Abstract
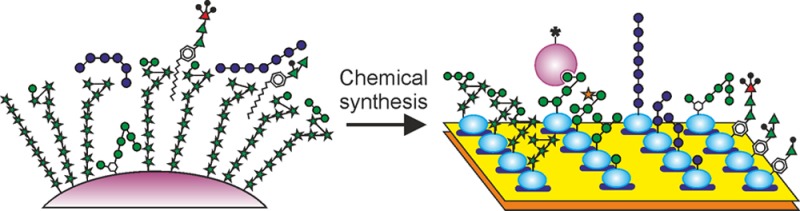
An array of homogeneous glycans representing all the major carbohydrate structures present in the cell wall of the human pathogen Mycobacterium tuberculosis and other mycobacteria has been probed with a panel of glycan-binding receptors expressed on cells of the mammalian innate immune system. The results provide an overview of interactions between mycobacterial glycans and receptors that mediate uptake and survival in macrophages, dendritic cells, and sinusoidal endothelial cells. A subset of the wide variety of glycan structures present on mycobacterial surfaces interact with cells of the innate immune system through the receptors tested. Endocytic receptors, including the mannose receptor, DC-SIGN, langerin, and DC-SIGNR (L-SIGN), interact predominantly with mannose-containing caps found on the mycobacterial polysaccharide lipoarabinomannan. Some of these receptors also interact with phosphatidyl-myo-inositol mannosides and mannose-containing phenolic glycolipids. Many glycans are ligands for overlapping sets of receptors, suggesting multiple, redundant routes by which mycobacteria can enter cells. Receptors with signaling capability interact with two distinct sets of mycobacterial glycans: targets for dectin-2 overlap with ligands for the mannose-binding endocytic receptors, while mincle binds exclusively to trehalose-containing structures such as trehalose dimycolate. None of the receptors surveyed bind furanose residues, which often form part of the epitopes recognized by antibodies to mycobacteria. Thus, the innate and adaptive immune systems can target different sets of mycobacterial glycans. This array, the first of its kind, represents an important new tool for probing, at a molecular level, biological roles of a broad range of mycobacterial glycans, a task that has not previously been possible.
Glycan-binding receptors on the surfaces of macrophages, dendritic cells, and neutrophils form a key component of the innate immune system for rapidly detecting and responding to pathogens.1,2 The protective responses mediated by these lectins can take several forms. C-Type carbohydrate-recognition domains (CRDs) in many of the receptors target microorganisms for uptake by endocytosis and subsequent destruction.3 More recently, it has become clear that some glycan-binding receptors initiate signaling responses, often resulting in secretion of pro-inflammatory cytokines.4−6 Both of these responses can direct and facilitate the adaptive immune response, through presentation of antigens derived from pathogens that have been internalized and degraded as well as by cytokine stimulation.
The multiple roles of glycan-binding receptors in interactions with bacterial pathogens can be illustrated by the interactions of mycobacteria with macrophages.7 Both the mannose receptor and DC-SIGN bind tightly to lipoarabinomannan (LAM) found on the mycobacterial surface, and this binding can lead to efficient internalization.8,9 These receptors also bind to phosphatidyl-myo-inositol mannosides (PIMs).10 In addition, trehalose dimycolate, an unusual mycobacterial glycolipid, binds to the receptor mincle, initiating a signaling pathway leading to secretion of interleukin 6 and TNF-α.11 These responses together allow mycobacteria to enter the macrophage and appear to form part of the strategy used by the organism to survive instead of being degraded.12
An important approach to characterizing the ligand-binding activity of glycan-binding receptors has been to screen arrays of immobilized glycans using labeled receptor fragments.13,14 A primary function of many mammalian glycan-binding receptors is to interact with carbohydrates expressed on microbial pathogens. However, the vast majority of studies examining the glycan-binding properties of these receptors have focused on arrays populated with mammalian glycans. With respect to pathogen binding, such mammalian glycan arrays provide critical insights into binding of glycans found on viruses, because viral glycoproteins are glycosylated in the host. On the other hand, there are major differences between most bacterial and fungal glycans and their mammalian counterparts. Thus, while there is some overlap in oligosaccharide epitopes between mammalian and microbial glycans, the mammalian-based arrays do not present the full spectrum of potential microbial ligands for the receptors. Glycan arrays containing microbial glycans are therefore essential to understanding the biological roles of glycan-binding receptors in innate immunity and for characterizing the specificity of anti-glycan antibodies arising from microbial infection.
Previously, arrays containing oligosaccharides released from lipopolysaccharides and synthetic versions of such glycans have been used to characterize interactions between glycans of Gram-negative bacteria and host receptors and antibodies.15−17 However, to date, an understanding of glycan–receptor interactions between mycobacteria, including Mycobacterium tuberculosis, which causes tuberculosis, and mammalian receptor proteins and antibodies has mostly been obtained through testing individual glycoconjugates, often isolated from natural sources. An array displaying homogeneous glycans from the mycobacterial surface, obtained through chemical synthesis, will provide a broader, more detailed picture of their interaction with host proteins. Such an array would reveal novel glycan–receptor interactions and would provide a means of evaluating their relative importance. Preliminary versions of such arrays have already proven to be useful in monitoring the antibody response during various phases of mycobacterial infection.18−20
Mycobacteria have surface glycoconjugates very different from those of most other bacteria.21 In addition to LAM and its delipidated form, arabinomannan, and PIMs, important mycobacterial surface glycoconjugates include phenolic glycolipids, glycopeptidolipids, trehalose mycolates and trehalose-containing lipooligosaccharides, and capsular α-glucans. Many of these compounds are difficult to obtain from natural sources in sufficient quantities, in pure enough form, or with appropriate linker motifs to create glycan arrays. In addition, structurally defined fragments of these molecules, which allow more precise detailing of glycan–receptor interactions, are difficult or impossible to obtain from nature. However, knowledge of their structures provides a basis for chemical synthesis of molecules that represent the glycan portions of such glycoconjugates.21−24 With the development of appropriate synthetic strategies, preparation and testing of a broad range of different classes of glycans is possible.
Generation of an array containing 60 chemically synthesized glycans, representing all known classes of mycobacterial surface carbohydrates, is described in this paper. Screening of the array with a panel of eight glycan-binding receptors demonstrates that the receptors interacting with subsets of these glycans are DC-SIGN, the mannose receptor, langerin, dectin-2, DC-SIGNR (L-SIGN), and mincle. The diversity of the array components allows novel insights into the specificity of these glycan–receptor interactions to be uncovered.
Results and Discussion
Development of a Mycobacterial Glycan Array
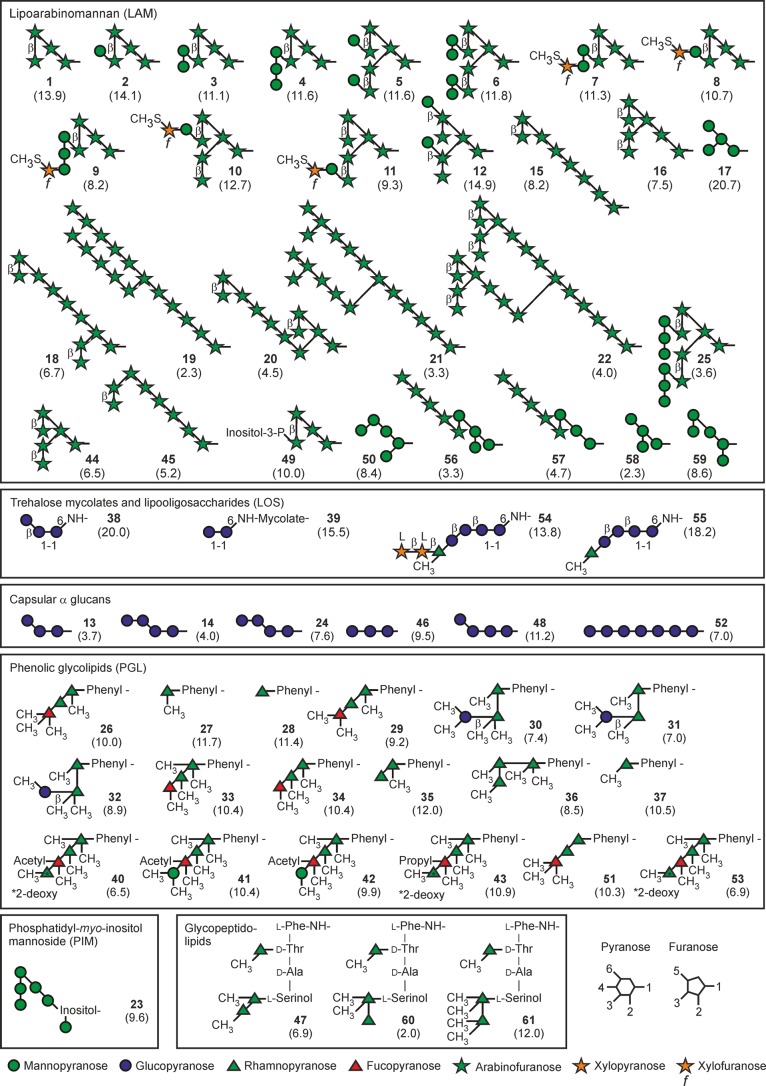
To develop an array that provides insight into a range of mycobacterium–host interactions, target glycans were selected from multiple classes of surface molecules found on various mycobacteria (Figure 1). Unless otherwise indicated, the monosaccharide residues referenced in the discussion below are in the pyranose form. The largest group of glycans represents fragments of LAM, including core mannose oligosaccharides, the arabinan domain, terminal arabinan fragments bearing additional mannose and 5-methylthio-xylofuranose modifications, and PIMs, which are present in all mycobacteria. Most of the rest of the structures are glycan-containing portions of smaller, often species-specific, extractable glycolipids, including the phenolic glycolipids, glycopeptidolipids, lipooligosaccharides, and trehalose monomycolate. Fragments of capsular α-glucans are also present.
