Abstract
BACKGROUND
To examine the association between ipsilateral breast tumor recurrence (IBTR) and timing of radiation therapy (RT) in women with ductal carcinoma in situ (DCIS) undergoing breast-conserving surgery (BCS).
METHODS
Women with DCIS treated with BCS and RT from 1980–2010 were identified from a prospectively-maintained database. IBTR rates, measured from RT completion, were compared between those who began RT ≤8 weeks, >8–12 weeks, and >12 weeks after completion of surgery. Association between RT timing and IBTR was evaluated by Kaplan-Meier and log-rank analysis; Cox modeling was used for multivariable analysis.
RESULTS
1323 women met inclusion criteria. Median follow-up was 6.6 years, with 311 patients followed for ≥10 years. 129 IBTR events occurred. Patients were categorized by RT timing: 806 (61%) ≤8 weeks, 386 (29%) >8–12 weeks, and 131 (10%) >12 weeks. 5- and 10-year IBTR rates were: 5.8% and 13.0% RT starting ≤8 weeks, 3.8% and 7.6% RT >8–12 weeks, and 8.8% and 23.0% with RT delayed >12 weeks after surgery, respectively (p=0.004). On multivariable analysis, menopause (HR 0.54, p=0.0009) and endocrine therapy (HR 0.45, p=0.002) were IBTR-protective, whereas delay in RT >12 weeks compared to ≤8 weeks was associated with higher risk of IBTR (HR 1.92, p=0.014). There was no difference in IBTR between RT initiation ≤8 weeks and >8–12 weeks (p=0.3).
CONCLUSIONS
Delay in RT >12 weeks is associated with a significantly higher risk of IBTR in women undergoing BCS for DCIS. Efforts should be made to avoid delay in starting RT to minimize risk of recurrence.
Keywords: ductal carcinoma in situ, radiotherapy, recurrence, breast cancer
INTRODUCTION
Ductal carcinoma in situ (DCIS), or stage 0 breast cancer, is a noninvasive breast lesion that comprises approximately 20% of all breast cancer diagnoses. In 2017 it is estimated that nearly 53,000 women will be diagnosed with DCIS in the United States.1, 2 The majority (60–77%) of these women will undergo breast–conserving surgery (BCS), with or without adjuvant therapy.3–6 Survival is excellent following BCS for DCIS, with 10-year breast cancer-specific mortality rates of 1–4%.7–10 However, rates of ipsilateral breast tumor recurrence (IBTR) are not insignificant, with risk of IBTR reported as 1–3% per year,5, 8 with long-term recurrence rates ranging from 25% to 35% following BCS alone in four large, prospective, randomized controlled trials.9, 11–13
A marked decrease in risk of IBTR in DCIS patients has been seen with the utilization of adjuvant radiation therapy (RT) after BCS. The Early Breast Cancer Trialists’ Collaborative Group meta-analysis of 3729 women with DCIS from four randomized controlled trials revealed a 10-year relative risk reduction of 54% and 10-year absolute risk reduction of 15% for IBTR with the addition of RT.8 Despite the demonstration of clear benefit for the use of RT in DCIS patients, evidence regarding optimal timing of adjuvant RT is lacking. Of the four mature randomized controlled trials, only two specified timing of RT therapy in the protocol: patients enrolled in National Surgical Adjuvant Breast and Bowel Project (NSABP) B-17 and European Organisation for Research and Treatment of Cancer (EORTC) 10853 initiated RT within 8 and 12 weeks, respectively.14, 15 However, it is well documented that in patients with invasive breast cancer, RT delay of more than 8 to 12 weeks leads to an increased risk of IBTR.16–18 The aim of this study was to examine the association between timing of adjuvant RT and risk of IBTR in women with DCIS undergoing BCS.
METHODS
Women were identified from a prospectively maintained database of patients with DCIS undergoing BCS at Memorial Sloan Kettering Cancer Center (MSK). Patients were included if they were female, had undergone surgery between 1980 and 2010, and received adjuvant RT. Patients excluded were those with a previous breast cancer diagnosis, those with synchronous invasive breast cancer, those unknown RT total dose or with total RT dose less than 4240 cGy, and those missing both RT initiation and completion date. If the start or end date of RT was not known, it was inferred from the known corresponding end or start date of RT, respectively, using the median duration of RT therapy in the cohort (6 weeks). Of 1323 patients, 53 start dates were inferred from known RT completion dates, and one RT end time was inferred from a known start date. Four patients had non-synchronous bilateral DCIS and were included in the dataset as separate entries. The use of these data was approved by the MSK Institutional Review Board.
