Abstract
Study Objectives:
Several studies have reported an association between obstructive sleep apnea (OSA) and several extra-pulmonary issues, such as arterial hypertension and insulin resistance. In recent years, the associations between OSA, non-alcoholic fatty liver disease, and non-alcoholic steatohepatitis (NASH) have been published; however, there is a gap between experimental and clinical studies regarding the efficacy of continuous positive airway pressure (CPAP) treatment in patient populations with these conditions. This issue should be considered when deciding on CPAP treatment in patients with OSA, especially in patients with moderate OSA.
Methods:
We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) using the following databases: MEDLINE, Lilacs, and CENTRAL. Two independent reviewers performed the search, analysis, data extraction, and critical analysis.
Results:
From 622 identified studies, we included 5 RCTs that involved patients with OSA and NASH and who were treated with a CPAP device. After CPAP treatment, no changes in liver steatosis, liver fibrosis, and aminotransferase levels (alanine aminotransferase and aspartate aminotransferase) were found. Finally, the quality of evidence using the GRADE approach was low and very low for several outcomes.
Conclusions:
According to the current analysis, no data regarding the efficacy of CPAP in patients with NASH are available to make recommendations.
Systematic Review Registration:
PROSPERO; ID: CRD42015027981; URL: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42015027981
Citation:
Labarca G, Cruz R, Jorquera J. Continuous positive airway pressure in patients with obstructive sleep apnea and non-alcoholic steatohepatitis: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14(1):133–139.
Keywords: liver, non-alcoholic fatty liver disease, obstructive sleep apnea, transaminases
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic disease characterized by chronic intermittent hypoxia (CIH) due to an obstruction in the upper airway. OSA severity varies from mild, defined by an apnea-hypopnea index (AHI) of 5 to 15 events/h, to moderate (15 to 30 events/h) and severe (> 30 events/h).1,2
This disease is widespread; studies report a prevalence of 2% to 9% for women and 4% to 24% for men. The prevalence increases up to 40% in obese patients (body mass index of more than 30 kg/m2).3,4
New epidemiological studies suggest that there is a rise in the prevalence of OSA. According to the model created by the Wisconsin Sleep Cohort Study, OSA prevalence (defined as AHI ≥ 5) was estimated at 33.9% (range: 26.6% to 43.2%) for men and 17.4% (range: 8.7% to 27.8%) for women.5 However, prevalence is different based on race and world region. Chen et al. reported the prevalence of OSA in different racial/ethnic groups in the United States, and they found that Hispanic groups were reported to have an odds ratio (OR) of 2.14 (confidence interval [CI]: 1.40–2.47) compared to Caucasian groups.6
Epidemiological studies in Hispanic patients with OSA reported a prevalence of 25.8% for mild OSA.7 In Sao Paulo, Brazil, the prevalence is 32.8% (range: 29.6% to 36.3%), and risk is higher in men than women, OR = 4.1 (CI: 2.9–5.8). OSA risk is high in the obese population, OR = 10.5 (CI: 7.1–15.7), compared to normal weight. Age is another risk factor for the development of OSA, with an OR = 34.5 (CI: 18.5–64.2) for patients aged 60 to 89 years.8 Finally, in Chile, the estimated prevalence according to the Chilean national health survey is 22.9% (CI: 18.4–28.2) in people older than 65 years.9
OSA has been linked to several conditions in Latinos.10,11 Typical symptoms include a decrease in cognitive function, a decrease in quality of life scores, and daytime sleepiness. Respiratory involvement includes an increased risk of pulmonary hypertension and obesity hypoventilation syndrome and a decrease in pulmonary function test outcomes.12 In recent years, additional extra-pulmonary conditions associated with OSA have been identified. The most common conditions are arterial hypertension, heart failure, acute coronary syndrome, atrial fibrillation, a high risk of the development of diabetes mellitus, dyslipidemia, and increased cardiovascular mortality.13,14
