Abstract
Oomycetes are a diverse group of eukaryotes in terrestrial, limnic and marine habitats worldwide and include several devastating plant pathogens, for example Phytophthora infestans (potato late blight). The cytochrome c oxidase subunit 2 gene (cox2) has been widely used for identification, taxonomy and phylogeny of various oomycete groups. However, recently the cox1 gene was proposed as a DNA barcode marker instead, together with ITS rDNA. The cox1 locus has been used in some studies of Pythium and Phytophthora, but has rarely been used for other oomycetes, as amplification success of cox1 varies with different lineages and sample ages. To determine which out of cox1 or cox2 is best suited as a universal oomycete barcode, we compared these two genes in terms of (i) PCR efficiency for 31 representative genera, as well as for historic herbarium specimens, and (ii) sequence polymorphism, intra- and interspecific divergence. The primer sets for cox2 successfully amplified all oomycete genera tested, while cox1 failed to amplify three genera. In addition, cox2 exhibited higher PCR efficiency for historic herbarium specimens, providing easier access to barcoding-type material. Sequence data for several historic type specimens exist for cox2, but there are none for cox1. In addition, cox2 yielded higher species identification success, with higher interspecific and lower intraspecific divergences than cox1. Therefore, cox2 is suggested as a partner DNA barcode along with ITS rDNA instead of cox1. The cox2-1 spacer could be a useful marker below species level. Improved protocols and universal primers are presented for all genes to facilitate future barcoding efforts.
Keywords: barcoding, cytochrome oxidase, herbarium specimen, mtDNA, oomycete-specific primers
Introduction
Oomycetes belong to the kingdom Straminipila, a remarkably diverse group that includes brown algae and planktonic diatoms, although they have previously been classified under the kingdom Fungi with which they share several convergent traits, such as mycelial growth and osmotrophic nutrition. These organisms have evolved both saprophytic and pathogenic lifestyles, and more than 60% of the known species are pathogens on plants (Thines & Kamoun 2010). The majority of these plant pathogens are classified into the order Peronosporales (includes downy mildews, Phytophthora and Pythium), which cause important diseases of crops and ornamental plants, as well as forest trees. Recent phylogenetic investigations have revealed that the diversity of oomycetes has been largely underestimated. However, our knowledge of oomycete diversity is largely biased to the economically relevant plant pathogens, and little is known about saprobic or marine oomycetes (Hulvey et al. 2010; Nigrelli & Thines 2013). Consequently, even if all of the approximately 2000 published names were valid, the number of oomycetes is still only about one-tenth of the expected species number (Hawksworth 2001). Although morphology is the most valuable criterion for their identification and diversity, morphological species identification is time-consuming and in some groups very difficult, especially for nontaxonomists. DNA barcoding is a fast and reliable tool for the identification of species, enabling us to unravel the diversity and distribution of oomycetes.
Two regions, the internal transcribed spacer (ITS) rDNA and cytochrome oxidase (cox) 1 mtDNA, were recently suggested as standard DNA barcode markers for oomycetes (Robideau et al. 2011). However, there are several problems that arise from the choice of these loci. The use of the ITS rDNA gene is problematic for two reasons. First, for more than ten genera including the economically important genera Bremia and Plasmopara of the Peronosporales, the order with the highest diversity in Oomycetes, ITS-based studies are almost completely lacking. This is largely due to extremely long ITS sizes of up to more than 3000 bp, caused by long repetitive insertions, which make it difficult to amplify and sequence these loci (Thines 2007c). Second, there are certain cases where the ITS region exhibits insufficient variability for phylogenetic distinction in closely related species, for example in Phytophthora (Goodwin et al. 1999; Cooke et al. 2000; Jung & Burgess 2009) and Peronospora (Choi et al. 2007b; Voglmayr et al. 2014b). Due to these limitations of the ITS region, additional genes, cox1 and cox2, have been used as phylogenetic markers within oomycetes (Hudspeth et al. 2000, 2003; Cook et al. 2001). The cox1 locus has been used primarily in studies of Pythium and Phytophthora (de Cock & Levesque 2004; Kroon et al. 2004; Bala et al. 2010; Robideau et al. 2011; Schroeder et al. 2013), while the cox2 locus has been widely used in phylogenetic studies in the downy mildews (Göker et al. 2007; Thines 2007b, 2011; Thines et al. 2007, 2008, 2009b, 2010; Choi et al. 2009a,c, 2011c,d; Hulvey et al. 2010; Runge et al. 2011; Schröder et al. 2011; Telle et al. 2011; Telle & Thines 2012; Thines & Kummer 2013; Testen et al. 2014) and also in Pythium (Martin 2000; Villa et al. 2006; Senda et al. 2009; Uzuhashi et al. 2010), Phytophthora (Martin & Tooley 2003a,b; Villa et al. 2006), white blister rusts (Choi et al. 2006, 2007c, 2008, 2009b, 2011a,e; Thines et al. 2009a; Ploch et al. 2010, 2011; Choi & Thines 2011; Mirzaee et al. 2013) and basal oomycetes (Cook et al. 2001; Sekimoto et al. 2007; Beakes & Sekimoto 2009; Hulvey et al. 2010). The success of cox1 amplification has been variable in obligate plant pathogenic downy mildews, and as a result, the cox2 gene has been more widely used for molecular identification at fine taxonomical levels such as within a species complex (Choi et al. 2006, 2007c, 2008, 2009a, 2011a,d; Schröder et al. 2011; Thines & Kummer 2013) and also for population genetic studies (Bowers et al. 2007; Choi et al. 2011c; Runge et al. 2011; Quesada-Ocampo et al. 2012). With the recent development of DNA extraction and PCR amplification, the cox2 locus often successfully amplified from dried herbarium specimens over a hundred years old (Telle & Thines 2008; Choi & Thines 2011). Considering the widespread use of the cox2 gene, its ease of amplification and its good performance on historic specimens, the question arises as to whether this is a better DNA barcode gene than cox1 for oomycetes.
It is thus the aim of the present work to compare the utility of the cox1 and cox2 loci as DNA barcode markers for 31 representative genera of oomycetes, including some from old herbarium specimens. In addition, the cox2-1 spacer that sits between cox1 and cox2 is analysed and compared with these two genes. We describe new methods to improve PCR success with newly developed oomycete-specific primers in conjunction with previously published primers.
Materials and methods
Oomycete sampling
A total of 31 genera were selected to ensure representative coverage of many oomycetes, including all major lineages of downy mildews and other Peronosporales such as Pythium and Phytophthora, Albuginales, Haptoglossales, lagenidiaceous oomycetes and Saprolegniales. Obligate biotrophic oomycetes were collected or loaned from international herbaria, while culturable oomycetes were sampled, isolated and cultivated as described by Nigrelli & Thines (2013). Three hundred herbarium specimens including 110 species of Peronospora (one specimen for each species) and 33 species of Bremia (several specimens for each species) were also sequenced and analysed to compare species identification success, nucleotide diversity and overall mean intra- and interspecific divergence. In addition, 24 herbarium specimens of Albugo candida and 12 of Plasmopara viticola, collected between 1876 and 2003, were selected to test the PCR efficiency on old herbarium samples, which are often the only source of rare species and could be used to link a species to a sequence from its type. In this study, we regard Albugo lepigoni and A. ipomoeae-aquaticae as belonging to two distinct genus-level lineages that differ from other genera of the Albuginaceae, based on both phylogenetic inference and morphology (Voglmayr & Riethmüller 2006; Y.J. Choi & M. Thines, unpublished data). The collection information for each sample, including geographic origin, collection source and date, is listed in Table S1 (Supporting information).
DNA extraction
For obligate plant pathogens that cannot be grown in artificial media, 5–20 mg of infected plant tissue were taken from dried herbarium specimens, and disrupted in a mixer mill (MM2, Retsch, Hann, Germany), using three iron beads of 3 mm diam. per sample. From herbarium specimens collected before the year 1990, genomic DNA was extracted using the innuPREP Plant DNA Kit (Analytic Jena AG, Jena, Germany) with PTB (Telle & Thines 2008), while for younger herbarium specimens, the BioSprint 96 DNA Plant Kit (Qiagen, Hilden, Germany) was used on a Kingfisher Flex (Thermo Scientific, Dreieich, Germany). For culturable samples, genomic DNA was extracted from 0.25 cm2 agar plugs of pure cultures using a protocol (Nigrelli & Thines 2013) modified from May & Ristaino (2004). DNA of Haptoglossa and Myzocytiopsis was extracted as described by May & Ristaino (2004).
