Abstract
The EGF receptor is a classic receptor tyrosine kinase. It contains nine tyrosines in its C-terminal tail, many of which are phosphorylated and bind proteins containing SH2 or phosphotyrosine-binding (PTB) domains. To determine how many and which tyrosines are required to enable EGF receptor-mediated signaling, we generated a series of EGF receptors that contained only one tyrosine in their C-terminal tail. Assays of the signaling capabilities of these single-Tyr EGF receptors indicated that they can activate a range of downstream signaling pathways, including MAP kinase and Akt. The ability of the single-Tyr receptors to signal correlated with their ability to bind Gab1 (Grb2-associated binding protein 1). However, Tyr-992 appeared to be almost uniquely required to observe activation of phospholipase Cγ. These results demonstrate that multiply phosphorylated receptors are not required to support most EGF-stimulated signaling but identify Tyr-992 and its binding partners as a unique node within the network. We also studied the binding of the isolated SH2 domain of Grb2 (growth factor receptor-bound protein 2) and the isolated PTB domain of Shc (SHC adaptor protein) to the EGF receptor. Although these adapter proteins bound readily to wild-type EGF receptor, they bound poorly to the single-Tyr EGF receptors, even those that bound full-length Grb2 and Shc well. This suggests that in addition to pTyr-directed associations, secondary interactions between the tail and regions of the adapter proteins outside of the SH2/PTB domains are important for stabilizing the binding of Grb2 and Shc to the single-Tyr EGF receptors.
Keywords: epidermal growth factor (EGF), epidermal growth factor receptor (EGFR), growth factor receptor-bound protein 2 (GRB2), receptor tyrosine kinase, signal transduction, Gab1, Shc
Introduction
The EGF receptor is a widely expressed growth factor receptor that mediates the proliferative effects of EGF and other members of the EGF ligand family. The receptor is composed of an extracellular ligand-binding domain, an intracellular tyrosine kinase domain, and an ∼230-amino acid-long C-terminal tail (1) that is central to the signaling capabilities of the receptor (2).
Binding of EGF to the extracellular domain induces dimerization of the receptor (3, 4), leading to activation of the tyrosine kinase domain (5). The receptor then undergoes autophosphorylation through which multiple tyrosines on the C-terminal tail become phosphorylated (6–8). These tyrosines serve as sites for the binding of SH2 and PTB2 domain-containing proteins that mediate the downstream effects of the growth factor (9).
The C-terminal tail of the EGF receptor contains a total of nine tyrosine residues. Of these, seven have routinely been reported to be phosphorylated (10–15). These are Tyr-974, Tyr-992, Tyr-1045, Tyr-1068, Tyr-1086, Tyr-1148, and Tyr-1173. Of the remaining two, Tyr-1114 has occasionally been identified as being phosphorylated in proteomics analyses (13). Tyr-1101 has never been shown to become phosphorylated in response to EGF but does appear to be phosphorylated by pp60Src (16).
Although Tyr-1173 was initially thought to be the major site of EGF receptor phosphorylation (17), more recent studies have shown that Tyr-1148 is phosphorylated at a level up to five times higher than that of Tyr-1173 (10). Tyr-1148 also appears to be phosphorylated more rapidly than all but Tyr-1173 (14). As a result, phosphorylation of Tyr-1148 has become a standard marker for activation of the EGF receptor signaling pathways.
Tyr-1148 lies within a consensus sequence for the binding of the PTB domain of the Shc adaptor protein (18). Grb2, which binds to phospho-Shc, could thus be recruited to receptors containing this tyrosine, drawing in its cargo proteins such as SOS and Gab1. The question arises as to whether Tyr-1148 is necessary and sufficient to support the activation of the panoply of signaling pathways that lie downstream of the EGF receptor.
To address this question, we generated a series of EGF receptors, the single-Tyr EGF receptors, that contained only one of the nine tyrosines in their C-terminal tail. The other eight tyrosines were mutated to phenylalanine. Functional analyses of these receptors demonstrated that the presence of any single tyrosine, except Tyr-1045, yielded a receptor that was signaling-competent. Unexpectedly, the receptor with only Tyr-992 was found to be more broadly capable of signaling than any of the other single-Tyr receptors, including the one containing only Tyr-1148. The signaling capacity of these single-Tyr receptors correlated with their ability to bind Gab1, identifying this as a key node in the EGF receptor signaling network. These studies demonstrate the robust nature of the network and focus attention on the role of Tyr-992 and Gab1 in EGF receptor-mediated signaling. They also demonstrate that multiply phosphorylated EGF receptors are not required to support many of the downstream signaling events.
Results
Signaling via EGF receptors with a single tyrosine residue in their C-terminal tail
SH2 and PTB domain-containing proteins bind to phosphotyrosine residues in the context of a linear recognition motif. To determine which of the nine tyrosines in the C-terminal tail of the EGF receptor were principally responsible for mediating the activation of specific downstream signaling pathways, we generated a set of nine different EGF receptors in which all but one of the nine tyrosines were replaced with phenylalanine. This left only a single tyrosine residue available for phosphorylation and protein binding. We refer to these receptors as the single-Tyr series of EGF receptors and denote the receptors as, for example, the Y974-only EGF receptor, which is a receptor with only Tyr-974 in its C-terminal tail.
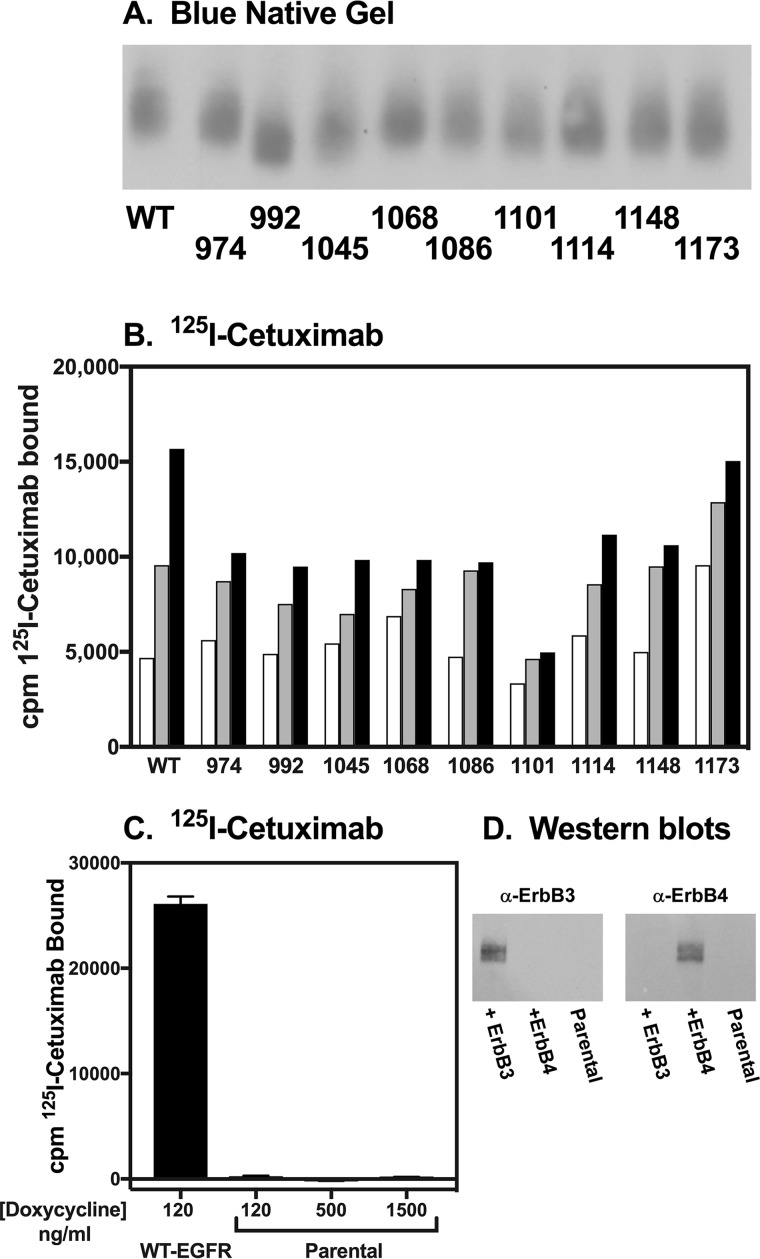
Nine stable CHO cell lines, each expressing one of the single-Tyr EGF receptors from a tet-inducible promoter, were generated. Fig. 1 shows a Western blot of a gel that was run on cells expressing the wild-type EGF receptor or each of the single-Tyr receptors. For this gel, the cells were solubilized in RIPA buffer but run on a blue native gel. This allowed us to blot with the monoclonal antibody, cetuximab, which recognizes an epitope on the extracellular domain of the receptor (19). We found that with the exception of the Y1068-only EGF receptor, our standard anti-EGF receptor antibody, which recognizes an epitope around Tyr-1068, did not recognize the single-Tyr EGF receptors. Apparently, Tyr-1068 is a major part of the epitope for this antibody, and phenylalanine is a poor substitute
Figure 1.
