Abstract
Objective
To determine the association between use of vaginal estrogen and risk of a global index event [GIE], defined as time to first occurrence of coronary heart disease (CHD), invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause.
Methods
For this prospective observational cohort study, we used data from participants of the Women's Health Initiative Observational Study who were recruited at 40 U.S. Clinical Centers, aged 50-79 years at baseline, and did not use systemic estrogen therapy during follow-up (n = 45,663, median follow-up 7.2 years). We collected data regarding incident CHD, invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, death, and self-reported use of vaginal estrogen (cream, tablet). We used Cox proportional hazards regression models to adjust for covariates.
Results
Among women with an intact uterus, the risks of stroke, invasive breast cancer, colorectal cancer, endometrial cancer, and pulmonary embolism/deep vein thrombosis were not significantly different between vaginal estrogen users and nonusers, while the risks of CHD, fracture, all-cause mortality, and GIE were lower in users than nonusers (GIE adjusted hazard ratio [aHR] 0.68; 95% confidence interval [CI] 0.55-0.86). Among hysterectomized women, the risks of each of the individual GIE components and of the overall GIE were not significantly different in users versus nonusers of vaginal estrogen (GIE aHR=0.94; 95% CI 0.70-1.26).
Conclusions
The risks of cardiovascular disease and cancer were not elevated among postmenopausal women using vaginal estrogens, providing reassurance about the safety of treatment.
Keywords: Breast cancer, Endometrial cancer, Cardiovascular diseases, Vaginal estrogen, Vaginal estradiol, Venous thromboembolism, Genitourinary syndrome of menopause, hormone therapy, pulmonary embolism, stroke, coronary heart disease
Introduction
The genitourinary syndrome of menopause encompasses a constellation of symptoms, including: genital symptoms of dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or pain, and impaired function; and urinary symptoms of urgency, dysuria and recurrent urinary tract infections.1 It is highly prevalent, occurring in 20-45% of menopausal women.1-3 Genital atrophic changes may have detrimental effects on a woman's quality of life.3, 4 Several clinical guidelines advocate local low-dose vaginal estrogen therapy, instead of systemic estrogen therapy, for the treatment of postmenopausal women with only vaginal symptoms (i.e. without hot flashes).1, 2, 5 Several formulations of low-dose vaginal estrogen therapy are available for treatment of the genitourinary syndrome of menopause, including topical creams, an intravaginal insert, and an intravaginal ring. In a recent Cochrane review, all appear to have similar efficacy and safety.6
The use of some formulations of low-dose vaginal estrogens acutely increases serum estrogen levels 7, 8, but maintenance levels remain in the normal postmenopausal range.9 Randomized trials have shown that systemic estrogen is linked to increased risks of venous thromboembolism, stroke, and (with combination estrogen + progestogen) invasive breast cancer.10-12 Despite the selection and confounding bias that apply to observational studies, these adverse health risks of systemic estrogen have been demonstrated in observational studies.13-17 Therefore, large prospective observational studies might demonstrate whether vaginal estrogen use confers similar risks. However, it is unknown whether low-dose vaginal estrogen confers similar risks. Potential systemic effects of vaginal estrogen are included as warnings on the package labeling for vaginal estrogen preparations. However, randomized controlled trials of vaginal estrogen therapy were not designed to examine major endpoints such as heart attack, stroke, pulmonary embolism, deep vein thrombosis, cancer, or hip fracture. Several observational studies have examined breast cancer and fracture risk in relation to vaginal estrogen therapy. Specifically, a Finnish observational study found that the use of vaginal estradiol was not associated with increased risk of breast cancer (although oral and transdermal estradiol use were associated with increased risk) 18, and a Swedish case-control study suggested that current users of low-potency vaginal estrogen had lower hip fracture risk compared with never-users of estrogen.19 In the recent American College of Obstetricians and Gynecologists Committee Opinion regarding route of estrogen administration and deep vein thrombosis, the conclusion was that vaginal estrogens (in contrast to oral systemic estrogens) are not associated with increased risk of venous thromboembolism20, but observational studies are lacking.9 Observational studies of associations of vaginal estradiol use with risk of other types of cardiovascular disease (CVD) and overall mortality are similarly lacking, though one study without a placebo comparison group reported decreased risk of coronary heart disease and stroke death in vaginal estrogen users compared with the background population.21 Long-term studies regarding endometrial safety of vaginal estrogen use are also needed6, 9; one large Danish registry study that did not exclude users of systemic hormone therapy found a two-fold increased endometrial cancer risk among users of vaginal estrogen.22 However, in our review of published studies, the overall balance of risks and benefits with the use of vaginal estrogens has not been studied in detail, even in observational studies.
The genitourinary syndrome of menopause does not improve without therapy and long duration of vaginal therapy is usually necessary.3 The high prevalence of the genitourinary syndrome of menopause and its adverse impact on sexual function and quality of life for postmenopausal women underscores the clinical relevance of assessing the safety and efficacy of local low-dose vaginal estrogen use. The Women's Health Initiative Observational Study (WHI-OS) offers an opportunity to examine the longitudinal data regarding associations between vaginal estrogen use and cardiovascular, cancer, and other health outcomes among a large cohort of U.S. women reporting information regarding vaginal estrogen therapy use and important covariates.
Methods
Study Population and Design
The Women's Health Initiative Observational Study (WHI-OS), conducted from 1993-2005, enrolled 93,676 postmenopausal women aged 50-79 years at one of 40 clinical centers nationwide to examine risk factors and biomarkers for disease in a large prospective cohort. Details of the inclusion and exclusion criteria and the recruitment process were previously described.23, 24 Inclusion criteria were age between 50 and 79 years and intention to maintain residence in the same geographic area for at least 3 years. Exclusion criteria included medical conditions conferring a predicted survival of less than 3 years, or conditions (e.g. alcohol or drug dependency, dementia, or medical illness) that would be expected to interfere with retention. Institutional review board approval was obtained at each clinical center. Each participant provided written informed consent. After the main WHI-OS ended, the WHI Extension Study (WHI Extension I, Ext 1) collected additional follow-up data from 2005-2010, reflecting 7.2 years of median follow-up.