Time to RT was defined as the interval between final definitive surgery, defined as date of surgery if only one excision was required, or date of final re-excision if multiple procedures were required, and the date of RT initiation. Patients were grouped and analyzed according to timing of RT initiation (≤ 8 weeks, > 8 to 12 weeks, > 12 weeks). Other factors included were age, menopausal status, family history of breast cancer (defined as one or more first- or second-degree family members with breast cancer), presentation (radiological or clinical), nuclear grade (low, intermediate, or high), presence of tumor necrosis, number of excisions required (≤ 2 or ≥ 3), margin status [positive/close (≤ 2 mm) or negative (> 2 mm)], endocrine therapy, total RT dose, and treatment time period (≤ 2000 or ≥ 2001). Number of surgical excisions was included as a surrogate for size/extent of DCIS.
The primary endpoint was time interval to first IBTR, calculated from the date of completion of RT to the date of histologically proven recurrence. Both biopsy-proven DCIS and invasive breast carcinoma in the ipsilateral treated breast were included as IBTR.
Whole breast radiation was used on all patients, generally prescribed with either the standard schedule of 5000cGy in 25 treatments, or the “hypofractionated” schedule of 4240 cGy in 16 treatments. The additional use of a boost was based on clinical judgment and the final margin status. A sequential boost to the lumpectomy cavity, ranging from 360cGy to 2160cGy, was delivered to 81% of patients (n = 1072). For the patients who received RT at an outside institution, treatment summaries documenting the dose and fraction size were obtained and reviewed. Follow-up consisted of annual mammograms, routine interval history, and physical examinations.
Differences in patient characteristics by time to RT initiation were assessed using the χ square test. The Kaplan-Meier method was used to estimate 5- and 10-year recurrence rates in the entire population as well as in each of the time-to-RT cohorts. Hazard ratios (HRs) and Wald test p-values for each variable were estimated from univariate and multivariable Cox regressions. A multivariable Cox regression model was used to assess the relationship between delay in RT initiation and IBTR while adjusting for patient clinicopathological factors that were associated with IBTR on univariate Cox regression analysis, or that varied between RT timing groups. RT dose was modeled as a continuous variable and was rescaled so that the hazard ratio (HR) corresponded to the effect of a 100 cGY increase in RT dose on the outcome. A sensitivity analysis was performed to ensure that the main finding on the relationship between RT timing and IBTR was robust with the inclusion of 54 patients with inferred start or end dates. All analyses were performed using SAS v9.4 and R 3.1.1. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