Figure 1.
Symbol representation of the mycobacterial glycans on the array. The degree of conjugation of each glycan to bovine serum albumin, in moles per mole, is indicated in parentheses below the glycan number. Glycans 14 and 24 have the same structure. Glycans 44 and 45 are present on the final version of the array but were not present in the version used for the screening results presented here.
Each glycan was synthesized with an amine-containing linker, which was coupled via a squarate linker to bovine serum albumin (BSA) (Figure 2A). Synthesis of the glycans has been described previously25−27 or is included in the Supporting Information. Table S1 details the linkers used for the various classes of molecules summarized in Table S2. Glycan loadings on BSA ranged from 3.3 to 14.9 glycans/protein (Figure 1). For the 28 glycans that give signals above background for one or more of the receptors, the degree of substitution is compared graphically in Figure 2B. Printing of BSA conjugates, as opposed to free glycans, was done to facilitate generation of the array, given that the 60 target glycans have a broad range of hydrophobicities and many have extremely low water solubility. The BSA conjugates provided materials that could reproducibly be printed on arrays from aqueous solutions, although even this strategy was insufficient to deal with exceptionally hydrophobic molecules such as trehalose dimycolate, necessitating the use of truncated analogues. The resulting neoglycoproteins were spotted in triplicate on epoxy-activated glass slides (Figure 2C).
Figure 2.
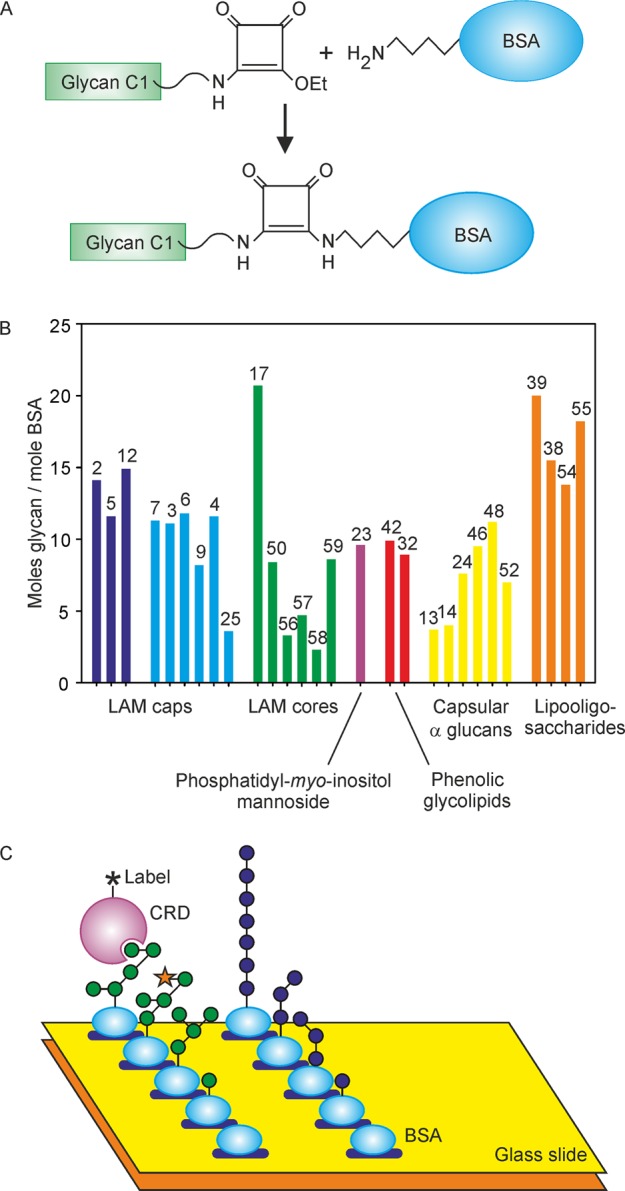
Array format. (A) Squaramide conjugation chemistry used to couple oligosaccharides to BSA. (B) Degree of conjugation of oligosaccharides to BSA for the glycans giving positive signals with one or more of the receptors. Profiles for array results in subsequent figures are shown in the same format, grouped on the basis of terminal structures. Glycan numbers from Figure 1 are indicated above the bars. (C) Screening of an array of immobilized BSA conjugates with soluble receptor fragments.
Conditions for Screening of the Array with Receptor Fragments
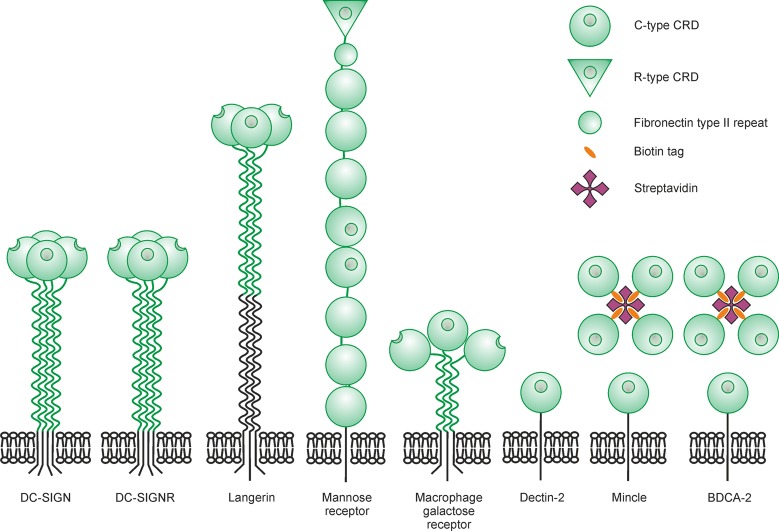
Eight glycan-binding proteins found on the surface of macrophages and/or dendritic cells were screened against the array (Figure 3). Each receptor is a C-type lectin containing a C-type CRD that is responsible for glycan-binding. DC-SIGN, the dendritic cell ICAM-grabbing nonintegrin, is expressed on dendritic cells,28 while langerin is found on Langerhans cells in skin.29 These receptors, as well as the mannose receptor, found on macrophages and sinusoidal endothelial cells,30 and the macrophage galactose receptor31,32 have endocytic activity and are involved in uptake of pathogens. In contrast, three receptors associate with the common FcRγ subunit and initiate intracellular signaling pathways: mincle from macrophages and other antigen-presenting cells,33 dectin-2 from macrophages and dendritic cells,34 and blood dendritic cell antigen 2 (BDCA-2), found exclusively on plasmacytoid dendritic cells.35 DC-SIGNR (L-SIGN) on liver and lymph node sinusoidal endothelial cells36 is less well understood, as it does not appear to have endocytic or signaling activity.
Figure 3.
Receptor fragments used to screen the array. Extracellular portions of the proteins face upward, with the portion of each receptor that was expressed colored green.
All of the receptors are composed of transmembrane polypeptides, and in each case, a fragment representing most or all of the extracellular portion of the protein was expressed. DC-SIGN and DC-SIGNR are tetramers, while langerin and the macrophage galactose receptor are trimers stabilized by coiled-coil domains. These oligomeric structures are preserved in the expressed fragments. The extracellular domain of the mannose receptor consists of multiple lectin-like domains in a single polypeptide. The CRD from mincle and BDCA-2 were expressed with biotin tags to facilitate array screening through streptavidin binding. The receptors tested were human forms, except for mincle. Bovine receptor mincle was used because of the difficulty of working with the human form. Ligand-binding characteristics of human and bovine mincle are similar.37
Several methods were used to detect binding to the array. Receptor fragments chemically labeled with fluorescent groups and fluorescently labeled streptavidin complexed with biotin-tagged receptor fragments were detected directly and also following secondary binding of fluorescently labeled antibodies to the initial fluorescent tags and to streptavidin. As shown below, similar results were obtained for proteins labeled in different ways, and the binding profiles were robust between replicates and at different dilutions. Because of the reproducibility of binding data using these different screening methods, results from different experiments can be compared with confidence.
