Abstract
Background
We have previously shown that in participants with major depressive disorder (MDD) trained to upregulate their amygdala hemodynamic response during positive autobiographical memory (AM) recall with real-time fMRI neurofeedback (rtfMRI-nf) training, depressive symptoms diminish. Here, we assessed the effect of rtfMRI-nf on amygdala functional connectivity during both positive AM recall and rest.
Method
The current manuscript consists of a secondary analysis on data from our published clinical trial of neurofeedback. Patients with MDD completed two rtfMRI-nf sessions (18 received amygdala rtfMRI-nf, 16 received control parietal rtfMRI-nf). One-week prior-to and following training participants also completed a resting-state fMRI scan. A GLM-based functional connectivity analysis was applied using a seed ROI in the left amygdala. We compared amygdala functional connectivity changes while recalling positive AMs from the baseline run to the final transfer run during rtfMRI-nf training, as well during rest from the baseline to the one-week follow-up visit. Finally, we assessed the correlation between change in depression scores and change in amygdala connectivity, as well as correlations between amygdala regulation success and connectivity changes.
Results
Following training, amygdala connectivity during positive AM recall increased with widespread regions in the frontal and limbic network. During rest, amygdala connectivity increased following training within the fronto-temporal-limbic network. During both task and resting-state analyses, amygdala-temporal pole connectivity decreased. We identified increased amygdala-precuneus and amygdala-inferior frontal gyrus connectivity during positive memory recall and increased amygdala-precuneus and amygdala-thalamus connectivity during rest as functional connectivity changes that explained significant variance in symptom improvement. Amygdala-precuneus connectivity changes also explain a significant amount of variance in neurofeedback regulation success.
Conclusions
Neurofeedback training to increase amygdala hemodynamic activity during positive AM recall increased amygdala connectivity with regions involved in self-referential, salience, and reward processing. Results suggest future targets for neurofeedback interventions, particularly interventions involving the precuneus.
Keywords: Neurofeedback, Amygdala, Functional connectivity, Depression, Positive memory recall, Resting-state
Highlights
-
•
Changes in amygdala functional connectivity following neurofeedback were examined.
-
•
Amygdala rtfMRI-nf training alters functional connectivity with prefrontal regions.
-
•
Increased amygdala-precuneus connectivity may underlie clinical improvements.
1. Introduction
The amygdala is a brain region consistently implicated in the pathophysiology of mood disorders, including major depressive disorder (MDD) (Whalen et al., 2002). This region responds to both positive and negative emotional stimuli (Sergerie et al., 2008) and is thought to influence the perceived salience of stimuli and events (Davis and Whalen, 2001). While much research in patients with MDD focuses on the exaggerated amygdala response to negative stimuli (e.g., (Drevets, 2003), emerging evidence suggests that in individuals with MDD the amygdala's hemodynamic response is ‘doubly dissociated’ from controls, showing an exaggerated responses to negative and attenuated response to positive stimuli (Suslow et al., 2010, Victor et al., 2010), including autobiographical memories (Young et al., 2016a). Real-time fMRI neurofeedback (rtfMRI-nf) training, in which blood-oxygen-level dependent (BOLD) fMRI data processing and display are performed concomitantly with image acquisition (Cox et al., 1995), has enabled rtfMRI-nf training in which individuals are able to see and regulate the BOLD signal from their brain (deCharms, 2008). Emerging evidence suggests rtfMRI-nf has clinical utility in reducing symptoms associated with chronic pain (deCharms et al., 2005), smoking cessation (Hartwell et al., 2016), anxiety (Zilverstand et al., 2015), post-traumatic stress disorder (PTSD) (Nicholson et al., 2017), and MDD (Linden et al., 2012). We developed rtfMRI-nf training to enhance the amygdala hemodynamic response during positive autobiographical memory recall (Zotev et al., 2011). This intervention significantly improves depressive symptoms and increases the processing of other positive stimuli in patients with MDD (Young et al., 2017a, Young et al., 2017b, Young et al., 2014).
The amygdala is strongly connected to a wide variety of brain regions which play key roles in controlling one's emotional, motivational, and social behavior (Bickart et al., 2014, Janak and Tye, 2015). Amygdala-medial prefrontal cortex (mPFC) connectivity is considered particularly important for emotion regulation both with respect to decreasing negative affect (Banks et al., 2007) and increasing reward behaviors (Seo et al., 2016). Furthermore, aberrant amygdala-prefrontal connectivity is prevalent in MDD (Ramasubbu et al., 2014, Satterthwaite et al., 2016) and may underlie impairments in affect regulation (Ramasubbu et al., 2014). Therefore, while our previous work suggests that increasing voluntary control of the amygdala via rtfMRI-nf training results in short-term clinical improvements, these improvements may be due to changes in how the brain communicates and regulates emotion. Therefore we seek to determine changes in the functional connectivity between the amygdala and other brain regions, particularly those within the default mode and salience networks as well as prefrontal regions important for emotional processing, following rtfMRI-nf training.
Amygdala functional connectivity changes may thus provide insight into neural mechanisms underlying neurofeedback training and recovery from MDD, and may allow us to test whether neuromodulatory effects of rtfMRI-nf persist beyond the training period. As many mental illnesses involve aberrant prefrontal-limbic connectivity, understanding how our rtfMRI-nf procedure specifically alters connectivity in this circuitry may support the use of rtfMRI-nf applications in the future that use network connectivity as the target instead of a single region. Our previous work in healthy men revealed that over the course of training, amygdala connectivity significantly increased with several prefrontal regions including the medial frontal polar cortex, dorsomedial prefrontal cortex (dMPFC), anterior cingulate cortex (ACC) and superior frontal gyrus (Zotev et al., 2011, Zotev et al., 2013). Additionally, correlations between amygdala hemodynamic activity and frontal EEG asymmetry during this rtfMRI-nf training protocol were found in patients with MDD, suggesting that correcting the amygdala response to positive memory recall normalized aberrant prefrontal-limbic connectivity (Zotev et al., 2016). While this provides important information on network changes during training/learning, changes required to maintain the learned response following training could not be established, as no baseline run was included. Examining the change from the baseline to transfer runs in which no neurofeedback information was provided allows us to determine whether changes in amygdala connectivity over the course of training are associated with the reduction in depressive symptoms that persist beyond the training period. The transfer condition is arguably more important clinically than the training phase as the transfer condition provides support that learned self-regulation along with the accompanying changes in connectivity can be voluntarily applied by the patient. Therefore, we first aim to determine the extent to which the change in amygdala-frontal connectivity that occurs during training in healthy participants is similarly required for maintaining the elevated amygdala response during transfer in MDD participants.
Furthermore, for neurofeedback-induced changes in brain activity and networks to be of clinical utility, it is essential that such changes persist beyond the scanning session in which training occurred. Therefore, another aim of the current study was to investigate whether rtfMRI-nf training to increase the amygdala hemodynamic response during positive memory recall resulted in changes in resting-state functional connectivity measured one week prior to and one week following rtfMRI-nf training. In a previous study we reported increased amygdala resting-state connectivity with the ACC and cuneus following our amygdala rtfMRI-nf procedure (Yuan et al., 2014). Changes in resting state were only measured after a single rtfMRI-nf training session, and therefore another goal of the current study was to examine whether similar changes were observed and maintained for at least one-week following 2 rtfMRI-nf sessions.
To examine our two aims; i.e., changes in amygdala functional connectivity pre- to post-neurofeedback training both while recalling positive autobiographical memories (the task employed during rtfMRI-nf training) and during rest, we conducted a secondary analysis on data from our published clinical trial of neurofeedback (Young et al., 2017b). We hypothesized that rtfMRI-nf would result in increased connectivity between the amygdala and prefrontal regions, particularly the ACC and medial prefrontal cortex, which are involved in emotional control and regulation, and that these changes will be evident both during both positive memory recall and at the one-week follow-up resting-state scan.
