Abstract
Background
Small bowel neuroendocrine tumors (SBNETs) present frequently with metastases, yet little is known about the molecular basis of this progression. This study sought to identify the serial differential expression of genes between normal small bowel (Nl), primary SBNETs (pSBTs), and liver metastases (lMets).
Methods
RNA isolated from matched Nl tissue, pSBTs, and lMets in 12 patients was analyzed with whole transcriptome expression microarrays and RNA-Seq. Chanes in gene expression between pSBTs and Nls, and lMets vs. pSBTs were calculated. Common genes that were differentially expressed serially (increasing or decreasing from Nl->pSBTs->lMets) were identified, and 10 were validated using qPCR.
Results
Use of two transcriptome platforms allowed for a robust discrimination of genes important in SBNET progression. Serial differential expression was validated in 7/10 genes, all of which had been described previously in abdominal cancers, and with several interacting with members of the AKT, MYC, or MAPK3 pathways. lMets had consistent underexpression of PMP22, while high expression of SERPINA10 and SYT13 was characteristic of both pSBTs and lMets.
Conclusion
Identification of the serial differential expression of genes from normal tissues to primary tumors to metastases lends insight into important pathways for SBNET progression. Differential expression of various genes, including PMP22, SYT13 and SERPINA10, are associated with the progression of SBNETs and warrant further investigation.
Background
Arising from the enterochromaffin (EC) cells of the small bowel, small bowel neuroendocrine tumors (SBNETs) have become the most common neoplasm of the small intestine,(1) and although they generally grow slowly, a substantial number of patients will progress to metastatic disease by the time of presentation. Despite the increased incidence of these neoplasms, little is known regarding the genetic steps accompanying the transformation of primary neoplasms and their progression to metastases. Improved understanding of these changes would aid in the identification of genes and pathways important to the evolution of SBNETs and assist potentially in the development of new diagnostic and therapeutic strategies.
Exome sequencing of SBNETs has revealed non-recurring mutations in a variety of genes as well as frequent sites of deletion or amplification involving genes in the AKT and SMAD pathways.(2) Francis et al. also reported a low frequency of somatic mutations in the cell cycle checkpoint gene CDKN1B,(2, 3), which was confirmed by others, with an incidence of 3–8.5%.(4, 5) Studies at the RNA level in neuroendocrine tumors (NETs) have shown utility for diagnosis,(6) identification of the sites of unknown primaries,(7) and discrimination of SBNETs from pancreatic NETs in primaries and metastases.(8, 9)
Transcriptome analysis also has the potential to improve our understanding of the pathways central to progression of primary neoplasmss to metastases. Recognition of genes serially over or underexpressed beginning with normal tissue and primary neoplasms, followed by even greater differential expression in metastases, could contribute to this understanding. In this study, we set out to compare changes in whole transcriptome expression between normal small bowel, primary SBNETs, and synchronous SBNET liver metastases using two different but complimentary platforms to identify genes associated with this progression.
Methods
RNA Isolation
Patients presenting to the University of Iowa with SBNETs were consented for genetic studies and entered into a tumor registry approverd by the institutional review board. Tissues collected during operative procedures performed on patients with SBNETs were placed in RNAlater solution (Thermo Fisher Scientific, Waltham, MA). Twelve patients who had histologic confirmation of SBNETs and tissue samples from normal small bowel (Nl), a primary SBNET (pSBT) and a SBNET liver metastasis (lMet) were selected for transcriptome analyses. RNA was isolated from tissues using the RNeasy® Plus Universal Mini Kit (Qiagen, Valencia, CA) with DNA digestion and resuspension in H2O per the protocol recommended by the manufacturer. RNA quality was then assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) with a requirement that samples have RNA integrity numbers (RIN) >6.
RNA Sequencing
RNA-Seq was performed at the University of Iowa Institute of Human Genetics (Iowa City, IA) using the Illumina TruSeq protocol (Illumina, Inc., San Diego, CA). Total RNA (500 ng) was fragmented, converted to cDNA, and ligated to sequencing adaptors. The molar concentrations of the indexed libraries were measured using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and combined equally into pools for sequencing. The concentration of each pool was determined using the Illumina Library Quantification Kit and sequenced on the Illumina HiSeq 4000 genome sequencer using a 75 bp paired-end sequencing-by-synthesis chemistry. The resulting fastq data were then aligned using the human hg19 genome assembly for mapping and annotation. TopHat (v. 2.1.0) was employed to perform mapping, Cuffquant for quantitation, and Cuffnorm and Cuffdiff for normalization and differential expression analysis.(10) The 10th percentile of the level of expression was added to the FPKM values reported by Cuffdiff to regularize the expression values in order to diminish artifacts of large or small-fold change values as a result of a measured value for expression being close to zero. Statistically significant expression change was determined by the false discovery rate (FDR) adjusted p-value (q-value), with significance defined as p<0.05.