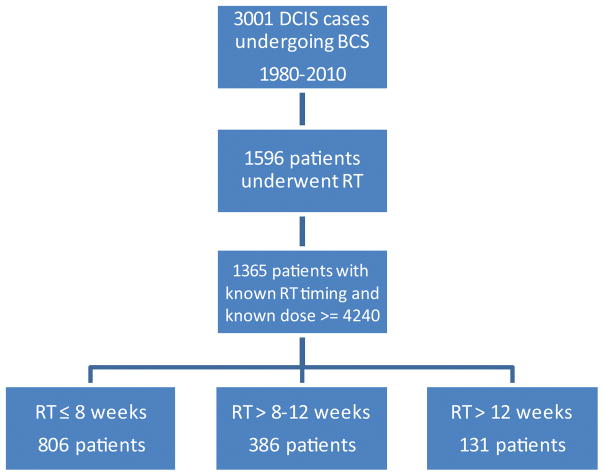
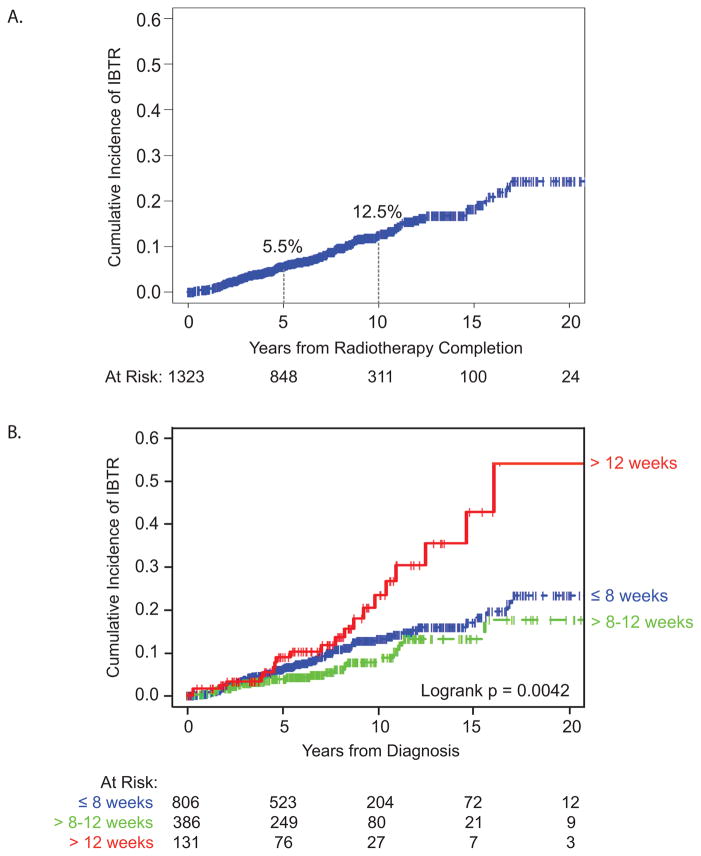
Of 3001 cases of DCIS treated with BCS, 1596 patients underwent RT and 1323 patients had known RT initiation or completion dates with RT dose ≥ 4240 cGy (See Figure 1). Median patient age was 56 years (range 27–86 years). Median follow-up was 6.6 years (range 0–30.7 years), with 311 women followed for at least 10 years. Of the 126 IBTR events, 56% were DCIS recurrences and 44% were invasive carcinoma. The overall cumulative incidence of IBTR at 5 and 10 years was 5.5% and 12.5%, respectively (Figure 2A).
Figure 1.
CONSORT Diagram. DCIS, ductal carcinoma in situ; BCS, breast-conserving surgery; RT, radiation therapy
Figure 2.
Cumulative incidence of ipsilateral breast tumor recurrence in (A) the overall patient cohort and patient cohort stratified by (B) timing of radiation therapy initiation.
Differences in Patient, Tumor, and Treatment Characteristics by RT Cohort
Radiation therapy was initiated within 8 weeks of surgery in 806 patients (61%), while 386 patients (29%) began RT between 8 and 12 weeks after surgery, and 131 patients (10%) experienced delay in RT more than 12 weeks after surgery. The median delay in the group that initiated RT more than 12 weeks after definitive surgery was 14.1 weeks, with a range of 12.1 to 62.4 weeks. Patient, tumor, and treatment characteristics are listed in Table 1. As compared to the longer time to RT cohorts, women starting RT within 8 weeks had a non-significantly higher proportion of pre/perimenopausal women (40% vs 35% and 33%, respectively, P = 0.10) and nonsignificant difference in the use of endocrine therapy between groups, with less frequent usage in women who experienced a delay in RT therapy (28% vs 25% and 19%, P = 0.07). DCIS with necrosis significantly varied between groups (78% vs 73% and 71%, respectively, P = 0.05). RT dose varied by time to RT (P < 0.0001), with mean (median) RT doses of 5778 cGy (6000 cGy), 5665cGy (6000 cGy), and 5595cGy (5980 cGy) in patients who initiated RT within 8 weeks, between 8 and 12 weeks after surgery, and more than 12 weeks after surgery, respectively. Patient age, family history of breast cancer, presentation, nuclear grade, number of surgical excisions, margin status, and year of surgery did not vary significantly between RT timing cohorts.
TABLE 1.
