Abstract
The overwhelming majority of people with chronic obstructive pulmonary disease (COPD) have at least one coexisting medical condition often conceptualized as ‘comorbidities’. These coexisting conditions vary in severity and impact; it is likely that for some patients, COPD is not their most important or severe condition. The concepts of multimorbidity and frailty may be useful to understand the broader needs of people with COPD undergoing pulmonary rehabilitation. Multimorbidity describes the coexistence of two or more chronic conditions, without reference to a primary condition. Best care for people with multimorbidity has been described as a shift from providing disease-focused to patient-centred care. Pulmonary rehabilitation is well placed to deliver such care as it focuses on optimizing function, encourages integration across care settings, values input from multidisciplinary teams and measures patient-important outcomes. When designing optimal pulmonary rehabilitation services for people with multimorbidity, the concept of frailty may be useful. Frailty focuses on impairments rather than medical conditions including impairments in mobility, strength, balance, cognition, nutrition, endurance, mood and physical activity. Emerging data suggest that frailty may be modifiable with pulmonary rehabilitation. The challenge for pulmonary rehabilitation clinicians is to broaden our perspective on the role and outcomes of pulmonary rehabilitation for people with multimorbidity.
Keywords: Chronic obstructive pulmonary disease, multimorbidity, frailty, pulmonary rehabilitation patient-centred care
Introduction
It is uncommon to meet an individual with chronic obstructive pulmonary disease (COPD) who does not have at least one other chronic health condition. Among Medicare beneficiaries in the United States, 18% of individuals with COPD have one to two coexisting conditions, 30% have three to four coexisting conditions and 49% have five or more.1 Among those referred for pulmonary rehabilitation, the proportion of patients with at least one coexisting condition varies from 51% to 96%.2 Coexisting conditions have important implications for outcomes in COPD. People with coexisting diabetes, hypertension or cardiovascular disease have increased risk of hospitalization and all-cause mortality compared to those with COPD alone, with greater risks in those with more severe lung disease.3 Similarly, depression and anxiety are associated with a greater risk of readmission to hospital and mortality following a COPD exacerbation.4,5
In recent years, several studies have addressed the impact of coexisting medical conditions on the outcomes of pulmonary rehabilitation for people with COPD, with some showing a positive impact and others a negative one. For example, for people with COPD and cardiometabolic disease, pulmonary rehabilitation outcomes have been reported as better by Walsh et al.6 but worse by Crisafulli et al. and Carreiro et al.7,8 Similarly, mood disturbance may either increase9 or decrease8 the likelihood of clinically significant gains with rehabilitation. There are also varying conclusions regarding the impact of obesity on pulmonary rehabilitation outcomes.10,11 This variability may arise in part from the complexity of participants in modern pulmonary rehabilitation programmes, who have coexisting conditions of varying severity and impact. All these studies have assumed that as participants in pulmonary rehabilitation, COPD is the primary and most important of the coexisting conditions; it is possible that for some patients, their other health conditions are more important or more severe. In some instances, pulmonary rehabilitation may not have met the broader needs of individual participants with significant coexisting health challenges.
Frailty is a concept that relates to comorbidity. Unlike comorbidity that focuses on medical conditions, frailty focuses on the impairments regardless of the conditions. Markers of frailty could include impairments in mobility, strength, balance, cognition, nutrition, endurance, mood and physical activity.12–14 Individuals with COPD and other chronic conditions often have impairments affecting numerous systems and are therefore more likely to meet the criteria for frailty.
Given that it is common for pulmonary rehabilitation candidates to have multiple chronic conditions, it is increasingly likely that many of their important clinical problems will not be directly related to respiratory disease. People with COPD have expressed their preference for individualized models of care that target the clinical problems they perceive to be most important.15 In this article, we will discuss how we could broaden our perspective on the role and outcomes of pulmonary rehabilitation, with particular reference to the concepts of multimorbidity and frailty.
Multimorbidity versus comorbidity – Does the label matter?
