Abstract
The gallbladder is the hepatobiliary organ for storing and secreting bile fluid, and is a synapomorphy of extant vertebrates. However, this organ has been frequently lost in several lineages of birds and mammals, including rodents. Although it is known as the traditional problem, the differences in development between animals with and without gallbladders are not well understood. To address this research gap, we compared the anatomy and development of the hepatobiliary systems in mice (gallbladder is present) and rats (gallbladder is absent). Anatomically, almost all parts of the hepatobiliary system of rats are topographically the same as those of mice, but rats have lost the gallbladder and cystic duct completely. During morphogenesis, the gallbladder–cystic duct domain (Gb–Cd domain) and its primordium, the biliary bud, do not develop in the rat. In the early stages, SOX17, a master regulator of gallbladder formation, is positive in the murine biliary bud epithelium, as seen in other vertebrates with a gallbladder, but there is no SOX17‐positive domain in the rat hepatobiliary primordia. These findings suggest that the evolutionary loss of the Gb–Cd domain should be translated simply as the absence of a biliary bud at an early stage, which may correlate with alterations in regulatory genes, such as Sox17, in the rat. A SOX17‐positive biliary bud is clearly definable as a developmental module that may be involved in the frequent loss of gallbladder in mammals.
Keywords: biliary tract, gallbladder, modularity, mouse, rat
Introduction
Evolutionary character loss is the most dramatic change among the regressive evolutionary processes, involving the complete loss of a physical entity, such as the loss of eyes in the cavefish or the forelimbs in snakes (Jeffery, 2005; Kvon et al. 2016; Leal & Cohn, 2016; reviewed by Fong et al., 1995; Cronk, 2009). The gallbladder is a bladder‐shaped organ of the hepatobiliary system, and is a well‐known example of evolutionary loss in vertebrates, because this organ is convergently absent in various lineages only in birds and placental mammals, although it is present among other extant vertebrate lineages (for review, see Siwe, 1937; Gorham & Ivy, 1938; Oldham‐Ott & Gilloteaux, 1997). To date, there is still no convincing explanation for the mechanism of the evolutionary loss of the gallbladder. One reason for this research gap is the lack of phenomenological data in comparative anatomy and development, probably due to difficulties in dissecting the whole hepatobiliary system and conducting interspecies comparisons.
In mammals, gallbladder loss has occurred frequently in Rodentia, whereas it is present in all outgroups in Euarchontoglires (i.e. Lagomorpha, Scandentia, Dermoptera and Primates; Figs 1A, S1; Gorham & Ivy, 1938; Oldham‐Ott & Gilloteaux, 1997). These losses are thought not to be correlated with ecological factors, such as disparities in diets (Schmidt & Ivy, 1937) or bile acid transitions (Haslewood, 1967; Hagey et al. 2010; Hofmann et al. 2010), and likely rely heavily on some intrinsic factor(s), such as changes in the developmental process, although this remains unknown. Differences in the presence/absence of a gallbladder even appear between mice (Mus musculus) and rats (Rattus norvegicus): a well‐constructed gallbladder is present in mice but is completely absent in rats (Mann et al. 1920; Higgins, 1926; Thomson, 1940; Greene, 1959; Martins & Neuhaus, 2007). These two animals are phylogenetically very close, and most of their anatomical features are quite similar to each other, so a comparative study of these two animals should provide some hints to clarify the evolutionary loss of the gallbladder.
Figure 1.
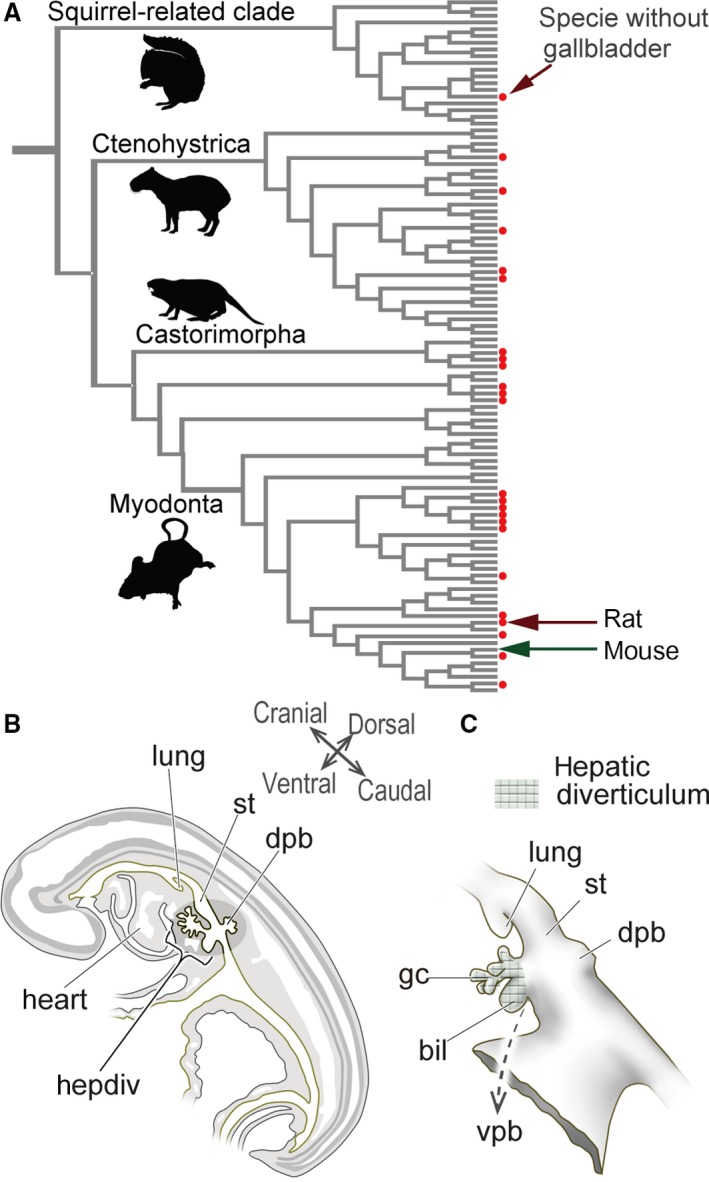
Diversity in the presence/absence of a gallbladder in rodents, and the definition of the mammalian hepatobiliary primordia. (A) Gallbladder evolution in rodents. The phylogenetic framework is based on Fabre et al. (2012), and the topology of the tree was arranged in Mesquite (Maddison & Maddison, 2011). The presence of a gallbladder is from Gorham & Ivy (1938) and Nzalak et al. (2010). The red dots indicate species that do not have gallbladders. The silhouette images are from PhyloPic (http://phylopic.org/). For more details, see Fig. S1. (B) Scheme of the left lateral view of the sagittal section of a pig embryo at the pharyngula stage. The hepatobiliary primordia arises from the foregut at the level of the septum transversum, caudal to the heart. (C) The hepatobiliary primordia in a pig embryo. The meshed domain is identified as the ‘hepatic diverticulum’ (sensu Patten, 1927). The diverticulum differentiates into the primordial liver, and biliary and ventral pancreatic buds. Of these, the biliary bud provides most of the extrahepatic biliary tract. The figures in (B) and (C) are redrawn from Patten (1927). bil, biliary bud; dpb, dorsal pancreatic bud; gc, glandular cord; hepdiv, hepatic diverticulum; lung, lung bud; st, stomach; vpb, ventral pancreatic bud. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
Anatomically, the biliary tract can be divided into two parts: the intrahepatic and extrahepatic bile duct. The former is a small network in liver tissue, which is derived from hepatoblasts; and the latter can be subdivided into several identifiable parts (e.g. gallbladder, cystic duct, common hepatic duct, common bile duct) that arise directly from the hepatic diverticulum, a morphologically definable epithelial diverticulum derived from the ventral wall of the caudal foregut in the pharyngula embryo (Fig. 1B,C; Maurer, 1906; Patten, 1927; Elias, 1955; Uemura et al. 2015). In many vertebrates, an obvious biliary bud that exclusively expresses Sox17 is found in the hepatic diverticulum (Zorn & Mason, 2001; Uemura et al. 2010, 2013). It is generally thought that the biliary bud gives rise to many parts of the extrahepatic duct, including the gallbladder, although the sequential developmental process remains unclear even in mice and rats.