Clinical Characteristics of Entire Patient Cohort, and Stratified by Radiation Therapy Timing
| Characteristic | Entire Population n = 1323 |
RT ≤ 8 weeks n = 806 |
RT > 8–12 weeks n = 386 |
RT > 12 weeks n = 131 |
P value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age (years) | P = 0.33 | ||||||||
| < 50 | 411 | 31% | 260 | 32% | 117 | 30% | 34 | 26% | |
| ≥ 50 | 912 | 69% | 546 | 68% | 269 | 70% | 97 | 74% | |
| Unknown | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Menopausal Status | P = 0.10 | ||||||||
| Pre/peri | 504 | 38% | 325 | 40% | 136 | 35% | 43 | 33% | |
| Post | 818 | 62% | 480 | 60% | 250 | 65% | 88 | 67% | |
| Unknown | 1 | < 1% | 1 | < 1% | 0 | 0% | 0 | 0% | |
| Family History | P = 0.34 | ||||||||
| No | 808 | 61% | 481 | 60% | 246 | 64% | 81 | 62% | |
| Yes | 509 | 39% | 323 | 40% | 137 | 36% | 49 | 38% | |
| Unknown | 6 | <1% | 2 | <1% | 3 | <1% | 1 | <1% | |
| Presentation | P = 0.69 | ||||||||
| Clinical | 140 | 11% | 87 | 11% | 42 | 11% | 11 | 8% | |
| Radiologic | 1182 | 89% | 718 | 89% | 344 | 89% | 120 | 92% | |
| Unknown | 1 | < 1% | 1 | < 1% | 0 | 0% | 0 | 0% | |
| Nuclear Grade | P = 0.16 | ||||||||
| Low | 106 | 8% | 55 | 7% | 38 | 10% | 13 | 10% | |
| Intermediate | 570 | 43% | 340 | 42% | 173 | 45% | 57 | 43% | |
| High | 609 | 46% | 390 | 48% | 164 | 42% | 55 | 42% | |
| Unknown | 38 | 3% | 21 | 3% | 11 | 3% | 6 | 5% | |
| Necrosis | P = 0.05 | ||||||||
| Absent | 295 | 22% | 162 | 20% | 101 | 26% | 32 | 24% | |
| Present | 1005 | 76% | 632 | 78% | 280 | 73% | 93 | 71% | |
| Unknown | 23 | 2% | 12 | 2% | 5 | <1% | 6 | 5% | |
| Number of Excisions | P = 0.48 | ||||||||
| ≤ 2 | 1182 | 89% | 716 | 89% | 345 | 89% | 121 | 92% | |
| ≥ 3 | 141 | 11% | 90 | 11% | 41 | 11% | 10 | 8% | |
| Unknown | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Margins | P = 0.28 | ||||||||
| Positive/close | 276 | 21% | 160 | 20% | 91 | 24% | 25 | 19% | |
| Negative | 995 | 75% | 616 | 76% | 279 | 72% | 100 | 76% | |
| Unknown | 52 | 4% | 30 | 4% | 16 | 4% | 6 | 5% | |
| Endocrine Therapy | P = 0.07 | ||||||||
| No | 970 | 73% | 576 | 72% | 289 | 75% | 105 | 80% | |
| Yes | 348 | 26% | 227 | 28% | 96 | 25% | 25 | 19% | |
| Unknown | 5 | < 1% | 3 | <1% | 1 | <1% | 1 | 1% | |
| Year of Surgery | P = 0.23 | ||||||||
| 1980–2000 | 342 | 26% | 216 | 27% | 88 | 23% | 38 | 29% | |
| 2001–2010 | 981 | 74% | 590 | 73% | 298 | 77% | 93 | 71% | |
| Unknown | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | |
Abbreviation: RT, radiation therapy
Chi-squared analysis based on complete data
Differences in IBTR Rates by Clinical, Pathologic, and Treatment Characteristics
Incidence of IBTR varied significantly by time to initiation of RT (P < 0.005, Figure 2B). Five-year IBTR rates for patients initiating RT within 8 weeks, between 8 to 12 weeks, and beyond 12 weeks were 5.8%, 3.8%, and 8.8%, respectively. Ten-year IBTR rates for patients starting RT within 8 weeks, between 8 to 12 weeks, and beyond 12 weeks were 13.0%, 7.6%, and 23.0%, respectively (Table 2).
TABLE 2.
Five- and 10-Year Ipsilateral Breast Tumor Recurrence Rate in Entire Population and by Time to Receipt of Radiation
| Total IBTR events | 5-year IBTR rate | 95% CI | 10-year IBTR rate | 95% CI | |
|---|---|---|---|---|---|
| Entire Population (n = 1365) | 126 | 5.5% | 4.3–7.0% | 12.5% | 10.2–15.2% |
| RT ≤ 8 weeks (n = 833) | 80 | 5.8% | 4.3–7.9% | 13.0% | 10.2–16.4% |
| RT > 8–12 weeks (n = 399) | 25 | 3.8% | 2.2–6.5% | 7.6% | 4.7–12.1% |
| RT > 12 weeks (n = 133) | 21 | 8.8% | 4.6–16.4% | 23.0% | 14.0–36.5% |
Abbreviations: IBTR, ipsilateral breast tumor recurrence; CI, confidence interval; RT, radiation therapy
On univariate regression analysis, delay in RT initiation more than 12 weeks was associated with a higher risk of IBTR (HR 1.82, 95% confidence interval [CI] 1.12–2.94, P = 0.015), whereas postmenopausal status (HR 0.50, 95% CI 0.35–0.71, P < 0.0001), radiologic presentation (HR 0.63, 95% CI 0.40–0.99, P = 0.048), and usage of endocrine therapy (HR 0.43, 95% CI 0.26–0.70, P = 0.0007) were associated with a lower risk of IBTR (Table 3). Of the patients taking hormonal therapy (n = 348), 329 (94.5%) did not experience a recurrence, 19 patients (5.5%) experienced a recurrence. This is in contrast to patients who did not take endocrine therapy (n = 970), where 863 (89.0%) did not experience a recurrence, whereas 107 (11.0%) had a documented recurrence. Family history of breast cancer, nuclear grade, necrosis, number of surgical excisions, margin status, total RT dose, and year of surgery were not significantly associated with IBTR in this cohort of women who received RT. As a sensitivity analysis, we performed the analysis excluding 54 patients with inferred RT start (n = 53) or end (n = 1) date to ensure that the main finding on the relationship between RT timing and IBTR was robust. The results from the sensitivity analyses were similar to those based on the full sample of 1323 patients. The logrank test for the full sample comparing IBTR by RT timing groups was 0.0042, and the logrank test for the sample without the 54 patients with inferred dates was P = 0.0015.
