Abstract
Purpose: Benzodiazepines are the drug of choice for alcohol withdrawal syndrome (AWS); however, phenobarbital is an alternative agent used with or without concomitant benzodiazepine therapy. In this systematic review, we evaluate patient outcomes with phenobarbital for AWS. Methods: Medline, Cochrane Library, and Scopus were searched from 1950 through February 2017 for controlled trials and observational studies using [“phenobarbital” or “barbiturate”] and [“alcohol withdrawal” or “delirium tremens.”] Risk of bias was assessed using tools recommended by National Heart, Lung, and Blood Institute. Results: From 294 nonduplicative articles, 4 controlled trials and 5 observational studies (n = 720) for AWS of any severity were included. Studies were of good quality (n = 2), fair (n = 4), and poor (n = 3). In 6 studies describing phenobarbital without concomitant benzodiazepine therapy, phenobarbital decreased AWS symptoms (P < .00001) and displayed similar rates of treatment failure versus comparator therapies (38% vs 29%). A study with 2 cohorts showed similar rates of intensive care unit (ICU) admission (phenobarbital: 16% and 9% vs benzodiazepine: 14%) and hospital length of stay (phenobarbital: 5.85 and 5.30 days vs benzodiazepine: 6.64 days). In 4 studies describing phenobarbital with concomitant benzodiazepine therapy, phenobarbital groups had similar ICU admission rates (8% vs 25%), decreased mechanical ventilation (21.9% vs 47.3%), decreased benzodiazepine requirements by 50% to 90%, and similar ICU and hospital lengths of stay and AWS symptom resolution versus comparator groups. Adverse effects with phenobarbital, including dizziness and drowsiness, rarely occurred. Conclusion: Phenobarbital, with or without concomitant benzodiazepines, may provide similar or improved outcomes when compared with alternative therapies, including benzodiazepines alone.
Keywords: alcohol withdrawal syndrome, phenobarbital
Introduction
Ethanol is the most frequently abused intoxicant in the United States.1 According to the National Institute of Alcohol Abuse and Alcoholism, nearly 88 000 deaths (approximately 62 000 males and 26 000 females) due to alcohol-related causes occur annually, making excessive alcohol consumption the fourth leading preventable cause of death in the United States.1 In 2010, more than 1 million people were hospitalized for alcohol dependence syndrome, and 3.6% of emergency department (ED) visits included an alcohol-related diagnosis.2 Mild symptoms frequently develop in patients withdrawing from alcohol and include autonomic and neuroexcitatory effects such as tremor, tachycardia, hypertension, anxiety, and agitation. Symptoms of severe alcohol withdrawal (AW) occur in fewer than 10% of patients who present to the ED with alcohol withdrawal syndrome (AWS) and include delirium tremens (DT) and seizures.3,4
The symptoms when alcohol is abruptly discontinued after chronic, exaggerated use occur because of a disturbance in the established homeostasis between excitatory and inhibitory neurotransmitter responses.5 Ethanol increases the inhibitory effects of the γ-aminobutyric acid type A (GABAA) receptor complex and decreases excitatory effects from glutamate on N-methyl-d-aspartate (NMDA) receptors.6-9 Chronic ingestion of ethanol causes downregulation of GABAA receptors, upregulation of NMDA receptors, decreased GABA release, and increased glutamate production. If ethanol is not present, the disequilibrium due to the lack of sufficient inhibitory neurotransmitter activity leads to the manifestation of clinical signs and symptoms of AWS.5,8,9
Alcohol abuse and withdrawal are associated with an increased risk for medical comorbidities (eg, infections, cardiopulmonary insufficiency, cardiac arrhythmia, bleeding disorders, need for mechanical ventilation). Withdrawal also leads to longer, more complicated hospital and intensive care unit (ICU) lengths of stays (LOS).10-12 Signs and symptoms of AW may occur within 8 hours after the last ingestion of ethanol but often appear after several days and reach peak intensity after 24 to 72 hours.3-5 Numerous medications in combination or as monotherapy are used for treatment of AW, including α-adrenergic agonists, barbiturates, benzodiazepines (BZDs), β-blockers, butyrophenones, carbamazepine, dexmedetomidine, gabapentin, propofol, and valproic acid.13-31 The primary goals of pharmacologic management of AWS are to minimize symptom severity and prevent major complications.6,7 The ideal agent should be cross-tolerant with alcohol. It should have a rapid onset, wide margin of safety, metabolism independent of liver function, low potential for abuse, and provide sedative, anxiolytic, and anticonvulsant effects without causing respiratory depression.6-9
Benzodiazepines have most of the properties of an ideal agent (rapid onset, wide margin of safety, and provide sedative, anxiolytic, and anticonvulsant effects without causing significant respiratory depression); thus, they have emerged as the cornerstone of AWS management. When symptom-triggered dosing of BZDs using a validated scale, such as the revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) scale,32 was compared with fixed schedule dosing alongside supportive care, symptom-triggered dosing demonstrated decreased BZD requirements, decreased time spent on mechanical ventilation, and decreased ICU LOS.6,22,33-35 However, in milder cases of AWS for which patients may be candidates for discharge from the ED rather than admission to the hospital, discharge with a medication with a longer half-life may prove beneficial.6,21,22 In patients with a history of AW and more severe cases of AWS, decreased GABAA receptor sensitivity to GABA and GABA agonists may cause BZD monotherapy to be ineffective.36 Patients may experience increased occurrence of morbidity, mortality, and severe adverse effects (eg, respiratory depression, ICU delirium, and oversedation) as doses of BZDs are escalated to manage resistant AW.14,37 Adjunctive agents or alternatives to BZDs may reduce these effects.13-15
Phenobarbital (PHB) has been studied for patients across the continuum of AWS over the last 40 years and offers potential advantages to BZDs.17-31 PHB exerts its effects in as few as 5 minutes with parenteral administration. With oral administration, absorption from the gastrointestinal tract is rapid and effects may be seen in 20 to 60 minutes. Barbiturates bind to the GABA receptor at a different binding site than BZDs, increasing the time the GABA-mediated chloride channels remain open by mimicking the stimulation provided by chronic alcohol use. Through a secondary, additive mechanism, barbiturates also may inhibit the excitatory NMDA receptor and reduce neuroexcitation commonly seen with AW. Respiratory depression, hypotension, and central nervous system depression may occur with supratherapeutic dosing, and severe hepatic impairment can develop after chronic, prolonged use.38
The objective of this systematic review was to evaluate PHB use with and without concomitant BZD therapy according to indication, dosing, and patient outcomes.