In the present study, we compared the anatomy and development of the hepatobiliary system in mice and rats and explored evolutionary mechanisms in the loss of the gallbladder.
Materials and methods
Animals and gross anatomical preparation
All animal experiments were performed in accordance with the Guidelines for Animal Use and Experimentation established by the University of Tokyo. The procedures were approved by the Institutional Animal Care and Use Committee of the Graduate School of Agricultural and Life Sciences at the University of Tokyo (approval ID: P13‐763, P15‐050).
For gross anatomy, we used 8‐week‐old adult mice (M. musculus) of the C57BL/6 NCr strain and 8‐week‐old adult rats (R. norvegicus) of the Sprague–Dawley strain. We used more than four animals in each experiment. For anaesthesia, we used intraperitoneal injections of pentobarbital sodium (50 mg kg−1, i.p. Somnopentyl, Kyoritsu Shoji). The biliary tract and blood vessels were visualised by the injection of latex, as described by Higashiyama et al. (2016), and made transparent with CUBIC solution (Susaki & Ueda, 2016). For embryology, we collected mice and rat embryos and fixed them in 4% paraformaldehyde in 0.1 m phosphate‐buffered saline (PFA/PBS) after removing extra‐embryonic membranes.
Histology and immunohistochemistry
Samples were fixed in modified 4% PFA/PBS, dehydrated and embedded in paraffin wax. Sections were cut at 6 μm for most samples, and 4 μm for 9.5 dpc mice and 11.5 dpc rats. For immunohistochemistry (IHC) staining, goat anti‐SOX17 (1/100 dilution; R&D Systems), mouse anti‐PDX1 (1/400 dilution; Abcam), anti‐HNF4α (1/400 dilution; Upstate), anti‐acetylated tubulin (1/100 dilution; Sigma) and anti‐SMA (1/400 dilution; α‐smooth muscle actin; Sigma) antibodies were used. The immunoreaction was visualised by secondary antibodies conjugated with horseradish peroxidase, alkaline phosphatase or fluorescent labels. The sections were stained with Alcian blue, then with haematoxylin and eosin, according to standard protocols. We used four samples for each experiment.
Reconstruction
The embryos were reconstructed graphically from serial sections. Light microscopic images of sectioned embryos were fed into a computer using an Olympus fluorescent microscope (BX51N‐34‐FL2). The series of photos of sections were aligned, and then structures were marked using Photoshop CS5 (Adobe). Next, we conducted hand drawings of the topographical relationships of the structures after observing the sections using the microscope. These traced pictures were fed into the computer via an image scanner and then coloured using Photoshop CS5. We also created three‐dimensional images using the ImageJ software (1.48V; National Institutes of Health, USA). We reconstructed three samples for each stage briefly, and checked that they all have the same topographical relationships.
Terminology
For nomenclature, we used the descriptions of murine gross anatomy by Higashiyama et al. (2016) for adults, and also referred to Patten (1927) and Hoshino (1960) for the embryos. We use the terms ‘hepatic diverticulum’ (sensu Patten, 1927) to describe the diverticulum in the ventral wall of the caudal foregut that gives rise to most of the hepatobiliary primordia (except the dorsal pancreatic bud) and ‘biliary bud’ to describe the obvious primordium that is suspected to give rise to the distal part of the extrahepatic biliary tract (Fig. 1B,C).
Results
Gross anatomy of the extrahepatic biliary tract and blood vessels
First, we describe the gross anatomy of the whole hepatobiliary system in mice and rats. In mice, the liver has five lobes: left and right medial lobes, left and right lateral lobes, and the caudate lobe. The gallbladder is located between the left and right medial lobes, and the extrahepatic biliary tract runs on the caudal surface of the liver and opens to the duodenum (Fig. 2A,A′). The branching pattern of the biliary tract is largely correlated with the portal vein and the arteries also follow the branching patterns. The pancreatic duct branches off from the point that is far distal from the duodenum, so there is a long hepatopancreatic duct. The topographical relationships of the biliary system in the mouse are the same as those in humans and many other placental mammals (Fig. 2C).
Figure 2.
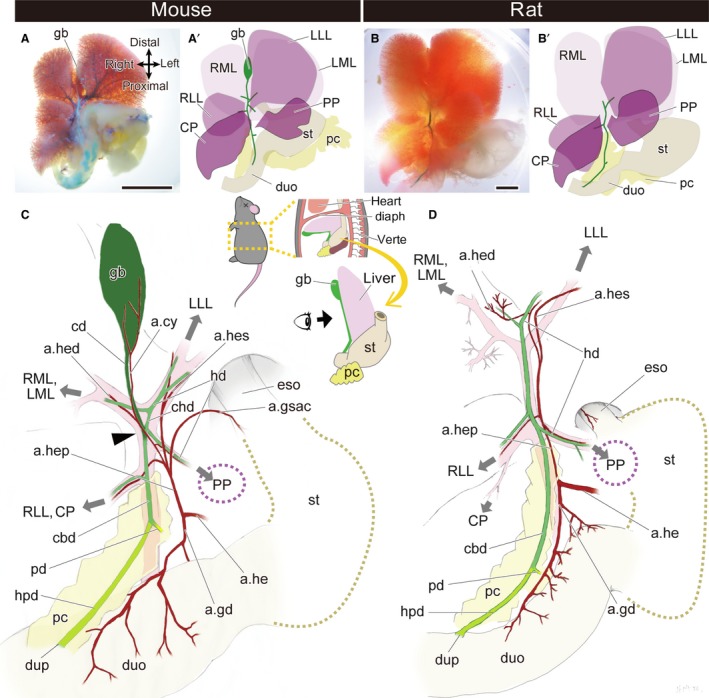
Gross anatomy of the extrahepatic biliary system in the mouse and rat. (A,A′) The morphology of the whole hepatobiliary system of the mouse. (B,B′) The morphology of the whole hepatobiliary system of the rat. (C,D) Anatomy of the hepatobiliary system of the mouse (C) and rat (D). The gallbladder and cystic duct are coloured in dark green, the hepatopancreatic duct is light green, and the other bile ducts are middle green. The arrowhead indicates the branching point of the cystic duct and the common hepatic duct. The grey arrows indicate the directions of the portal veins that supply the liver lobes. a.cy, arteria cystica; a.gd, arteria gastroduodenalis; a.gsac, arteria gastrica sinistra accessoria; a.he, arteria hepatica communis; a.hed, arteria hepatica dextra; a.hep, arteria hepatica propria; a.hes, arteria hepatica sinistra; cbd, common bile duct; cd, cystic duct; chd, common hepatic duct; CP, caudate process of the caudate lobe; diaph, diaphragm; duo, duodenum; dup, duodenal papilla; eso, oesophagus; gb, gallbladder; hd, hepatic duct; hpd, hepatopancreatic duct; LLL, left lateral lobe; LML, left medial lobe; pc, pancreas; pd, pancreatic duct; PP, papillary process of the caudate lobe; RLL, right lateral lobe; RML, right medial lobe; st, stomach; verte, vertebrae. Scale bar: 1 cm. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
In rats, the liver has five lobes, as seen in mice. The extrahepatic biliary tract runs on the caudal part of the liver and opens into the duodenum, whereas the gallbladder is completely absent, with no vestige (Fig. 2B,B′). In mice, the cystic duct and common hepatic duct branch off at the same level of the bifurcation of the portal veins, the branches of which enter the medial lobes and left lateral lobe of the liver (Fig. 2C). The cystic duct detaches from the liver from this point (arrowhead in Fig. 2C), and then continues to the gallbladder. However, in rats, the cystic duct does not arise from the level of the bifurcated point of the portal vein, and the distal part of the biliary tract enters the liver lobes, as seen in the common hepatic duct in mice (Fig. 2D). Thus, not only the gallbladder but also the cystic duct is completely absent in rats. Except for this absence of the gallbladder–cystic duct domain (the Gb–Cd domain; coloured dark green in Fig. 2C), the anatomy of the hepatobiliary system of rats is similar to that of mice: the biliary tract runs along the portal vein, the arterial system is produced from the hepatic artery, and the pancreatic duct branches far from the duodenal wall, so rats have long hepatopancreatic ducts, as do mice (Fig. 2C,D).