Interaction of DC-SIGN with Diverse Mycobacterial Surface Glycans
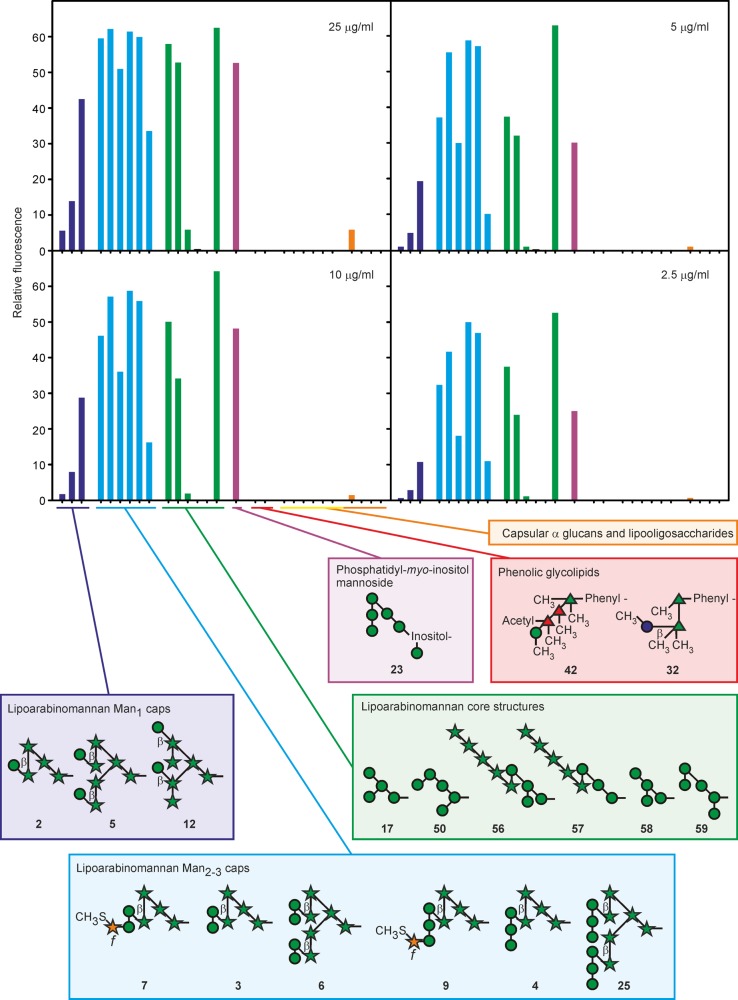
DC-SIGN directly labeled with fluorescein yielded consistent results over a 10-fold concentration range, with the differences between the amounts of bound ligand becoming less distinct at higher concentrations (Figure 4). Similar concentration independence of the binding results was observed for all of the other receptors. The relative intensities of the signals from bound receptors reflect the combined effects of different affinities for various glycans and different degrees of substitution of BSA with each glycan (Figure 2B). For instance, the low intensity of the signals for 56–58 reflects the relatively low degree of substitution in these three glycans. Similarly, substitution of 25 at roughly half the level of other glycans probably also contributes to the low intensity of the signal. Nevertheless, multiple glycans with similar degrees of substitution can be compared to gain a semiquantitative indication of specificity for one structure compared to others.
Figure 4.
Binding of the extracellular domain of DC-SIGN to mycobacterial glycans. After incubation with the glycan array at various concentrations, bound DC-SIGN labeled with Alexa Fluor 555 was detected by fluorescence. Data for all glycans and all receptors are provided in Table S3.
In agreement with previous studies, the predominant class of ligand for DC-SIGN in the mycobacterial cell wall is LAM.9,38 Comparison of related glycans representing part of the LAM structure provides insight into the mode of interaction of DC-SIGN with different portions of the polysaccharide. Strikingly, all of the glycans targeted by DC-SIGN contain mannose residues, and none consisting exclusively of arabinofuranose residues give signals above background. When the low levels of conjugation of 56–58 and 25 are taken into account, the results do not reveal a clear preference for particular types of glycans. Oligosaccharides bind better than simpler structures, but the binding site accepts many linkages.
Comparison of specific glycans reveals further details about binding of DC-SIGN to LAM. Glycans interact with the primary binding site in C-type CRDs through a conserved Ca2+ that ligates the equatorial 3- and 4-OH groups in mannose and other monosaccharides. Similar binding of 3 and 7 indicates that DC-SIGN must be able to bind internal mannose residues, because the 4-OH group of the reducing end mannose residue in 7 is blocked with a 5-methylthio-xylofuranose residue. A similar observation can be made comparing 4 and 9. Structural analysis of the CRD of DC-SIGN bound to oligosaccharide ligands suggests a mechanism for binding of subterminal mannose residues substituted on the 2-OH group,39,40 but the array results provide direct evidence of such binding in the context of the underlying arabinan polysaccharide. DC-SIGN also binds to single mannose residues at the nonreducing termini of 2, 5, and 12. However, the stronger signal for 12 suggests that the position of such mannose residues on the arabinan may affect accessibility to the DC-SIGN-binding site. Direct comparison of the binding for multiple substructures from LAM facilitated by the array thus reveals that DC-SIGN interacts with multiple different mannose cap structures found on LAM. Such precise information would be difficult or impossible to obtain from glycans isolated from nature and fractionated either before or after hydrolysis and/or depolymerization.
Other glycans on the array represent mannose core structures of LAM, some of which resemble portions of the high-mannose structures on N-linked glycans that mediate binding to viruses such as human immunodeficiency virus. A key motif for DC-SIGN binding is a cluster of three mannose residues that form a branch structure corresponding to 17.39 Given the high degree of conjugation of 17 to BSA, this glycan does not appear to be a good ligand compared to many of the terminal LAM structures. Importantly, many of the mannose residues in these core oligosaccharides in LAM may be inaccessible to binding because of substitution with arabinofuranose and because they are occluded by other carbohydrate structures in the cell wall.21
DC-SIGN also binds 23, a PIM derivative (PIM6), which has a terminal Manα1–2Man epitope like those in the LAM caps. The ability of DC-SIGN to interact effectively with ligands bearing single mannose residues such as those in 2, 5, and 12 is also consistent with reports of binding to mannose-containing O-linked glycans in glycoproteins of M. tuberculosis.41
Mannose Receptor Binding to Terminal Residues on Mycobacterial Glycans
DC-SIGN and the mannose receptor have been extensively studied as routes for internalization of mycobacteria into macrophages and dendritic cells.42,43 The array reveals key differences in the ligands bound by these receptors. The primary known target for the mannose receptor is LAM.44 On the array, the mannose receptor shows a binding pattern that overlaps with DC-SIGN (Figure 5). The observed binding is mediated through the C-type CRDs, because a truncated form of the mannose receptor lacking the N-terminal R-type CRD and the fibronectin type 2 repeat shows a binding pattern that is very similar to that of the full extracellular domain. The R-type CRD binds primarily to sulfated glycans that are not similar to any of the glycans present on the array and would thus not be expected to contribute to binding.45
Figure 5.
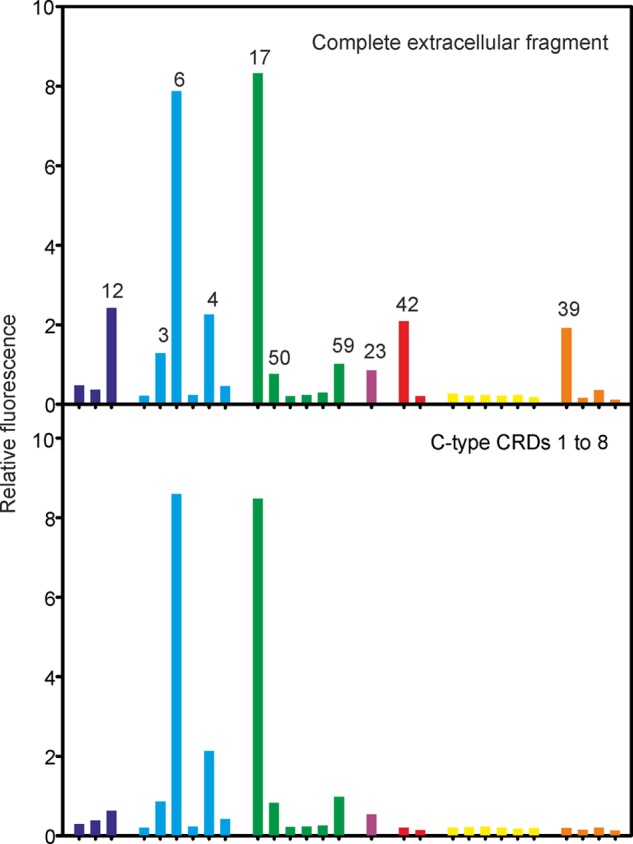
Comparison of full length and truncated mannose receptor binding to the glycan array. Both the full extracellular domain fragment and the extracellular domain lacking the N-terminal R-type CRD and the fibronectin type II repeat were labeled with Alexa Fluor 555. The array was screened at 10 μg mL–1.
