, sex, socioeconomic status, season of admission
ematched by age, sex, and CD4
PLWH people living with HIV, SA South Africa, ART antiretroviral therapy, LP lumbar puncture, ID infectious disease, DRC Democratic Republic of Congo, ICH intracerebral hemorrhage, WHO World Health Organization, aOR adjusted odds ratio, HTN hypertension
HIV infection is frequently reported among patients enrolled in studies that assessed new stroke patients. Almost 3% of all inpatient admissions were due to stroke in a Malawi cohort comprising 70% PLWH [112]. A prospective cohort of young stroke patients (<46 years) admitted to a South African stroke referral hospital demonstrated HIV infection in 23%, more than twice the population’s HIV prevalence (11%) [113]. In a hospital-based South African cohort study of PLWH with stroke, 57% of patients presented with stroke as their initial presentation of HIV [114]. Although morbidity and mortality remain very high among patients after stroke in SSA, outcomes of PLWH versus the general population post-stroke did not differ substantially in terms of case-fatality rates or disabilities [112, 115].
When comparing stroke patients with and without HIV, some studies found no differences in risk factors or outcomes [116]. Other studies found that PLWH with stroke were more likely to be younger and have fewer traditional CVD risk factors than stroke patients without HIV [112, 113, 115, 117]. Such results suggest a possible role of HIV infection itself or other nontraditional risk factors in conferring stroke risk among PLWH in this setting. However, such studies have predominantly assessed patients who are ART-naïve and often recently diagnosed with HIV; these patients are more likely to be immunosuppressed and at increased risk for opportunistic infections which could impact the central nervous system. Infectious etiologies of stroke are more common in PLWH, and rigorous diagnostic workup in one study revealed infectious causes in 23% of PLWH compared to none of the HIV-uninfected [112]. Other studies found a similarly high probability of infectious etiology for stroke when comprehensive diagnostic testing was performed [113, 114].
Two studies that used a case-control study design to compare stroke patients with non-stroke controls from the community demonstrated an independent association between HIV and stroke, as well as an association between stroke and traditional CVD risk factors [118, 119]. In one study, all PLWH were ART-naïve [118]; in the other study, an association between the timing of ART initiation (within the past 6 months) and stroke was significant, suggesting a possible role for immune reconstitution syndrome [119]. Neither study outlined a thorough diagnostic workup for other infectious causes of stroke, which could be an important, unmeasured confounder.
Data regarding stroke in PLWH on ART for more than a year and therefore at reduced risk for infectious etiologies will be essential to extend our understanding of CVD in PLWH in SSA.
Discussion
Summary of literature and current knowledge gaps
Although our review of the published literature underscores the increasing availability of data on this topic (Fig. 2), substantial limitations in existing data remain. Published data still frequently include small sample sizes and are likely under-powered to detect differences. Additionally, the patient populations are frequently younger than 50 years and therefore do not reflect the aging of the epidemic, and many studies do not evaluate the impact of socio-economic status on CVD risk factors and outcomes. Many studies continue to present data on mixed populations of ART-naïve and ART-experienced patients so that the impact of immunosuppression and longitudinal treatment with ART cannot be assessed. Moreover, in case-control studies that include an HIV-uninfected control group, patients are rarely proactively tested for HIV, so the control group likely includes a mixed population of people living with and without HIV. Studies that include ART-naïve PLWH often do not include a thorough diagnostic workup for infectious etiologies of stroke. CVD outcomes, particularly ACS, peripheral arterial disease, and venous thromboembolic disease, are infrequently identified and reported, so the impact of any increasing prevalence of CVD risk factors remains uncertain. Most studies report cross-sectional data, which do not capture risks, exposures, and outcomes over time. Case-control studies often include unmeasured confounders, including infectious co-morbidities such as tuberculosis or syphilis. Last, data are often from clinical cohorts or trials, which might not be representative of PLWH engaged in care in large public health programs.
Larger cohort studies among PLWH and HIV-uninfected patients in SSA during the ART era will clarify the incidence, prevalence, and attributable morbidity and mortality of CVD risk factors and outcomes in these populations. Focused investigation regarding possible interactions of traditional CVD risk factors with HIV infection, ART, and chronic inflammation can elucidate potential pathophysiologic mechanisms for further comparison with data from the US and Europe to guide prevention and management strategies. Innovative models of integrated care, which are appropriate and scalable for enhanced CVD risk management, should be extensively studied in SSA. Such models should leverage successful HIV programs, where feasible.
Prospective ascertainment of best practices for CVD risk factor screening and CVD management in SSA will identify future interventions for PLWH. Abundant opportunities for CVD risk factor screening exist for PLWH given frequent interactions with community testing campaigns and clinical care [120, 121], but subsequent linkage to and retention in clinical care after diagnosis is essential in order to optimize both HIV and CVD outcomes [36]. The identification of optimal ART, anti-hypertensive, lipid, and diabetes management strategies for PLWH is needed, as well as implementation of effective methods of lifestyle modification, nutrition education, smoking cessation, and expansion of opportunities to exercise. The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) trial, with its sites in SSA, will provide new data on CVD risk reduction in this setting [122].
