Abstract
Fungi are ubiquitous transient or persistent human colonisers, and form the mycobiome with shifts in niche specific mycobiomes (dysbiosis) being associated with various diseases. These complex interactions of fungal species with the human host can be viewed as a spectrum of symbiotic relationships (i.e. commensal, parasitic, mutualistic, amensalistic). The host relevant outcome of the relationship is the damage to benefit ratio, elegantly described in the damage response framework. This review focuses on Candida albicans, which is the most well studied human fungal symbiont clinically and experimentally, its transition from commensalism to parasitism within the human host, and the factors that influence this relationship.
Introduction
Fungi are ubiquitous within the environment. However, only a few species are routinely found associated with humans and are capable of causing disease. A handful of these fungi are considered true pathogens, causing disease in healthy individuals (Histoplasma and Paracoccidioides), while the majority of fungi are often classified as opportunistic pathogens (i.e. Candida and Cryptococcus), causing disease primarily in immunosuppressed individuals [1]. However, it is apparent that some opportunistic fungal pathogens also cause disease in otherwise healthy individuals (i.e. Candida vaginitis or Cryptococcus gattii outbreaks) [2,3]. To understand and categorize the complexity of these different interactions between host and pathogen, Casadevall and Pirofski proposed the damage response framework (DRF), which defines microbial virulence as a function of host damage [4]. Within this framework, host damage (and therefore disease) can occur at either end of the host immune response spectrum (i.e. weakened or hyperactive immune defences). Along this spectrum there is a continuum between pathogen-mediated and host-mediated damage, which results in disease only when damage impairs the normal function of the host.
Host damage also varies as a function of time, with several possible outcomes of host-pathogen interactions. For example, as the amount of damage increases over time and surpasses a threshold, disease ensues [4]. Fungal pathogens that are able to exist within the host for extended periods of time with limited damage lead to other outcomes, including latency (Cryptococcus) or commensalism. In addition, many fungal pathogens cause chronic (long-term) infections, and therefore could also be classified as symbionts, albeit with different host/microbe damage to benefit ratios. The most well studied fungal commensal is Candida albicans, which colonises the oral, genital and gastrointestinal tracts, but can cause host damage and disease in the context of weakened or uncontrolled immune responses. An environmental reservoir of C. albicans has not yet been identified. Therefore, C. albicans is considered an obligate commensal fungus. This and its ability to fit within all six classes of the DRF [5] have made C. albicans the model fungus for studying the transition from commensal to pathogen and will be the main focus of this review.
Symbiosis within the human ecosystem
Symbiosis is a type of long-term close biological relationship between two or more species. These relationships can be mutualistic (all organisms benefit), commensalistic (one organism benefits without affecting the other), parasitic (one organism benefits at the expense of the other), or amensalistic (one organism is inhibited or obliterated, while the other is unaffected). For example, most host-pathogen interactions are parasitic with the pathogen causing damage to the host. However, as mentioned above, periods of latency during an infection may be classed as a commensal relationship as damage to the host is minimal during this period. Interactions between members of the microbiome may be described as amensalistic as the presence of some species prevent the growth of others through nutrient depletion and the secretion of chemical mediators. This is an important factor as dysbiosis of the microbiome has been identified as a major contributor for several fungal infections (i.e. vulvovaginal candidiasis, atopic dermatitis). Although common for bacterial species, the idea of mutualistic relationships between fungi and the human host is a new concept as the potential benefit to the host of harbouring fungi is still unknown.
Niche-specific fungal symbionts
Fungal symbionts vary in route of acquisition, niche/s inhabited, and ultimately, the type of symbiotic relationship formed. Some species are acquired at birth by direct transmission, leading to colonization of the skin and mucosal surfaces, while many fungal pathogens are ubiquitous within the environment, leading to constant exposure by oral or respiratory routes, as well as skin contact. Most of these exposures are transient, due to clearance by host immune responses or out competition for resources by the microbiome. However, changes in immune status or dysbiosis could lead to chronic colonisation and eventual host damage.