Whole Transcriptome Microarrays
A total of 10 ng of total RNA was extracted and converted to cRNA utilizing the GeneChip® WT Pico Reagent Kit (Affymetrix, Inc., Santa Clara, CA); then cRNA was hybridized to the GeneChip® Human Transcriptome Array 2.0 (HTA; Affymetrix), and fluorescence was measured using the GeneChip® Scanner 3000 (Affymetrix). Data were processed using the Affymetrix Expression and Transcriptome Analysis consoles, and comparisons were tested using analysis of variance (ANOVA) with significant differential expression defined as ANOVA p-value and FDR p-value < 0.05.
Expression Data Analysis
Genes with significant differential expression between pSBTs and Nls, lMets and Nls, and lMets and pSBTs by RNA-Seq were identified using a regularized, log-fold change greater than 1 or less than -1 (approximately 2 fold and -2 fold, respectively). Common genes expressed differentially in pSBT vs. Nl, lMet vs. Nl, and lMet vs. pSBT analyses were identified, and genes with either significant serially increased expression from normal tissue to liver mets (lMet > pSBT > Nl) or serially decreased expression (Nl > pSBT > lMet) were selected. The data from the HTA microarrays were analyzed in a similar fashion, and we complied a list of genes satisfying the criteria of significant differential expression of greater than 2-fold increase or decrease in serial expression (from Nls to pSBTs then lMets). The lists obtained from RNA-Seq and HTA expression studies were analyzed, and genes common to both lists were identified.
PCR Validation
Genes were selected for qPCR validation based on a combination of the magnitude of the differences in expression observed and involvement in cancer formation or progression as identified by Ingenuity Pathway Analysis (IPA; Ingenuity System Inc., Qiagen). Total RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA), then used as a template for qPCR reactions with Taqman primers from 10 genes meeting the criteria outlined above, as well as the control genes POL2RA and HPRT1 using a 7900HT Fast Real-Time PCR System (Applied Biosystems). These validation assays were carried out using RNA from all three tissue sites in 40 additional patients. Assays were performed in quadruplicate, dCt calculated for each gene, and ddCt calculated for each tissue comparison. The concordance of qPCR results with HTA and RNA-Seq results was assessed by confirming statistically significant differential expression between tissue sites (pSBT vs. Nl, lMet vs. Nl, and lMet vs. pSBT) using paired t-tests. Gene expression levels were also assessed for the ability to discriminate between pSBTs and lMets using classification trees.
Results
RNA-Seq analysis revealed 1270 genes in the pSBT vs. Nl list that met criteria for presumed clinically relevant upregulation (p <0.05 and regularized log-fold change >1), with 1136/1270 of these genes also meeting criteria in the lMet vs. Nl analysis (Table 1). There were 727 genes in the pSBT vs. Nl groups that met criteria for clinically relevant downregulation (p < 0.05 and regularized log fold change <−1), and 598/727 were also downregulated in the lMet vs. Nl results. When the same selection criteria of log-fold changes were applied to the lMet vs. pSBT list, there were 157 upregulated genes and 565 downregulated genes. A total of 34 of the 157 genes were serially upregulated and were also seen in the 1136 genes ly common to the upregulation of pSBT vs. Nl and lMet vs. Nl (and thus were not highly expressed specifically in the liver or small bowel). Serial downregulation was seen in 143 of the 565 genes differentially expressed between pSBT and lMet, from the 598 common downregulated genes identified in the SBT and lMet vs. Nl comparisons(EDITOR THIS SENTENCE DOES NOT SEEM TO MAKE SENSE TO ME- ASK THE AUTHORS TO REWRITE THE SENTENCE TO MAKE GRAMMATICAL SENSE). Thus, the final numbers for further consideration were 34 serially upregulated genes (expression 2-fold greater in lMet than pSBT, and 2-fold greater in pSBT than Nl) and 143 serially downregulated genes(EDITOR ASK THE AUTHOR IF THE WAY THIS SETENCE WAS EDITED IS CORRECT.
Table 1.
Number of significantly differentially expressed genes for each comparison
| pSBT vs. Nl | lMet vs. Nl | Common to first two Comparisons | lMet vs. pSBT | Common to all three Comparisons | Common to both platforms | |
|---|---|---|---|---|---|---|
| RNA-Seq | ||||||
| Significantly overexpressed | 1270 | 2346 | 1136 | 157 | 34 | 9 |
| Significantly Underexpressed | 727 | 2678 | 598 | 565 | 143 | 31 |
| HTA | ||||||
| Significantly overexpressed | 1837 | 3540 | 401 | 333 | 34 | 9 |
| Significantly Underexpressed | 1354 | 3248 | 871 | 482 | 119 | 31 |
HTA analysis identified more differentially expressed genes (p<0.05 and fold change <−2 or >2) for both the pSBT and lMet vs. Nl comparisons than seen with RNA-Seq. In the pSBT vs. Nl analysis, 1837 upregulated genes and 1354 downregulated genes were identified (Table 1). Inspection of the lMet vs. Nl list established that 401/3540 upregulated genes discovered also met criteria in the pSBT vs. Nl analysis, and 871/3248 downregulated genes were also present in both lists. The lMet vs. pSBT results were 333 upregulated and 482 downregulated genes. A search for common genes to all three comparisons identified 34 serially upregulated and 119 serially downregulated genes. The RNA-Seq list (34 upregulated, 143 downregulated) was compared with the list generated from the HTA analysis; the result was 9 common genes that were serially overexpressed and 31 that were serially underexpressed (Supplemental Figure 1).