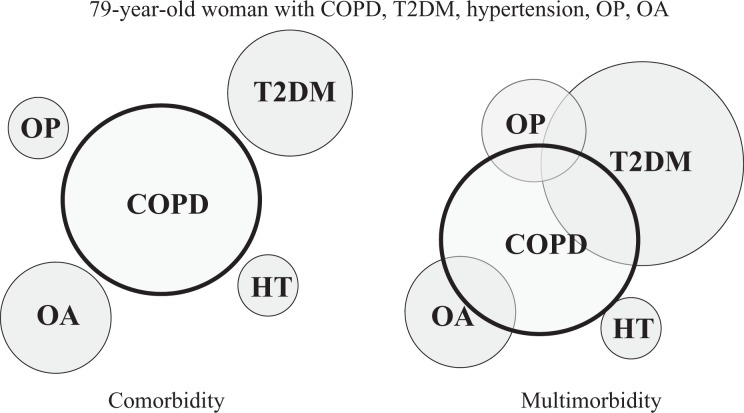
Traditionally, coexisting conditions have been described using the term ‘comorbidity’, defined as the presence of one or more additional disorders co-occurring with a primary disorder, which in this case is COPD. However, such a definition presupposes that one condition is ‘primary’ and remains so over time. More recently, the term multimorbidity has been used to describe the coexistence of two or more chronic conditions in the same individual, without reference to a primary condition.16 The concept of multimorbidity acknowledges that chronic conditions may overlap, may vary in severity and may change in importance or burden over time. A conceptual framework comparing comorbidity and multimorbidity in a typical patient who might present to pulmonary rehabilitation is presented in Figure 1. For this individual at this particular time, COPD is not the dominant problem and there is interaction between coexisting chronic conditions; this is better reflected by a multimorbidity model. Multimorbidity is now the most common chronic condition experienced by adults, affecting almost three in four individuals aged 65 years and older.16 It is heavily influenced by health inequalities, occurring 10–15 years earlier in those who live in more deprived areas compared to those who live in the most affluent areas.17 Individuals with multimorbidity are at greater risk of adverse outcomes and treatment complications than their individual conditions would confer, are more likely to receive ineffective care, have higher health care costs, and have worse survival.18,19
Figure 1.
Concepts of comorbidity and multimorbidity. Comorbidity refers to coexisting chronic conditions, whereas multimorbidity acknowledges that there may not be a ‘dominant’ problem that conditions interact and vary in severity, importance and burden. COPD: chronic obstructive pulmonary disease; HT: hypertension; OA: osteoarthritis; OP: osteoporosis; T2DM: type 2 diabetes mellitus.
Delivery of health care to people with multimorbidity is challenging and not well supported by clinical practice guidelines. This was well illustrated in 2005 in an article that detailed the application of the most recent clinical guidelines to a hypothetical 79-year-old woman with osteoporosis, osteoarthritis, type 2 diabetes mellitus, hypertension and COPD.20 The five relevant disease-specific clinical practice guidelines recommended 12 separate medications, taken in 19 doses on five occasions throughout the day, as well as 14 non-pharmacological activities, some of which were contradictory (e.g. weight-bearing exercise for osteoporosis vs. non-weight bearing exercise for type 2 diabetes with peripheral neuropathy). Such a treatment regimen is unlikely to be safe, effective or efficient, and its burden is unlikely to promote adherence. Only two of the five clinical practice guidelines directly addressed multimorbidity. More recently, qualitative studies have described dispiriting experiences of care for people with multimorbidity. Patients and carers report poor communication with and between health care providers, a lack of care coordination, long wait times for services, difficulty making decisions about health care, being unsure how to prioritize, and feeling alone.21 Family physicians described poor communication and lack of care coordination across services, concerns regarding the ability of patients to adhere to complex treatment regimens, difficulty quantifying the harms and benefits of guideline-directed care, concerns regarding adverse events when following multiple guidelines, unrealistic expectations of patients and families and insufficient time or reimbursement to deal with the complexities of multimorbidity in everyday practice.21,22
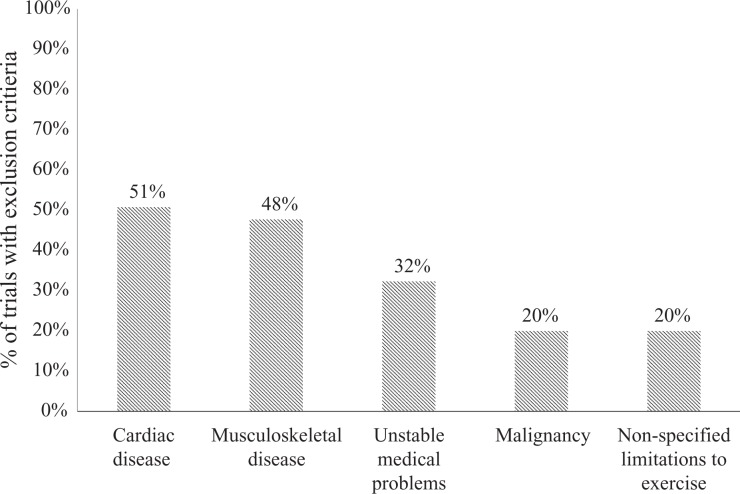
While important efforts are underway to make evidence-based care more accessible to people with multimorbidity,23 current guidelines do not meet the challenges of multimorbidity and, as a result, pulmonary rehabilitation clinicians still face significant challenges. A review of seven recent guidelines relevant to rehabilitation for people with chronic disease24–30 reveals that three guidelines do not mention coexisting conditions, while another three guidelines make only passing mention of minor programme adaptations such as commencing exercise training at low workloads and progressing slowly or being as physically active as possible. The most extensive discussion is in the American Thoracic Society/European Respiratory Society pulmonary rehabilitation statement,24 which suggests additions to existing assessments to improve safety and efficacy (e.g. use of cardiopulmonary exercise test and electrocardiograms, assessment of anxiety and depression); use of specialized equipment, particularly for bariatric patients; and modifications to exercise prescription for those who cannot tolerate the usual training protocols, including consideration of interval training and inspiratory muscle training. Broadening of education for pulmonary rehabilitation providers is suggested, to ensure recognition of relevant signs and symptoms across a broad range of chronic conditions. The authors conclude that further research is needed to better understand the pulmonary rehabilitation outcomes in this group. This recommendation reflects the paucity of clinical trial data in pulmonary rehabilitation for patients with multimorbidity. Analysis of studies included in a recent Cochrane review of pulmonary rehabilitation for COPD31 reveals that of 65 randomized controlled trials, 51% excluded people with cardiac disease and 48% excluded those with musculoskeletal disease (Figure 2), conditions that are present in 43% and 42% of people with COPD, respectively.23 It will remain difficult for clinical guideline developers and clinicians to adequately address multimorbidity, while there is insufficient research to guide their decisions.