Current evidence from laboratory studies suggests that CIH increases molecular cytokine levels and endothelial dys-function and increases oxidative stress.14 This fact could be linked to an increased risk of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).15,16 Observational studies and systematic reviews include studies linking OSA to NAFLD with an OR of 2.01 (CI: 1.36–2.97; P < .05) based on histology and an OR of 2.99 (CI: 1.79–4.99; P < .05) based on radiology. The same studies report an OR of 2.37 (CI: 1.59–3.51; P < .05) for NASH and 2.16 (CI: 1.45–3.20; P < .05) for hepatic fibrosis observed via histology.17
OSA treatment includes several medical and non-medical interventions. For all patients with OSA, improvements in sleep habits, physical activity, and weight-reduction therapies are suggested.18 Medical treatment includes the use of continuous positive airway pressure (CPAP) devices; CPAP involves keeping the upper airway open, thus preventing complete and partial occlusion and eliminating obstructive events. The American College of Physicians recommends CPAP treatment as an initial therapy for patients in whom severe OSA (AHI > 30 events/h) and moderate OSA (AHI 15–30 events/h) was diagnosed (GRADE: strong recommendation; moderate-quality evidence). CPAP treatment is also recommended in patients with mild OSA and increased cardiovascular risk, symptoms, and comorbidities.4,12 This intervention improves symptoms and decreases cardiovascular risk and mortality.19,20 Due to a lack of available data, NAFLD is not an indication to treat patients with OSA.2,21
According to the available data, we hypothesize that patients with OSA and NASH treated with CPAP should show improvements in their liver histology and liver enzyme function, especially those patients with severe or moderate OSA. The aim of this systematic review is to evaluate the efficacy of CPAP treatment for OSA in patients with NASH.
METHODS
Search Strategy
The full protocol of this systematic review and meta-analysis is available in PROSPERO recorded under the registry ID: CDR42015027981. A literature search was performed by two independent reviewers (GL and RC) in July 2015, and an updated search was performed in December 2016. This systematic review was performed according to the recommendations published in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.22
The search strategy using MEDLINE (MeSH) criteria is available in the supplemental material. The following databases were included: MEDLINE, Lilacs, ClinicalTrials.gov, and CENTRAL. We also performed specific meta-searches in TripDatabase and Epistemonikos.23 Potential studies were evaluated without language restrictions, and corresponding authors were contacted via personal communication by the principal author to obtain data concerning changes in transaminases or liver change assessment. We also performed a manual search of the references and included conference abstracts from the European Respiratory Society, American Thoracic Society, American College of Chest Physicians, and Associated Professional Sleep Societies from 2010 to 2015. Finally, disagreements between reviewers were discussed and resolved via discussion. If disagreement continued, we consulted a third reviewer to resolve disagreements.
Inclusion Criteria
Potential studies were included according to the following criteria. (1) Patients were older than 18 years, had confirmed diagnoses of OSA and NASH, and OSA was diagnosed by a respiratory polygraph or polysomnography showing an AHI of more than 5 events/h. For NASH diagnoses, patient must show abnormal aminotransferase levels (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) in association with proven hepatic steatosis via a radiological study (hepatic ultrasound, computed tomography, or magnetic resonance imaging of the liver) or a histological biopsy, and other liver diseases must be ruled out (eg, infections, inflammatory diseases, alcohol-related diseases). (2) We restricted this review to randomized controlled trials (RCTs) comparing patients with CPAP treatment versus no treatment or sham CPAP and a follow-up of 4 weeks or more with changes in liver enzymes (aminotransferases) and changes in liver structure determined via biopsy or changes in liver steatosis and liver fibrosis shown by liver elastography (FibroTest, SteatoTest, NASH test).24
Quality Assessment
The included studies were evaluated by two independent reviewers (JJ and GL) using the quality assessment method reported by the Cochrane collaboration for systematic reviews of intervention studies.25 Disagreements between reviewers were resolved via discussion to reach a consensus.