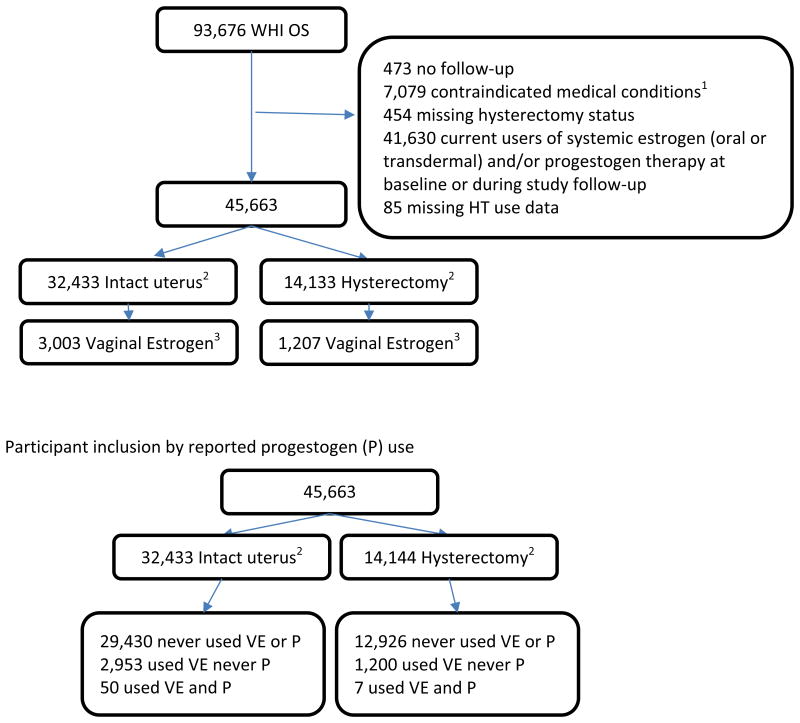
For the current analyses, of the 93,676 WHI-OS participants, we excluded data from women with prior breast, endometrial, or ovarian cancer (n = 7,079), women for whom information was missing regarding hysterectomy status (n = 454), women currently using oral or transdermal estrogen or progestogen any time during follow-up (n = 41,630, censoring beginning at baseline for prevalent users or at time of subsequent initiation), women for whom data regarding menopausal hormone therapy (HT) use was missing (n = 85), and women who did not provide follow-up data (n = 473), resulting in an analytic sample of 45,663 women (Figure 1). Study participants could have initiated vaginal estrogen, or experienced hysterectomy, during the study follow-up, resulting in a sample size of 32,433 women without hysterectomy (of whom 3,003 women were users of vaginal estrogen during follow-up) and 14,133 women with hysterectomy (of whom 1,207 women were users of vaginal estrogen during follow-up).
Figure 1. Algorithm of Study Participants.
1 contraindicated medical conditions include past history of breast cancer, endometrial cancer or ovarian cancer.
2numbers don't add up to 45,663 due to the time varying nature of hysterectomy status: 903 change from no hysterectomy to hysterectomy and are counted in both cells
3 vaginal estrogen cream or vaginal tablet at some time during follow-up (Estradiol ring was only ascertained during the Extension Study; users are not included in this analysis)
Outcomes
Study outcomes were assessed using annual self-assessment questionnaires. The following events were adjudicated based on medical record review by WHI physicians: myocardial infarction, stroke, death from any cause, hip fracture, and any cancer. Incident venous thromboembolic events (deep vein thrombosis, hospitalization for pulmonary embolism) were ascertained based on self-report using annual questionnaires. Cause of death was determined at the Clinical Coordinating Center after medical record or death certificate review. The National Death Index, conducted serially, provided additional information on cause of death.
As for the WHI Hormone Therapy trials, the global index event (GIE) was defined as the time to first occurrence of CHD (nonfatal myocardial infarction or CHD death), invasive breast cancer, stroke, pulmonary embolism, hip fracture, colorectal cancer, endometrial cancer, or death from any cause through the end of WHI Extension I.25
Assessment of Vaginal Estrogen Use
Use of vaginal estrogen was ascertained using self-assessment questionnaires at baseline, annually from years 3 through 8 and at Ext 1 year 4 (Table 1). Information regarding specific type of vaginal estrogen (cream, ring, or tablet) or dose was not collected. (Use of the vaginal estradiol ring was only assessed at the end of the follow-up period in the Extension Study, so users are not included in this analysis).
Table 1. Questionnaire assessment of vaginal estrogen use.
| Annual follow-up visit # | Form # | Questionnaire item | Response choices |
|---|---|---|---|
| Baseline | 43 | Did you ever take any type of estrogen, such as Premarin, progesterone, such as Provera, testosterone, or any other hormone medications…were these hormones in the form of a vaginal cream or suppository? | Yes, no |
| Did you take them were 3 straight months or more? | Yes, no | ||
| What is the name of the vaginal cream or suppository you used? | Name, # times per day/week/month/year | ||
| 3 | 143 | In the past 2 years, did you use a vaginal cream or suppository containing estrogen which was prescribed by a doctor? | Yes, no, don't know |
| In the past 2 years, how many months did you use the vaginal cream of suppository | <1 month, 1-6 months, 7-10 months, 11-12 months, 13-18 months, 19-24 months | ||
| 4, 5, 6, 7, 8 | 144, 145, 146, 147, 148 | In the past year, did you use a vaginal cream or suppository containing estrogen which was prescribed by a doctor? | Yes, no, don't know |
| In the past year, how many months did you use the vaginal cream of suppository | <1 month, 1-6 months, 7-10 months, 11-12 months | ||
| Extension 1 | 153 | Medication and Supplement Inventory. For each of the prescription medications you are currently taking, please answer the questions below using the label on the prescription bottle. | Name of medicationStrength of medicationMedication type |
We imputed missing information regarding vaginal estrogen use from the previous year's questionnaire if the same preparation was reported in the year before, compared with the year after, the missing questionnaire.
Other Measures
We used baseline questionnaires to collect information regarding age, race/ethnicity, education level, household income, medical history (previous cancer, cardiovascular disease, deep vein thrombosis, pulmonary embolism, fracture, hypertension, or diabetes mellitus), reproductive history (oophorectomy, hysterectomy, age at menopause, age at first birth), medication use (past HT use, oral contraceptive use, aspirin use, or statin use), breast cancer risk factors, cigarette smoking, and alcohol intake (servings/week). Gail breast cancer risk score was calculated for each participant.26 Physical activity (total MET-hours per week) was assessed using the WHI validated physical activity questionnaire.27, 28
At baseline, using a standardized protocol, blood pressure, height, and weight were directly measured. Body mass index (BMI) was calculated as body weight in kilograms (kg) divided by the square of height in meters.
Role of the Funding Source
The WHI Observational Study was funded by National Institutes of Health. Representatives from the National Institutes of Health participated in the design, monitoring, and conduct of the WHI Observational Study.