Expression and cell surface localization of the “only” EGF receptors. A, Western blot of cell lysates run on a blue native gel. Cells expressing wild-type or one of the single-Tyr EGF receptors were lysed in RIPA buffer containing 750 mm ϵ-amino-caproic acid. Lysates were run on a blue native gel, and EGF receptors were visualized using cetuximab. B, cells stably transfected with each of the single-Tyr receptors were treated with increasing doses of doxycycline and subjected to 125I-cetuximab binding. 125I-Cetuximab was used rather than 125I-EGF to avoid variations in binding because of alterations in EGF affinity in the different single-Tyr EGF receptors. Increasing concentrations of doxycycline are denoted by increasing color from white to gray to black of the bars. The concentrations of doxycycline used ranged from 25 to 1000 ng for the different lines. C, parental (untransfected) CHO cells were subjected to 125I-cetuximab binding as in B and are shown in comparison to 125I-cetuximab binding to cells transfected with wild-type EGF receptors. D, anti-ErbB3 or anti-ErbB4 Western blots of parental CHO cells or CHO cells transfected with ErbB3 (left panel) or ErbB4 (right panel).
As can be seen in Fig. 1A, all nine of the single-Tyr EGF receptors were expressed in CHO cells. All of the receptors ran at roughly the same position as the wild-type EGF receptor, suggesting a similar overall structure and level of glycosylation.
Each cell line was stimulated with several increasing concentrations of doxycycline to induce increased levels of receptor expression. Subsequently, intact cells were subjected to 125I-cetuximab binding to determine whether the single-Tyr receptors were transported to the cell surface. As shown in Fig. 1B, increasing concentrations of doxycycline led to increasing levels of 125I-cetuximab bound in all cell lines. This indicates that the single-Tyr receptors are expressed and present on the cell surface. Although expression levels varied among cell lines, it was possible to identify concentrations of doxycycline that yielded similar levels of expression of each single-Tyr receptor.
The parental CHO cells do not express any EGF receptors as judged by the absence of 125I-cetuximab binding in these cells (Fig. 1C). Nor do they express ErbB3 or ErbB4 as indicated by Western blots (Fig. 1D). Thus, any signaling in cells expressing the single-Tyr EGF receptors cannot be due to interaction with these other ErbB receptors. CHO cells do express a minimal number of ErbB2 (∼5000 receptors/cell). To prevent signaling caused by dimerization of the mutant EGF receptors with the low levels of endogenous ErbB2, all experiments were carried out in the presence of 5 μg/ml pertuzumab, which blocks the dimerization of ErbB2 with the EGF receptor (20, 21).
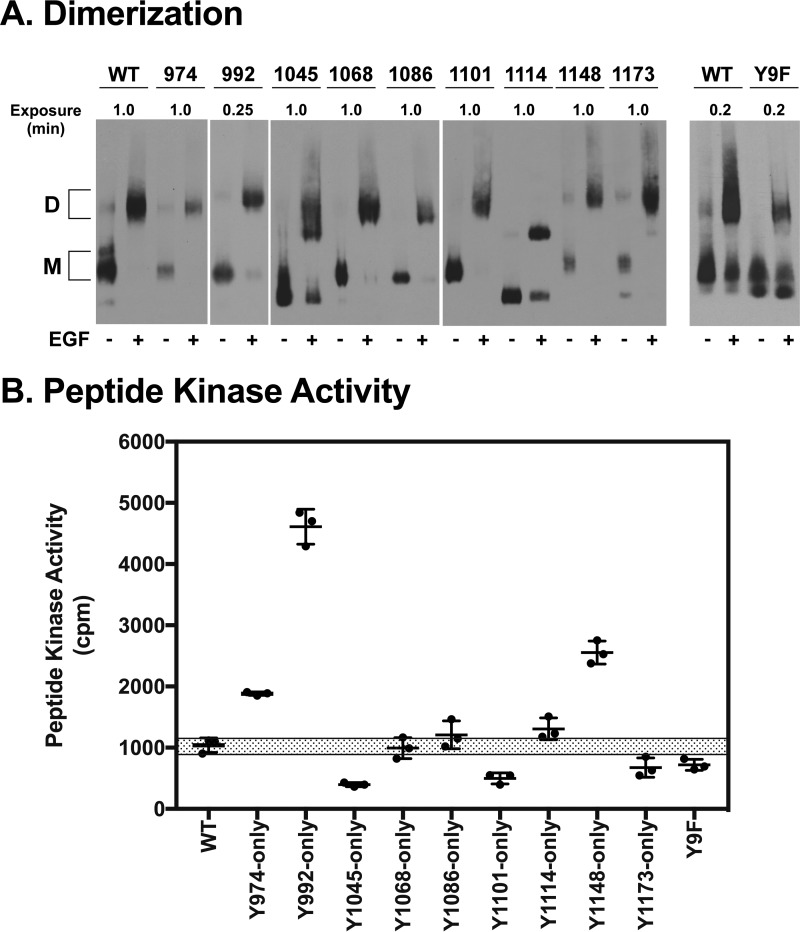
To determine whether the single-Tyr EGF receptors retained their ability to dimerize in response to EGF, membranes were prepared from cells expressing each of the single-Tyr EGF receptors as well as cells expressing the Y9F-EGF receptor, which lacks all nine tyrosines in its C-terminal tail. Membranes were treated with or without EGF in vitro. The EGF receptors were then solubilized with dodecylmaltoside, run on a blue native gel, and blotted with cetuximab. As shown in Fig. 2A, addition of EGF to membranes containing the wild-type EGF receptor led to a shift of the receptor band from a fast moving monomeric species to a slow moving dimeric species. All of the single-Tyr EGF receptors, as well as the Y9F-EGF receptor, also showed this EGF-dependent shift from fast moving monomer to slow moving dimer. Interestingly, the Y1045-only, Y1114-only, and Y9F-EGF receptor monomers ran slightly faster than the monomer species of the other receptors. Because the receptors all ran at a similar position when solubilized in a denaturing buffer (Fig. 1A), this difference is likely due to conformational differences in the tails of these receptors.
Figure 2.
EGF-stimulated dimerization and kinase activity of the single-Tyr EGF receptors. A, cells expressing wild-type or each of the single-Tyr EGF receptors or EGF receptors lacking all nine tyrosines (Y9F) were treated without or with 100 nm EGF and lysed in blue native gel sample buffer containing 0.5% dodecylmaltoside. Samples were run on blue native gels and subjected to Western blotting with cetuximab. D, dimer; M, monomer. B, membranes were prepared from cells expressing wild-type or each of the single-Tyr EGF receptors or EGF receptors lacking all nine tyrosines. Membranes were incubated without or with EGF in the presence of [γ-32P]ATP and the Arg-Arg-Src substrate peptide for 15 min, and the EGF-stimulated incorporation of 32P into the peptide was quantitated as described under “Experimental procedures.” Assays were performed in triplicate. The graph shows the individual replicates plus the mean and standard deviation. The shaded bar represents the range of activity observed with membranes from cells expressing wild-type EGF receptors.
In addition to inducing dimerization of all the single-Tyr EGF receptors, EGF also induced the activation of their tyrosine kinase activity. For the experiment shown in Fig. 2B, membranes from cells expressing each of the single tyrosine EGF receptors or the Y9F receptor were incubated with the exogenous peptide substrate Arg-Arg-Src and [γ-32P]ATP in the absence or presence of EGF (22). The incorporation of 32P into the peptide was then measured, and the EGF-stimulated activity is shown in Fig. 2B. All of the single-Tyr receptors, as well as the Y9F-EGF receptor, exhibited EGF-stimulated tyrosine kinase activity. Most showed activity in a range that was similar to that seen for the wild-type EGF receptor. However, the Y974-only, Y1148-only, and especially the Y992-only EGF receptors all showed peptide kinase activity that was significantly higher than that of the wild-type EGF receptor. By contrast, the Y1045-only and Y1101-only EGF receptors exhibited peptide kinase activity that was approximately half that seen for the wild-type receptor. These data indicate that the single tyrosine EGF receptors are expressed on the cell surface, bind EGF, and dimerize in response to ligand binding. In addition, they all retain EGF-stimulated tyrosine kinase activity.