Materials and Methods
Search Strategy and Selection Criteria
Studies were retrieved from the PubMed, Cochrane Controlled Trial Registry, and Scopus databases from 1950 through February 2017 to address the question, “Does use of PHB in adult patients at risk for developing AWS affect clinical outcomes compared to nonuse of PHB?” Controlled and observational studies were considered for inclusion. The search strategy used was [“phenobarbital” or “barbiturate”] and [“alcohol withdrawal” or “delirium tremens.”] References from the bibliographies of the studies retrieved from the literature search were reviewed to identify additional studies. After these initial studies were selected, additional studies that cited a selected study were reviewed for potential inclusion. Studies written in languages other than English and those presented solely as abstracts at scientific conferences were not considered for inclusion. Case studies were excluded due to the lack of rigor.30,31 This systematic review was registered with PROSPERO (CRD42017056990).
Study Selection
Two authors (Drayton A. Hammond & Jordan M. Rowe) independently reviewed the abstracts for all studies. A study was considered eligible for inclusion if patients received PHB for treatment of AWS of any severity and clinical outcomes (ie, BZD usage, location-specific lengths of stay, symptomatology, and adverse events) were evaluated. When relevant data for our review were unavailable, original study authors were contacted for further details whenever possible.
Data Extraction
Data extracted from the identified studies included study design, inclusion and exclusion criteria, clinical setting, patient population, number of study participants, age, severity of illness, receipt and dosing of PHB and comparator therapies for treatment of AWS, and patient outcomes, if available. Two reviewers independently used quality assessment tools from the National Heart, Lung, and Blood Institute to assess study quality.39 Discrepancies were resolved through discussion with all study investigators.
Analyzed Outcomes
Outcomes that were analyzed included frequency of ICU admission, first intubation for mechanical ventilation, continuous infusion BZD, and breakthrough BZD or PHB use. In addition, differences in the duration of ICU, hospital, and ED stay and mechanical ventilation were evaluated. Differences in cumulative amounts and maximum doses of BZD and PHB as well as serum concentrations of PHB were calculated. Changes in AWS symptoms and development of adverse effects with PHB were determined.
Results
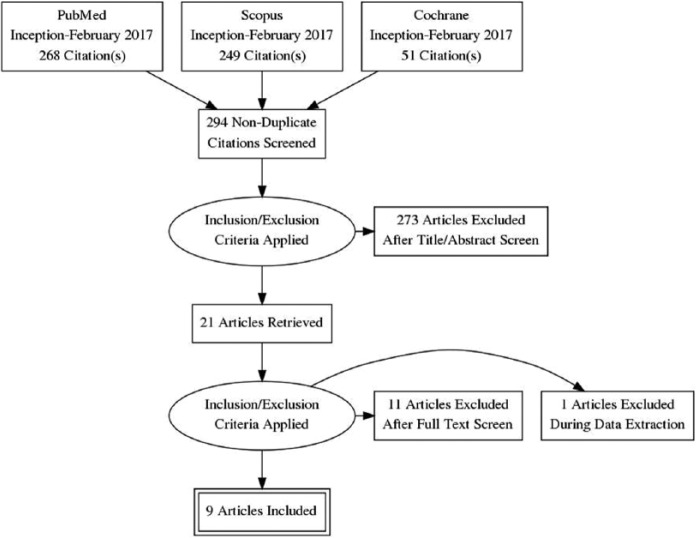
Our literature search identified 294 studies from which 21 studies were reviewed and 9 were included in the systematic review17-25 (Table 1). Four of these studies included patients who received concomitant BZD therapy in the PHB group,17-20 and 5 studies included patients who received PHB as monotherapy.21-25 The comparator groups included BZD therapy (n = 6),17-21,23 carbamazepine (n = 1),23 gabapentin (n = 1),25 propofol (n = 1),19 PHB (n = 3),18,19,25 and no comparator group (n = 2).21,24 Three studies included more than 1 agent in a comparator group.18,19,24 Three of the studies included patients with severe AWS (33%-100%) as designated by the study authors or because patients received care in the ICU,17-19 and 6 included patients with mild-to-moderate AWS (40-100%) as designated by the study authors.20-25 When evaluated according to quality of study, 2 were good quality,17,21 4 were fair quality,18-20,22 and 3 were poor quality.23-25 Four trials were excluded because they either used barbital or did not include enough patients or describe PHB use with enough detail to identify exposure with outcomes.26-29 Two case reports were excluded30,31 (Figure 1).