PCR amplification and sequencing
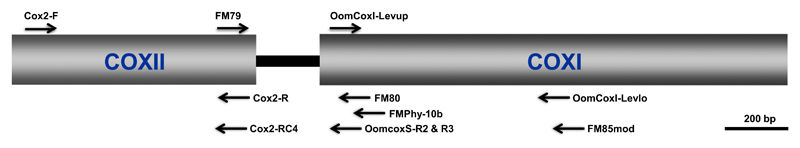
Four mitochondrial regions, cox1, cox2, cox2-1 spacer and a region spanning cox2 and cox2-1 spacer, were amplified by PCR using nine combinations of eleven primers (Fig. 1; Tables 1 and 2): primers OomCox1-levup and OomCox1-levlo or FM85mod (Robideau et al. 2011) for the cox1 region, Cox2-F and Cox2-R (Hudspeth et al. 2000) or Cox2-RC4 (designed here) for cox2, FM79-C1 (designed here) and FM80 (Martin & Tooley 2003a), FMPhy-10b (Martin et al. 2004) or OomCoxS-R2 (designed here) for cox2-1 spacer, Cox2-F and FMPhy-10b or OomCoxS-R3 (designed here) for a region spanning the cox2 and cox2-1 spacer. Amplification reactions were carried out in 25 μL with genomic DNA from 1 to 3 ng, 1x Mango PCR Buffer, 0.2 mM dNTPs, 2 mM MgCl2, 0.8 mg/mL BSA, 0.4 μm forward and reverse primers, and 0.5 Unit Mango Taq Polymerase (Bioline GmbH, Luckenwalde, Germany). PCR conditions were as follows: an initial denaturation step of 95 °C for 4 min; 36 cycles of 95 °C for 40 s, primer set-specific annealing temperature for 40 s (see Table 2) and 72 °C extension for 60 s; and final extension of 5 min at 72 °C. For a spanning region of cox2 and cox2-1 spacer, the annealing time was extended to 60 s. For all primer sets, annealing temperatures were optimized through gradient PCR. To compare the effects of the annealing temperature on PCR success for cox1 and cox2 genes, amplifications were performed at different temperatures. All PCR products were evaluated for successful amplification using gel electrophoresis (8 V/cm) on a 1.5% agarose gel for 1 h, with reference to a DNA marker, HyperLadder I (Bioline GmbH). PCR products were directly sequenced with the primers identical to those used for amplifications using the ABI BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) with the following modifications: a reaction volume of 20 μL, with 0.0625× ready reaction premix, 1× BigDye sequencing Buffer, 3.2 pmol primer, 2–5 ng of PCR template and ddH2O. Cycle sequencing was as follows: an initial denaturation step of 95 °C for 3 min; 40 cycles of 95 °C for 30 s, 50 °C for 40 s and 60 °C for 4 min. The sequencing products were purified using the BigDye Xterminator Purification Kit (Applied Biosystems). Amplicon sequencing was performed on the Applied Biosystems 3730 DNA Analyzer (Applied Biosystems) at the DNA sequencing laboratory of the Biodiversity and Climate Research Centre (BiK-F). All sequences were blasted at http://ncbi.nlm.nih.gov/blast/Blast.cgi, using the megablast algorithm and default search parameters (Altschul et al. 1990) to confirm their identity. GenBank Accession numbers are listed in Table S1 (Supporting information).
Fig. 1.
Gene organization of the mitochondrial cox2-1 gene cluster and location of PCR primers. Arrows above or below the fragment indicate the primers that has been used for amplification.
Table 1.
Previously published and newly developed primers
| Primer | Sequence (5′ →3′) | Reference |
|---|---|---|
| OomCox1-levup | TCAWCWMGATGGCTTTTTTCAAC | Robideau et al. (2011) |
| OomCox1-levlo | CYTCHGGRTGWCCRAAAAACCAAA | Robideau et al. (2011) |
| FM85mod | RRHWACKTGACTDATRATACCAAA | Robideau et al. (2011) |
| FM79-C1 | GGNCAATGTAGYGAAATHTG | This study |
| FM80 | AATATCTTTATGATTTGTTGAAA | Martin & Tooley (2003a) |
| FMPhy-10b | GCAAAAGCACTAAAAATTAAATATAA | Martin et al. (2004) |
| OomCoxS-R2 | GTWGAAAAAABCCAHCKDKWTGACC | This study |
| OomCoxS-R3 | GTWGAAAAAABCCAHCKDKWTGAC | This study |
| Cox2-F | GGCAAATGGGTTTTCAAGATCC | Hudspeth et al. (2000) |
| Cox2-R | CCATGATTAATACCACAAATTTCACTAC | Hudspeth et al. (2000) |
| Cox2-RC4 | TGATTWAYNCCACAAATTTCRCTACATTG | This study |
Table 2.
Combinations of primers used for PCR amplification
| Target gene | Forward primer | Reverse primer | T (°C)‡ |
|---|---|---|---|
| cox1 | OomCox1-levup | OomCox1-levlo | 50, 55 |
| FM85mod | 50, 55 | ||
| cox2 | Cox2-F | Cox2-R | 50, 55 |
| Cox2-RC4 | 50 | ||
| cox2-1 spacer† | FM79-C1 | FM80 | 52 |
| FMPhy-10b | 52 | ||
| OomCoxS-R2 | 52 | ||
| cox2+ cox2-1 spacer | Cox2-F | FMPhy-10b | 54 |
| OomCoxS-R3 | 54 |
The spacer region between cox2 and cox1.
Annealing temperature during amplification.
Evaluation of sequence polymorphism and distance
Sequences were edited using the dnastar computer package (DNAStar, Inc., Madison, Wis., USA), version 5.05. An alignment of cox1, cox2 and cox2-1 spacer sequences for three data sets, representing Oomycetes, Peronospora and Bremia (Table S1, Supporting information), was performed using mafft 7 (Katoh & Standley 2013). dnasp (Librado & Rozas 2009) was used to calculate DNA polymorphism statistics for each loci. Interspecific (between species pairs) sequence divergence was calculated by uncorrected ‘p’ (percentage) distance model using mega 5.0 (Tamura et al. 2011). Minimum evolution analysis was used to construct the phylogenetic tree in mega 5.0, with the Tamura-Nei model. The reproducibility of the internal branches from the resulting trees was tested by bootstrap analysis using 1000 replications. Species identification, intra- and interspecific distance analyses for the Bremia data set were carried out using TaxonDNA/SpeciesIdentifier 1.7.8 (Meier et al. 2006). The frequency of intra- and interspecific uncorrected ‘p’ distances was plotted to check whether intraspecific and interspecific variation were overlapping. Species identification success was evaluated using two different criteria, Best Match (BM) and Best Close Match (BCM), as outlined by Meier et al. (2006). The BM criterion assigns a species name to the query sequence based on its best barcode match. The identification is considered ‘success’ when the query sequence and the matching one are from the same species, mismatched names are considered ‘misidentified’, and equally good matches from different species are considered ‘ambiguous’. The BCM is similar to BM except that the relative frequency of all intraspecific distances is plotted to determine a threshold calculated from pairwise metrics for each marker. All sequences without a match below the threshold value remain ‘no match’.
Primer design
To design oomycete-specific primers, alignments were generated using clustalx (Thompson et al. 1997). Using primerselect software (DNASTAR, Inc.), the new primers, Cox2-RC4, FM79-C1, OomCoxS-R2 and OomCoxS-R3 (Table 2), were newly designed or modified from previously published ones, to optimize them for successful amplification for all oomycete genera sequenced. The primer Cox-RC4 was modified from Cox2-R (Hudspeth et al. 2000), by considering a few potentially critical nucleotide differences found throughout different oomycete genera, which are described in Appendix S1 (Supporting information). Primer oligonucleotides were ordered from Sigma-Aldrich (Taufkirchen, Germany).
Results
Comparison of PCR success at different annealing temperatures
Previously, two different annealing temperatures were used for PCR amplification of cox1 (55 °C; Robideau et al. (2011)) and cox2 (50 °C; Hudspeth et al. (2000)). In this study, better PCR performance was achieved at 50 °C, for each two primer sets of cox1 and cox2 (Table 2). This optimal annealing temperature for both the cox1 and cox2 primer sets in this study was also confirmed through gradient PCR; the amplification performance gradually decreased with increase in annealing temperature from 50 to 55 °C. The barcoding primer set of cox1, OomCox1-levup and FM85mod failed to amplify most samples at 55 °C, excluding a few genera with a faint band, although the success was slightly improved at 50 °C. As no unspecific band was observed even at 50 °C, we used this annealing temperature on subsequent experiments for both genes. For cox2-1 spacer and a region spanning cox2 and the cox2-1 spacer, optimal annealing temperatures were 52 and 54 °C, respectively (Table 2).