TABLE 3.
Univariate Cox Regression Analysis of Time to Ipsilateral Breast Tumor Recurrence
| Variable | n | Events (IBTR) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Time to Initiation of RT | |||||
| ≤ 8 weeks | 806 | 80 | 1.00 | ||
| > 8 weeks–12 weeks | 386 | 25 | 0.70 | 0.45–1.10 | 0.12 |
| > 12 weeks | 131 | 21 | 1.82 | 1.12–2.94 | 0.015 |
| Menopausal Status | |||||
| Pre/peri | 504 | 69 | 1.00 | ||
| Post | 818 | 57 | 0.50 | 0.35–0.71 | < 0.0001 |
| Family History | |||||
| No | 808 | 74 | 1.00 | ||
| Yes | 509 | 51 | 1.13 | 0.79–1.62 | 0.49 |
| Presentation | |||||
| Clinical | 140 | 23 | 1.00 | ||
| Radiologic | 1182 | 103 | 0.63 | 0.40–0.99 | 0.048 |
| Nuclear Grade | |||||
| Low | 106 | 6 | 1.00 | ||
| Intermediate | 570 | 48 | 1.61 | 0.68–3.76 | 0.27 |
| High | 609 | 62 | 1.62 | 0.70–3.74 | 0.26 |
| Necrosis | |||||
| Absent | 295 | 23 | 1.00 | ||
| Present | 1005 | 95 | 1.25 | 0.79–1.97 | 0.34 |
| Number of Excisions | |||||
| ≤ 2 | 1182 | 109 | 1.00 | ||
| ≥ 3 | 141 | 17 | 1.27 | 0.76–2.11 | 0.37 |
| Margins | |||||
| Positive/close | 276 | 28 | 1.00 | ||
| Negative | 995 | 89 | 1.08 | 0.71–0.66 | 0.71 |
| RT dose | |||||
| per 100 cGy increase in RT dose | 1.00* | 1.00–1.00 | 0.55 | ||
| Endocrine Therapy | |||||
| No | 970 | 107 | 1.00 | ||
| Yes | 348 | 19 | 0.43 | 0.26–0.70 | 0.0007 |
| Year of Surgery | |||||
| 1980–2000 | 342 | 62 | 1.00 | ||
| 2001–2010 | 981 | 64 | 0.82 | 0.56–1.21 | 0.31 |
Abbreviations: IBTR, ipsilateral breast tumor recurrence; HR, hazard ratio; CI, confidence interval; RT, radiation therapy
HR is expressed per 100cGy increase in RT dose
Multivariable Model for IBTR
On multivariable analysis, after adjustment for clinicopathologic and treatment factors significantly associated with IBTR on univariate analysis (menopausal status, clinical versus radiologic presentation, use of endocrine therapy), as well as factors that varied between RT timing groups (menopausal status, necrosis, endocrine therapy, RT dose), delay in RT initiation remained a significant risk factor for IBTR (Table 4, overall P < 0.02). Patients who received RT more than 12 weeks after surgery had an increased risk of IBTR compared to patients initiating RT within 8 weeks after surgery (HR 1.92, 95% CI 1.14–3.24, P = 0.014). There was no significant difference in IBTR between patients who initiated RT within 8 weeks compared to those who initiated RT between 8 to 12 weeks after surgery (HR 0.80, 95% CI 0.51–1.27, P = 0.35). Lower risk of IBTR was observed in patients who were postmenopausal (HR 0.54, 95% CI 0.37–0.78, P = 0.0009) and in those who received endocrine therapy (HR 0.45, 95% CI 0.27–0.74, P = 0.0018). Radiologic presentation, presence of necrosis, and total RT dose were not statistically significant predictors of IBTR on multivariable analysis.