Figure 2.
Top five exclusion criteria in randomized controlled trials of pulmonary rehabilitation. Source: Data from McCarthy et al.31
Why pulmonary rehabilitation is well placed to improve outcomes for people with multimorbidity
Providing best care for people with multimorbidity has been described as a shift from disease-focused interventions to patient-focused care:
To align with the clinical reality of multimorbidity, care should evolve from a disease orientation to a patient goal orientation, focused on maximizing the health goals of individual patients with unique sets of risks, conditions, and priorities.16 (p. 2494)
This involves identifying patient and family goals and preferences for care; identifying disease-related and other modifiable barriers to goal achievement, including social and environmental circumstances; understanding and communicating the likely effect of treatments on goal attainment; and facilitating shared decision-making.
In practical terms, best care for people with multimorbidity has the following features:
focussed on optimizing function;
measures patient-centred outcomes;
avoids inappropriate, excessive and non-beneficial care;
ensures integration and coordination across disease conditions;
ensures integration between clinicians and settings of care; and
has coordinated input from multidisciplinary health care teams, assembled to meet each patient’s needs.16,18
These features will be familiar to health professionals in pulmonary rehabilitation. Pulmonary rehabilitation programmes have always had a strong focus on improving function, and measurement of patient-important outcomes is considered essential to best practice care.24,32 Pulmonary rehabilitation programme coordinators frequently assume a role in care coordination, ensuring that members of the multidisciplinary team are appropriately involved to meet the needs of individual patients. Recently, pulmonary rehabilitation has been acknowledged as a core component of integrated care for people with COPD.24,33 However to date, these activities have occurred within a respiratory disease framework. To ensure that we are well equipped to provide patient-centred care to the growing number of individuals with multimorbidity, broader thinking may be required.
A key shift in thinking might be around how we describe, assess and measure outcomes for patients undergoing pulmonary rehabilitation. For patients with COPD, severity of disease is frequently described in terms of forced expiratory volume in 1 second or Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage; assessments and outcome tools are frequently disease specific (e.g. Chronic Respiratory Disease questionnaire and St George’s Respiratory Questionnaire) or may not adequately describe the range of functional impairments experienced by people with multimorbidity (e.g. 6-minute walk test or incremental shuttle walk test). New measurement tools that accurately describe the range of impacts on physical, psychological and social function in people with multimorbidity are needed, to guide patient-centred care. An ideal measurement tool for patients with multimorbidity would be applicable regardless of underlying diagnoses, sensitive to changes with pulmonary rehabilitation, relevant across other settings of care, capture patient-important outcomes and facilitate shared decision-making about best care for the individual.18 The concept of frailty and its associated measurement tools has the potential to provide such a comprehensive assessment for patients with multimorbidity in pulmonary rehabilitation.
What is frailty?
A consensus on the definition of frailty does not currently exist. In the geriatric literature, physical frailty is based on a definition provided by ‘the interventions on frailty working group’ including mobility, strength, balance, motor processing, cognition, nutrition, endurance and physical activity.12 Until recently, frailty had not been considered in individuals with COPD, now two main models appear to exist. Frieds’ five markers of frailty focus on the physiological components of frailty including gait speed, weight loss, exhaustion, grip strength and physical activity.13 Gobbens et al.’s definition of frailty is dynamic and multidimensional, describing frailty as a decline in one or more domains of human function. Specifically, Gobbens et al. noted that frailty also includes psychological and social elements in additional to physical factors.14 Frailty is not dependent on the underlying diagnosis; is common in people with respiratory disorders, especially those with coexisting medical conditions; and describes key elements of function, many of which are addressed by pulmonary rehabilitation. As a result, measures of frailty may be useful to describe, assess and measure outcomes in pulmonary rehabilitation programmes for individuals with comorbidities.
Frailty in COPD
On average, 11% of community-dwelling older persons are classified as frail.34 Although frailty is associated with aging, individuals with COPD have a twofold increase in prevalence of frailty compared to their ‘healthy’ elderly counterparts.35 In individuals with COPD living in the community, the prevalence of frailty has been reported to be 58%.36 As those enrolled in pulmonary rehabilitation (PR) often present with coexisting chronic conditions, the prevalence of frailty may even be higher. In a recent study, over 60% of patients attending a PR programme were reported to exhibit some level of frailty.37 Indeed, a key component of frailty is suggested to be a reduction in exercise capacity and those referred to PR nearly always complain of reduced exercise tolerance.