Developmental process of the extrahepatic biliary system in mice and rats
To clarify the developmental sequences of the whole biliary system in mice, histological sections were prepared and reconstruction analyses were conducted (Figs 3and4). At 10.5 dpc, the biliary and pancreatic buds had grown in the caudal foregut, and the primordial liver was obvious (Fig. 3A–A′′). The umbilical vein supplied the primordial liver and passed through the left lateral side of the biliary bud. At the level of the proximal limit of the biliary bud, a small glandular cord had branched and entered into the primordial liver (Fig. 3A). At 11.5 dpc, the umbilical and vitelline veins were clearly formed (Fig. 3B). The biliary bud extended beside the umbilical vein and formed the extrahepatic biliary tract. The tract started from the duodenum and extended cranially, and made a 90° turn at the same position as the curve of the umbilical vein, just caudal to the liver. Distal to the curve, the biliary bud had extended ventrally to form the primordial gallbladder in the mesenchymal tissue caudal and adjacent to the liver. The common hepatic duct, the derivative of the glandular cord, was clearly apparent at the point of the 90° curve of the tract (Fig. 3B′,B′′). Thus, the Gb–Cd domain could be identified clearly as the distal part from the branching point of the common hepatic duct (arrowheads in Fig. 3) at this stage morphologically. The dorsal and ventral pancreatic buds fused into the single primordial pancreas (Fig. 3B). At 13.5 dpc, the posterior vena cava disappeared and the vena umbilicalis began to degenerate. The biliary tract proceeded to extend through the portal vein (Fig. 3C). At 15.5 dpc, the distal portion of the vena umbilicalis and vena vitelline became very thin and they largely lost the connection with the umbilical cord. The distal portion of the Gb–Cd domain became bladder‐shaped (Fig. 4D′), and the gallbladder was differentiated in the domain (Fig. 3D). The arteria cystica was formed beside the gallbladder and cystic duct (Fig. S2).
Figure 3.
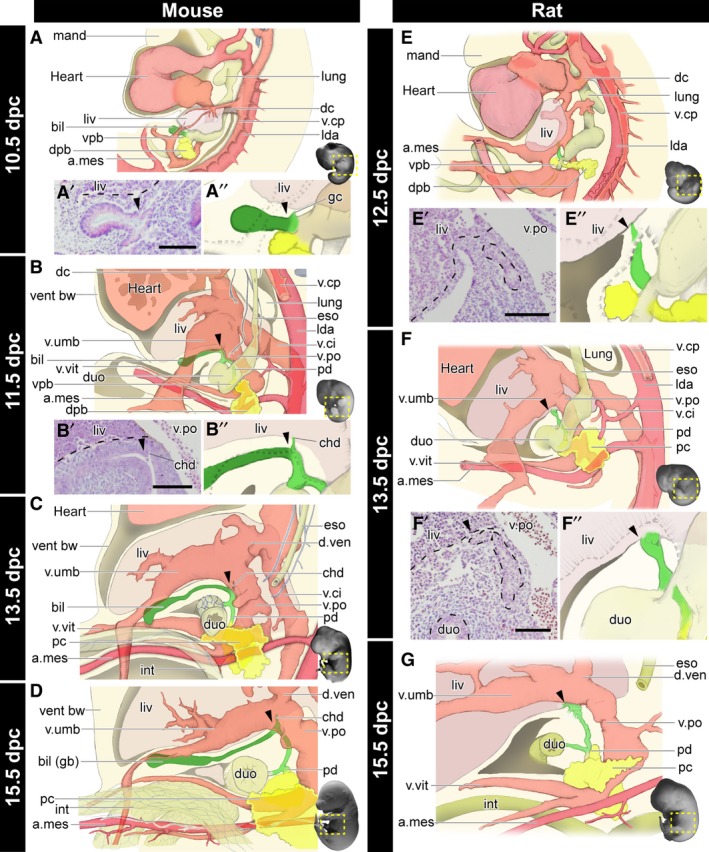
Morphogenesis of the biliary system in the mouse and rat. All panels are left lateral views. See also Figs 4 and S2 for the histological sections, digitally reconstructed models and latex‐injected models. (A–D) Developmental scheme of the hepatobiliary system in mice. The biliary bud (green), pancreas (yellow), artery (magenta), and vein (pink) are drawn. The gallbladder–cystic duct (Gb–Cd) domain (dark green) can be identified as the distal domain from the branching point (arrowheads) of the common hepatic duct (chd). Details of the common hepatic ducts are shown in panels (A’), (A’’) and (B’), (B’’). (E–G) The developmental scheme in rats. The arrowheads indicate the landmarks comparable with mice. The Gb–Cd domain is clearly absent. a.mes, arteria mesenterica cranialis; bil, biliary bud; chd, common hepatic duct; dc, ductus cuvieri; dpb, dorsal pancreatic bud; duo, duodenum; d.ven, ductus venosus; eso, oesophagus; gb, gallbladder; gc, glandular cord; int, intestine; lda, left dorsal aorta; liv, liver or liver primordium; lung, lung bud; mand, mandibular process; pc, pancreas; pd, pancreatic duct; v.ci, vena cava inferior; v.cp, vena cava posterior; vent bw, ventral bodywall; vpb, ventral pancreatic bud; v.po, vena portae; v.umb, vena umbilcailis; v.vit, vena vitelline. Scale bar: 500 μm. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
Figure 4.
