Abstract
Background
Potassium iodide (KI) is often prescribed prior to thyroidectomy for Graves’ disease, but the effect of KI on the ease and safety of thyroidectomy for Graves’ is largely unknown.
Methods
We conducted a prospective cohort study of patients with Graves’ disease undergoing thyroidectomy. For the first 8-months no patients received KI; for the next 8-months, KI was added to the pre-operative protocol for all patients. Outcomes included operative difficulty (based on the Thyroidectomy Difficulty Scale) and complications.
Results
A total of 31 patients in the no-KI group and 28 in the KI group were included. According to the Thyroidectomy Difficulty Scale, gland vascularity decreased in the KI group (mean score 2.6 vs. 3.3, p=0.04), but there were no significant differences in thyroid friability, fibrosis, size, or overall difficulty (p=NS for all). Despite similar operative difficulty, patients prescribed KI were less likely to experience transient hypoparathyroidism (7.1% vs. 25.9%, p=0.018) and transient hoarseness (0% vs. 16.1%, p=0.009) compared with the no-KI group.
Conclusion
KI administration decreases gland vascularity but does not change the overall difficulty of thyroidectomy. However, KI was associated with less transient hypoparathyroidism and transient hoarseness, suggesting that KI improves the safety of thyroidectomy for Graves’ disease.
Keywords: Graves’ Disease, Thyroidectomy, Potassium Iodide, Patient Safety, Surgical Complications
Introduction
Graves’ disease is the most common cause of hyperthyroidism in adults, and treatment options include radioactive iodine ablation, anti-thyroidal medications, and surgery.1,2 Clinical factors often dictate the patients’ ultimate treatment choice, but surgery is becoming more common.3,4 Total thyroidectomy is now the preferred operation for those desiring surgery instead of subtotal thyroidectomy due to its improved efficacy without increased complications.5–10 Despite the increasing utilization of surgery and the refined approach with a greater focus on patient safety and operative outcomes, total thyroidectomy remains technically challenging in patients with hyperthyroidism.11
Administration of potassium iodide solution (KI) is recommended prior to thyroidectomy for Graves’ because it is thought to make surgery easier and safer.7,10,12 Historically, KI in combination with propranolol effectively controlled hyperthyroidism even in the absence of currently available anti-thyroidal medications.13 Now that newer anti-thyroidal medications are available, KI is rarely used as a means to control hyperthyroidism.14 Instead, KI is now administered because it is generally thought to decrease vascularity and make surgery easier. Recently, a randomized study demonstrated that KI causes a measurable decrease in gland vascularity after noticing a significant decrease in blood flow on Doppler ultrasonography from 138 ml/min before treatment to 75 ml/min after treatment.12 This trial as well as another reported 50 to 100 ml less operative blood loss for patients administered KI compared with controls which presumably improves visibility and facilitates surgical extirpation.12,15
Despite these findings, recent studies call into question the necessity of KI in preparing patients for surgery based on the absence of an observed increase in complications when KI is not used.16,17 Yet neither of these latter studies compared outcomes to a group of Graves’ patients prepared for surgery with KI.16,17 Ultimately, the impact of KI on the ease and safety of total thyroidectomy for Graves’ disease remains largely unknown. Therefore, we aimed to quantify the difficulty of total thyroidectomy for Graves’ disease based upon preoperative preparation with KI. Our secondary aim was to evaluate if KI affects the safety of thyroidectomy by comparing complications in this same population.
Methods
We performed a prospective cohort study of patients presenting for surgical treatment of Graves’ disease during the 16-month period from May 2015 through August 2016. We excluded any patients younger than 18 years of age or with a history of prior partial thyroidectomy. In an effort to streamline care protocols, all surgeons agreed to similar preparation strategies for patients with Graves’ disease. Per the clinical protocol, all patients were given 1000 mg calcium carbonate three times daily for 10 days prior to surgery and for 2 weeks following surgery.18 For the first 8 months, no patients were prescribed KI. During the more recent 8 months, KI was added to the preoperative preparation for all patients. Patients were instructed to place one drop (0.05 ml) of a 1 g per mL KI solution on their tongue three times daily for 10 days prior to surgery. All data were gathered prospectively and outcomes included operative difficulty and surgical complications. This study was in compliance with the Health Insurance Portability and Accountability Act and approved by the University of Wisconsin Institutional Review Board.