2. Methods
2.1. Participants
Thirty-six right-handed, unmedicated adults ages 18–55 who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000) criteria for MDD in a current major depressive episode participated. Volunteers, recruited from the community via general advertisements for studies at the Laureate Institute for Brain Research (LIBR), underwent screening evaluations at LIBR, including the Structural Clinical Interview for DSM-IV disorders (First et al., 2002). Exclusion criteria included current pregnancy, general MRI exclusions, serious suicidal ideation, psychosis, major medical or neurological disorders, exposure to any medication likely to influence cerebral function or blood flow within three weeks, and meeting DSM-IV criteria for drug/alcohol abuse within the previous year or for alcohol/drug dependence (except for nicotine) within the lifetime. All volunteers were naive to rtfMRI-nf. Participants gave written informed consent to participate in the study and received financial compensation. The research protocol was carried out in accordance with the Declaration of Helsinki for experimental involving humans, approved by the Western Institutional Review Board, and registered on clinicaltrials.gov (CONSORT diagram Supplementary Fig. SF1).
2.2. Procedure
Participants were randomly assigned under double-blind conditions to the experimental group receiving amygdala rtfMRI-nf (n = 18) or to the control group receiving rtfMRI-nf from the left horizontal segment of the intraparietal sulcus (n = 16) and completed four study visits. During the Baseline Visit (Visit 1) participants were administered our primary outcome measure, the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and completed a resting state scan. Participants also completed other fMRI and behavioral tasks at this visit, the results of which have been published previously (Young et al., 2017a). Between 5 and 7 days later participants completed their first rtfMRI-nf session (Visit 2), followed 5–7 days later by their second rtfMRI-nf session (Visit 3). The follow-up visit (Visit 4) was completed 5–7 days following Visit 3 and was identical to the baseline Visit 1. All imaging was conducted using a General Electric Discovery MR750 whole-body 3 Tesla MRI scanner. Pulse-oximetry and respiration waveforms were recorded for each fMRI run.
2.3. Resting state paradigm
During Visits 1 and 4, participants completed a 6 min 24 s resting state scan during which they were instructed to open their eyes, fixate on a cross, relax, and not think about anything in particular. For fMRI, a receive-only 32-channel head coil was used and gradient-recalled, echoplanar imaging (EPI) with sensitivity encoding (SENSE) with the following parameters: repetition/echo time = 2000/27 ms, SENSE acceleration = 2, flip angle = 78°, matrix = 96 × 96, field-of-view/slice thickness = 240/2.9 mm, 39 axial slices. High-resolution T1-weighted anatomical MRI scans (repetition/echo time = 5.0/2.0 ms, inversion/delay time = 725/1400 ms, flip angle = 8°, matrix = 256 × 256, field-of-view/slice thickness = 240/0.9 mm, 186 axial slices) also were acquired for co-registration with the EPI series.
2.4. rtfMRI-nf paradigm
During Visits 2 and 3, participants completed rtfMRI-nf training as described previously (Bodurka and Bandettini, 2008, Zotev et al., 2011). Participants were instructed to retrieve positive memories while attempting to increase the hemodynamic activity in the assigned region to that of a blue bar representing the target level of activation. Participants were informed to maintain this strategy of positive memory recall even if they felt it was ineffective at increasing their brain activity, though they could change the specific positive memories utilized or the aspects of the memories focused on, and it was suggested that they focus on a) the positive aspects of the memory; what was happy about it, b) why the memory is important to them; how it affected their life and c) how it's related to their self-concept.
Each neurofeedback run consisted of alternating 40 s blocks of: Rest, Happy Memories (upregulate condition; red bar shown), and Count (backwards from 300 by a given one-digit integer). Each rtfMRI-nf session consisted of six fMRI runs each lasting 8 min 40 s: a baseline run in which no neurofeedback information was provided, a practice run, three training runs, and a final transfer run in which no neurofeedback information was provided. The neurofeedback signal for each Happy Memory condition was computed as the fMRI percent signal change relative to the average fMRI signal for the preceding Rest block, updated every 2 s and displayed as a red bar. To reduce bar fluctuations due to noise in the fMRI signal, the bar height was computed at every time point as a moving average of the current and two preceding fMRI percent signal change values.
Imaging was acquired with a receive-only 8-channel head coil, and similar parameters as in the resting state paradigm (EPI sequence with SENSE = 2, repetition/echo time = 2000/30 ms, SENSE acceleration = 2, acquisition matrix = 96 × 96, flip angle = 90°, field-of-view/slice thickness = 240/2.9 mm, 34 axial slices). The EPI images were reconstructed into a 128 × 128 matrix, in which the resulting fMRI voxel volume was 1.875 × 1.875 × 2.9 mm3. High-resolution whole–brain T1-weighted anatomical MRI scans were collected (voxel volume: 0.9 × 0.9 × 1.2 mm3) to provide an anatomical reference for the fMRI analysis.
2.5. Data processing and analysis
fMRI analysis was performed using AFNI (http://afni.nimh. nih.gov/afni; build date 23 Sep 2016). Single-subject analysis steps for both task and resting based analyses consisted of suppression of cardiorespiratory artifacts using recorded respiratory and pulse-oximeter cardiac waveforms and AFNI implementation of the RETROICOR method (Glover et al., 2000), slice timing correction, within-subject realignment, coregistration between anatomical and functional images, spatial normalization to the stereotaxic array of Talairach and Tournoux (Talairach and Tournoux, 1988), spatial smoothing (Gaussian kernel, 4 mm full width at half maximum), and finally the voxel time series were low band pass filtered (cutoff 0.10 Hz).
Standard general linear model (GLM) analysis was applied separately for each fMRI run. The following regressors were included in the GLM model: two block stimulus conditions for the task based analysis (Happy Memories, Count), six motion parameters as nuisance covariates to take into account possible artifacts caused by head motion, and five Legendre polynomial terms (used to model and regress out baseline signal changes, which is approximately equivalent to using a high-pass filter with cutoff frequency of 0.005 Hz) for modeling baseline signal drift. The regressors were convolved with the canonical hemodynamic response function provided with AFNI. For the task-based analysis the hemodynamic response estimates (GLM β coefficients) were then computed for each voxel using the 3dDeconvolve AFNI program and then converted to percent signal changes (done with the mean signal voxel-wise such that s = x/mean ∗ 100, where x is a raw signal and s is a signal scaled to percent change) for Happy versus Rest conditions.
For both the rest and task based analyses, the voxel-wise percent signal change data were averaged within the left amygdala ROI as defined during neurofeedback (7 mm sphere centered at Talairach coordinates − 21, − 5, − 16). The time course of the mean BOLD signal from the amygdala seed during positive memory recall blocks was used as the stimulus regressor for the task-based connectivity analysis censoring all other time points by omitting them, and the analogous time course from the entire resting state run was used as the stimulus regressor for the resting-based connectivity analysis. For both task and resting analyses, mean frame wise displacement (FD) was included as a covariate to implement group-level motion correction in addition to the subject-level corrections described earlier. The GLM-based R2 statistics were converted to correlation coefficients by taking the square root of the R2 value multiplied by the sign of the beta weight, and then to z scores using the Fisher r-to-z transformation. AFNI's 3dANOVA was used to determine how the functional connectivity pattern changed from initial baseline to final transfer run in the amygdala relative to parietal rtfMRI-nf group during the task and from the baseline to follow-up resting state scan (completed 1 week before and after rtfMRI-nf training). Separate Group × Time ANOVAs were performed for the resting-state and task-based connectivity. The significance criterion was set at p < 0.05 corrected (determined using AFNI 3dClustSim at voxel p < 0.001 and the Spatial AutoCorrelation Function which uses a mixture of exponential functions to model more realistic noise distribution (Cox et al., 2017) to address recent criticisms of the cluster method (Eklund et al., 2016), cluster size > 30 voxels).
To determine how specific the functional connectivity changes were to the amygdala rtfMRI-nf procedure, and to rule out non-specific effects of learning to regulate hemodynamic activity or the strategy or recalling positive autobiographical memories more generally, we also performed the connectivity analyses described above using the parietal control region as the ROI. This ROI was also defined using the same coordinates as during rtfMRI-nf training (Talairach coordinates − 42, − 48, 48; 7 mm sphere).