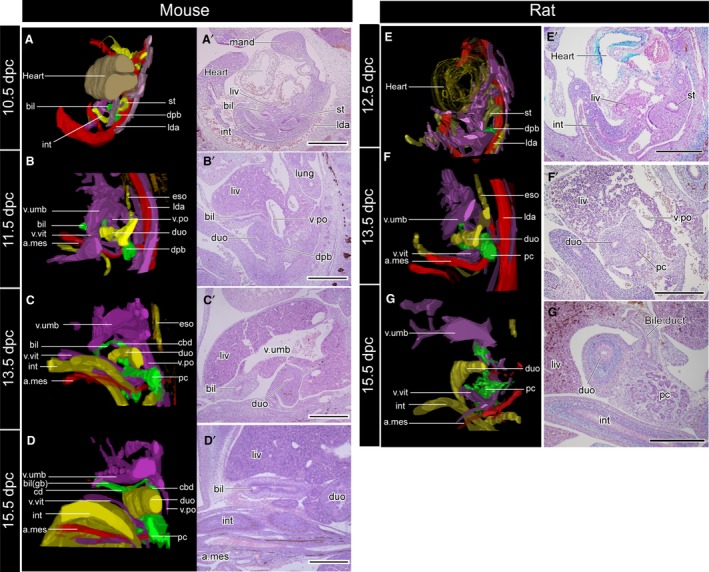
Three‐dimensional reconstructed models (A–D) and the underlying histological sections (A′–D′). The topographical relationships of all samples are the same as in Fig. 3. The sections were subjected to immunohistochemical (IHC) staining for acetylated tubulin to visualise the peripheral nerves. The biliary tract is innervated by the intestinal part of the vagus, but the nerve supplies had not been formed at the developmental stages examined in the present study. a.mes, arteria mesenterica cranialis; bil, biliary bud; cbd, common bile duct; cd, cystic duct; dpb, dorsal pancreatic bud; duo, duodenum; eso, oesophagus; gb, gallbladder; int, intestine; lda, left dorsal aorta; liv, liver or liver primordium; lung, lung bud; mand, mandibular process; pc, pancreas; v.po, vena portae; v.umb, vena umbilcailis; v.vit, vena vitelline. Scale bar: 500 μm. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
In 12.5 dpc rats, comparable with 10.5 dpc mice, the extrahepatic bile duct was observed on the right of the portal vein, which connected the primordial liver and the caudal foregut, whereas the biliary bud was not found morphologically (Fig. 3E,E′). In 13.5 dpc rats, the morphology of the blood vessels and digestive tract was very similar to that of 11.5 dpc mice (Fig. 3B,F), and the extrahepatic biliary tract had obviously formed, whereas the Gb–Cd domain was completely absent (Fig. 3F,F′′). Considering the topographical relationship with the blood vessels, the distal part of the biliary tract that enters the liver is obviously a structure homologous to the common hepatic duct in mice. Thus, the homologous position of the proximal end of the Gb–Cd domain was identified in rats (arrowheads), although there was no sign of the domain. The Gb–Cd domain had formed in the mesenchymal tissue, caudal to the liver in mice (Figs 3B–B′′ and 4A–D′); however, this mesenchymal region did not appear in rats (Figs 3F–F′′ and 4E–G′). At 15.5 dpc in rats, the major part of the extrahepatic biliary tract was formed. The distal part of the duct formed many branches, as though making a plexus around the portal vein. The Gb–Cd domain did not appear at any point throughout development (Fig. 3G).
Early molecular patterning of the biliary bud in mice and rats
To clarify the patterning of the primordial biliary bud in early‐stage embryos, we conducted IHC to detect HNF4α, SOX17 and PDX1, which are known generally as molecules specific for the hepatic primordia, biliary and pancreatic bud, respectively (Zorn & Mason, 2001; Uemura et al. 2010). We prepared 9.5 dpc mouse and 11.5 dpc rat embryos; the morphologies of the pharyngeal arches and heart were similar to each other (Fig. 5A,I). Obvious hepatic diverticula were found in these embryos, just caudal to the ductus cuvieri and at the level of the septum transversum (Fig. 5B,J). In mice, the primordial liver, biliary bud and ventral pancreatic bud appeared obviously on the ventral side of the caudal foregut, and the dorsal pancreatic bud was in the dorsal side of the foregut (Fig. 5B). As is known, HNF4α and PDX1 were positive in the liver primordium and epithelia of the pancreatic bud, respectively (Figs 5D–F and 6). A SOX17‐positive domain was found in the biliary bud epithelium (Figs 5E and 6). In rats, histologically, the separation of the buds was unclear in comparison with that in mice (Fig. 5K). The HNF4α signal was positive in the primordial liver and the cranial half of the hepatic diverticulum (Figs 5L and 6). However, SOX17 was negative in the hepatic diverticulum in rats, whereas it was positive in the endoderm of blood vessels and primordial thyroid (Figs 5M and S3). PDX1 was positive in a comparable domain with mouse embryos: the dorsal and ventral pancreatic buds (Fig. 5N). At later stages, SOX17 was strongly positive in the distal portion of the biliary bud, which corresponds with the future Gb–Cd domain in mouse embryos (Fig. 5G,H), while it was negative in any hepatobiliary structure in rat embryos during development (Fig. 5O,P).
Figure 5.
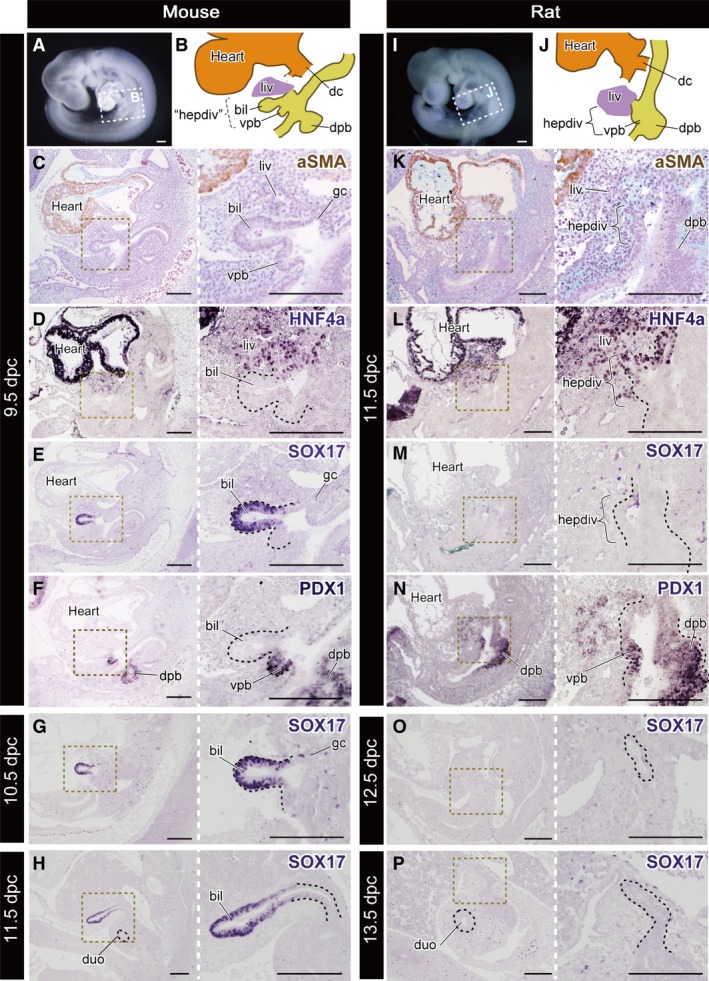
Molecular patterning of the hepatobiliary primordia. All panels are left lateral views. (A) The mouse embryo at 9.5 dpc. The scheme of the hepatobiliary primordia is shown in (B). ‘hepdiv’ in (B) indicates the presumptive domain that corresponds to the hepatic diverticulum of the 11.5 dpc rat in (J). (C–F) Histological sections of the same 9.5‐dpc embryo with immunohistochemical (IHC) staining for aSMA, HNF4a, SOX17 and PDX1. (G,H) Sections of 10.5‐ and 11.5‐dpc mice stained for SOX17. The SOX17‐positive domain is localised in the distal portion of the biliary bud. (I) A rat embryo at 11.5 dpc. The scheme of the hepatobiliary primordia is shown in (J). (K–N) Sections of the same 11.5‐dpc embryo with IHC staining in the same manner as the mouse. (M,N) Sections of 12.5‐ and 13.5‐dpc rats stained for SOX17. The black dotted line in the higher‐magnification panels indicates the extrahepatic biliary tract. bil, biliary bud; dc, ductus cuvieri; dpb, dorsal pancreatic bud; duo, duodenum; gc, glandular cord; hepdiv, hepatic diverticulum; liv, liver or liver primordium; vpb, ventral pancreatic bud. Scale bars: 200 μm. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption].