Evaluation of primers
Nine combinations of 11 primers (Table 2), including four new oomycete-specific primers, were compared for PCR success and efficiency. For cox1, the reverse primer OomCox1-levlo considerably increased the average PCR success rate, compared with FM85mod, regardless of the annealing temperature, although the resulting amplicon of 680 bp was slightly smaller than 727 bp produced with FM85mod. The primer set, OomCox1-levup and FM85mod, may not be useful for herbarium specimens with relatively low DNA content, compared to DNA extracted from cultures.
For cox2, two different reverse primers Cox2-R and Cox2-RC4, together with the forward primer Cox2-F, successfully amplified the region for all samples and genera tested. Amplification with the new primer Cox-RC4 was more robust than with Cox2-R and generated higher amounts of amplified product, in particular for Bremia, Graminivora and Pustula. While the nucleotide sequence divergence between the two primers is minimal, there are a few potentially critical nucleotide differences that may explain the better performance of the Cox2-RC4 primer (see Appendix S1, Supporting information).
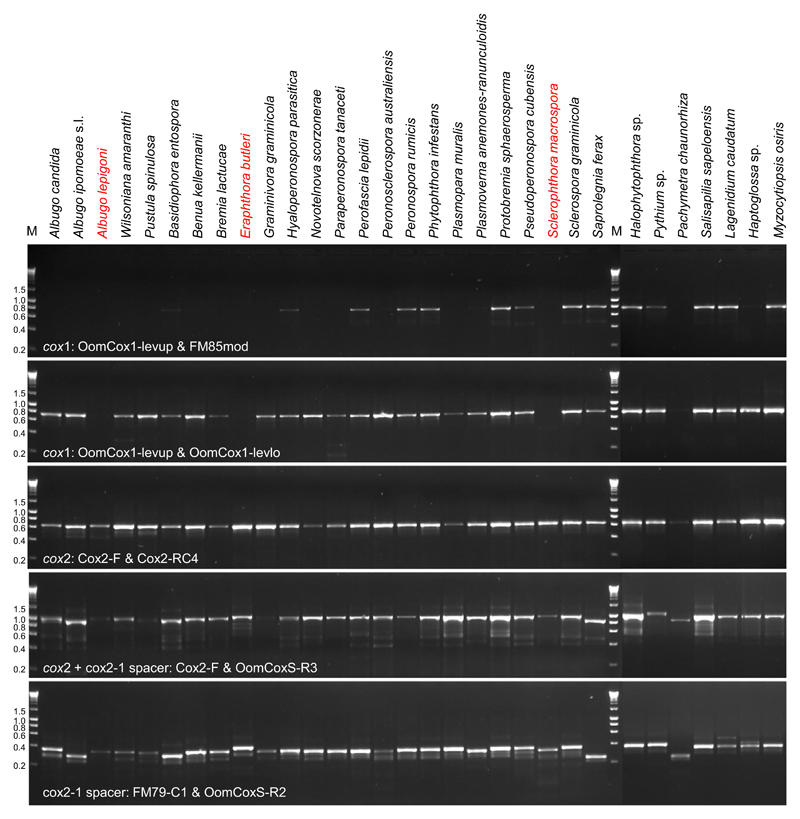
For the cox2-1 spacer region, three different reverse primers, FM80, FMPhy-10b and OomCoxS-R2, were tested together with the forward primer FM79-C1. The primer FM80 failed to amplify 12 of 31 samples, while the latter two primers successfully amplified the region in all samples. Although the latter two primers showed similar PCR success and efficiency, we prefer to use the FMPhy-10b, as the resulting sequences were 47 bp longer than OomCoxS-R2 and overlapped partially with the sequences resulting from cox1 primer set. Primers for cox2-1 spacer often produced a faint, nonspecific band below the target amplicon, which did not affect sequencing. To amplify a region spanning cox2 and the cox2-1 spacer, two reverse primers, OomCoxS-R3 and FMPhy-10b, were used together with the forward primer Cox2-F. Both primers led to successful amplification of all of the oomycete lineages (Fig. 2), but FMPhy-10b generated lower amounts of amplified product for Albugo lepigoni, Pustula, Wilsoniana and Graminivora than OomCoxS-R3.
Fig. 2.
Gel electropherograms for the amplification of cox1, cox2, cox2 + cox2-1 spacer and cox2-1 spacer regions on 31 representative oomycete lineages. DNA HyperLadder I (Bioline) was used as a molecular size marker (kb) (M), and fragment sizes are indicated to the right.
The newly developed primers, Cox2-RC4 (targeting cox2 gene), OomCoxS-R2 (cox2-1 spacer), OomCoxS-R3 (cox2 and cox2-1 spacer) and FM79-C1 (cox2-1 spacer), are oomycete specific. Although we extracted the DNA from infected plant tissue that may have also contained plant, insect, fungal or bacterial DNA, no co-amplification of nontarget DNA was observed. The newly developed primers improved the overall results when compared with previously published primers with respect to amplification performance (data not shown).
Cox1 vs. cox2 as a barcoding locus
The primer set specific for cox2 successfully amplified all oomycete genera included in our study, while the primer sets for cox1 did not amplify Eraphthora, Sclerophthora and Albugo lepigoni (Fig. 2). The PCR failure of the three lineages by the cox1 primer sets was confirmed by a further study including four Eraphthora, five Sclerophthora, one A. lepigoni and ten A. occidentalis samples (a species closely related with A. lepigoni) (data not shown). This indicated that the cox1 primer sets are not useful for at least these groups. The cox1 primer set amplified a single fragment of 680 bp in the other oomycete samples tested. The size of cox2 products is slightly different, mostly 581 bp, but 572 bp in Pachymetra and 593 bp in Albuginales with the exception of A. lepigoni (599 bp).
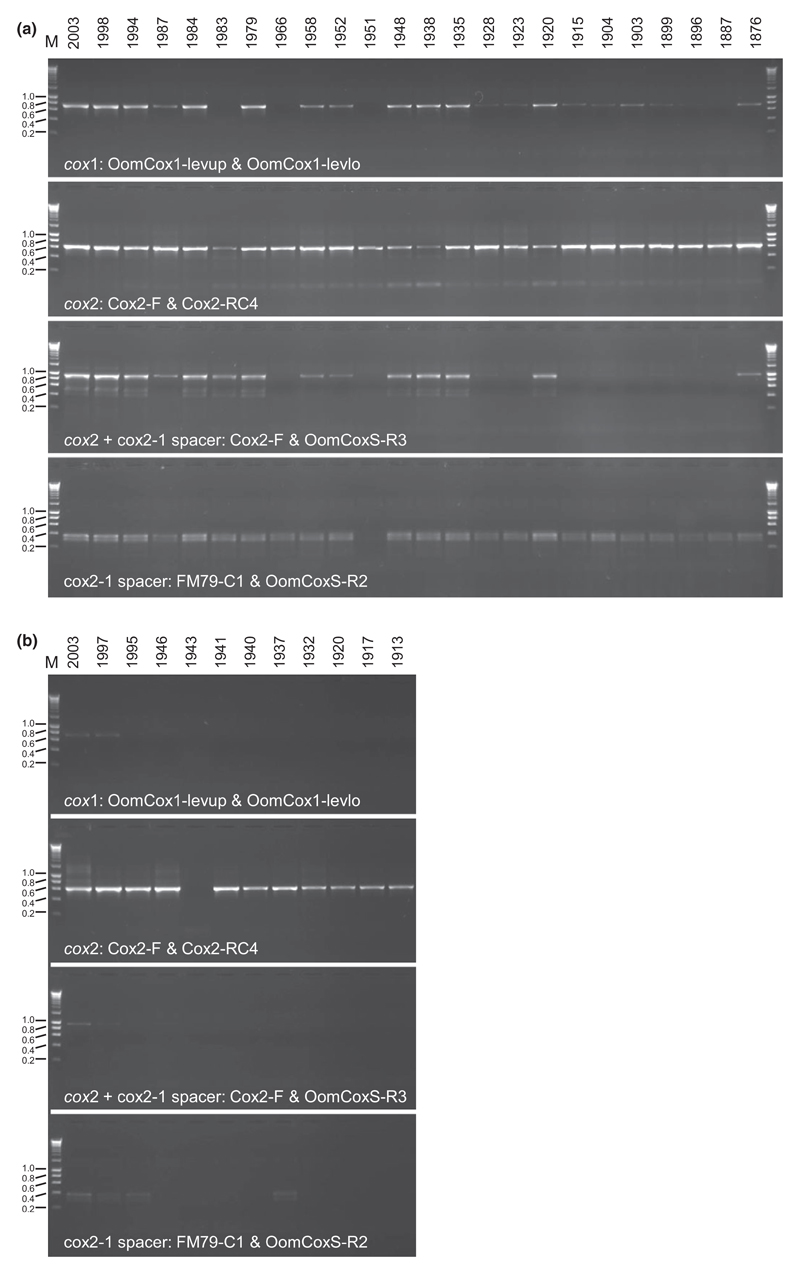
For historic herbarium specimens of A. candida and Plasmopara viticola, the utility of cox1 and cox2 genes was compared with regard to amplification and sequencing. The cox2 primers successfully amplified all herbarium specimens regardless of the specimen ages of A. candida and generated higher amounts of amplified product than with cox1 primers (Fig. 3). However, cox1 could not be amplified from four samples (collected in 1983, 1966, 1951 and 1887) and showed only a faint band for several 100-year-old samples. The PCR success for a spanning region of cox2 and cox2-1 spacer decreased with the ages of the specimens. Interestingly, all primer sets resulted in a bright band for the oldest herbarium specimen collected in 1876. To confirm whether recent contamination was the source of amplification as might be expected from old material with supposedly low DNA concentration (Andreasen et al. 2009), the sequence of the amplicon was determined and compared with other sequences of A. candida. The resulting sequences were identical to a previously published sequence of A. candida from Berteroa incana, with the same two nucleotide positions that are different from other Albugo candida sequences. This indicated that the amplicon originated from the herbarium specimen and not from contamination. Compared to A. candida, PCR amplification was less robust for the herbarium specimens of P. viticola. However, the cox2 primers successfully amplified 11 of the 12 up to 100-year-old specimens, but the primer sets for cox1 amplified only two samples collected very recently, in 1997 and 2003.
