Abstract
While prophylactic vaccines provide protective humoral immunity against infectious agents, vaccines that elicit potent CD8 T cell responses are valuable tools to shape and drive cellular immunity against cancer and intracellular infection. In particular, IFNγ-polarized cytotoxic CD8 T cell immunity is considered optimal for protective immunity against intracellular antigens. SOCS1 is a cross-functional negative regulator of TLR and cytokine receptor signaling via degradation of the receptor-signaling complex. We hypothesized that loss of SOCS1 in dendritic cells would improve T cell responses by accentuating IFNγ-directed immune responses. We tested this hypothesis using a recombinant Listeria monocytogenes vaccine platform that targets CD11c+ dendritic cells in mice where SOCS1 is selectively deleted in all CD11c+ cells. Unexpectedly, in mice lacking SOCS1 expression in CD11c+ cells, we observed a decrease in CD8+ T cell response to the Listeria monocytogenes vaccine. NK cell responses were also decreased in mice lacking SOCS1 expression in CD11c+ cells, but did not explain the defect in CD8+ T cell immunity. We found that DC lacking SOCS1 expression were functional in driving antigen-specific CD8+ T cell expansion in vitro, but that this process was defective following infection in vivo. Instead monocyte-derived innate TNFα and iNOS-producing DC (TipDC) dominated the anti-bacterial response. Thus, loss of SOCS1 in CD11c+ cells skewed the balance of immune response to infection by increasing innate responses while decreasing antigen-specific adaptive responses to infectious antigens.
Keywords: SOCS1, Dendritic cell, IFNγ, Listeria monocytogenes, vaccine
Introduction
L. monocytogenes is a ubiquitous Gram-positive facultative intracellular pathogen typically found in soil and food. We and others have been developing live-attenuated L. monocytogenes-based vaccine platforms for application to both cancer and infectious disease. The systemic infection model of listeriosis in mice has provided important insights into host-pathogen interactions and the adaptive immune response. Both a functional innate response and an adaptive immune response are critical for eradicating the pathogen (1-3). L. monocytogenes elicits a potent CD8+ T cell response in mice, attributed to direct infection of dendritic cells (DC) in the spleen and delivery of L. monocytogenes–associated antigen directly to the host-cell cytosol (4, 5). CD8α+ DC are the primary reservoir for live bacteria within the first few hours of systemic infection (6), and these cells play a critical role in priming L. monocytogenes-specific T cells (6, 7). These DC are an early source of interleukin-12 (IL-12), which in turn induces interferon-γ (IFNγ) release by NK, NKT, and T cells (8). Importantly, these inflammatory cytokines also elicit negative feedback loops through regulatory proteins that limit cellular activation by these potent cytokines.
The suppressor of cytokine signaling (SOCS) family proteins (SOCS1-7 and CIS) are a group of structurally related proteins characterized by a central SH2 docking motif for interaction with tyrosine phosphorylated proteins. SOCS1 is induced by cellular activation and serves as a negative feedback mechanism for cytokines sharing the common gamma chain (IL-2, IL-4, IL-7, IL-15), IFNα, IFNγ, and IL-12. While the SH2 domain targets the SOCS proteins to specific molecules within the JAK-STAT pathway, the SOCS-box functions as an E3 ubiquitin ligase, promoting degradation of the cytokine receptor complex. SOCS1 knockout (SOCS1-/-) mice are normal at birth; however, they exhibit slow growth and die within 3 weeks of birth, with activation of peripheral T cells, necrosis of the liver, and macrophage infiltration of major organs (9, 10). The neonatal defects exhibited by SOCS1-/- mice appear to occur primarily as a result of unchecked IFNγ signaling, since SOCS1-/- mice that also lack the IFNγ gene or the IFNγ receptor gene avoids neonatal lethality (11). The major source of this IFNγ has been shown to be T cells, since Rag-/- mice do not display SOCS1-/- lethality (12). SOCS1 is involved in the suppression of inflammation by regulating cytokine signaling in innate immune cells, including macrophages and DC, as well as non-immune cells. Deficiency of SOCS1 in macrophages was shown to result in hyper-responsiveness to lipopolysaccharide (LPS) (13-16), and silencing SOCS1 in DC was shown to enhance antigen presentation, T cell priming, lupus-like autoimmune diseases and anti-tumor immunity (17, 18).
For these reasons, we hypothesized that SOCS1 knockout in DC would be a means to increase antigen-specific T cell responses to L. monocytogenes-based vaccines and therefore potentiate their ability to generate therapeutic T cells targeting infectious diseases or cancer. We tested our primary hypothesis using Socs1fl/fl mice crossed with mice expressing Cre recombinase under the control of the CD11c promoter. This resulted in a strain where CD11c+ cells selectively lack SOCS1 expression and activity. DC demonstrated prolonged signal transduction following IFNγ stimulation, but surprisingly vaccination of mice where DCs lack SOCS1 resulted in a deficient CD8+ T cell response to bacterial antigens. While NK cells were also negatively affected in these mice, NK cells were not responsible for the poor CD8+ T cell responses generated in these animals. Instead, infection in these mice instead resulted in an increase in the number of iNOS producing DC (TipDC) in the spleen resulting in increased innate control of the bacterium. Our data demonstrates that blocking negative feedback of cytokine signaling via deletion of SOCS1 in DC, rather than increasing CD8 T cell responses to a pathogen, instead suppressed T cell responses and increased innate control of bacterial infection.
Materials and Methods
Murine models
To generate mice in which SOCS1 was specifically deleted in CD11c+ cells, CD11c-Cre-GFP transgenic mice (19) were obtained from The Jackson Laboratory (Stock#007567, Bar Harbor, ME) and bred with SOCS1fl/fl mice (15) (generously provided by Dr. Yoshimura, Keio University). Five to ten week old sex-matched Cre-SOCS1f/f and CD11c-Cre+ SOCS1f/f littermates were used for all experiments. C57BL/6 mice, OT-1 mice bearing a TCR specific for the SIINFEKL epitope of ovalbumin on a Rag1-/- background, and Rag1-/- knockout mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animal protocols were approved by the Earle A. Chiles Research Institute IACUC (Animal Welfare Assurance No. A3913-01).
Bacterial and viral strains
L. monocytogenes strains used for these studies, wt and ΔactA ActA-QV (ΔactA-QV, expressing the class I-restricted vaccinia virus derived epitopes B8R20–27, C4L125–132, A42R88–96 and K3L6–15, in addition to OVA257–264), (20) were grown to stationary phase in brain-heart infusion broth, washed in PBS, and injected intravenously (retro-orbital route) in 200μL total volume. Unless expressed in the text, the following infectious doses were used for L. monocytogenes strains: wt - 1×104 for survival and infectious studies and 1×105 for challenge and cell sorting; ΔactA-QV - 1×105 for infection and immune response. When bacterial counts were determined in various cell types, mice were infected with 1×105 cfu wt L. monocytogenes. 15 hr post-infection spleens were harvested and half of each spleen was directly homogenized, while the other half was dissociated and flow sorted for specific cell subpopulations. All spleen samples were then lysed and plated on BHI plates to calculate bacterial cfu in the source material. Vaccinia virus WR expressing full-length chicken ovalbumin (VV-OVA) were grown in HeLa cells and frozen. Thawed cell lysates were treated for 30-minutes with 1.25 μg/mL trypsin at 37°C. Virus was diluted in HBSS and injected intraperitoneally as 1×106 PFU in 200μL. For non-infectious vaccination, mice were immunized intraperitoneally with 5μg of anti-DEC-205-OVA (generously provided by CellDex Therapeutics, Hampton, NJ) together with 25μg of anti-CD40 (clone FGK4.5, BioXCell, West Lebanon, NH) in 200μL total volume.