Figure 6.
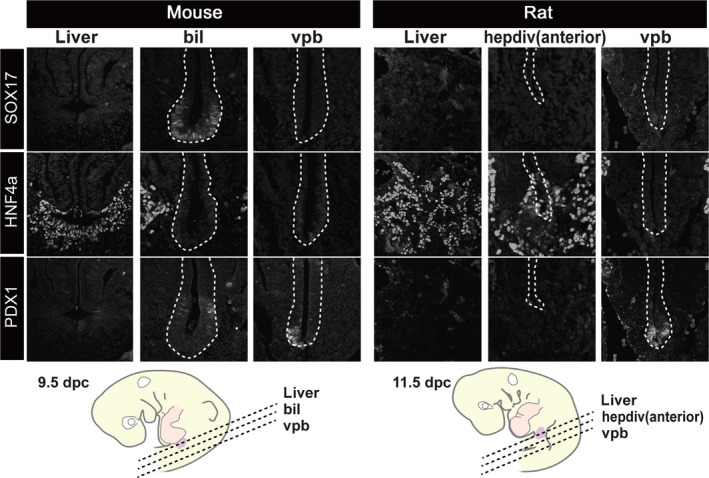
Patterns of molecular markers that are common in the hepatobiliary primordia. Transverse sections were made at the levels shown in the scheme. The SOX17‐positive domain was clearly localised in the distal portion of the biliary bud in the mouse, but there was no equivalent signal in the rat embryo. HNF4a (liver tissue) and PDX1 were positive in the liver and pancreatic primordia in both animals. bil, biliary bud; hepdiv, hepatic diverticulum; vpb, ventral pancreatic bud. cale bars: 200 μm. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
Discussion
In the present study, we compared the anatomy and development of the hepatobiliary system between mice and rats. Anatomically, the Gb–Cd domain was completely absent in rats, while the topographical relationships between other hepatobiliary domains were quite similar in the two animals. The Gb–Cd domain did not appear at any stage in development in rats, and this was because of the lack of a biliary bud in early embryos. This absence appears to be correlated with the loss of Sox17 expression in the hepatic diverticulum, which plays a role in the differentiation of the gallbladder in mice and other vertebrates that have complete gallbladders (see below).
Gb–Cd domain may be lost with no correlation with remodelling of any other extrahepatic biliary structure
In this study, we confirmed that the topographical relationship of the hepatobiliary system in rats is quite similar to the typical pattern in mice, with the exception of the Gb–Cd domain. Because the topographical relationship of the biliary system in mouse represents a synapomorphy in mammals (Higashiyama et al. 2016), the pattern in rats should be translated as a simple loss in the local Gb–Cd domain, with no correlation with dynamic remodelling of the remaining extrahepatic hepatobiliary system from the ancestral condition in mammals. This is consistent with previous suggestions from the anatomical descriptions of other mammalian species, such as the horse, deer and camel: specifically, the presence or absence of a gallbladder is not correlated with either qualitative (e.g. branching pattern) or quantitative traits (e.g. length or diameter) of the biliary tract (Ellenberger & Baum, 1903; Mann et al. 1920; Stevenson, 1921; Thomson, 1940).
According to descriptions of the embryonic hepatobiliary primordial morphology in various mammals, the developmental pattern of the biliary tract in mice should be conserved among the mammals with gallbladders, even in echidna, whereas the budding position of the ventral pancreatic bud varies slightly (Keibel, 1904; Elias, 1955; Godlewski et al. 1997). Also, the molecular background of the primordial hepatobiliary system seems to be shared among vertebrates including non‐mammalian groups (see below). Thus, the developmental mechanisms of the hepatobiliary system may be conserved at least among mammals. The present study found that the hepatobiliary development of the rat is quite similar to that of the mouse with the exception of the Gb–Cd domain. This result is consistent with a previous report comparing human and rat embryos (Godlewski et al. 1997). The developmental process of a mammal that does not have a gallbladder has been rarely reported, except the rat (Higgins, 1926). Thus, whether the rat pattern is shared among other mammals without gallbladders or not remains unclear. But, according to the adult anatomy, the topographical relationship of the major part of the extrahepatic biliary tract is largely conserved in rats and several other mammals without gallbladders (Ellenberger & Baum, 1903; Mann et al. 1920; Stevenson, 1921; Schmaltz, 1927; Thomson, 1940). Thus, despite the presence or absence of the Gb–Cd domain, the morphology of a large part of the extrahepatic biliary tract and its development is normally conserved, at least among mammals. Considering this, the evolutionary loss of the gallbladder may be caused by the same mechanism in mammals.
The pattern of the biliary system in rats is similar to some case reports of human patients describing the congenital absence of the gallbladder and cystic duct (Gerwig et al. 1961; Richards, 1966). Thus, the rat might be a suitable model for these patients. However, the branching point of the pancreatic duct in mice and rats is distal from the duodenal wall, and thus rats have long hepatopancreatic ducts, as reported previously (Higgins, 1926; Thomson, 1940; Kararli, 1995). This trait differs from humans; the human pancreatic duct usually opens to the bile duct in the duodenal wall, so the physiological state of mice and rats may differ from that of humans. In most rodents, the pancreatic duct forms an anastomosis with the biliary tract, as the same pattern in humans (Schwegler & Boyden, 1937; Breazile & Brown, 1976). Thus, the long hepatopancreatic duct may be a synapomorphy of mice and rats, or perhaps of Murinae.