Fig. 3.
Gel electropherograms for the amplification of cox1, cox2, cox2 + cox2-1 spacer and cox2-1 spacer regions on herbarium specimens of Albugo candida (a) and Plasmopara viticola (b). DNA HyperLadder I (Bioline) was used as a molecular size marker (kb) (M), and fragment sizes are indicated to the right.
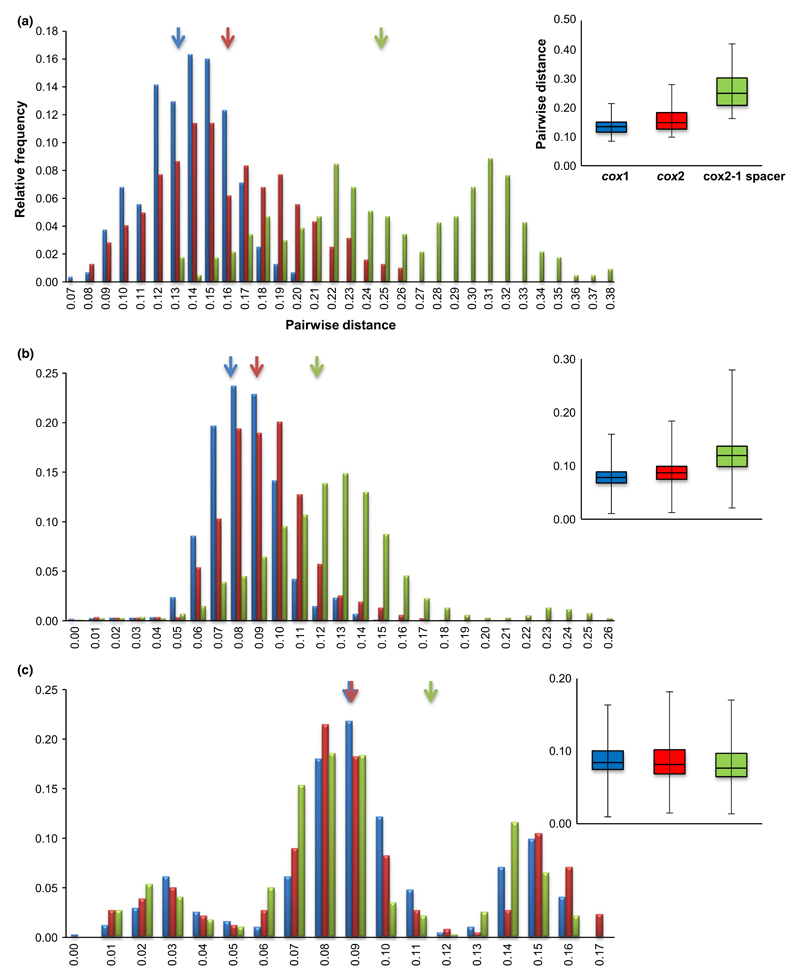
The nucleotide diversity and the interspecific (between species) variation of cox1 and cox2 genes for three data sets, representing 26 Oomycete genera, 110 Peronospora and 33 Bremia species, represented with one sample, are presented in Table 3. For these three data sets, cox2 gene presents consistently higher nucleotide diversity than cox1: 0.15563 ± 0.000405 for Oomycete genera, 0.0959 ± 0.013 for Peronospora species and 0.09696 ± 0.03804 for Bremia species in cox2 gene, while in cox1 0.13067 ± 0.0000122, 0.08581 ± 0.010 and 0.09668 ± 0.03641, respectively (t-test: P < 0.001 in Oomycete and Peronospora, but P > 0.05 in Bremia data sets). For Peronospora species, cox2 yielded a slightly higher level of species identification success of 98.2% (108 of 110 species), than cox1 of 96.4% (106 of 110 species). This difference is due to a discriminatory power for four related species of Peronospora parasitic on Caryophyllaceae, P. scleranthi, P. honckenyae, P. parva and P. cerastii-brachypetali. In the cox1 gene, these cannot be distinguished from one another. In contrast, the cox2 gene discriminated them into three genotypes (Fig. S1, Supporting information). For the Bremia data set, cox2 successfully discriminated all species, but cox1 failed to distinguish one species. Mean pairwise distances of cox1 and cox2 varied with the three data sets, but cox2 always presented higher interspecific divergences of 0.1586, 0.0877 and 0.0890, respectively, than cox1 with 0.1331, 0.0786 and 0.0881 (Table 3, Fig. 4).
Table 3.
Summary statistics and interspecific divergences of oomycete taxa represented with one sample
| Oomycetes | Gene | Size (bp) | L† (bp) | K‡ | Par§ | Pi¶ ± SD | Distinguishable taxa | Overall mean pairwise distance |
|---|---|---|---|---|---|---|---|---|
| 26 genera | cox1 | 641 | 641 | 83.756 | 263 | 0.13067 ± 0.0000122 | 26 | 0.1331 |
| cox2 | 605 | 572 | 89.022 | 291 | 0.15563 ± 0.000405 | 26 | 0.1586 | |
| cox spacer** | 260–420 | 208 | 46.290 | 198 | 0.22255 ± 0.08745 | 26 | 0.2500 | |
| 110 species of Peronospora | cox1 | 680 | 660 | 56.634 | 278 | 0.08581 ± 0.010 | 106 | 0.0786 |
| cox2 | 577 | 546 | 52.360 | 268 | 0.09590 ± 0.013 | 108 | 0.0877 | |
| cox spacer | 340–415 | 226 | 24.504 | 148 | 0.10843 ± 0.05909 | 106 | 0.1197 | |
| 33 species of Bremia | cox1 | 629 | 629 | 60.810 | 149 | 0.09668 ± 0.03641 | 32 | 0.0881 |
| cox2 | 539 | 539 | 52.261 | 171 | 0.09696 ± 0.03804 | 33 | 0.0890 | |
| cox spacer | 355–400 | 434 | 50.743 | 141 | 0.11692 ± 0.05239 | 29 | 0.1153 |
Number of sites compared after removing gaps and misaligned regions.
Average number of nucleotide difference.
Number of parsimony informative sites.
Nucleotide diversity.
A spacer region between cox2 and cox1.
Fig. 4.
Distance histograms of the interspecific comparisons of cox1, cox2 and cox2-1 spacer sequences. Histograms display interspecific variation for 26 genera of Oomycete (a), 110 species of Peronospora (b) and 33 species of Bremia (c). Arrows point the overall mean pairwise distances for cox1 (blue), cox2 (red) and cox2-1 spacer (green). Box plots depict the variation for intra- and interspecific differences for the three markers. Black bar in the box indicates the median.