Data Synthesis
Data extraction and synthesis were performed by two independent reviewers (GL and JJ), and the data were tabulated using an Excel database (Microsoft, Redmond, Washington, United States). Disagreements were resolved via discussion.
We defined an improvement in liver structure as the primary outcome, as defined by histological changes shown in post-CPAP treatment liver biopsies according to histological criteria previously reported by Bell et al.26
Secondary outcomes were changes in aminotransferase levels (AST or ALT) reported as the mean differences in units/ liter (U/L). We also explored differences between treatment efficacy and OSA severity (mild/moderate/severe).
We described the data from the included studies, and for outcomes with enough data (more than two studies), we performed a meta-analysis using a randomized-effects model with the DerSimonian and Laird method following the intention-to-treat principle. The data were analyzed using the Cochrane Review Manager software (RevMan) version 5.3 (Cochrane, London, United Kingdom).
Heterogeneity was evaluated using a visual inspection of a forest plot and using Q statistics and chi-square tests with I2 tests. We considered an I2 of more than 50% as representing high heterogeneity. A value of P < .05 was considered statistically significant. Finally, publication bias was evaluated using visual inspection of a funnel plot.
For the quantitative meta-analysis, we used mean difference (MD) and standard deviation; for qualitative data, we used relative ratio (RR). All analyses included 95% CIs, with values of P < .05 indicating significance.
Summaries of results and evidence grading were performed using the GRADE method. We evaluated the quality of evidence, with downgrading or upgrading performed according to the following: risk of bias, indirectness, imprecision, inconsistency and publication bias. A table summarizing the findings was created using GRADEpro software (Evidence Prime Inc., Ontario, Canada).27
RESULTS
Summary of Results
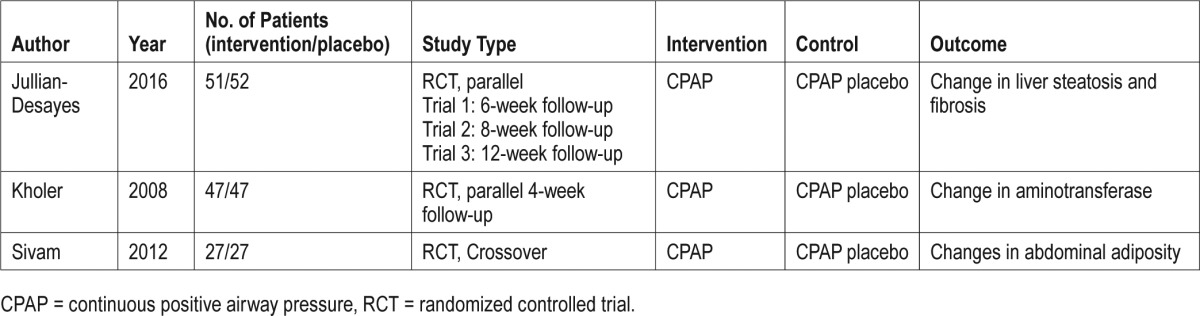
A total of 529 studies were identified from different sources. Ultimately, 3 of the studies (including a total of 5 RCTs) were suitable for inclusion in our review.28–30 Jullian-Desayes et al. reported 3 RCTs in the same manuscript.29 A total of 4 studies were excluded from the analysis31–34; all of the excluded studies were observational and did not report on a specific NASH population. The summary of the literature search and PRISMA flow chart is shown in Figure 1, and characteristics from the included and excluded studies are reported in Table 1 and Table S1 in the supplemental material.
Figure 1. PRISMA Study flow diagram.