Mycobiome studies have been performed for most mucosal surfaces including the oral cavity [6], GI tract [7,8], vagina [9,10], lung [11,12], and skin [13,14]. Over 75 genera of fungi have been identified in the oral cavity alone, which can seed the respiratory or GI tract mycobiome. The most common fungal species associated with the GI tract are Candida, Saccharamyces and Cladosporium, although DNA from more than 50 genera of fungi has been detected. Stability and fluctuations in population dynamics of the GI mycobiome is highly dependent on diet and host immune status. For example, Saccharomyces cerevisiae is commonly acquired from dietary sources and is considered to be a harmless or transient commensal. However, inflammatory bowel diseases [15] and some autoimmune diseases [16,17] are associated with increased levels of circulating S. cerevisiae antibodies (ASCA), which recognise fungal cell wall components [18]. While it has been documented that C. albicans is an immunogen for development of ASCA [19], dietary S. cerevisiae can exacerbate symptoms in ASCA positive IBD patients [20]. In this situation, S. cerevisiae may be considered a parasitic symbiont. On the other end of the spectrum, a related species, S. boulardii, is used clinically as a probiotic treatment for gastroenteritis, acting as a mutualistic symbiont [21].
The vaginal mucosa is colonised by a variety of fungi and bacteria. Although Candida spp. are thought to be the predominate fungal colonisers of the vaginal mucosa, more recent microbiome studies have detected up to 20 genera of fungi in the vaginal niche, including Candida, Aspergillus and Cladosporium [22]. In healthy women, these fungal colonisers likely behave as commensal symbionts. However, many host and environmental factors including pregnancy, antibiotic usage and uncontrolled diabetes influence the vaginal microbiota and can result in dysbiosis and infection.
The dominant fungal commensal of the skin is Malassezia spp [23], a lipophilic genus that consists mostly of plant pathogens. The lipid composition of the skin plays a role in controlling fungal colonisation, with Malassezia globasa being the predominate coloniser of the scalp and forehead and Malassezia restricta colonising the back [24]. However, Malassezia colonisation levels also correlate with a variety of skin diseases including psoriasis, dandruff, atopic dermatitis/eczema, seborrheic dermatitis, and pityriasis versicolor [25]. This suggests that above a certain threshold, these commensals act as parasitic symbionts, possibly due to increases in antigenic load. As evidence of this, it was shown that M. sympodialis secretes allergen-loaded exosomes that induce pro-inflammatory responses [26].
Candida albicans: the ubiquitous fungal symbiont
C. albicans is a commensal fungus of the oral, gastrointestinal and genital tracts in up to 80% of healthy individuals. Under specific host and environmental conditions, C. albicans can transition from its commensal state to a parasitic state causing mucosal (oral and vaginal candidiasis) and life-threatening systemic disease. The polymorphic nature of C. albicans is a key virulence factor playing essential roles in the transition from commensalism to parasitism [27]. Several yeast-like morphologies aid C. albicans commensalism. For example, gastrointestinally induced transition (GUT) cells promote commensalism of C. albicans within the GI tract [28], grey cells enable colonisation of the tongue [29] and opaque cells have increased fitness on skin [30]. This repertoire of cell morphologies demonstrates the plasticity of C. albicans and its evolution as a human commensal.
Transition to damage-causing pathogen and disease
Commensalism of C. albicans on mucosal surfaces is thought to occur with yeast cells. In agreement with this, epithelial cells do not readily recognise the yeast form of C. albicans [31]. However, under periods of immune suppression, trauma and dysbiosis, abiotic and biotic cues induce C. albicans morphogenesis resulting in epithelial activation and induction of a proinflammatory response [31]. In vulvovaginal candidiasis (VVC), the vaginal epithelium becomes sensitised to C. albicans, and its activation results in significant neutrophil recruitment [2]. This hyperactivation of the innate immune system generates a strong non-protective immune response that drives the immunopathology associated with VVC [2]. One question that arises is why do healthy women maintain C. albicans in a commensal relationship and what is the selective pressure that promotes persistent colonisation in the vagina? An answer to this may lie in the location of the vagina between the rectum and urethra. The antagonistic relationships C. albicans exerts on many bacterial species, including those that cause potentially life threatening urinary tract infections, (UTIs), might inhibit migration of these pathogens from the rectum to the urethra, providing a selective pressure to maintain C. albicans in the vagina as a mutualistic symbiont. The primary etiological agent responsible for UTIs is uropathogenic Escherichia coli (>80% cases) [32]. Candida colonization of the vagina increases in puberty, coinciding with hormonal changes that promote fungal adherence and growth [33]. Prepubescent girls also have the highest occurrence of Gram-negative bacteria in the vagina (mainly E. coli), while the highest occurrence of Candida species is in women of child-bearing age [34]. This suggests that Candida inhibits E. coli in the vaginal environment either directly, or via colonization resistance, providing a microbiological barrier between the anus and urethra.