Policy implications
As more PLWH are successfully linked to clinical care and treated with ART, attention must turn to maintaining their restored health in order to realize the full health benefits of ART. The existing and expanding health care infrastructure developed for HIV presents an opportunity to incorporate additional preventative interventions for chronic disease complications to decrease morbidity and improve quality of life for PLWH.
Some argue that integration of non-HIV medical services with HIV clinical care could decrease quality of care and dilute the effectiveness of current HIV programs. Concerns that investment in HIV care has detracted from investments in other forms of health care, such as immunizations [123], have tempered enthusiasm for expanding HIV health systems to include additional health care directives.
However, the potential benefits of an integrated approach to medical care for PLWH in SSA and other resource-limited settings could be profound, and support for this approach has been fast-growing [20, 124–126]. The established and expanding HIV infrastructure could be an ideal foundation on which to build additional interventions and management strategies for other chronic diseases [29, 127]. An emphasis on HIV as a chronic disease that requires life-long management has also influenced the paradigm of care [128–130] and encourages the use of current HIV infrastructure as a platform for management of chronic non-infectious co-morbidities. In regions where primary care services are already more established, existing clinical services could alternatively serve as a platform for decentralizing HIV care and integrating with existing NCD prevention and management efforts [131].
Conclusions
The limited data available from PLWH in SSA regarding atherosclerotic CVD outcomes suggests an increased risk of early atherosclerosis and stroke. Given ongoing gaps in our current understanding of CVD in PLWH in SSA, now is the time to advance targeted research priorities and determine the burden of CVD outcomes, strategies for intervention, and best practices to enhance the health of this vulnerable population.
Additional files
Search Strategy. (PDF 10 kb)
Additional References. The following references primarily focused on CVD risk factors (n = 123) and biomarkers of immune status and endothelial activation (n = 12). These publications were assessed for eligibility but were excluded from the final qualitative analysis. (PDF 63 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by the National Institutes of Health [R01AI058736 (EPH and RPW); R37AI093269 (EPH and RPW); R01HL132786 (VAT); R56HL125029 (VAT); R21HL122138 (VAT); K01HL123349 (EPH); T32AI 007433 (EPH); Harvard University Center for AIDS Research (HU CFAR) NIH/NIAID 5P30AI060354 (EPH and MM)], by the Lily and Ernst Hausmann Research Trust (BMM) and by the Steve and Deborah Gorlin MGH Research Scholars Award (RPW). The content is solely the responsibility of the authors, and the study’s findings and conclusions do not necessarily represent the official views of the NIH.
Availability of data and materials
The original research articles included in this systematic review are publicly available. The complete search strategy is available in Additional file 1.
Abbreviations
- ABI
Ankle-brachial index
- ACC
American College of Cardiology
- ACS
Acute coronary syndrome
- AHA
American Heart Association
- AI
Aortic augmentation index
- ART
Antiretroviral therapy
- BMI
Body mass index
- cIMT
Carotid intima media thickness
- CVD
Cardiovascular disease
- FMD
Flow-mediated dilation
- HDL
High-density lipoprotein
- IGT
Impaired glucose tolerance
- LDL
Low-density lipoprotein
- NCD
Non-communicable disease
- PLWH
People living with HIV
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PWV
Pulse wave velocity
- SSA
Sub-Saharan Africa
Authors’ contributions
EPH and VAT designed the study and developed the methodology. EPH and EBM performed the literature review. EPH wrote the first draft of the manuscript; BMM, KM, EBM, MM, RPW, LGB, and VAT provided critical revisions of the manuscript. All authors have read and approved the final version of the paper.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12889-017-4940-1) contains supplementary material, which is available to authorized users.
Contributor Information
Emily P. Hyle, Phone: 617-643-3903, Email: ehyle@mgh.harvard.edu
Bongani M. Mayosi, Email: bongani.mayosi@uct.ac.za
Keren Middelkoop, Email: Keren.Middelkoop@hiv-research.org.za.
Mosepele Mosepele, Email: mosepele.mosepele@gmail.com.
Emily B. Martey, Email: emartey@mgh.harvard.edu
Rochelle P. Walensky, Email: rwalensky@mgh.harvard.edu
Linda-Gail Bekker, Email: Linda-Gail.Bekker@hiv-research.org.za.