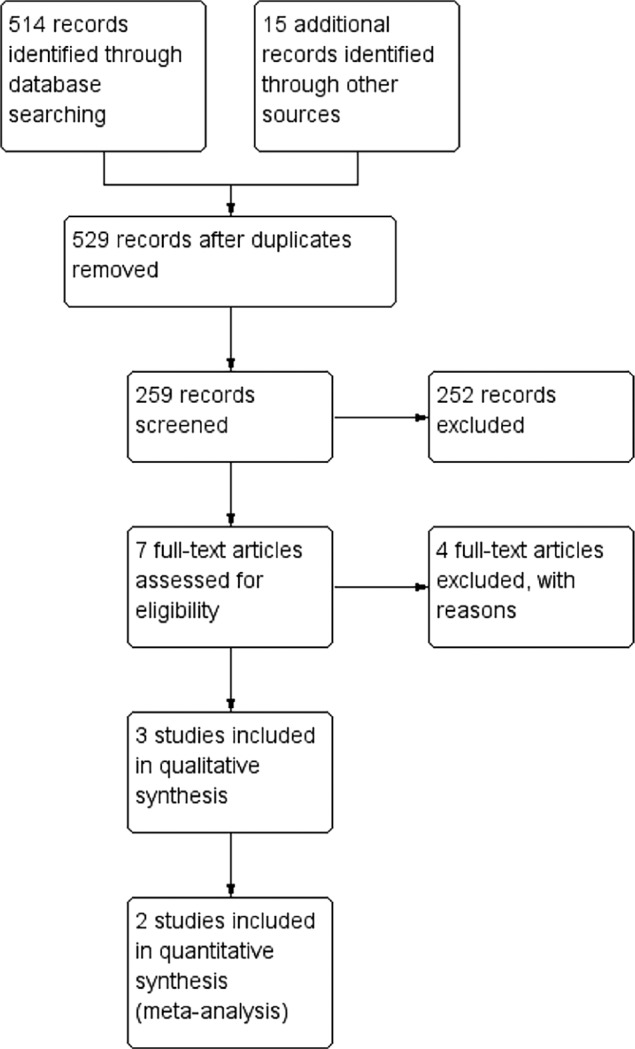
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Table 1.
Characteristic of included studies.
We found a high risk of bias regarding the blinding of outcome assessments and incomplete outcome data and an unclear risk of bias regarding allocation concealment and blinding of participants and personnel. A full quality assessment for the included studies using the Cochrane approach is shown in Figure 2.
Figure 2. Risk of bias summary: judgements about each risk of bias item for each included study.
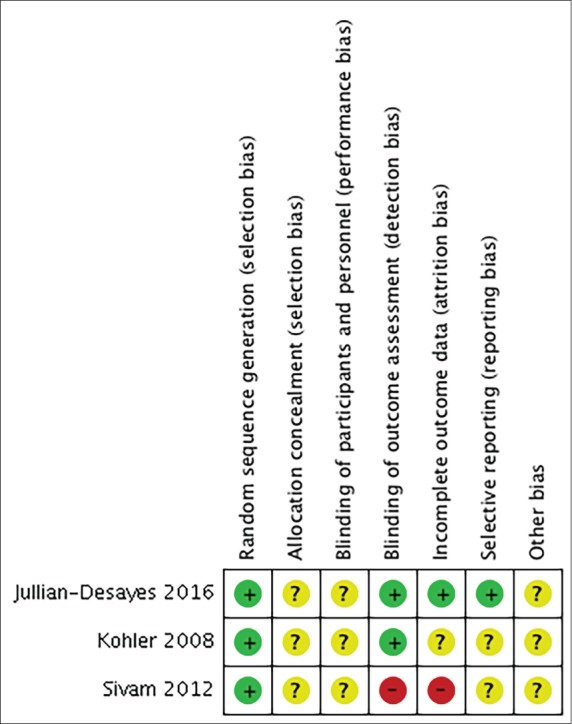
Green (+) = low risk of bias, yellow (?) = unclear risk of bias, red (-) = high risk of bias.