Bone marrow dendritic cell culture and stimulation
Bone marrow derived dendritic cells (BMDC) were generated according to a standard protocol (21). Briefly, 2×106 bone marrow cells were seeded per 100mm Petri dish in RPMI 1640 supplemented with 10% fetal bovine serum and 20ng/ml recombinant murine GM-CSF (R&D Systems, Minneapolis, MN) with or without 10ng/ml IL-4 (PeproTech, Rocky Hill, NJ). On day 3, 10 ml of an RPMI1640 medium containing 20ng/ml mGM-CSF was added to the plates. On day 6 half of the culture supernatant was collected and centrifuged, and the cell pellet was resuspended in 10 ml of a fresh RPMI1640 medium containing 20ng/ml mGM-CSF and returned to the original plate. When needed 10ng/ml IL-4 was added at the same time. In general 7-8 day cultured BMDCs were used for the experiments unless otherwise specified. Flow cytometric analysis showed that these DC subsets contained >90% CD11c+ cells, data not shown.
BMDC (5×104) were stimulated with 100ng/ml lipopolysaccharide (LPS, InvivoGen, San Diego, CA), 10ng/ml IFN-γ (R&D Systems, Minneapolis, MN), or a combination of both reagents for 18hs at 37°C. Supernatants were removed and used for cytokine analysis, and cells were washed and stained for flow cytometric analysis as described. For western blot analysis and RNA extraction, cells were lysed and processed as described below.
For in vitro antigen presentation studies, BMDC were plated in 96-well plates (Costar-Corning) at 5×103 cells per well with α-DEC-205-OVA, soluble Endo-Free OVA (InvivoGen, San Diego, CA) or OVA257–264 (SIINFEKL) synthetic peptide for 45 min at 37°C in complete medium. BMDC were washed three times and resuspended in 200μl of complete medium containing 5×104 CFSE-labeled OT-1 CD8+ T cells. Proliferation was analyzed after 65–72 h of culture by flow cytometry (22). For isolation of splenic CD11c+ cells, spleens were dissociated and CD11c+ cells purified by positive selection (EasySep™ Mouse CD11c Positive selection isolation kit, StemCell Technologies, Vancouver, Canada) and purity check for flow cytometry. Each determination was performed in triplicate.
For RNA extraction and quantitative Real time-PCR (qRT-PCR), BMDCs were plated in a 6-well plate (2×106 cells per well) and stimulated as described above. At 18 hours, cells were harvested and RNA was purified using Qiazol and RNeasy Mini kit (Qiagen, Valencia, CA). DNase-treated RNA was used as template for cDNA synthesis using SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA) and qRT-PCR was performed using PowerUP SYBR Green Master Mix (Applied Biosystems, Foster City, CA) and the following primers: β-Actin-For; 5′-CCCTGTGCTGCTCACCGA-3′, β-Actin-Rev; 5′-ACAGTGTGGGTGACCCCGTC-3′, SOCS1-For; 5′-CACCTTCTTGGTGCGCG-3′, SOCS1-Rev; 5′-AAGCCATCTTCACGCTGAGC-3′. Reactions were carried out and analyzed in a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA). Fold change was expressed as 2-ΔΔCt, where the internal control is the β-Actin gene and the gene of interest is SOCS1.
For western blot analysis, cells were lysed in RIPA buffer in the presence of protease and phosphatase inhibitor (Thermo Fisher Scientific, Waltham, MA) and denatured in SDS loading buffer containing β 2-mercaptoethanol, electrophoresed on 10% SDS-PAGE gels and transferred to PVDF membrane (EMD Millipore, Billerica, MA). Blocked blots were probed overnight at 4°C with anti-STAT-1 (Cell Signaling Technology, Danvers, MA), anti-Phospho-STAT-1 (Tyr701) (#9171, Cell Signaling Technology) or anti-β-actin (#A2228, Sigma-Aldrich, St. Louis, MO) primary antibodies (Cell Signaling Technology, Danvers, MA) diluted 1:1000 followed by goat α-rabbit HRP-conjugated secondary antibody (1:20000) (Sigma, St. Louis, MO). Binding was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA) and images acquired with FluorChem E System (ProteinSimple, San Jose, CA).
Flow cytometry and cytokine analysis
Fluorochrome-conjugated antibodies specific for CD11c (clone N418), CD11b (clone M1/70), Ly-6C (clone HK1.4), Ly-6G (clone 1A8), MHCII I-A/I-E (clone M5/114.15.2), CD90.1 (clone HIS51), CD3 (clone 17A2), iNOS (clone CXNFT), IL12-p40 (clone C17.8), CD19 (clone eBio1D3), IL-2 (clone JES6-5H6), CD86 (clone GL1), CD27 (clone LG.7F9), NK1.1 (clone PK136), CD49b (clone DX5), NKp46 (clone 29A1.4), CD45.1 (clone A20), CD45.2 (clone 104), IFN-γ (clone XMG1.2), (eBioscience, San Diego, CA) CD4 (clone RM4-4), CD8α (clone 53-6.7), TNF (clone MP6-XT22) (BD Bioscience) and XCR1 (clone ZET) (Biolegend, San Diego, CA) were used at optimal titers as determined in our laboratory.
Serum cytokines were determined using the Mouse Inflammation BD Cytometric Bead Array (CBA, BD Biosciences, San Jose, CA). Samples were acquired on an LSRII flow cytometer and the exported data were analyzed using the CBA Analysis Plugin for Excel.
T cell function and analysis
For analysis of T cell responses, spleens were dissociated and filtered through a 70μm cell strainer. Red blood cells were lysed with Red Blood Cell Lysing Buffer (Sigma, St. Louis, MO). For peptide stimulation assays, splenocytes were stimulated for 4 hours with 1μM OVA257–264 (SIINFEKL), B8R20-27, A42R88–96 or LLO190–201 peptide in the presence of brefeldin A (GolgiPlug, BD Biosciences, San Jose, CA). Peptides for stimulation were obtained from A&A Labs (San Diego, CA, USA) and reconstituted in DMSO. Unstimulated controls (DMSO only) were used to assess nonspecific protein production for each animal. Cells were stained for surface antigens, and then fixed (Cytofix/Cytoperm buffer, BD Bioscience) and stored at -80C (in Cytofix/Cytoperm buffer) until further analysis. For intracellular cytokine staining, frozen cells were thawed, permeabilized (Perm Wash buffer, BD Biosciences), and stained for intracellular IFN-γ. To assess TipDC/DC activation, splenocytes were processed as described above and incubated 4hs at 37C, 5% CO2 with or without 107 cfu/ml heat killed L. monocytogenes (HKLm). Cells were stained and iNOS intracellular staining was performed as described above. Samples were acquired on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo™ 10.2 (FlowJo LLC, Ashland, OR).
Spleens were harvested from donor mice and either CD8+ or total T cells purified by negative selection (EasySep™ Mouse CD8+ T cell and EasySep™ Mouse T cell isolation kits, StemCell Technologies, Vancouver, Canada). Prior to adoptive transfer, cells were stained to confirm purity of CD8+ T cells and total T cells (>90%).