There are likely a number of factors contributing to the increased prevalence of frailty in individuals with COPD. The high prevalence of multimorbidity is a key factor – coexisting conditions including diabetes, peripheral vascular disease, heart failure and osteoarthritis are also associated with an increased prevalence of frailty.14,38–41 Loss of muscle mass is an important contributor to frailty13 and peripheral muscle weakness is common in those with COPD as a likely consequence of systemic inflammation or the use of corticosteroids.13,42,43 The debilitating nature of COPD also affects an individuals’ ability to remain physically active44 and reductions in physical activity levels have been associated with an increase in frailty prevalence.45 Both peripheral muscle strength and physical activity are further impacted following an acute exacerbation.46–48 Acute exacerbations, defined as an increase in symptoms, become increasingly common as the disease progresses and as such, they are likely to contribute to the process of frailty. Self-reported shortness of breath has been shown to be the greatest predictor of frailty36 and frailty is associated with a reduction in peak oxygen consumption.49
Consequences of frailty
Individuals who are recognized as frail have a marked reduction in activities of daily living (ADLs), increased healthcare utilization36 and are at a greater risk of mortality.50 In fact, frailty increases the risk of long-term (12 years) mortality by 80% in individuals with COPD compared to 34% in those without COPD.50 In addition, findings from a recent qualitative study describe the frustration and fear felt by older, frail people at the prospect of losing their independence, highlighting the impact of frailty on psychological well-being.51
Assessment of frailty
Multicomponent assessment
It is now readily acknowledged that the assessment of frailty needs to include multiple components. Three multicomponent assessments have been applied in COPD including Frieds’ definition of frailty,37,52 the frailty staging system (FSS)50 and the Tilburg frailty indicator.53
Frieds’ five markers of frailty
Frieds’ model focuses on the physical aspect of frailty. The model includes five criteria displayed in Table 1. Individuals who meet two of these five criteria are defined as pre-frail and those who meet three are classified as frail. According to Fried et al., those classified as pre-frail are at an elevated risk for falls, disability, death and hospitalization, but three items had greater predictive power for these adverse outcomes.13
Table 1.
Frieds’ five markers of frailty.
| Item | Type of measure | Criteria |
|---|---|---|
| Weight loss | Direct measurement of weight | Unintentional loss of ≥10 pounds in the previous year. |
| Weakness | Handheld dynamometer | Maximum grip strength (kilograms) of the dominant hand adjusted for gender and BMI. (For example, a male with a BMI of 26.1–28 would require grip strength at least 30 kg to be defined as not frail.) |
| Exhaustion | Two questions taken from the Center for Epidemiological Studies – Depression scale (‘I felt that everything I did was an effort’ and ‘I could not get going’). | A score of 2 or 3 (felt this way for a moderate amount of the time in the last week (3–4 days) or most of the time). |
| Physical activity | Minnesota leisure time activity questionnaire | Kilocalories per week expended are calculated using standardized algorithm and stratified by gender. Men with kilocalories of physical activity per week less than 383 are frail and women with kilocalories per week less than 270 are frail. |
| Slowness (or gait speed) | 15-Foot walk test | Time taken to walk 15 ft adjusting for gender and standing height (For example, a female with height greater than 159 cm would be required to walk 15 ft in 6 seconds or less to be identified as not frail.) |
CES-D: Center for Epidemiological Studies – Depression scale; BMI: body mass index.
The FSS
An alternative measure of frailty is the FSS54 consisting of domains of function: visual function, hearing function, arm and leg function, urinary continence, nutritional status, mental state, depression, ADL, home environment and social support. Individuals are classified as frail or not frail in each domain, a score of one is given when the function is lost. This tool was designed to be a pragmatic assessment of frailty that could be easily applied within the clinical setting and it was designed to be flexible in terms of the targets to be assessed and the manner in which assessment is conducted. Galizia et al.50 applied seven domains of the FSS in individuals with COPD, which are displayed in Table 2. These authors further classified the severity of frailty into mild, moderate and severe. Those who were classified as frail in one domain were considered to be mild, those who were classified as frail in two or three domains were classified as moderate and people who had a loss of function in four or more domains were considered severe. The risk or mortality was highest in those who were classified as severely frail.50
Table 2.
The FSS as applied by Galizia et al.50
| Item | Type of measure | Criteria |
|---|---|---|
| Visual function | Self-report | Could not recognize a friend across the street. |
| Hearing function | Self-report | Need people to raise their voices to hear and understand them. |
| Mobility | Self-report | Having great difficulty or being unable to walk around the house, walk outside, climb stairs or walk half a mile. |
| Urinary function | Self-report | Total incontinence |
| Cognitive function | MMSE | A score less than 24 |
| Disability | BADL | Need assistance with at least one BADL |
| Social support | Social support scale used in an elderly population (Mazella et al. 2010). | A score of 13–17 |
BADL: basic activities of daily living; MMSE: mini mental state examination; FSS: frailty staging system.