Intervention Effect
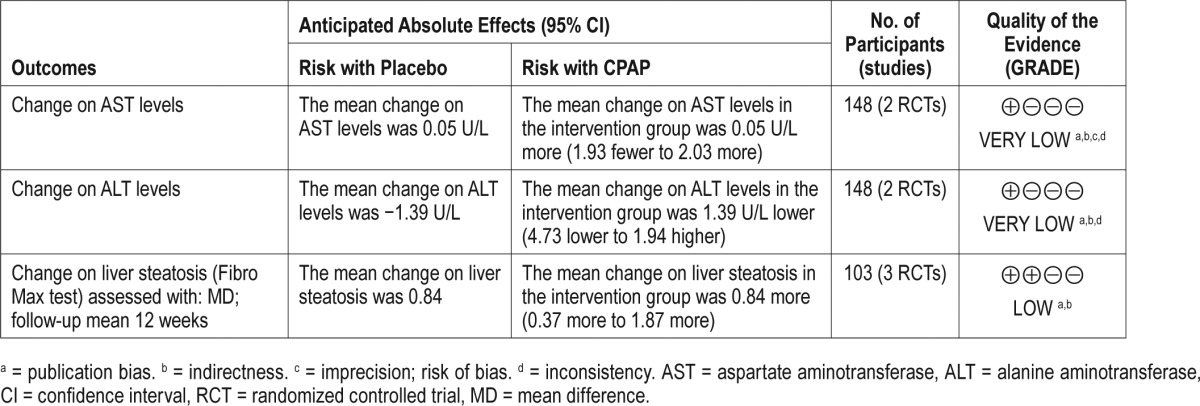
The summary of findings and evidence of gradings are reported in Table 2. The main reasons to downgrade the evidence were as follows: risk of bias from primary studies, presence of indirectness, and publication bias. Funnel plots of comparisons included in the current meta-analysis are shown in Figure S1 and Figure S2 in the supplemental material.
Table 2.
Summary of finding table using the GRADE approach.
Primary Outcome
We found no data regarding histological improvements or changes in liver biopsy results; none of the included studies evaluated this outcome.
Secondary Outcome
Hepatic Steatosis
Data regarding improvements in liver steatosis were measured using radiological approaches, and 3 studies evaluated hepatic steatosis via hepatic elastography using the FibroTest, SteatoTest, and NASH test. Fifty-one patients were allocated to CPAP intervention groups, and 52 patients were allocated to sham-CPAP groups. After 6–12 weeks of follow-up and following an intention to treat analysis, CPAP treatment resulted in SteatoTest outcomes of ≥ S2 with an MD of 0.84 (CI: 0.37–1.87; P value not significant); for the NASHTest, the changes in N1 or N2 showed an MD of 0.050 (CI: –0.022, 0.122; P value not significant); and the changes for the FibroTest (≥ F0) had an MD of 88.6 (CI: 66.5–118.0; P value not significant). We rated this evidence as low because of indirectness and risk of bias.
Aminotransferase Levels
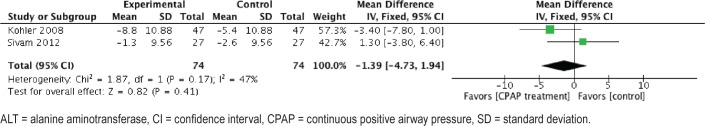
We found 2 studies evaluating the efficacy of CPAP in patients with OSA and NAFLD. Changes in ALT and AST levels post-CPAP treatment were reported by Kohler et al. and Sivam et al.28,30 For ALT levels, the meta-analysis included 74 patients in the intervention group and 74 in the sham-CPAP group. After CPAP treatment, no difference in ALT levels was found, with an MD of −1.39 U/L (CI: −4.73, 1.67; I2 = 0%; P < .05) (Figure 3). We rated this evidence as very low because of publication bias and indirectness.
Figure 3. Forest plot of change on ALT levels after CPAP treatment.
ALT = alanine aminotransferase, CI = confidence interval, CPAP = continuous positive airway pressure, SD = standard deviation.