For in vivo antigen presentation experiments, single-cell suspensions of purified OT-1 CD8+ T cells were stained with 5, 6-carboxy-succinimidyl-fluoresceine-ester (CFSE, Molecular Probes, Eugene, OR) 10 minutes at 37C. Reactions were stopped with cold PBS and resuspended in the desired volume. Mice were injected with the CFSE-CD8+ T cells and spleens removed and processed after 3 days. Staining was performed as described and analysis conducted with FlowJo 10.2. Mitotic events were determined as described (22).
To evaluate the immune response, 10,000 purified OT-1 CD8+ T cells were transferred into Cre-SOCS1f/f and CD11c- Cre+ SOCS1f/f mice 1 day prior to immunization with ΔactA-QV. Spleens were harvested and processed for flow cytometry 7 days later.
For reconstitution of RAG1-/- knockout hosts, 2×107 purified total T cells from Cre-SOCS1f/f or CD11c- Cre+ SOCS1f/f spleens and the following day they were infected with ΔactA-QV. Seven days after the immunization, spleens were removed and processed for staining.
NK cells function and analysis
NK cells were purified from mouse splenocytes by using EasySep Mouse NK cell isolation kit (StemCell Techonologies, Vancouver, Canada) as described by the manufacturer. Cell purity was over 90% as confirmed by flow cytometry. Cells were plated and stimulated with IL-18 (R&D Systems, Minneapolis, MN) and/or IL-12 (PeproTech, Rocky Hill, NJ) for 6 hs before removing the supernatant for CBA analysis and staining the cells for ICS as described above.
For in vivo NK depletion, 100μg of α-NK1.1 antibody (Clone PK136, BioXCell, West Lebanon, NH) was intraperitoneally injected and NK cell depletion confirmed by analyzing blood samples 24hr later. Mice were primed with ΔactA-QVac, serum IFN-γ levels measured at 24hr post-infection and spleens removed and processed for ICS 7 days later as described.
Statistics
Data were analyzed and graphed using Prism (GraphPad Software, La Jolla, CA). Individual data sets were compared using Student's T-test and analysis across multiple groups was performed using ANOVA with individual groups assessed using Tukey's comparison.
Results
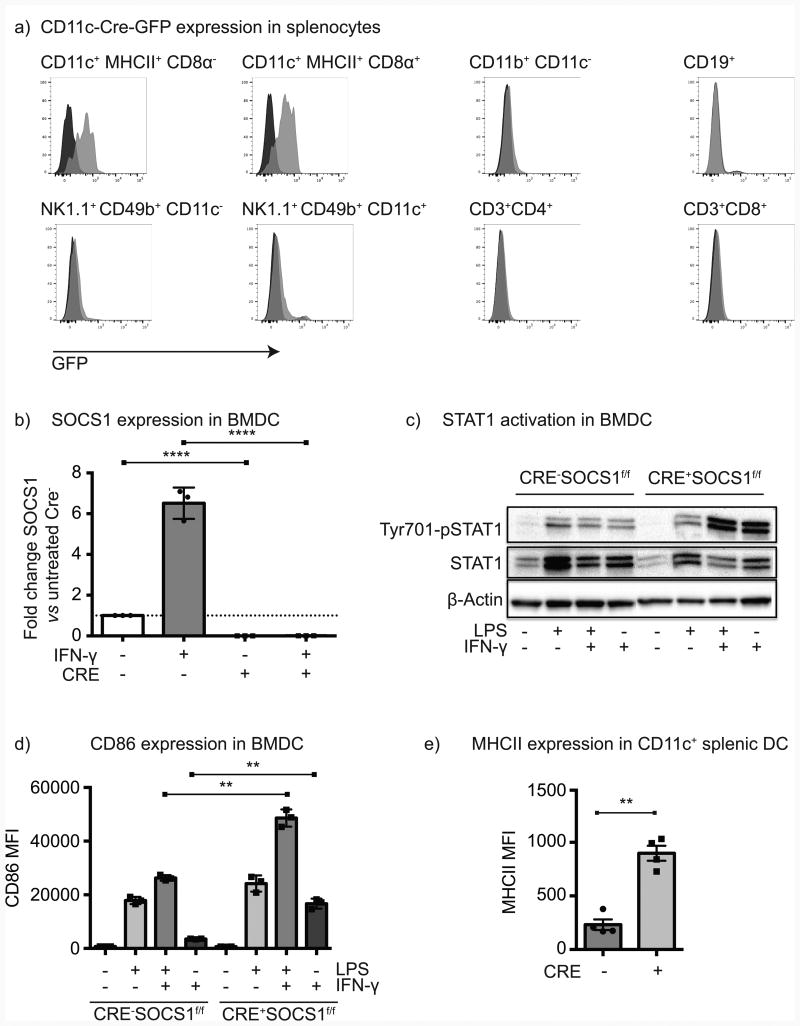
To achieve loss of SOCS1 in dendritic cells, mice expressing Cre recombinase under the control of the CD11c promoter were bred with a floxed socs1 gene (SOCS1fl/fl mice) to generate Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates for study. These mice were healthy, avoiding the postnatal lethality of SOCS1-/- mice (9, 10); however, Cre+SOCS1fl/fl mice exhibited some degree of splenomegaly and females developed psoriatic symptoms at 2-3 months, males after 4-5 months. To confirm cell specific expression of Cre, we took advantage of bicistronic expression of GFP along with Cre under control of the CD11c promoter (19). Consistent with prior reports, GFP was clearly detected in CD11c+MHCII+ cells in the spleen, including both the CD8α- and CD8α + subsets (Figure 1a). CD11b+ cells lacking CD11c were GFP-, as were CD19+ B cells and CD3+ T cells of both the CD4 and CD8 compartments. NK cell subsets can express CD11c; however, we could detect only weak GFP expression in these cells, mostly in the NK1.1+CD49b+ subset (Figure 1a). To confirm loss of SOCS1, BMDC derived from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were stimulated with IFNγ and the socs1 transcript was detected by qRT-PCR. While Cre-SOCS1fl/fl BMDC robustly increased expression of socs1 transcript following IFNγ treatment, no socs1 transcript was detectable in Cre+SOCS1fl/fl BMDC after cytokine priming (Figure 1b). To confirm functional loss of SOCS1 activity, STAT1 phosphorylation was measured in BMDC following prolonged stimulation with LPS, IFNγ or the combination. BMDC lacking SOCS1 activity exhibited increased IFNγ-mediated STAT1 phosphorylation compared to control littermates (Figure 1c). The increased response to IFNγ had phenotypic consequences, since while untreated Cre-SOCS1fl/fl and Cre+SOCS1fl/fl BMDC exhibited similar expression of costimulatory molecules, BMDC lacking SOCS1 activity exhibited a greater upregulation of CD86 following IFNγ treatment (Figure 1d). Naïve Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates exhibited similar proportions and absolute numbers of CD11c+MHCII+, CD11c+MHCII+CD8α+ and CD11c+MHCII+CD11b+CD8α- splenic DC (Supplementary Figure 1a-b). However, SOCS1 deficient DC showed higher basal levels of expression of MHCII in CD11c+ cells (Figure 1e) and higher basal levels of CD86 in CD11c+MHCII+CD8α+ and CD11c+MHCII+CD11b+CD8α- DC (Supplementary Figure 1ci-ii). Furthermore, XCR1 expression levels were significantly higher in CD11c+MHCII+CD8α+ DC when SOCS1 was ablated (Supplementary Figure 1ci-ii). These data demonstrate that mice lacking SOCS1 are highly responsive to IFNγ treatment and exhibit increased basal activation in vivo.