TABLE 4.
Multivariate Cox Regression Analysis of Time to Ipsilateral Breast Tumor Recurrence*
| Variable | n | Events (IBTR) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Time to Initiation of RT | 0.015† | ||||
| ≤ 8 weeks | 790 | 75 | 1.00 | ||
| > 8 weeks–12 weeks | 380 | 25 | 0.80 | 0.51–1.27 | 0.35 |
| > 12 weeks | 124 | 18 | 1.92 | 1.14–3.24 | 0.014 |
| Menopausal Status | |||||
| Pre/peri | 494 | 64 | 1.00 | ||
| Post | 800 | 54 | 0.54 | 0.37–0.77 | 0.0009 |
| Presentation | |||||
| Clinical | 134 | 20 | 1.00 | ||
| Radiologic | 1160 | 98 | 0.68 | 0.42–1.10 | 0.12 |
| Necrosis | |||||
| Absent | 293 | 23 | 1.00 | ||
| Present | 1001 | 95 | 1.23 | 0.77–1.94 | 0.39 |
| RT dose | |||||
| Per 100 cGy | 1.01 | 0.97–104 | 0.77 | ||
| Endocrine Therapy | |||||
| No | 949 | 100 | 1.00 | ||
| Yes | 345 | 18 | 0.45 | 0.27–0.74 | 0.0018 |
Abbreviations: IBTR, ipsilateral breast tumor recurrence; HR, hazard ratio; CI, confidence interval; RT, radiation therapy
The multivariable model included 1294 patients with complete data and 118 IBTR events.
Type III Wald test for overall difference in IBTR event rate across the three RT timing groups.
DISCUSSION
In this population of patients with DCIS undergoing BCS with adjuvant RT, we observed higher IBTR rates among those who experienced a delay in initiation of RT following surgical excision, with a 1.9 times increased risk of IBTR in women who initiated RT more than 12 weeks after surgery as compared to patients who started RT within 8 weeks of surgery. This relationship persisted after adjusting for other variables correlated with time to RT or associated with IBTR in this population.
The overall 10-year IBTR rates for patients receiving RT within 8 weeks and between 8 to 12 weeks after surgery were 13.0% and 7.6%, respectively, which align with previous studies delineating an IBTR rate of approximately 1% per year in patients with DCIS undergoing BCS and RT.5, 8, 10, 19 Conversely, 10-year recurrence rates for patients in whom RT was delayed more than 12 weeks was 23.0%—nearly double than expected based on previous studies. Interestingly, IBTR rates in the cohort of patients starting RT more than 12 weeks from definitive surgery mirrors that seen in DCIS patients treated at our institution with BCS without adjuvant treatment.20 This suggests a smaller benefit from initiation of RT beyond 12 weeks after surgery.
While the literature shows that positive margins are associated with a higher risk of IBTR12, 21–23, we have previously demonstrated that positive or close margins were not associated with IBTR in this population of women undergoing adjuvant RT after BCS for DCIS (P = 0.95).19 Our current study reiterates these findings with the addition of RT timing in the analysis. It is likely that the observed lack of effect of positive/close margins on IBTR in this population is due to the fact that few women had positive margins. Furthermore, most “positive” or close margins were only focally positive rather than across a broad front, and most were positive or close at the fascia or the dermis rather than at a radial margin. Therefore, our patients with positive/close margins probably had a lower residual disease burden than some other studied populations.