Table 1.
Evidence for Phenobarbital in Alcohol Withdrawal Syndrome.
| Study authors, type, and quality | Setting and severity | Sample size | PB dosing | Comparator | BZD dosing | Efficacy and safety outcome(s) |
|---|---|---|---|---|---|---|
| Concomitant BZD therapy | ||||||
| Rosenson et al.17
Prospective, randomized, double-blind, placebo-controlled trial Good quality |
ED then admitted to the hospital Severe AWS |
N = 102 (n = 51 PB and n = 51 placebo) | 10 mg/kg IV x1 | Placebo | Yes Symptom-guided LZ-based AWS protocol |
PB: decreased ICU admission (8% vs 25%, 17% difference, 95% CI 4-32%) PB: decreased continuous infusion LZ (4% vs 31%, 27% difference, 95% CI, 14-41%) PB: decreased total LZ requirements (26 mg vs 49 mg, difference 23 mg, 95% CI, 7-40 mg) PB: no adverse effects No difference in ICU or hospital LOS No difference in admission to other locations in the hospital |
| Duby et al.18
Retrospective, cohort study Fair quality |
ICU Severe AWS |
N = 135 (n = 65 preguideline: physician preference and n = 70 postguideline: protocol of escalating doses of BZD and PB to RASS goal of -2 to 0) | Escalating doses (route unknown) of 60 mg, 120 mg, and 240 mg after max DZ (120 mg) based on RASS Median PB doses in both groups was 0 |
Physician preference (no protocol) | Escalating doses based on RASS (max 120 mg DZ) | Post-guideline care associated with: Decreased ICU LOS (9.6 d vs 5.2 d, P = .0004) Decreased ventilator days (5.6 d vs 1.31 d, P < .0001) Decreased DZ (319 mg vs 93 mg, P = .002) Decreased sedation time (10.8 d vs 3.5 d, P < .001) Decreased need for continuous sedation (33 [55%] vs 18 [24%], P < .001) Decreased intubation (13 [22%] vs 4 [5%], P < .001) |
| Gold et al.19
Retrospective, cohort study Fair quality |
ICU Severe AWS |
N = 95 (n = 54 DZ ± PB preguideline, n = 41 DZ ± PB post-guideline) | Escalating IV doses (65 mg, 130 mg, 260 mg) | No protocol for intermittent DZ | Yes Escalating doses of DZ to max of 150 mg/dose |
Symptom-guided DZ ± PB for adequate sedation associated with decreased complications Post-guideline: Increased max individual dose DZ (32 mg vs 86 mg; P = .001), total DZ (248 mg vs 562 mg; P = .001), and PB use (17% vs 58%; P = .01) Post-guideline: Decreased mechanical ventilation (47% vs 22%; P = .008) Post-guideline: reduction in mechanical ventilation rate (21.9% vs 47.3%, P = .008) Post-guideline: ICU LOS and total amount of BZD received correlated (r = .48, P = .008) No difference in ICU LOS or nosocomial complications between groups |
| Gashlin et al.20
Retrospective, cohort study Fair quality |
Hospitalized, non-ICU Mild-to-moderate AWS |
N = 28 (n = 7 PB, n = 21 BZD) | PB (65 mg IV ×1, then 130 mg IV ×1, then 260 mg IV until AWS symptom resolution) Median PB dose 455 mg (IQR: 309-618) or 6.3 mg/kg (IQR: 3.5-10.3) |
BZD | BZD (DZ 20 mg PO or equivalent dose of LZ; doses reduced for age and liver dysfunction) | No difference in percentage of CIWA-Ar scores <10 at 24 hours between PB and BZD (28.6% vs 23.8%, P = .588) Reduction in cumulative BZD dose in DZ equivalents in PB group [25 mg (IQR: 20-226) vs 326 mg (160-550), P = .02] No difference in intubation requirement (14.3% vs 4.8%) or ICU admission (14.3% vs 19%) |
| No concomitant BZD therapy | ||||||
| Hendey et al.21
Prospective, randomized, double-blind trial Good quality |
ED (only 6 patients admitted to the hospital) Mild-to-moderate AWS |
N = 44 (n = 25 PB, n = 19 LZ) | 260 mg IV ×1, 130 mg IV thereafter at physician’s discretion) Mean PB dose 509 mg (range: 260-910) |
LZ | 2 mg IV as needed + CDP PO as needed Mean LZ dose 4.2 mg (range: 2-8) |
PB and LZ both reduced the average CIWA-Ar score from baseline to discharge (15.0 to 5.4 and 16.8 to 4.2, P < .0001) More doses of PB given (2.9 doses (range: 1-6) vs 2.1 doses (1-4), P = .03) No difference in hospital admission rate (12% vs 16%, P = .8) No difference in ED LOS (267 m vs 256 m, P = .8) |
| Young et al.22
Prospective, uncontrolled trial Fair quality |
ED (only 5 patients admitted to hospital) Mild-to-moderate AWS |
N = 62 PB | 260 mg IV ×1 then 130 mg IV until clinical end point of light sedation or adverse effect noted | None | None | Safe discharge from ED was achieved in 92% of patients Average ED LOS was 3 h, 47 min No discharged patients returned to ED during the following week Adverse effect in 6% of patients (none were admitted to hospital): 1 hypotension, 1 ataxia, 2 lethargy after final bolus doses |
| Hjermø et al.23
Retrospective, cohort study Poor quality |
Psychiatric department in 2 hospitals Mild-to-moderate AWS |
N = 194 106 PB (n = 53 PB at hospital 1, n = 53 PB at hospital 2) n = 88 DZ at hospital 2 |