Statistical Analysis
We used Cox proportional hazards models to examine associations between vaginal estrogen use and individual endpoints and GIE risk. The main predictor was time-varying exposure to vaginal estrogen. The time to outcome was computed from the date of enrollment to the date of the first event (among the events included in the GIE index), or last available follow-up visit, or end of study follow-up period, whichever came first. The end of the study follow-up period was defined as 2.5 years after the last ascertainment of vaginal estrogen use. Because of the different risk profiles of estrogen alone (in women with hysterectomy) and estrogen plus progestogen (in women without hysterectomy), we made the a priori decision to present the results separately for women with intact uterus and women who have had hysterectomy. Hysterectomy status was time-varying. Covariates were chosen a priori based on previously published studies and included: age, education, past estrogen use, history of cancer other than breast, endometrial or ovarian cancer before study baseline, history of cardiovascular disease before study baseline, history of deep vein thrombosis or pulmonary embolism before study baseline, race/ethnicity, baseline body mass index, baseline diagnosis of diabetes, baseline physical activity (total MET-hours/week), hypertension, Gail breast cancer risk score, fracture after age 55 prior to study enrollment, smoking, income, and alcohol use (servings/week) All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Mean follow-up duration was 6.4 years; median f/u was 7.2 years. Among women who initiated vaginal estrogen during the follow-up period, median duration of use was 2 years. Among women already using vaginal estrogen at baseline, median duration of use was 3 years before enrollment in WHI. Among vaginal estrogen users, on average, use encompassed 40% of the time during follow-up. At baseline, mean (standard deviation) age of participants was 64.8 (7.4) years in women who did not use vaginal estrogen at any time during follow-up and 65.5 (7.0) years in women who used vaginal estrogen during follow-up. In the overall analytic sample (women with hysterectomy and women without hysterectomy combined), compared with non-users of vaginal estrogen, vaginal estrogen users were less likely to be black or African American (4.9% vs. 11.2%), Hispanic (4.4% vs. 2.3%), current smokers (3.6% vs. 7.4%), diabetic (3.0% vs. 5.5%), and to have BMI ≥30kg/m2 (19.1% vs. 29.9%); vaginal estrogen users were more likely to be white (89.2% vs. 79.8%), college graduates (46.3% vs. 37.8%), have household income ≥$100,000, and to have BMI <25 kg/m2 (45.5% vs. 35.9%) (Table 2). The same pattern was true when participants were stratified by hysterectomy status. At baseline, the frequencies of other cardiovascular risk factors, cardiovascular disease events before study enrollment, and previous cancer diagnosis were similar among users and non-users of vaginal estrogen. Bilateral oophorectomy, BMI ≥30 kg/m2, treated hypertension, history of cardiovascular disease, and past use of HT were more frequent in women who had undergone hysterectomy than among women without hysterectomy. The frequencies of specific types of cardiovascular events and cardiovascular risk factors at baseline were similar in women with versus without hysterectomy.
Table 2. Baseline characteristics by vaginal estrogen use* in those with or without hysterectomy (n=45,663).
| Intact Uterus† | Hysterectomy | Overall2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No VE1 | Yes VE1 | No VE1 | Yes VE1 | No VE1 | Yes VE1 | |
|
| ||||||
| N | 29,430 | 3,003 | 12,926 | 1,207 | 41,563 | 4,100 |
|
| ||||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
|
| ||||||
| Age at baseline, years | ||||||
| Mean(SD) | 64.7 (7.3) | 65.3 (7.0) | 65.1 (7.4) | 66.0 (7.1) | 64.8 (7.4) | 65.5 (7.0) |
| <60 | 7,914 (26.9) | 667 (22.2) | 3,134 (24.2) | 238 (19.7) | 10,873 (26.2) | 881 (21.5) |
| 60-69 | 12,983 (44.1) | 1,419 (47.3) | 5,747 (44.5) | 556 (46.1) | 18,323 (44.1) | 1,912 (46.6) |
| ≥70 | 8,533 (29.0) | 917 (30.5) | 4,045 (31.3) | 413 (34.2) | 12,367 (29.8) | 1,307 (31.9) |
| Ethnicity | ||||||
| Asian or Pacific Islander | 885 (3.0) | 64 (2.1) | 237 (1.8) | 28 (2.3) | 1,107 (2.7) | 91 (2.2) |
| Black or African-American | 2,526 (8.6) | 122 (4.1) | 2,176 (16.9) | 80 (6.7) | 4,653 (11.2) | 201 (4.9) |
| Hispanic/Latino | 1,223 (4.2) | 66 (2.2) | 639 (5.0) | 32 (2.7) | 1,829 (4.4) | 95 (2.3) |