Virginia A. Triant, Email: vtriant@mgh.harvard.edu
References
- 1.World Health Organization . HIV/AIDS fact sheet. 2016. [Google Scholar]
- 2.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 3.Wester CW, Koethe JR, Shepherd BE, Stinnette SE, Rebeiro PF, Kipp AM, et al. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011;25(12):1471–1479. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remais JV, Zeng G, Li G, Tian L, Engelgau MM. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol. 2013;42(1):221–227. doi: 10.1093/ije/dys135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Global atlas on cardiovascular disease prevention and control. 2011. [Google Scholar]
- 6.Reddy KS. Cardiovascular disease in non-western countries. N Engl J Med. 2004;350(24):2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 7.Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. The epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31(6):642–648. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 9.Stringhini S, Sinon F, Didon J, Gedeon J, Paccaud F, Bovet P. Declining stroke and myocardial infarction mortality between 1989 and 2010 in a country of the african region. Stroke. 2012;43(9):2283–2288. doi: 10.1161/STROKEAHA.112.658468. [DOI] [PubMed] [Google Scholar]
- 10.Roth GA, Murray CJ. The global burden of disease study 2010 does not show a rise in the age-standardized mortality rate for cardiovascular disease in sub-Saharan Africa. Prog Cardiovasc Dis. 2013;56(3):278–280. doi: 10.1016/j.pcad.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934–947. doi: 10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- 12.Joshi R, Jan S, Wu Y, MacMahon S. Global inequalities in access to cardiovascular health care: our greatest challenge. J Am Coll Cardiol. 2008;52(23):1817–1825. doi: 10.1016/j.jacc.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Chin JH. Stroke in sub-Saharan Africa: an urgent call for prevention. Neurology. 2012;78(13):1007–1008. doi: 10.1212/WNL.0b013e318248df95. [DOI] [PubMed] [Google Scholar]
- 14.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 15.Ortegon M, Lim S, Chisholm D, Mendis S. Cost effectiveness of strategies to combat cardiovascular disease, diabetes, and tobacco use in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e607. doi: 10.1136/bmj.e607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, et al. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370(9604):2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 17.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloomfield GS, Mwangi A, Chege P, Simiyu CJ, Aswa DF, Odhiambo D, et al. Multiple cardiovascular risk factors in Kenya: evidence from a health and demographic surveillance system using the WHO STEPwise approach to chronic disease risk factor surveillance. Heart. 2013;99(18):1323–1329. doi: 10.1136/heartjnl-2013-303913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendavid E, Ford N, Mills EJ. HIV and Africa's eldery: the problems and possibilities. AIDS. 2012;26(S1):S85–S91. doi: 10.1097/QAD.0b013e3283558513. [DOI] [PubMed] [Google Scholar]
- 21.Nyirenda M, Newell M-L, Mugisha J, Mutevedzi PC, Seeley J, Scholten F, et al. Health, wellbeing, and disability among older people infected or affected by HIV in Uganda and South Africa. Glob Health Action. 2013;6(1):19201. doi: 10.3402/gha.v6i0.19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negin J, Martiniuk A, Cumming RG, Naidoo N, Phaswana-Mafuya N, Madurai L, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS. 2012;26(Suppl 1):S55–S63. doi: 10.1097/QAD.0b013e3283558459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nat Clin Pract Cardiovasc Med. 2009;6(2):120–127. doi: 10.1038/ncpcardio1437. [DOI] [PubMed] [Google Scholar]
- 24.Sliwa K, Carrington MJ, Becker AC, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the heart of Soweto study cohort. Eur Heart J. 2012;33(7):866–874. doi: 10.1093/eurheartj/ehr398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oni T, Youngblood E, Boulle A, McGrath N, Wilkinson RJ, Levitt NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis. 2015;15:20. doi: 10.1186/s12879-015-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(6):1754–1771. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart. 2013;99(16):1146–1153. doi: 10.1136/heartjnl-2012-303177. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield GS, Khazanie P, Morris A, Rabadan-Diehl C, Benjamin LA, Murdoch D, et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S40–S53. doi: 10.1097/QAI.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV-AIDS on a collision course: relevance for health care delivery, particularly in low-resource settings—insights from South Africa. Am J Clin Nutr. 2011;94(6):1690S–1696S. doi: 10.3945/ajcn.111.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutimura E, Crowther NJ, Stewart A, Cade WT. The human immunodeficiency virus and the cardiometabolic syndrome in the developing world: an African perspective. J Cardiometab Syndr. 2008;3(2):106–110. doi: 10.1111/j.1559-4572.2008.07584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS One. 2016;11(3):e0150970. doi: 10.1371/journal.pone.0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayne ES, George JA. Mortal allies: human immunodeficiency virus and noncommunicable diseases. Curr Opin HIV AIDS. 2017;12(2):148–156. doi: 10.1097/COH.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 33.Naidu S, Ponnampalvanar S, Kamaruzzaman SB, Kamarulzaman A. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDs. 2017;31(1):1–13. doi: 10.1089/apc.2016.0140. [DOI] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher D, Waswa L, Baisley K, Karabarinde A, Unwin N. Epidemiology of hypertension in low-income countries: a cross-sectional population-based survey in rural Uganda. J Hypertens. 2011;29(6):1061–1068. doi: 10.1097/HJH.0b013e3283466e90. [DOI] [PubMed] [Google Scholar]
- 36.Pastakia SD, Ali SM, Kamano JH, Akwanalo CO, Ndege SK, Buckwalter VL, et al. Screening for diabetes and hypertension in a rural low income setting in western Kenya utilizing home-based and community-based strategies. Glob Health. 2013;9:21. doi: 10.1186/1744-8603-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owolabi LF, Ibrahim A. Stroke in young adults: a prospective study from northwestern Nigeria. ISRN Neurol. 2012;2012:468706. doi: 10.5402/2012/468706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy GA, Asiki G, Ekoru K, Nsubuga RN, Nakiyingi-Miiro J, Young EH, et al. Sociodemographic distribution of non-communicable disease risk factors in rural Uganda: a cross-sectional study. Int J Epidemiol. 2013;42(6):1740–1753. doi: 10.1093/ije/dyt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall V, Thomsen R, Henriksen O, Lohse N. Diabetes in sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization . WHO report on the global tobacco epidemic. 2011. [Google Scholar]
- 41.Jaquet A, Ekouevi DK, Aboubakrine M, Bashi J, Messou E, Maiga M, et al. Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in west African countries. Int J Tuberc Lung Dis. 2009;13(11):1433–1439. [PMC free article] [PubMed] [Google Scholar]
- 42.International Diabetes Federation. IDF Diabetes Atlas, 7th ed. Brussels: International Diabetes Federation; 2015. p. 55.