Asymptomatic oral carriage of Candida in the oral cavity is mediated via non-specific host barriers (saliva, antimicrobial peptides etc.) and strong immune defences. However, the onset of AIDS or use of cancer chemotherapy results in oropharyngeal candidiasis (OPC). The role of host factors in the predisposition to OPC is discussed below.
Entry of C. albicans into the bloodstream, via implanted medial devices, translocation from the gut during abdominal surgery or neutropenia, results in systemic. Key target organs include the kidney, spleen and liver, where C. albicans exerts excessive damage. Device associated infections permit the formation of drug and host resistant biofilms, which continually seed infections if not removed. Due to the lack of appropriate diagnostics and non-specific symptoms, infections are often misdiagnosed resulting in increased mortality rates.
Candidiasis and Dysbiosis
Shifts in fungal populations leading to dysbiosis are associated with various diseases [35,36]. In this situation, members of the mycobiome transition from commensal to parasitic symbionts. Whether these shifts are causative or indicative of disease is likely a sliding scale as host and symbionts influence each other within the eco-system. Prolonged administration of antibiotic therapy predisposes women to VVC, suggesting that dysbiosis of the vaginal flora is a prerequisite for symptomatic Candida colonisation. Initially it was suggested that antibiotic treatment removed lactobacilli from the vaginal mucosa, increasing vaginal pH, and promoting Candida hyphal formation. In agreement with this, lactobacilli have been proposed to reduce Candida colonisation [37], and have been explored as a potential probiotics [38]. However, other studies suggest that vaginal pH, and the lactobacilli population are not significantly altered in VVC [39], and that Candida colonisation is more common in women with a lactobacillus dominated microflora [40]. Therefore, the role of this amensalistic relationship warrants further investigation.
Treatment with oral antifungals perturbs the intestinal mycobiome, reducing the populations of Candida, whilst promoting the expansion of Aspergillus, Wallemia and Epicoccum, and causing increased allergic airway disease [41]. On the other hand, the use of oral antibiotics, depleted the bacterial composition of the GI microbiome, promoting the expansion of Candida [42]. The consequence of Candida overgrowth in the GI tract is still controversial. However, individuals with high Candida burdens appear to be more prone to intestinal inflammatory diseases [43], although the role of Candida remains to be determined.
Role of the Host: Genetic mutations
Genetic susceptibility to C. albicans infections varies according to site of infection, and not all infections are associated with genetic mutation. Systemic infection is associated with defects in innate immunity (neutrophil function) while oropharyngeal candidiasis (OPC) is associated with defects in cellular immunity (primarily CD4+ T cells). A major focus of recent genetic linkage studies is chronic mucocutaneous candidiasis (CMC), which is an infectious phenotype in patients with inherited or acquired T cell deficiency (primarily IL-17 immunity). CMC is an extremely rare familial disorder, with a current worldwide cohort <300 patients, and is characterized by chronic infection of skin, nails, and oral/genital mucosa [44]. Defects in STAT1 result in CMC due to defects in IL-17, IFN-γ and IL-22 signalling [45]. IL-17 signalling is essential for controlling oral carriage of Candida, with defects in many of the signalling components resulting in OPC [46]. OPC can also result from an autoimmune disease where the patient generates antibodies to IL-17A, IL-17F and IL-22 [47].
Genetic predisposition to VVC is still an emerging topic. One study found higher frequencies in mutations associated with increased expression of mediators involved in mucosal tolerance (IL-22 and IDO1) in controls vs. RVVC patients [48]. This study also found higher frequencies of a mutation causing a premature stop codon in the C-type lectin receptor Dectin-1 in women with RVVC, which mediates IL-22 production [48]. Polymorphisms in mannose binding lectin (MBL), a component of innate immunity that triggers complement activation, are also found at higher frequencies in RVVC patients and are associated with leading reduced vaginal MBL levels [49,50]. Overall, these studies support the concept that pathways important in mucosal tolerance that may limit innate responsiveness to a commensal symbiont promote resistance to RVVC.