The Tilburg frailty indicator
The Tilburg frailty indicator developed by Gobbens et al. includes physical, psychological and social domains.14,53 Physical frailty is assessed via eight questions, four questions ask about psychological well-being and three questions are assigned to the social domain. Individuals can answer yes, sometimes or no and the maximum score able to be obtained is 15. A score of five or greater is indicative of frailty.
Park et al.36 used this framework to assess frailty in individuals with self-reported COPD. Data was taken from the National Health and Nutrition Examination Survey (NHANES) survey and as not all the criteria included in the Tilburg frailty indicator were available (e.g. balance, endurance, mood and coping), frailty was assessed using nine criteria across the three domains (Table 3). Frailty assessed using these nine criteria provided a total frailty score, which was found to demonstrate internal consistency (0.66). A cut-off point of 2 was used to define frailty. Those individuals with COPD identified as being frail had greater disabilities.36
Table 3.
The Tilburg frailty indicator as applied by Park et al.36
| Item | Type of measure | Criteria | |
|---|---|---|---|
| Physical frailty | Nutrition | A direct measure of weight | Unintentional weight loss of more than 10 pounds over the previous year. |
| Mobility | Self-report | Difficulty walking without any special equipment or having at least moderate difficulty walking up 10 steps. | |
| Physical activity | Actigraph (ActiGraph Model 7164 accelerometer, LLC, Ft. Walton Beach, FL, USA) | Less than 85.35 counts/minute. | |
| Strength | Self-report | Some difficulty carrying or lifting something weighing 10 pounds. | |
| Vision | Self-report | Poor vision, even when wearing corrective eyewear. | |
| Hearing | Self-report | Moderate trouble hearing without a hearing aid. | |
| Psychological frailty | Cognition | Self-report | Difficulties in remembering or experiencing periods of confusion |
| Social frailty | Social support | Self-report | Not having anyone to provide emotional support. |
| Social relations | Self-report | Having no close friends. | |
Single-item assessment of frailty
The application of a multicomponent model of frailty can be considered cumbersome, especially in a busy clinical setting and there has been an increasing focus on identifying single-item assessments that may be used as surrogate markers of frailty. To date, the most frequent factors used in the assessment of frailty in older adults include gait speed, physical function and cognition.55 In individuals with COPD, the value of gait speed and physical activity as indicators of frailty have been considered.
Gait speed
Gait speed, defined as the time it takes to walk a short distance, takes very little time and space to assess. It is a component of Frieds’ five factors of frailty and has been associated with a number of important health outcomes in elderly individuals including hospitalizations,56,57 falls58 and mortality.59
Usual gait speed over 4 m (4MGS) provides a global assessment of functional capacity in community-dwelling adults. The test involves walking a 4-m course at ‘usual’ walking speed from a standing start. The test is performed twice without resting between repetitions and the faster time is used to calculate the 4MGS in metres/second.60 A gait speed less than 0.8 m/second is considered ‘slow’ and has been associated with adverse health outcomes.59 The 4MGS has good convergent validity with the 6-minute walk test (6MWT) (r = 0.77–0.82).61 Importantly, 4MGS has been shown to be an indicator of future readmissions in individuals with COPD following an acute exacerbation.62 The 4MGS is responsive to improvements with pulmonary rehabilitation, with the largest effects seen in patients with the slowest gait speed (effect size 1.0) and the evidence of a ceiling effect in those with well-preserved gait speed (effect size 0.2).63
To date, the only measure of gait speed to be directly compared with a multicomponent assessment of frailty in individuals with COPD is 100-foot walk time. One hundred-foot walk time has been applied in a population undergoing pulmonary rehabilitation and was found to be a good indicator of physical frailty assessed using Frieds’ five factors.52 The test was shown to be responsive to pulmonary rehabilitation at 6 weeks with a mean increase of 8.4 m/minute observed, although no additional improvement was detected at week 12.52
Physical activity
A low level of physical activity, assessed using the Minnesota leisure time activity questionnaire, is one of Fried’s five markers for frailty. Recently, Valenza et al. have modified the Fried criterion and reclassified low physical activity in individuals with COPD as <150 minute/week.45 Physical activity levels less than this threshold were identified using the Baecke physical activity questionnaire, which includes items about household activities, sport, and leisure time activities with values less than nine considered to be sedentary. A total physical activity score of 3.54 and 3.88, respectively, for individuals following an acute exacerbation and for those with stable COPD was reported to be predictive of frailty.45
Recently, objectively measured physical activity has been included as part of a multicomponent assessment of frailty; however, a deficit in this domain alone was not indicative of clinical frailty.36 A multicomponent assessment of frailty, including low levels of physical activity defined as less than 85.35 counts/minute assessed using the Actigraph (ActiGraph Model 7164 accelerometer, LLC, Ft. Walton Beach, FL, USA), has had some success in predicting greater healthcare utilization.36
How does frailty impact on pulmonary rehabilitation?