- 43.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60(4):627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuesch R, Wang Q, Elzi L, Bernasconi E, Weber R, Cavassini M, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV-infected patients: Swiss HIV cohort study (SHCS) J Acquir Immune Defic Syndr. 2013;62(4):396–404. doi: 10.1097/QAI.0b013e3182847cd0. [DOI] [PubMed] [Google Scholar]
- 45.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 46.Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS. 2013;27(3):381–389. doi: 10.1097/QAD.0b013e32835abcc9. [DOI] [PubMed] [Google Scholar]
- 47.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201(2):285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 49.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 50.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 51.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in western Kenya. PLoS One. 2011;6(7):e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parikh SM, Obuku EA, Walker SA, Semeere AS, Auerbach BJ, Hakim JG, et al. Clinical differences between younger and older adults with HIV/AIDS starting antiretroviral therapy in Uganda and Zimbabwe: a secondary analysis of the DART trial. PLoS One. 2013;8(10):e76158. doi: 10.1371/journal.pone.0076158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okello S, Kanyesigye M, Muyindike WR, Annex BH, Hunt PW, Haneuse S, et al. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens. 2015;33(10):2039–2045. doi: 10.1097/HJH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31(7):1372–1378. doi: 10.1097/HJH.0b013e328360de1c. [DOI] [PubMed] [Google Scholar]
- 55.Mercier S, Gueye NF, Cournil A, Fontbonne A, Copin N, Ndiaye I, et al. Lipodystrophy and metabolic disorders in HIV-1-infected adults on 4- to 9-year antiretroviral therapy in Senegal: a case-control study. J Acquir Immune Defic Syndr. 2009;51(2):224–230. doi: 10.1097/QAI.0b013e31819c16f4. [DOI] [PubMed] [Google Scholar]
- 56.Omech B, Sempa J, Castelnuovo B, Opio K, Otim M, Mayanja-Kizza H, et al. Prevalence of HIV-associated metabolic abnormalities among patients taking first-line antiretroviral therapy in Uganda. ISRN AIDS. 2012;2012:960178. doi: 10.5402/2012/960178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in south African HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57(4):284–289. doi: 10.1097/QAI.0b013e318221863f. [DOI] [PubMed] [Google Scholar]
- 58.Maganga E, Smart LR, Kalluvya S, Kataraihya JB, Saleh AM, Obeid L, et al. Glucose metabolism disorders, HIV and antiretroviral therapy among Tanzanian adults. PLoS One. 2015;10(8):e0134410. doi: 10.1371/journal.pone.0134410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Julius H, Basu D, Ricci E, Wing J, Basu JK, Pocaterra D, et al. The burden of metabolic diseases amongst HIV positive patients on HAART attending the Johannesburg hospital. Curr HIV Res. 2011;9(4):247–252. doi: 10.2174/157016211796320360. [DOI] [PubMed] [Google Scholar]
- 60.Muronya W, Sanga E, Talama G, Kumwenda JJ, van Oosterhout JJ. Cardiovascular risk factors in adult Malawians on long-term antiretroviral therapy. Trans R Soc Trop Med Hyg. 2011;105(11):644–649. doi: 10.1016/j.trstmh.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Zannou DM, Denoeud L, Lacombe K, Amoussou-Guenou D, Bashi J, Akakpo J, et al. Incidence of lipodystrophy and metabolic disorders in patients starting non-nucleoside reverse transcriptase inhibitors in Benin. Antivir Ther. 2009;14(3):371–380. doi: 10.1177/135965350901400307. [DOI] [PubMed] [Google Scholar]
- 62.Wensink GE, Schoffelen AF, Tempelman HA, Rookmaaker MB, Hoepelman AI, Barth RE. Albuminuria is associated with traditional cardiovascular risk factors and viral load in HIV-infected patients in rural South Africa. PLoS One. 2015;10(8):e0136529. doi: 10.1371/journal.pone.0136529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabkin M, Mutiti A, Chung C, Zhang Y, Wei Y, El-Sadr WM. Missed opportunities to address cardiovascular disease risk factors amongst adults attending an urban HIV clinic in South Africa. PLoS One. 2015;10(10):e0140298. doi: 10.1371/journal.pone.0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrahams Z, Dave JA, Maartens G, Levitt NS. Changes in blood pressure, glucose levels, insulin secretion and anthropometry after long term exposure to antiretroviral therapy in south African women. AIDS Res Ther. 2015;12:24. doi: 10.1186/s12981-015-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomo ZA, Hakim JG, Walker SA, Tinago W, Mandozana G, Kityo C, et al. Impact of second-line antiretroviral regimens on lipid profiles in an African setting: the DART trial sub-study. AIDS Res Ther. 2014;11(1):32. doi: 10.1186/1742-6405-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.George JA, Venter WDF, Van Deventer HE, Crowther NJ. A longitudinal study of the changes in body fat and metabolic parameters in a south African population of HIV-positive patients receiving an antiretroviral therapeutic regimen containing stavudine. AIDS Res Hum Retrovir. 2009;25(8):771–781. doi: 10.1089/aid.2008.0308. [DOI] [PubMed] [Google Scholar]