| White (not of Hispanic origin) | 24,198 (82.5) | 2,706 (90.5) | 9,569 (74.2) | 1,041 (86.5) | 33,085 (79.8) | 3,645 (89.2) |
| Other | 510 (1.7) | 33 (1.1) | 270 (2.1) | 22 (1.8) | 769 (1.9) | 53 (1.3) |
|
| ||||||
| Education | ||||||
| High school or less | 6,897 (23.6) | 531 (17.8) | 3,846 (30.1) | 262 (22.0) | 10,521 (25.5) | 773 (19.0) |
| Some college or vocational training | 10,320 (35.4) | 999 (33.5) | 5,057 (39.5) | 447 (37.5) | 15,110 (36.7) | 1,409 (34.7) |
| College grad or more | 11,959 (41.0) | 1,450 (48.7) | 3,892 (30.4) | 484 (40.6) | 15,556 (37.8) | 1,882 (46.3) |
|
| ||||||
| Family Income | ||||||
| Less than $50,000 | 17,477 (64.9) | 1,532 (55.4) | 8,535 (72.2) | 712 (63.6) | 25,521 (67.2) | 2,183 (57.7) |
| $50,000 to $99,999 | 7,131 (26.5) | 898 (32.5) | 2,626 (22.2) | 280 (25.0) | 9,573 (25.2) | 1,146 (30.3) |
| $100,000 or more | 2,303 (8.6) | 337 (12.2) | 659 (5.6) | 127 (11.3) | 2,910 (7.7) | 453 (12.0) |
|
| ||||||
| Any Insurance | ||||||
| No | 1,258 (4.3) | 75 (2.5) | 642 (5.0) | 26 (2.2) | 1,867 (4.6) | 100 (2.5) |
| Yes | 27,805 (95.7) | 2,905 (97.5) | 12,110 (95.0) | 1,162 (97.8) | 39,162 (95.4) | 3,961 (97.5) |
|
| ||||||
| Mammogram (last 2 years) | ||||||
| Mammogram within 2 years | 22,479 (79.2) | 2,650 (90.3) | 9,715 (78.6) | 1,022 (87.7) | 31,561 (79.0) | 3,575 (89.6) |
| No mammogram within 2 years | 5,887 (20.8) | 285 (9.7) | 2,650 (21.4) | 143 (12.3) | 8,397 (21.0) | 417 (10.4) |
|
| ||||||
| Bilateral Oophorectomy | ||||||
| No | 29,046 (99.4) | 2,972 (99.5) | 7,341 (60.7) | 755 (64.9) | 35,606 (87.9) | 3,617 (89.5) |
| Yes | 165 (0.6) | 15 (0.5) | 4,761 (39.3) | 409 (35.1) | 4,922 (12.1) | 424 (10.5) |
|
| ||||||
| Alcohol servings/week | ||||||
| Mean(SD) | 2.5 (5.2) | 2.7 (4.9) | 1.9 (4.6) | 2.1 (4.5) | 2.3 (5.0) | 2.5 (4.8) |
| None | 12,693 (43.2) | 1,111 (37.1) | 6,822 (52.9) | 580 (48.2) | 19,158 (46.2) | 1,644 (40.2) |
| <2 servings/week | 8,223 (28.0) | 881 (29.4) | 3,281 (25.5) | 306 (25.4) | 11,281 (27.2) | 1,157 (28.3) |
| 2 or more servings/week | 8,433 (28.7) | 1,004 (33.5) | 2,787 (21.6) | 318 (26.4) | 11,009 (26.6) | 1,290 (31.5) |
|
| ||||||
| Smoking status | ||||||
| Never Smoked | 15,039 (51.8) | 1,545 (52.1) | 6,665 (52.5) | 654 (55.0) | 21,305 (52.1) | 2,139 (52.9) |
| Past Smoker | 11,915 (41.1) | 1,318 (44.4) | 5,004 (39.4) | 493 (41.5) | 16,586 (40.5) | 1,763 (43.6) |
| Current Smoker | 2,058 (7.1) | 103 (3.5) | 1,018 (8.0) | 42 (3.5) | 3,021 (7.4) | 145 (3.6) |
|
| ||||||
| Physical Activity (total MET-hours/week) | ||||||
| Mean(SD) | 13.4 (14.4) | 15.5 (14.7) | 11.7 (13.7) | 13.2 (12.9) | 12.9 (14.2) | 14.9 (14.3) |
|
| ||||||
| Body mass index, kg/m2 | ||||||
| Mean(SD) | 27.6 (6.1) | 26.2 (5.1) | 28.9 (6.5) | 27.2 (5.4) | 28.0 (6.3) | 26.5 (5.2) |
| <25 | 11,137 (38.4) | 1,412 (47.7) | 3,850 (30.2) | 469 (39.2) | 14,722 (35.9) | 1,843 (45.5) |
| 25-<30 | 9,891 (34.1) | 1,036 (35.0) | 4,422 (34.7) | 444 (37.2) | 14,017 (34.2) | 1,433 (35.4) |
| ≥30 | 8,005 (27.6) | 513 (17.3) | 4,486 (35.2) | 282 (23.6) | 12,267 (29.9) | 774 (19.1) |
|
| ||||||
| Diabetes treated (pills or shots) | ||||||
| No | 28,050 (95.4) | 2,931 (97.6) | 11,932 (92.5) | 1,154 (95.7) | 39,213 (94.5) | 3,977 (97.0) |
| Yes | 1,343 (4.6) | 72 (2.4) | 972 (7.5) | 52 (4.3) | 2,292 (5.5) | 122 (3.0) |
|
| ||||||
| Systolic blood pressure | ||||||
| Mean(SD) | 128 (18.3) | 126 (17.6) | 130 (18.6) | 128 (18.3) | 128 (18.4) | 127 (17.9) |
|
| ||||||
| Diastolic blood pressure | ||||||
| Mean(SD) | 74.8 (9.5) | 73.8 (9.1) | 75.5 (9.8) | 74.9 (9.5) | 75.0 (9.6) | 74.1 (9.2) |
|
| ||||||
| Hypertension | ||||||
| Never hypertensive | 19,496 (67.6) | 2,105 (71.6) | 7,383 (58.5) | 717 (60.7) | 26,357 (64.8) | 2,746 (68.4) |
| Untreated hypertensive | 2,334 (8.1) | 243 (8.3) | 1,209 (9.6) | 123 (10.4) | 3,470 (8.5) | 356 (8.9) |
| Treated hypertensive | 7,004 (24.3) | 590 (20.1) | 4,039 (32.0) | 342 (28.9) | 10,866 (26.7) | 910 (22.7) |
|
| ||||||
| Age at Menopause | ||||||
| Mean (SD) | 50.2 (4.8) | 50.6 (4.7) | 45.3 (7.4) | 46.0 (7.1) | 48.8 (6.1) | 49.3 (5.8) |
|
| ||||||
| Fracture after Age 55+ | ||||||
| No | 19,612 (72.5) | 1,987 (72.3) | 8,560 (72.3) | 811 (73.4) | 27,626 (72.4) | 2,722 (72.6) |
| Yes | 4,396 (16.2) | 518 (18.9) | 1,921 (16.2) | 209 (18.9) | 6,193 (16.2) | 706 (18.8) |
| Age <55 | 3,052 (11.3) | 243 (8.8) | 1,360 (11.5) | 85 (7.7) | 4,351 (11.4) | 321 (8.6) |
|
| ||||||
| Menopausal hormone therapy usage at baseline | ||||||