The correlation coefficients for regions that significantly changed connectivity from pre- to post-training in the amygdala relative to parietal rtfMRI-nf group were extracted and follow-up t-tests were performed using SPSS to determine which group/visit was driving the ANOVA effect. To determine whether any connectivity change accounted for additional variance in the treatment response after regressing out the effects of amygdala change and baseline MADRS score, we used AFNI's 3dfim + to calculate the correlation between the final MADRS score and amygdala connectivity changes, with amygdala change and MADRS baseline score regressed out. A final analysis examined whether any connectivity changes were significantly related to regulation success (defined as the average amygdala signal averaged over all training blocks). We used AFNI's 3dfim + to calculate the correlation between regulation success and amygdala connectivity changes, with amygdala change regressed out. The significance criterion was set at p < 0.05 corrected (determined using AFNI 3dClustSim at voxel p < 0.001, cluster size > 30).
3. Results
3.1. Demographic, clinical characteristics, and results of the randomized clinical trial
The clinical results of the trial have been published previously (Young et al., 2017b). Briefly, the amygdala and parietal rtfMRI-nf groups did not differ at baseline on measured demographic or clinical characteristics (Table 1). At the one-week follow-up the amygdala group had significantly lower MADRS scores than the parietal rtfMRI-nf group, with six participants in the amygdala group and one in the parietal rtfMRI-nf group meeting criteria for remission at study end, yielding a Number Needed to Treat = 4. Participants in the amygdala rtfMRI-nf group were able to successfully increase their amygdala response both from baseline and relative to the parietal rtfMRI-nf group. Participants in the parietal rtfMRI-nf group were able to successfully increase the parietal response over the course of the study to the same extent as the amygdala group was able to increase their amygdala activity. All but one participant in the experimental group was able to increase their amygdala response from baseline to the final transfer run, while 10 of the 15 participants increased their parietal response from baseline to the final transfer run. The difference between the number who increased activity in their assigned ROI is not significant (Fisher's exact test = 2.18, p = 0.11). Clinical change was correlated with amygdala, but not parietal, regulation success. One participant in each group withdrew from the study due to physical discomfort during imaging.
Table 1.
Clinical and demographic characteristics for each group.
| Sample characteristics |
|||||
|---|---|---|---|---|---|
| Amygdala group | Parietal group | ||||
| Total N | N female | Total N | N female | Fisher's value | |
| N (# of participants) | 19 | 13 | 17 | 13 | 0.72 |
| Mean | SD | Mean | SD | t value | |
| Age | 32 | 12 | 31 | 9 | 0.28 |
| MDE length in months | 30 | 56 | 34 | 49 | 0.23 |
| Time since Last Antidepressant (months) | 33 | 34 | 31 | 35 | 0.17 |
| Baseline MADRS | 23.5 | 9.9 | 23.8 | 6.7 | 0.11 |
| Final MADRS | 11.9 | 9 | 21.9 | 8.1 | 3.49a |
| Number | Percent | Number | Percent | Fisher's value | |
| Number of episodes | |||||
| 1 | 5 | 26% | 2 | 12% | 0.41 |
| 2 | 2 | 11% | 2 | 12% | 1.00 |
| 3 or more | 12 | 63% | 13 | 76% | 0.48 |
| Previous number of antidepressants | |||||
| None | 8 | 42% | 4 | 24% | 0.30 |
| 2-Jan | 6 | 32% | 7 | 41% | 0.73 |
| 3 or more | 5 | 26% | 6 | 35% | 0.72 |
| Co-morbid diagnosis | |||||
| None | 7 | 39% | 7 | 41% | 1.00 |
| PTSD | 6 | 32% | 3 | 18% | 0.45 |
| GAD | 5 | 26% | 7 | 41% | 0.48 |
| Social phobia | 3 | 16% | 6 | 25% | 0.26 |
Abbreviations: GAD = generalized anxiety disorder; MADRS = Montgomery-Asberg Depression Rating Scale; MDE = major depressive episode; PTSD = post-traumatic stress disorder.
Indicates a significant difference between groups at p < 0.05.
3.2. Task-based amygdala connectivity changes
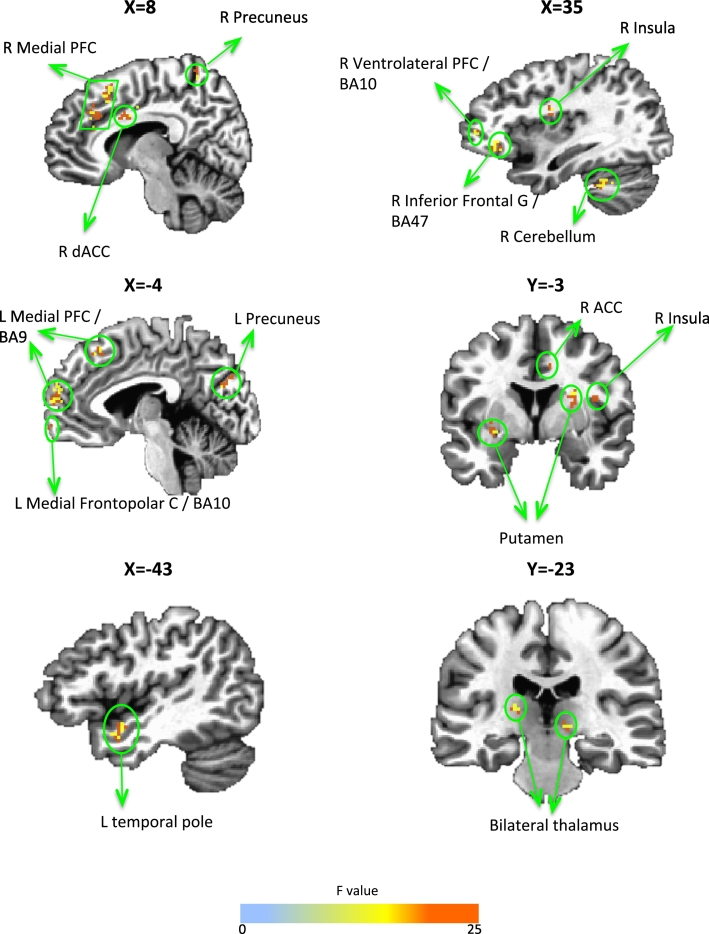
Table 2 and Fig. 1 show the results of the Group × Visit ANOVA on amygdala connectivity during positive memory recall. Increased connectivity with the amygdala in the amygdala compared to the parietal rtfMRI-nf group was observed in multiple prefrontal cortical (right inferior frontal gyrus/lateral orbital cortex, dorsal ACC and ventrolateral PFC, left medial frontopolar cortex, bilateral medial PFC) and striatal regions (bilateral putamen, right caudate), as well as the right insula, cerebellum, and bilateral thalamus and precuneus. For all clusters in Table 2a, there were no differences at baseline. The only region to show decreased connectivity with the amygdala following amygdala compared to the parietal rtfMRI-nf training was the right temporal pole. Table 2b shows regions that showed a Group × Time interaction, but were either different at baseline between groups (right dorsolateral PFC and lingual gyrus, left precentral gyrus, and posterior cingulate cortex) or did not differ in the magnitude of change between groups (right caudate). When the control parietal region was used as the seed, no regional connectivity changes survived cluster correction.
Table 2.
Regions where connectivity with the amygdala changed during positive memory recall following rtfMRI-nf.
(a) Regions that showed a Group × Time interaction reflecting a differential change between groups.