- 67.Kiage JN, Heimburger DC, Nyirenda CK, Wellons MF, Bagchi S, Chi BH, et al. Cardiometabolic risk factors among HIV patients on antiretroviral therapy. Lipids Health Dis. 2013;12:50. doi: 10.1186/1476-511X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirigo AT, Tesfaye DY. Influences of gender in metabolic syndrome and its components among people living with HIV virus using antiretroviral treatment in Hawassa, southern Ethiopia. BMC Res Notes. 2016;9:145. doi: 10.1186/s13104-016-1953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anastos K, Ndamage F, Lu D, Cohen MH, Shi Q, Lazar J, et al. Lipoprotein levels and cardiovascular risk in HIV-infected and uninfected Rwandan women. AIDS Res Ther. 2010;7:34. doi: 10.1186/1742-6405-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu E, Armstrong C, Spiegelman D, Chalamilla G, Njelekela M, Hawkins C, et al. First-line antiretroviral therapy and changes in lipid levels over 3 years among HIV-infected adults in Tanzania. Clin Infect Dis. 2013;56(12):1820–1828. doi: 10.1093/cid/cit120. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong C, Liu E, Okuma J, Spiegelman D, Guerino C, Njelekela M, et al. Dyslipidemia in an HIV-positive antiretroviral treatment-naive population in Dar es salaam, Tanzania. J Acquir Immune Defic Syndr. 2011;57(2):141–145. doi: 10.1097/QAI.0b013e318219a3d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iliyasu Z, Gajida AU, Abubakar IS, Shittu O, Babashani M, Aliyu MH. Patterns and predictors of cigarette smoking among HIV-infected patients in northern Nigeria. Int J STD AIDS. 2012;23(12):849–852. doi: 10.1258/ijsa.2012.012001. [DOI] [PubMed] [Google Scholar]
- 73.Adewole OO, Eze S, Betiku Y, Anteyi E, Wada I, Ajuwon Z, et al. Lipid profile in HIV/AIDS patients in Nigeria. Afr Health Sci. 2010;10(2):144–149. [PMC free article] [PubMed] [Google Scholar]
- 74.van Rooyen JM, Fourie CM, Steyn HS, Koekemoer G, Huisman HW, Schutte R, et al. Cardiometabolic markers to identify cardiovascular disease risk in HIV-infected black south Africans. S Afr Med J. 2014;104(3):195–199. doi: 10.7196/SAMJ.7739. [DOI] [PubMed] [Google Scholar]
- 75.Daniyam C, Iroezindu M. Lipid profile of anti-retroviral treatment-naïve HIV-infected patients in Jos, Nigeria. Ann Med Health Sci Res. 2013;3(1):26–30. doi: 10.4103/2141-9248.109468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fourie CM, Van Rooyen JM, Kruger A, Schutte AE. Lipid abnormalities in a never-treated HIV-1 subtype C-infected African population. Lipids. 2010;45(1):73–80. doi: 10.1007/s11745-009-3369-4. [DOI] [PubMed] [Google Scholar]
- 77.Lam C, Martinson N, Hepp L, Ambrose B, Msandiwa R, Wong ML, et al. Prevalence of tobacco smoking in adults with tuberculosis in South Africa. Int J Tuberc Lung Dis. 2013;17(10):1354–1357. doi: 10.5588/ijtld.13.0016. [DOI] [PubMed] [Google Scholar]
- 78.Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240(1):154–160. doi: 10.1016/j.atherosclerosis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 80.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gleason RL, Jr, Caulk AW, Seifu D, Parker I, Vidakovic B, Getenet H, et al. Current Efavirenz (EFV) or ritonavir-boosted lopinavir (LPV/r) use correlates with elevate markers of atherosclerosis in HIV-infected subjects in Addis Ababa, Ethiopia. PLoS One. 2015;10(4):e0117125. doi: 10.1371/journal.pone.0117125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bestawros M, Chidumayo T, Blevins M, Canipe A, Bala J, Kelly P, et al. Increased systemic inflammation is associated with cardiac and vascular dysfunction over the first 12 weeks of antiretroviral therapy among undernourished, HIV-infected adults in southern Africa. J AIDS Clin Res. 2015;6(3):431. doi: 10.4172/2155-6113.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hudson CL, Zemlin AE, Ipp H. The cardiovascular risk marker asymmetric dimethylarginine is elevated in asymptomatic, untreated HIV-1 infection and correlates with markers of immune activation and disease progression. Ann Clin Biochem. 2014;51(Pt 5):568–575. doi: 10.1177/0004563213505848. [DOI] [PubMed] [Google Scholar]