A SOX17‐positive biliary bud is completely lost in rats
The major molecular background of the hepatobiliary primordia is thought to be conserved among vertebrates. For example, Pdx1 expression in the embryonic pancreas is found not only in mice, but also in Xenopus (Pearl et al. 2009) and zebrafish (Biemar et al. 2001). Sox17 is expressed in the biliary bud in mice (Matsui et al. 2006; Spence et al. 2009), Xenopus (Zorn & Mason, 2001) and zebrafish (Shin et al. 2012). A complete gallbladder even exists in cyclostomes (Youson, 1993), and Sox17/18, the gene orthologous to Sox17, is expressed in the visceral endoderm in lamprey, and it is thought to play a role in the patterning of the visceral organs (Takeuchi et al. 2009). At least in mice, Sox17 insufficiency causes an immature gallbladder, resulting in a congenital biliary atresia‐like syndrome (Uemura et al. 2010, 2013), and its conditional knockout can result in complete loss of the gallbladder (Spence et al. 2009; Higashiyama et al. 2017). Thus, Sox17 is known to be a key gene for the formation of the gallbladder. In the present study, we found that SOX17 was negative in the hepatic diverticulum in rats. Phylogenetically, it should be a derived trait in the rat lineage, which may be synchronised with the loss of the Gb–Cd domain (Fig. 7). In contrast, the other hepatobiliary domains of rats were formed quite similarly to those in mice. This finding suggests that a large part of the extrahepatic duct is derived from the surrounding region of the biliary bud, which is not under Sox17 regulation.
Figure 7.
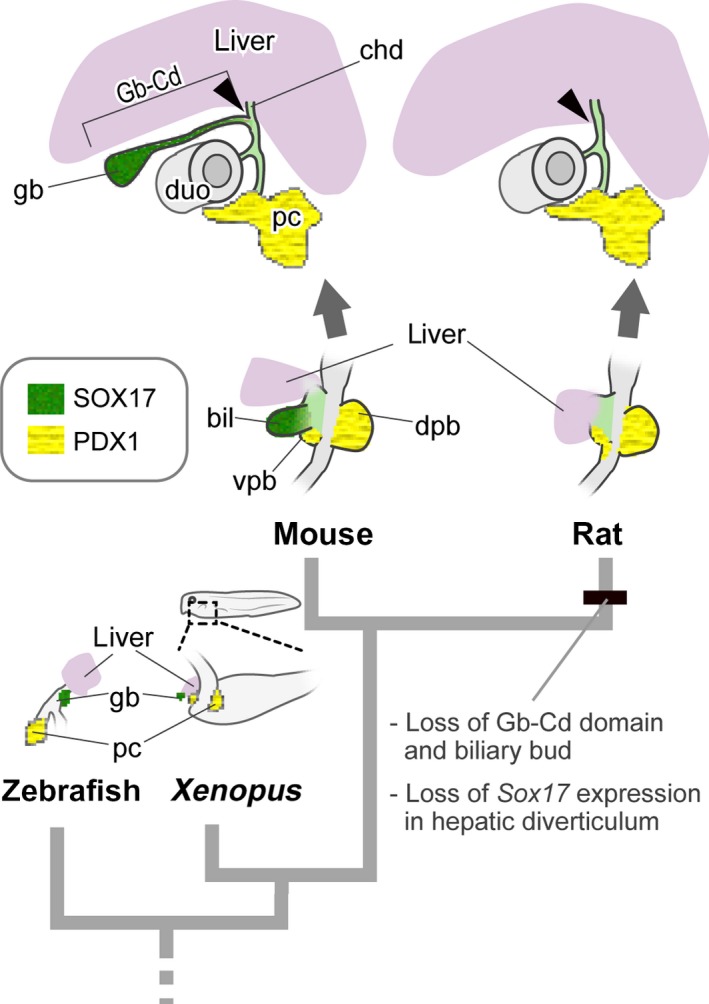
Evolutionary scenario of gallbladder loss in the rat. The schemes for zebrafish and Xenopus are drawn after Shin et al. (2012) and Zorn & Mason (2001), respectively. An obvious hepatic diverticulum is found in the ventral foregut in the rat, as seen in many other vertebrates. However, the SOX17‐positive biliary bud is completely lost in rats, while Sox17 is expressed in other animals that have gallbladders. bil, biliary bud; cd, cystic duct; chd, common hepatic duct; dpb, dorsal pancreatic bud; duo, duodenum; gb, gallbladder; pc, pancreas; vpb, ventral pancreatic bud. [Correction added on 24 October 2017, after first online publication: the abbreviations cited on this figure was added on figure caption]
It remains unclear whether the loss of Sox17 in the hepatic diverticulum in rats is caused by a mutation in Sox17 itself or by the changes in regulation further upstream. In mice, the normal differentiation of the Sox17‐positive biliary bud has been suggested to be induced by signals such as Fgf10, which is expressed in the adjacent mesenchyme of the septum transversum (Saito et al. 2013). Because the sizes of the mesenchymal domain caudal and adjacent to the liver are very different between mouse and rat embryos, the loss of Gb–Cd domain in the rat may be caused by the lack of its surrounding mesenchyme that induces the expression of Sox17. This is consistent with recent studies with interspecific chimerism, with the injection of rat pluripotent stem cells (PSCs) into a murine blastocyst, or murine PSCs into a rat blastocyst. In the former case, the gallbladder never develops in the host (i.e. rat), whereas in the latter case, the gallbladder developed and the rat cells contributed its epithelium (Kobayashi et al. 2010; Wu et al. 2017). These results may support the idea that the arising of the biliary bud is firstly induced by signals from the neighbouring mesenchyme.
The lack of Sox17 expression in the hepatic diverticulum might be relevant with the development of the peribiliary glands (PBGs), the tubulo‐alveolar glands found within the biliary tract walls in the rats. Recent studies have revealed that the multipotent stem/progenitor cells exist in the PBGs, and they expressed Sox17 in human and mouse (Cardinale et al. 2011; Carpino et al. 2012). On the other hand, the gene expression of the PBGs in rats remains unclear, while the obvious PBGs are found among the biliary wall in the rats (Berthoud et al. 1992). Considering the development of the biliary tract of rats is free from the Sox17 regulation, Sox17 might not be expressed in the PBGs. Further studies are required to determine whether Sox17 is activated or not in PBGs in rats.
Modularity and evolutionary loss of the gallbladder
In mice, the Gb–Cd domain is derived from the biliary bud, but the primordium is completely absent in rats. Thus, the evolutionary loss of the gallbladder occurs in a different context than secondary degeneration during development, such as eye loss in the cavefish (Jeffery, 2005), but it should be comparable with the loss of all morphological traces, including the primordia, such as the pelvic apparatus in sticklebacks (Chan et al. 2010) and the limb bud in snakes (Kvon et al. 2016; Leal & Cohn, 2016).
Similar to the famous example such as the limb bud, the biliary bud should be considered as a developmental module at the organ level, which is the developmentally well‐integrated unit that can develop semi‐autonomously from another body part of the embryo (Raff, 1996; Schlosser & Wagner, 2004). This is supported by the following evidence: (i) the biliary bud is derived in the same position in the ventral caudal foregut at the level of the septum transversum and can be identified as a homologous structure in terms of comparative anatomy among many vertebrates (Elias, 1955); (ii) Sox17 and its downstream genes are expressed specifically in the biliary bud, and its conditional deletion can delete the gallbladder and cystic duct (Higashiyama et al. 2017); and (iii) the isolated biliary bud can be cultured from the embryonic stage to a gallbladder with well‐constructed smooth muscle layer without any exogenous signalling from other body parts in vitro (Uemura et al. 2013; Higashiyama et al. 2017).