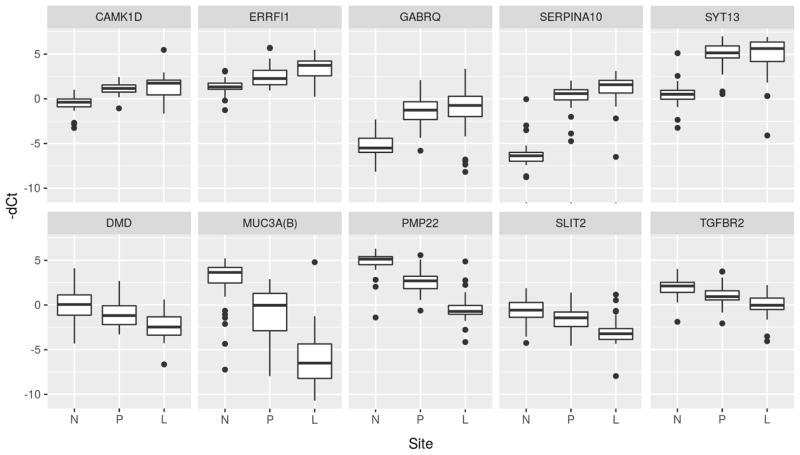
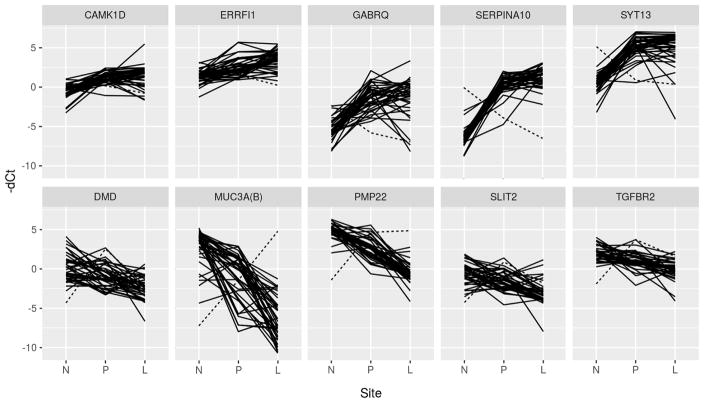
From this group, 10 genes were selected for validation, 5 of which were overexpressed and 5 were underexpressed (Table 2). Genes were selected based on a combination of the level of gene expression, direction of expression, availability of quality primers, and published reports of their involvement in cancer pathways. Of these 10 genes, 7 were confirmed to maintain their serial differential expression between all 3 tissue types when validated in 40 additional SBNET patients. Two of these genes (ERRFI1, SERPINA10) had serially increasing expression, while five (DMD, MUC3A, PMP22, SLIT2, TGFBR2) had serially decreasing expression. The serial changes in expression between tissue sites are depicted in box plots with increased expression corresponding with increased −dCt (Figure 1), while the individual patient levels of gene expression are demonstrated by spaghetti plots (Figure 2). There was one patient who was an outlier for multiple genes despite unremarkable tumor and clinical characteristics, and this individual is indicated by a dotted line in the spaghetti plots.
Table 2.
Gene Description, Location, Expression, and Function
| Gene | Chr | Expression | Name | Cellular Location | Function |
|---|---|---|---|---|---|
| CAMK1D | 10 | Increased | Calcium/Calmodulin Dependent Protein Kinase 1D | Cytoplasm | Regulation of granulocyte function, differentiation and activation of neutrophils and activation of CREB- dependent gene transcription. |
| ERRFI1 | 1 | Increased | ERBB Receptor Feedback Inhibitor 1 | Cytoplasm | Upregulated with cell growth. Negative regulator of several EGFR members. |
| GABRQ | X | Increased | GABA Receptor Subunit Theta | Plasma Membrane | Part of a multisubunit chloride channel mediating inhibitory synaptic transmission. |
| SERPINA10 | 14 | Increased | Serine Protease Inhibitor, Clade A, Member 10 | Extracellular Space | Primarily expressed in liver and excreted into plasma. Inhibits factors Xa and XIa. |
| SYT13 | 11 | Increased | Synaptotagmin 13 | Plasma Membrane | Membrane trafficker. Calcium- dependent neurotransmitter exocytosis. Vesicle transport. |
| DMD | X | Decreased | Dystrophin | Plasma Membrane | Involved in the dystrophin-glycoprotein complex (DGC) that bridges the cytoskeleton and extracellular matrix. High quantities at neuron synapses |
| MUC3A | 7 | Decreased | Mucin 3A | Extracellular Space | Epithelial Glycoprotein. May protect mucosal surfaces from foreign particles and infectious agents. |
| PMP22 | 17 | Decreased | Peripheral Myelin Protein 22 | Plasma Membrane | Integral membrane protein, involved in demyelinating disease and apoptosis. |
| SLIT2 | 4 | Decreased | Slit Guidance Ligand 2 | Extracellular Space | Role in migration of neurons and other cells as well as guidance of axons. Decreases growth and migration in cancer cell lines. |
| TGFBR2 | 3 | Decreased | Transforming Growth Factor Beta Receptor 2 | Plasma Membrane | Transmembrane binder of TGF-β. Regulates transcription of genes related to cell proliferation. Negative regulator of cellular proliferation. |
Location and function information derived from GeneCards: www.genecards.org
Figure 1.