| Never used | 23,535 (80.0) | 2,226 (74.1) | 8,106 (62.7) | 742 (61.5) | 30,980 (74.5) | 2,884 (70.3) |
| Past user | 5,895 (20.0) | 777 (25.9) | 4,820 (37.3) | 465 (38.5) | 10,583 (25.5) | 1,216 (29.7) |
|
| ||||||
| Age at First Birth, years | ||||||
| Never had term pregnancy | 4,096 (15.7) | 373 (13.8) | 1,422 (12.6) | 125 (11.7) | 5,445 (14.9) | 490 (13.3) |
| < 20 | 2,665 (10.2) | 191 (7.1) | 2,030 (17.9) | 123 (11.5) | 4,618 (12.6) | 307 (8.4) |
| 20-29 | 16,573 (63.6) | 1,851 (68.7) | 7,123 (63.0) | 736 (68.7) | 23,231 (63.4) | 2,518 (68.6) |
| 30+ | 2,721 (10.4) | 281 (10.4) | 737 (6.5) | 88 (8.2) | 3,372 (9.2) | 358 (9.7) |
|
| ||||||
| Gail breast cancer risk score | ||||||
| Mean (SD) | 1.8 (1.0) | 2.0 (1.0) | 1.8 (1.1) | 2.0 (1.1) | 1.8 (1.1) | 2.0 (1.1) |
|
| ||||||
| Female relative had breast cancer | ||||||
| No | 22,426 (80.8) | 2,272 (79.5) | 9,693 (79.8) | 912 (79.0) | 31,521 (80.5) | 3,092 (79.2) |
| Yes | 5,344 (19.2) | 586 (20.5) | 2,452 (20.2) | 243 (21.0) | 7,636 (19.5) | 813 (20.8) |
|
| ||||||
| Oral contraceptive use ever | ||||||
| No | 19,445 (66.1) | 1,877 (62.5) | 8,893 (68.8) | 810 (67.1) | 27,805 (66.9) | 2,620 (63.9) |
| Yes | 9,985 (33.9) | 1,126 (37.5) | 4,033 (31.2) | 397 (32.9) | 13,758 (33.1) | 1,480 (36.1) |
|
| ||||||
| Aspirin use med inventory | ||||||
| No | 23,428 (79.6) | 2,288 (76.2) | 10,290 (79.6) | 932 (77.2) | 33,105 (79.7) | 3,141 (76.6) |
| Yes | 6,002 (20.4) | 715 (23.8) | 2,636 (20.4) | 275 (22.8) | 8,458 (20.3) | 959 (23.4) |
|
| ||||||
| Statin use | ||||||
| No | 26,975 (91.7) | 2,766 (92.1) | 11,630 (90.0) | 1,070 (88.6) | 37,885 (91.2) | 3,738 (91.2) |
| Yes | 2,455 (8.3) | 237 (7.9) | 1,296 (10.0) | 137 (11.4) | 3,678 (8.8) | 362 (8.8) |
|
| ||||||
| Stroke ever | ||||||
| No | 28,952 (98.4) | 2,961 (98.6) | 12,584 (97.4) | 1,184 (98.3) | 40,755 (98.1) | 4,035 (98.5) |
| Yes | 469 (1.6) | 41 (1.4) | 335 (2.6) | 21 (1.7) | 793 (1.9) | 62 (1.5) |
|
| ||||||
| Myocardial infarction ever | ||||||
| No | 28,713 (97.6) | 2,958 (98.5) | 12,376 (95.9) | 1,170 (96.9) | 40,314 (97.1) | 4,020 (98.0) |
| Yes | 695 (2.4) | 45 (1.5) | 535 (4.1) | 37 (3.1) | 1,212 (2.9) | 80 (2.0) |
|
| ||||||
| Coronary artery bypass graft or percutaneous transluminal coronary artery angioplasty ever | ||||||
| No | 28,386 (98.1) | 2,919 (98.6) | 12,293 (97.2) | 1,158 (97.6) | 39,917 (97.8) | 3,974 (98.4) |
| Yes | 537 (1.9) | 42 (1.4) | 360 (2.8) | 28 (2.4) | 881 (2.2) | 65 (1.6) |
|
| ||||||
| History of cardiovascular disease* | ||||||
| No | 28,053 (95.3) | 2,898 (96.5) | 11,952 (92.5) | 1,140 (94.4) | 39,248 (94.4) | 3,933 (95.9) |
| Yes | 1,377 (4.7) | 105 (3.5) | 974 (7.5) | 67 (5.6) | 2,315 (5.6) | 167 (4.1) |
|
| ||||||
| Deep vein thrombosis or pulmonary embolism before study enrollment | ||||||
| No | 28,232 (95.9) | 2,861 (95.3) | 11,969 (92.6) | 1,108 (91.8) | 39,433 (94.9) | 3,864 (94.2) |
| Yes | 1,198 (4.1) | 142 (4.7) | 957 (7.4) | 99 (8.2) | 2,130 (5.1) | 236 (5.8) |
|
| ||||||
| History of cancer* | ||||||
| No | 27,687 (94.1) | 2,834 (94.4) | 11,650 (90.1) | 1,115 (92.4) | 38,585 (92.8) | 3,841 (93.7) |
| Yes | 1,743 (5.9) | 169 (5.6) | 1,276 (9.9) | 92 (7.6) | 2,978 (7.2) | 259 (6.3) |
Vaginal estrogen (cream or suppository) use is time-varying
Hysterectomy status is time-varying
History of cardiovascular disease includes a past history of myocardial infarction, stroke, or revascularization prior to study enrollment.
Not including breast, endometrial, or ovarian cancers (which were exclusion criteria)
In the study cohort overall, after adjustment for age, education, past HT use, history of cancer, history of CVD, history of venous thromboembolism, race/ethnicity, BMI, diabetes, physical activity level, HTN, Gail breast cancer risk score, previous fracture, smoking, household income, and alcohol intake level, the risk of invasive breast cancer, death, stroke, colorectal cancer, and venous thromboembolism were similar among users versus nonusers of vaginal estrogen (Table 3). Compared with non-users of vaginal estrogen, users of vaginal estrogen had 48% lower risk of CHD (aHR 0.52, 95% 0.31-0.85), and 60% lower risk of hip fracture (aHR 0.40, 95% CI 0.19-0.85). The risk of GIE was lower in users than in nonusers of vaginal estrogen (adjusted hazard ratio [aHR] 0.76, 95% confidence interval [CI] 0.64-0.91).
Table 3. Hazard Ratio (HR) and 95% confidence interval (CI) for Global index events (GIE) and components by Vaginal estrogen (VE) use.