Pulmonary rehabilitation targets many of the components of frailty, including slowness, weakness, fatigue and physical inactivity. To date, there has been little exploration of the utility of frailty measures in pulmonary rehabilitation or whether rehabilitation can alter frailty. One study from the United Kingdom examined 816 individuals with COPD who were assessed for outpatient pulmonary rehabilitation and found that 26% met Fried’s criteria for frailty.64 Those with frailty had twice the odds of programme non-completion compared to their non-frail counterparts (adjusted odds ratio 2.2, 95% confidence interval 1.39 to 3.46). However, individuals with frailty who completed pulmonary rehabilitation (defined as undertaking 50% of planned sessions) had better outcomes than non-frail individuals for exercise performance, subjectively measured physical activity, symptoms and health status. More than 60% of programme completers who were assessed as frail at baseline were no longer frail at the end of pulmonary rehabilitation. A reduction in frailty following rehabilitation was also seen in a smaller study (n = 41); however, results were less consistent, perhaps because of the smaller sample size.52
It is not yet known whether reductions in frailty following pulmonary rehabilitation can be sustained over time or whether these reductions impact on health outcomes such as hospital admission and mortality. It is notable that only 55% of frail individuals were able to complete an outpatient programme; this signals a clear need for new ways to support individuals with complex needs to attain the benefits of pulmonary rehabilitation.
How can pulmonary rehabilitation meet the needs of individuals with multimorbidity in the future?
A patient-focused approach to multimorbidity in pulmonary rehabilitation might have the following features:
Broad inclusion criteria
Admission to a pulmonary rehabilitation programme should be based on symptoms, function limitation and consideration of frailty. Diagnosis provides useful information but should be a secondary criterion when considering eligibility. Coexisting health conditions should not exclude individuals from pulmonary rehabilitation, except where there are concerns regarding the safety of exercise. Referrals and history taking in pulmonary rehabilitation should acknowledge the impact of multimorbidity on patient symptoms, function and presence of frailty.
Goal focused
Patient and family goals and preferences for care must be central to programme design and outcome assessment. Effective goal setting is critical to rehabilitation practice and is not a new concept in pulmonary rehabilitation65 but is even more critical if there is to be an explicit focus on patient-centred care.
Modular approach to rehabilitation content
Pulmonary rehabilitation includes exercise training education and behaviour change24; beyond this, the ideal programme content is not known. In the context of multimorbidity, the content of a patient-centred pulmonary rehabilitation programme will vary. Pulmonary rehabilitation practitioners need to feel comfortable proving rehabilitation for impairments in other systems beyond respiratory, targeting the components of frailty. This is likely to require a sound understanding of rehabilitation for heart failure, cancer and musculoskeletal disorders as well as appropriate structures to provide psychological and social support. Membership of the multidisciplinary team should be determined by the needs of the individual patient rather than ideology or tradition. For instance, the diabetes educator or pain specialist may be a key team member in some cases. Financial and other incentives should be considered to ensure that delivery of pulmonary rehabilitation directly addresses patient goals and measures whether these goals are achieved.
Broader training for pulmonary rehabilitation practitioners
As suggested in the American Thoracic Society (ATS)/European Respiratory Society (ERS) pulmonary rehabilitation statement,24 broader training will be required for health professionals delivering pulmonary rehabilitation. This will ensure that important symptoms of coexisting conditions are recognized and can be adequately addressed. Pulmonary rehabilitation practitioners need an in-depth understanding of the role and nature of rehabilitation across a range of chronic diseases as well as sophisticated skills in adapting the exercise component to address individual patient needs and goals. Training in goal setting will be essential for all new pulmonary rehabilitation practitioners.
Outcome assessment aligned with individual goals and preferences
While respiratory-specific outcomes are excellent for capturing respiratory symptoms such as dyspnoea and cough, other important domains such as fatigue may not be adequately covered. Concepts such as frailty may be useful to understand and measure the impacts of multiple health conditions upon individuals, regardless of underlying diagnoses. The multiple chronic conditions measurement framework18 proposes an individualized measurement framework for people with multimorbidity. As well as measures of health and well-being, other important process measures for the care of people with multimorbidity can be included such as the degree of care coordination, the extent of shared decision-making and the cost of care.
Ensure pulmonary rehabilitation research reflects patient populations
While research includes patients with more diverse characteristics is bigger, messier and more complex, it better reflects the patients who are admitted to pulmonary rehabilitation programmes. Both researchers and funders should explicitly consider multimorbidity when new trials are proposed. Patients who are frail (e.g. with slow gait speeds) should not be excluded from pulmonary rehabilitation trials.
Clinical practice guidelines
Recently, important efforts have been made to highlight important considerations for pulmonary rehabilitation in people with mulitmorbidity;24 however, further advances are needed in this area. Future clinical guidelines should consider multimorbidity early in the document development process, directly address it where possible including statements regarding confidence in treatment effects for common coexisting conditions, acknowledge cost-benefit trade-offs that may influence treatment decisions and outline gaps where future research is needed.