- 84.Haissman JM, Vestergaard LS, Sembuche S, Erikstrup C, Mmbando B, Mtullu S, et al. Plasma cytokine levels in Tanzanian HIV-1-infected adults and the effect of antiretroviral treatment. J Acquir Immune Defic Syndr. 2009;52(4):493–497. doi: 10.1097/QAI.0b013e3181b627dc. [DOI] [PubMed] [Google Scholar]
- 85.Mutevedzi PC, Rodger AJ, Kowal P, Nyirenda M, Newell ML. Decreased chronic morbidity but elevated HIV associated cytokine levels in HIV-infected older adults receiving HIV treatment: benefit of enhanced access to care? PLoS One. 2013;8(10):e77379. doi: 10.1371/journal.pone.0077379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siedner MJ, Kim JH, Nakku RS, Bibangambah P, Hemphill L, Triant VA, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis. 2016;213(3):370–378. doi: 10.1093/infdis/jiv450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graham SM, Rajwans N, Jaoko W, Estambale BB, McClelland RS, Overbaugh J, et al. Endothelial activation biomarkers increase after HIV-1 acquisition: plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS. 2013;27(11):1803–1813. doi: 10.1097/QAD.0b013e328360e9fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunt PW, Cao HL, Muzoora C, Ssewanyana I, Bennett J, Emenyonu N, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sherer R, Solomon S, Schechter M, Nachega JB, Rockstroh J, Zuniga JM. HIV provider-patient communication regarding cardiovascular risk: results from the AIDS treatment for life international survey. J Int Assoc Provid AIDS Care. 2014;13(4):342–345. doi: 10.1177/2325957414530473. [DOI] [PubMed] [Google Scholar]
- 90.Ekali LG, Johnstone LK, Echouffo-Tcheugui JB, Kouanfack C, Dehayem MY, Fezeu L, et al. Fasting blood glucose and insulin sensitivity are unaffected by HAART duration in Cameroonians receiving first-line antiretroviral treatment. Diabetes Metab. 2013;39(1):71–77. doi: 10.1016/j.diabet.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Mosepele M, Hemphill LC, Palai T, Nkele I, Bennett K, Lockman S, et al. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) atherosclerotic cardiovascular disease (ASCVD) risk score among HIV-infected patients in sub-Saharan Africa. PLoS One. 2017;12(2):e0172897. doi: 10.1371/journal.pone.0172897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lazar JM, Wu X, Shi Q, Kagame A, Cohen M, Binagwaho A, et al. Arterial wave reflection in HIV-infected and HIV-uninfected Rwandan women. AIDS Res Hum Retrovir. 2009;25(9):877–882. doi: 10.1089/aid.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fourie C, van Rooyen J, Pieters M, Conradie K, Hoekstra T, Schutte A. Is HIV-1 infection associated with endothelial dysfunction in a population of African ancestry in South Africa? Cardiovasc J Afr. 2011;22(3):134–140. doi: 10.5830/CVJA-2010-056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ngatchou W, Lemogoum D, Ndobo P, Yagnigni E, Tiogou E, Nga E, et al. Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naive HIV+ patients from Cameroon. Vasc Health Risk Manag. 2013;9:509–516. doi: 10.2147/VHRM.S42350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ssinabulya I, Kayima J, Longenecker C, Luwedde M, Semitala F, Kambugu A, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One. 2014;9(2):e89537. doi: 10.1371/journal.pone.0089537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Awotedu KO, Mbeza BL, Awotedu AA, Ekpebegh C. Arterial stiffness in HIV patients in a semi urban area of South Africa. Clin Microbiol. 2015;4(3):207. [Google Scholar]
- 97.Siedner MJ, Kim JH, Nakku RS, Hemphill L, Triant VA, Haberer JE, et al. HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS. 2016;30(4):667–670. doi: 10.1097/QAD.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feinstein MJ, Kim JH, Bibangambah P, Sentongo R, Martin JN, Tsai AC, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Res Hum Retrovir. 2017;33(1):49–56. doi: 10.1089/aid.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid intima media thickness in mainly female HIV-infected subjects in rural South Africa: association with cardiovascular but not HIV-related factors. Clin Infect Dis. 2015;61(10):1606–1614. doi: 10.1093/cid/civ586. [DOI] [PubMed] [Google Scholar]