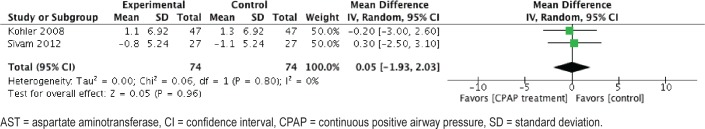
Regarding the changes in AST levels post-CPAP treatment, the meta-analysis included 74 patients in the intervention group and 74 in the sham-CPAP group. After CPAP treatment, no differences in AST levels were found with a 0.05 U/L (CI: −1.93, 2.03; I2 = 47%; P < .05) (Figure 4). We rated this evidence as very low because of publication bias, imprecision, and indirectness.
Figure 4. Forest plot of change on AST levels after CPAP treatment.
AST = aspartate aminotransferase, CI = confidence interval, CPAP = continuous positive airway pressure, SD = standard deviation.
DISCUSSION
The effects of CPAP treatment in patients with OSA have been evaluated and reported in several RCTs and meta-analyses.12,18,20 CPAP treatment has shown positive effects in decreasing apnea severity, CIH, and several inflammatory markers. Clinically, patients treated with CPAP experience improved cognitive function tests, level of sleepiness, and quality of life.18 Secondary effects included extra-pulmonary involvement. Current evidence confirms this fact, showing decreased cardiovascular risks and cardiovascular mortality, slight improvements in blood pressure, increased cardiac output, and other clinically relevant effects.12 Based on these data, current guidelines recommend the use of CPAP for patients with severe or moderate OSA and a high cardiovascular risk.18 Other clinical conditions linked to OSA and metabolic disorders described in previous years are type 2 diabetes mellitus and NAFLD or NASH. These conditions are not current indications for CPAP treatment.18
According to our systematic review and meta-analysis, data regarding NASH in patients with OSA were insufficient to obtain a recommendation regarding the efficacy and safety of CPAP treatment, and we are unable to report potential conditions related to the use of CPAP treatment in patients with OSA, especially those with moderate OSA.
We found several methodological biases in the design of these studies; first, researchers must define a gold standard for liver improvement in patients with NASH. The study from Bell et al. evaluated the histological changes after the intervention (vitamin E) and included follow-up with liver biopsies showing histopathological changes26; this procedure is associated with a high risk of complications. Pathological analysis is the gold standard for the diagnosis of NASH; however, in recent years, several noninvasive approaches have been published, such as hepatic elastography.24,35 This method is highly sensitive and should be used to evaluate progression from steatohepatitis to cirrhosis or changes in liver steatosis. In our review, we found 2 RCTs evaluating changes in those methods; however, none of those studies reported liver biopsies with histological changes in patients with NASH, and no evidence regarding steatohepatitis (abnormal aminotransferase levels) relative to baseline values was found. We believe that the populations included in these studies represented patients with NAFLD and not NASH.29
Second, we did not find changes in surrogate outcomes, such as aminotransferase levels, in the meta-analysis by Chen et al., which included data from patients with OSA and NAFLD or NASH from randomized and observational studies. They performed the meta-analysis with all included studies and reported an improvement in aminotransferase levels after CPAP treatment in their conclusion.36 According to the Cochrane handbook of systematic reviews of intervention, this approach was wrong because of different study designs having different inclusion criteria and methodological approaches. Other biases in this study were found regarding the quality assessment. No data about the risk of bias from the RCTs and observational studies were reported,25,37 likely resulting in incorrect conclusions and applications of these results.
Third, the efficacy of CPAP therapy depends on some factors, such as adherence to CPAP treatment. It is well known that CPAP effect is “dose-dependent,” and adherence levels > 4 h/night are associated with better outcomes. When analyzing CPAP efficacy by using OSA severity, the treatment is more effective for severe OSA. In our study, due to the lack of data reported in primary studies and the limited number of RCTs, we were unable to evaluate these issues in a subgroup analysis or meta-regression.