Pulmonary rehabilitation – So what’s in a name?
There is ample evidence that pulmonary rehabilitation is a highly successful intervention, delivering meaningful improvements for patients with respiratory disease, their communities and the health system. We should not give it up in favour of an untested, generic model of rehabilitation. The challenge is not in the name of pulmonary rehabilitation; the challenge is for our model to evolve, building on existing successes to more comprehensively address the needs of people with multimorbidity. This presents an exciting opportunity to place pulmonary rehabilitation at the forefront of person-centred care.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chronic conditions amongst medicare beneficiaries, chart book: 2012 ed Baltimore: Centers for Medicare and Medicaid Services, 2012. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions/downloads/2012chartbook.pdf [Google Scholar]
- 2. Franssen FM, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev 2014; 23: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32: 962–969. [DOI] [PubMed] [Google Scholar]
- 4. Iyer AS, Bhatt SP, Garner JJ, et al. Depression is associated with readmission for acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2016; 3: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrams TE, Vaughan-Sarrazin M, Van der Weg MW. Acute exacerbations of chronic obstructive pulmonary disease and the effect of existing psychiatric comorbidity on subsequent mortality. Psychosomatics 2011; 52: 441–449. [DOI] [PubMed] [Google Scholar]
- 6. Walsh JR, McKeough ZJ, Morris NR, et al. Metabolic disease and participant age are independent predictors of response to pulmonary rehabilitation. J Cardiopulm Rehabil Prev 2013; 33: 249–256. [DOI] [PubMed] [Google Scholar]
- 7. Crisafulli E, Costi S, Luppi F, et al. Role of comorbidities in a cohort of patients with COPD undergoing pulmonary rehabilitation. Thorax 2008; 63: 487–492. [DOI] [PubMed] [Google Scholar]
- 8. Carreiro A, Santos J, Rodrigues F. Impact of comorbidities in pulmonary rehabilitation outcomes in patients with chronic obstructive pulmonary disease. Rev Port Pneumol 2013; 19: 106–113. [DOI] [PubMed] [Google Scholar]
- 9. Mesquita R, Vanfleteren LE, Franssen FM, et al. Objectively identified comorbidities in COPD: impact on pulmonary rehabilitation outcomes. Eur Respir J 2015; 46: 545–548. [DOI] [PubMed] [Google Scholar]
- 10. Vagaggini B, Costa F, Antonelli S, et al. Clinical predictors of the efficacy of a pulmonary rehabilitation programme in patients with COPD. Resp Med 2009; 103: 1224–30. [DOI] [PubMed] [Google Scholar]
- 11. Greening NJ, Evans RA, Williams JE, et al. Does body mass index influence the outcomes of a walking-based pulmonary rehabilitation programme in COPD? Chronic Respir Dis 2012; 9: 99–106. [DOI] [PubMed] [Google Scholar]
- 12. Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc 2004; 52: 625–634. [DOI] [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Medi Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 14. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, et al. Toward a conceptual definition of frail community dwelling older people. Nurs Outlook 2010; 58: 76–86. [DOI] [PubMed] [Google Scholar]
- 15. McDonald VM, Higgins I, Gibson PG. Insight into older peoples’ healthcare experiences with managing COPD, asthma, and asthma-COPD overlap. J Asthma 2013; 50: 497–504. [DOI] [PubMed] [Google Scholar]
- 16. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition – multimorbidity. JAMA 2012; 307: 2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 18. Multiple Chronic Conditions Measurement Framework, 2012. National Quality Forum, www.qualityforum.org (accessed 23 June 2016).