Fungal factors
The most studied and important virulence attribute of C. albicans is its ability to reversibly switch morphologies, between yeast, pseudohyphal and true hyphal growth. Temperature, CO2, pH, serum, quorum sensing molecules, nutrient availability and hypoxia all regulate morphogenesis [51]. As outlined above, each of these morphologies play distinct roles in colonisation and parasitism, and inhibition of morphogenesis dramatically inhibits fungal virulence. Other important virulence factors include the expression of adhesins (i.e. ALS family) and cell wall proteins (i.e. Hwp1) that enable attachment to host cells [52]. The Als5 adhesin is expressed during commensal growth, but repressed during pathogenicity. In agreement with Als5 playing a role in fungal commensalism, expression of C. albicans Als5 in S. cerevisiae promotes colonisation of the nematode gastrointestinal tract, without affecting survival [53]. Therefore, Als5 may be a critical factor for C. albicans commensalism. Once attached, C. albicans can actively penetrate mucosal barriers. The expression of SAPs are thought to be essential to this process, degrading proteins on host cells, and disrupting the host’s protective barriers (i.e. degradation of mucin) [54]. Due to the role of SAPs in epithelial damage SAP expression is correlated with virulence rather and commensalism [29]. Finally, C. albicans induces epithelial damage through the secretion of candidalysin, a novel pore forming peptide. Candidalysin is predominately expressed by hyphal cells and is responsible for the majority of host damage exerted by C. albicans [55]. The role of candidalysin in commensalism has not been extensively studied, but deletion of candidalysin does reduce colonisation levels in murine models of OPC [51], suggesting that candidalysin may play roles in commensalism and well as pathogenesis. In addition to this repertoire of fungal virulence factors, C. albicans can also modulate the structure of its cell wall to either conceal or reveal immune stimulatory PAMPs on its surface [56–58]. This pliability of the cell wall architecture enables C. albicans to control the innate immune response. Therefore, C. albicans has uniquely positioned itself as a commensal symbiont, with a plethora of ways to sense and respond to changes within the host that ensure successful transition from commensalism to parasitism and initiation of disease during periods of weakened host defences.
Conclusion
Fungi form an important part of our microbiome, with symbiotic relationships ranging from commensal to parasitic. The symbiotic relationship between humans and fungi is greatly underappreciated and under researched. Given that many fungi are opportunistic pathogens, understanding the balance and transitions between these relationships is vital for the future of medial mycology.
Figure 1. Transition of C. albicans from commensal to parasite.
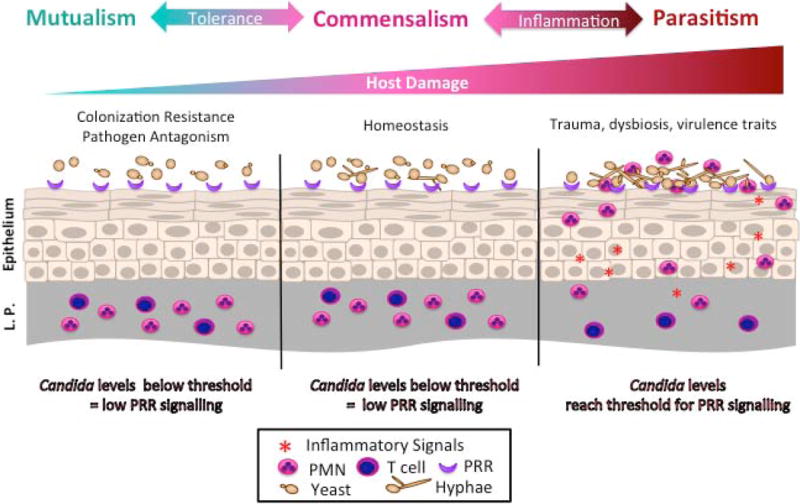
During commensal growth C. albicans grows predominately as yeast and is tolerated by the host, and does not activate innate immune responses. Periods of dysbiosis and immune suppression permit C. albicans to proliferate, induce hyphal formation, and activate innate immune responses. At the vaginal mucosal, epithelial cells become sensitised resulting in hyperactivation of innate immune responses and neutrophil influx. This non-protective hyperactivation of neutrophils results in damage to the vaginal mucosa and symptomatic vaginal candidiasis.
Highlights.