Finally, the maximum duration of CPAP therapy in the studies was 12 weeks. This duration may not be long enough to result in hepatic changes, and this point should have been presented in the discussion section of these studies. Multiple studies have examined the efficacy and duration of different treatments in patients with NAFLD or NASH. According to pharmacological studies, a follow-up comprising 6 to 12 months of active treatment is reasonable to obtain an accurate assessment.38 These facts have been evaluated in several pharmacologic and non-pharmacologic studies and in systematic reviews.39 For example, in studies that evaluate the efficacy of lifestyle modification,40 surgical intervention such as bariatric surgery in patients with NASH includes more than 6 months of follow-up.41 Finally, in a Cochrane systematic review of pharmacological intervention in NAFLD, follow-up ranged between 1 to 24 months.42
Despite the data reported in our systematic review and meta-analysis, further research about CPAP treatment in patients with OSA and NASH is needed to improve the current evidence.
This systematic review was developed according to the Cochrane collaboration approach and was thorough in several aspects.25 Regarding the limitations of this study, this systematic review did not include subgroup analyses such as an analysis by OSA severity. Another limitation is the difference in follow-up among the primary studies. These issues are secondary to the limited number of trials and the risk of bias of each trial. However, we think this review presents the main evidence available regarding the use of CPAP treatment in patients with OSA and NASH, and we found no comparable published reviews or published studies directly on this topic in the PROSPERO database. Evidence was ranked as low according to the GRADE approach; the main reasons to downgrade the evidence were the number of primary studies and publication bias. In addition, some outcomes were reported only in one small study.
CONCLUSIONS
No data regarding the efficacy of CPAP in patients with NASH are available to make treatment recommendations. CPAP treatment does not improve liver histology or surrogate outcomes, such as aminotransferase levels, and should not be considered as an alternative indicator for patients with OSA. However, the body of evidence supporting this conclusion is weak, and further research and well-designed RCTs are needed.
DISCLOSURE STATEMENT
All authors have read and approved the final manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Gabriel Labarca: Principal investigator, conception and design of the study, acquisition of data, data analysis, preparation of the manuscript, and final proof read. Technical support, register in PROSPERO. Dr. Rodrigo Cruz (RC): Data analysis, critical analysis, manuscript correction, final proof read, and gastroenterology supervisor. Dr. Jorge Jorquera (JJ): Data analysis, critical analysis, manuscript correction, final proof read, and respiratory medicine and sleep medicine supervisor.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- CPAP
continuous positive airway pressure
- MD
mean difference
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OR
odds ratio
- OSA
obstructive sleep apnea
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- RCT
randomized controlled trial
- RR
relative ratio
REFERENCES
- 1.Qaseem A, Dallas P, Owens DK, et al. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(3):210–220. doi: 10.7326/M12-3187. [DOI] [PubMed] [Google Scholar]
- 2.Lloberes P, Duran-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47(3):143–156. doi: 10.1016/j.arbres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;18(3):335–344. doi: 10.1164/rccm.201309-1735OC. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Carrillo J, Vargas C, Cisternas A, Olivares-Tirado P. Obstructive sleep apnea: findings from the Chilean National Health Survey 2010. Poster pressented at: European Respiratory Society ERS International Congress 2017; September 9-13, 2017; Milan, Italy. Available at: http://www.ers-education.org/events/international-congress/milan-2017.aspx?idParent=183717. [Google Scholar]
- 10.Ramos AR, Tarraf W, Rundek T, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology. 2015;84(4):391–398. doi: 10.1212/WNL.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chopra A, Jung M, Kaplan RC, et al. Sleep apnea is associated with hearing impairment: the Hispanic Community Health Study/Study of Latinos. J Clin Sleep Med. 2016;12(5):719–726. doi: 10.5664/jcsm.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labarca G, Cruz NR, Descalzi F. [Multisystemic involvement in obstructive sleep apnea] Rev Med Chil. 2014;142(6):748–757. doi: 10.4067/S0034-98872014000600009. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 14.Vicente E, Marin JM, Carrizo SJ, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J. 2016;48(4):1108–1117. doi: 10.1183/13993003.00234-2016. [DOI] [PubMed] [Google Scholar]
- 15.Kheirandish-Gozal L, Sans Capdevila O, Kheirandish E, Gozal D. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest. 2008;133(1):92–99. doi: 10.1378/chest.07-0773. [DOI] [PubMed] [Google Scholar]
- 16.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring) 2011;19(11):2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14(5):417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, Shekelle P. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(7):471–483. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 19.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Nadeem R, Loomba RS, Nida M, Vieira D. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep-disordered breathing and heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol. 2014;37(1):57–65. doi: 10.1002/clc.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rada G, Pérez D, Capurro D. Epistemonikos: a free, relational, collaborative, multilingual database of health evidence. Stud Health Technol Inform. 2013;192:486–490. [PubMed] [Google Scholar]
- 24.Lupsor-Platon M, Badea R, Gersak M, et al. Noninvasive assessment of liver diseases using 2D shear wave elastography. J Gastrointestin Liver Dis. 2016;25(4):525–532. doi: 10.15403/jgld.2014.1121.254.lup. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available from www.handbook.cochrane.org.