100-200 mg PO or IV up to 4 times/h | DZ + carbamazepine 200 mg TID (41% of patients) | 10-20 mg IV up to 4 times/h + CDP as needed to achieve sleep | No difference in hospital LOS (PB hospital 1: 5.85 ± 6.3 d, PB hospital 2: 5.30 ± 2.6 d, DZ hospital 2: 6.64 ± 4.2 d) No difference in rate of ICU admission (16%, 9%, 14%) Adverse effect in 1 PB patient: respiratory depression |
| Rosenthal et al.24
Prospective, randomized trial Poor quality |
Inpatient detoxification unit Mild-to-moderate AWS |
N = 42 patients (unknown how many each of PB and valproate) | PB (Day 1: 60 mg PO QID; Day 2: 60 mg PO TID; Day 3: 60 mg PO BID; Day 4: 30 mg PO ×1) | Valproate + PB 60 mg PO as needed for breakthrough AWS symptoms | Unknown | Both decreased AWS symptoms (P < .00001) PB group: more frequent use of as-needed PB (39 doses vs 20 doses, P < .05) |
| Mariani et al.25
Prospective, randomized trial Poor quality |
Inpatient detoxification unit Mild-to-moderate AWS |
N = 21 (n = 10 PB, n = 11 GP) | PB (Day 1: 60 mg PO QID; Day 2: 60 mg PO TID; Day 3: 60 mg PO BID; Day 4: 30 mg PO BID PB 60 mg PO PRN breakthrough AWS symptoms) |
GP (Day 1: 1200 mg PO ×1, 600 mg PO BID for 2400 mg; Day 2: 600 TID; Day 3: 600 BID; Day 4: 600 ×1) and PB PRN | None | No difference in treatment failure (PB 38% vs GP 29%, P = .70) – 3+ PB as needed doses, intolerable adverse effect, or left against medical advice No difference in requirement for as-needed PB (38% vs 57%, P = .45) |
Note. AWS = alcohol withdrawal syndrome; BID = twice daily; BZD = benzodiazepine; CDP = chlordiazepoxide; CI = confidence interval; CIWA-Ar = clinical institute withdrawal assessment score, revised; DZ = diazepam; ED = emergency department; GP = gabapentin; ICU = intensive care unit; IQR = interquartile range; IV = intravenous; LOS = length of stay; LZ = lorazepam; PO = by mouth; PB = phenobarbital; QID = 4 times daily; TID = 3 times daily; RASS = Richmond Agitation and Sedation Scale; PRN = as needed.
Figure 1.
Article Inclusion/Exclusion.
PHB With Concomitant BZD Therapy
Severe AWS
Rosenson and colleagues performed a randomized, double-blind, controlled trial in which they evaluated the effectiveness of single-dose PHB (10 mg/kg) compared with placebo (n = 51 in each group) in nonintubated patients with AWS at ED admission.17 A modified CIWA score was used for evaluation of AWS. The primary outcome was the initial level of hospital admission from the ED. The study found a decreased ICU admission rate with PHB treatment compared with placebo (8.0% vs 25.0%, no P value provided). Patients in the PHB group required less total lorazepam (26.0 mg vs 49.0 mg, no P value provided) and fewer requirements for lorazepam infusions (4.0% vs 31.0%, no P value provided). There were no differences in ICU or hospital LOS or adverse outcomes (eg, intubation) between the 2 groups. There were 48 patients who met inclusion criteria who were not included in the study based on ED provider judgment, which may have affected results. In addition, 8 (15.7%) patients in the placebo group still received PHB. The use of PHB appeared to decrease BZD requirements and need for ICU admission in this study.
Duby and colleagues performed a pre-post intervention study at a single-center ICU in patients with a diagnosis of AWS.18 Patients in the preintervention phase were treated per provider discretion. The intervention was the inclusion of a protocol that used escalating BZD doses and PHB though routes were not provided. Diazepam doses were initiated at 10 mg escalated in 10 to 20 mg increments every 15 to 30 minutes to a target sedation level: Richmond Agitation Sedation Scale score of 0 to −2. Once diazepam doses reached 120 mg, PHB was administered as an adjunct every 30 minutes to a maximum dose of 240 mg. Routes of administration for diazepam and PHB were not provided. The primary outcome was the ICU LOS. A total of 135 patients (preintervention: n = 60; postintervention: n = 75) were included in the analysis. There was a significant decrease in the mean ICU LOS in the postintervention group (5.2 days vs 9.6 days, P = .0004), compared with the preintervention group. There were also significant decreases in mean duration of mechanical ventilation (1.3 days vs 5.6 days, P < .001) and need for continuous sedation in the postintervention group (24.0% vs 55.0%, P < .001). There was a substantial decrease in mean BZD requirements in the postintervention group (P = .0002), although PHB was still rarely used even in the postintervention group. This study largely compared nonprotocolized care versus protocolized care. The benefits of PHB treatment are difficult to extrapolate, given its limited use in this study.