In addition to the known clinicopathologic and treatment factors associated with increased risk of IBTR among women with DCIS, such as premenopausal status and lack of adjuvant endocrine therapy, delay in RT initiation of more than 12 weeks appears to be associated with worse outcomes. Our findings are similar to those of numerous studies demonstrating increased IBTR with RT delay more than 8 to 12 weeks after surgery for invasive breast cancer.16–18 In a meta-analysis by Huang et al examining 7401 breast cancer patients, patients who initiated RT between 9 to 16 weeks after BCS had a 62% increased risk of local recurrence at 5 years compared to patients who initiated RT within 8 weeks; however, the analysis is limited, as use of chemotherapy was not analyzed as a possible confounding variable.16 Similarly, using the Quebec Tumor Registry, Hébert-Croteau et al studied 1062 patients with stage I–II breast cancer with negative lymph nodes undergoing BCS with RT. With a mean follow-up of 7.1 years and while controlling for multiple clinicopathologic factors, such as age, comorbidity, adjuvant systemic therapies, and margins, this study found a 75% increased risk of local recurrence in women who initiated RT more than 12 weeks after surgery compared to those who initiated RT within 8 weeks.18 Delay in RT also has been shown to be associated with breast cancer-specific survival among patients with invasive breast cancer. In a SEER-based study of 13,907 women older than 65 years of age with stage I-II invasive breast cancer undergoing BCS with RT, Hershman et al found close to a four-fold increased risk of disease-specific mortality for women who initiated RT more than 12 weeks after surgery compared to patients who received RT within 12 weeks.24
The reason for an association between RT delay and IBTR is likely complex and multifactorial. Retrospective series highlight a number of variables associated with a delay in RT that may also impact IBTR risk. Madubata and colleagues examined the relationship between time to RT initiation and race among 9138 women diagnosed with DCIS between 1996 and 2011 in the Missouri Cancer Registry. While there was no difference in rates of RT usage by race, Black women experienced significant delay in initiation of RT after BCS (8.1 weeks versus 6.3 weeks in White women; odds ratio 1.9, P < 0.0001).25 In addition to a delay in RT, Black women also experienced an increased risk of IBTR, though a causal relationship could not be determined.25
Race may also affect risk of disease-specific mortality in women with DCIS. In a SEER-based review of 108,196 women, Narod et al examined breast cancer-specific mortality in patients with first primary DCIS. With a median follow-up of 7.5 years, this study revealed that Black women had a 2.4-fold increased risk of death following a diagnosis of stage 0 breast cancer when compared to White non-Hispanic women (P < 0.001), whereas other patient ethnicities included in the analysis (White Hispanic, Asian, other) did not show significantly increased risk.7 However, this retrospective study did not examine the association of race with use of or delay in adjuvant therapies.
Other factors may also affect both outcomes and timing to RT. In a study of nearly 25,000 women with invasive breast cancer, of whom more than 13,000 underwent BCS with adjuvant RT, longer time to RT initiation was associated with older age, advanced stage, being unmarried, and increased comorbidities, in addition to Black race.24 Warren et al used a two-year SEER sample of 1103 women with DCIS, of whom 7% had a Charlson comorbidity index of one or more, and found a 62% increased risk of IBTR with the presence of patient comorbidities compared to patients without comorbidities.26 In a study of 1026 women with DCIS by Gold et al, patients with higher comorbidity burden experienced 1.8 times increased odds of experiencing RT delay more than 8 weeks compared to those without the presence of comorbidities.27 Lack of data on race and comorbidities in our study cohort is a limitation, as these factors may influence timely receipt of RT and IBTR.
Another possibility is that the association between delay in RT initiation and increased IBTR risk may be related to a biologic difference in effectiveness of RT at different time points. If the effect of RT after surgery is time-dependent secondary to the inflammatory state and acute changes in the tissue microenvironment postoperatively, then one would expect a difference in therapeutic effectiveness with RT delay.
The major strengths of this study are the large study population and prospectively recorded data with long-term follow-up. Although the follow-up for this cohort ranged up to 30 years, with 311 patients being followed for at least 10 years, additional long-term follow-up is indicated for a disease process with a long-term risk of recurrence. These findings should be generalizable to other populations given that previous MSK studies20 using the same database reveal prognoses and outcomes that closely match those of other American, European, and Asian populations.28–32 Furthermore, these data have the advantage of robust clinical data as compared to population-based databases that use coded data. However, this work is subject to the same limitations of all retrospective analyses, including the inability to determine causality versus association.
CONCLUSIONS
Delay in initiation of RT more than 12 weeks following BCS for women with DCIS is associated with a significantly higher risk of IBTR. Future research should aim to understand the reasons for RT delay and to explore whether the relationship between RT delay and IBTR is causal or simply associative. While confounding factors may underlie this relationship, until a causal relationship can be excluded, efforts should be made to avoid delay in starting RT to minimize risk of recurrence.
Acknowledgments
Funding: This study was presented in part in podium format at the 2017 Society of Surgical Oncology Annual Cancer Symposium, March 15–18, Seattle, WA, and the preparation of this manuscript was supported by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of interest information
The authors have no conflict of interest disclosures to report, and this manuscript is not under consideration elsewhere.
AUTHOR CONTRIBUTIONS: Elizabeth Shurell: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, and writing–review and editing. Cristina Olcese: Formal analysis, data curation, writing–original draft, and writing–review and editing. Sujata Patil: Formal analysis, data curation, writing–original draft, and writing–review and editing. Beryl McCormick: Investigation, writing–original draft, and writing–review and editing. Kimberly J. Van Zee: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, and writing–review and editing. Melissa L. Pilewskie: Conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, and writing–review and editing.