Figure 1. Selective elimination of SOCS1 activity in dendritic cells.
a) CD11c-Cre-GFP expression in splenocytes from Cre-SOCS1fl/fl (black histogram) and Cre+SOCS1fl/fl (grey histogram) littermates gated on key immune cell populations. b) BMDC were stimulated with IFNγ or left untreated for 18hr and RNA extracted and analyzed for SOCS1 expression using qRT-PCR. c) BMDC from the indicated strains were left untreated or stimulated with LPS, IFNγ or the combination for 18 hr and Western blotted forpSTAT1, STAT1 and β-actin as a loading control. d) BMDC stimulated as in c) were analyzed for CD86 expression by flow cytometry. e) Splenic CD11c+ DC from naïve Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice were analyzed for MHCII expression by flow cytometry. Results shown are representative of two to four independent experiments. Statistics calculated by Student's t test; * = p<0.05, ** = p<0.01, **** = p<0.0001.
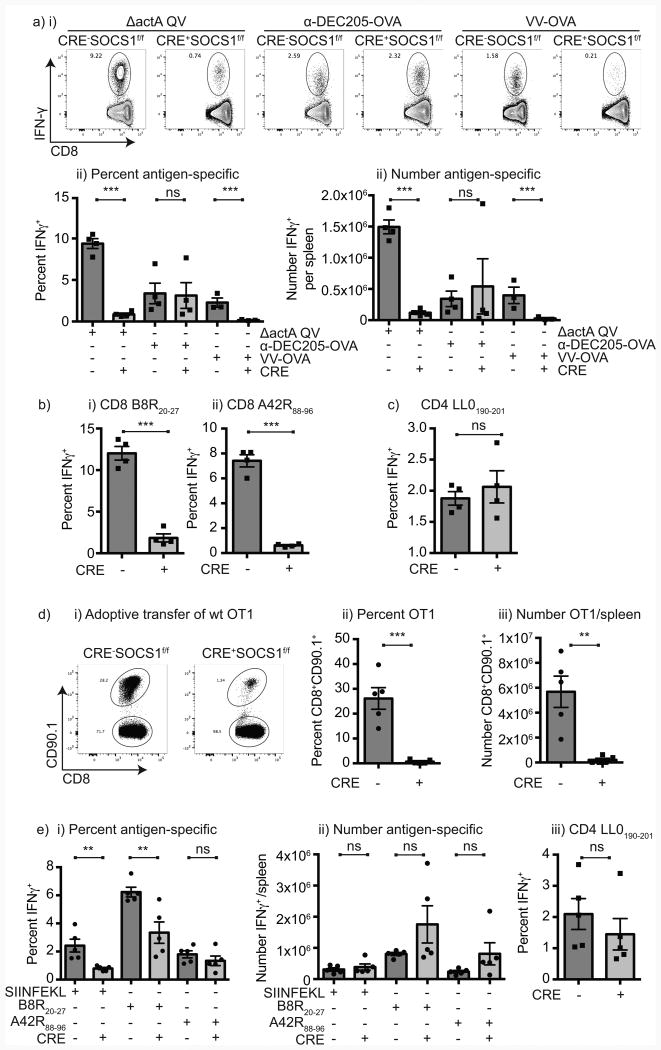
To determine whether vaccine-driven antigen-specific responses are improved in mice with DC-specific loss of SOCS1 expression we used a L. monocytogenes–based vaccine. L. monocytogenes vaccines directly infects dendritic cells in the spleen following systemic administration, and these dendritic cells are required to initiate CD8+ T cell responses to bacterial antigens (23). Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were vaccinated with ΔactA QV L. monocytogenes expressing 4 different well-characterized T cell epitopes, including the SIINFEKL epitope of ovalbumin. Mice with wild-type SOCS1 expression made a strong antigen-specific T cell response to vaccination, with IFN-γ+ SIINFEKL-specific CD8 T cells readily detectable in the spleen 7 days following vaccination; however, unexpectedly mice with DCs lacking SOCS1 demonstrated a significantly lower CD8 T cell response to antigen (Figure 2a). Similarly, CD8 T cell response to the other epitopes in the vaccine B8R and A42R were significantly reduced in Cre+SOCS1fl/fl mice (Figure 2b). To determine whether the T cell response to other infectious agents was similarly compromised in these mice, mice were vaccinated with a recombinant vaccine strain of Vaccinia virus expressing ovalbumin. Again, mice with wild-type SOCS1 expression made a strong response to ovalbumin, while mice with DCs lacking SOCS1 demonstrated a significantly lower CD8+ T cell response to ovalbumin (Figure 2a), indicating that this failure is not unique to L. monocytogenes. To determine whether this response was specific to infectious agents, mice were vaccinated with α-DEC205-OVA and α-CD40, which delivers ovalbumin to splenic DCs and drives efficient cross-presentation to T cells to generate robust ovalbumin-specific CD8+ T cell expansion (24). Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates made equivalent responses to vaccination with anti-DEC205-OVA and anti-CD40 (Figure 2a), suggesting that DC function is intact when antigen is delivered via this route. Furthermore, the success of this vaccination strategy was not due to the activity of anti-CD40, since delivery of anti-CD40 to mice vaccinated with ΔactA QV L. monocytogenes was not able to restore T cell responses in Cre+SOCS1fl/fl mice (Supplementary Figure 2). Interestingly, the CD4+ T cell response to Listeria-associated antigen was not diminished in Cre+SOCS1fl/fl mice (Figure 2c), suggesting that the failure in response was associated with intracellular antigens from the infectious agents that are cross-presented by DC to CD8+ T cells. To exclude the possibility that CD8+ T cell function was broadly diminished in Cre+SOCS1fl/fl mice, Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were adoptively transferred with a low dose of TCR transgenic CD8+ OT1 T cells, which are specific for the SIINFEKL epitope of ovalbumin presented on H2Kb, and have wild-type SOCS1 expression. Vaccination of these mice with ΔactA QV L. monocytogenes resulted in significantly lower OT1 CD8+ T cell responses in mice where DCs lack SOCS1 expression (Figure 2d). To assess the function of T cells from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice independent of DC function, T cells from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were adoptively transferred to Rag1-/- mice with wild-type SOCS1 expression, and these mice were vaccinated with ΔactA QV L. monocytogenes. While the proportion of CD8+ T cells from Cre+SOCS1fl/fl mice responding to antigens was slightly lower than CD8+ T cells from Cre-SOCS1fl/fl, there was no difference in the number of responding T cells after vaccination (Figure 2ei-ii). Again, the CD4+ T cell response to LLO was equivalent between strains (Figure 2eiii). These data demonstrate that when DCs lack SOCS1 expression, antigen-specific CD8+ T cell responses to infectious agents are significantly decreased.