Box plot of expression levels of candidate genes. N = Normal Small Bowel; P = Primary Tumor; L = Liver Metastasis.
Figure 2.
Plot of gene expression for each individual patient. Dotted line represents gene expression of a single patient who had discordant expression for multiple genes. N = Normal Small Bowel; P = Primary Tumor; L = Liver Metastasis.
ERRFI1 and SERPINA10 were both confirmed to have serial overexpression by qPCR in the validation cohort. While these genes were more highly expressed in lMets vs. pSBTs, their expression was less than a 2-fold overexpression (1.91 and 1.75, respectively). In the three genes where significant serial expression was not confirmed in the validation group (CAMK1D, GABRQ, SYT13), there were significant differences for pSBTs and lMets versus Nls, but expression levels in lMets and pSBTs were similar (Table 3).
Table 3.
Results of qPCR Expression Validation
| Gene | Comparison | ddCt | Fold Change | p-value |
|---|---|---|---|---|
| Increasing Expression | ||||
| CAMK1D | ||||
| Primary - Normal | 1.67 | 3.19 | <0.001 | |
| Liver – Normal | 1.86 | 3.64 | <0.001 | |
| Liver - Primary | 0.19 | 1.14 | 0.36* | |
| ERRFI1 | ||||
| Primary - Normal | 1.17 | 2.25 | <0.001 | |
| Liver – Normal | 2.10 | 4.30 | <0.001 | |
| Liver - Primary | 0.93 | 1.91 | <0.001 | |
| GABRQ | ||||
| Primary - Normal | 3.88 | 14.76 | <0.001 | |
| Liver – Normal | 3.96 | 15.53 | <0.001 | |
| Liver - Primary | 0.07 | 1.05 | 0.86* | |
| SERPINA10 | ||||
| Primary - Normal | 6.56 | 94.54 | <0.001 | |
| Liver – Normal | 7.37 | 165.72 | <0.001 | |
| Liver - Primary | 0.81 | 1.75 | <0.01 | |
| SYT 13 | ||||
| Primary - Normal | 4.55 | 23.41 | <0.001 | |
| Liver – Normal | 4.38 | 20.89 | <0.001 | |
| Liver - Primary | −0.16 | 0.89 | 0.61* | |
| Decreasing Expression | ||||
| DMD | ||||
| Primary - Normal | −1.04 | −2.06 | <0.01 | |
| Liver – Normal | −2.51 | −5.70 | <0.001 | |
| Liver - Primary | −1.47 | −2.76 | <0.001 | |
| MUC3A | ||||
| Primary - Normal | −3.72 | −13.18 | <0.001 | |
| Liver – Normal | −8.70 | −415.73 | <0.001 | |
| Liver - Primary | −4.98 | −31.54 | <0.001 | |
| PMP22 | ||||
| Primary - Normal | −2.12 | −4.35 | <0.001 | |
| Liver – Normal | −5.26 | −38.28 | <0.001 | |
| Liver - Primary | −3.14 | −8.80 | <0.001 | |
| SLIT2 | ||||
| Primary - Normal | −0.78 | −1.72 | 0.02 | |
| Liver – Normal | −2.31 | −4.98 | <0.001 | |
| Liver - Primary | −1.53 | −2.89 | <0.001 | |
| TGFBR2 | ||||
| Primary - Normal | −0.92 | −2.79 | <0.01 | |
| Liver – Normal | −2.06 | −4.17 | <0.001 | |
| Liver - Primary | −1.31 | −2.20 | <0.001 | |
Did not reach significance
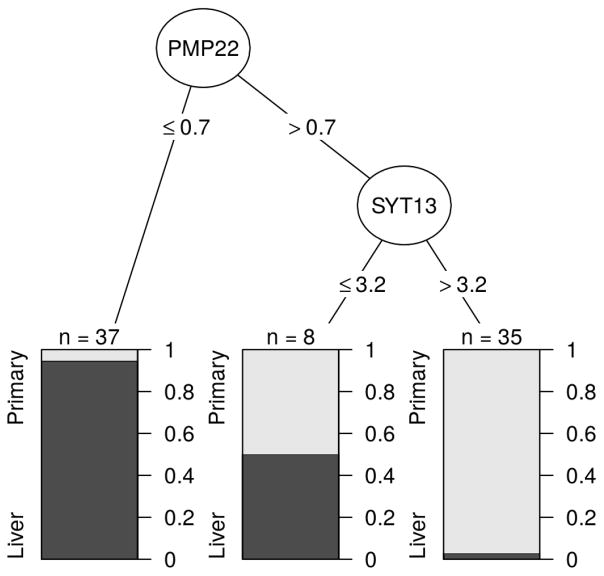
All five genes with serial underexpression remained significantly under expressed on qPCR validation. MUC3A had some of the greatest fold changes, with the difference in expression between lMets and Nls being −415.73, and a 31-fold between pSBTs and lMets. PMP22 was the gene that was most consistently underexpressed in lMets, where 36/37 tumors had qPCR expression levels less that 0.7 −dCt, with only one pSBT belonging in this group, to a patient who was a significant outlier for several genes (dotted line in Figure 2)(EDITORS THIS SENTENCE ALSO DOESN’T MAKE SENSE TO ME!). Gene expression levels of PMP22 predicted accurately 38/40 pSBTs and 35/40 lMets for an overall accuracy rate of 91%. The addition of a SYT13 expression threshold of 3.2 −dCt resulted in correct characterization of 69/72 (96%) primary tumors and metastases, with 8 others being inconclusive, demonstrating the robustness of these serial gene expression studies (Figure 3).