(b) Regions that showed a Group × Time interaction but either showed differential baseline activity between or changed similarly in both groups.
| Areaa | r values |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinatesb | Cluster size | F value | Pre-rtfMRI-nf |
Post-rtfMRI-nf |
rtfMRI-nf-induced change |
||||
| Amygdala | Parietal | Amygdala | Parietal | Amygdala | Parietal | ||||
| a) | |||||||||
| R inferior frontal G/lateral orbital C/BA47 | 35, 29, − 8 | 33 | 7.46 | 0.01 (0.19) | 0.03 (0.22) | 0.23 (0.13) | 0.06 (0.12)⁎ | 0.23 (0.16)# | 0.03 (0.13)⁎ |
| L medial PFC/BA9 | − 3, 53, 22 | 63 | 9.77 | 0.02 (0.19) | 0.16 (0.22) | 0.26 (0.10) | 0.10 (0.05)⁎ | 0.24 (0.19)# | − 0.06 (0.15)⁎ |
| R medial PFC | 13, 57, 22 | 61 | 14.0 | − 0.003 (0.18) | 0.10 (0.14) | 0.24 (0.16) | 0.08 (0.14)⁎ | 0.24 (0.17)# | − 0.03 (0.15)⁎ |
| L medial frontopolar C/BA10 | − 1, 63, − 6 | 34 | 9.37 | − 0.03 (0.16) | − 0.01 (0.08) | 0.25 (0.16) | 0.05 (0.11)⁎ | 0.27 (0.16)# | 0.06 (0.10)⁎ |
| R ventrolateral PFC/BA10 | 43, 49, 4 | 65 | 7.46 | 0.06 (0.23) | 0.09 (0.19) | 0.17 (0.15) | − 0.02 (0.18)⁎ | 0.11 (0.16)# | − 0.11 (0.18)⁎ |
| R medial frontal G | 3, 27, 34 | 214 | 16.8 | 0.05 (0.07) | 0.07 (0.16) | 0.18 (0.07) | 0.08 (0.10)⁎ | 0.13 (0.07)# | 0.02 (0.09)⁎ |
| R dACC | 9, 5, 32 | 34 | 24.8 | 0.09 (0.16) | 0.11 (0.10) | 0.26 (0.16) | 0.13 (0.09)⁎ | 0.17 (0.16)# | 0.02 (0.09)⁎ |
| R mid insula | 35, − 7, 22 | 45 | 19.3 | 0.01 (0.18) | 0.04 (0.14) | 0.21 (0.06) | 0.12 (0.04)⁎ | 0.21 (0.12)# | 0.08 (0.14)⁎ |
| L ventral striatum | − 27, − 3, − 4 | 47 | 9.95 | 0.01 (0.20) | 0.09 (0.13) | 0.24 (0.13) | 0.14 (0.08)⁎ | 0.23 (0.15)# | 0.04 (0.08)⁎ |
| R putamen | 23, 3, 12 | 33 | 10.1 | − 0.005 (0.21) | 0.07 (0.17) | 0.23 (0.09) | 0.14 (0.14)⁎ | 0.24 (0.18)# | 0.07 (0.11)⁎ |
| L thalamus | − 17, − 23, 10 | 37 | 19.3 | 0.01 (0.17) | 0.06 (0.11) | 0.24 (0.11) | 0.10 (0.10)⁎ | 0.24 (0.13)# | 0.03 (0.05)⁎ |
| R thalamus | 11, − 21, 0 | 36 | 13.4 | 0.08 (0.20) | 0.14 (0.10) | 0.32 (0.11) | 0.14 (0.15)⁎ | 0.24 (0.16)# | 0.01 (0.17)⁎ |
| L precuneus | − 5, − 71, 22 | 34 | 20.1 | 0.02 (0.21) | 0.12 (0.25) | 0.23 (0.06) | 0.04 (0.09)⁎ | 0.22 (0.18)# | − 0.08 (0.19)⁎ |
| R precuneus | 1, − 52, 56 | 39 | 18.9 | 0.09 (0.18) | 0.16 (0.14) | 0.32 (0.13) | 0.14 (0.16)⁎ | 0.22 (0.15)# | − 0.02 (0.19)⁎ |
| R cerebellum | 35, − 43, − 32 | 32 | 10.9 | − 0.03 (0.20) | 0.01 (0.15) | 0.23 (0.08) | 0.08 (0.13)⁎ | 0.26 (0.12)# | 0.07 (0.11)⁎ |
| R temporal pole | − 43, 4, − 18 | 40 | 13.7 | 0.09 (0.18) | 0.09 (0.19) | − 0.22 (0.17) | 0.09 (0.15)⁎ | − 0.31 (0.18)# | − 0.01 (0.25)⁎ |
| b) | |||||||||
| R caudate | 17, − 13, 22 | 39 | 20.1 | − 0.05 (0.16) | − 0.04 (0.16) | 0.19 (0.06) | 0.10 (0.17)⁎ | 0.23 (0.14)# | 0.14 (0.15)# |
| R dorsolateral PFC/BA6 | 39, 3, 46 | 148 | 31.2 | 0.22 (0.12) | 0.05 (0.13)⁎ | 0.17 (0.11) | 0.12 (0.11) | − 0.05 (0.21) | 0.07 (0.20)# |
| L precentral G/BA6 | − 45, − 3, 26 | 43 | 10.7 | 0.13 (0.09) | 0.24 (0.16)⁎ | 0.004 (0.18) | 0.12 (0.10)⁎ | − 0.13 (0.15)# | − 0.12 (0.15) |
| L PCC | − 5, − 25, 28 | 31 | 8.30 | 0.23 (0.12) | 0.03 (0.22)⁎ | − 0.05 (0.13) | 0.07 (0.21) | − 0.27 (0.17)# | 0.04 (0.12)⁎ |
| R lingual G | 15, − 75, − 2 | 33 | 16.5 | 0.27 (0.11) | 0.02 (0.14)⁎ | 0.02 (0.22) | 0.02 (0.19) | − 0.26 (0.25)# | 0.01 (0.22)⁎ |
Abbreviations: BA = Brodmann area; c = cortex; dACC = dorsal anterior cingulate cortex; G = gyrus; L = left; PCC = posterior cingulate cortex; PFC = prefrontal cortex; R = right.
Area refers to the anatomical location of the peak voxel, according to AFNI's whereami function.
Coordinates correspond to the stereotaxic array by Talairach and Tournoux and are listed as x, y, z, with each designating mm from the anterior commissure, such that positive x = left, positive y = anterior, and positive z = dorsal.
Significant difference from the amygdala group at p < 0.05.
Significant change from the Pre rtfMRI-nf Baseline at p < 0.05.
Fig. 1.
Task-based amygdala connectivity changes.
Regions from Table 1a that significantly changed connectivity with the amygdala during positive memory recall following rtfMRI-nf in the amygdala relative to parietal rtfMRI-nf group at pcorrected < 0.05.
3.3. Resting state amygdala connectivity changes
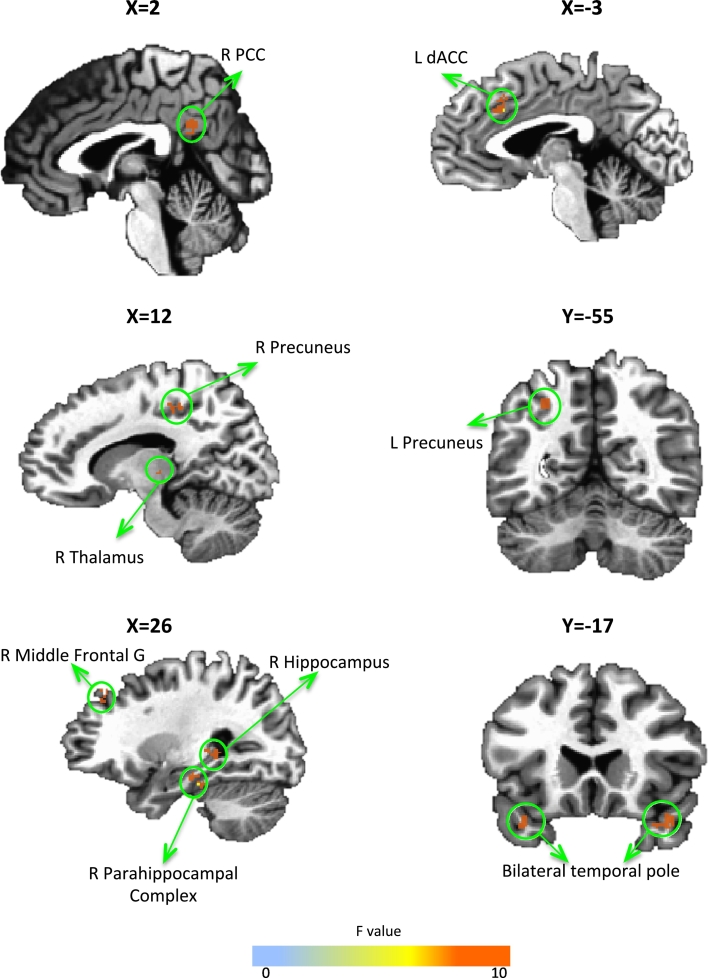
Table 3 and Fig. 2 show the results of the Group × Visit ANOVA on amygdala connectivity during rest. Increased connectivity with the amygdala in the amygdala compared to parietal rtfMRI-nf group was observed in the right middle frontal gyrus, hippocampus, parahippocampal gyrus, thalamus, left dorsal ACC, and bilateral precuneus. For all clusters in Table 3a, there were no differences at baseline and the amygdala group had increased connectivity changes relative to the parietal rtfMRI-nf group. The only region to show decreased connectivity with the amygdala following amygdala rtfMRI-nf training was the bilateral temporal pole. Table 3b shows regions that were significant in the overall ANOVA, but were either different at baseline between groups (left insula and parahippocampal gyrus) or did not change significantly in the amygdala group (left middle frontal gyrus). When the control parietal region was used as the seed, no regional connectivity changes survived cluster correction.