The variation in hepatobiliary anatomy may indicate modularity in the Gb–Cd domain or the biliary bud. As described above, the presence or absence of a gallbladder does not correlate with the remodelling of the remaining parts of the extrahepatic biliary tract. On the other hand, the branching pattern of the tract is also changed markedly in some animals that have complete gallbladders, such as the network‐like tract in the chinchilla (Nowak et al. 2015). The length of the structures in the biliary system and the branching pattern of the small arteries also vary, even in the same species (Higashiyama et al. 2016). Thus, the evolutionary changes in the Gb–Cd domain and other biliary tract structures may have occurred quasi‐independently, and the evolutionary loss of gallbladder may have occurred without remodelling of the rest of the hepatobiliary system in mammals.
The modularity of the Gb–Cd domain or the biliary bud may act as a module of evolution. Phylogenetically, the Gb–Cd domain has been lost repeatedly without being obviously associated with the modification of other body parts in rodents (Figs 1a and S1). This evolutionary trend appears to follow a pattern of dissociated co‐evolution: the frequent loss of a module without affecting the context (Schlosser, 2004).
In conclusion, the Gb–Cd domain or the biliary bud is a developmental module that develops semi‐autonomously from other body parts. Moreover, as seen in the gallbladder agenesis in human patients or in other mammals that normally have a gallbladder, the complete absence of the domain may not be lethal in mammals while it sometimes induces diseases (Higgins, 1927; Kamishina et al. 2010). There are also a few species reported where the presence or absence of the gallbladder varies by individual, such as in giraffe and a rodent species, Oxymycterus dasythrichus (Wakuri & Hori, 1970; Geise et al. 2004). Thus, the Gb–Cd domain may have relatively high flexibility among the organs, and high evolvability, at least in mammals. It is still unclear what mechanism(s) cause(s) the evolutionary loss of gallbladder repeatedly only in avians and placental mammals. Based on previous reports, avians may lose the gallbladder by a different mechanism, because the biliary bud has been reported to exist in the pigeon embryo, which does not have a gallbladder (Scammon, 1916). Developmental and anatomical studies of the hepatobiliary system in various organisms will help clarify this long‐standing issue.
Conflict of interest
The authors declare no conflict of interest in relation to the research, results, their interpretation or publication.
Author contributions
The research design and most of the experiments and observations were conducted by HH. The IHC staining of anti‐SOX17, ‐PDX1 and ‐HNF4α was performed by MU. The draft manuscript was written by HH, and all authors provided critical revisions of the manuscript.
Funding
This work was supported mainly by financial grants from the Ministry of Education, Science, Sports and Culture of Japan to Y. Kanai (S‐24228005).
Supporting information
Fig. S1. The presence and absence of a gallbladder in phylogenetic tree of rodents.
Fig. S2. Whole‐mount anatomy of the abdominal cavity murine fetus at 15.5 dpc.
Fig. S3. SOX17‐positive structures in mouse and rat embryos.
Acknowledgements
The authors are grateful to Ms Yuki Uchiyama for her kind support and advice on treatment of mice, Dr Takao Suzuki for the critical advice on the concepts of modularity, and Ms Kana Hayakawa for providing some information for the discussion. The authors also thank Dr Aisa Ozawa, Mr Ko Fujino and Mr Montri Pattarapanawan for checking the manuscript; and Ms Itsuko Yagihashi for secretarial assistance.
Contributor Information
Hiroki Higashiyama, Email: h-hiroki@m.u-tokyo.ac.jp.
Yoshiakira Kanai, Email: aykanai@mail.ecc.u-tokyo.ac.jp.
References
- Berthoud HR, Kressel M, Neuhuber W (1992) An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol 186, 431–442. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, et al. (2001) Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol 230, 189–203. [DOI] [PubMed] [Google Scholar]
- Breazile JE, Brown EM (1976) Anatomy In: The Biology of the Guinea Pig. (eds Wagner JE, Manning PJ.), pp. 53–62. New York, NY: Academic Press. [Google Scholar]
- Cardinale V, Wang Y, Carpino G, et al. (2011) Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 54, 2159–2172. [DOI] [PubMed] [Google Scholar]
- Carpino G, Cardinale V, Onori P, et al. (2012) Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 220, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Marks ME, Jones FC, et al. (2010) Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 327, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk QC (2009) Evolution in reverse gear: the molecular basis of loss and reversal. Cold Spring Harb Symp Quant Biol 74, 259–266. [DOI] [PubMed] [Google Scholar]
- Elias H (1955) Origin and early development of the liver in various vertebrates. Acta Hepatol 3, 1–56. [Google Scholar]
- Ellenberger W, Baum H (1903) Handbuch der vergleichenden Anatomie der Haustiere, Zehnte Auflage. Berlin: Verlag von August Hirschwald. [Google Scholar]
- Fabre PH, Hautier L, Dimitrov D, et al. (2012) A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol 12, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DW, Kane TC, Culver DC (1995) Vestigialization and loss of nonfunctional characters. Annu Rev Eco Syst 26, 249–268. [Google Scholar]
- Geise L, Weksler M, Bonvicino C (2004) Presence or absence of gall bladder in some Akodontini rodents (Muridae, Sigmodontinae). Mamm Biol (Z Säugetierkunde) 69, 210–214. [Google Scholar]
- Gerwig WH Jr, Countryman LK, Gomez AC (1961) Congenital absence of the gallbladder and cystic duct: report of six cases. Ann Surg 153, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Gaubert‐Cristol R, Rouy S, et al. (1997) Liver development in the rat and in man during the embryonic period (Carnegie stages 11–23). Microsc Res Tech 39, 314–327. [DOI] [PubMed] [Google Scholar]
- Gorham F, Ivy AC (1938) General function of the gall bladder from the evolutionary standpoint. Zool Ser Field Mus Nat Histo 22, 159–213. [Google Scholar]
- Greene EC (1959) The Anatomy of the Rat. New York, NY: Hafner. [Google Scholar]
- Hagey LR, Vidal N, Hofmann AF, et al. (2010) Evolutionary diversity of bile salts in reptiles and mammals, including analysis of ancient human and extinct giant ground sloth coprolites. BMC Evol Biol 10, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslewood G (1967) Bile salt evolution. J Lipid Res 8, 535–550. [PubMed] [Google Scholar]
- Higashiyama H, Sumitomo H, Ozawa A, et al. (2016) Anatomy of the murine hepatobiliary system: a whole‐organ‐level analysis using a transparency method. Anat Rec 299, 161–172. [DOI] [PubMed] [Google Scholar]
- Higashiyama H, Ozawa A, Sumitomo H, et al. (2017) Embryonic cholecystitis and defective gallbladder contraction in the Sox17‐haploinsufficient model of biliary atresia. Development 144, 1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GM (1926) The biliary tract of certain rodents with and those without a gall bladder. Anat Rec 32, 89–111. [Google Scholar]
- Higgins GM (1927) The extrahepatic biliary tract in the guinea‐pig. Anat Rec 36, 129–147. [Google Scholar]