Figure 2. Diminished CD8 T cell responses to infection in mice lacking SOCS1 in DC.
a) Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were primed with 1×105 cfu ΔactA QV, 5μg anti-DEC205-OVA with 25 μg anti-CD40 or 1×106 pfu VV-OVA. Spleens were harvested 7 days post priming and IFNγ+ SIINFEKL-specific CD8 T cells determined by using ICS. i) Representative flow-cytometry plots of IFNγ expression in response to SIINFEKL peptide. Quantitation of ii) the percent and iii) absolute numbers of IFNγ+ SIINFEKL-specific CD8 T cells per spleen. b) Quantitation of the percent of IFNγ+ i) B8R20–27-specific or ii) A42R88–96-specific CD8 T cells in spleens from mice immunized with ΔactA QV. c) Percent IFNγ+ LLO190-201–specific CD4 T cells 7 days post immunization with ΔactA QV. d) OVA-specific CD90.1+ OT1 CD8 T cells were isolated from OT1 transgenic mice and 10,000 cells per mouse adoptively transferred to Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice. The following day mice were immunized with 1×105 cfu ΔactA QV as above, spleens harvested 7 days later, cells stained and CD90.1+ (OT1) CD8 T cells analyzed. i) Representative flow-cytometry plot of CD90.1+ CD8 T cell expansion in Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice. ii) The percent and iii) total number of OT1 cells per mouse. e) CD8 T cells from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice were purified and transferred to Rag1-/- mice. 24hr later mice were immunized with 1×105 cfu ΔactA QV. Spleens were harvested 7 post infection and splenocytes stimulated in vitro with the indicated peptide. i) Percent and ii) absolute numbers of antigen-specific CD8 T cells as well as iii) percent of LLO190-201 CD4 T cell responses are shown. Each symbol represents one mouse, n=3-5 mice per group. Data represents the mean ± SEM of each group. The displayed experiments are representative of 3 to 5 independent repeats. Statistics calculated by Student's t test; ns = no significant differences observed between the groups analyzed; ** = p<0.01; *** = p<0.001.
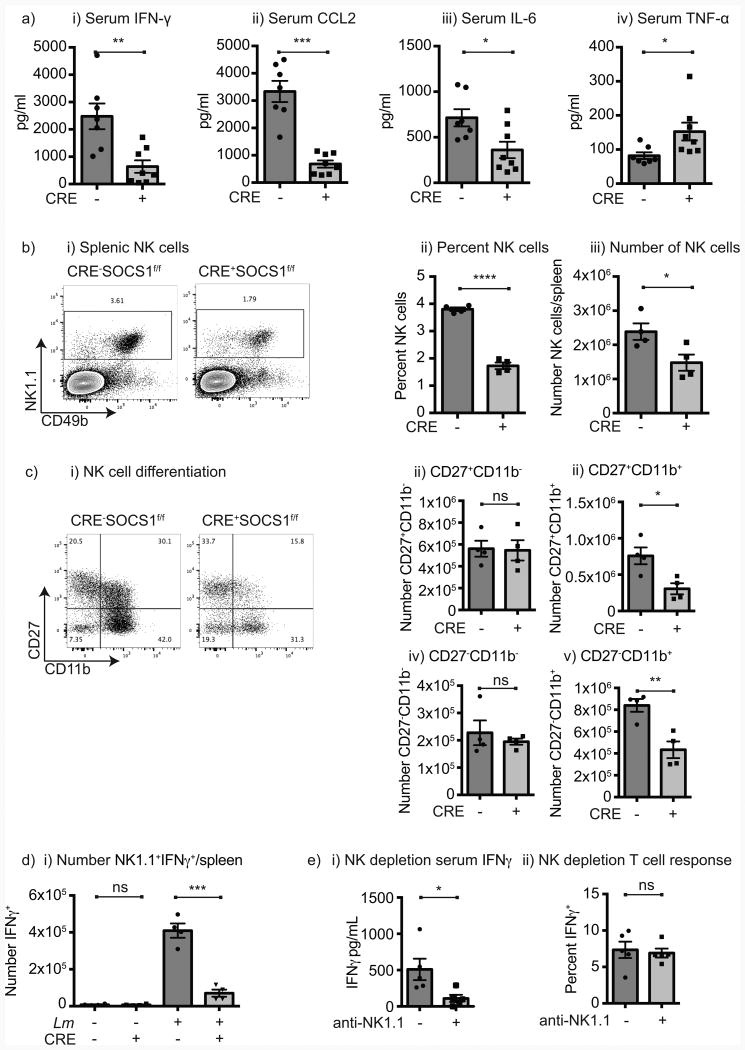
L. monocytogenes generates both an innate and adaptive immune response that each contribute to clearance of the bacterium. To determine whether the innate response was altered in mice where DCs lack SOCS1, we examined cytokine expression in the serum 24 hours following infection with wt L. monocytogenes. In mice where DCs lack SOCS1 there was a significantly lower expression of IFNγ, CCL2, and IL-6, but a significantly higher level of TNFα (Figure 3a). Consistent with prior reports (25), infection with ΔactA L. monocytogenes did not result in IL-10 secretion in control mice or in mice lacking SOCS1 in DC (not shown). Since NK cells can be an important source of IFNγ in early innate responses to infectious agents including L. monocytogenes (8), and NK cells expressed low levels of Cre (Figure 1a), we investigated the role of NK cells in Cre+SOCS1fl/fl mice. NK cells were decreased in number and proportion in Cre+SOCS1fl/fl mice (Figure 3b), mostly as a result of decreased number and proportion of mature CD11b+CD27+ and CD11b+CD27- NK cells (Figure 3c). Following infection with L. monocytogenes, IFNγ production by NK cells was significantly decreased in Cre+SOCS1fl/fl mice (Figure 3d), indicating a failure of NK activation in response to infection. However, NK cells isolated from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were equally able to produce IFNγ in response to stimulation in vitro (Supplementary Figure 3) suggesting the defect was not intrinsic to the NK cell but rather their response to infection. To determine whether NK cells were required for T cell antigen-specific responses to infection, wild-type mice were depleted of NK cells prior to vaccination with ΔactA QV L. monocytogenes using a depleting NK1.1 antibody (Figure 3ei and Supplementary Figure 3). These mice demonstrated loss of NK cells and significantly decreased IFNγ in the serum following infection, but antigen-specific T cell responses were unchanged (Figure 3eii). These data demonstrate that in vivo NK cell function is changed in Cre+SOCS1fl/fl mice, but that early NK cell production of IFNγ is not required for CD8+ T cell responses and so does not explain the poor response in mice where DCs lack SOCS1.
Figure 3. Decreased NK cell activity in mice lacking SOCS1 in DC.
a) Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice were infected with 1×104 cfu wt L. monocytogenes and 24hr later serum cytokines analyzed by multiplex cytokine bead assay. Values shown are from two combined experiments. b) i) Representative flow-cytometry plot of the NK1.1+CD49b+ NK cells in gated CD3- naïve Cre-SOCS1fl/fl and Cre+SOCS1fl/fl splenocytes; ii) percentages and iii) absolute numbers of splenic NK cells. c) Characterization of NK cell activation/maturation in naïve mice; i) Representative flow-cytometry plot of CD27 and CD11b expressing in gated NK cells in representative Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice; ii) absolute numbers of CD27+CD11b-; iii) CD27+CD11b+; iv) CD27-CD11b- and v) CD27-CD11b+ NK cells. d) Mice were left untreated or immunized with 1×104 cfu wt L. monocytogenes, spleens harvested 24 hr later and splenocytes incubated with brefeldin A for 4 hr before ICS staining to identify absolute numbers of NK1.1+ IFNγ+ cells. e) Cre-SOCS1fl/fl mice were left untreated or treated with anti-NK1.1 antibody to deplete NK cells and 24hr later immunized with 1×105 cfu ΔactA QV. i) One day post-infection serum was removed from both groups and IFNγ determined by cytokine bead assay. ii) Seven days post-infection spleens were removed and IFNγ+ SIINFEKL-specific CD8 T cell determined by ICS. Each symbol represents one mouse, n=3-5 mice per group. Data represents the mean ± SEM of each group. The displayed experiments are representative of 2 to 4 independent repeats. Statistics calculated by Student's t test; ns = no significant differences observed between the groups analyzed; * = p<0.05; ** = p<0.01; *** = p<0.001, **** = p<0.0001.