Table 3.
Regions where connectivity with the amygdala changed during rest following rtfMRI-nf.
(a) Regions that showed a Group × Time interaction reflecting a differential change between groups.
(b) Regions that showed a Group × Time interaction but either showed differential baseline activity between or changed similarly in both groups.
| Areaa | r values |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinatesb | Cluster size | F value | Pre-rtfMRI-nf |
Post-rtfMRI-nf |
rtfMRI-nf-induced change |
||||
| Amygdala | Parietal | Amygdala | Parietal | Amygdala | Parietal | ||||
| a) | |||||||||
| R middle frontal G | 25, 38, 37 | 58 | 8.03 | 0.01 (0.02) | − 0.001 (0.01) | 0.13 (0.10) | − 0.004 (0.01)⁎ | 0.13 (0.10)# | 0.01 (0.01)⁎ |
| L dACC | − 3, 20, 32 | 46 | 6.45 | 0.01 (0.04) | − 0.01 (0.02) | 0.13 (0.03) | − 0.01 (0.02)⁎ | 0.09 (0.05)# | 0.003 (0.02)⁎ |
| R PCC | 1, − 48, 28 | 47 | 7.37 | 0.02 (0.05) | − 0.001 (0.05) | 0.09 (0.02) | 0.01 (0.02)⁎ | 0.07 (0.05)# | 0.01 (0.06)⁎ |
| R thalamus | 15, − 24, 0 | 32 | 6.18 | − 0.004 (0.01) | − 0.01 (0.02) | 0.08 (0.02) | − 0.01 (0.04)⁎ | 0.08 (0.07)# | 0.003 (0.04)⁎ |
| L precuneus | − 27, − 55, 41 | 72 | 6.87 | − 0.002 (0.01) | − 0.02 (0.04) | 0.09 (0.03) | 0.01 (0.04)⁎ | 0.11 (0.06)# | 0.01 (0.04)⁎ |
| R precuneus | 11, − 34, 46 | 43 | 5.83 | − 0.02 (0.03) | − 0.01 (0.02) | 0.07 (0.12) | − 0.002 (0.02)⁎ | 0.08 (0.02)# | 0.01 (0.02)⁎ |
| R PHG | 27, − 25, − 19 | 65 | 5.56 | 0.01 (0.03) | 0.04 (0.07) | 0.17 (0.10) | 0.06 (0.04)⁎ | 0.16 (0.10)# | 0.02 (0.06)⁎ |
| R hippocampus | 27, − 26, 0 | 49 | 7.17 | − 0.02 (0.05) | − 0.004 (0.01) | 0.06 (0.05) | 0.006 (0.03)⁎ | 0.09 (0.06)# | 0.01 (0.03)⁎ |
| L temporal pole/BA 38 | − 41, 3, − 18 | 75 | 6.58 | 0.06 (0.05) | 0.07 (0.05) | − 0.10 (0.04) | 0.02 (0.03)⁎ | − 0.15 (0.10)# | − 0.03 (0.06)⁎ |
| R temporal pole/BA 38 | 41, 17, − 21 | 135 | 10.1 | 0.07 (0.04) | 0.06 (0.05) | − 0.09 (0.03) | 0.02 (0.05)⁎ | − 0.16 (0.10)# | − 0.03 (0.07)⁎ |
| b) | |||||||||
| L PHG | − 25, − 4, − 14 | 92 | 9.05 | 0.01 (0.01) | − 0.004 (0.01)⁎ | 0.10 (0.14) | 0.03 (0.09) | 0.11 (0.08)# | 0.02 (0.08)⁎ |
| L insula | − 36, 18, 9 | 37 | 9.12 | − 0.002 (0.01) | − 0.02 (0.03)⁎ | 0.09 (0.05) | 0.02 (0.02)⁎ | 0.11 (0.06)# | 0.02 (0.06)⁎ |
| L middle frontal G | − 29, 10, 44 | 38 | 6.58 | 0.02 (0.04) | 0.01 (0.01) | 0.05 (0.07) | − 0.001 (0.03)⁎ | 0.04 (0.01) | − 0.001 (0.03)⁎ |
Abbreviations: BA = Brodmann area; dACC = dorsal anterior cingulate cortex; G = gyrus; L = left; PCC = posterior cingulate cortex; R = right.
Area refers to the anatomical location of the peak voxel, according to AFNI's whereami function.
Coordinates correspond to the stereotaxic array by Talairach and Tournoux.
Significant difference from the amygdala group at p < 0.05.
Significant change from the Pre rtfMRI-nf Baseline at p < 0.05.
Fig. 2.
Resting state amygdala connectivity changes.
Regions from Table 2b that significantly changed connectivity with the amygdala during rest following rtfMRI-nf in the amygdala relative to parietal rtfMRI-nf group at pcorrected < 0.05.
3.4. Association with clinical change
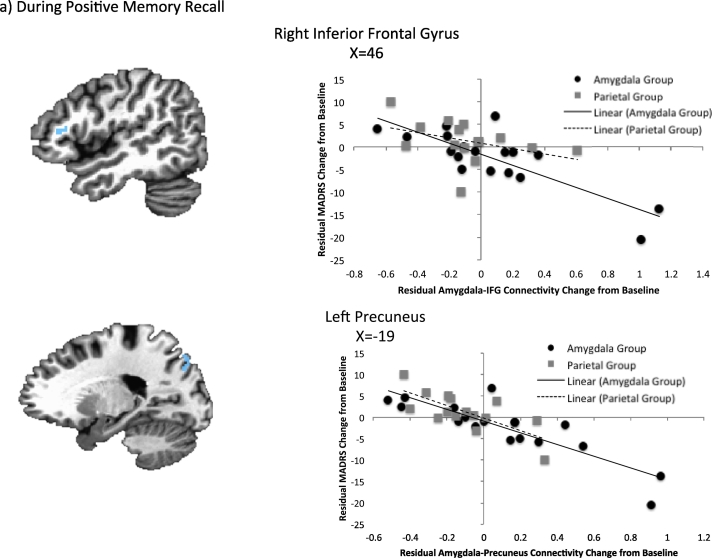
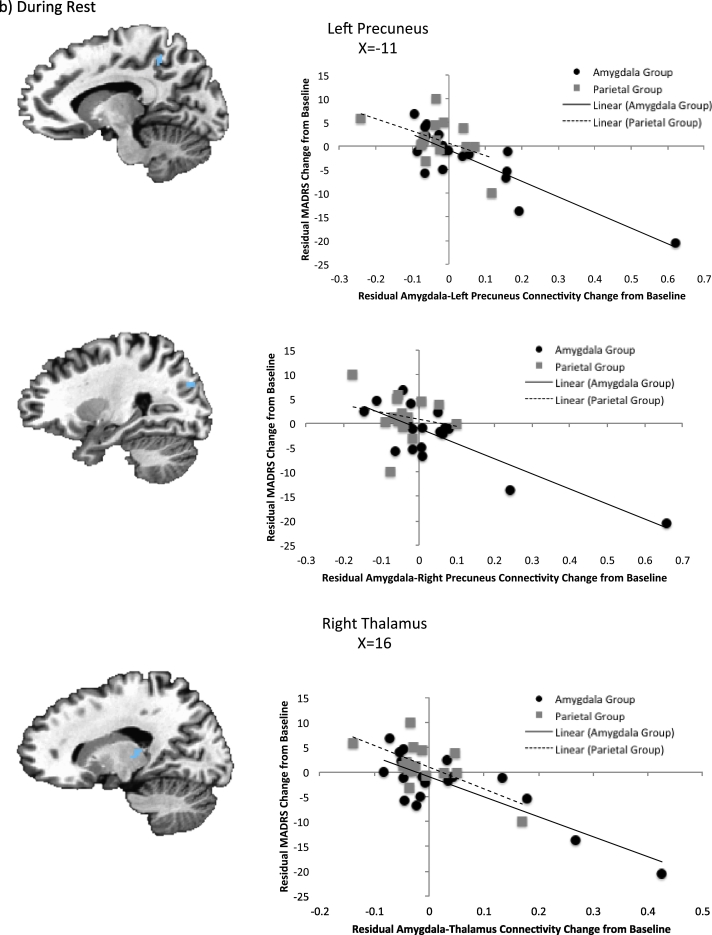
The results of the 3dfim + analysis in AFNI for treatment response and connectivity changes are presented in Table 4 and Fig. 3. During positive memory recall, amygdala-left precuneus and amygdala-right inferior frontal gyrus connectivity were significantly correlated with improvement in depressive symptoms after controlling for baseline severity and amygdala activity change. During rest, bilateral amygdala-precuneus and amygdala-right thalamus connectivity significantly explained variance in the treatment response after controlling for amygdala change and baseline MADRS scores. However, there were several outliers present in the resting state data (though not the task data), and when these outliers were removed, only the relationship between clinical change and amygdala-left precuneus activity remained significant (r = − 0.46, p = 0.002).