A model of symbiosis as a function of host damage is proposed
Transition from commensalism to parasitism is dependent on host, environmental and fungal factors
Candida albicans is considered as a true fungal symbiont of humans
The potential of fungal symbionts as mutualists is considered
Acknowledgments
RAH is supported by an MRC career Development Award ((MR/L00903X/1). MCN is funded by NIH grants from NIAID (1R01DE022069-01A1) and NIDCR (1 R01 AI116025-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Fidel PL, Jr, Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72:2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrnes EJ, 3rd, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010;6:e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Pirofski L-A. The damage-response framework of microbial pathogenesis. Nat Rev Micro. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Jabra-Rizk MA, Kong EF, Tsui C, Nguyen MH, Clancy CJ, Fidel PL, Noverr M. Candida albicans Pathogenesis: Fitting within the Host-Microbe Damage Response Framework. Infection and Immunity. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. A detailed overview of the damage response framework and how the fungal pathogen Candida albicans fits within all six classifications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra J, Retuerto M, Mukherjee PK, Ghannoum M. The Fungal Biome of the Oral Cavity. Methods Mol Biol. 2016;1356:107–135. doi: 10.1007/978-1-4939-3052-4_9. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhr MJ, Hallen-Adams HE. The human gut mycobiome: pitfalls and potentials–a mycologist’s perspective. Mycologia. 2015;107:1057–1073. doi: 10.3852/15-147. [DOI] [PubMed] [Google Scholar]
- 9.Bradford LL, Ravel J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence. 2017;8:342–351. doi: 10.1080/21505594.2016.1237332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspollu A, Vain E, Saarma I, Salumets A, Donders GG, Metsis M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One. 2013;8:e54379. doi: 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willger SD, Grim SL, Dolben EL, Shipunova A, Hampton TH, Morrison HG, Filkins LM, O’Toole GA, Moulton LA, Ashare A, et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome. 2014;2:40. doi: 10.1186/2049-2618-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L, Lucht L, Tipton L, Rogers MB, Fitch A, Kessinger C, Camp D, Kingsley L, Leo N, Greenblatt RM, et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am J Respir Crit Care Med. 2015;191:932–942. doi: 10.1164/rccm.201409-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Jo JH, Kennedy EA, Kong HH. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence. 2017;8:324–333. doi: 10.1080/21505594.2016.1249093. Detailed microbiome study of the respiratory tract in healthy and HIV patients with and without chronic obstructive pulmonary disease (COPD). This study confims that fungi become over represented in the lung during HIV and COPD). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemoto A, Cho O, Morohoshi Y, Sugita T, Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J Dermatol. 2015;42:166–170. doi: 10.1111/1346-8138.12739. [DOI] [PubMed] [Google Scholar]
- 15.Kaul A, Hutfless S, Liu L, Bayless TM, Marohn MR, Li X. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18:1872–1884. doi: 10.1002/ibd.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mankai A, Sakly W, Thabet Y, Achour A, Manoubi W, Ghedira I. Anti-Saccharomyces cerevisiae antibodies in patients with systemic lupus erythematosus. Rheumatol Int. 2013;33:665–669. doi: 10.1007/s00296-012-2431-3. [DOI] [PubMed] [Google Scholar]
- 17.Shor DB, Orbach H, Boaz M, Altman A, Anaya JM, Bizzaro N, Tincani A, Cervera R, Espinosa G, Stojanovich L, et al. Gastrointestinal-associated autoantibodies in different autoimmune diseases. Am J Clin Exp Immunol. 2012;1:49–55. [PMC free article] [PubMed] [Google Scholar]
- 18.Poulain D, Sendid B, Standaert-Vitse A, Fradin C, Jouault T, Jawhara S, Colombel JF. Yeasts: neglected pathogens. Dig Dis. 2009;27(Suppl 1):104–110. doi: 10.1159/000268129. [DOI] [PubMed] [Google Scholar]
- 19.Standaert-Vitse A, Jouault T, Vandewalle P, Mille C, Seddik M, Sendid B, Mallet JM, Colombel JF, Poulain D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology. 2006;130:1764–1775. doi: 10.1053/j.gastro.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Barclay GR, McKenzie H, Pennington J, Parratt D, Pennington CR. The effect of dietary yeast on the activity of stable chronic Crohn’s disease. Scand J Gastroenterol. 1992;27:196–200. doi: 10.3109/00365529208999948. [DOI] [PubMed] [Google Scholar]
- 21.Szajewska H, Konarska Z, Kolodziej M. Probiotic Bacterial and Fungal Strains: Claims with Evidence. Dig Dis. 2016;34:251–259. doi: 10.1159/000443359. [DOI] [PubMed] [Google Scholar]