Binding to the mannose receptor is determined predominantly by the presence of exposed mannose residues at the nonreducing termini of glycans. While DC-SIGN gives similar signals for 3 and 7, the mannose receptor shows very little binding to 7, in which the terminal mannose residue is blocked by a 5-methylthio-xylofuranose residue. Similarly, 9 binds well to DC-SIGN but not to the mannose receptor. Several glycans bearing a single terminal mannose residue, including a phenolic glycolipid (42), also bind to the mannose receptor. Glycan 42 is the only phenolic glycolipid with a terminal mannose residue in which both 3- and 4-OH groups are exposed; 41 does not bind because the 3-OH group is methylated. Addition of methyl groups and addition of capping 5-methylthio-xylofuranose residues are potential mechanisms by which bacteria can modulate binding to receptors, which could form part of escape mechanisms analogous to those used to evade antibody binding.46 Strong signals for both 6 and 17, which do not share any common linkages, support the hypothesis that the presence of nonreducing terminal mannose residues is the predominant factor determining binding to the mannose receptor. Binding is roughly proportional to the number of exposed nonreducing terminal mannose residues.
Additional Mannose-Binding Receptors That Interact with Mycobacteria
Three additional mannose-binding receptors, langerin, dectin-2, and DC-SIGNR, show distinct recognition patterns on the array (Figure 6). Nevertheless, many of the ligands giving the strongest signals are common to all three receptors and to the mannose receptor. In each case, there is little binding to 7 and 9, with 5-methylthio-xylofuranose caps, compared to the uncapped versions, underscoring binding largely to exposed mannose residues at the nonreducing ends of glycans.
Figure 6.
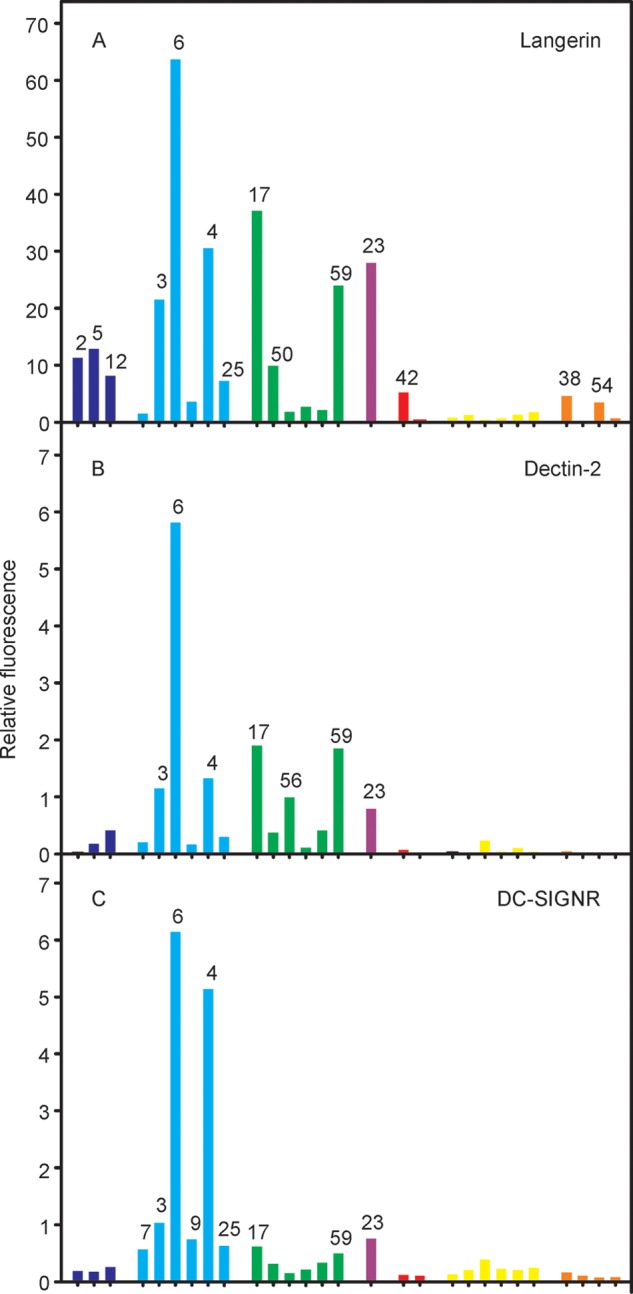
Comparison of glycan array results for mannose-binding receptors. Binding was detected for (A) langerin labeled with Alexa Fluor 555, at 10 μg mL–1; (B) dectin-2 labeled with Alexa Fluor 555, at 5 μg mL–1; and (C) DC-SIGNR labeled with fluorescein, at 5 μg mL–1, followed by detection with a Cy3-labeled antibody to fluorescein.
Langerin shows significant binding to 2, 5, 12, and 42, which bear single terminal mannose residues, although it also binds more complex LAM structures (Figure 6A). The array results provide evidence for binding to both PIMs and mannose-containing phenolic glycolipids in addition to LAM. Interaction of Mycobacterium leprae with Langerhans cells is mediated in part by binding of langerin to mannose-containing O-linked glycans on M. leprae superoxide dismutase.47 The relatively strong signals observed for simple, terminal mannose residues are consistent with binding of langerin to the small glycans associated with mycobacterial glycoproteins.48
Dectin-2, which binds to mycobacterial LAM,49 interacts with the Manα1–2Man disaccharide.50 Structural analysis, combined with the ability of dectin-2 to bind yeast mannans and selected bacterial polysaccharides, indicates that this disaccharide motif can be either at a nonreducing terminus or internally in a polysaccharide. The binding site can accommodate terminal mannose residues in other linkages, but at reduced affinity. These features are consistent with enhanced binding of cap structures on LAM that contain Manα1–2Man (3, 4, and 6) with lower levels of binding to other mannose-containing compounds (Figure 6B). The absence of binding to 7 and 9 is consistent with structural data showing that derivatization of the 4-OH group of the nonreducing end mannose in Manα1–2Man results in a steric clash.50
The sinusoidal endothelial cell receptor DC-SIGNR binds to a specific subset of mannose-containing glycans (Figure 6C). All of the strongest signals are for glycans with α1–2-linked mannose units, consistent with evidence that Manα1–2Man is the preferred disaccharide ligand51 and that DC-SIGNR shows restricted binding to mammalian oligosaccharides compared to DC-SIGN.39 The difference in specificity likely derives from subtle differences in the binding site that restrict access by many oligosaccharides in DC-SIGNR.
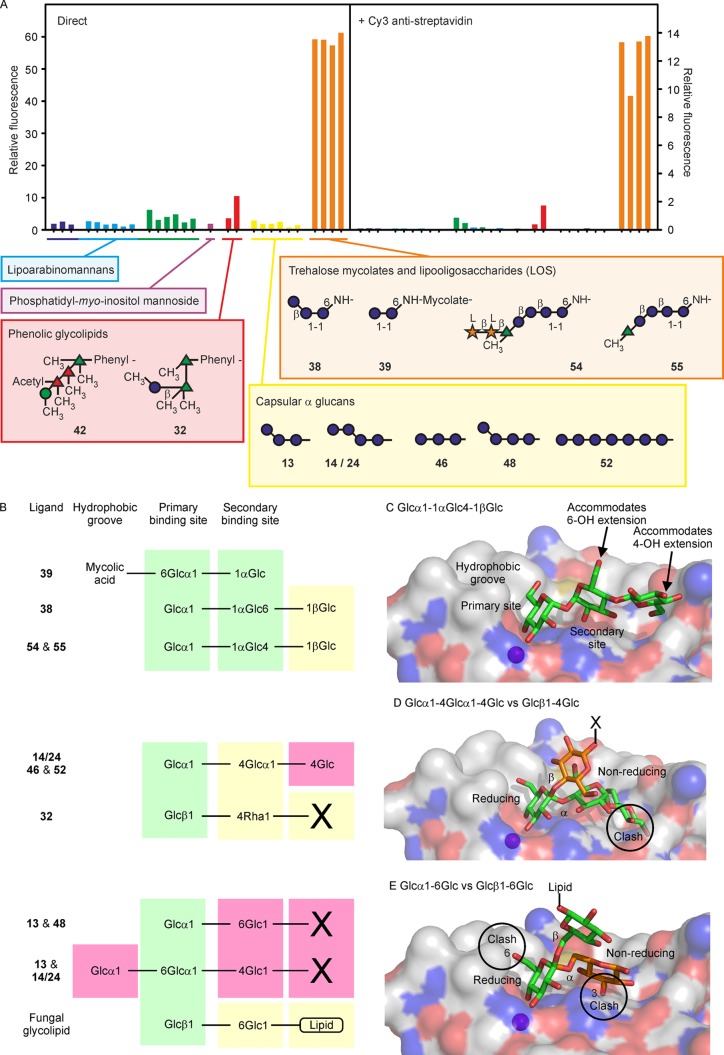
Mincle Binding to a Distinct Set of Mycobacterial Glycans
The fluorescently labeled mincle–streptavidin complex can be detected directly or following the addition of a secondary antibody, with similar results (Figure 7A). The signals for multiple glycans on the array that bear one or more nonreducing terminal mannose or glucose residues are very small compared to those for ligands containing trehalose. Thus, the binding specificity cannot be simply described on the basis of a single terminal monosaccharide residue but depends on the presence of the trehalose disaccharide.52 The importance of binding of mincle to trehalose dimycolate (cord factor) is well-documented, but screening against the full array provides several novel insights that are summarized in Figure 7B. The trehalose-containing glycans 38, 39, 54, and 55 bind strongly despite the variation in substituents.