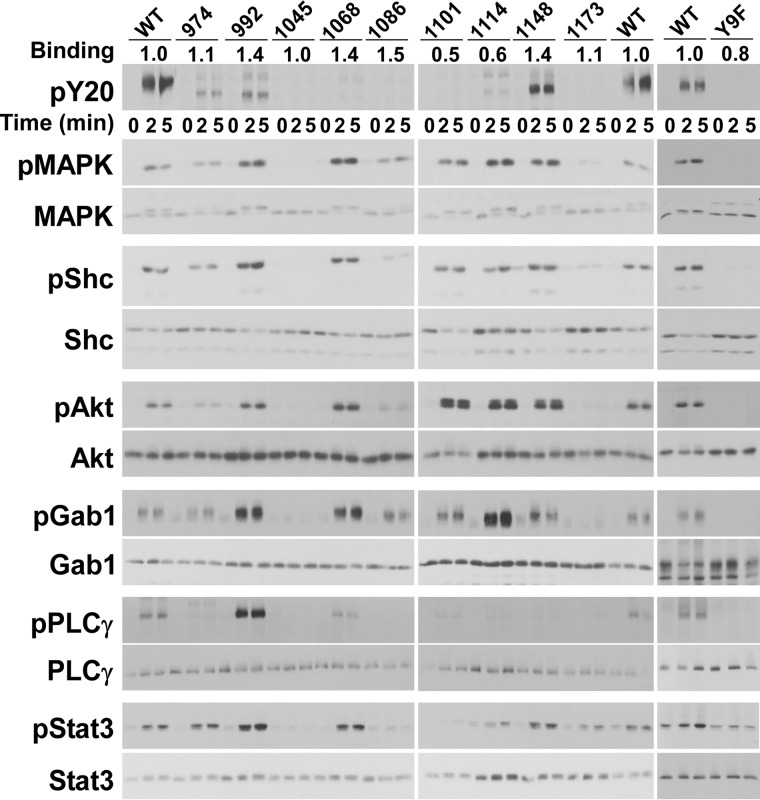
To examine signaling by the single-Tyr EGF receptors, cells expressing each of the receptors were stimulated with EGF and lysates analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting (Fig. 3). The behavior of the single-Tyr receptors was compared with that of the Y9F-EGF receptor, which would not be expected to signal because it lacks all nine tyrosines in its tail (far-right panels of Fig. 3). Receptor levels were determined based on 125I-cetuximab binding and are shown in the row marked Binding at the top of the Western blots. Binding for the single-Tyr receptors was normalized to that seen for the wild-type EGF receptor.
Figure 3.
Signaling by the single-Tyr receptors. Cells stably expressing wild-type or one of the single-Tyr EGF receptors were treated with 10 nm EGF for 1, 2, or 5 min at 37 °C as indicated. RIPA lysates were prepared and separated by SDS-PAGE. For the anti-phosphotyrosine blots, the protein load for the wild-type receptor was one-third that of the single-Tyr receptors. For all other blots, equal protein was loaded for the wild-type and the single-Tyr receptors. The values at the top of the Western blots in the line labeled Binding represent the binding of 125I-cetuximab to each cell line in that experiment, normalized to that observed for cells expressing the wild-type EGF receptor.
We first examined the ability of the single-Tyr EGF receptors to undergo autophosphorylation. Because these receptors have just one tyrosine as opposed to the nine tyrosines found in the wild-type receptor, the mutant receptors would be expected to be phosphorylated at only a fraction of the level of the wild-type receptor. Therefore, for the anti-phosphotyrosine blots, the wild-type receptor was loaded at one-third of the standard load to allow easier comparison with the more weakly phosphorylated single-Tyr receptors. As can be seen in the top panel of Fig. 3, the Y974-only, Y992-only, Y1114-only, and Y1148-only EGF receptors were detectably phosphorylated. The apparent lack of phosphorylation of the other single-Tyr receptors appears to be due to the failure of the PY20 antibody to recognize the individual phosphotyrosines because EGF-stimulated phosphorylation of the Y1068-only and Y1173-only receptors was readily detected using phosphosite-specific antibodies (supplemental Fig. S1). In addition, the PY20 antibody was able to detect phosphorylation of the Y1101-only EGF receptor if twice as much lysate was loaded on the SDS gel (supplemental Fig. S1). Thus, these anti-phosphotyrosine Western blots cannot be used to determine whether, and how extensively, each of the different single-Tyr EGF receptors are phosphorylated, but they do indicate that the receptors are autophosphorylated. As expected, the Y9F receptor was not detectably phosphorylated.
To determine the extent to which these single-Tyr EGF receptors could mediate cell signaling, we assessed the ability of EGF to stimulate the activity of several downstream signaling pathways. Eight of the nine single-Tyr receptors, including those carrying the rarely phosphorylated Tyr-1101 and Tyr-1114, supported activation of MAP kinase (second and third panels from the top in Fig. 3). Only the Y1045-only EGF receptor and the Y9F-EGF receptors failed to stimulate the activity of MAP kinase. For many of the single-Tyr EGF receptors, the level of MAP kinase activation was greater in cells expressing the mutant receptors than in cells expressing the wild-type receptor. This may be due to the absence of normal feedback inhibitory mechanisms in these receptors giving rise to an unopposed stimulatory signal.
A similar pattern of activation was seen for the EGF-stimulated phosphorylation of Shc, Akt, Gab1, and Stat3. All of the single-Tyr EGF receptors, with the exception of the Y1045-only receptor, supported these downstream signaling responses. Thus, despite the presence of just a single tyrosine residue, these receptors were remarkably catholic in their ability to engage downstream signaling molecules. The Y9F-EGF receptor was completely inactive in these assays, indicating that tyrosines in other parts of the EGF receptor structure were not able to substitute for any of the tyrosines in the C-terminal tail. It also suggests that the single-Tyr EGF receptors are not signaling because they have dimerized with another receptor. If they were, the Y9F-EGF receptor that is kinase-active and capable of dimerizing would have been able to elicit some downstream response, but it did not.
A strikingly different pattern was observed for the phosphorylation of phospholipase Cγ. In this case, the Y992-only EGF receptor robustly stimulated this signaling event to levels significantly higher than what was observed for the wild-type EGF receptor. In addition, the Y1068-only EGF receptor supported weak activation of phospholipase Cγ. These data indicate that although some signaling events, such as Gab1 phosphorylation, are readily supported by most of the single-Tyr EGF receptors, other pathways, like activation of phospholipase Cγ, have a much more specific requirement for the phosphorylated tyrosine.
Binding of signaling proteins to the “only” EGF receptors
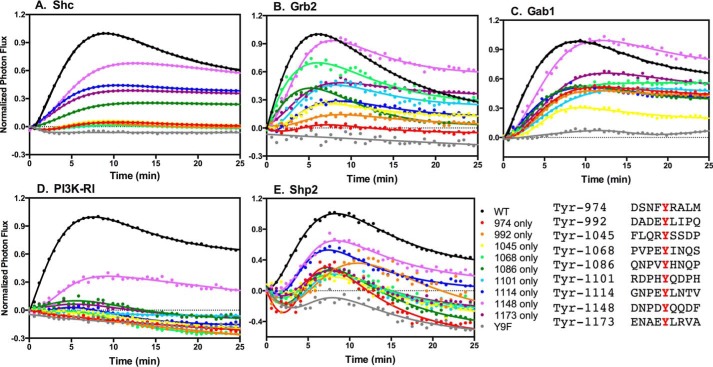
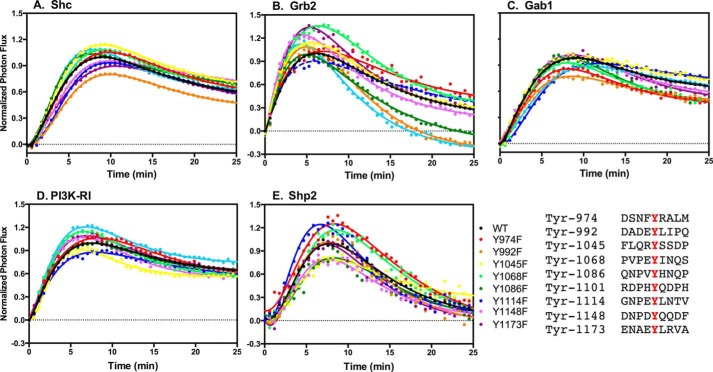
We have previously used luciferase fragment complementation imaging to visualize the association of multiple downstream signaling proteins with wild-type EGF receptors in response to different EGF receptor agonists (23). We used this luciferase complementation system to determine how the presence of a single tyrosine residue in the C-terminal tail of the EGF receptor affected its ability to bind Shc, Grb2, Gab1, PI3K-RI, and Shp2. To do this, the single-Tyr EGF receptors were C-terminally fused to the N-terminal fragment of firefly luciferase (NLuc). The receptors were transiently transfected into CHO cells stably expressing Shc, Grb2, Gab1, PI3K-RI, or Shp2 C-terminally fused to the C-terminal fragment of firefly luciferase (C-Luc).
Fig. 4A shows the ability of wild-type and the single-Tyr EGF receptors to bind p52Shc. The Y1148-only EGF receptor exhibited the highest level of binding to Shc of any of the single-Tyr receptors. This is consistent with the fact that Y1148 lies within an NPXY consensus sequence for the binding of the Shc PTB domain (18). The Y1114-only and the Y1173-only EGF receptors bound Shc about half as well as the wild-type EGF receptor. The Y1086-only EGF receptor also exhibited modest binding of Shc. Tyr-1086 and Tyr-1114 both lie within an NPXY consensus sequence that would enable them to bind to the Shc PTB domain, possibly explaining their strong showing in this assay.