- 22.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Program NCS, et al. Human Skin Fungal Diversity. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Kong HH, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, Gabrielsson S, Scheynius A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses–novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobsen ID, Wilson D, Wächtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Review of Anti-infective Therapy. 2012;10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 28.Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao L, Du H, Guan G, Dai Y, Nobile CJ, Liang W, Cao C, Zhang Q, Zhong J, Huang G. Discovery of a “White-Gray-Opaque” Tristable Phenotypic Switching System in Candida albicans: Roles of Non-genetic Diversity in Host Adaptation. PLoS Biology. 2014;12:e1001830. doi: 10.1371/journal.pbio.1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie J, Tao L, Nobile CJ, Tong Y, Guan G, Sun Y, Cao C, Hernday AD, Johnson AD, Zhang L, et al. White-Opaque Switching in Natural MTLa/α Isolates of Candida albicans: Evolutionary Implications for Roles in Host Adaptation, Pathogenesis, and Sex. PLOS Biology. 2013;11:e1001525. doi: 10.1371/journal.pbio.1001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al. A Biphasic Innate Immune MAPK Response Discriminates between the Yeast and Hyphal Forms of Candida albicans in Epithelial Cells. Cell Host & Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foxman B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infectious Disease Clinics of North America. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Barousse MM, Theall KP, Van Der Pol B, Fortenberry JD, Orr DP, Fidel PL. Susceptibility of Middle Adolescent Females to Sexually Transmitted infections: Impact of Hormone Contraception and Sexual Behaviors on Vaginal Immunity. American Journal of Reproductive Immunology. 2007;58:159–168. doi: 10.1111/j.1600-0897.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 34.Tibaldi C, Cappello N, Latino MA, Masuelli G, Marini S, Benedetto C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: risk factors and rates of occurrence. Clinical Microbiology and Infection. 2009;15:670–679. doi: 10.1111/j.1469-0691.2009.02842.x. [DOI] [PubMed] [Google Scholar]
- 35.Huseyin CE, O’Toole PW, Cotter PD, Scanlan PD. Forgotten fungi-the gut mycobiome in human health and disease. FEMS Microbiol Rev. 2017 doi: 10.1093/femsre/fuw047. [DOI] [PubMed] [Google Scholar]
- 36*.Hoarau G, Mukherjee PK, Gower-Rousseau C, Hager C, Chandra J, Retuerto MA, Neut C, Vermeire S, Clemente J, Colombel JF, et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. MBio. 2016;7 doi: 10.1128/mBio.01250-16. Characterisation of the fungal and bacterial microbiomes of families with Chron’s Disease (CD), identifing that Candida tropicalis, Serratia marcescens and E. coli are associated with CD dysbiosis. The presence of C. tropicalis was associated with high circulatory levels of anti-Saccharomyces cerevisiae antibodies, a known biomarker of CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Frontiers in Microbiology. 2017;8:564. doi: 10.3389/fmicb.2017.00564. Identifies the antifungal properties of vaginal Lactobacillus isolates on Candida albicans. Lactobacillus cell free supernatnats were able to reduce C. albicans growth up up to 60% and reduce the yeast to hyphal transition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsubara VH, Bandara HMHN, Mayer MPA, Samaranayake LP. Probiotics as Antifungals in Mucosal Candidiasis. Clinical Infectious Diseases. 2016;62:1143–1153. doi: 10.1093/cid/ciw038. [DOI] [PubMed] [Google Scholar]
- 39.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 40.van de Wijgert JHHM, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Wheeler Matthew L, Limon Jose J, Bar Agnieszka S, Leal Christian A, Gargus M, Tang J, Brown J, Funari Vincent A, Wang Hanlin L, Crother Timothy R, et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host & Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. Investigates the role of the gut mycobiome in controling immune responses. Depletion of the gut mycobiome through antifungal treatment increased symtpoms in acute and chronic models of colitis, and increase allergic airway disease. Therefore, disruption of the normal gut mycobiome can enhance relevent disease states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erb Downward JR, Falkowski NR, Mason KL, Muraglia R, Huffnagle GB. Modulation of Post-Antibiotic Bacterial Community Reassembly and Host Response by Candida albicans. Scientific Reports. 2013(3):2191. doi: 10.1038/srep02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ott SJ, Kühbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, Drews O, Weichert W, Timmis KN, Schreiber S. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scandinavian Journal of Gastroenterology. 2008;43:831–841. doi: 10.1080/00365520801935434. [DOI] [PubMed] [Google Scholar]