- Hofmann AF, Hagey LR, Krasowski MD (2010) Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res 51, 226–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino G (1960) Studies on the morphological changes of v. umbilicalis in the liver of embryonic stage. J Showa Med Ass 20, 400–415 (in Japanese). [Google Scholar]
- Jeffery WR (2005) Adaptive evolution of eye degeneration in the Mexican blind cavefish. J Hered 96, 185–196. [DOI] [PubMed] [Google Scholar]
- Kamishina H, Katayama M, Okamura Y, et al. (2010) Gallbladder agenesis in a Chihuahua. J Vet Med Sci 72, 959–962. [DOI] [PubMed] [Google Scholar]
- Kararli TT (1995) Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16, 351–380. [DOI] [PubMed] [Google Scholar]
- Keibel F (1904) Zur Entwickelung der Leber, des Pankreas und der Milz bei Echidna aculeata var. typica. Jena: Verlag von Gustav Eschen. [Google Scholar]
- Kobayashi T, Yamaguchi T, Hamanaka S, et al. (2010) Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 142, 787–799. [DOI] [PubMed] [Google Scholar]
- Kvon EZ, Kamneva OK, Melo US, et al. (2016) Progressive loss of function in a limb enhancer during snake evolution. Cell 167, 633–642 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal F, Cohn MJ (2016) Loss and re‐emergence of legs in snakes by modular evolution of Sonic hedgehog and HOXD enhancers. Curr Biol 26, 1–8. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2011) Mesquite: a Modular System for Evolutionary Analysis. Version 2.75 http://mesquiteproject.org
- Mann F, Brimhall S, Foster J (1920) The extrahepatic biliary tract in common domestic and laboratory animals. Anat Rec 18, 47–66. [Google Scholar]
- Martins PNA, Neuhaus P (2007) Surgical anatomy of the liver, hepatic vasculature and bile ducts in the rat. Liver Int 27, 384–392. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kanai‐Azuma M, Hara K, et al. (2006) Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci 119, 3513–3526. [DOI] [PubMed] [Google Scholar]
- Maurer F (1906) Die Entwickelung des Darmsystems In: Handbuch der Vergleichenden und Experimentellen Entwickelungslehre der Wirbeltiere, Band II, Teil 1. (eds Hertwigs O.), pp. 109–252. Jena: Gustav Fischer. [Google Scholar]
- Nowak E, Kuchinka J, Szczurkowski A, et al. (2015) Extrahepatic biliary tract in Chinchilla (Chinchilla laniger, Molina). Anat Histol Embryol 44, 236–240. [DOI] [PubMed] [Google Scholar]
- Nzalak J, Ibe C, Dauda S, et al. (2010) Weight assessment of some accessory digestive organs in the adult african giant pouched rat (Cricetomys gambianus, Waterhouse‐1840). Niger Vet J 31, 300–301. [Google Scholar]
- Oldham‐Ott CK, Gilloteaux J (1997) Comparative morphology of the gallbladder and biliary tract in vertebrates: variation in structure, homology in function and gallstones. Microsc Res Tech 38, 571–597. [DOI] [PubMed] [Google Scholar]
- Patten BM (1927) Embryology of the Pig. Philadelphia, PA: Blakiston. [Google Scholar]
- Pearl EJ, Bilogan CK, Mukhi S, et al. (2009) Xenopus pancreas development. Dev Dyn 238, 1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff RA (1996) The Shape of Life: Genes, Development, and the Evolution of Animal Form. London and Chicago: University of Chicago Press. [Google Scholar]
- Richards RN (1966) Congenital absence of the gallbladder and cystic duct associated with primary carcinoma of the common bile duct. Can Med Assoc J 94, 859–860. [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Kojima T, Takahashi N (2013) The septum transversum mesenchyme induces gall bladder development. Biol Open 2, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammon RE (1916) On the development of the biliary system in animals lacking a gall‐bladder in postnatal life. Anat Rec 10, 543–558. [Google Scholar]
- Schlosser G (2004) The role of modules in development and evolution In: Modularity in Development and Evolution. (eds Schlosser G, Wagner GP.), pp. 519–582. London and Chicago: University of Chicago Press. [Google Scholar]
- Schlosser G, Wagner GP (2004) Modularity in Development and Evolution. London and Chicago: University of Chicago Press. [Google Scholar]
- Schmaltz R (1927) Atlas der Anatomie des Pferdes. 4 Teil: Die Eingeweide in topographischen und Einzeldarstellungen. Berlin: Verlagsbuchhandlung R. Schoetz. [Google Scholar]
- Schmidt C, Ivy A (1937) The general function of the gall bladder. Do species lacking a gall bladder possess its functional equivalent? The bile and pigment output of various species of animals. J Cell Comp Physiol 10, 365–383. [Google Scholar]
- Schwegler RA, Boyden EA (1937) The development of the pars intestinalis of the common bile duct in the human fetus, with special reference to the origin of the ampulla of Vater and the sphincter of Oddi I The involution of the ampulla. Anat Rec 67, 441–467. [Google Scholar]
- Shin D, Weidinger G, Moon RT, et al. (2012) Intrinsic and extrinsic modifiers of the regulative capacity of the developing liver. Mech Dev 128, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwe SA (1937) V. Die großen Drüsen des Darmkanals. A. Die Leber In: Handbuch der vergleichenden Anatomie der Wirbeltiere, Band 3 (eds Bolk L, Göppert E, Kallius E, Lubosch W.), pp. 725–774. Berlin: Urban & Schwarzenberg. [Google Scholar]
- Spence JR, Lange AW, Lin SC, et al. (2009) Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell 17, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PH (1921) The extrahepatic biliary tract of the camel. Anat Rec 22, 84–95. [Google Scholar]
- Susaki EA, Ueda HR (2016) Whole‐body and whole‐organ clearing and imaging techniques with single‐cell resolution: toward organism‐level systems biology in mammals. Cell Chem Biol 23, 137–157. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Takahashi M, Okabe M, et al. (2009) Germ layer patterning in bichir and lamprey; an insight into its evolution in vertebrates. Dev Biol 332, 90–102. [DOI] [PubMed] [Google Scholar]
- Thomson S (1940) Studies of the anatomy of the extrahepatic biliary tract in Mammalia. Field Zool 22, 415–430. [Google Scholar]
- Uemura M, Hara K, Shitara H, et al. (2010) Expression and function of mouse Sox17 gene in the specification of gallbladder/bile‐duct progenitors during early foregut morphogenesis. Biochem Biophys Res Commun 391, 357–363. [DOI] [PubMed] [Google Scholar]
- Uemura M, Ozawa A, Nagata T, et al. (2013) Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development 140, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Igarashi H, Ozawa A, et al. (2015) Fate mapping of gallbladder progenitors in posteroventral foregut endoderm of mouse early somite‐stage embryos. J Vet Med Sci 77, 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakuri H, Hori H (1970) On the gall bladder of a giraffe. J Mamm Soc Japan 5, 41–43 (in Japanese). [Google Scholar]
- Wu J, Platero‐Luengo A, Sakurai M, et al. (2017) Interspecies chimerism with mammalian pluripotent stem cells. Cell 168, 473–486 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youson JH (1993) Biliary atresia in lampreys. Adv Vet Sci Comp Med 37, 197–255. [PubMed] [Google Scholar]
- Zorn AM, Mason J (2001) Gene expression in the embryonic Xenopus liver. Mech Dev 103, 153–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The presence and absence of a gallbladder in phylogenetic tree of rodents.
Fig. S2. Whole‐mount anatomy of the abdominal cavity murine fetus at 15.5 dpc.
Fig. S3. SOX17‐positive structures in mouse and rat embryos.