- 100.Sliwa K, Wilkinson D, Hansen C, Ntyintyane L, Tibazarwa K, Becker A, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the heart of Soweto study): a cohort study. Lancet. 2008;371(9616):915–922. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 101.Becker AC, Sliwa K, Stewart S, Libhaber E, Essop AR, Zambakides CA, et al. Acute coronary syndromes in treatment-naive black south africans with human immunodeficiency virus infection. J Interv Cardiol. 2010;23(1):70–77. doi: 10.1111/j.1540-8183.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 102.Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109(3):316–319. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- 103.Becker AC, Jacobson B, Singh S, Sliwa K, Stewart S, Libhaber E, et al. The thrombotic profile of treatment-naive HIV-positive black south Africans with acute coronary syndromes. Clin Appl Thromb Hemost. 2011;17(3):264–272. doi: 10.1177/1076029609358883. [DOI] [PubMed] [Google Scholar]
- 104.Becker AC, Libhaber E, Sliwa K, Singh S, Stewart S, Tikly M, et al. Antiphospholipid antibodies in black south africans with hiv and acute coronary syndromes: prevalence and clinical correlates. BMC Res Notes. 2011;4:379. doi: 10.1186/1756-0500-4-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Redman LA, Naidoo P, Biccard BM. HIV, vascular surgery and cardiovascular outcomes: a south African cohort study. Anaesthesia. 2014;69(3):208–213. doi: 10.1111/anae.12521. [DOI] [PubMed] [Google Scholar]
- 106.Kolapo KO, Vento S. Stroke: a realistic approach to a growing problem in sub-Saharan Africa is urgently needed. Tropical Med Int Health. 2011;16(6):707–710. doi: 10.1111/j.1365-3156.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 107.Kengne AP, Ntyintyane LM, Mayosi BM. A systematic overview of prospective cohort studies of cardiovascular disease in sub-Saharan Africa. Cardiovasc J Afr. 2011;22:1–10. doi: 10.5830/CVJA-2010-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80(3 Supplement 2):S5–S12. doi: 10.1212/WNL.0b013e3182762397. [DOI] [PubMed] [Google Scholar]
- 109.Ovbiagele B, Nath A. Increasing incidence of ischemic stroke in patients with HIV infection. Neurology. 2011;76(5):444–450. doi: 10.1212/WNL.0b013e31820a0cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK. V.A. T. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–358. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011;25(13):1637–1646. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 112.Kumwenda JJ, Mateyu G, Kampondeni S, van Dam AP, van Lieshout L, Zijlstra EE. Differential diagnosis of stroke in a setting of high HIV prevalence in Blantyre, Malawi. Stroke. 2005;36(5):960–964. doi: 10.1161/01.STR.0000162585.97216.ef. [DOI] [PubMed] [Google Scholar]
- 113.Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry. 2007;78(12):1320–1324. doi: 10.1136/jnnp.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mochan A, Modi M, Modi G. Stroke in black south African HIV-positive patients: a prospective analysis. Stroke. 2003;34(1):10–15. doi: 10.1161/01.STR.0000043821.35051.FA. [DOI] [PubMed] [Google Scholar]
- 115.Heikinheimo T, Chimbayo D, Kumwenda JJ, Kampondeni S, Allain TJ. Stroke outcomes in Malawi, a country with high prevalence of HIV: a prospective follow-up study. PLoS One. 2012;7(3):e33765. doi: 10.1371/journal.pone.0033765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel VB, Sacoor Z, Francis P, Bill PL, Bhigjee AI, Connolly C. Ischemic stroke in young HIV-positive patients in Kwazulu-Natal, South Africa. Neurology. 2005;65(5):759–761. doi: 10.1212/01.wnl.0000174434.00402.b5. [DOI] [PubMed] [Google Scholar]
- 117.Hoffmann M, Berger JR, Nath A, Rayens M. Cerebrovascular disease in young, HIV-infected, black Africans in the KwaZulu Natal province of South Africa. J Neuro-Oncol. 2000;6(3):229–236. doi: 10.3109/13550280009015825. [DOI] [PubMed] [Google Scholar]
- 118.Walker R, Jusabani A, Aris E, Gray W, Unwin N, Swai M, et al. Stroke risk factors in an incident population in urban and rural Tanzania: a prospective, community-based, case-control study. Lancet Glob Health. 2013;1(5):e282–e288. doi: 10.1016/S2214-109X(13)70068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–890. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Govindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, et al. Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in cape town, South Africa. PLoS One. 2013;8(11):e80017. doi: 10.1371/journal.pone.0080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med. 2015;23(4):146–149. [PMC free article] [PubMed] [Google Scholar]
- 123.Grépin KA. HIV donor funding has both boosted and curbed the delivery of different non-HIV health services in sub-Saharan Africa. Health Aff (Millwood) 2012;31(7):1406–1414. doi: 10.1377/hlthaff.2012.0279. [DOI] [PubMed] [Google Scholar]