Gold and colleagues performed a pre-post intervention study at a single-center medical ICU in patients with an admission diagnosis of AWS.19 Requirement for ICU admission was >200 mg of diazepam within 4 hours or an individual dose of >40 mg of diazepam for management of AWS. All subjects were treated using a symptom-triggered approach, which depended on the presence of a Riker Sedation-Agitation Scale score ≥5 given a goal of 3 to 4. The authors believe the preguideline dosing strategy was leading to underdosing of patients; therefore, the postguideline intervention was an escalating BZD dosing protocol for patients who did not have control of agitation from a dose of BZD for ≥1 hour. Doses of diazepam were escalated until agitation was controlled for ≥1 hour, and intravenous PHB was added in a similar fashion (65 mg with doses doubled up to a 260 mg maximum dose) to patients who required diazepam doses of >100 mg. This study was conducted to describe the outcomes of patients with severe AWS and whether dose escalation of BZDs in combination with PHB improves those outcomes. A total of 95 patients were included in the analysis (preguideline: n = 54; postguideline: n = 41). Significant increases in PHB use and total diazepam dose administered were found in the postguideline group (58.0% vs 17.0%, P < .001; 562 mg vs 248 mg, P < .001, respectively). There was a significant reduction in the use of mechanical ventilation postguideline (21.9% vs 47.3%, P = .008). Mechanical ventilation was most commonly used for airway protection for all patients. There were no differences in ICU LOS or the overall incidence of nosocomial pneumonia. ICU LOS was greater in patients who required mechanical ventilation, regardless of type of guideline used. Although PHB was used after escalating doses of diazepam and in only 58% of patients in the postguideline group compared with 17% in the preguideline group, its use may have contributed to decreasing mechanical ventilation in this study. The reduction in BZD requirements likely was a result of the protocolized guideline rather than PHB.
Mild-to-moderate AWS
Gashlin and colleagues performed a retrospective cohort study at a single-center using 2 AWS cohorts: treatment with BZDs alone and treatment with BZDs plus PHB.20 To qualify for the BZD-only group, patients had to receive at least 3 oral diazepam doses of 20 mg (or equivalent) within 6 hours. All other patients were in the BZD and PHB group, but the authors did not indicate the strategy for selection of PHB use in patients. All patients had at least 1 CIWA-Ar score greater than 10. Patients directly admitted to the ICU for AWS treatment initiation were excluded. The primary outcome was the proportion of patients with a CIWA-Ar score less than 10 at 24 hours after AWS treatment initiation. A total of 28 patients were evaluated (BZD-only: n = 21; BZD and PHB: n = 7). There was no difference in the primary outcome between the BZD-only and BZD plus PHB groups (23.8% vs 28.6%, respectively; P = .588). The median duration of AWS symptoms was also similar between groups. Median BZD requirements were lower in the BZD plus PHB group (25 mg vs 326 mg; P = .02). The median cumulative PHB dose was 455 mg (interquartile range, 309- 618 mg), which equated to a median 6.3 mg/kg dose per patient. Evaluation of safety end points (eg, requirement for intubation or ICU admission) was similar between the 2 groups. PHB appears to only reduce BZD requirements in this study, while maintaining a good safety profile compared with BZDs only.
Summary
Use of intravenous PHB with concomitant BZDs appears to offer some potential benefits, although it is difficult to determine whether these benefits are due to more intentional and protocolized care or due to the administration of PHB itself. Although data are limited, when used as a single, large dose in the ED as part of a front-loading strategy in order to gain control of AWS symptoms, PHB appears to reduce the need for ICU admission rates and is well tolerated. When added to dose escalation of BZDs, decreases in length of mechanical ventilation and ICU LOS have been shown. However, this may be due to just more intentional treatment with GABA agonists and not solely due to the benefits of PHB itself. Despite an overall small sample of patients were evaluated, more consistent findings were the decrease in BZD requirements when PHB was used in addition to BZD and the relative safety of PHB in these studies.
PHB Monotherapy
Mild-to-moderate AWS
Hendey and colleagues performed a randomized, double-blind trial at a single center, evaluating adult patients in the ED with known or suspected AWS.21 Patients were randomized to enteral lorazepam 2 mg or enteral PHB (260 mg initial dose, 130 mg for subsequent doses), which were administered per a modified CIWA score. The primary outcome was the change in AWS scores from baseline to discharge or hospital admission. A total of 44 patients were included in the analysis (BZD: n = 19; PHB: n = 25). The mean dose of PHB administered was 509 mg (range, 260-910 mg) and 4.2 mg lorazepam (range, 2-8 mg) in the BZD group. PHB and BZD were found to significantly decrease the CIWA score from baseline to discharge (15-5.4 and 16.8-4.2; P < .0001 for both groups). There were no differences between the 2 groups in the extent of CIWA reduction (P = .40). Patients who were discharged from the ED were asked to return within 48 hours for AWS symptom evaluation. Nine patients in each group returned, with no difference found in AWS symptoms or tolerability of discharge medications (placebo for PHB, chlordiazepoxide for BZD group).