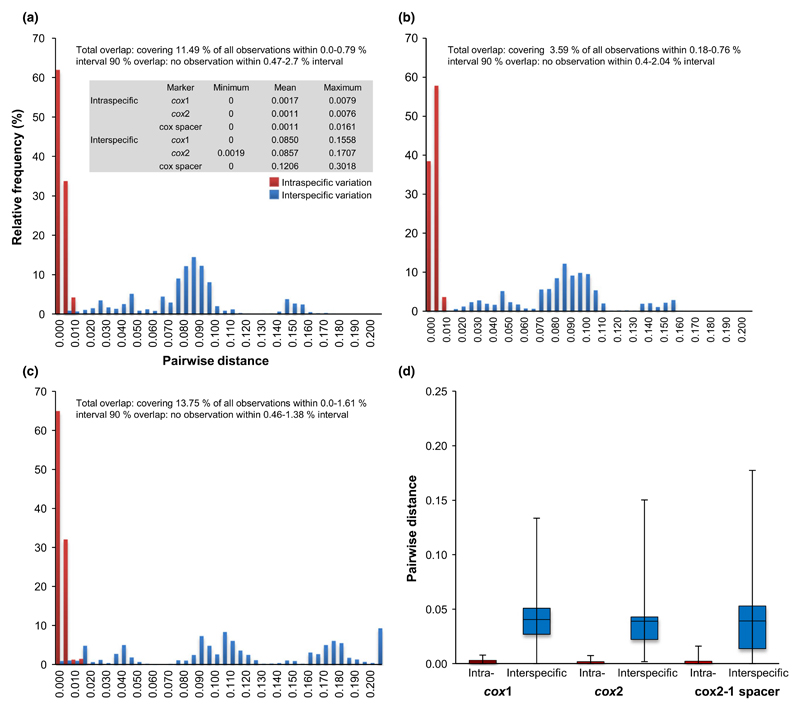
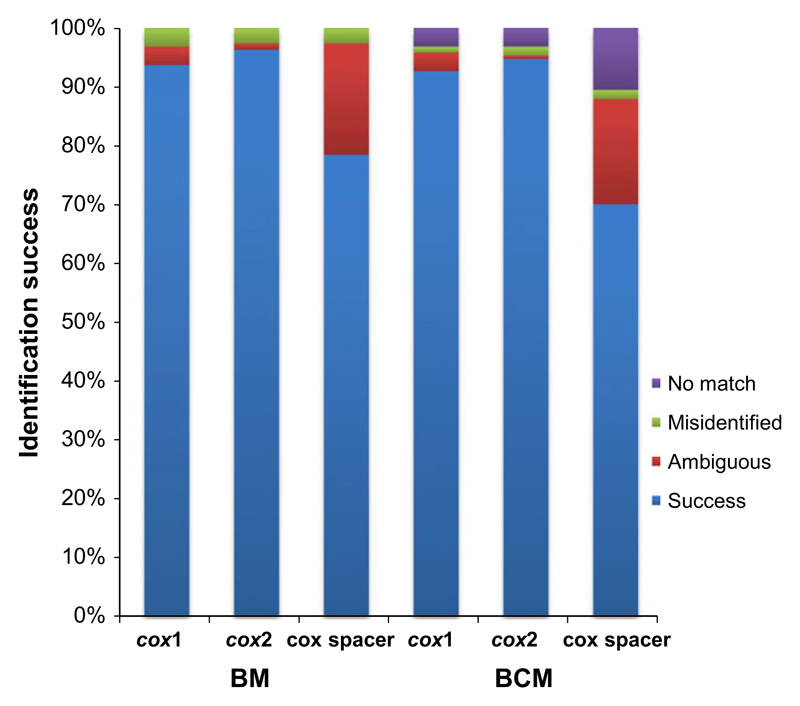
For 190 samples representing 33 species of the Bremia, intraspecific (within species) and interspecific (between species) distances for each marker were calculated and plotted in Fig. 5. The mean intraspecific variations of all markers were low, ranging from 0.0011 (cox2, cox2-1 spacer) to 0.0017 (cox1), and the mean interspecific variation was higher for the cox2-1 spacer than cox1 and cox2. The maximum intra- and interspecific variations of cox2-1 spacer were much higher than cox2 and cox1. As the minimum interspecific variation was zero for both cox1 and cox2-1 spacer and very low (0.0019) for cox2, intra- and interspecific distances overlapped for all markers. However, the cox2 represents the least observations for interval overlap (3.59% of all observations), while the cox1 and cox2-1 spacer showed a higher percentage of overlap observations, 11.49% and 13.75%, respectively. At 90% overlap, excluding the largest 5% of the intraspecific and the lowest 5% of the interspecific distances, however, no overlap was found for any of the markers. Species identification results using the two criteria, Best Match (BM) and Best Close Match (BCM), are displayed in Fig. 6 and Table S2 (Supporting information). Both BM and BCM criteria showed similar results for the three markers. However, the best identification success was obtained for the cox2 (96.31% for BM and 94.73% for BCM), followed by the cox1 (93.68% for BM and 92.63% for BCM), while the cox2-1 spacer obtained least success (78.42% for BM and 70.00% for BCM) with much higher score of no match (10.52%).
Fig. 5.
Distance histograms of the intra- and interspecific comparisons of cox1 (a), cox2 (b) and cox2-1 spacer (c) for Bremia species and boxplots (d) depicting the variation for the three markers. A table summarizes distance data. Black bar in the box indicates the median. Total overlap range (with corresponding percentage of observations it represents) and 90% overlap (largest 5% of the intraspecific and lowest 5% of the interspecific excluded) are indicated.
Fig. 6.
Identification success based on Best Match (BM) and Best Close Match (BCM) criteria for cox1, cox2 and cox2-1 spacer. Success: correct identifications; Ambiguous: ambiguous identifications; Misidentified: incorrect identifications; and No match: sequences without any match closer than a threshold value below 95% of intraspecific distances.
Cox2-1 spacer
The region spanning cox2 and the cox2-1 spacer is diverse in the size of the amplicons; in two genera, Saprolegnia and Pachymetra, it was much smaller (789 bp and 825 bp, respectively) than in other genera, in which it ranged from 911 bp (Basidiophora) to 985 bp (A. lepigoni). The variation observed between the genera is mostly due to length differences in the cox2-1 spacer. The primer set for the cox2-1 spacer region resulted in fragments smaller than 420 bp., which were highly polymorphic in both nucleotide differences and lengths (Table 3). The insertion of repeat sequences, which were commonly found in this region, requires many gaps during the process of alignment, leading to poor alignments, especially for different oomycete genera. In addition, despite higher nucleotide diversity and interspecific divergence for all data sets (Table 3, Figs 4 and 5), identification success of cox2-1 spacer was much lower than cox1 and cox2 (Table S2, Fig. 6).
Discussion
Numerous oomycete species are pathogenic to many different host plants, and while most obligate biotrophic pathogens are highly host specific, the hemibiotrophic and necrotrophic species have broader host ranges (Beakes & Sekimoto 2009; Thines & Kamoun 2010; Lara & Belbahri 2011; Beakes et al. 2012). Due to the ability to spread rapidly to new areas or countries, especially by global trade with seeds and plants, but also by ballast water, pathogenic oomycetes pose a significant threat for horticulture, forestry, agriculture and aquaculture (Brasier 2008; Grunwald et al. 2012). Their prompt detection and precise identification are essential prerequisites for implementation of proper control strategies. DNA barcode markers will improve the success rate of identification of such potential pathogens. For the oomycetes, some loci have been useful for barcoding, for example the nuclear ribosomal internal transcribed spacer (ITS) and the mitochondrial loci cytochrome c oxidase 1 (cox1) and 2 (cox2) genes. However, the applicability of these loci has not yet been tested systematically. As the ITS region has large insertions for some oomycete species (exceeding 2500 bp), which can affect both ITS1 (Voglmayr 2003; García-Blázquez et al. 2008) and ITS2 (Thines et al. 2005; Choi et al. 2007a; Thines 2007a), this locus is not suitable as a general barcode. When the ITS rDNA and cox1 mtDNA genes were suggested as barcode markers (Robideau et al. 2011), no comparison was made to the performance of the cox2 mtDNA locus, which has widely been used for phylogenetic studies at the species level and has shown a high resolution (Martin 2000; Cook et al. 2001; Hudspeth et al. 2003; Martin & Tooley 2003a,b; Choi et al. 2006, 2007c, 2008, 2009a,b,c, 2011a,c,d,e; Villa et al. 2006; Göker et al. 2007; Sekimoto et al. 2007; Thines 2007b, 2011; Thines et al. 2007, 2008, 2009a,b, 2010; Beakes & Sekimoto 2009; Senda et al. 2009; Hulvey et al. 2010; Ploch et al. 2010, 2011; Uzuhashi et al. 2010; Choi & Thines 2011; Runge et al. 2011; Schröder et al. 2011; Telle et al. 2011; Telle & Thines 2012; Mirzaee et al. 2013; Thines & Kummer 2013; Testen et al. 2014; Voglmayr et al. 2014a,b). For the largest group of oomycetes, the obligate biotrophic plant pathogens (downy mildews and white blister rusts), cox1 has only rarely been used (40 species with this locus in GenBank in Dec. 2014), but a larger database is already present for cox2 (170 species with this locus in GenBank in Dec. 2014). For studies of early diverging oomycetes, cox2 has been proven to be a suitable locus (Cook et al. 2001; Hakariya et al. 2007, 2009; Sekimoto et al. 2007, 2008; Hulvey et al. 2010), whereas there are only a few cox1 sequences of some of these available.