| Intact uterus | Hysterectomy | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N (rate/1000 person- years)a |
Adjusted Model 1b HR (95% CI) |
Adjusted Model 2c HR (95% CI) |
N (rate/1000 person- years) |
Adjusted Model 1 HR (95% CI) |
Adjusted Model 2 HR (95% CI) |
N (rate/1000 person- years) |
Adjusted Model 1 HR (95% CI) |
Adjusted Model 2 HR (95% CI) |
|
| N | 32,433 | 32,156 | 26,069 | 14,133 | 13,988 | 11,303 | 45,663 | 45,251 | 36,629 |
|
| |||||||||
| GIEd | |||||||||
| No VE | 4,110 (19.9) | 1 (ref) | 1 (ref) | 1796 (24.1) | 1 (ref) | 1 (ref) | 5,906 (21.0) | 1 (ref) | 1 (ref) |
| VE | 96 (12.0) | 0.63 (0.51-0.77) | 0.68 (0.55-0.86) | 57 (19.7) | 0.83 (0.63-1.08) | 0.94 (0.70-1.26) | 153 (14.0) | 0.69 (0.59-0.81) | 0.76 (0.64-0.91) |
|
| |||||||||
| Invasive Breast cancer | |||||||||
| No VE | 858 (4.0) | 1 (ref) | 1 (ref) | 327 (4.2) | 1 (ref) | 1 (ref) | 1,185 (4.1) | 1 (ref) | 1 (ref) |
| VE | 26 (3.2) | 0.78 (0.53-1.15) | 0.79 (0.51-1.22) | 14 (4.7) | 1.06 (0.61-1.85) | 1.23 (0.68-2.21) | 40 (3.6) | 0.86 (0.62-1.18) | 0.91 (0.64-1.29) |
|
| |||||||||
| Death | |||||||||
| No VE | 1683 (7.7) | 1 (ref) | 1 (ref) | 909 (11.3) | 1 (ref) | 1 (ref) | 2,592 (8.7) | 1 (ref) | 1 (ref) |
| VE | 32 (3.9) | 0.58 (0.41-0.82) | 0.62 (0.41-0.93) | 27 (9.0) | 0.85 (0.58-1.25) | 1.04 (0.68-1.58) | 59 (5.3) | 0.67 (0.52-0.87) | 0.78 (0.58-1.04) |
|
| |||||||||
| CHDe | |||||||||
| No VE | 865 (4.0) | 1 (ref) | 1 (ref) | 446 (5.6) | 1 (ref) | 1 (ref) | 1,311 (4.5) | 1 (ref) | 1 (ref) |
| VE | 10 (1.2) | 0.35 (0.19-0.65) | 0.39 (0.19-0.78) | 10 (3.4) | 0.61 (0.33-1.15) | 0.74 (0.37-1.50) | 20 (1.8) | 0.44 (0.29-0.69) | 0.52 (0.31-0.85) |
|
| |||||||||
| Stroke | |||||||||
| No VE | 619 (2.9) | 1 (ref) | 1 (ref) | 283 (3.6) | 1 (ref) | 1 (ref) | 902 (3.1) | 1 (ref) | 1 (ref) |
| VE | 9 (1.1) | 0.41 (0.21-0.80) | 0.54 (0.28-1.04) | 11 (3.7) | 1.08 (0.59-1.99) | 1.27 (0.67-2.40) | 20 (1.8) | 0.63 (0.40-0.98) | 0.78 (0.49-1.24) |
|
| |||||||||
| Colorectal cancer | |||||||||
| No VE | 342 (1.6) | 1 (ref) | 1 (ref) | 144 (1.8) | 1 (ref) | 1 (ref) | 486 (1.6) | 1 (ref) | 1 (ref) |
| VE | 9 (1.1) | 0.70 (0.36-1.36) | 0.63 (0.28-1.41) | 4 (1.3) | 0.76 (0.28-2.07) | 0.82 (0.26-2.62) | 13 (1.2) | 0.72 (0.41-1.25) | 0.67 (0.35-1.31) |
|
| |||||||||
| Hip Fracture | |||||||||
| No VE | 409 (1.9) | 1 (ref) | 1 (ref) | 173 (2.2) | 1 (ref) | 1 (ref) | 582 (2.0) | 1 (ref) | 1 (ref) |
| VE | 6 (0.7) | 0.42 (0.19-0.94) | 0.40 (0.16-0.96) | 4 (1.3) | 0.67 (0.25-1.81) | 0.42 (0.10-1.69) | 10 (0.9) | 0.49 (0.26-0.92) | 0.40 (0.19-0.85) |
|
| |||||||||
| Endometrial cancer | |||||||||
| No VE | 222 (1.0) | 1 (ref) | 1 (ref) | NAa | NA | NA | NA | NA | NA |
| VE | 11 (1.3) | 1.27 (0.69-2.33) | 1.47 (0.75-2.90) | NA | NA | NA | NA | NA | NA |
|
| |||||||||
| PE/DVTb | |||||||||
| No VE | 385 (1.8) | 1 (ref) | 1 (ref) | 215 (2.7) | 1 (ref) | 1 (ref) | 600 (2.0) | 1 (ref) | 1 (ref) |
| VE | 9 (1.1) | 0.68 (0.35-1.31) | 0.72 (0.34-1.53) | 3 (1.0) | 0.43 (0.14-1.33) | 0.57 (0.18-1.81) | 12 (1.1) | 0.59 (0.33-1.05) | 0.68 (0.36-1.28) |
Rates are crude rates per 1000 person-years (N= 45,663)
Model 1 is adjusted for age, education, past estrogen use, history of cancer before study baseline, history of cardiovascular disease before study baseline, history of deep vein thrombosis or pulmonary embolism before study baseline. Model 1 in the overall sample also includes hysterectomy status
Model 2 is adjusted for variables in Model 1 and race/ethnicity, baseline body mass index, baseline diagnosis of diabetes, baseline physical activity (total MET-hours/week), hypertension, Gail breast cancer risk score, fracture after age 55 prior to study enrollment, smoking, income, and alcohol use (servings/week)
GIE denotes Global Index Event, defined as time to first coronary heart disease, breast cancer, stroke, pulmonary embolism, hip fractures, colorectal cancer, endometrial cancer, or death.
CHD denotes coronary heart disease
Endometrial cancer analysis was not conducted in participants with hysterectomy or in the overall sample
PE denotes pulmonary embolism; DVT denotes deep vein thrombosis
When we analyzed results based on hysterectomy status, the risk of invasive breast cancer, stroke, colorectal cancer, endometrial cancer, and venous thromboembolism were not significantly different in vaginal estrogen users than in nonusers of vaginal estrogen. In women with prior hysterectomy, the aHR estimates for GIE and for each of the individual components of the global index, were not significantly different in users of vaginal estrogen than in nonusers of vaginal estrogen. In contrast, in women without hysterectomy, the risk of several outcomes was significantly lower in vaginal estrogen users than in nonusers of vaginal estrogen: GIE aHR 0.68 (95% CI 0.55-0.86), death aHR 0.62 (95% CI 0.41-0.93), CHD aHR 0.39 (95% CI 0.19-0.78), and hip fracture aHR 0.40 (95% CI 0.16-0.96). The aHRs are illustrated graphically in Figure 2.
Figure 2. Hazard Ratio (HR) and 95% Confidence Interval (CI) for Global Index Events (GIE) and Components by Vaginal Estrogen (VE) Use Overall and by Hysterectomy Status1.