Table 4.
Regions for which amygdala connectivity change following rtfMRI-nf was associated with residual depression scores at the one-week follow-up.
| Area | Coordinatesa | Cluster size | Correlation (r) | Correlation after removal of outliers |
|---|---|---|---|---|
| Task based amygdala connectivity | ||||
| R inferior frontal G | 47, 27, 10 | 35 | − 0.66 | No outliers |
| L precuneus | − 19, − 77, 42 | 35 | − 0.67 | No outliers |
| Resting state amygdala connectivity | ||||
| L precuneus | − 13, − 45, 46 | 53 | − 0.62 | − 0.42 |
| R precuneus | 27, − 73, 26 | 271 | − 0.65 | − 0.31 |
| R thalamus | 18, − 27, 9 | 39 | − 0.59 | − 0.17 |
Abbreviations: G = gyrus; L = left; R = right.
Coordinates correspond to the stereotaxic array by Talairach and Tournoux.
Fig. 3.
Association between change in MADRS scores and amygdala connectivity changes controlling for baseline MADRS and amygdala activity change during (a) positive memory recall and (b) rest.
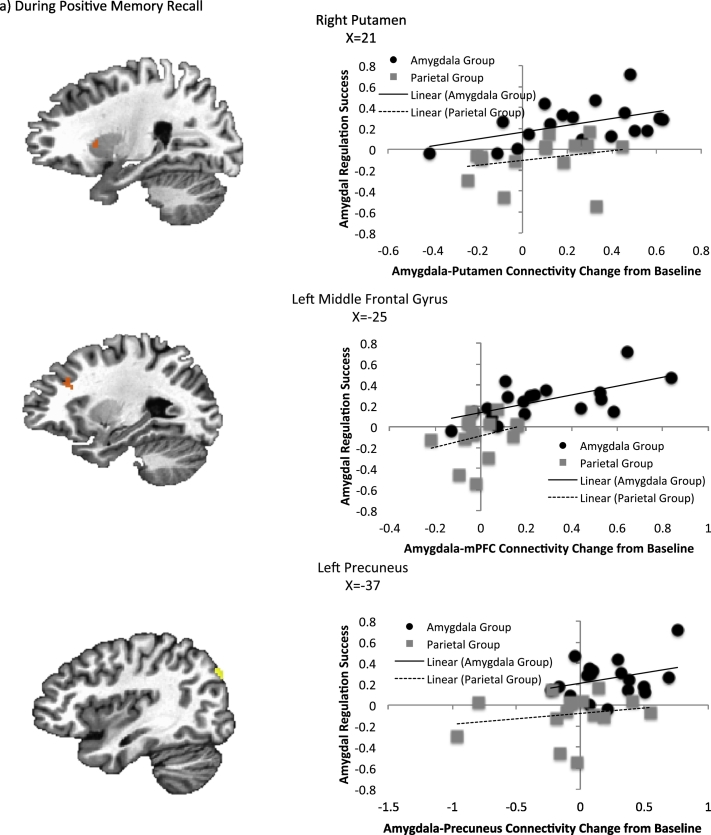
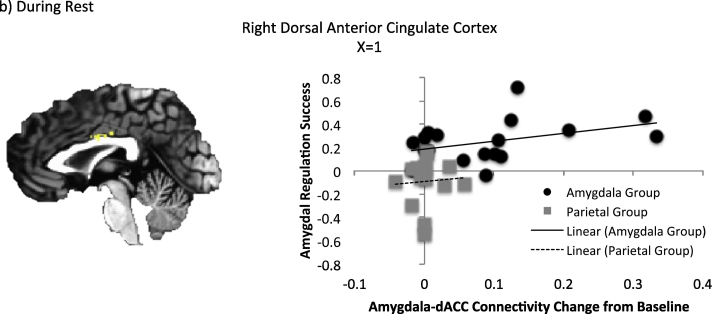
3.5. Association with regulation success
The results of the 3dfim + analysis in AFNI for regulation success and connectivity changes are presented in Table 5 and Fig. 4. During positive memory recall, amygdala-right putamen, amygdala left middle frontal gyrus, and amygdala-left precuneus connectivity were significantly correlated with regulation success after controlling for amygdala activity change. During rest, the amygdala-right dorsal ACC connectivity was significantly correlated with regulation success. No outliers were present in any of these variables.
Table 5.
Regions for which amygdala connectivity change following rtfMRI-nf was associated with amygdala regulation success.
| Area | Coordinatesa | Cluster Size | Correlation (r) |
|---|---|---|---|
| Task based amygdala connectivity | |||
| R putamen | 21, 9, 14 | 48 | 0.66 |
| L middle frontal G | − 25, 27, 26 | 31 | 0.5 |
| L precuneus | − 37, − 79, 36 | 38 | 0.49 |
| Resting state amygdala connectivity | |||
| R dACC | 1, − 8, 30 | 25 | 0.56 |
Abbreviations: dACC = dorsal anterior cingulate gyrus; G = gyrus; L = left; R = right.
Coordinates correspond to the stereotaxic array by Talairach and Tournoux.
Fig. 4.
Association between amygdala regulation success and amygdala connectivity changes controlling for amygdala activity change during (a) positive memory recall and (b) rest.
4. Discussion
In this secondary analysis of our published clinical trial of neurofeedback data (Young et al., 2017b), we found real-time fMRI neurofeedback training to upregulate the amygdala during positive memory recall resulted in amygdala functional connectivity changes both during positive memory recall and during rest. As hypothesized, amygdala connectivity significantly increased with prefrontal regions following training in the amygdala relative to parietal rtfMRI-nf group. Enhanced amygdala-prefrontal and amygdala-precuneus connectivity was observed following rtfMRI-nf both while participants recalled positive memories as well as during a separate resting-state task performed one week prior to and following completion of rtfMRI-nf training. No significant functional connectivity changes were observed when the control parietal region was selected as the seed, suggesting that the amygdala rtfMRI-nf procedure resulted in significant and lasting (at least in the short term) changes in brain connectivity that were not due to non-specific effects of learning to regulate hemodynamic activity or the strategy or recalling positive autobiographical memories more generally.
During positive memory recall in the absence of neurofeedback information (change from initial baseline to final transfer run), amygdala connectivity significantly increased with regions in implicated in self-referential and reward processing, as well as in nodes of the salience network (Menon, 2011). Moreover, in the amygdala relative to the parietal rtfMRI-nf group several functional hubs of the default mode network, including the medial prefrontal cortex and precuneus (Buckner et al., 2008), significantly increased connectivity with the amygdala following training. As these regions are involved with self-referential processing (Nejad et al., 2013), we hypothesize that one way in which amygdala rtfMRI-nf training results in clinical improvements is by making these positive memories seem more salient or relevant to the participant than prior to training. Incorporating positive events into one's self-schema may contribute to the antidepressant effects of this intervention.