Figure 7.
Binding of mincle to mycobacterial glycans. (A) Mincle complexed with Alexa Fluor 488-conjugated streptavidin was used to probe the array at 5 μg mL–1 and was detected directly by measurement of fluorescence (left) or after further incubation with a Cy3-labeled anti-streptavidin antibody (right). (B) Schematic diagram of the binding sites in mincle and the positions occupied by individual monosaccharide residues in oligosaccharide ligands. X represents either additional monosaccharide residues or BSA to which the oligosaccharide is conjugated. Residues in green shaded sites make favorable interactions with the surface of mincle; residues in yellow regions project away from the surface, and residues in red regions would clash with the surface. (C) Model for binding of ligands containing trehalose extended on the 6-OH group. (D) Model of Glc1–4Glc di- and trisaccharides bound to mincle. (E) Model of Glc1–6Glc disaccharides bound to mincle. The crystal structure of trehalose monobutyrate bound to bovine mincle (Protein Data Bank entry 4ZRV) was used to model trehalose derivatives bound to mincle using PyMOL. Conformations of glycans, taken from small molecule databases, were not modified, but irrelevant regions were removed. Superpositions of individual monosaccharide residues, described in detail in Supporting Information 1, were performed manually. In panels D and E, regions of positive potential on the surface of mincle are colored blue, regions of negative potential are colored red, and the bound Ca2+ is colored magenta. In the ligands, carbon atoms are colored green or orange and oxygen atoms are colored red.
Glycans 54 and 55 represent surface lipooligosaccharides found in Mycobacterium kansasii, an opportunisitic pathogen, but not in M. tuberculosis.22 Binding of these glycans by mincle suggests that the binding site can accommodate additions to the 4-OH of one of the glucose residues in trehalose. The 4-OH of the glucose residue in the secondary site is accessible, and simple modeling shows that an additional glucose residue could be accommodated without a steric clash (Figure 7C). In M. kansasii, the trehalose headgroups can be acylated on the 4-OH group of the glucose residue that is not extended with further monosaccharides.22 Therefore, in nature, these headgroups would be ligands only if forms not acylated at this position are present.
The strong signal for 38 demonstrates that binding still occurs when a carbohydrate residue is attached at the 6-OH in place of one of the acyl groups found in trehalose dimycolate. The 6-OH of the glucose residue in the secondary binding site is accessible for substitution (Figure 7C). This glucose trisaccharide forms the core of several lipooligosaccharides found in Mycobacterium smegmatis, and in these cases, the acyl groups are in positions that would not interfere with binding.22 In addition to identifying alternative ligands for mincle in different species of mycobacteria, these results suggest possible sites at which trehalose can be modified to generate synthetic ligands that target this lectin. These results would be of interest in the development of improved adjuvants that bind to mincle, and they suggest that the array platform may be useful in screening for additional trehalose modifications that are tolerated.
Very weak signals are detected for binding of mincle to some of the other glucose-containing glycans, including phenolic glycolipid glycan 32. Because of the requirement for free 3- and 4-OH groups on glucose residues to ligate Ca2+, it is possible for 32 to bind, while the closely related 30 and 31, in which the 3-OH group is methylated, do not. Other terminal glucose residues with exposed 3- and 4-OH groups in the α-glucan fragments 13, 14/24, 46, 48, and 52 do not bind. These glucose residues are all α-linked, while the glucose in 32 is β-linked. Modeling the nonreducing end monosaccharide residue of a β1–4-linked glucose disaccharide in the primary binding site of mincle shows the reducing end residue projected away from the surface of the protein (Figure 7D), accommodating linkage to another monosaccharide, for example, rhamnose in 32. In contrast, the reducing end of an α1–4-linked disaccharide from α-glucan would be too close to the protein surface for attachment to a larger glycan or to a carrier. A clash with the reducing end monosaccharide of an α1–6-linked glucose disaccharide also prevents binding (Figure 7E), although the β1–6-linked disaccharide in a fungal glycolipid ligand for mincle can be accommodated.49
Mincle binds α-methyl mannoside at least as well as it binds α-methyl glucoside, but mannose-containing glycans on the array give only very weak signals. Modeling suggests that clashes resulting from the α-linkage, as seen with the α-glucan fragments, preclude binding. Thus, there is complete non-overlap between mycobacterial ligands for endocytic receptors DC-SIGN, the mannose receptor, and langerin that facilitate the entry of mycobacteria into cells and mincle, which initiates signaling.
Receptors That Do Not Bind Any Mycobacterial Glycans on the Array
No ligands were identified for the macrophage galactose receptor or BDCA-2, in spite of the fact that these protein preparations are purified by sugar affinity chromatography and bind ligands on mammalian glycan arrays.53,54 The lack of binding to the array glycans is consistent with preferential binding of the macrophage galactose receptor to GalNAc, which is absent from the array.53,55 The lack of binding to any of the array glycans provides evidence that this specificity is quite strict. Consequently, although the macrophage galactose receptor can bind to some bacteria and parasites,53,55 it does not likely interact with mycobacteria.
In the case of BDCA-2, although the primary binding site does bind mannose, high-affinity binding requires additional binding of a galactopyranose residue in a secondary binding site,54 and no appropriate ligands are present on the array. Murine dendritic cell activating receptor, DCAR, binds to PIMs.56 In the absence of a clear human ortholog of DCAR, it was suggested that BDCA-2 performs a similar function. However, the array results rule out this possibility. If BDCA-2 had carbohydrate-binding specificity like mouse DCAR, it would bind to several array glycans bearing terminal mannose residues, such as 23, the glycan portion of PIM6, to which no binding is observed.
Mycobacterial Glycans Not Bound by Any Receptors
Roughly half of the array glycans, including glycans containing 6-deoxy-l-pyranoses fucose and rhamnose, do not bind to any of the receptors tested. Several of the mannose-binding receptors, including DC-SIGN, langerin, and the mannose receptor, bind mammalian oligosaccharides containing fucose through either the 2- and 3-OH groups or the 3- and 4-OH groups.15,39,57,58 Among the fucose-containing glycans, only 33 and 34 contain fucose residues with adjacent free OH groups. The 2-O-methyl group in these monosaccharide residues may prevent binding to these proteins.
A lack of binding must be interpreted with some caution, because the density of BSA-conjugated oligosaccharides does not exactly match the density found on the surface of mycobacteria. However, structural information can provide an explanation for the lack of binding in some cases. For example, although 27, 28, 35, and 60 bear rhamnose residues with adjacent free OH groups, none of these glycans bind to any of the receptors. The vicinal OH groups in rhamnose and fucose are twisted in opposite senses (Figure 8A) and thus do not have the correct geometry to interact with the primary binding site in mannose-binding C-type CRDs.59 Rhamnose can be accommodated in a galactose-binding site (Figure 8B). However, attachment of a rhamnose residue at the nonreducing end of a glycan would result in a clash with the tryptophan residue that forms an essential part of galactose-binding sites in C-type CRDs.60 No instance of binding of rhamnose to any C-type lectin has been reported, but it appears that a rhamnose-binding site could be created in a CRD lacking this tryptophan.
Figure 8.
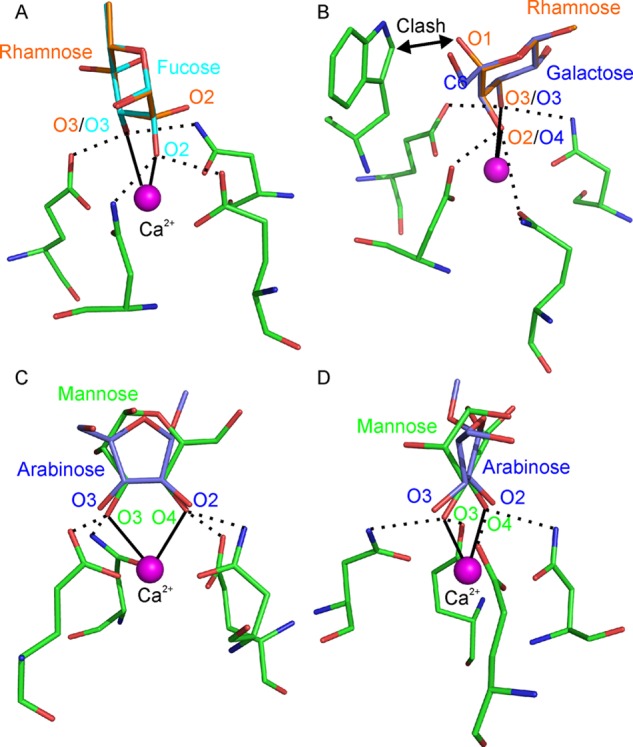
Modeling of glycans that fail to bind to any of the receptors tested. (A) α-l-Rhamnose, superposed on a fucose residue in the primary binding site of langerin. The 2- and 3-OH groups of rhamnose are aligned with the 2- and 3-OH groups of fucose, which are ligated to Ca2+. (B) α-l-Rhamnose superposed on a galactose residue in the primary binding site of the scavenger receptor C-type lectin. The 2- and 3-OH groups of rhamnose are aligned with the 4- and 3-OH groups of galactose, which are ligated to Ca2+. (C and D) Two views of methyl α-d-arabinofuranoside overlaid on a d-mannopyranose residue in the primary binding site of langerin. Hydrogen bonds between carbohydrate OH groups and amino acid side chains are indicated by dotted lines, and coordination bonds between glycan OH groups and Ca2+ are shown as solid lines.