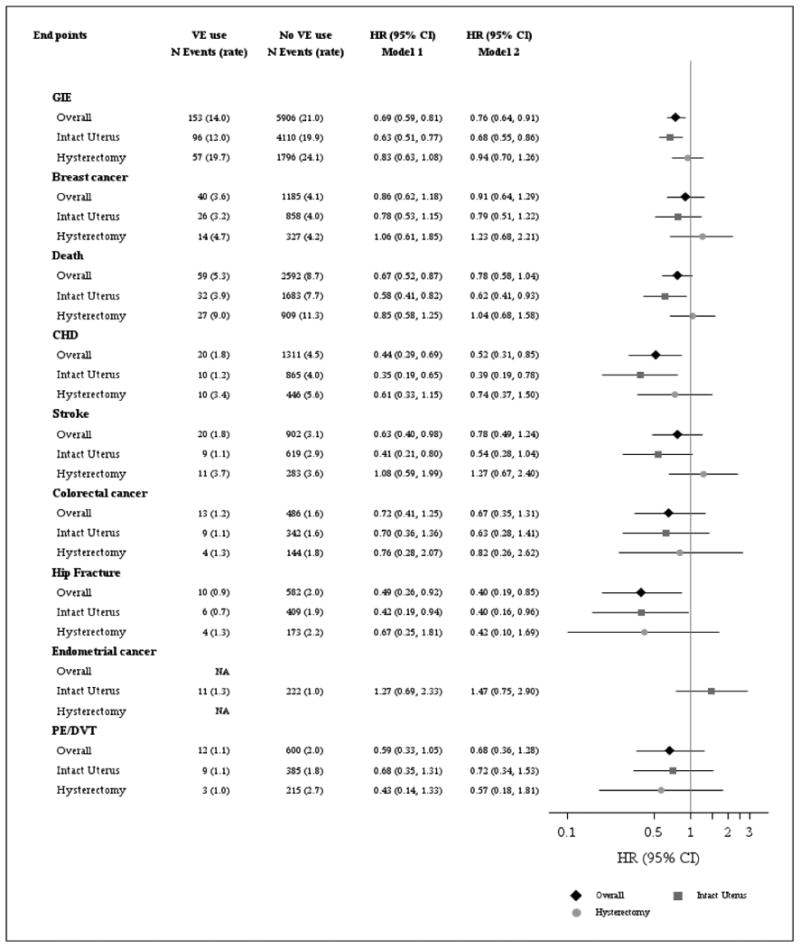
1 VE includes vaginal cream or vaginal tablet. VE and hysterectomy status were included in the model as time-varying covariates. Global Index Event (GIE) is defined as time to first coronary heart disease, breast cancer, stroke, pulmonary embolism, hip fractures, colorectal cancer, endometrial cancer, or death. Analysis for endometrial cancer was not conducted in participants with hysterectomy or in the overall analytic sample. Rate are crude rates per 1000 person-years (N= 45,663). Model 1 is adjusted for age, education, past estrogen use, history of cancer before study baseline, history of cardiovascular disease before study baseline, history of deep vein thrombosis or pulmonary embolism before study baseline. Model for overall analytic sample also includes hysterectomy status. (N=45,251). Model 2 was adjusted for variables in Model 1 and race/ethnicity, baseline body mass index, baseline diagnosis of diabetes, baseline physical activity (total MET-hours/week), hypertension, Gail breast cancer risk score, fracture after age 55 prior to study enrollment, smoking, income, and alcohol use (servings/week) (N=36,629)
Discussion
In this large prospective cohort study, postmenopausal women who used vaginal estrogen had similar risks of invasive breast cancer, stroke, colorectal cancer, endometrial cancer, and pulmonary embolism/deep vein thrombosis than nonusers of vaginal estrogen. We did not find evidence for elevated risk of CHD or death in vaginal estrogen users compared with non-users.
Previously published studies of vaginal estrogen preparations have not focused on summary indices of risks vs. benefits of vaginal estrogen preparations, nor have they focused on colorectal cancer, pulmonary embolism, or hip fracture as specific individual outcomes. However, our results are consistent with a few prospective observational studies that evaluated CHD, stroke, breast cancer, and endometrial cancer risk among vaginal estrogen users.
First, a nationwide cohort study in Finland from 1994-2009 found a lower risk for CHD death and for stroke death in vaginal estrogen users, compared with the background population.21 (Of note, the background population of the Finnish study included women using both vaginal estrogen and systemic HT, and the study lacked a placebo group).
A second Finnish observational study with 648,022 women-years of follow-up found that the use of vaginal estradiol was not associated with increased risk of breast cancer.18
Finally, with regard to endometrial cancer, two randomized controlled trials documented no increase in endometrial hyperplasia or cancer after 12 months of treatment with 10 microgram estradiol vaginal tablets.29, 30. However, in a study of all Danish women aged 50-79 years followed from 1995-2009 (representing 9 million person-years of follow-up), the relative risk of endometrial cancer was increased with vaginal estrogen: 1.96 (1.77-2.17).22 The differences in results between the Danish study and the current study might be related to differing doses or formulations of vaginal estrogen preparations in the two studies, or due to the fact that we censored data from vaginal estrogen users if they initiated oral or transdermal progestogen.
Our findings help to fill important knowledge gaps regarding the safety of vaginal estradiol. Randomized trials have shown that systemic estrogen is linked to increased risks of venous thromboembolism, stroke, and (with combination estrogen + progestogen) invasive breast cancer.10-12, 31, 32. These risks of systemic estrogen and estrogen + progestogen were adopted into estrogen class labeling that is applied to all estrogen preparations, despite the lack of clinical trial evidence demonstrating that vaginal estrogens confer these same risks. (Clinical trials of vaginal estrogens were not designed to evaluate most key clinical outcomes such as CVD and fracture). Low-dose vaginal estrogen preparations approved by the U.S. Food and Drug Administration carry the same boxed warning about health risks as systemic formulations of estrogen alone and combination estrogen plus progestogen. The boxed warning, which reflects estrogen class labeling, states: “WARNING: endometrial cancer, cardiovascular disorders, breast cancer and probable dementia”. This warning is based on extrapolations of data from clinical trials of systemic hormone therapy, which involved substantially higher levels of systemic exposure. The boxed warning is not based on evidence from clinical trials of vaginal estrogen and may discourage the use of a highly effective local treatment for a common condition with adverse effects on quality of life.33 Currently, the FDA is considering a proposal to modify package labelling so that it better reflects the safety profile of vaginal estrogen.
In the short-term, serum levels of estrogens may rise with use of vaginal estrogen. A study using modern mass spectrometry assays of estrogen levels showed that the use of conjugated equine estrogens vaginal cream (Premarin 0.625 mg) caused a 5-fold increase in serum estradiol after 1 week of daily treatment in postmenopausal women.7 However, estrogen levels do not increase above the postmenopausal range with initial daily dosing during use of the estradiol vaginal insert (Vagifem 10 mcg).34, and during the maintenance phase of low-dose vaginal estrogen therapy, blood levels remain in the normal postmenopausal range.9
Our study has limitations. Although we adjusted for multiple potential confounders, there could have been residual confounding in this observational study. There have been reports of alterations in serum lipoproteins and increased bone density associated with low-dose vaginal estradiol rings.35, 36 While the lower CHD risk that we observed in vaginal users was consistent with other observational studies of oral HT, clinical trials have not addressed the effects of vaginal estrogen on clinical cardiovascular events. In addition, we could not compare health outcomes among the individual types of vaginal estrogen preparations (e.g. estradiol ring, vaginal estradiol tablet, estrogen cream). Study strengths include the prospective data collection, the large sample size, the availability of detailed information regarding relevant confounders, and the adjudication of all GIE events except PE/DVT. To our knowledge, this is the first large prospective study to evaluate the overall health risks and benefits of vaginal estrogen therapy.