- 44**.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, Ouachee-Chardin M, Fouyssac F, Girisha KM, Etzioni A, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127:3154–3164. doi: 10.1182/blood-2015-11-679902. A study that looks at the role of STAT1 gain of function mutations in 274 patients from across the world. Chronic mucocutaneous candidiasis occurred in 98% of the patients with C. albicans being the causitive agent in 95% of cases. This study highlight the important roel of STAT1 in controling mucosal and invasive fungal infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LAB, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CAA, et al. STAT1 Mutations in Autosomal Dominant Chronic Mucocutaneous Candidiasis. New England Journal of Medicine. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 46.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity*. Science (New York, NY) 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. The Journal of Experimental Medicine. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human Dectin-1 Deficiency and Mucocutaneous Fungal Infections. The New England journal of medicine. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wojitani MDKH, de Aguiar LM, Baracat EC, Linhares IM. Association between mannose-binding lectin and interleukin-1 receptor antagonist gene polymorphisms and recurrent vulvovaginal candidiasis. Archives of Gynecology and Obstetrics. 2012;285:149–153. doi: 10.1007/s00404-011-1920-z. [DOI] [PubMed] [Google Scholar]
- 50.Nedovic B, Posteraro B, Leoncini E, Ruggeri A, Amore R, Sanguinetti M, Ricciardi W, Boccia S. Mannose-binding lectin codon 54 gene polymorphism and vulvovaginal candidiasis: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:738298. doi: 10.1155/2014/738298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Micro. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 52.Staab JF, Bahn Y-S, Tai C-H, Cook PF, Sundstrom P. Expression of Transglutaminase Substrate Activity on Candida albicans Germ Tubes through a Coiled, Disulfide-bonded N-terminal Domain of Hwp1 Requires C-terminal Glycosylphosphatidylinositol Modification. Journal of Biological Chemistry. 2004;279:40737–40747. doi: 10.1074/jbc.M406005200. [DOI] [PubMed] [Google Scholar]
- 53.Bois M, Singh S, Samlalsingh A, Lipke PN, Garcia MC. Does Candida albicans Als5p Amyloid Play a Role in Commensalism in Caenorhabditis elegans? Eukaryotic Cell. 2013;12:703–711. doi: 10.1128/EC.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Höfs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. Journal of Microbiology. 2016;54:149–169. doi: 10.1007/s12275-016-5514-0. [DOI] [PubMed] [Google Scholar]
- 55**.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. Identification of the first fungal peptide attributed to causing host damage. The secretion of candidalysin causes damage to host membranes and activiates damage response signalling pathways resulting in the activation of mucosal innate immune responses. Deletion of candidalysin inhibits the ability of C. albicans to cause host damage, but does not affect hyphal formation, highlighting candidalysin as the major virulence factor of C. albicans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Sherrington SL, Sorsby E, Mahtey N, Kumwenda P, Lenardon MD, Brown I, Ballou ER, MacCallum DM, Hall RA. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLOS Pathogens. 2017;13:e1006403. doi: 10.1371/journal.ppat.1006403. Identifies that expsoure to low environmental pH induces significant cell wall remodelling which results in the exposure of beta-glucan a highly immune stimulatory carbohydrate. This increased exposure of beta-glucan, drives a strong proinflammaorty innate immune response characterisitic of vaginal thrush, an environment where C. albicans is continously exposed to low mucosal pH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Ballou ER, Avelar GM, Childers DS, Mackie J, Bain JM, Wagener J, Kastora SL, Panea MD, Hardison SE, Walker LA, et al. Lactate signalling regulates fungal β-glucan masking and immune evasion. Nature Microbiology. 2016;2:16238. doi: 10.1038/nmicrobiol.2016.238. Identifies that lactate, an alternative carbon source found in the human host, drives concelment of the highly innue stimualtory beta-glucan permitting C. albicans to evade the innate immune system. Lactate dependent cell wall remodelling is regulated via Crz1 and is sensed by Gpr1, leading to the discovery of a novel pathway controling cell wall remodelling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, Morphotype-Specific Candida albicans β-Glucan Exposure during Infection and Drug Treatment. PLoS Pathogens. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. Provides the first glance at how the fungal cell wall changes during infection. [DOI] [PMC free article] [PubMed] [Google Scholar]