Figure 4.
Binding of signaling proteins to the single-Tyr receptors. Cells stably expressing one of five different signaling proteins fused to CLuc were transiently transfected with cDNA encoding the wild-type EGF receptor or one of the single-Tyr EGF receptors C-terminally fused to NLuc. The concentration of EGF that yielded optimal complementation of the EGF receptor with that particular protein (23) was added, and light production in the presence of luciferin was measured at 30-s intervals for 25 min. A, p52 Shc. B, Grb2. C, Gab1. D, PI3K-RI. E, Shp2.
All other members of the single-Tyr series of receptors, including Y974-only, Y992-only, Y1045-only, Y1068-only, and Y1101-only, failed to bind Shc, even though most of these receptors clearly phosphorylated Shc (Fig. 3). Because the proximal tyrosines in the EGF receptor tail (Tyr-974, Tyr-992, Tyr-1045, and Tyr-1068) are not in a consensus sequence for the direct binding of the Shc PTB domain, these data suggest that Shc is binding indirectly to these sites to become phosphorylated. Steric issues may prevent complementation of the luciferase fragments. As expected, the Y9F-EGF receptor failed to bind Shc.
Grb2 exhibited a somewhat different pattern of binding to the single-Tyr EGF receptors (Fig. 4B). Like Shc, Grb2 bound to the Y1148-only EGF receptor at essentially wild-type levels and bound about half as well as wild type to Y1173-only EGF receptor. Because neither of these sites are known to bind Grb2 directly, it is likely that the assay is detecting Grb2 binding to Shc, which was bound to the EGF receptor and phosphorylated.
Grb2 also bound well to the Y1068-only EGF receptor, the Y1114-only EGF receptor, and also fairly well to the Y1101-only EGF receptor. Both Tyr-1068 and Tyr-1114 lie within a canonical binding consensus sequence (YXNX) (24) for the Grb2 SH2 domain, and Tyr-1068 has been shown to bind Grb2 (25, 26). Thus, Grb2 is likely binding directly to these two receptors. The sequence surrounding Tyr-1101 (pYQDPH) does not fit the consensus sequence for the binding of the Grb2 SH2 domain, so it is difficult to explain why Grb2 is binding to this receptor. It is possible that another adapter protein binds to this tyrosine and, after phosphorylation, provides a site for Grb2 binding. We could not detect Grb2 binding to the proximal tyrosines (Tyr-974, Tyr-992, and Tyr-1045). Grb2 also failed to bind to the Y9F-EGF receptor, indicating the need for at least one tyrosine for the binding of this adapter protein to the EGF receptor.
Gab1 is a major scaffolding protein recruited to the EGF receptor via Grb2 (27). Because of this relationship, we anticipated that the ability of Gab1 to associate with the EGF receptor would parallel that of Grb2. Indeed, Gab1 bound well to the Y1148-only EGF receptor and to the other single-Tyr receptors to which Grb2 bound, including Y1068-only, Y1086-only, Y1101-only, Y1114-only, and Y1173-only (Fig. 4C). However, Gab1 also showed significant binding to single-Tyr receptors carrying proximal tyrosines-the Y974-only and Y992-only EGF receptors. These data are consistent with the ability of all of these single-Tyr receptors to phosphorylate Gab1 (Fig. 3). Like Shc and Grb2, Gab1 failed to bind to the Y9F-EGF receptor, demonstrating that its association with the EGF receptor was dependent upon the presence of at least one phosphotyrosine.
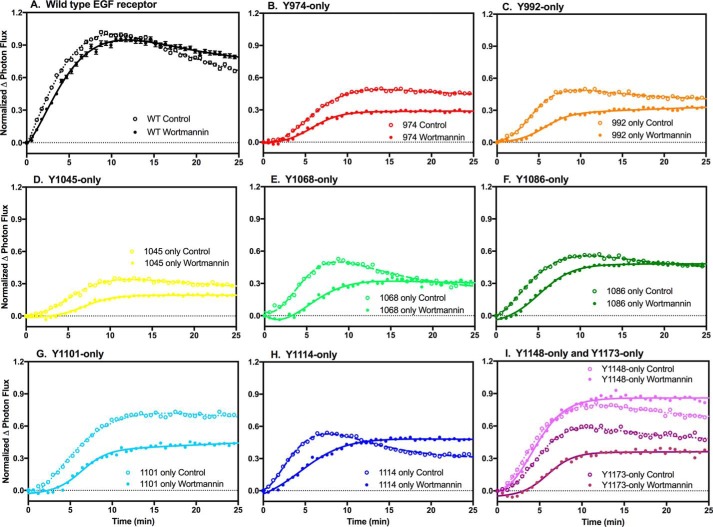
Gab1 contains a PH domain that could recruit the protein to the plasma membrane upon stimulation with EGF, thereby promoting the recruitment Gab1 to the EGF receptor (28). To determine the extent to which its PH domain contributes to the recruitment of Gab1 to the EGF receptor, we assessed the effect of wortmannin on the binding of Gab1 to wild-type EGF receptors, as well as to the single-Tyr series of receptors. Wortmannin is an inhibitor of the PI 3-kinase (29) that generates the PI 3,4,5-P3, through which the Gab1 PH domain is recruited to the membrane (30). As shown in Fig. 5, pretreatment of cells with wortmannin did not affect the ability of Gab1 to bind to the wild-type EGF receptor or the Y1148 only-EGF receptor. However, wortmannin treatment slowed the kinetics of Gab1 association with the other single-Tyr EGF receptors and, in some cases, also reduced the extent of binding. This suggests that under non-optimal conditions, the PH domain-dependent localization of Gab1 to the membrane contributes to the association of Gab1 with the EGF receptor.
Figure 5.
Effect of wortmannin on the binding of Gab1 to the single-Tyr EGF receptors. Cells stably expressing Gab1 C-terminally fused to CLuc were transiently transfected with cDNA encoding wild-type or one of the single-Tyr EGF receptors fused to NLuc. The cells were treated without or with 100 nm wortmannin for 40 min at 37 °C and then stimulated with 10 nm EGF and assayed for light production in the presence of luciferin. Open circles in each panel denote assays in the absence of wortmannin, whereas closed circles represent data from assays done in the presence of wortmannin.
PI3K-RI can be recruited to the EGF receptor signaling complex by binding to the YMXM motifs present in Gab1 (24), and like Gab1, PI3K-RI did bind to the Y1148-only EGF receptor (Fig. 4D). However, unlike Gab1, PI3K-RI did not associate with any of the other single-Tyr EGF receptors. Given the observation that all but the Y1045-only EGF receptors supported Akt activation (Fig. 3) and hence likely mediated activation of the PI 3-kinase, it is possible that the failure of PI3K-RI to show significant complementation with the other single-Tyr receptors is due to steric issues.
Shp2 can also be recruited to the EGF receptor through Gab1 (31). The data in Fig. 4E demonstrate that Shp2 was recruited reasonably well to the Y1148-only and Y1114-only EGF receptors. The other single-Tyr receptors showed substantially less Shp2 binding than these two single-Tyr receptors.
Given the observation that Tyr-1148 appeared to be capable of mediating the association of all five signaling proteins with the EGF receptor, we wondered whether the removal of this, or any other single tyrosine, would substantially affect the ability of these proteins to bind to the EGF receptor. We therefore generated a set of EGF receptors in which only one of the nine tyrosines in the tail was mutated to a phenylalanine. We refer to these as the “Y-to-F” series and denote the specific mutation as, for example, Y1148F-EGF receptor. The Y-to-F EGF receptors were C-terminally fused to NLuc and transiently transfected into CHO cells expressing Shc, Grb2, Gab1, PI3K-RI, or Shp2 C-terminally fused to the CLuc.
Substitution of any one tyrosine in the C-terminal tail of the EGF receptor with phenylalanine (the Y-to-F series) did not affect the ability of Shc to bind to the EGF receptor (Fig. 6). In particular, Shc bound normally to the Y1148F- and Y1173F-EGF receptors, despite reports that these two tyrosines represent the major Shc binding sites on the EGF receptor (25, 32). Thus, in the absence of its major binding site, Shc still binds to the EGF receptor to the same extent and with similar kinetics as it does to wild-type EGF receptor. Similarly, removal of any single tyrosine residue did not affect the ability of Grb2, Gab1, PI3K-RI, or Shp2 to bind to the EGF receptor. Thus, EGF receptor signaling appears to incorporate significant redundancy so that the system is not substantially affected by the loss of any one particular tyrosine, even if it represents a major site of phosphorylation and protein binding.
Figure 6.