Figure 3.
Classification tree for differentiation of primary tumors from liver metastases using expression levels of PMP22 and SYT13. Cutoff limits expressed as −dCt values. Dark grey= Liver metastasis. Light Grey = Primary tumor.
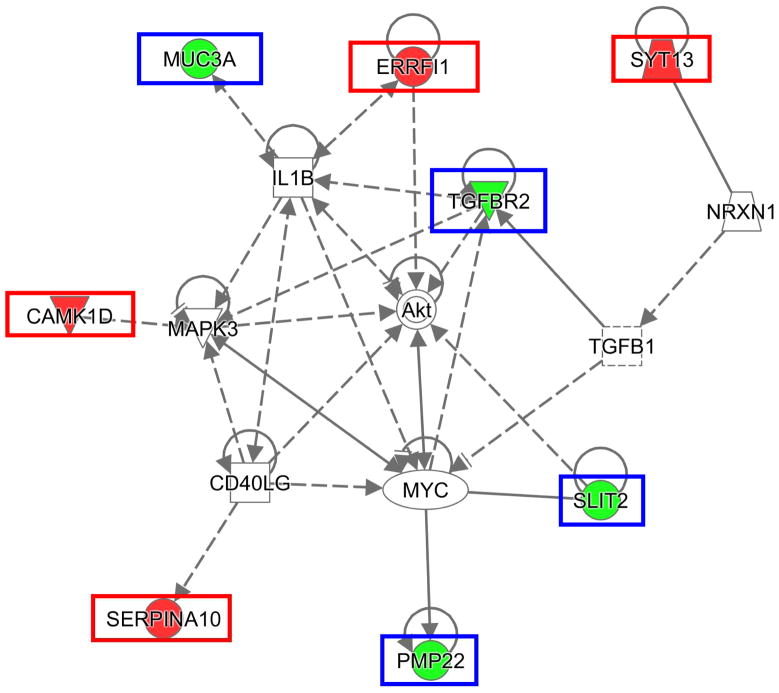
Of all the disease and function categories, there were 3 somewhat redundant categories identified by IPA that encompassed all 10 genes; these categories were digestive system cancer, abdominal cancer, and epithelial cancer (Table 4). The top IPA disease groups were cancer, organismal injury, and abnormalities, as well as reproductive system disease. Notable disease and function subcategories where analysis indicated possible upregulation were mammary tumor invasion, vascularization, and cell movement. A network was constructed using 8 of the genes (Figure 4). Some of the more recognizable central nodes in this network were AKT (which interacts directly with ERRFI1, SLIT2, and TGFBR2), MYC, and MAPK3.
Table 4.
Effects of Serial Gene Expression on Disease and Function
| Disease or Function | Overexpressed Genes | Underexpressed Genes |
|---|---|---|
| Abdominal cancer | CAMK1D, ERRFI1, GABRQ, SERPINA10, SYT13 | DMD, MUC3A, PMP22, SLIT2, TGFBR2 |
| Cell movement | CAMK1D, ERRFI1 | DMD, PMP22, SLIT2, TGFBR2 |
| Cell movement of microvascular endothelial cells | N/A | SLIT2, TGFBR2 |
| Digestive system cancer | CAMK1D, ERRFI1, GABRQ, SERPINA10, SYT13 | DMD, MUC3A, PMP22, SLIT2, TGFBR2 |
| Epithelial cancer | CAMK1D, ERRFI1, GABRQ, SERPINA10, SYT13 | DMD, MUC3A, PMP22, SLIT2, TGFBR2 |
| Invasion of mammary tumor cells | N/A | SLIT2, TGFBR2 |
| Migration of cells | CAMK1D, ERRFI1 | PMP22, SLIT2, TGFBR2 |
| Vascularization | ERRFI1 | SLIT2, TGFBR2 |
Effect of the expression seen in our study on the listed disease or function as predicted by IPA: Bold = Increased Activity/Formation;
Normal = Affected but direction unknown; Underline = Decreased Activity/Formation
Figure 4.