None of the receptors bind to arabinofuranose or xylofuranose residues on the array. Although the binding of furanoses to C-type CRDs has not been extensively investigated, none have been identified as ligands.14 All of the xylofuranose residues and the majority of the arabinofuranose residues in the array glycans contain adjacent free OH groups. However, these groups have a disposition significantly different from those of the pyranoses (Figure 8C,D). The altered geometry of the vicinal OH groups in the furanose configuration is apparently incompatible with the Ca2+ coordination geometry required for CRD binding.
Synthetic Glycan Arrays for Dissecting Host–Pathogen Interactions
The results reported here provide new insights into mycobacterium–host interactions and underscore the utility of using synthetic glycans to populate arrays. An advantage of this approach is that the synthetically accessible quantities of glycans ensure a continuing, reproducible supply of arrays. For many glycans, the quantities available from synthesis vastly exceed what can be purified from nature, in turn allowing testing of a much wider range of ligand–receptor combinations. Moreover, the purity of the individual glycans makes it possible to reach reliable conclusions about binding specificity. Finally, the preparation of a series of well-defined fragments of polysaccharides such as LAM is difficult if not impossible using material isolated from nature. Far better insights can be obtained using arrays with well-defined synthetic structures, justifying the effort required to synthesize these materials. While quantitative comparisons must be made with caution and do not reflect simply affinity for specific glycans, unambiguous distinction between ligands that bind and those that do not can generally be obtained.
With respect to biologically important interactions of receptors with mycobacteria, a striking outcome of this work is the finding that largely distinct sets of glycans are recognized by receptors that mediate internalization and receptors that initiate signaling. The glycan array results for the mannose receptor and DC-SIGN confirm that these receptors bind to oligosaccharides derived from LAM and PIM6, which is consistent with evidence that binding to such structures mediates internalization of mycobacteria in macrophages.8−10 In contrast to these mannose-binding endocytic receptors, the signaling receptor mincle appears to be a common receptor for different trehalose-containing ligands in various mycobacterial species. However, in keeping with the demonstration that dectin-2 expressed in macrophages binds to LAM,49 binding of this receptor to a subset of LAM fragments is consistent with the suggestion that mannose-containing glycans can also initiate signaling.
A significant advantage of using arrays to define the glycan-binding specificity of receptors, in addition to the speed at which results can be obtained, is the large amount of information about nonbinding ligands. The results described here illustrate this point in two ways. First, the presence of monosaccharide units that have not been commonly tested for binding to C-type CRDs indicates that these glycans are not likely ligands for this class of carbohydrate-binding domain. In the absence of such data, there is always the possibility that important classes of ligands have been overlooked simply because they have not been tested. Key examples of biologically relevant monosaccharides that fail to bind are rhamnose, arabinofuranose, and xylofuranose. In each case, examination of the orientation of adjacent OH groups in these monosaccharides provides an explanation for their failure to bind. Taken together, these results provide compelling evidence that the primary determinant of binding to C-type CRDs is the presence of a pair of vicinal OH groups that have the appropriate diequatorial orientation, such as the 3- and 4-OH groups of mannose, in a pyranose ring.
The lack of binding of the receptors to furanose residues is also noteworthy because arabinofuranose residues are highly immunogenic.18−20,61−64 The results presented here together with structural and binding information for anti-LAM monoclonal antibodies and antibodies in serum18−20,65 suggest that the innate and adaptive arms of the immune system have evolved to recognize different sets of mycobacterial glycans. Given that many microorganisms produce cell surface glycans rich in furanoses, mammals could potentially have evolved innate immune system lectins capable of binding these residues. To date, the only mammalian lectins described that bind to furanoses are the intelectins, which interact with β-galactofuranose residues via the α-diol side chain.66 Furanoses may be targeted by additional, yet to be discovered, mammalian lectins. The array described here will facilitate detection of such carbohydrate-binding activity, providing a rapid method for mapping the specificity of novel proteins.
In summary, this array represents an important novel resource for probing the biological role of mycobacterial glycans, in particular with regard to host–pathogen interactions. This tool allows rapid screening of a broad range of mycobacterial glycans against any glycan-binding receptor of interest, such as the receptors of the innate immune system examined here, a task that was heretofore impossible on this scale. It should also be noted that a smaller subset of this array containing only LAM fragments has been demonstrated to provide important insights into the specificity of antibodies generated upon infection and vaccination.19,20 More generally, this paper illustrates how screening of arrays populated with chemically synthesized pathogen-specific glycans can provide a comprehensive overview of their interactions with host receptors. In addition, screening of the receptors against broader ranges of glycans provides novel insights into their binding specificity beyond what can be learned with mammalian glycans.
Methods
The Supporting Information contains details about materials, glycan synthesis, protein expression and labeling, and molecular modeling.
Synthesis of BSA Conjugates
To a solution of the amine-functionalized glycan (5 mg, 1 equiv) in ethanol (1 mL) and H2O (1 mL) was added diethyl squarate (1.5 equiv). A 1 M aqueous solution of sodium carbonate was added slowly (1 μL every 1 min) to adjust the pH to 8.0–8.5 as determined by pH paper. The solvent was evaporated under reduced pressure, and the residue was purified by C18 chromatography using 20% methanol in water as the eluent. The resulting glycan squarate derivative (20 equiv) was dissolved in 0.5 M borate buffer (pH 9), followed by addition of BSA (1 equiv). The reaction mixture was stirred for 48–72 h at room temperature. The mixture was transferred to dialysis tubing (6000–8000 molecular weight cutoff) and dialyzed against water five times (4 L of water, every 4 h). The BSA conjugate was lyophilized, and glycan loading was assessed by matrix-assisted laser desorption ionization mass spectrometry using sinapinic acid as the matrix.
Generation of the Glycan Array
Microarray slides were printed at Engineering Arts LLC (Phoenix, AZ). BSA conjugates were non-contact printed on Schott type E slides (Schott North America, Inc., Elmsford, NY) using an Engineering Arts au301 Rainmaker microarray printer and dispensed at 100 μg mL–1 in buffer [1:10 phosphate-buffered saline with 0.005% (v/v) Triton X-100]. Antigens were dispensed at 360 pL per spot to produce 140–160 μm diameter spots in triplicate. The relative humidity was maintained at 65%; slides were subjected to white light for 100% inline drop inspection. Printing was performed in a HEPA-filtered environment at room temperature. Printed slides were stored at −20 °C until they were used.
Screening of the Glycan Array
Slides were prewetted in buffer A [25 mM Tris-HCl (pH 7.8), 0.15 mM NaCl, 2 mM CaCl2, and 0.05% Tween 20] for 5 min, rinsed with buffer B [25 mM Tris-HCl (pH 7.8), 0.15 mM NaCl, and 2 mM CaCl2] three times, and blocked overnight with buffer C [1% BSA in 25 mM Tris-HCl (pH 7.8), 0.15 mM NaCl, and 2 mM CaCl2] at 4 °C. Aliquots (500 μL) of serial dilutions of protein samples (Table S1) in buffer C were transferred to wells of the slide module immediately after aspiration of the blocking buffer. Wells were sealed with an adhesive seal and incubated for 60 min at 37 °C. Protein was removed by aspiration, and slides were washed 10 times with buffer A and three times with buffer B. Fluorescence was measured directly or after addition of a secondary antibody in buffer C (1:1000 dilution). Slides were incubated with a secondary antibody at room temperature for 40 min before being washed repeatedly with buffer A and deionized water. Before being scanned, slides were dried by centrifugation.
Array Imaging and Data Analysis
Microarrays were scanned at 5 μm resolution with a GenePix 4000B scanner (Molecular Devices, Sunnyvale, CA). The fluorescent signal was detected at 532 nm for Cy3 or Alexa Fluor 555 and 488 nM for Alexa Fluor 488. The laser power was 100%, and the photomultiplier tube gain was 400. The fluorescent signals were analyzed by quantifying the pixel density (intensity) of each spot using GenePix ProMicroarray Image Analysis Software version 6.1. Fluorescence intensity values for each spot and its background were calculated. The local background signal was automatically subtracted from the signal of each separate spot, and the mean signal intensity of each spot was used for data analysis. Averages of triplicate experiments and standard deviations were calculated using Microsoft Excel.