By analysing 31 representative oomycete genera and old historic herbarium specimens, we determined that the cox2 sequence yielded better PCR performance than cox1. While 3 of 31 representative oomycete lineages were not showing satisfactory amplification for cox1, all 31 lineages could be amplified for the cox2 locus. This is most likely due to highly conserved regions that enable stable anchoring of oligonucleotide primers, which seems to be more variable for cox1 as the two reverse primers of cox1 were not conserved throughout some oomycete lineages. Although we designed several new primers for conserved regions of cox1, none of them could improve the PCR performance (data now shown). In addition, cox2 was more effective in identifying the largest genus of Oomycete, Peronospora, at the species level than cox1, due to its higher nucleotide diversity and interspecific divergence. Our study also showed that a region covering the cox2 gene, together with the hyper-variable cox2-1 spacer, can be amplified from all oomycete lineages tested with a newly developed primer, highlighting its potential as a powerful tool for identification, population genetics and phylogenetic studies in a variety of oomycetes. Due to interspecific sequence variation, the cox2-1 spacer was previously considered useful for the development of species-specific markers and sequence-based identification of Phytophthora species (Martin & Tooley 2003a; Martin et al. 2004; Grunwald et al. 2011). The availability of the spacer region for a large panel of oomycetes will allow future intraspecific genetic studies that may provide much needed insights into host plant specialization, colonization history and biogeography of these plant pathogens.
For the past three centuries, thousands of oomycete specimens have been deposited in 3400 internationally recognized herbaria around the world (Thiers 2014). These specimens are an invaluable resource, especially for the study of obligate plant pathogens, which are unculturable in artificial media, for example white blister rust and downy mildew pathogens. This is not only because many species are rare and not easily collected, but also because the name of a species is ultimately linked to its type specimen. Thus, for the purpose of species identification by barcoding, it is also advantageous if historic type specimens are accessible along with barcoding primers. Although it is probably more complicated to amplify a target gene by PCR from DNA extracted from historic herbarium specimens than to sequence and map its whole genome (Yoshida et al. 2013) because of DNA degradation and fragmentation, there are many cases in which the cox2 sequence has value. Notably, cox2 may be used for accurate species identification and phylogenetic studies of herbarium specimens, especially in the two oomycete groups, Albuginales (Choi et al. 2007c, 2008, 2009b, 2011a,e; Thines et al. 2009a; Ploch et al. 2010; Choi & Thines 2011) and Peronosporales (Thines et al. 2008, 2009b, 2010; Hulvey et al. 2010; Choi et al. 2011d; Runge et al. 2011; Schröder et al. 2011; Thines 2011; Telle & Thines 2012). These studies include several cases of extracting DNA from specimens from the 19th century (Choi et al. 2007c, 2011a,c; Telle & Thines 2008; Thines et al. 2009a; Runge et al. 2011) and type specimens from the 19th century and the first half of the 20th century (Choi et al. 2009b,c, 2011b,e). In the present study, the cox2 primer set successfully amplified old herbarium collections, including four specimens collected in the late 19th century, an intensive period of research of oomycete systematics, while cox1 could not be amplified from most of these using the same conditions. In the case of the P. viticola specimens tested, cox1 could not be amplified from any specimen older than 16 years. Even if amplification failed for the full cox2 region of old specimens, it may be possible to obtain a smaller fragment in semi-nested PCRs, as Telle & Thines (2008) have shown that this approach often yields amplification from DNA from which the complete fragment could not be obtained. However, this approach is not available for the cox1 locus. In conclusion, our results suggest we should reconsider the usefulness of cox1 as a universal barcode for oomycetes and we provide evidence that cox2 is better suited to this because of its ease of amplification among oomycete lineages, better performance on herbarium specimens, higher discriminatory power at the species level and the availability of a large taxonomically diverse database that already includes many species of oomycetes, especially the downy mildews. A cox2-based identification system will be more effective in understanding the biodiversity and distribution of oomycetes. The increased availability of historic and sometimes well-referenced herbarium specimens should facilitate species identification and resolve taxonomic issues, as well as provide insights into evolutionary questions through a comparative analysis between extant and historic pathogens.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Additional methods – cox2 primer design.
Minimum Evolution trees of Peronospora species based on cox1 (A) and cox2 (B) sequences.
Oomycete samples used in this study.
Identification success based on Best Match (BM) and Best Close Match (BCM) criteria for cox1, cox2, and cox2-1 spacer. Success: correct identifications; Ambiguous: ambiguous identifications; Misidentified: incorrect identifications; No match: sequences without any match closer than a threshold value below 95% of intraspecific distances.
Acknowledgements
YJC was supported by a fellowship from the Alexander von Humboldt foundation and HV by the Austrian Science Fund (FWF; project P22739-B20). This study was supported by the LOEWE excellence programme of the German state of Hessen, in the framework of the Integrative Fungal Research Cluster (IPF) and the Biodiversity and Climate Research Centre (BiK-F).
Footnotes
Y.J.C. and M.T. conceived the study and designed the experiments; GB, S.G., J.K., H.D.S., R.G.S., H.V. and Y.J.C. provided materials; Y.J.C. conducted experiments with contributions from B.N., L.N., S.P. and S.T.; Y.J.C. and M.T. analysed and interpreted the data; and YJC and M.T. wrote the manuscript with contributions from G.B., S.G., H.D.S., R.G.S. and H.V.