Young and colleagues prospectively evaluated patients with acute AWS in the ED.22 Patients with acute drug intoxication were excluded. Patients received an initial PHB infusion of 260 mg, followed by additional 130 mg boluses until light sedation was reached or adverse effects (eg, altered mental status, hypotension) were recognized. After the last dose of PHB, a serum concentration was drawn 30 minutes later, and patients were observed for at least 1 hour. A total of 62 patients were included in the analysis. The mean serum PHB concentration was 13.9 µg/mL, with an approximate increase in serum concentration per mg/kg increase in dose of 1.65 µg/mL. The majority of patients were safely discharged after a mean ED stay of 3.78 hours. Only 4 patients did not respond to PHB therapy, evidenced by DT. Therapy was generally well tolerated, although hypotension (n = 1), ataxia (n = 1), and lethargy (n = 2) were identified in patients.
Hjermø and colleagues evaluated the effect of PHB therapy in patients with DT at 2 psychiatric departments.23 PHB was administered at a dose of 100 to 200 mg hourly, while intravenous diazepam was administered at a dose of 10 to 20 mg hourly. A total of 194 patients were included in the analysis. There were no differences between groups in hospital LOS or duration of DT (no P values provided). In addition, there was no difference in development of pneumonia between groups (P > .05), although respiratory depression was numerically more common in the PHB-treated group (3.8% vs 1.1%, P > .05).
Rosenthal and colleagues performed a randomized, open-label, controlled trial in an inpatient detoxification unit at a single center.24 Patients with a non–substance-related psychiatric disorder, or opioid (except methadone) or long elimination half-life BZDs use (eg, diazepam and flurazepam) were excluded. Patients were randomized to a 5-day detoxification schedule of either PHB or valproate. PHB was dosed 60 mg enterally 4 times daily on the first day and then tapered off by day 5. PHB (enteral or intramuscular) was available as a rescue medication. Outcomes evaluated were based on symptom severity assessments, including the Modified Selective Severity Assessment score, which is an objective scale performed by nurses evaluating symptoms such as tremor, sleep disturbance, and agitation. This interim analysis included 42 patients (n in each group not provided). There was no difference found in the Modified Selective Severity Assessment score between groups. Patients in the PHB group received twice as many rescue medications as the valproate group (39 vs 20, P < .05).
Mariani and colleagues performed a randomized, open-label, controlled study in an inpatient detoxification unit at a single center.25 Patients with AW-associated delirium, a non–substance-related psychiatric disorder, or opioid (except methadone) or sedative-hypnotic use were excluded. Patients were randomized into a 4-day detoxification schedule with either PHB or gabapentin. PHB was dosed 60 mg enterally 4 times daily on the first day and then tapered down to 30 mg twice daily on day 4, and gabapentin was dosed 1200 mg once and 600 mg twice on day 1 then tapered to 600 mg once on day 4. Patients in the gabapentin group were able to receive rescue PHB as needed. The primary outcome was the proportion of treatment failure in each group, defined as the requirement of ≥3 doses of as-needed PHB. A total of 27 patients were included in the analysis (gabapentin: n = 14, PHB: n = 13). Two patients in each group were defined as treatment failures (P value not provided). There was no difference in daily withdrawal scores between the groups throughout the study period. Therapy was well tolerated, with no patients developing seizures or DT related to AWS.
Summary
The use of oral and intravenous PHB as monotherapy for AWS has been studied in heterogeneous patient samples in numerous studies with variable study designs and comparator groups. In summary, it appears that PHB by either route is as effective as other GABA agonists for the management of AW and appears to have a clear dose-response relationship in regard to serum levels; however, specific serum levels have not been associated with clinical outcomes and may require patient individualization. These results are only applicable to patients with mild-to-moderate AWS and represent surrogate end points.
Clinical Applications and Future Directions
In total, 9 studies that evaluated PHB in the treatment of mild, moderate, or severe AWS were included in our analysis.17-25 Despite the heterogeneity of these data, when the studies were separated based on AWS severity, more meaningful conclusions on the roles PHB can play in mild-to-moderate and severe AWS were formed. This heterogeneity prevented both performing a meta-analysis to evaluate the effect of PHB in treatment of AWS and providing a strong recommendation for PHB in specific patients.
PHB is a reasonable adjunctive agent in patients who are not responsive to BZDs alone. The addition of a weight-based or dose-escalation protocol was associated with improved outcomes, including need for ICU admission and reduction in BZD doses, and was well tolerated. The methods in these studies seem to be relatively easily translated into an institutional guideline for PHB administration in patients not responding to BZD monotherapy. Data suggest that approximately 75% of patients with severe AWS require intubation to protect their airway due to a decreased Glasgow coma score.34 Therefore, front-loading of PHB may be preferred to prevent progression of AWS symptoms. Historically, clinicians have been hesitant to use higher doses of barbiturates; however, the doses provided in this trial are similar to the doses patients received in other studies.22,23,27 BZDs were administered following the loading dose of PHB, which may increase the risk of respiratory depression and sedative effects.6,40,41 Although the studies were not specifically powered to evaluate safety outcomes, this regimen appeared to be safe as no adverse effects related to PHB were reported. As there is no comparison between a dose escalation, route of administration, or a single-dose approach to administering PHB, institutional preference and comfort with administration of these approaches would be an appropriate deciding factor until this comparison has been performed.