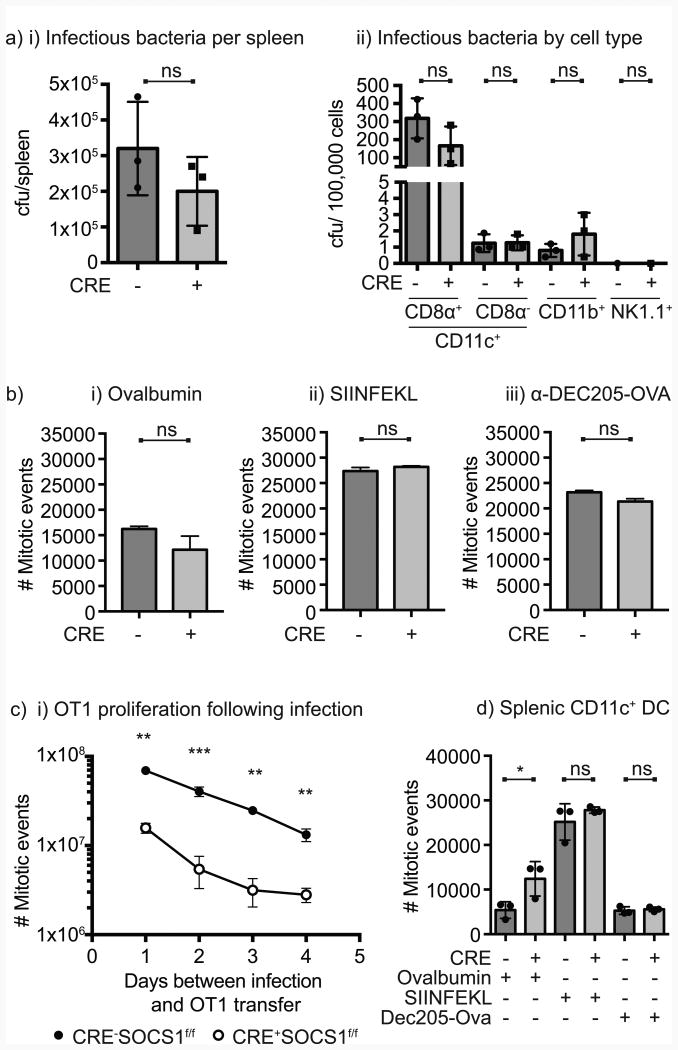
Infection of CD8α+ DC is critical to generate antigen-specific responses following infection with L. monocytogenes. To determine whether this infection was deficient in Cre+SOCS1fl/fl mice, we analyzed infection of a range of cell populations at early time points following in vivo infection with L. monocytogenes wt strain. Similar amounts of bacteria could be detected at this early time point in the spleen of Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates (Figure 4ai), and as anticipated, the majority of L. monocytogenes was present in CD11c+CD8α+ DC with small amounts of the bacterium in CD11c+CD8α- DC and CD11b+ monocytes (Figure 4aii). The number of bacteria in dendritic cells was not different between Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates (Figure 4aii), indicating that the bacteria is similarly infecting CD11c+CD8α+ DC despite a failure to generate T cell responses. To determine whether DCs from these animals have a defect in functional antigen presentation, bone marrow-derived DC from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were pulsed with ovalbumin, SIINFEKL peptide or anti-DEC205-OVA then tested for their ability to stimulate proliferation of OT1 CD8+ T cells in vitro. In each case, OT1 CD8+ T cell proliferation was equivalent in each group (Figure 4b). To assess antigen presentation in vivo, Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were challenged with ΔactA QV L. monocytogenes (which also contains the SIINFEKL epitope) 4, 3, 2 or 1 day prior to adoptive transfer of a high dose of OT1 CD8+ T cells, and assessed for OT1 CD8+ T cell proliferation 3 days later. OT1 CD8+ T cell proliferation was significantly decreased in the Cre+SOCS1fl/fl mice at each time point following infection (Figure 4c), indicating a significantly decreased capacity to functionally present antigen in vivo. To determine whether this functional difference in the cross-presenting capacity of splenic DC was true ex vivo, we sorted CD11c+ splenic DC from Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates and pulsed these with ovalbumin, SIINFEKL peptide or anti-DEC205-OVA then tested for their ability to stimulate proliferation of OT1 CD8+ T cells in vitro. Cre+SOCS1fl/fl DC showed slightly increased capacity to stimulate OT1 proliferation following cross-presentation of ovalbumin and were equally able to stimulate T cells with pulsed peptide and anti-DEC205-OVA (Figure 4d). These data demonstrate that DC lacking SOCS1 are functional in classic in vitro assays of cross-presentation, but dysfunctional in stimulating adaptive immunity following infection in vivo.
Figure 4. Decreased in vivo but equivalent in vitro T cell activation activity in mice lacking SOCS1 in DC.
a) Mice were infected with 1×105 cfu wt L. monocytogenes and 15 hr post-infection spleens were harvested. i) Half of each spleen was homogenized, cells lysed, samples plated on BHI plates and incubated overnight at 37°C; ii) the other half of the spleens was dissociated, splenocytes stained and the indicated cells flow sorted, lysed and plated on BHI plates. After 24hr incubation at 37°C, cfu were counted. Each symbol represents one mouse, n=3 mice per group. b) BMDC generated with GM-CSF plus IL-4 were pulsed with the indicated antigens for 45 min at 37°C, then washed and co-cultured with purified CFSE-labeled OT1 CD8 T cells. Proliferation was analyzed by CFSE dilution after 3 days of incubation and converted to mitotic events. c) Individual groups of mice were immunized with 1×105 cfu ΔactA QV for four consecutive days, and on the fifth day CFSE-labeled OT1 CD8 T cells were adoptively transferred into the mice and spleens harvested 72 hs later. Proliferation was analyzed by CFSE dilution and converted to mitotic events. Data represents the mean ± SEM of each group. d) CD11c+ DC were flow sorted from naïve Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice and pulsed in vitro with the indicated antigens for 45 min at 37°C, then washed and co-cultured with purified CFSE-labeled OT1 CD8 T cells. Proliferation was analyzed by CFSE dilution after 3 days of incubation and converted to mitotic events. Results shown are representative of two to four independent experiments. Statistics calculated by Student's t test; ns, no significant differences observed between the groups analyzed; * = p<0.05; ** = p<0.01; *** = p<0.001, **** = p<0.0001.