The amygdala functions as a key hub within the salience network (Seeley et al., 2007). Other nodes of this network, including the dACC and insula (Seeley et al., 2007), notably increased their connectivity with the amygdala following training in the amygdala relative to parietal rtfMRI-nf group. Neuronal activity within these regions increases when salient stimuli are encountered. We hypothesize that by increasing salience network connectivity, positive memories become more attended to relative to memories that are discounted or negatively valenced, thereby reversing the negative processing bias associated with MDD (Gotlib and Joormann, 2010). As the amygdala is also associated with increased arousal irrespective of valence (Lewis et al., 2007), another possible interpretation is that increased activation in the amygdala and salience network is the result of the mismatch between the current mood state and the positive emotional content of the memories. However, improved clinical scores would not be expected if amygdala rtfMRI-nf was increasing self-discrepancy during memory recall, and we consider increased attention and relevance of positive stimuli more in line with the current findings. Furthermore, an examination of emotional processing biases following our rtfMRI-nf procedure found enhanced attention to positive and reduced attention to negative stimuli in a battery of tasks performed outside of the scanner (Young et al., 2017a), further supporting a reversal of the negative processing bias.
Increased amygdala connectivity with striatal regions involved in reward processing also was observed (Schultz, 2000), although the change in amygdala-striatal connectivity did not differ significantly between the amygdala and parietal rtfMRI-nf groups. Consistent with this observation, the results of a recent meta-analysis showed that the striatum and insula consistently increased hemodynamic activity during neurofeedback training involving a variety of target regions (Emmert et al., 2016), and increased striatal-amygdala connectivity was correlated with regulation success but not treatment response in the current analysis. Thus, the changes in hemodynamic activity in these regions do not appear specific to the amygdala rtfMRI-nf intervention.
Our previous work examining differences between healthy and depressed individuals in amygdala connectivity during positive autobiographical memory recall found decreased connectivity between the amygdala and precuneus, as well as between the amygdala and ACC, in MDD relative to control participants (Young et al., 2016b). Taken together with the increased amygdala-precuneus and amygdala-ACC connectivity found following amygdala rtfMRI-nf training, these findings suggest that the rtfMRI-nf procedure normalizes amygdala connectivity towards that seen in healthy individuals while recalling positive memories.
The only region to show significantly decreased connectivity with the amygdala during memory recall following training in the amygdala rtfMRI-nf group was the temporal pole; all other significant differences in connectivity demonstrated an increase following rtfMRI-nf training. Amygdala-temporal polar cortical connectivity also significantly decreased during rest in the amygdala relative to parietal rtfMRI-nf group. The temporal pole has been reported to have abnormally increased connectivity with the amygdala in depressed patients relative to controls during rest (Ramasubbu et al., 2014). While the function of the temporal pole is not well understood, several studies have implicated it in theory of mind, as this region is activated when considering the emotions and thoughts of others' (Farrow et al., 2001, Vollm et al., 2006). It also links perceptual representations to visceral emotional responses (Olson et al., 2007), and is correlated with personal distress scores (Moriguchi et al., 2006). Decreased amygdala-temporal pole connectivity following rtfMRI-nf training may therefore indicate that the emotions experienced during positive AM recall (and rest) are more cognitive/intellectually driven as opposed to bottom up/automatically driven, or that participants are thinking less about others and more about their selves when recalling positive AMs. Though the exact functional significance of this change is unknown, the finding that this was the only region to show decreased connectivity with the amygdala, during both task and rest, suggests an important role of amygdala-temporal pole connectivity in MDD (Price and Drevets, 2010).
In the current study, we replicated our previous results in healthy individuals that amygdala rtfMRI-nf training increased amygdala functional connectivity with the medial prefrontal cortex, ACC, insula, and thalamus (Zotev et al., 2011). Importantly, in this previous study, there was no baseline run and therefore only the difference in connectivity during the transfer run between the amygdala and parietal rtfMRI-nf groups was examined. By including the baseline run we confirmed that the functional connectivity between these regions and the amygdala changes over time, and may play a role in sustaining neurofeedback learning effects.
These findings also are compatible with the reciprocal anatomical projections between the amygdala and mPFC regions including the ACC, through which amygdala activity is modulated by activity within the PFC, allowing the modulation of emotional processes by mPFC circuits involved in higher cognitive processes, such as autobiographical memory recall (Phillips et al., 2003, Price and Drevets, 2010). While many studies have demonstrated functional roles for these connections during emotional regulation within the context of applying cognitive strategies to alter amygdala activity during processing of negative emotional stimuli (Ochsner and Gross, 2005, Ochsner et al., 2004), including a rtfMRI-nf study in patients with post-traumatic stress disorder trained to down regulate their amygdala activity during trauma-related words (Nicholson et al., 2017), our results converge with other literature showing that increased amygdala-prefrontal connectivity is also important during the processing of positive emotional stimuli and increased connectivity between these regions allows for adaptive responses to emotional stimuli more generally. The finding that amygdala regulation success was also correlated with increased amygdala-mPFC connectivity further supports the hypothesis that prefrontal-amygdala connectivity allows for adaptive responding, not just a suppression of limbic activity more generally. Further support for the role of the PFC in maintaining positive emotional states comes from rtfMRI-nf training of patients with MDD to upregulate regions determined to be active (via a localizer scan) while viewing positively valenced pictures; this training reportedly resulted in increased activity in several prefrontal regions in the medial PFC (including ACC) and temporal lobe (Linden et al., 2012). Collectively, these results support the existence of a valence-general affective workspace in which regional limbic and prefrontal activity (e.g., amygdala, ACC, insula, medial PFC) activation is observed when affective valence is being represented during the experience and perception of emotion, regardless of whether that emotion is positive or negative (Lindquist et al., 2016).
Similar amygdala connectivity changes were observed during a resting-state paradigm employed both one week prior to and one week following completion of rtfMRI-nf training. Increased amygdala connectivity during rest in the amygdala relative to parietal rtfMRI-nf group was observed with regions of the self-referential processing/default mode network (medial prefrontal cortex, precuneus, PCC) and salience network (dACC). Increased amygdala connectivity with the hippocampus/parahippocampal gyrus was also observed. These results are consistent with our previous findings of altered resting state amygdala connectivity following a single rtfMRI-nf session. Increased connectivity pre- to post-training was observed in the parahippocampus, precuneus, ACC, and medial frontal gyrus (Yuan et al., 2014).
The current study could have important implications for potential improvements of rtfMRI-nf training. The selection of the amygdala as the target for rtfMRI-nf was based on empirical evidence of the amygdala's critical role in MDD. While this indeed produced antidepressant effects and altered hemodynamic activity towards that seen in healthy individuals, targeting connectivity via rtfMRI-nf may yield additional antidepressant interventions. Indeed, amygdala-precuneus and amygdala-right IFG connectivity accounted for a significant amount of the variance in residual symptom improvement after controlling for baseline MADRS scores and amygdala change. The right IFG is active during autobiographical memory retrieval relative to semantic retrieval (Greenberg et al., 2005) and plays a general role in identifying salient stimuli (Downar et al., 2002, Hampshire et al., 2010). Decreased amygdala-IFG connectivity has been reported in depressed relative to healthy participants as they process emotional faces (Carballedo et al., 2011), as has decreased amygdala-precuneus functional connectivity during rest (Wang et al., 2016). Increased amygdala-precuneus connectivity also was significantly correlated with amygdala regulation success, further implicating the importance of this structure in our amygdala rtfMRI-nf procedure. Therefore, it is possible that training to increase amygdala-precuneus or amygdala-IFG connectivity may afford additional targets for antidepressant interventions.