It is difficult to offer a recommendation for PHB monotherapy given the limited data available in the studies evaluated. PHB does appear to be as effective and safe as other GABA agonists (eg, gabapentin and valproate). Administration of PHB seems to have a relatively linear relationship with regard to expected serum levels, which may be a potential target for future studies. Frequently, the symptoms of patients who present to the ED or a psychiatric facility with mild-to-moderate AWS may be controlled using PHB without requiring hospital admission. A chief concern when discharging patients who present with AWS from the ED is that the patient may progress to severe AWS symptoms (ie, seizure or DT) following discharge and either require readmission or experience irreversible neurological damage.6,42 As the elimination half-life of PHB may be between 5 and 6 days, patients who receive PHB in the ED should not require a prescription for a BZD or barbiturate at discharge and would be at decreased risk for failing treatment.43
Future Studies
We believe that future directions for studying the utility of PHB therapy should include the creation of studies solely evaluating PHB and BZDs (ie, no other adjunctive treatments used) and dose-finding studies for appropriate dosing and routes of administration of PHB as monotherapy, as well as adjunctive therapy to BZDs. AWS literature, especially in severe AWS, has commonly included many medications in addition to the primary medication of evaluation in the study. Due to the lack of standardization of institutional protocols and lack of available guidelines for therapy in these patients, many individual medications and classes of medications are used for control of AWS symptoms. As previously mentioned, there have been no studies comparing different dosing strategies of PHB to one another. Although it is likely that specific patients may benefit from individualized PHB dosing, closer identification of the safety of PHB use in addition to BZDs needs to be evaluated. Future studies involving PHB should target a clinically relevant, objective end point related to AWS such as time to resolution of symptoms. Of utmost importance remains the ability to identify patients at risk for AWS and those early on in their AWS symptomatology to prevent progression to more severe AWS.
Conclusions
In conclusion, PHB may have a role in AWS treatment alongside BZDs or as monotherapy; however, there is not enough evidence to recommend a general, nonindividualized regimen based on AWS severity at this time. The most favorable results for patients with severe AWS or mild-to-moderate AWS who presented to the hospital were seen when PHB was administered using an early and aggressively dosed strategy. Patients with severe AWS who received PHB required less escalation of their care, and those with mild-to-moderate AWS spent less time in the ED and did not require further care following discharge. To better delineate the role of PHB, clinical trials of adequate size and appropriate design should be conducted. Specific areas of investigation include front-loading PHB in various severities of AWS and head-to-head comparisons between adjuvant therapies in patients with resistant AWS already admitted to the ICU.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. National Institute of Alcohol Abuse and Alcoholism. http://pubs.niaaa.nih.gov/publications/AlcoholFacts&Stats/AlcoholFacts&Stats.htm. Accessed October 27, 2016.
- 2. U.S. alcohol epidemiologic data reference manual, volume 9. http://pubs.niaaa.nih.gov/publications/NEDS&NIS-DRM9/NEDS&NIS-DRM9.pdf. Accessed October 27, 2016.
- 3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 4. Bayard M, McIntyre J, Hill KR, Woodside J., Jr. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69(60):1443-1450. [PubMed] [Google Scholar]
- 5. Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World.1998;22(1):61-66. [PMC free article] [PubMed] [Google Scholar]
- 6. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22(2):100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38:S494-S501. [DOI] [PubMed] [Google Scholar]
- 8. Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152(3):332-340. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman PL, Grant KA, Snell LD, Reinlib L, Iorio K, Tabakoff B. NMDA receptors: role in ethanol withdrawal seizures. Ann N Y Acad Sci. 1992;654:52-60. [DOI] [PubMed] [Google Scholar]
- 10. O’Brien JM, Jr, Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345-350. [DOI] [PubMed] [Google Scholar]
- 11. Awissi D, Lebrun G, Coursin DB, Riker RR, Skrobik Y. Alcohol withdrawal and delirium tremens in the critically ill: a systematic review and commentary. Intensive Care Med. 2013;39(1):16-30. [DOI] [PubMed] [Google Scholar]
- 12. Genther D, Gourin C. The effect of alcohol abuse and alcohol withdrawal on short-term outcomes and cost of care after head and neck cancer surgery. Laryngoscope. 2012;122(8):1739-1747. [DOI] [PubMed] [Google Scholar]
- 13. Brotherton AL, Hamilton EP, Kloss HG, Hammond DA. Propofol for treatment of refractory alcohol withdrawal syndrome: a review of the literature. Pharmacotherapy. 2016;36(4):433-442. [DOI] [PubMed] [Google Scholar]