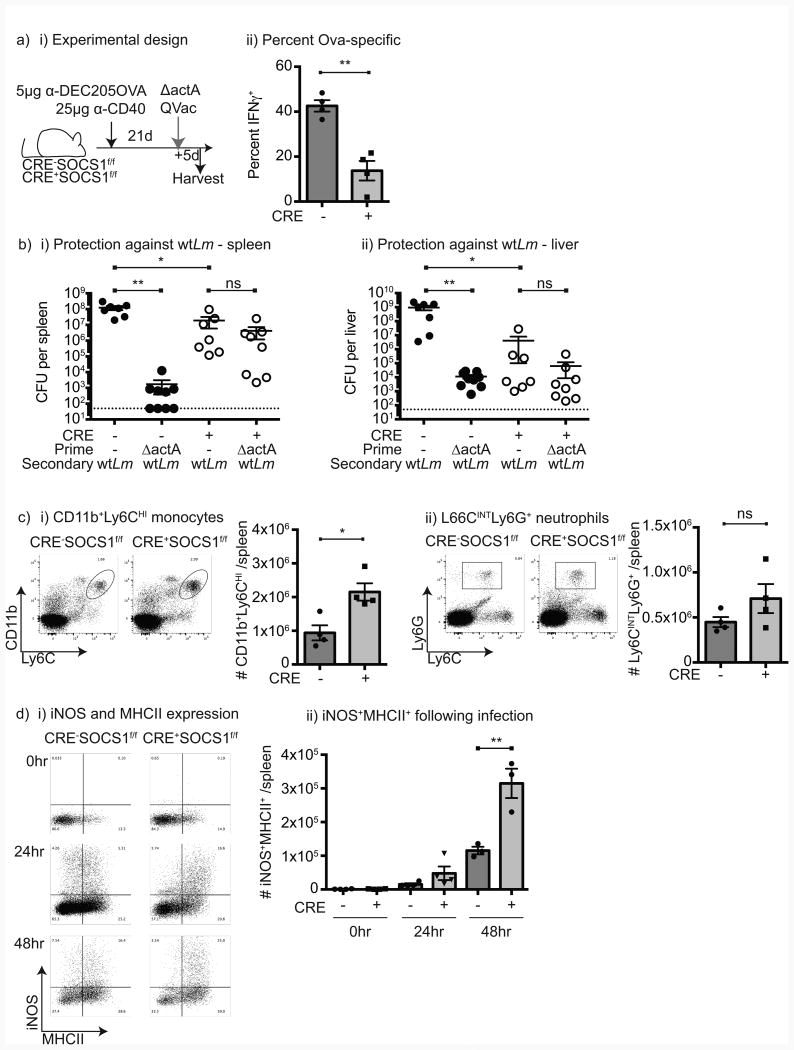
To determine whether the failure in mice lacking SOCS1 in DCs is limited to priming of naïve T cell responses, Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were vaccinated with anti-DEC205-OVA plus anti-CD40 to generate an equivalent and functional in vivo response, then challenged 21 days later with ΔactA QV L. monocytogenes. Mice lacking SOCS1 in DCs exhibited a significantly lower CD8+ T cell response following rechallenge (Figure 5a), indicating that CD8+ T cell memory expansion is also impaired following L. monocytogenes rechallenge. To determine whether a failure in CD8 T cell responses impacted bacterial clearance, naïve mice or mice that had been vaccinated with ΔactA QV L. monocytogenes 21 days prior were challenged with 1LD50 (1×105 cfu) wild-type L. monocytogenes and bacterial load in the spleen was determined 3 days later. Cre-SOCS1fl/fl mice that were vaccinated demonstrated significantly improved bacterial clearance compared to naïve mice (Figure 5b). By contrast, Cre+SOCS1fl/fl littermates were not protected by vaccination (Figure 5b). However, naïve mice lacking SOCS1 in DCs surprisingly appear to exhibit improved innate control of the infection at 3 days post-infection compared to mice with normal SOCS1 expression (Figure 5b). Daily analysis of bacterial load in infected mice demonstrated that wt L. monocytogenes was able to infect and replicate in Cre+SOCS1fl/fl mice and while bacterial counts are initially lower, the mice are similarly susceptible to progressive infection as control mice (Supplementary Figure 4a). Similarly, ΔactA QV L. monocytogenes showed initially lower bacterial counts in the spleen and livers of Cre+SOCS1fl/fl mice, but similar clearance of the attenuated ΔactA QV L. monocytogenes strain by day 5 post-infection (Supplementary Figure 4b) Thus, the differing T cell response to infection cannot be adequately explained by differential clearance or persistence of bacteria in mice. To examine cell populations that may be participating in the innate clearance of L. monocytogenes, Cre-SOCS1fl/fl and Cre+SOCS1fl/fl littermates were analyzed for their monocyte and neutrophil populations. Naïve Cre+SOCS1fl/fl mice have higher numbers of CD11b+Ly6Chi monocytes in the spleen than Cre-SOCS1fl/fl mice, and equivalent numbers of Ly6GhiLy6Chi neutrophils (Figure 5ci-ii). Monocytes have been shown to be recruited into the spleen of infected mice and differentiate into TNFα and iNOS-producing DC (TipDC), which participate in innate bacterial clearance (26). As we demonstrated, wt L. monocytogenes infected Cre+SOCS1fl/fl mice display elevated TNFα compared to control littermates (Figure 3a), suggestive of increased TipDC activity. To determine whether there was increased TipDC activity, iNOS expression was measured in splenic cells following infection. We observed increases in TipDC over time following infection with wt L. monocytogenes, and the number of these cells was significantly elevated in Cre+SOCS1fl/fl mice compared to control littermates (Figure 5d). These data suggest that mice lacking SOCS1 in DCs exhibit a dual phenotype. These mice generate an increased innate response associated with increased induction of iNOS in TipDC, but an impaired T cell response due to poor expansion of CD8+ T cells.
Figure 5. Increased innate response and TipDC activity in mice lacking SOCS1 in DC.
a) i) Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice were immunized with anti-DEC205-OVA and anti-CD40 to generate an equivalent priming event then boosted 21 days later with 1×105 cfu ΔactA QV. ii) Spleens were harvested 5 days post-boost and IFNγ+ SIINFEKL-specific CD8 T cells determined by using ICS. b) Cre-SOCS1fl/fl and Cre+SOCS1fl/fl mice were left untreated or primed with 1×105 cfu ΔactA QV and challenged 21 days later with 1×105 cfu wt L. monocytogenes. Three days later i) spleens and ii) livers were harvested, homogenized, plated on BHI plates and cfu numbers determined after overnight incubation at 37°C. c) Spleens from naïve mice were harvested, stained and analyzed by flow-cytometry. i) Summary of the absolute number of CD11b+Ly6Chigh cells and ii) the absolute number of Ly6G+Ly6Cint cells gated in the population. d) Mice were left untreated (0hr) or immunized with 1×104 cfu wt, spleens removed at 24hr and 48hr post-infection, dissociated, stained and iNOS synthesis determined by ICS. i) Representative expression of iNOS and MHCII in CD3-CD19-CD11b+Ly6Chigh monocytes over time, and ii) summary of the number of iNOS+ MHCII+ Tip-DC/spleen. Each symbol represents one mouse. Data represents the mean ± SEM of each group. Results shown are representative of three independent experiments. Statistics calculated by Student's t test; ns = no significant differences observed between the groups analyzed; * = p<0.05; ** = p<0.01.
Discussion
We demonstrate that SOCS1 deficiency in CD11c+ cells results in poor activation of CD8 T cell responses to antigens in bacterial and viral vaccines. This deficiency is caused by poor expansion of CD8 T cells by the critical CD8α+ DC population. Prior publications have described improved antigen presentation in DC with SOCS1 deficiency; however, these experiments were performed with ex vivo derived BMDC and not in an intact animal (18, 27). We demonstrate for the first time that restricted cell-specific loss of SOCS1 in dendritic cells in vivo has the function of redirecting the immune response following L. monocytogenes infection away from an adaptive and towards an innate response. We found that SOCS1 deficiency causes an increase in TNFα secretion in serum as well as an increase in iNOS+ CD11bint Ly6Chigh CD11cint MHCII+ TipDCs in spleens during the infection that could improve innate rather than adaptive control of infection.