- 124.Narayan KM, Miotti PG, Anand NP, Kline LM, Harmston C, Gulakowski R, 3rd, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low- and middle-income country settings. J Acquir Immune Defic Syndr. 2014;67 Suppl 1:S2–S7. doi: 10.1097/QAI.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 125.Oni T, McGrath N, BeLue R, Roderick P, Colagiuri S, May CR, et al. Chronic diseases and multi-morbidity--a conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health. 2014;14:575. doi: 10.1186/1471-2458-14-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davis S, Patel P, Sheikh A, Anabwani G, Tolle MA. Adaptation of a general primary care package for HIV-infected adults to an HIV centre setting in Gaborone, Botswana. Tropical Med Int Health. 2013;18(3):328–343. doi: 10.1111/tmi.12041. [DOI] [PubMed] [Google Scholar]
- 127.Topp SM, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, Reid SE. Integrating HIV treatment with primary care outpatient services: opportunities and challenges from a scaled-up model in Zambia. Health Policy Plan. 2013;28(4):347–357. doi: 10.1093/heapol/czs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rabkin M, Kruk ME, El-Sadr WM. HIV, aging and continuity care: strengthening health systems to support services for noncommunicable diseases in low-income countries. AIDS. 2012;26(S1):S77–S83. doi: 10.1097/QAD.0b013e3283558430. [DOI] [PubMed] [Google Scholar]
- 129.Rabkin M, Melaku Z, Bruce K, Reja A, Koler A, Tadesse Y, et al. Strengthening health systems for chronic care: leveraging HIV programs to support diabetes services in Ethiopia and Swaziland. J Trop Med. 2012;2012:137460. doi: 10.1155/2012/137460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katende D, Mutungi G, Baisley K, Biraro S, Ikoona E, Peck R, et al. Readiness of Ugandan health services for the management of outpatients with chronic diseases. Tropical Med Int Health. 2015;20(10):1385–1395. doi: 10.1111/tmi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsima BM, Setlhare V, Nkomazana O. Developing the Botswana primary care guideline: an integrated, symptom-based primary care guideline for the adult patient in a resource-limited setting. J Multidiscip Healthc. 2016;9:347–354. doi: 10.2147/JMDH.S112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Botha S, Fourie CM, van Rooyen JM, Kruger A, Schutte AE. Cardiometabolic changes in treated versus never treated HIV-infected black south Africans: the PURE study. Heart Lung Circ. 2014;23(2):119–126. doi: 10.1016/j.hlc.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 133.Ngatchou W, Lemogoum D, Ndobo P, Yiagnigni E, Tiogou E, Nga E, et al. Effects of antiretroviral therapy on arterial stiffness in Cameroonian HIV-infected patients. Blood Press Monit. 2013;18(5):247–251. doi: 10.1097/MBP.0b013e328363ee43. [DOI] [PubMed] [Google Scholar]
- 134.Longo-Mbenza B, Mashi ML, Tshikwela ML, Mokondjimobe E, Gombet T, Ellenga-Mbolla B, et al. Relationship between younger age, autoimmunity, cardiometabolic risk, oxidative stress, HAART, and ischemic stroke in Africans with HIV/AIDS. ISRN Cardiology. 2011;2011:897908. doi: 10.5402/2011/897908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16(1):1243. doi: 10.1186/s12889-016-3916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86(4):324–333. doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Asiki G, Stockdale L, Kasamba I, Vudriko T, Tumwekwase G, Johnston T, et al. Pilot study of antibodies against varicella zoster virus and human immunodeficiency virus in relation to the risk of developing stroke, nested within a rural cohort in Uganda. Tropical Med Int Health. 2015;20(10):1306–10. doi: 10.1111/tmi.12556. [DOI] [PubMed] [Google Scholar]
- 138.Mochan A, Modi M, Modi G. Protein S deficiency in HIV associated ischaemic stroke: an epiphenomenon of HIV infection. J Neurol Neurosurg Psychiatry. 2005;76(10):1455–1456. doi: 10.1136/jnnp.2004.059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy. (PDF 10 kb)
Additional References. The following references primarily focused on CVD risk factors (n = 123) and biomarkers of immune status and endothelial activation (n = 12). These publications were assessed for eligibility but were excluded from the final qualitative analysis. (PDF 63 kb)
Data Availability Statement
The original research articles included in this systematic review are publicly available. The complete search strategy is available in Additional file 1.