Conclusions
In conclusion, we did not observe an increased risk of cardiovascular disease or cancer among women using vaginal estrogen compared to non-users. These findings should provide reassurance to women and their health providers regarding the safety of vaginal estrogen and will help to inform menopausal hormone therapy clinical decision-making.
Acknowledgments
The authors thank the WHI investigators, staff, and the study participants for their outstanding dedication and commitment.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A.Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski -Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
Funding: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C
AMK serves as a consultant for: Allergan, Inc; AMAG, Pfizer Inc, and Shionogi. He receives research grants (funds paid to University of Florida) from TherapeuticsMD. He receives royalties from: UpToDate
RTC serves as consultant for: Amgen, Genentech, Novartis, Astra-Zeneca and Pfizer. He also is on speaker's panel for Genentech and Novartis.
JLS serves as a research consultant to the New England Research Institutes.
Footnotes
Conflicts of interest: The following authors have no conflicts to disclose: CJC, KMH, JEM, CC, DSL, JAC, CA, MLS
Contributor Information
Carolyn J. Crandall, Dept. of Medicine, University of California at Los Angeles, Los Angeles, CA.
Kathleen M. Hovey, Dept. of Epidemiology and Environmental Health, University at Buffalo, The State University of New York, Buffalo, New York.
Christopher A. Andrews, Dept. of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI.
Rowan T. Chlebowski, Dept. of Medical Oncology and Therapeutics, City of Hope National Medical Center, Duarte, CA.
Marcia L. Stefanick, Departments of Medicine and Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California.
Dorothy S. Lane, Department of Family, Population and Preventive Medicine, Stony Brook University School of Medicine, Stony Brook, New York.
Jan Shifren, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Chu Chen, Program in Epidemiology, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington.
Andrew M. Kaunitz, Dept. of Obstetrics and Gynecology, University of Florida College of Medicine, Jacksonville, FL.
Jane A. Cauley, Dept. of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA.
JoAnn E. Manson, Division of Preventive Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA.
References
- 1.Portman DJ, Gass ML Vulvovaginal Atrophy Terminology Consensus Conference P. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. J Sex Med. 2014;11(12):2865–72. doi: 10.1111/jsm.12686. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–16. doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 3.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902. doi: 10.1097/GME.0b013e3182a122c2. quiz 3-4. [DOI] [PubMed] [Google Scholar]
- 4.American College of O, Gynecologists Committee on Practice B-G. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996–1007. doi: 10.1097/AOG.0b013e31821921ce. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Menopause: diagnosis and management. Available at: https://www.nice.org.uk/guidance/ng23. [PubMed]
- 6.Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD001500.pub3. CD001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. 2009;16(1):30–6. doi: 10.1097/gme.0b013e31817b6132. [DOI] [PubMed] [Google Scholar]
- 8.Kunovac Kallak T, Baumgart J, Stavreus Evers A, et al. Higher than expected estradiol levels in aromatase inhibitor-treated, postmenopausal breast cancer patients. Climacteric. 2012;15(5):473–80. doi: 10.3109/13697137.2011.642427. [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric. 2015;18(2):121–34. doi: 10.3109/13697137.2014.947254. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353–68. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 2015;(3) doi: 10.1002/14651858.CD002229.pub4. CD002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med. 2012;157(2):104–13. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- 13.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 14.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168(8):861–6. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergendal A, Kieler H, Sundstrom A, Hirschberg AL, Kocoska-Maras L. Risk of venous thromboembolism associated with local and systemic use of hormone therapy in peri- and postmenopausal women and in relation to type and route of administration. Menopause. 2016;23(6):593–9. doi: 10.1097/GME.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 16.Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Hu FB, Manson JE. Exogenous Hormone Use: Oral Contraceptives, Postmenopausal Hormone Therapy, and Health Outcomes in the Nurses' Health Study. Am J Public Health. 2016;106(9):1631–7. doi: 10.2105/AJPH.2016.303349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estrogen-only therapy. Obstet Gynecol. 2006;108(6):1354–60. doi: 10.1097/01.AOG.0000241091.86268.6e. [DOI] [PubMed] [Google Scholar]
- 19.Michaelsson K, Baron JA, Farahmand BY, Ljunghall S. Use of low potency estrogens does not reduce the risk of hip fracture. Bone. 2002;30(4):613–8. doi: 10.1016/s8756-3282(01)00701-3. [DOI] [PubMed] [Google Scholar]
- 20.American College of O, Gynecologists ACOG committee opinion no. 556: Postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstetrics and gynecology. 2013;121(4):887–90. doi: 10.1097/01.AOG.0000428645.90795.d9. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estradiol use and the risk for cardiovascular mortality. Hum Reprod. 2016;31(4):804–9. doi: 10.1093/humrep/dew014. [DOI] [PubMed] [Google Scholar]
- 22.Morch LS, Kjaer SK, Keiding N, Lokkegaard E, Lidegaard O. The influence of hormone therapies on type I and II endometrial cancer: A nationwide cohort study. Int J Cancer. 2016;138(6):1506–15. doi: 10.1002/ijc.29878. [DOI] [PubMed] [Google Scholar]
- 23.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 25.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 26.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 27.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women's Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41(3):530–8. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettee Gabriel K, McClain JJ, Lee CD, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc. 2009;41(7):1403–12. doi: 10.1249/MSS.0b013e31819b2482. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich LS, Naessen T, Elia D, Goldstein JA, Eugster-Hausmann M investigators VAGt. Endometrial safety of ultra-low-dose Vagifem 10 microg in postmenopausal women with vaginal atrophy. Climacteric. 2010;13(3):228–37. doi: 10.3109/13697137.2010.481058. [DOI] [PubMed] [Google Scholar]
- 30.Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol. 2010;116(4):876–83. doi: 10.1097/AOG.0b013e3181f386bb. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Goldstein SR, Kagan R, et al. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–6. doi: 10.1097/GME.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 34.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric. 2010;13(3):219–27. doi: 10.3109/13697137.2010.483297. [DOI] [PubMed] [Google Scholar]
- 35.Naessen T, Berglund L, Ulmsten U. Bone loss in elderly women prevented by ultralow doses of parenteral 17beta-estradiol. Am J Obstet Gynecol. 1997;177(1):115–9. doi: 10.1016/s0002-9378(97)70448-4. [DOI] [PubMed] [Google Scholar]
- 36.Naessen T, Rodriguez-Macias K, Lithell H. Serum lipid profile improved by ultra-low doses of 17 beta-estradiol in elderly women. J Clin Endocrinol Metab. 2001;86(6):2757–62. doi: 10.1210/jcem.86.6.7524. [DOI] [PubMed] [Google Scholar]