Total thyroidectomy was planned in all patients through a transverse cervical incision. The operating surgeon graded the difficulty of each thyroidectomy with the Thyroidectomy Difficulty Scale.19 With this scale, the operating surgeon grades gland vascularity, friability, mobility (fibrosis), and size on 5 point Likert scales with 1 representing the easiest thyroidectomy and 5 representing the most difficult. These grades are assigned at the conclusion of the case, and the sum of these 4 values is the overall thyroidectomy difficulty score.11,19 Additionally, this scale has an excellent inter-rater reliability and correlates with operative times and complications.19
Routine parathyroid hormone levels obtained in the recovery room defined transient hypoparathyroidism if less than 10 pg/mL. We also considered patients who required calcitriol or large amounts of calcium beyond what was prescribed to have transient hypoparathyroidism. If patients required calcitriol or more than 2 g of calcium daily to prevent symptoms of hypocalcemia 6 months after surgery, we considered their hypoparathyroidism permanent. Transient hoarseness was defined as significant voice changes at the 2-week post-operative appointment. Recurrent laryngeal nerve injuries were diagnosed based on clinical voice and swallowing symptoms often coupled with intraoperative nerve monitoring and laryngoscopy when appropriate. If patients had a loss of signal on a nerve monitor and voice changes they were classified as having a nerve injury. Not all patients with post-operative voice changes underwent laryngoscopy. Larngoscopy was generally obtained selectively based on symptom severity and time to resolution. Any persistent hoarseness after 6-months was considered permanent.
We compared patients in the first 8 months to patients from the last 8 months with an intention to treat analysis. The intention to treat analysis compared the practice of prescribing KI to not prescribing KI and indicated how a change in practice ultimately affects patient outcomes. We also compared patients who took KI as prescribed to those who did not take KI with a per protocol analysis. Descriptive statistics were reported as mean with standard deviation or as frequency with percent. Chi squared tests compared nominal variables, and Mann-Whitney U tests compared ordinal and continuous variables. P values 0.05 or less defined statistical significance. All analyses were performed using SPSS 23 (IBM, Chicago, IL).
Results
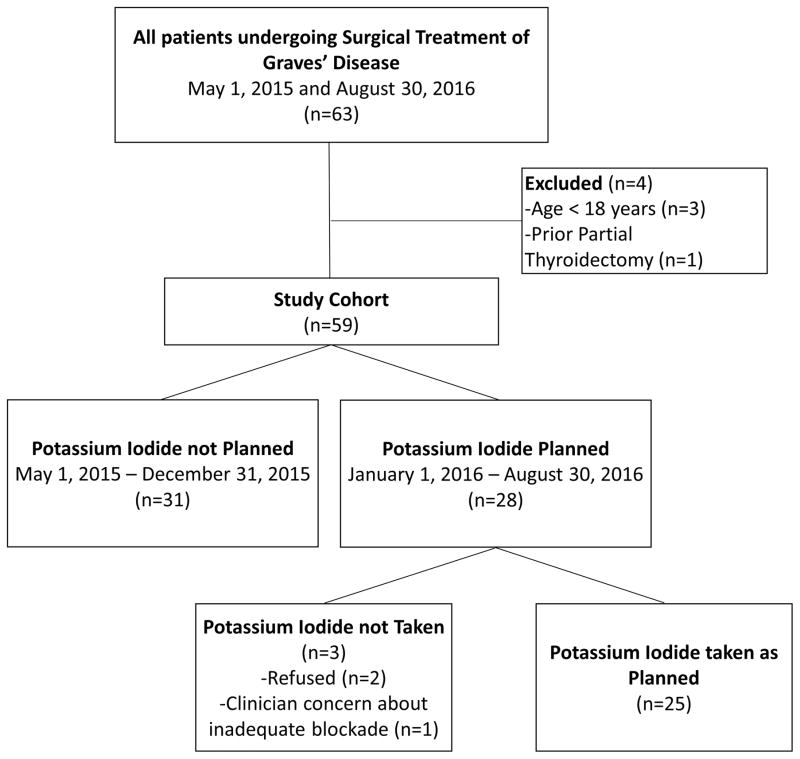
The final study cohort included 59 patients undergoing surgery for Graves’ disease with 31 (52.5%) in the group not given KI and 28 (47.5%) in the KI group (Figure 1). Of those in the KI group, 25 (89.3%) took the medication as directed. Patients in the no KI group were slightly older than those in the KI group (mean age 46.6 years versus 38.4 years, p = 0.016), but other demographics were similar between the two groups (Table 1). Patients a were equally likely to receive anti-thyroidal medication (83.9% in the no KI group versus 89.3% in the KI group, p = 0.54), and the degree of pre-operative blockade appeared similar based on patient hemodynamics (mean pulse 75 in the no KI group versus 77 in the KI group, p = 0.41) and free thyroxine levels (mean 1.3 in the no KI group versus 1.1 in the KI group, p = 0.40).
Figure 1.