Binding of signaling proteins to the Y-to-F EGF receptor mutants. Cells stably expressing one of five different signaling proteins fused to CLuc were transiently transfected with cDNA encoding the wild-type EGF receptor or the indicated Y-to-F single point mutation EGF receptors C-terminally fused to NLuc. The concentration of EGF that yielded optimal complementation of the EGF receptor with that particular signaling protein (23) was added, and light production in the presence of luciferin was measured for 25 min. A, p52 Shc. B, Grb2. C, Gab1. D, PI3K-RI. E, SHP2.
Binding of mutants of Grb2 to the single-Tyr EGF receptors
Grb2 is an adapter protein composed of one SH2 domain and two SH3 domains (33). The SH2 domain is responsible for binding phosphotyrosine residues, whereas the SH3 domains bind polyproline regions in proteins that are then recruited to the EGF receptor. If the binding of Grb2 to the EGF receptor is mediated entirely by its SH2 domain, one would predict that the binding of the isolated Grb2 SH2 domain to the EGF receptor mutants should recapitulate the binding of full-length Grb2 to the single-Tyr EGF receptors.
To test this hypothesis, we generated a construct containing the isolated SH2 domain of Grb2 C-terminally fused to CLuc. As shown in Fig. 7A, the isolated Grb2 SH2 domain bound well to the wild-type EGF receptor. However, it failed to bind to any of the single-Tyr EGF receptors. This was somewhat surprising because full-length Grb2 bound well to several of the single-Tyr EGF receptors, including the Y1148-only, Y1068-only, and Y1173-only EGF receptors (Fig. 4B). This suggested that the SH3 domains of full-length Grb2 contributed to its ability to bind to the single-Tyr EGF receptors.
Figure 7.
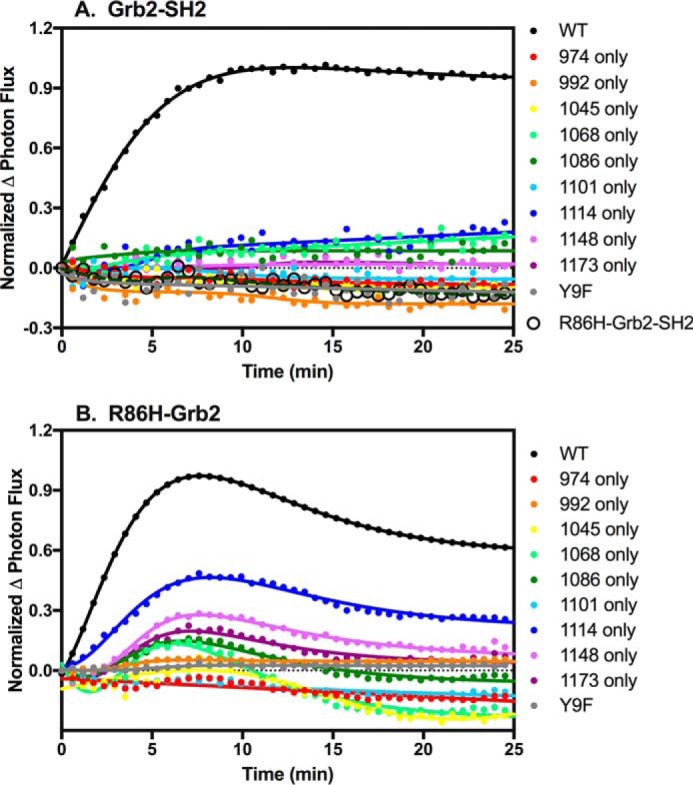
Binding of Grb2 mutants to EGF receptors. A, cells stably expressing the isolated Grb2-SH2 domain or the R86H-Grb2SH2 domain C-terminally fused to CLuc were transiently transfected with cDNA encoding the wild-type EGF receptor or one of the single-Tyr EGF receptors fused to NLuc. The cells were stimulated with 10 nm EGF, and light production in the presence of luciferin was measured. B, cells stably expressing the isolated Grb2 SH2 domain containing the R86H mutation and fused to CLuc were transiently transfected with cDNA encoding the wild-type EGF receptor or one of the single-Tyr EGF receptors. Cells were stimulated with 10 nm EGF, and light production in the presence of luciferin was measured.
To test this, we first generated a cDNA that encoded the Grb2 SH2 domain containing an R86H mutation C-terminally fused with CLuc. The R86H mutation has been reported to abolish the ability of the SH2 domain to bind to phosphotyrosine residues (34–37). As shown in Fig. 7A, whereas the Grb2 SH2 domain bound well to the wild-type EGF receptor (filled black circles), the R86H-Grb2-SH2 domain did not bind at all (open black circles). These data demonstrate that the R86H mutation does in fact ablate the phosphotyrosine binding activity of the Grb2 SH2 domain.
We then assessed whether a full-length Grb2 construct containing the R86H mutation fused to CLuc could bind to the wild-type EGF receptor or any of the single-Tyr EGF receptors. As shown in Fig. 7B, only the Y1114-only EGF receptor supported a significant level of binding of R86H-Grb2. The other single-Tyr EGF receptors bound R86H-Grb2 very poorly. Thus, neither the isolated SH2 domain nor the SH3 domains by themselves are able to mediate the efficient binding of Grb2 to the single-Tyr receptors. This suggests that the ability of full-length Grb2 to bind to the single-Tyr EGF receptors involves a collaboration of the SH2 and SH3 domains of Grb2.
Binding of Shc SH2 and PTB domains to the single-Tyr EGF receptors
Shc contains an N-terminal PTB domain and a C-terminal SH2 domain. Theoretically, either domain is capable of binding to phosphotyrosine in the appropriate consensus sequence. To determine whether the association of Shc with the single-Tyr EGF receptors was mediated through the SH2 or PTB domain of the protein, we generated two constructs: one containing the Shc-PTB domain fused to CLuc and one encoding the Shc-SH2 domain fused to C-Luc. When transiently transfected into cells stably expressing the wild-type EGF receptor fused to NLuc, the isolated PTB domain was found to bind nearly as well to the wild-type EGF receptor as full-length Shc. However, the isolated SH2 domain failed to associate with the receptor (Fig. 8A). This confirms prior reports that the PTB domain, but not the SH2 domain, is responsible for the ability of Shc to bind to the EGF receptor (32).
Figure 8.
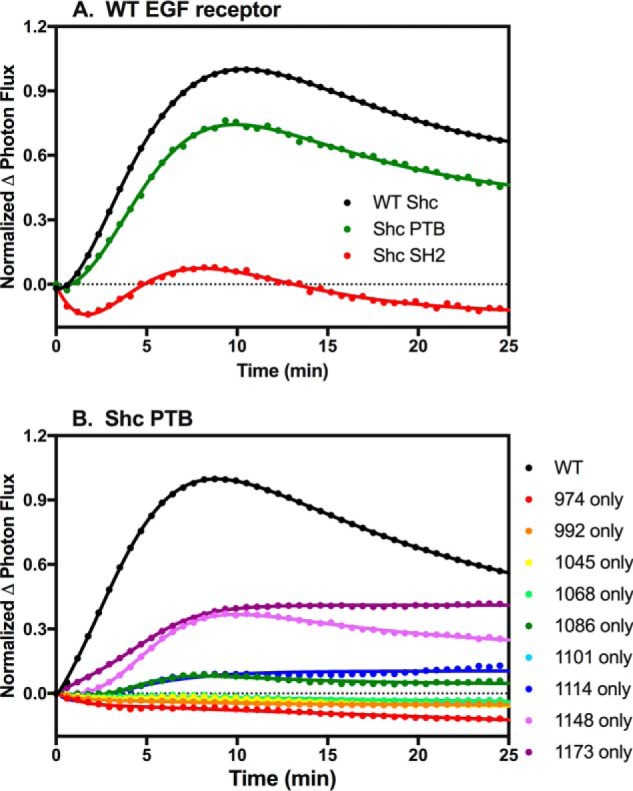
Binding of Shc SH2 and PTB domains to EGF receptors. A, cells stably expressing wild-type EGF receptor fused to NLuc were transiently transfected with full-length wild-type Shc (WT Shc), the isolated Shc PTB domain (Shc PTB), or the isolated Shc SH2 domain (Shc SH2) fused to CLuc. Cells were stimulated with 1 nm EGF, and light production in the presence of luciferase measured. B, cells stably expressing the Shc PTB domain fused to CLuc were transiently transfected with cDNA encoding wild-type EGF receptor or one of the single-Tyr EGF receptors. Cells were stimulated with 1 nm EGF, and light production in the presence of luciferin was measured.
Although the isolated PTB domain bound well to the wild-type EGF receptor, it bound relatively poorly to the single-Tyr EGF receptors (Fig. 8B). As can be seen in the figure, the Shc PTB domain showed significant binding only to the Y1148-only and Y1173-only EGF receptors. For the Y1148-only receptor, the binding of the Shc PTB domain was substantially less than the binding of full-length Shc to this receptor (Fig. 4A). Thus, as was the case for Grb2, the ability of full-length Shc to bind to the single-Tyr EGF receptors appears to involve more than simply the pTyr/PTB domain interaction.