Invitation indication: Not invited
References
- 1.American Cancer Society. Cancer Facts and Figures. 2017. [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts and Figures. 2015–2016. [Google Scholar]
- 3.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 4.Ho A, Goenka A, Ishill N, et al. The effect of age in the outcome and treatment of older women with ductal carcinoma in situ. Breast. 2011;20:71–77. doi: 10.1016/j.breast.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Subhedar P, Olcese C, Patil S, Morrow M, Van Zee KJ. Decreasing Recurrence Rates for Ductal Carcinoma In Situ: Analysis of 2996 Women Treated with Breast-Conserving Surgery Over 30 Years. Ann Surg Oncol. 2015;22:3273–3281. doi: 10.1245/s10434-015-4740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Fernandez A, Chabrera C, Garcia-Font M, et al. A study comparing two consecutive historical periods in breast cancer with a focus on surgical treatment, loco-regional recurrence, distant metastases and mortality. Clin Transl Oncol. 2015;17:296–305. doi: 10.1007/s12094-014-1227-1. [DOI] [PubMed] [Google Scholar]
- 7.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1:888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart KE, Houssami N, Taylor R, Hayen A, Boyages J. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer. 2015;15:890. doi: 10.1186/s12885-015-1904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32:3613–3618. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 12.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355:528–533. doi: 10.1016/s0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 17.Tsoutsou PG, Koukourakis MI, Azria D, Belkacemi Y. Optimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectives. Crit Rev Oncol Hematol. 2009;71:102–116. doi: 10.1016/j.critrevonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hebert-Croteau N, Freeman CR, Latreille J, Rivard M, Brisson J. A population-based study of the impact of delaying radiotherapy after conservative surgery for breast cancer. Breast Cancer Res Treat. 2004;88:187–196. doi: 10.1007/s10549-004-0594-7. [DOI] [PubMed] [Google Scholar]
- 19.Van Zee KJ, Subhedar P, Olcese C, Patil S, Morrow M. Relationship Between Margin Width and Recurrence of Ductal Carcinoma In Situ: Analysis of 2996 Women Treated With Breast-conserving Surgery for 30 Years. Ann Surg. 2015;262:623–631. doi: 10.1097/SLA.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28:3762–3769. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ER, Costantino J, Fisher B, Palekar AS, Redmond C, Mamounas E. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The National Surgical Adjuvant Breast and Bowel Project Collaborating Investigators. Cancer. 1995;75:1310–1319. doi: 10.1002/1097-0142(19950315)75:6<1310::aid-cncr2820750613>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists’ Collaborative G. Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinovich ML, Azizi L, Macaskill P, et al. The Association of Surgical Margins and Local Recurrence in Women with Ductal Carcinoma In Situ Treated with Breast-Conserving Therapy: A Meta-Analysis. Ann Surg Oncol. 2016;23:3811–3821. doi: 10.1245/s10434-016-5446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Madubata CC, Liu Y, Goodman MS, et al. Comparing treatment and outcomes of ductal carcinoma in situ among women in Missouri by race. Breast Cancer Res Treat. 2016;160:563–572. doi: 10.1007/s10549-016-4030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren JL, Weaver DL, Bocklage T, et al. The frequency of ipsilateral second tumors after breast-conserving surgery for DCIS: a population based analysis. Cancer. 2005;104:1840–1848. doi: 10.1002/cncr.21406. [DOI] [PubMed] [Google Scholar]
- 27.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer. 2008;113:3108–3115. doi: 10.1002/cncr.23923. [DOI] [PubMed] [Google Scholar]
- 28.Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20:1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Li H, Tan PH, et al. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin Oncol (R Coll Radiol) 2014;26:684–691. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Sedloev T, Vasileva M, Kundurzhiev T, Hadjieva T. Validation of the Memorial Sloan-Kettering Cancer Center nomogram in the prediction of local recurrence risks after conserving surgery for Bulgarian women with DCIS of the breast. 2nd World Congress on Controversies in Breast Cancer; Barcelona, Spain. 2016. [Google Scholar]
- 31.Collins LC, Achacoso N, Haque R, et al. Risk Prediction for Local Breast Cancer Recurrence Among Women with DCIS Treated in a Community Practice: A Nested, Case-Control Study. Ann Surg Oncol. 2015;22(Suppl 3):S502–508. doi: 10.1245/s10434-015-4641-x. [DOI] [PubMed] [Google Scholar]
- 32.Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30:600–607. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]