Gene network constructed from candidate genes using IPA. Red boxes = Increased expression in our data set. Blue boxes= Decreased expression in our data set.
Discussion
The identification of genes that are serially up or downregulated in the progression from normal tissues to primary neoplasmss to metastases has the potential for helping us to understand the molecular pathways important for SBNET tumor progression. One of the challenges of transcriptome data is the large number of genes that are differentially expressed between tissues and how to best sort out candidate genes in an unbiased manner. We utilized the two, separate, transcriptome analysis platforms HTA and RNA-Seq to identify genes with serially increasing (lMet>pSBT>Nl) or serially decreasing expression (Nl>pSBT>lMet). The requirement that candidate genes for validation needed to be serially up or down-regulated using both platforms decreased the number of upregulated gene candidates from 34 (in each HTA and RNA-Seq) to 9 (seen with both), and the number of downregulated genes from 153 for HTA and 177 for RNA-Seq to 40. This approach resulted in a decrease of 74–77% in the number of candidate genes, thereby facilitating the selection of genes more likely to have biologic importance rather than spurious changes in expression. From this group of 49 genes, we selected 5 that were serially upregulated and 5 that were downregulated, based on either the greatest differences in expression or those of biologic interest for further validation by qPCR.
Seven of these ten genes remained significantly differentially expressed in serial fashion by qPCR in an additional 40 SBNET patients. The three genes that did not hold up on validation failed due to the lack of a significant increase in lMets compared to pSBTs (Table 3). These 3 genes, however, were still significantly increased in both pSBTs and lMets relative to Nls, suugesting that these 3 genes may still play important roles in aggressiveness of SBNET tumors and could be potential targets for therapy. The two genes that did meet our requirements for validation showed significant expression differences of just less than a 2-fold increase between pSBTs and lMets. This observation is in contrast to the downregulated genes which were all confirmed on qPCR validation.
At first glance, the importance of each of these genes is not obvious, because they have not been described as being important in previous exome sequencing, comparative genomic hybridization, or other gene expression studies. Some genes, however, were able to discriminate between pSBTs and lMets based soley on expression levels, while others were noted to interact with familiar pathways such as AKT, and several have been described to be involved in the progression or formation of other cancers.
While the ability to discriminate between pSBT and lMet using gene expression levels does not have particular clinical value, it does help to confirm biologic differences that may be important. The expression of PMP22 below 0.7 −dCt was seen in 35/40 lMets and only 1 pSBT (which was in the one patient who had a significant outlier) indicates that loss of this gene is a common characteristic of lMets. Furthermore, these expression differences indicate that loss/downregulation of PMP22 is important in SBNET progression or a downstream consequence of other critical changes. Why loss of expression of an integral membrane protein that is important in myelin sheaths would be involved in tumor progression of PMP22 is unclear but could relate to the observation that overexpression results in apoptosis in HEK-293 cells.(11)
To attempt to identify interactions of serially differentially expressed genes, a gene network was constructed using IPA (Figure 4). Three of the genes chosen for validation (ERRFI1, SLIT2, and TGFBR2) were noted to have interaction with AKT, which is of particular interest given the current use of Everolimus for treatment of metastatic NETs. The biologic effects of ERRFl1 overexpression are not entirely clear, because ERRFI1 may negatively regulate receptor signaling of epidermal growth factor and the upregulation of ERRFI1 has been described to inhibit cell growth and promote apoptosis.(12) SLIT2 overexpression has been associated with increased vascularization of tumors in mice, cell movement of microvascular endothelial cells, and decreased mammary cell invasion.(13–15) TGFBR2 has an intermediary role in TGF-β induced AKT signaling(16) and appears to play an important role in cancer. Mutation of this gene has been well described in colon cancer, where TGFBR2 provides a selective growth advantage,(17) and its downregulation has been described in neoplastic EC cells.(18) The downregulation of this plasma membrane protein may be secondary to MYC expression, which also increases migration of breast and colorectal cancer cells,(19–21) and may provide SBNETs a mechanism of avoiding the cytostatic effects of TGFBI.(18, 22) TGFBR2 is located on the plasma membrane, which is ideal for therapeutic targets but may be less valuable, because TGFBR2 is downregulated in the progression to metastasis.