Discussion
In the current paradigm for EGF receptor-mediated signaling, phosphorylation of tyrosine residues in the C-terminal tail of the receptor provides docking sites for the binding of signaling proteins that contain SH2 or PTB domains. The EGF receptor contains nine tyrosines in its tail, raising the question of whether all these tyrosines are needed to support EGF receptor-mediated signaling. Here we present data indicating that EGF receptors with only a single tyrosine in their C-terminal tail are able to recapitulate a large number of the signaling activities of the EGF receptor.
All of the single-Tyr EGF receptors dimerized in response to EGF, indicating that the binding and dimerization functions of the receptor were intact, despite the changes in the C-terminal tail. All of the receptors also exhibited EGF-stimulated tyrosine kinase activity as measured by the phosphorylation of an exogenous peptide substrate. We were also able to detect autophosphorylation of all but the Y1045-only and Y1086-only EGF receptors. The former likely was not phosphorylated because it did not signal, but the latter did support some signaling, indicating that it must have been autophosphorylated. It appears that the pan-anti-phosphotyrosine PY20 antibody cannot detect this phosphorylation. The fact that the PY20 antibody failed to detect tyrosine phosphorylation in several of the single-Tyr EGF receptors that were later found to be phosphorylated when site-specific antibodies were used is a cautionary tale. It is clear that use of this pan-anti-phosphotyrosine antibody does not guarantee the detection of all phosphotyrosines in a sample.
All of the single-Tyr EGF receptors, except the Y1045-only receptor, were able to support the activation of multiple downstream signaling pathways. Notably, the ability of a receptor to activate signaling did not correlate with its peptide kinase activity. For example, the Y1173-only EGF receptor exhibited nearly wild-type levels of peptide kinase activity but showed relatively weak signaling in all assays. By contrast, the Y1148-only EGF receptor exhibited peptide kinase activity that was two to three times higher than the wild-type EGF receptor and yet showed only wild-type levels of activation of Gab1 and Stat3 and did not stimulate the activation of phospholipase Cγ at all. Thus, the observed signaling differences among the single tyrosine receptors must reflect differences in how, or whether, the remaining tyrosine residue interacts with different signaling proteins.
The ability of the single-Tyr EGF receptors to bind downstream signaling molecules was variable, possibly because of the steric requirements necessary to obtain complementation of the two luciferase fragments. However, all of the single tyrosine receptors, except the Y1045-only receptor, bound Gab1. The ability of the single-Tyr EGF receptors to bind Gab1 probably explains why these receptors were all able to activate signaling pathways such as MAP kinase and Akt.
Tyr-1148 is typically reported to be the major site of tyrosine phosphorylation on the EGF receptor (10, 11). Consequently, the observation that the Y992-only EGF receptor mediated activation of all six downstream pathways examined, whereas the Y1148-only EGF receptor activated only five of the pathways, was unexpected. Tyr-992 is known to represent the binding site for phospholipase C γ (38). This is probably why the Y992-only EGF receptor was almost unique in its ability to activate phospholipase Cγ. This specificity suggests that, unlike the other signaling pathways, activation of phospholipase Cγ is highly dependent on the nature or position of the phosphorylated tyrosine and suggests that Tyr-992 plays a unique role in EGF receptor-mediated signaling.
Data from several studies using different approaches suggest that Tyr-992 does indeed represent a distinctive site in the EGF receptor tail. Tyr-992 is proximal to the kinase domain, and coarse-grain simulations suggest that the available chain length beyond the kinase domain is not long enough to permit phosphorylation of this residue in trans within an asymmetric kinase dimer (39). Rather, Koland (39) speculated that Tyr-992 may be phosphorylated in cis. Consistent with this view, Huang et al. (40) presented evidence that phosphorylation of Tyr-992 was most efficient when the EGF receptor could form higher-order oligomers. In contrast to dimers where only one kinase domain is activated, a greater fraction of the kinase domains in oligomers are activated, which would allow more phosphorylation in cis of Tyr-992. The phosphorylation of Tyr-992 is uniquely increased after irradiation (41) and after cholesterol depletion (42), and Tyr-992 phosphorylation is selectively stimulated after treatment of glioblastoma cells with an agonist of the formyl-peptide receptor (43). Finally, the amino acid sequence surrounding Tyr-992 is conserved in all EGF receptors from insect to human, as well as in vertebrate ErbB2 and ErbB4. Together, these findings suggest that Tyr-992 may occupy a special niche in EGF receptor-mediated signaling that has evolutionarily deep roots.
It is perhaps not surprising that the single-Tyr receptors are able to signal as widely as the wild-type EGF receptor. Studies have shown that the stoichiometry of phosphorylation of the EGF receptor is low (44), and a single molecule approach suggested that the receptor is only rarely multiphosphorylated (45). Thus, in vivo, the typical receptor may contain only a single phosphorylated tyrosine on its tail. The difference for the single-Tyr receptors is that unlike a population of singly phosphorylated wild-type receptors that would present an ensemble of all possible phosphosites, the single-Tyr EGF receptors present one specific phosphorylated tyrosine. Thus, any signaling through these receptors has to proceed utilizing that one phosphotyrosine.
A noteworthy observation in our study is the ability of Tyr-1101 and Tyr-1114 to support downstream signaling. These two tyrosines are not normally reported to be phosphorylated in response to EGF, yet they clearly are able to support signaling when they represent the only tyrosine in the C-terminal tail. It is possible that in the wild-type EGF receptor, the phosphorylation of other tyrosines is kinetically preferred, and the subsequent binding of signaling proteins to those phosphotyrosines precludes the phosphorylation of and signaling through Tyr-1101 or Tyr-1114. This implies that steric considerations limit the number of sites that can be phosphorylated on any given receptor and is consistent with the low stoichiometry of phosphorylation reported for the EGF receptor (10, 44, 45).
We used the luciferase fragment complementation system to explore the structural features of Grb2 and Shc that enabled them to bind to the single-Tyr EGF receptors. Grb2 binds to the EGF receptor via its SH2 domain, and it is generally accepted that Shc binds to the EGF receptor through its PTB domain rather than through its SH2 domain (32). Consistent with this conclusion, the isolated Grb2 SH2 domain and the isolated Shc PTB domain, both bound well to wild-type EGF receptor, whereas the Shc SH2 domain did not bind.
Whereas full-length Grb2 bound well to several of the single-Tyr EGF receptors, the isolated SH2 domain of Grb2 did not bind to any of the single-Tyr EGF receptors. This suggests that regions of Grb2 outside of the SH2 domain contribute to the ability of full-length Grb2 to bind to the single-Tyr EGF receptors. R86H-Grb2, which has an inactivated SH2 domain, bound poorly to most of the single-Tyr EGF receptors and not at all to the Y9F-EGF receptor. Thus, the SH3 domains alone are also insufficient for stable binding of Grb2 to the single-Tyr EGF receptors. These observations imply that the combination of an SH2 domain-mediated interaction and an SH3 domain-dependent interaction enabled the binding of full-length Grb2 to the single-Tyr EGF receptors. A similar effect may be occurring in Shc as the full-length protein bound strongly to the Y1148-only EGF receptor, but the isolated PTB domain bound relatively poorly to this receptor.
We speculate that in Grb2, the SH2 domain initiates the interaction of the adapter protein with the tyrosine-phosphorylated EGF receptor but that additional contacts between the tail and the Grb2 SH3 domains, or its recruited cargo protein, further stabilize the association of Grb2 with the single-Tyr EGF receptors. The structural plasticity of the intrinsically disordered C-terminal tail could facilitate the formation of such secondary interactions, thereby enhancing the affinity and specificity of the binding proteins for the EGF receptor tail.
In summary, we have shown that EGF receptors bearing only a single tyrosine in their C-terminal tail are capable of activating a broad range of downstream signaling pathways, demonstrating the adaptability of the EGF signaling network. However, the strong dependence of phospholipase Cγ activation on the presence of Tyr-992 suggests that at least some signaling pathways exhibit strong site specificity and may be regulated differently than other more generally activatable pathways, like MAP kinase. The enhanced phosphorylation of Tyr-992 by EGF receptor oligomers (40) and the strong requirement for Tyr-992 for activation of phospholipase Cγ suggest the possibility that different oligomeric states of the receptor may mediate the activation of specific downstream responses. This offers a new opportunity for the design of selective inhibitors of particular EGF-stimulated signaling pathways. This could also explain the biased signaling that is apparent in the EGF receptor system (23) if different EGF receptor agonists induce different levels of higher-order receptor oligomers.