Several limitations merit comment. The entrance criteria resulted in a large proportion of patients being excluded (primarily due to medication status), limiting the generalizability of our findings. Additionally, the relatively small sample size limited statistical power to examine behavioral, demographic, or biomarker parameters that might moderate neurofeedback success. Further testing in larger samples is necessary to a) replicate the results that amygdala rtfMRI-nf training improves symptoms and alters connectivity with salience and self-referential processing regions and b) determine the sub-populations for whom this intervention may be best suited. It has also been shown that the amygdala signal changes may reflect signal changes in the adjacent Basal Vein of Rosenthal, which drains from large regions located a long distance from the amygdala. We have minimized this issue by optimizing our imaging parameters for detecting the hemodynamic signal in the medial temporal lobe region (including the amygdala) (Bellgowan et al., 2006). Furthermore, the resting-state data collected on the baseline and follow-up days had different parameters from the data collected during the neurofeedback days. This makes it more difficult to make direct comparisons of resting and task based connectivity changes. However, this was not the goal of the current analysis. Finally, patients were only followed for one-week after the final rtfMRI-nf session, while acute treatments trials more commonly include follow-up periods lasting 2–8 weeks. This short follow-up period was selected as a first step in determining whether behavioral/clinical/neural changes will persist beyond the training period. Therefore, while we were able to show that amygdala rtfMRI-nf resulted in significant and large clinical improvements, the duration of this improvement beyond one-week was not assessed and additional studies with longer follow-up periods are warranted, as are additional transfer rtfMRI-nf sessions to assess whether amygdala regulation and connectivity changes are sustained long-term.
4.1. Conclusions
In conclusion, amygdala rtfMRI-nf training relative to parietal rtfMRI-nf resulted in increased connectivity between the amygdala and several regions implicated in self-referential and salience processing. As most psychiatric disorders exhibit abnormal brain networks, targeting these networks instead of single regions may lead to additional interventions.
Furthermore, these results hold implications for modifications that can be made to current cognitive therapies which might increase efficacy based on the function of associated regions. For example, instead of simply having patients recall positive events, a different therapeutic strategy may involve instructing patients to think about how the positive event fits into their current self-representation, why it is important, and what was rewarding about it.
The following is the supplementary data related to this article.
CONSORT Flow Diagram Flow diagram of the progress through the phases of the parallel randomized clinical trial of two groups, including enrollment, intervention, allocation, follow-up, and data analysis.
Funding
Research reported in this publication was supported by the National Institutes of Health/National Institute of Mental Health under Award Number K99MH101235 and by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (22347). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial disclosures
WCD is currently an employee of Janssen Research and Development, LLC, of Johnson & Johnson, Inc., and a stockholder in Johnson & Johnson, Inc. The other authors have no financial conflicts of interest or disclosures to report.
References
- American Psychological Association (APA) Fourth edition. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgowan P.S., Bandettini P.A., van Gelderen P., Martin A., Bodurka J. Improved BOLD detection in the medial temporal region using parallel imaging and voxel volume reduction. NeuroImage. 2006;29:1244–1251. doi: 10.1016/j.neuroimage.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Dickerson B.C., Barrett L.F. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodurka J., Bandettini P. Real-time software for monitoring MRI scanner operation. NeuroImage. 2008;41:S85. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carballedo A., Scheuerecker J., Meisenzahl E., Schoepf V., Bokde A., Moller H.J., Doyle M., Wiesmann M., Frodl T. Functional connectivity of emotional processing in depression. J. Affect. Disord. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Jesmanowicz A., Hyde J.S. Real-time functional magnetic resonance imaging. Magn. Reson. Med. 1995;33:230–236. doi: 10.1002/mrm.1910330213. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- deCharms R.C. Applications of real-time fMRI. Nat. Rev. Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- deCharms R.C., Maeda F., Glover G.H., Ludlow D., Pauly J.M., Soneji D., Gabrieli J.D., Mackey S.C. Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J. Neurophysiol. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging abnormalities in the amygdala in mood disorders. Ann. N. Y. Acad. Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert K., Kopel R., Sulzer J., Bruhl A.B., Berman B.D., Linden D.E., Horovitz S.G., Breimhorst M., Caria A., Frank S., Johnston S., Long Z., Paret C., Robineau F., Veit R., Bartsch A., Beckmann C.F., Van De Ville D., Haller S. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: how is brain regulation mediated? NeuroImage. 2016;124:806–812. doi: 10.1016/j.neuroimage.2015.09.042. [DOI] [PubMed] [Google Scholar]
- Farrow T.F., Zheng Y., Wilkinson I.D., Spence S.A., Deakin J.F., Tarrier N., Griffiths P.D., Woodruff P.W. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Patient edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York, NY: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. [Google Scholar]
- Glover G.H., Li T.Q., Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D.L., Rice H.J., Cooper J.J., Cabeza R., Rubin D.C., Labar K.S. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell K.J., Hanlon C.A., Li X., Borckardt J.J., Canterberry M., Prisciandaro J.J., Moran-Santa Maria M.M., LeMatty T., George M.S., Brady K.T. Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J. Psychiatry Neurosci. 2016;41:48–55. doi: 10.1503/jpn.140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Critchley H.D., Rotshtein P., Dolan R.J. Neural correlates of processing valence and arousal in affective words. Cereb. Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E., Habes I., Johnston S.J., Linden S., Tatineni R., Subramanian L., Sorger B., Healy D., Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Ohnishi T., Lane R.D., Maeda M., Mori T., Nemoto K., Matsuda H., Komaki G. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–1482. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Theberge J., Neufeld R.W., McKinnon M.C., Reiss J., Jetly R., Lanius R.A. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum. Brain Mapp. 2017;38:541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R., Konduru N., Cortese F., Bray S., Gaxiola-Valdez I., Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front. Psych. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Cook P.A., Bruce S.E., Conway C., Mikkelsen E., Satchell E., Vandekar S.N., Durbin T., Shinohara R.T., Sheline Y.I. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivity. Mol. Psychiatry. 2016;21:894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D.O., Funderburk S.C., Bhatti D.L., Motard L.E., Newbold D., Girven K.S., McCall J.G., Krashes M., Sparta D.R., Bruchas M.R. A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior. J. Neurosci. 2016;36:10831–10842. doi: 10.1523/JNEUROSCI.1164-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Suslow T., Konrad C., Kugel H., Rumstadt D., Zwitserlood P., Schoning S., Ohrmann P., Bauer J., Pyka M., Kersting A., Arolt V., Heindel W., Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol. Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System—an Approach to Cerebral Imaging. [Google Scholar]
- Victor T.A., Furey M.L., Fromm S.J., Ohman A., Drevets W.C. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch. Gen. Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollm B.A., Taylor A.N., Richardson P., Corcoran R., Stirling J., McKie S., Deakin J.F., Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wang Y.L., Yang S.Z., Sun W.L., Shi Y.Z., Duan H.F. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav. Brain Res. 2016;298:301–309. doi: 10.1016/j.bbr.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., Somerville L.H., McLean A.A., Kim H. Functional neuroimaging studies of the amygdala in depression. Semin. Clin. Neuropsychiatry. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Young K.D., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Siegle G.J., Bodurka J., Drevets W.C. Amygdala activity during autobiographical memory recall in depressed and vulnerable individuals; association with symptom severity and autobiographical overgenerality. Am. J. Psychiatry. 2016;173:78–89. doi: 10.1176/appi.ajp.2015.15010119. [DOI] [PubMed] [Google Scholar]
- Young K.D., Misaki M., Harmer C.J., Victor T., Zotev V., Phillips R., Siegle G.J., Drevets W.C., Bodurka J. 2017. Real-Time fMRI Amygdala Neurofeedback Changes Positive Information Processing in Major Depressive Disorder. (Biol Psychiatry Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Siegle G.J., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am. J. Psychiatry. 2017;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Young K.D., Phillips R., Zotev V., Misaki M., Bodurka J. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 2014;4:690–701. doi: 10.1089/brain.2014.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A., Sorger B., Sarkheil P., Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front. Behav. Neurosci. 2015;9:148. doi: 10.3389/fnbeh.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Krueger F., Phillips R., Alvarez R.P., Simmons W.K., Bellgowan P., Drevets W.C., Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Young K.D., Drevets W.C., Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Yuan H., Misaki M., Phillips R., Young K.D., Feldner M.T., Bodurka J. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage. 2016;11:224–238. doi: 10.1016/j.nicl.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Flow Diagram Flow diagram of the progress through the phases of the parallel randomized clinical trial of two groups, including enrollment, intervention, allocation, follow-up, and data analysis.