- 14. Wong A, Smithburger PL, Kane-Gill SL. Review of adjunctive dexmedetomidine in the management of severe acute alcohol withdrawal syndrome. Am J Drug Alcohol Abuse. 2015;41(5):382-391. [DOI] [PubMed] [Google Scholar]
- 15. Wong A, Benedict NJ, Kane-Gill SL. Multicenter evaluation of pharmacologic management and outcomes associated with severe resistant alcohol withdrawal. J Crit Care. 2015;30(2):405-409. [DOI] [PubMed] [Google Scholar]
- 16. Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy. 2014;34(9):910-917. [DOI] [PubMed] [Google Scholar]
- 17. Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44(3):592-598. [DOI] [PubMed] [Google Scholar]
- 18. Duby JJ, Berry AJ, Ghayyem P, Wilson MD, Cocanour CS. Alcohol withdrawal syndrome in critically ill patients: protocolized versus nonprotocolized management. J Trauma Acute Care Surg. 2014;77(6):938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gashlin LZ, Groth CM, Wiegand TJ, Ashley ED. Comparison of alcohol withdrawal outcomes in patients treated with benzodiazepines alone versus adjunctive phenobarbital: a retrospective cohort study. Asia Pac J Med Toxicol. 2015;4:31-36. [Google Scholar]
- 21. Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized, trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med. 2011;29(4):382-385. [DOI] [PubMed] [Google Scholar]
- 22. Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med. 1987;16(8):847-850. [DOI] [PubMed] [Google Scholar]
- 23. Hjermø I, Anderson JE, Fink-Jensen A, Allerup P, Ulrichsen J. Phenobarbital versus diazepam for delirium tremens—a retrospective study. Dan Med Bull. 2010;57(8):1-5. [PubMed] [Google Scholar]
- 24. Rosenthal RN, Perkkel C, Singh P, et al. A pilot open randomized trial of valproate and phenobarbital in the treatment of acute alcohol withdrawal. Am J Addict. 1998;7:189-197. [DOI] [PubMed] [Google Scholar]
- 25. Mariani JJ, Rosenthal RN, Tross S, Singh P, Anand OP. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15:76-84. [DOI] [PubMed] [Google Scholar]
- 26. Tangmose K, Nielsen M, Allerup P, Ulrichsen J. Linear correlation between phenobarbital dose and concentration in alcohol withdrawal patients. Dan Med Bull. 2010;57(8):1-4. [PubMed] [Google Scholar]
- 27. Kramp P, Rafaelsen OJ. Delirium tremens: a double-blind comparison of diazepam and barbital treatment. Acta Psychiatr Scand. 1978;58(2):174-190. [DOI] [PubMed] [Google Scholar]
- 28. Flygenring J, Hansen J, Holst B, Petersen E, Sørensen A. Treatment of alcohol withdrawal symptoms in hospitalized patients. Acta Psychiatr Scand.1984;69(5):398-408. [DOI] [PubMed] [Google Scholar]
- 29. Askgaard G, Hallas J, Fink-Jensen A, Molander AC, Madsen KG, Pottegård A. Phenobarbital compared to benzodiazepines in alcohol withdrawal treatment: a register-based cohort study of subsequent benzodiazepine use, alcohol recidivism and mortality. Drug Alcohol Depend. 2016;161:258-264. [DOI] [PubMed] [Google Scholar]
- 30. Hayner C, Wuestefeld N, Bolton P. Phenobarbital treatment in a patient with resistant alcohol syndrome. Pharmacotherapy. 2009;29(7):875-878. [DOI] [PubMed] [Google Scholar]
- 31. Ives TJ, Mooney AJ, III, Gwyther RE. Pharmacokinetic dosing of phenobarbital in the treatment of alcohol withdrawal syndrome. South Med J. 1991;84(1):18-21. [DOI] [PubMed] [Google Scholar]
- 32. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353-1357. [DOI] [PubMed] [Google Scholar]
- 33. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121. [DOI] [PubMed] [Google Scholar]
- 34. Spies CD, Otter HE, Huske B, et al. Alcohol withdrawal severity is decreased by symptom-orientated adjusted bolus therapy in the ICU. Intensive Care Med. 2003;29:2230-2238. [DOI] [PubMed] [Google Scholar]
- 35. Cassidy EM, O’Sullivan I, Bradshaw P, Islam T, Onovo C. Symptom-triggered benzodiazepine therapy for alcohol withdrawal syndrome in the emergency department: a comparison with the standard fixed dose benzodiazepine regimen. Emerg Med J. 2012;29(10):802-804. [DOI] [PubMed] [Google Scholar]
- 36. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson MT, Yamanaka TT, Fraidenburg DR, Kane SP. Benzodiazepine misadventure in acute alcohol withdrawal: the transition from delirium tremens to ICU delirium. J Anesth. 2013;27(1):135-136. [DOI] [PubMed] [Google Scholar]
- 38. Brunton L.L., Chabner BA, Knollmann BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011. [Google Scholar]
- 39.Study quality assessment tools. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools. Accessed September 16, 2016.
- 40. Hartshorn EA. Interactions of CNS drugs psychotherapeutic agents—the anxiety drugs. Ann Pharmacother. 1975;9:26-35. [Google Scholar]
- 41. Levy RH, Mattson RH, Meldrum BS, Perucca E. Antiepileptic Drugs. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 42. Carlson RW, Kumar NN, Wong-Mckinstry E, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28:549-585. [DOI] [PubMed] [Google Scholar]
- 43. Nelson E, Powell JR, Conrad K, et al. Phenobarbital pharmacokinetics and bioavailability in adults. J Clin Pharmacol. 1982;22(2-3):141-148. [DOI] [PubMed] [Google Scholar]