Acknowledgments
This work was supported by the Alberta Glycomics Centre, the Bill and Melinda Gates Foundation (Grant OPP1039665), and the Canadian Institutes of Health Research (Grant 118908) (T.L.L.) and by the Wellcome Trust (Grant 093599) and the Biotechnology and Biological Sciences Research Council (Grant BB/K007718/1) (K.D. and M.E.T.).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.7b00797.
Author Contributions
R.B.Z., S.A.F.J., and M.J. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Dambuza I. M.; Brown G. D. (2015) C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27. 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar R. L. (2015) J. Allergy Clin. Immunol. 135, 609–615. 10.1016/j.jaci.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal E. P.; Miller J. L.; Gordon S. (2005) Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 17, 18–24. 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond R. A.; Brown G. D. (2013) Signalling C-type lectins in antimicrobial immunity. PLoS Pathog. 9, e1003417. 10.1371/journal.ppat.1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley M. S.; Crocker P. R.; Paulson J. C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666. 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K.; Taylor M. E. (2015) Recent insights into structures and functions of C-type lectins in the immune system. Curr. Opin. Struct. Biol. 34, 26–34. 10.1016/j.sbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick K. E.; Ní Cheallaigh C.; O’Farrelly C.; Hokamp K.; MacHugh D. E.; Harris J. (2013) Receptor-mediated recognition of mycobacterial pathogens. Cell. Microbiol. 15, 1484–1495. 10.1111/cmi.12161. [DOI] [PubMed] [Google Scholar]
- Kang P. B.; Azad A. K.; Torrelles J. B.; Kaufman T. M.; Beharka A.; Tibesar E.; DesJardin L. E.; Schlesinger L. S. (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202, 987–999. 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N.; Nigou J.; Herrmann J.-L.; Jackson M.; Amara A.; Lagrange P. H.; Puzo G.; Gicquel B.; Neyrolles O. (2003) The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J. Biol. Chem. 278, 5513–5516. 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- Torrelles J. B.; Azad A. K.; Schlesinger L. S. (2006) Fine discrimination in the recognition of individual species of phophatidyl-myo-inositol mannoside from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 177, 1805–1816. 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- Ishikawa E.; Ishikawa T.; Morita Y. S.; Toyonaga K.; Yamada H.; Takeuchi O.; Kinoshita T.; Akira S.; Yoshikai Y.; Yamasaki S. (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206, 2879–2888. 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. (2013) Recognition of the mycobacterial cord factor by mincle: relevance for granuloma formation and resistance to tuberculosis. Front. Immunol. 4, 1–7. 10.3389/fimmu.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O.; Head S.; Mondala T.; Scanlan C.; Huflejt M. E.; Alvarez R.; Bryan M. C.; Fazio F.; Calarese D.; Stevens J.; Razi N.; Stevens D. J.; Skehel J. J.; van Die I.; Burton D. R.; Wilson I. A.; Cummings R.; Bovin N.; Wong C.-H.; Paulson J. C. (2004) Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U. S. A. 101, 17033–17038. 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. E.; Drickamer K. (2009) Structural insights into what glycan arrays tell us about how glycan-binding proteins interact with their ligands. Glycobiology 19, 1155–1162. 10.1093/glycob/cwp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H.; Taylor M. E.; Razi N.; McBride R.; Knirel Y. A.; Graham S. A.; Drickamer K.; Weis W. I. (2011) Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J. Mol. Biol. 405, 1027–1039. 10.1016/j.jmb.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanske J.; Schulze J.; Aretz J.; McBride R.; Loll B.; Schmidt H.; Knirel Y.; Rabsch W.; Wahl M. C.; Paulson J. C.; Rademacher C. (2017) Bacterial polysaccharide specificity of the pattern recognition receptor langerin is highly species-dependent. J. Biol. Chem. 292, 862–871. 10.1074/jbc.M116.751750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissner A.; Pereira C. L.; Leddermann M.; Anish C.; Seeberger P. H. (2016) Deciphering antigenic determinants of Streptococcus pneumoniae serotype 4 capsular polysaccharide using synthetic oligosaccharides. ACS Chem. Biol. 11, 335–344. 10.1021/acschembio.5b00768. [DOI] [PubMed] [Google Scholar]
- Tong M.; Jacobi C. E.; van de Rijke F. M.; Kuijper S.; van de Werken S.; Lowary T. L.; Hokke C. H.; Appelmelk B. J.; Nagelkerke N. J. D.; Tanke H. J.; van Gijlswijk R. P. M.; Veuskens J.; Kolk A. H. J.; Raap A. K. (2005) A multiplexed and miniaturized serological tuberculosis assay identifies antigens that discriminate maximally between TB and non-TB sera. J. Immunol. Methods 301, 154–163. 10.1016/j.jim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Chen T.; Blanc C.; Eder A. Z.; Prados-Rosales R.; Oliveira-Souza A. C.; Kim R. S.; Glatman-Freedman A.; Joe M.; Bai Y.; Lowary T. L.; Tanner R.; Brennan M. J.; Fletcher H. A.; McShane H.; Casadevall A.; Achkar J. M. (2016) Association of human antibodies to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J. Infect. Dis. 214, 300–310. 10.1093/infdis/jiw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados-Rosales R.; Carreño L.; Cheng T.; Blanc C.; Weinrick B.; Malek A.; Lowary T. L.; Baena A.; Joe M.; Bai Y.; Kalscheuer R.; Batista-Gonzalez A.; Saavedra N. A.; Sampedro L.; Tomas J.; Anguita J.; Hung S.-C.; Tripathi A.; Xu J.; Glatman-Freedman A.; Jacobs W. R. Jr.; Chan J.; Porcelli S. A.; Achkar J. M.; Casadevall A. (2017) Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 13, e1006250. 10.1371/journal.ppat.1006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angala S. K.; Belardinelli J. M.; Huc-Claustre E.; Wheat W. H.; Jackson M. (2014) The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 49, 361–399. 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B.; Chu C.; Lowary T. L. (2015) Lipooligosaccharides from mycobacteria: structure, function, and synthesis. Isr. J. Chem. 55, 360–372. 10.1002/ijch.201400194. [DOI] [Google Scholar]
- Chatterjee D.; Khoo K. (2001) The surface glycopeptidolipids of mycobacteria: structures and biological of properties. Cell. Mol. Life Sci. 58, 2018–2042. 10.1007/PL00000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw G. J., and Appelmelk B. J. (2006) Mycobacterial glycolipids and the host: role of phenolic glycolipids and lipoarabinomannan. In Protein–Carbohydrate Interactions in Infectious Diseases (Bewley C., Ed.) pp 6–29, RSC Publishing, Cambridge, U.K. [Google Scholar]
- Gadikota R. R.; Callam C. S.; Appelmelk B. J.; Lowary T. L. (2003) Synthesis of oligosaccharide fragments of mannosylated lipoarabinomannan appropriately functionalized for neoglycoconjugate preparation. J. Carbohydr. Chem. 22, 459–480. 10.1081/CAR-120025322. [DOI] [Google Scholar]
- Sahloul K.; Lowary T. L. (2015) Development of an orthogonal protection strategy for the synthesis of mycobacterial arabinomannan fragments. J. Org. Chem. 80, 11417–11434. 10.1021/acs.joc.5b02083. [DOI] [PubMed] [Google Scholar]
- Joe M.; Bai Y.; Nacario R. C.; Lowary T. L. (2007) Synthesis of the docosanasaccharide arabinan domain of mycobacterial arabinogalactan and a proposed octadecasaccharide biosynthetic precursor. J. Am. Chem. Soc. 129, 9885–9901. 10.1021/ja072892+. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T. B. H.; Kwon S. S.; Torensma R.; van Vliet S. J.; van Duijnhoven G. C. F.; Middel J.; Cornelissen I. L. M. H. A.; Nottet H. S. L. M.; KewalRamani V. N.; Littman D. R.; Figdor C. G.; van Kooyk Y. (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597. 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Valladeau J.; Ravel O.; Dezutter-Dambuyant C.; Moore K.; Kleijmeer M.; Liu Y.; Duvert-Frances V.; Vincent C.; Schmitt D.; Davoust J.; Caux C.; Lebecque S.; Saeland S. (2000) Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces formation of Birbeck granules. Immunity 12, 71–81. 10.1016/S1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D. (1990) The macrophage mannose receptor: current status. Am. J. Respir. Cell Mol. Biol. 2, 317–318. 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- Higashi N.; Fujioka K.; Denda-Nagai K.; Hashimoto S.; Nagai S.; Sato T.; Fujita Y.; Morikawa A.; Tsuiji M.; Miyata-Takeuchi M.; Sano Y.; Suzuki N.; Yamamoto K.; Matsushima K.; Irimura T. (2002) The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J. Biol. Chem. 277, 20686–20693. 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- Ng W. C.; Liong S.; Tate M. D.; Irimura T.; Denda-Nagai K.; Brooks A. G.; Londrigan S. L.; Reading P. C. (2014) The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J. Virol. 88, 1659–1672. 10.1128/JVI.02014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M.; Tanaka T.; Kaisho T.; Sanjo H.; Copeland N. G.; Gilbert D. J.; Jenkins N. A.; Akira S. (1999) A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163, 5039–5048. [PubMed] [Google Scholar]