Data accessibility
DNA sequences: GenBank Accession nos KJ654007-KJ654304 & KP684521-KP684912 (in Table S1). Sequence alignments and phylogenetic trees: TreeBase Study Accession no. 16949; URL: http://purl.org/phylo/tree-base/phylows/study/TB2:S16949.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andreasen K, Manktelow M, Razafimandimbison S. Successful DNA amplification of a more than 200-year-old herbarium specimen: recovering genetic material from the Linnaean era. Taxon. 2009;58:959–962. [Google Scholar]
- Bala K, Robideau GP, Desaulniers N, de Cock AWAM, Levesque CA. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia. 2010;25:22–31. doi: 10.3767/003158510X524754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beakes GW, Sekimoto S. The evolutionary phylogeny of Oomycetes-insights gained from studies of holocarpic parasites of algae and invertebrates. In: Lamour K, Kamoun S, editors. Oomycete Genetics and Genomics. John Wiley & Sons Inc; New York: 2009. pp. 1–24. [Google Scholar]
- Beakes GW, Glockling SL, Sekimoto S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma. 2012;249:3–19. doi: 10.1007/s00709-011-0269-2. [DOI] [PubMed] [Google Scholar]
- Bowers JH, Martin FN, Tooley PW, Luz EDMN. Genetic and morphological diversity of temperate and tropical isolates of Phytophthora capsici. Phytopathology. 2007;97:492–503. doi: 10.1094/PHYTO-97-4-0492. [DOI] [PubMed] [Google Scholar]
- Brasier CM. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathology. 2008;57:792–808. [Google Scholar]
- Choi YJ, Thines M. Morphological and molecular confirmation of Albugo resedae (Albuginales; Oomycota) as a distinct species from A. candida. Mycological Progress. 2011;10:143–148. [Google Scholar]
- Choi YJ, Hong SB, Shin HD. Genetic diversity within the Albugo candida complex (Peronosporales, Oomycota) inferred from phylogenetic analysis of ITS rDNA and COX2 mtDNA sequences. Molecular Phylogenetics and Evolution. 2006;40:400–409. doi: 10.1016/j.ympev.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Hong SB, Shin HD. Extreme size and sequence variation in the ITS rDNA of Bremia lactucae. Mycopathologia. 2007a;163:91–95. doi: 10.1007/s11046-007-0092-7. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Hong SB, Shin HD. Re-consideration of Peronospora farinosa infecting Spinacia oleracea as distinct species, Peronospora effusa. Mycological Research. 2007b;111:381–391. doi: 10.1016/j.mycres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Shin HD, Hong SB, Thines M. Morphological and molecular discrimination among Albugo candida materials infecting Capsella bursa-pastoris world-wide. Fungal Diversity. 2007c;27:11–34. [Google Scholar]
- Choi YJ, Shin HD, Ploch S, Thines M. Evidence for uncharted biodiversity in Albugo candida complex, with the description of a new species. Mycological Research. 2008;112:1327–1334. doi: 10.1016/j.mycres.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Kiss L, Vajna L, Shin HD. Characterization of a Plasmopara species on Ambrosia artemisiifolia, and notes on P. halstedii, based on morphology and multiple gene phylogenies. Mycological Research. 2009a;113:1127–1136. doi: 10.1016/j.mycres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Shin HD, Thines M. The host range of Albugo candida extends from Brassicaceae through Cleomaceae to Capparaceae. Mycological Progress. 2009b;8:329–335. [Google Scholar]
- Choi YJ, Shin HD, Thines M. Two novel Peronospora species are associated with recent reports of downy mildew on sages. Mycological Research. 2009c;113:1340–1350. doi: 10.1016/j.mycres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Shin HD, Ploch S, Thines M. Three new phylogenetic lineages are the closest relatives of the widespread species Albugo candida. Fungal Biology. 2011a;115:598–607. doi: 10.1016/j.funbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Shin HD, Voglmayr H. Reclassification of two Peronospora species parasitic on Draba in Hyaloperonospora based on morphological molecular and phylogenetic data. Mycopathologia. 2011b;171:151–159. doi: 10.1007/s11046-010-9340-3. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Thines M, Han JG, Shin HD. Mitochondrial phylogeny reveals intraspecific variation in Peronospora effusa, the spinach downy mildew pathogen. Journal of Microbiology. 2011c;49:1039–1043. doi: 10.1007/s12275-011-1069-2. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Thines M, Runge F, et al. Evidence for high degrees of specialisation, evolutionary diversity, and morphological distinctiveness in the genus Bremia. Fungal Biology. 2011d;115:102–111. doi: 10.1016/j.funbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Thines M, Shin HD. A new perspective on the evolution of white blister rusts: Albugo s. str. (Albuginales; Oomycota) is not restricted to Brassicales but also present on Fabales. Organisms Diversity & Evolution. 2011e;11:193–199. [Google Scholar]
- de Cock AWAM, Levesque CA. New species of Pythium and Phytophthora. Studies in Mycology. 2004;50:481–487. [Google Scholar]
- Cook KL, Hudspeth DSS, Hudspeth MES. A cox2 phylogeny of representative marine peronosporomycetes (Oomycetes) Nova Hedwigia. 2001;122:231–243. [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genetics and Biology. 2000;30:17–32. doi: 10.1006/fgbi.2000.1202. [DOI] [PubMed] [Google Scholar]
- García-Blázquez G, Göker M, Voglmayr H, et al. Phylogeny of Peronospora, parasitic on Fabaceae, based on ITS sequences. Mycological Research. 2008;112:502–512. doi: 10.1016/j.mycres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Göker M, Voglmayr H, Riethmüller A, Oberwinkler F. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genetics and Biology. 2007;44:105–122. doi: 10.1016/j.fgb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Goodwin SB, Legard DE, Smart CD, Levy M, Fry WE. Gene flow analysis of molecular markers confirms that Phytophthora mirabilis and P. infestans are separate species. Mycologia. 1999;91:796–810. [Google Scholar]
- Grunwald NJ, Martin FN, Larsen MM, et al. Phytophthora-ID.org: a sequence-based Phytophthora identification tool. Plant Disease. 2011;95:337–342. doi: 10.1094/PDIS-08-10-0609. [DOI] [PubMed] [Google Scholar]
- Grunwald NJ, Garbelotto M, Goss EM, Heungens K, Prospero S. Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends in Microbiology. 2012;20:131–138. doi: 10.1016/j.tim.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Hakariya M, Hirose D, Tokumasu S. A molecular phylogeny of Haptoglossa species, terrestrial peronosporomycetes (oomycetes) endoparasitic on nematodes. Mycoscience. 2007;48:169–175. [Google Scholar]
- Hakariya M, Hirose D, Tokumasu S. Molecular phylogeny of terrestrial holocarpic endoparasitic peronosporomycetes, Haptoglossa spp., inferred from 18S rDNA. Mycoscience. 2009;50:130–136. [Google Scholar]
- Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycological Research. 2001;105:1422–1432. [Google Scholar]
- Hudspeth DSS, Nadler SA, Hudspeth MES. A cox2 molecular phylogeny of the Peronosporomycetes. Mycologia. 2000;92:674–684. [Google Scholar]
- Hudspeth DSS, Stenger D, Hudspeth MES. A cox2 phylogenetic hypothesis for the downy mildews and white rusts. Fungal Diversity. 2003;13:47–57. [Google Scholar]
- Hulvey J, Telle S, Nigrelli L, Lamour K, Thines M. Salisapiliaceae - a new family of oomycetes from marsh grass litter of southeastern north America. Persoonia. 2010;25:109–116. doi: 10.3767/003158510X551763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Burgess TI. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species. Phytophthora plurivora sp. nov. Persoonia. 2009;22:95–110. doi: 10.3767/003158509X442612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon LPNM, Bakker FT, van den Bosch GBM, Bonants PJM, Fliera WG. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genetics and Biology. 2004;41:766–782. doi: 10.1016/j.fgb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Lara E, Belbahri L. SSU rRNA reveals major trends in oomycete evolution. Fungal Diversity. 2011;49:93–100. [Google Scholar]
- Librado P, Rozas J. DNASP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Martin FN. Phylogenetic relationships among some Pythium species inferred from sequence analysis of the mitochondrially encoded cytochrome oxidase II gene. Mycologia. 2000;92:711–727. [PubMed] [Google Scholar]
- Martin FN, Tooley PW. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia. 2003a;95:269–284. [PubMed] [Google Scholar]
- Martin FN, Tooley PW. Phylogenetic relationships of Phytophthora ramorum, P. nemorosa, and P. pseudosyringae, three species recovered from areas in California with sudden oak death. Mycological Research. 2003b;107:1379–1391. doi: 10.1017/s0953756203008785. [DOI] [PubMed] [Google Scholar]
- Martin FN, Tooley PW, Blomquist C. Molecular detection of Phytophthora ramorum, the causal agent of sudden oak death in california, and two additional species commonly recovered from diseased plant material. Phytopathology. 2004;94:621–631. doi: 10.1094/PHYTO.2004.94.6.621. [DOI] [PubMed] [Google Scholar]
- May KJ, Ristaino JB. Identity of the mtDNA haplotype(s) of Phytophthora infestans in historical specimens from the Irish potato famine. Mycological Research. 2004;108:471–479. doi: 10.1017/s0953756204009876. [DOI] [PubMed] [Google Scholar]