- 19. St John PD, Tyas SL, Menec V, et al. Multimorbidity, disability, and mortality in community-dwelling older adults. Can Fam Physician 2014; 60: e272–e280. [PMC free article] [PubMed] [Google Scholar]
- 20. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294: 716–724. [DOI] [PubMed] [Google Scholar]
- 21. Gill A, Kuluski K, Jaakkimainen L, et al. Where do we go from here? Health system frustrations expressed by patients with multimorbidity, their caregivers and family physicians. Healthc Policy 2014; 9: 73–89. [PMC free article] [PubMed] [Google Scholar]
- 22. Fried TR, Tinetti ME, Iannone L. Primary care clinicians experiences with treatment decision making for older persons with multiple conditions. Arch Intern Med 2011; 171: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson KC, Gould MK, Krishnan JA, et al. An official American thoracic society workshop report. A framework for addressing multimorbidity in clinical practice guidelines for pulmonary disease, critical illness, and sleep disorders. Ann Am Thorac Soc 2016; 13: S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 25. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128: 873–934. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014; 63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes A. Standards of medical care in diabetes – 2014. Diabetes Care 2014; 37(Suppl 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 28. Society for Vascular Surgery Lower Extremity Guidelines Writing G, Conte MS, Pomposelli FB, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015; 61: 2S–41S. [DOI] [PubMed] [Google Scholar]
- 29. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res 2012; 64: 465–474. [DOI] [PubMed] [Google Scholar]
- 30. Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45: 2532–2553. [DOI] [PubMed] [Google Scholar]
- 31. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013; 68 (Suppl 2): ii1–ii30. [DOI] [PubMed] [Google Scholar]
- 33. Wagg K. Unravelling self-management for COPD: what next? Chron Respir Dis 2012; 9: 5–7. [DOI] [PubMed] [Google Scholar]
- 34. Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soci 2012; 60: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 35. Lahousse L, Ziere G, Verlinden VJ, et al. Risk of frailty in elderly With COPD: a population-based study. J Gerontol A, Biol Sci Med Sci. 2016; 71: 689–695. [DOI] [PubMed] [Google Scholar]
- 36. Park SK, Richardson CR, Holleman RG, et al. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006). Heart Lung 2013; 42: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mittal N, Raj R, Islam EA, et al. The frequency of frailty in ambulatory patients with chronic lung diseases. J Prim Care Community Health 2016; 7: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romero-Ortuno R, Walsh CD, Lawlor BA, et al. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr 2010; 10: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sourial N, Wolfson C, Bergman H, et al. A correspondence analysis revealed frailty deficits aggregate and are multidimensional. J Clin Epidemiol 2010; 63: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang SS, Weiss CO, Xue QL, et al. Patterns of comorbid inflammatory diseases in frail older women: the women’s health and aging studies I and II. J Gerontol A Biol Sci Med Sci 2010; 65: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strawbridge WJ, Shema SJ, Balfour JL, et al. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53: S9–S16. [DOI] [PubMed] [Google Scholar]
- 42. Maltais F, LeBlanc P, Jobin J, et al. Peripheral muscle dysfunction in chronic obstructive pulmonary disease. Clin Chest Med 2000; 21: 665–677. [DOI] [PubMed] [Google Scholar]
- 43. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Resp Crit Care Med 1994; 150: 11–60. [DOI] [PubMed] [Google Scholar]
- 44. Gimeno-Santos E, Raste Y, Demeyer H, et al. The proactive instruments to measure physical activity in patients with chronic obstructive pulmonary disease. Eur Respir J 2015; 46: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Valenza MC, Torres-Sanchez I, Cabrera-Martos I, et al. Physical activity as a predictor of absence of frailty in subjects with stable COPD and COPD exacerbation. Respir Care 2016; 61: 212–219. [DOI] [PubMed] [Google Scholar]
- 46. Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58: 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borges RC, Carvalho CR. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD 2012; 9: 596–602. [DOI] [PubMed] [Google Scholar]
- 48. Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129: 536–544. [DOI] [PubMed] [Google Scholar]
- 49. Bastone Ade C, Ferriolli E, Teixeira CP, et al. Aerobic fitness and habitual physical activity in frail and nonfrail community-dwelling elderly. J Phys Act Health 2015; 12: 1304–1311. [DOI] [PubMed] [Google Scholar]
- 50. Galizia G, Cacciatore F, Testa G, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Agin Clin Exp Res 2011; 23: 118–125. [DOI] [PubMed] [Google Scholar]
- 51. Kendall M, Carduff E, Lloyd A, et al. Different experiences and goals in different advanced diseases: comparing serial interviews with patients with cancer, organ failure, or frailty and their family and professional carers. J Pain Symp Manage 2015; 50: 216–224. [DOI] [PubMed] [Google Scholar]
- 52. Mittal N, Raj R, Islam E, et al. Pulmonary rehabilitation improves frailty and gait speed in some ambulatory patients with chronic lung diseases. Southwest Respir Crit Care Chron 2015; 3: 2–10. [Google Scholar]
- 53. Gobbens RJ, van Assen MA, Luijkx KG, et al. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010; 11: 344–355. [DOI] [PubMed] [Google Scholar]
- 54. Lachs MS, Feinstein AR, Cooney LM, et al. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med 1990; 112: 699–706. [DOI] [PubMed] [Google Scholar]
- 55. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011; 59: 2129–2138. [DOI] [PubMed] [Google Scholar]
- 56. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51: 314–322. [DOI] [PubMed] [Google Scholar]
- 57. Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci 2005; 60: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 58. Biderman A, Cwikel J, Fried AV, et al. Depression and falls among community dwelling elderly people: a search for common risk factors. J Epidemiol Community Health 2002; 56: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kon SS, Patel MS, Canavan JL, et al. Reliability and validity of 4-metre gait speed in COPD. Eur Respir J 2013; 42: 333–340. [DOI] [PubMed] [Google Scholar]
- 61. Karpman C, Benzo R. Gait speed as a measure of functional status in COPD patients. Int J Chron Obstruct Pulmon Dis 2014; 9: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kon SS, Jones SE, Schofield SJ, et al. Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax 2015; 70: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 63. Kon SS, Canavan JL, Nolan CM, et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J 2014; 43: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 64. Maddocks M, Kon SSC, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; 71(11): 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garrod R, Marshall J, Jones F. Self efficacy measurement and goal attainment after pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis 2008; 3: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]