- 26.Bell LN, Wang J, Muralidharan S, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56(4):1311–1318. doi: 10.1002/hep.25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78(2):141–146. doi: 10.1159/000170785. [DOI] [PubMed] [Google Scholar]
- 29.Jullian-Desayes I, Tamisier R, Zarski JP, et al. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: data from randomized trials. Respirology. 2016;21(2):378–385. doi: 10.1111/resp.12672. [DOI] [PubMed] [Google Scholar]
- 30.Sivam S, Phillips CL, Trenell MI, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40(4):913–918. doi: 10.1183/09031936.00177011. [DOI] [PubMed] [Google Scholar]
- 31.Chin K, Nakamura T, Takahashi K, et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med. 2003;114(5):370–376. doi: 10.1016/s0002-9343(02)01570-x. [DOI] [PubMed] [Google Scholar]
- 32.Cifci N, Uyar M, Elbek O, Suyur H, Ekinci E. Impact of CPAP treatment on cardiac biomarkers and pro-BNP in obstructive sleep apnea syndrome. Sleep Breath. 2010;14(3):241–244. doi: 10.1007/s11325-009-0306-y. [DOI] [PubMed] [Google Scholar]
- 33.Shpirer I, Copel L, Broide E, Elizur A. Continuous positive airway pressure improves sleep apnea associated fatty liver. Lung. 2010;188(4):301–307. doi: 10.1007/s00408-009-9219-6. [DOI] [PubMed] [Google Scholar]
- 34.Buttacavoli M, Gruttad'Auria CI, Olivo M, et al. Liver steatosis and fibrosis in OSA patients after long-term CPAP treatment: a preliminary ultrasound study. Ultrasound Med Biol. 2016;42(1):104–109. doi: 10.1016/j.ultrasmedbio.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(23):7392–7402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen LD, Lin L, Zhang LJ, et al. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: a meta-analysis. Clin Respir J. 2016 Sep 10; doi: 10.1111/crj.12554. [Epub ahead of print]. doi: 10.1111/crj.12554. [DOI] [PubMed] [Google Scholar]
- 37.Sterne JA, Hernán MA, Reeves BC, et al. The Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) assessment tool. [Accessed March 10, 2016]. http://www.riskofbias.info. Published August 2016.
- 38.Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1183–1195. doi: 10.1016/j.metabol.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Sawangjit R, Chongmelaxme B, Phisalprapa P, et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): a PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 2016;95(32):e4529. doi: 10.1097/MD.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Nostedt JJ, Switzer NJ, Gill RS, et al. The effect of bariatric surgery on the spectrum of fatty liver disease. Can J Gastroenterol Hepatol. 2016;2016:2059245. doi: 10.1155/2016/2059245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lombardi R, Onali S, Thorburn D, Davidson BR, Gurusamy KS, Tsochatzis E. Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011640. doi: 10.1002/14651858.CD011640.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.