SYT13 is a plasma membrane bound protein involved in the trafficking of neurotransmitters and though it was not overexpressed in lMets vs. pSBTs, it was significantly overexpressed in both tumor sites when compared to normal. The expression levels in our tumors, >300 fragments per kilobase of transcript per million mapped reads (FPKM) for pSBT and > 600 for lMet, were almost 10x greater than the greatest average expression levels reported in normal tissues, which are found in the cerebral cortex, pituitary, and cerebellum; are all the sites afeprotected by the blood-brain barrier which makes the SYT13 gene product potentially an excellent target for therapeutics and imaging. Cisplatin decreases phosphorylation of SYT13(23) and may warrant further investigation in SBNETs. Another highly overexpressed gene found in this study was SERPINA10, a serine protease that inhibits factor Xa and X1a. Although not located on the plasma membrane, SERPINA10 has been described previously as being upregulated in both SBNETs and PNETs (24, 25) and thus could play a role in therapy or diagnosis for both types of these NETs.
An important technical issue for studies of differential gene expression is the selection of controls. In the qPCR and HTA experiments, housekeeping genes can be selected to calculate relative levels of gene expression, but this approach is not a reliable method for RNA-Seq. In our study, we had the benefit of having matched normal small bowel tissue from each patient, facilitating comparisons of all genes in the transcriptome. One problem with this strategy is that normal small bowel is a mixture of cell types, and the cells of origin of SBNETs, the EC cells, represent <1% of all cells present. Although these cells could be microdissected by laser-capture and RNA-Seq performed on a more pure precursor cell population, our attempts at these methods has not yielded suitable RNA concentration or quality for genome wide expression studies, and no EC cell lines are available to use as controls. There were advantages to using matched normal samples from individual patients, however. First, this approach gave a frame of reference for comparing pSBTs to lMets, and second, it helped to separate out genes that were highly expressed specifically either in the small bowel or in the liver, rather than being associated with the progression to metastases. For example, transferrin and thrombin were found to be highly expressed in lMets but not Nls or pSBTs. These genes are highly expressed in normal liver tissue, and despite the fact that our liver metastases are generally homogeneous populations of tumor cells without many contaminating hepatocytes, the fact that these liver genes are highly expressed in these NET metastases suggests that just being present in the liver microenvironment leads to this increased expression. The pSBT versus normal comparisons helped similarly to exclude120/342 (35%) genes identified by The Human Protein Atlas (www.proteinatlas.org) as being highly expressed in the small bowel, such as mucin 17 and fatty acid binding protein 2, and which were found at high levels in both pSBTs and Nl tissues in our analysis. These liver and small bowel genes may have otherwise added further noise to a straight comparison of pSBT vs. lMet, and thus, using the normal small bowel as a control allowed for further exclusion of genes unlikely to be involved in tumor progression from the final list of candidates.
This comparison of matched tissue samples from 12 patients for gene discovery and an additional 40 patients for gene validation has allowed us to identify a number of genes important in the progression to metastasis. The genes were vetted carefully by requiring serial increasing or decreasing expression from Nl to pSBT to lMet and by demanding that these gene candidates be found by two completely different platforms of gene expression using the same RNA samples. As might be expected, not all samples were confirmed to be differentially expressed in serial fashion on validation, but in each of these cases, these genes remained highly differentially expressed in pSBTs and lMets relative to Nl. These results lend further support to the importance of SERPINA10 overexpression and TGFBR2 underexpression in NETs.(18, 24, 25) We also report the novel findings of expression changes in PMP22, SLIT2, and SYT13, with decreased PMP22 expression being a reliable characteristic of lMets, and SYT13 representing a promising imaging and therapeutic target. Our study also sheds light on the importance of the tumor microenvironment on gene expression in tumors, which was suggested through using matched Nl, pSBT, and lMet tissues from each patient. While the results of these expression analyses are of considerable interest, validation of additional genes will also be important. For each of these genes, further evaluation will be important to confirm their role in the sequence of SBNET progression, and their potential utility as diagnostic, imaging, or therapeutic targets.
Supplementary Material
Figure 1: Heat map of 40 genes identified as having serial differential expression. Increasing intensity of red demonstrates increasing magnitude of gene underexpression and increasing intensity of blue demonstrates increasing magnitude gene overexpression. Values are fold-changes for primary tumor and liver metastases in comparison to normal small bowel. SBNET = Primary tumor; SBLivMet = Liver metastasis.
Acknowledgments
T32: T32CA148062 (KK) and SPORE: P50CA174521 (JH, JD, PB, GL, TO, AB).
RNA-Seq data presented herein were obtained at the Genomics Division of the Iowa Institute of Human Genetics, which is supported, in part, by the University of Iowa Carver College of Medicine and the Holden Comprehensive Cancer Center (National Cancer Institute of the National Institutes of Health under Award Number P30CA086862).