Experimental procedures
Materials
EGF was purchased from Biomedical Technologies. Cetuximab and pertuzumab were obtained from the Barnes–Jewish Hospital pharmacy. 125I-Cetuximab was generated using the ICl procedure described previously (46). The PY-20 anti-phosphotyrosine antibody was from BD Transduction Laboratories. The anti-MAP kinase, anti-Shc, and anti-Gab1 antibodies were from Millipore. The phospho-MAP kinase antibody was from Promega. The anti-Akt, phospho-Akt, phospho-Gab1, phospholipase Cγ, phosphophospholipase Cγ, Stat3, and phosho-Stat3 Tyr-709 antibodies were from Cell Signaling. The Tyr-239/240 phospho-Shc antibodies were obtained from Promega. FetalPlex was from Gemini Bioproducts. Na125I and [γ-32P]ATP were from PerkinElmer. Wortmannin was purchased from Sigma–Aldrich. The substrate peptide Arg-Arg-Leu-Ile-Glu-Asp-Ala-Glu-Tyr-Ala-Ala-Arg-Gly was synthesized and purified in-house.
DNA constructs
The single-Tyr series of EGF receptors was generated through multiple rounds of site-directed mutagenesis that ultimately substituted a phenylalanine for all but one tyrosine. To reduce the number of rounds of mutagenesis, the codon encoding Ala-1078 was altered to generate an FseI site while retaining Ala at that position. This allowed us to mutate the four tyrosines N-terminal to the FseI site and the five tyrosines C-terminal to the FseI site independently and then ligate the appropriate fragments together to obtain the desired single-Tyr receptor. The Y-to-F series of EGF receptors were generated by site-directed mutagenesis of the wild-type EGF receptor using primers that converted a single tyrosine to a phenylalanine. All constructs were generated in the pBI-Tet vector, which allowed induction of the mutant receptor with doxycycline. For luciferase complementation experiments, constructs were fused with the NLuc fragment of firefly luciferase as described previously (47).
The R86H mutation was introduced into Grb2 by site-directed mutagenesis. The Grb2-SH2 domain, without or with the R86H mutation, was generated by introducing a BsiWI site at amino acid position 153 of Grb2, allowing fusion in-frame with the CLuc fragment of firefly luciferase in the pcDNA 3.1 Zeo vector.
The constructs encoding the PTB and SH2 domains of Shc were generated by site-directed mutagenesis of wild-type Shc using primers that flanked the region of deletion. The PTB domain construct retained only those residues N-terminal of Pro-210. The SH2 construct retained the residues C-terminal of Pro-365.
Cell lines
The stable cell lines constitutively expressing the five signaling proteins fused to CLuc (Shc, Grb2, Gab1, PI3K-RI, and SHP2) were derived as described previously (23). CHO cells were transfected with cDNAs encoding each of the nine single-Tyr receptors without the luciferase fragment plus the pTK hygromycin vector (Clontech) for purposes of selection. Stable clonal lines were selected by growth in 100 μg/hygromycin. The cells were maintained in DMEM with 10% Fetalplex plus 100 μg/ml G418 and 100 μg/ml hygromycin.
Blue native gel electrophoresis
Blue native gels were run according to the protocol of Wittig et al. (48). For the experiment in Fig. 1, samples were prepared by scraping cells from a 35-mm well into 0.3 ml of RIPA buffer containing 750 mm ϵ-amino-caproic acid to disrupt receptor oligomers. For the experiment in Fig. 2, membranes were prepared as outlined below and solubilized in sample preparation buffer (48) containing 0.50% dodecylmaltoside by passing the material through a 25-gauge needle 10 times. Undissolved material was pelleted, loading buffer was added, and the supernatant was applied to the gel. Samples were run overnight at 4 °C, and gels were transferred to PVDF. EGF receptors were visualized by Western blotting with cetuximab.
Phosphorylation of the exogenous peptide substrate
For each cell line, the membranes were prepared from three D150 tissue culture plates by scraping the cells into 5 ml of 10 mm Tris, pH 7.2, 75 mm NaCl plus protease inhibitor mixture and lysing with 20 strokes in a Dounce homogenizer. Unbroken cells and heavy organelles were removed by a low-speed spin, and purified membranes were recovered by centrifuging the supernatant at 17,000 rpm for 20 min in an SS34 rotor in an RC-5B centrifuge. The membranes were resuspended in ∼150 μl 70 mm β-glycerophosphate, pH 7.2, 200 mm NaCl. 125I-Cetuximab binding was performed on the membranes, and membrane volume was subsequently adjusted to obtain an equal number of EGF receptors per 30-μl aliquot. Pertuzumab was added to the membranes at a final concentration of 8.3 μg/ml.
Kinase assays contained 30-μl membranes, 5 μl of 20 mm Arg-Arg-Src peptide plus 5 μl of 250 nm EGF or buffer. Assays were started by the addition of 10 μl of ATP mix containing 250 μm [γ-32P]ATP, 60 mm MgCl2, 10 mm MnCl2, 100 mm p-nitrophenol, and 500 μm sodium orthovanadate (49). Assays were incubated for 15 min at 30 °C and stopped by the addition of 50 μl of 10% trichloroacetic acid. Precipitated material was pelleted by centrifugation, and 75 μl of the supernatant was spotted onto small squares of phosphocellulose paper. The papers were washed in 75 mm H3PO4 for 30 min with three changes of buffer. After drying, the papers were counted for 32P in a liquid scintillation counter.
Receptor autophosphorylation and downstream signaling
Cells stably expressing each of the single-Tyr EGF receptors were cultured in concentrations of doxycycline determined to give approximately equal levels of expression of the different receptors. After 24 h, cultures were treated without or with 10 nm EGF for the indicated time in the presence of 5 μg/ml pertuzumab to preclude any contribution of the low number of ErbB2 receptors present in CHO cells. RIPA lysates were prepared, and equal amounts of total protein were separated on SDS gels and then blotted for phosphotyrosine, MAP kinase, phospho-MAP kinase, Akt, phospho-Akt, Shc, phospho-Shc, Gab, phospho-Gab, Stat3, phospho-Stat3 and phospholipase Cγ, phosphophospholipase Cγ.
125I-Cetuximab binding
Cell surface EGF receptor levels were assessed by incubating triplicate wells of a 48-well dish with 120 pm 125I-cetuximab. The plates were incubated overnight at 4 °C and then washed three times in phosphate-buffered saline. Cell monolayers were dissolved in 1 n NaOH, and the solution was counted for 125I. Nonspecific binding was determined in replicate wells to which 60 nm unlabeled cetuximab had been added.
Luciferase assays
CHO cells stably expressing Shc, Grb2, Gab1, PI3K-RI, or Shp2 fused to CLuc (23) were plated in 96-well black-walled dishes, and 24 h later were transiently transfected with cDNA encoding one of the single-Tyr or Y-to-F EGF receptors or wild-type EGF receptor each fused to the NLuc. Three different concentrations of wild-type EGF receptor DNA and two different concentrations of mutant EGF receptor DNA were transfected in every experiment to allow comparisons to be made between cells expressing similar amounts of wild-type and mutant EGF receptor. After an additional 24 h of growth in the presence of the appropriate concentration of doxycycline, the cells were assayed for luciferase activity in the absence or presence of EGF. Cell surface EGF receptor expression levels were assessed by measuring the binding of 125I-cetuximab in parallel wells.
Luciferase assays were performed in hextuplicate as described previously (23). The lines through the data were drawn using an equation that represents the sum of a logistics association equation and an exponential dissociation equation (23).
Author contributions
L. J .P. conceived of this study and wrote the paper. K. G. and J. O. designed, performed, and analyzed the experiments shown in Figs. 1–8. All authors reviewed the results, edited the manuscript, and approved the final version.
Supplementary Material
This work was supported by National Institutes of Health Grants R01 GM099695 and R01 GM108785 (to L. J. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Fig. S1.
- PTB
- phosphotyrosine-binding
- RIPA
- radioimmune precipitation assay
- PH
- pleckstrin homology.