- Meier R, Shiyang K, Vaidya G, Ng PKL. DNA barcoding and taxonomy in diptera: a tale of high intraspecific variability and low identification success. Systematic Biology. 2006;55:715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- Mirzaee MR, Ploch S, Runge F, et al. A new presumably wide-spread species of Albugo parasitic to Strigosella spp. (Brassicaceae) Mycological Progress. 2013;12:45–52. [Google Scholar]
- Nigrelli L, Thines M. Tropical oomycetes in the German Bight - Climate warming or overlooked diversity? Fungal Ecology. 2013;6:152–160. [Google Scholar]
- Ploch S, Choi YJ, Rost C, et al. Evolution of diversity in Albugo is driven by high host specificity and multiple speciation events on closely related Brassicaceae. Molecular Phylogenetics and Evolution. 2010;57:812–820. doi: 10.1016/j.ympev.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Ploch S, Telle S, Choi YJ, et al. The molecular phylogeny of the white blister rust genus Pustula reveals a case of underestimated biodiversity with several undescribed species on ornamentals and crop plants. Fungal Biology. 2011;115:214–219. doi: 10.1016/j.funbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Quesada-Ocampo LM, Granke LL, Olsen J, et al. The genetic structure of Pseudoperonospora cubensis populations. Plant Disease. 2012;96:1459–1470. doi: 10.1094/PDIS-11-11-0943-RE. [DOI] [PubMed] [Google Scholar]
- Robideau GP, de Cock AWAM, Coffey MD, et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Molecular Ecology Resources. 2011;11:1002–1011. doi: 10.1111/j.1755-0998.2011.03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge F, Choi YJ, Thines M. Phylogenetic investigations in the genus Pseudoperonospora reveal overlooked species and cryptic diversity in the P. cubensis species cluster. European Journal of Plant Pathology. 2011;129:135–146. [Google Scholar]
- Schröder S, Telle S, Nick P, Thines M. Cryptic diversity of Plasmopara viticola (Oomycota, Peronosporaceae) in North America. Organisms Diversity & Evolution. 2011;11:3–7. [Google Scholar]
- Schroeder KL, Martin FN, de Cock AWAM, et al. Molecular detection and quantification of Pythium species: evolving taxonomy, new tools, and challenges. Plant Disease. 2013;97:4–20. doi: 10.1094/PDIS-03-12-0243-FE. [DOI] [PubMed] [Google Scholar]
- Sekimoto S, Hatai K, Honda D. Molecular phylogeny of an unidentified Haliphthoros-like marine oomycete and Haliphthoros milfordensis inferred from nuclear-encoded small- and large-subunit rRNA genes and mitochondrial-encoded cox2 gene. Mycoscience. 2007;48:212–221. [Google Scholar]
- Sekimoto S, Yokoo K, Kawamura Y, Honda D. Taxonomy, molecular phylogeny, and ultrastructural morphology of Olpidiopsis porphyrae sp. nov. (Oomycetes, straminipiles), a unicellular obligate endoparasite of Bangia and Porphyra spp. (Bangiales, Rhodophyta) Mycological Research. 2008;112:361–374. doi: 10.1016/j.mycres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Senda M, Kageyama K, Suga H, Levesque CA. Two new species of Pythium, P. senticosum and P. takayamanum, isolated from cool-temperate forest soil in Japan. Mycologia. 2009;101:439–448. doi: 10.3852/08-104. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telle S, Thines M. Amplification of cox2 ~620 bp from 2 mg of up to 129 years old herbarium specimens, comparing 19 extraction methods and 15 polymerases. PLoS ONE. 2008;3:e3584. doi: 10.1371/journal.pone.0003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telle S, Thines M. Reclassification of an enigmatic downy mildew species on lovegrass (Eragrostis) to the new genus Eraphthora, with a key to the genera of the Peronosporaceae. Mycological Progress. 2012;11:121–129. [Google Scholar]
- Telle S, Shivas RG, Ryley MJ, Thines M. Molecular phylogenetic analysis of Peronosclerospora (Oomycetes) reveals cryptic species and genetically distinct species parasitic to maize. European Journal of Plant Pathology. 2011;130:521–528. [Google Scholar]
- Testen AL, Jimenez-Gasco MD, Ochoa JB, Backman PA. Molecular detection of Peronospora variabilis in quinoa seeds and phylogeny of the quinoa downy mildew pathogen in South America and the United States. Phytopathology. 2014;104:379–386. doi: 10.1094/PHYTO-07-13-0198-R. [DOI] [PubMed] [Google Scholar]
- Thiers B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. 2014 Available at http://sweetgum.nybg.org/ih/, Retrieved June 2014.
- Thines M. Characterisation and phylogeny of repeated elements giving rise to exceptional length of ITS2 in several downy mildew genera (Peronosporaceae) Fungal Genetics and Biology. 2007a;44:199–207. doi: 10.1016/j.fgb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Thines M. A first molecular phylogeny for the graminicolous downy mildews. In: Lebeda A, Spencer-Phillips PTN, editors. Advances in Downy Mildew Research. Vol. 3. Palacký University and JOLA, v.o.s; Olomouc and Kostelec na Hané: 2007b. pp. 25–29. [Google Scholar]
- Thines M. Repeats of the ITS2 of Plasmopara species and their relevance for phylogenetic studies. In: Lebeda A, Spencer-Phillips PTN, editors. Advances in Downy Mildew Research. Vol. 3. Palacký University and JOLA, v.o.s; Olomouc and Kostelec na Hané: 2007c. pp. 31–35. [Google Scholar]
- Thines M. Recent outbreaks of downy mildew on grape ivy (Parthenocissus tricuspidata, Vitaceae) in Germany are caused by a new species of Plasmopara. Mycological Progress. 2011;10:415–422. [Google Scholar]
- Thines M, Kamoun S. Oomycete-plant coevolution: recent advances and future prospects. Current Opinion in Plant Biology. 2010;13:427–433. doi: 10.1016/j.pbi.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Thines M, Kummer V. Diversity and species boundaries in floricolous downy mildews. Mycological Progress. 2013;12:321–329. [Google Scholar]
- Thines M, Komjáti H, Spring O. Exceptional length of ITS in Plasmopara halstedii is due to multiple repetitions in the ITS-2 region. European Journal of Plant Pathology. 2005;112:395–398. [Google Scholar]
- Thines M, Göker M, Oberwinkler F, Spring O. A revision of Plasmo-para penniseti, with implications for the host range of the downy mildews with pyriform haustoria. Mycological Research. 2007;111:1377–1385. doi: 10.1016/j.mycres.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Thines M, Göker M, Telle S, et al. Phylogenetic relationships of graminicolous downy mildews based on cox2 sequence data. Mycological Research. 2008;112:345–351. doi: 10.1016/j.mycres.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Thines M, Choi YJ, Kemen E, et al. A new species of Albugo parasitic to Arabidopsis thaliana reveals new evolutionary patterns in white blister rusts (Albuginaceae) Persoonia. 2009a;22:123–128. doi: 10.3767/003158509X457931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines M, Telle S, Ploch S, Runge M. Identity of the downy mildew pathogens of basil, coleus, and sage with implications for quarantine measures. Mycological Research. 2009b;113:532–540. doi: 10.1016/j.mycres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Thines M, Runge F, Telle S, Voglmayr H. Phylogenetic investigations in the downy mildew genus Bremia reveal several distinct lineages and a species with a presumably exceptional wide host range. European Journal of Plant Pathology. 2010;128:81–89. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTALX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzuhashi S, Tojo M, Kakishima M. Phylogeny of the genus Pythium and description of new genera. Mycoscience. 2010;51:337–365. [Google Scholar]
- Villa NO, Kageyama K, Asano T, Suga H. Phylogenetic relationships of Pythium and Phytophthora species based on ITS rDNA, cyto-chrome oxidase II and beta-tubulin gene sequences. Mycologia. 2006;98:410–422. doi: 10.3852/mycologia.98.3.410. [DOI] [PubMed] [Google Scholar]
- Voglmayr H. Phylogenetic relationships of Peronospora and related genera based on nuclear ribosomal ITS sequences. Mycological Research. 2003;107:1132–1142. doi: 10.1017/s0953756203008438. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Riethmüller A. Phylogenetic relationships of Albugo species (white blister rusts) based on LSU rDNA sequence and oospore data. Mycological Research. 2006;110:75–85. doi: 10.1016/j.mycres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Choi YJ, Shin HD. Multigene phylogeny, taxonomy and reclassification of Hyaloperonospora on Cardamine . Mycological Progress. 2014a;13:131–144. doi: 10.1007/s11557-013-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Montes-Borrego M, Landa BB. Disentangling Peronospora on Papaver: phylogenetics, taxonomy, nomenclature and host range of downy mildew of opium poppy (Papaver somniferum) and related species. PLoS ONE. 2014b;9:e96838. doi: 10.1371/journal.pone.0096838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Schuenemann VJ, Cano LM, et al. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. Elife. 2013;2:e00731. doi: 10.7554/eLife.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional methods – cox2 primer design.
Minimum Evolution trees of Peronospora species based on cox1 (A) and cox2 (B) sequences.
Oomycete samples used in this study.
Identification success based on Best Match (BM) and Best Close Match (BCM) criteria for cox1, cox2, and cox2-1 spacer. Success: correct identifications; Ambiguous: ambiguous identifications; Misidentified: incorrect identifications; No match: sequences without any match closer than a threshold value below 95% of intraspecific distances.