Footnotes
Study presented at the 38th Annual Meeting of the American Association of Endocrine Surgeons, Orlando, Florida, April 2–4, 2017
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 2.Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR, et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123(6):2502–8. doi: 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis JM, Kiezun A, Ramos AH, Serra S, Pedamallu CS, Qian ZR, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45(12):1483–6. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crona J, Gustavsson T, Norlen O, Edfeldt K, Akerstrom T, Westin G, et al. Somatic Mutations and Genetic Heterogeneity at the CDKN1B Locus in Small Intestinal Neuroendocrine Tumors. Ann Surg Oncol. 2015;22(Suppl 3):S1428–35. doi: 10.1245/s10434-014-4351-9. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JE, Sherman SK, Li G, Choi AB, Bellizzi AM, O’Dorisio TM, et al. Somatic alterations of CDKN1B are associated with small bowel neuroendocrine tumors. Cancer Genet. 2015;208(11):564–70. doi: 10.1016/j.cancergen.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modlin IM, Kidd M, Bodei L, Drozdov I, Aslanian H. The clinical utility of a novel blood-based multi-transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am J Gastroenterol. 2015;110(8):1223–32. doi: 10.1038/ajg.2015.160. [DOI] [PubMed] [Google Scholar]
- 7.Kerr SE, Schnabel CA, Sullivan PS, Zhang Y, Huang VJ, Erlander MG, et al. A 92-gene cancer classifier predicts the site of origin for neuroendocrine tumors. Mod Pathol. 2014;27(1):44–54. doi: 10.1038/modpathol.2013.105. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell JE, Sherman SK, Stashek KM, O’Dorisio TM, Bellizzi AM, Howe JR. A practical method to determine the site of unknown primary in metastatic neuroendocrine tumors. Surgery. 2014;156(6):1359–65. doi: 10.1016/j.surg.2014.08.008. discussion 65–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman SK, Maxwell JE, Carr JC, Wang D, Bellizzi AM, Sue O’Dorisio M, et al. Gene expression accurately distinguishes liver metastases of small bowel and pancreas neuroendocrine tumors. Clin Exp Metastasis. 2014;31(8):935–44. doi: 10.1007/s10585-014-9681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson HL, McFie PJ, Roesler WJ. Different transcription factor binding arrays modulate the cAMP responsivity of the phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem. 2002;277(46):43895–902. doi: 10.1074/jbc.M203169200. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Zhu S, Zhou Y, Jin Y, Dai H, Wang X. Upregulation of mitogen-inducible gene 6 triggers antitumor effect and attenuates progesterone resistance in endometrial carcinoma cells. Cancer Gene Ther. 2015;22(11):536–41. doi: 10.1038/cgt.2015.52. [DOI] [PubMed] [Google Scholar]
- 13.Gohrig A, Detjen KM, Hilfenhaus G, Korner JL, Welzel M, Arsenic R, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74(5):1529–40. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Fernandis AZ, Rao Y, Ganju RK. Slit protein-mediated inhibition of CXCR4- induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem. 2004;279(10):9115–24. doi: 10.1074/jbc.M308083200. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Xiao Y, Ding BB, Zhang N, Yuan X, Gui L, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4(1):19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 16.Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells. J Biol Chem. 2003;278(5):3275–85. doi: 10.1074/jbc.M204623200. [DOI] [PubMed] [Google Scholar]
- 17.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 18.Kidd M, Modlin IM, Pfragner R, Eick GN, Champaneria MC, Chan AK, et al. Small bowel carcinoid (enterochromaffin cell) neoplasia exhibits transforming growth factor-beta1- mediated regulatory abnormalities including up-regulation of C-Myc and MTA1. Cancer. 2007;109(12):2420–31. doi: 10.1002/cncr.22725. [DOI] [PubMed] [Google Scholar]
- 19.Jackstadt R, Roh S, Neumann J, Jung P, Hoffmann R, Horst D, et al. AP4 is a mediator of epithelial-mesenchymal transition and metastasis in colorectal cancer. J Exp Med. 2013;210(7):1331–50. doi: 10.1084/jem.20120812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7(1):51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S, et al. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2. J Biol Chem. 2013;288(25):18121–33. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weeks BH, He W, Olson KL, Wang XJ. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001;61(20):7435–43. [PubMed] [Google Scholar]
- 23.Pines A, Kelstrup CD, Vrouwe MG, Puigvert JC, Typas D, Misovic B, et al. Global phosphoproteome profiling reveals unanticipated networks responsive to cisplatin treatment of embryonic stem cells. Mol Cell Biol. 2011;31(24):4964–77. doi: 10.1128/MCB.05258-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capurso G, Lattimore S, Crnogorac-Jurcevic T, Panzuto F, Milione M, Bhakta V, et al. Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer. 2006;13(2):541–58. doi: 10.1677/erc.1.01153. [DOI] [PubMed] [Google Scholar]
- 25.Leja J, Essaghir A, Essand M, Wester K, Oberg K, Totterman TH, et al. Novel markers for enterochromaffin cells and gastrointestinal neuroendocrine carcinomas. Mod Pathol. 2009;22(2):261–72. doi: 10.1038/modpathol.2008.174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Heat map of 40 genes identified as having serial differential expression. Increasing intensity of red demonstrates increasing magnitude of gene underexpression and increasing intensity of blue demonstrates increasing magnitude gene overexpression. Values are fold-changes for primary tumor and liver metastases in comparison to normal small bowel. SBNET = Primary tumor; SBLivMet = Liver metastasis.