References
- 1. Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., and Schlessinger J. (1984) Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309, 418–425 [DOI] [PubMed] [Google Scholar]
- 2. Lemmon M. A., and Schlessinger J. (2010) Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yarden Y., and Schlessinger J. (1987) Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry 26, 1443–1451 [DOI] [PubMed] [Google Scholar]
- 4. Yarden Y., and Schlessinger J. (1987) Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry 26, 1434–1442 [DOI] [PubMed] [Google Scholar]
- 5. Zhang X., Gureasko J., Shen K., Cole P. A., and Kuriyan J. (2006) An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 [DOI] [PubMed] [Google Scholar]
- 6. Downward J., Parker P., and Waterfield M. D. (1984) Autophosphorylation sites on the epidermal growth factor receptor. Nature 311, 483–485 [DOI] [PubMed] [Google Scholar]
- 7. Hsuan J. J., Totty N., and Waterfield M. D. (1989) Identification of a novel autophosphorylation site (P4) on the epidermal growth factor receptor. Biochem. J. 262, 659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolis B. L., Lax I., Kris R., Dombalagian M., Honegger A. M., Howk R., Givol D., Ullrich A., and Schlessinger J. (1989) All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. J. Biol. Chem. 264, 10667–10671 [PubMed] [Google Scholar]
- 9. Schlessinger J., and Lemmon M. A. (2003) SH2 and PTB domains in tyrosine kinase signaling. Sci. STKE 191, RE12. [DOI] [PubMed] [Google Scholar]
- 10. Curran T. G., Zhang Y., Ma D. J., Sarkaria J. N., and White F. M. (2015) MARQUIS: a multiplex method for absolute quantification of peptides and posttranslational modifications. Nat. Commun. 6, 5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dengjel J., Akimov V., Olsen J. V., Bunkenborg J., Mann M., Blagoev B., and Andersen J. S. (2007) Quantitative proteomic assessment of very early cellular signaling events. Nat. Biotechnol. 25, 566–568 [DOI] [PubMed] [Google Scholar]
- 12. Guo L., Kozlosky C. J., Ericsson L. H., Daniel T. O., Cerretti D. P., and Johnson R. S. (2003) Studies of ligand-induced site-specific phosphorylation of epidermal growth factor receptor. J. Am. Soc. Mass Spectrom. 14, 1022–1031 [DOI] [PubMed] [Google Scholar]
- 13. Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., and Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 14. Kim Y., Li Z., Apetri M., Luo B., Settleman J. E., and Anderson K. S. (2012) Temporal resolution of autophosphorylation for normal and oncogenic forms of EGFR and differential effects of gefitinib. Biochemistry 51, 5212–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong J., Taylor P., Peterman S. M., Prakash A., and Moran M. F. (2009) Epidermal growth factor receptor phosphorylation sites Ser991 and Tyr998 are implicated in the regulation of receptor endocytosis and phosphorylations at Ser1039 and Thr1041. Mol. Cell. Proteomics 8, 2131–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biscardi J. S., Maa M.-C., Tice D. A., Cox M. E., Leu T.-H., and Parsons S. J. (1999) c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274, 8335–8343 [DOI] [PubMed] [Google Scholar]
- 17. Sorkin A., Helin K., Waters C. M., Carpenter G., and Beguinot L. (1992) Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. J. Biol. Chem. 267, 8672–8678 [PubMed] [Google Scholar]
- 18. Songyang Z., Margolis B., Chaudhuri M., Shoelson S. E., and Cantley L. C. (1995) The phosphotyrosine interaction domain of SHC recognizes tyrosine-phosphorylated NPXY motif. J. Biol. Chem. 270, 14863–14866 [DOI] [PubMed] [Google Scholar]
- 19. Li S., Schmitz K. R., Jeffrey P. D., Wiltzius J. J., Kussie P., and Ferguson K. M. (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 [DOI] [PubMed] [Google Scholar]
- 20. Franklin M. C., Carey K. D., Vajdos F. F., Leahy D. J., de Vos A. M., and Sliwkowski M. X. (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 [DOI] [PubMed] [Google Scholar]
- 21. Macdonald-Obermann J. L., and Pike L. J. (2014) Different epidermal growth factor (EGF) ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J. Biol. Chem. 289, 26178–26188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pike L. J., Gallis B., Casnellie J. E., Bornstein P., and Krebs E. G. (1982) Epidermal growth factor stimulates the phosphorylation of synthetic tyrosine-containing peptides by A431 cell membranes. Proc. Natl. Acad. Sci. U.S.A. 79, 1443–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ronan T., Macdonald-Obermann J. L., Huelsmann L., Bessman N. J., Naegle K. M., and Pike L. J. (2016) Different EGF receptor agonists produce unique signatures for the recruitment of downstream signaling proteins. J. Biol. Chem. 291, 5528–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., and Lechleider R. J. (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 25. Batzer A. G., Rotin D., Ureña J. M., Skolnik E. Y., and Schlessinger J. (1994) Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 14, 5192–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward C. W., Gough K. H., Rashke M., Wan S. S., Tribbick G., and Wang J. (1996) Systematic mapping of potential binding sites for Shc and Grb2 SH2 domains on insulin receptor substrate-1 and the receptors for insulin, epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor. J. Biol. Chem. 271, 5603–5609 [DOI] [PubMed] [Google Scholar]
- 27. Gu H., and Neel B. G. (2003) The 'Gab' in signal transduction. Trends Cell Biol. 13, 122–130 [DOI] [PubMed] [Google Scholar]
- 28. Wöhrle F. U., Daly R. J., and Brummer T. (2009) Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun. Signal. 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powis G., Bonjouklian R., Berggren M. M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W. F., Dodge J., and Grindey G. (1994) Wortmannin, a potent and selective inhibitor of phosphatidylinositol 3-kinase. Cancer Res. 54, 2419–2423 [PubMed] [Google Scholar]
- 30. Rodrigues G. A., Falasca M., Zhang Z., Ong S. H., and Schlessinger J. (2000) A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20, 1448–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simister P. C., and Feller S. M. (2012) Order and disorder in large multi-site docking proteins of the Gab family: implications for signalling complex formation and inhibitor design strategies. Mol. Biosyst. 8, 33–46 [DOI] [PubMed] [Google Scholar]
- 32. Sakaguchi K., Okabayashi Y., Kido Y., Kimura S., Matsumura Y., Inushima K., and Kasuga M. (1998) Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation. Mol. Endocrinol. 12, 536–543 [DOI] [PubMed] [Google Scholar]
- 33. Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., and Schlessinger J. (1992) The SH2 and SH# domain-containing protein GRB2 links receptor tyrosine kinases to Ras signaling. Cell 70, 431–442 [DOI] [PubMed] [Google Scholar]
- 34. Chen Z., Kolokoltsov A. A., Wang J., Adhikary S., Lorinczi M., Elferink L. A., and Davey R. A. (2012) Grb2 interaction with the ecotropic murine leukemia virus receptor, mCAT-1, controls virus entry and is stimulated by virus binding. J. Virol. 86, 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahankali M., Peng H.-J., Cox D., and Gomez-Cambronero J. (2011) The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association. Cell. Signal. 23, 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., and Schlessinger J. (1993) Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature 363, 85–88 [DOI] [PubMed] [Google Scholar]
- 37. Su J., Yang L.-T., and Sap J. (1996) Association between receptor protein-tyrosine phosphatase RPTPα and the Grb2 adaptor. J. Biol. Chem. 271, 28086–28096 [DOI] [PubMed] [Google Scholar]
- 38. Rotin D., Margolis B., Mohammadi M., Daly R. J., Daum G., Li N., Fischer E. H., Burgess W. H., Ullrich A., and Schlessinger J. (1992) SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase Cγ. EMBO J. 11, 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koland J. G. (2014) Coarse-grained molecular simulation of epidermal growth factor receptor protein tyrosine kinase multi-site self-Phosphorylation. PLoS Comput. Biol. 10, e1003435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang Y., Bharill S., Karandur D., Peterson S. M., Marita M., Shi X., Kaliszewski M. J., Smith A. W., Isacoff E. Y., and Kuriyan J. (2016) Molecular basis for multimerization in the activation of the epidermal growth factor receptor. eLife 5, e14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sturla L.-M., Amorino G., Alexander M. S., Mikkelsen R. B., Valerie K., and Schmidt-Ullrich R. K. (2005) Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. J. Biol. Chem. 280, 14597–14604 [DOI] [PubMed] [Google Scholar]
- 42. Westover E. J., Covey D. F., Brockman H. L., Brown R. E., and Pike L. J. (2003) Cholesterol depletion results in site-specific increases in EGF receptor phosphorylation due to membrane level effects: studies with cholesterol enantiomers. J. Biol. Chem. 278, 51125–51133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang J., Hu J., Bian X., Chen K., Gong W., Dunlop N. M., Howard O. M., and Wang J. M. (2007) Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Res. 67, 5906–5913 [DOI] [PubMed] [Google Scholar]
- 44. Reddy R. J., Gajadhar A. S., Swenson E. J., Rothenberg D. A., Curran T. G., and White F. M. (2016) Early signaling dynamics of the epidermal growth factor receptor. Proc. Natl. Acad. Sci. U.S.A. 113, 3114–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim K. L., Kim D., Lee S., Kim S.-J., Noh J. E., Kim J.-H., Chae Y. C., Lee J.-B., and Ryu S. H. (2016) Pairwise detection of site-specific receptor phosphorylation using single-molecule blotting. Nat. Commun. 7, 11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doran D. M., and Spar I. L. (1980) Oxidative iodine monochloride iodination technique. J. Immunol. Methods 39, 155–163 [DOI] [PubMed] [Google Scholar]
- 47. Yang K. S., Ilagan M. X., Piwnica-Worms D., and Pike L. J. (2009) Luciferase fragment complementation imaging of conformational changes in the EGF receptor. J. Biol. Chem. 284, 7474–7482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wittig I., Braun H.-P., and Schägger H. (2006) Blue native PAGE. Nat. Protocols 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 49. Pike L. J. (1987) Assay of growth factor-stimulated tyrosine kinases using synthetic peptide substrates. Methods Enzymol. 146, 353–362 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.