L. monocytogenes LLO-mediated entry into the cytoplasm of DC is required for efficient cross-presentation to CD8 T cells (28). L. monocytogenes produces cyclic dinucleotides, which are critical for bacterial function (29), but also activate the cytoplasmic sensor STING (STimulator of INterferon Genes) resulting in type I IFN production (30). Archer et al. demonstrated that over-activation of STING can result in excess IFN production that limits CD8 T cell responses to L. monocytogenes (31). Similarly, over-activation of STING using exogenous administration of cyclic dinucleotides has resulted in type I IFN-mediated suppression of CD8 T cell responses (32). However, despite decreased adaptive immune responses, L. monocytogenes that over-activate STING do not have increased virulence, and in fact can exhibit decreased virulence in vivo (33). This would be consistent with our data demonstrating an increased innate control of infection concomitant with decreased adaptive responses. TNFα and iNOS are essential for defense against infection with L. monocytogenes (26, 34, 35). In addition, IFNγ secretion from NK cells has been described as crucial for activation of monocytes to differentiate to TipDCs (8). Although in the mice lacking SOCS1 in DC, the levels of NK cell-derived IFNγ are low during the first 24hs post infection, it may be sufficient to induce iNOS production due to the higher sensitivity to IFNγ (17, 18). Thus, the balance of innate and adaptive responses to L. monocytogenes infection can be varied and still control the infection, but to achieve strong protective T cell-mediated immunity requires carefully controlled inflammation at challenge (36, 37).
The CD8a+ subpopulation of DC are critical to generate CD8 T cell responses via cross-presentation of viral and bacterial-associated antigens (4, 5, 38). This same DC population is required for cross-presentation of cell-associated antigens (39), and thus these cells direct adaptive immune responses to cancer (40). Importantly, over-exuberant inflammatory responses have similarly been shown to diminish immune control of cancer through “rebound immune suppression” (41). Thus, as with bacterial and viral vaccination approaches, strategies that aim to generate CD8 T cell mediated immunity to tumors may similarly need to avoid over-activation of cross-presenting dendritic cells in order to optimize the adaptive immune response.
Supplementary Material
Acknowledgments
Grant support: This work was supported by CDMRP CA110297 (KSB), NIH R01CA182311 (MJG), NIH R21AI126151 (MRC).
References
- 1.Stavru F, Archambaud C, Cossart P. Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol Rev. 2011;240:160–184. doi: 10.1111/j.1600-065X.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Schmidt RL, Lenz LL. Early events regulating immunity and pathogenesis during Listeria monocytogenes infection. Trends Immunol. 2012;33:488–495. doi: 10.1016/j.it.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pamer EG. Immune responses to Listeria monocytogenes. Nature reviews Immunology. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 4.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8 + Dendritic Cells Selectively Present MHC Class I-Restricted Noncytolytic Viral and Intracellular Bacterial Antigens In Vivo. The Journal of Immunology. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, Jung S, Hochrein H, Russmann H, Brocker T, Busch DH. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Campisi L, Soudja SM, Cazareth J, Bassand D, Lazzari A, Brau F, Narni-Mancinelli E, Glaichenhaus N, Geissmann F, Lauvau G. Splenic CD8alpha(+) dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8(+) T-cell memory. Eur J Immunol. 2011;41:1594–1605. doi: 10.1002/eji.201041036. [DOI] [PubMed] [Google Scholar]
- 8.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of Hierarchical Clustering and Activation of Innate Immune Cells by Dendritic Cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proceedings of the National Academy of Sciences. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proceedings of the National Academy of Sciences. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TWH, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 Is a Critical Inhibitor of Interferon γ Signaling and Prevents the Potentially Fatal Neonatal Actions of this Cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 12.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. SOCS1 Deficiency Causes a Lymphocyte-Dependent Perinatal Lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 Participates in Negative Regulation of LPS Responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 14.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB Is a Negative Regulator of LPS-Induced Macrophage Activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Ayada T, Kinjyo I, Hiwatashi K, Yoshida H, Okada Y, Kobayashi T, Yoshimura A. Silencing of SOCS1 in macrophages suppresses tumor development by enhancing antitumor inflammation. Cancer science. 2009;100:730–736. doi: 10.1111/j.1349-7006.2009.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachithanandan N, Graham KL, Galic S, Honeyman JE, Fynch SL, Hewitt KA, Steinberg GR, Kay TW. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60:2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 18.Hanada T, Tanaka K, Matsumura Y, Yamauchi M, Nishinakamura H, Aburatani H, Mashima R, Kubo M, Kobayashi T, Yoshimura A. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. Journal of immunology. 2005;174:4325–4332. doi: 10.4049/jimmunol.174.7.4325. [DOI] [PubMed] [Google Scholar]
- 19.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauer P, Hanson B, Lemmens EE, Liu W, Luckett WS, Leong ML, Allen HE, Skoble J, Bahjat KS, Freitag NE, Brockstedt DG, Dubensky TW., Jr Constitutive Activation of the PrfA regulon enhances the potency of vaccines based on live-attenuated and killed but metabolically active Listeria monocytogenes strains. Infect Immun. 2008;76:3742–3753. doi: 10.1128/IAI.00390-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. The Journal of clinical investigation. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Unutmaz D, Wong P, Sano GI, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. The Journal of experimental medicine. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bahjat KS, Meyer-Morse N, Lemmens EE, Shugart JA, Dubensky TW, Brockstedt DG, Portnoy DA. Suppression of cell-mediated immunity following recognition of phagosome-confined bacteria. PLoS Pathog. 2009;5:e1000568. doi: 10.1371/journal.ppat.1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 28.Bahjat KS, Liu W, Lemmens EE, Schoenberger SP, Portnoy DA, Dubensky TW, Jr, Brockstedt DG. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect Immun. 2006;74:6387–6397. doi: 10.1128/IAI.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. MBio. 2013;4:e00282–00213. doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, Barber GN, Hayakawa Y, McGaha TL, Ravishankar B, Munn DH, Mellor AL. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. Journal of immunology. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect Immun. 2012;80:1537–1545. doi: 10.1128/IAI.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to IMF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 36.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nature immunology. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 37.Sckisel GD, Bouchlaka MN, Monjazeb AM, Crittenden M, Curti BD, Wilkins DE, Alderson KA, Sungur CM, Ames E, Mirsoian A, Reddy A, Alexander W, Soulika A, Blazar BR, Longo DL, Wiltrout RH, Murphy WJ. Out-of-Sequence Signal 3 Paralyzes Primary CD4(+) T-Cell-Dependent Immunity. Immunity. 2015;43:240–250. doi: 10.1016/j.immuni.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nature reviews Immunology. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 39.Schnorrer P, Behrens GMN, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proceedings of the National Academy of Sciences. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, Chen M, Kol A, Shiao SL, Reddy A, Perks JR, Culp WT, Sparger EE, Canter RJ, Sckisel GD, Murphy WJ. Blocking Indolamine-2,3-Dioxygenase Rebound Immune Suppression Boosts Antitumor Effects of Radio-Immunotherapy in Murine Models and Spontaneous Canine Malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-15-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.