- Gavino A. C.; Chung J. S.; Sato K.; Ariizumi K.; Cruz P. D. J. (2005) Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp. Dermatol. 14, 281–288. 10.1111/j.0906-6705.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- Dzionek A.; Fuchs A.; Schmidt P.; Cremer S.; Zysk M.; Miltenyi S.; Buck D. W.; Schmitz J. (2000) BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037–6046. 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- Bashirova A. A.; Geijtenbeek T. B.; van Duijnhoven G. C.; van Vliet S. J.; Eilering J. B.; Martin M. P.; Wu L.; Martin T. D.; Viebig N.; Knolle P. A.; KewalRamani V. N.; van Kooyk Y.; Carrington M. (2001) A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193, 671–678. 10.1084/jem.193.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégouzo S. A. F.; Harding E. C.; Acton O.; Rex M. J.; Fadden A. J.; Taylor M. E.; Drickamer K. (2014) Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology 24, 1291–1300. 10.1093/glycob/cwu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis S. I.; den Dunnen J.; Litjens M.; van der Vlist M.; Geijtenbeek T. B. H. (2009) Carbohydrate-specific signalling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 10, 1081–1088. 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Feinberg H.; Conroy E.; Mitchell D. A.; Alvarez R.; Blixt O.; Taylor M. E.; Weis W. I.; Drickamer K. (2004) Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11, 591–598. 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- Feinberg H.; Castelli R.; Drickamer K.; Seeberger P. H.; Weis W. I. (2007) Multiple modes of binding enhance the affinity of DC-SIGN for high-mannose N-linked glycans found on viral glycoproteins. J. Biol. Chem. 282, 4202–4209. 10.1074/jbc.M609689200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitarque S.; Herrmann J.-L.; Duteyrat J.-L.; Jackson M.; Stewart G. R.; Lecointe F.; Payre B.; Schwartz O.; Young D. B.; Marchal G.; Lagrange P. H.; Puzo G.; Gicquel B.; Nigou J.; Neyrolles O. (2005) Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem. J. 392, 615–624. 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. K.; Schlesinger L. S. (1998) Characterization of mannose receptor-dependent phagocytosis mediated by Mycobcterium tuberculosis lipoarabinomannan. Infect. Immun. 66, 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailleux L.; Schwartz O.; Herrmann J.-L.; Pivert E.; Jackson M.; Amara A.; Legres L.; Dreher D.; Nicod L. P.; Gluckman J. C.; Lagrange P. H.; Gicquel B.; Neyrolles O. (2003) DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127. 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S.; Kaufman T. M.; Iyer S.; Hull S. R.; Marciando L. K. (1996) Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 157, 4568–4575. [PubMed] [Google Scholar]
- Liu Y.; Chirino A. J.; Misulovin Z.; Leteux C.; Feizi T.; Nussenzweig M. C.; Bjorkman P. J. (2000) Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 191, 1105–1115. 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng Y.-L.; Thomas J.; Stephens D. S. (2016) Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 42, 759–772. 10.3109/1040841X.2015.1022507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J.; Brennan P. J.; Heaslip D.; Udey M. C.; Modlin R. L.; Belisle J. T. (2015) Carbohydrate-dependent binding of langerin to SodC, a cell wall glycoprotein of Mycobacterium leprae. J. Bacteriol. 197, 615–625. 10.1128/JB.02080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. T.; Sweredoski M. J.; Hess S. (2014) O-linked glycosylation sites profiling in Mycobacterium tuberculosis culture filtrate proteins. J. Proteomics 97, 296–306. 10.1016/j.jprot.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa A.; Saijo S.; Hoshino Y.; Miyake Y.; Ishikawa E.; Suzukawa M.; Inoue H.; Tanaka M.; Yoneyama M.; Oh-Hora M.; Akashi K.; Yamasaki S. (2014) Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity 41, 402–413. 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Feinberg H.; Jégouzo S. A. F.; Rex M. J.; Drickamer K.; Weis W. I.; Taylor M. E. (2017) Mechanism of pathogen recognition by human dectin-2. J. Biol. Chem. 292, 13402–13414. 10.1074/jbc.M117.799080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H.; Mitchell D. A.; Drickamer K.; Weis W. I. (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Feinberg H.; Jégouzo S. A. F.; Rowntree T. J. W.; Guan Y.; Brash M. A.; Taylor M. E.; Weis W. I.; Drickamer K. (2013) Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 288, 28457–28465. 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégouzo S. A. F.; Quintero-Martínez A.; Ouyang X.; dos Santos Á.; Taylor M. E.; Drickamer K. (2013) Organization of the extracellular portion of the macrophage galactose receptor: a trimeric cluster of simple binding sites for N-acetylgalactosamine. Glycobiology 23, 853–864. 10.1093/glycob/cwt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégouzo S. A. F.; Feinberg H.; Dungarwalla T.; Drickamer K.; Weis W. I.; Taylor M. E. (2015) A Novel mechanism for binding of galactose-terminated glycans by the C-type carbohydrate-recognition domain in blood dendritic cell antigen 2. J. Biol. Chem. 290, 16759–16771. 10.1074/jbc.M115.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet S. J.; van Liempt E.; Saeland E.; Aarnoudse C. A.; Appelmelk B.; Irimura T.; Geijtenbeek T. B.; Blixt O.; Alvarez R.; van Die I.; van Kooyk Y. (2005) Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int. Immunol. 17, 661–669. 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- Toyonaga K.; Torigoe S.; Motomura Y.; Kamichi T.; Hayashi J. M.; Morita Y. S.; Noguchi N.; Chuma Y.; Kiyohara H.; Matsuo K.; Tanaka H.; Nakagawa Y.; Sakuma T.; Ohmuraya M.; Yamamoto T.; Umemura M.; Matsuzaki G.; Yoshikai Y.; Yano I.; Miyamoto T.; Yamasaki S. (2016) C-Type lectin receptor DCAR recognizes mycobacterial phosphatidyl-inositol mannosides to promote a Th1 response during infection. Immunity 45, 1245–1257. 10.1016/j.immuni.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Somers W. S.; Tang J.; Shaw G. D.; Camphausen R. T. (2000) Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SleX and PSGL-1. Cell 103, 467–479. 10.1016/S0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Ng K. K.-S.; Kolatkar A. P.; Park-Snyder S.; Feinberg H.; Clark D. A.; Drickamer K.; Weis W. I. (2002) Orientation of bound ligands in mannose-binding proteins: implications of multivalent ligand recognition. J. Biol. Chem. 277, 16088–16095. 10.1074/jbc.M200493200. [DOI] [PubMed] [Google Scholar]
- Iobst S. T.; Wormald M. R.; Weis W. I.; Dwek R. A.; Drickamer K. (1994) Binding of sugar ligands to Ca2+-dependent animal lectins: I. Analysis of mannose binding by site-directed mutagenesis and NMR. J. Biol. Chem. 269, 15505–15511. [PubMed] [Google Scholar]
- Iobst S. T.; Drickamer K. (1994) Binding of sugar ligands to Ca2+-dependent animal lectins: II Generation of high affinity galactose binding by site-directed mutagenesis. J. Biol. Chem. 269, 15512–15519. [PubMed] [Google Scholar]
- Misaki A.; Seto N.; Azuma I. (1974) Structure and immunological properties of D-arabino-D-galactans isolated from cell-walls of Mycobacterium species. J. Biochem. 76, 15–27. 10.1093/oxfordjournals.jbchem.a130540. [DOI] [PubMed] [Google Scholar]
- Misaki A.; Azuma I.; Yamamura Y. (1977) Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J. Biochem. 82, 1759–1770. 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- Kotani S.; Kato T.; Matsuda T.; Kato K.; Misaki A. (1971) Chemical structure of the antigenic determinants of cell wall polysaccharide of Mycobacterium tuberculosis strain H37Rv. Biken J. 14, 379–387. [PubMed] [Google Scholar]
- Kaur D.; Lowary T. L.; Vissa V.; Crick D.; Brennan P. (2002) Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology 148, 3049–3057. 10.1099/00221287-148-10-3049. [DOI] [PubMed] [Google Scholar]
- Murase T.; Zheng R. B.; Joe M.; Bai Y.; Marcus S. L.; Lowary T. L.; Ng K. K. S. (2009) Structural insights into antibody recognition of mycobacterial polysaccharides. J. Mol. Biol. 392, 381–392. 10.1016/j.jmb.2009.06.074. [DOI] [PubMed] [Google Scholar]
- Wesener D. A.; Wangkanont K.; McBride R.; Song X.; Kraft M. B.; Hodges H. L.; Zarling L. C.; Splain R. A.; Smith D. F.; Cummings R. D.; Paulson J. C.; Forest K. T.; Kiessling L. L. (2015) Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol. 22, 603–610. 10.1038/nsmb.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.