Study cohort inclusion and exclusion flowchart. This diagram depicts how the final study cohort was derived.
Table 1.
Patient Demographics and Pre-Operative Management of Graves’ Disease
| Variable | KI not planned N=31 | KI planned N=28 | P |
|---|---|---|---|
| Age, mean ± SD, years | 46.6 ± 12.8 | 38.4 ± 14.1 | 0.02 |
| Sex, n (%) | 0.50 | ||
| Female | 22 (71.0) | 22 (78.6) | |
| Male | 9 (29.0) | 6 (21.4) | |
| Body Mass Index, mean ± SD | 32.6 ± 22.5 | 27.2 ± 5.0 | 0.42 |
| Smoker, n (%) | 6 (19.4) | 4 (14.3) | 0.60 |
| Prior treatment with radioactive iodine, n (%) | 1 (3.2) | 2 (7.1) | 0.49 |
| Anti-thyroidal Medication, n (%) | 0.71 | ||
| Methimazole | 24 (77.4) | 24 (85.7) | |
| Propylthiouracil | 2 (6.5) | 1 (3.6) | |
| None | 5 (16.1) | 3 (10.7) | |
| Pre-operative Beta-Blockade, n (%) | 12 (38.7) | 17 (60.7) | 0.09 |
| Pre-operative Pulse, mean ± SD | 75 ± 15 | 77 ± 13 | 0.41 |
| Pre-operative Systolic Blood Pressure, mean ± SD, mmHg | 122 ± 16 | 123 ± 16 | 0.85 |
| Pre-operative TSH, mean ± SD, uIU/mL | 0.49 ± 1.0 | 0.57 ± 1.1 | 0.33 |
| Pre-operative free T4, mean ± SD, ng/dL | 1.3 ± 0.6 | 1.1 ± 0.5 | 0.40 |
| Neve monitor, n (%) | 26 (83.9) | 28 (100) | 0.009 |
KI- Potassium Iodide, SD- standard deviation, TSH- Thyroid stimulating hormone, T4- thyroxine.
Thyroid Difficulty
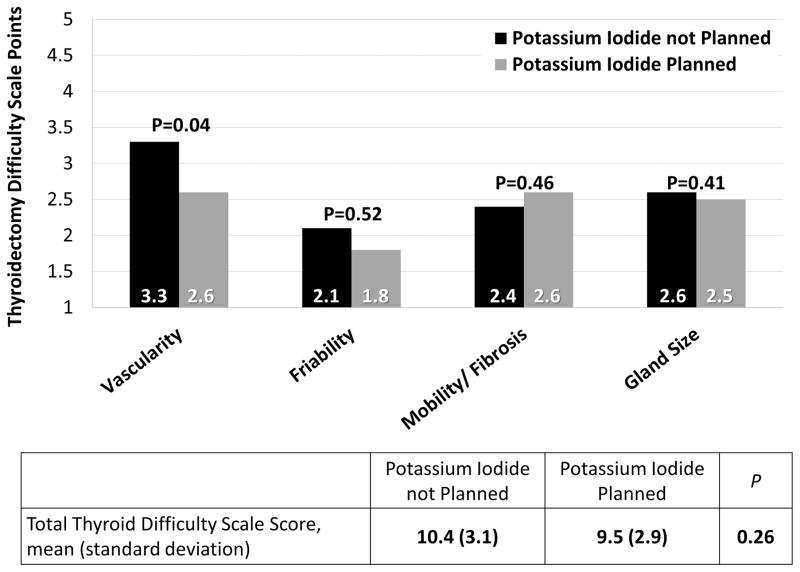
Operating surgeons calculated Thyroidectomy Difficulty Scale scores at the conclusion of the operation. Thyroid difficulty scores were available for 50 patients (25 in each group). Overall, the difficulty level of thyroidectomy was similar between groups (mean score 10.4 without KI vs. 9.5 with KI, p=0.26). Using a score of 10 on the total scale as a cut-off for a difficult thyroidectomy as previously described11, the proportion of difficult thyroidectomies in each group was not significantly different (56% in the no KI group versus 44% in the KI group, p=0.40). When breaking down the score into its component parts, the group not given KI demonstrated significantly higher gland vascularity (mean score 3.3 vs. 2.6, p=0.04), but there were no significant differences in mean scores for thyroid friability, mobility and fibrosis, or size (Figure 2).
Figure 2.
Thyroidectomy Difficulty Based on whether or not Potassium Iodide was planned. This intention to treat analysis graphically displays the mean scores assigned to thyroidectomies in the groups of patients based on whether or not potassium iodide was planned as a part of the preoperative preparation.
Surgical Complications
Even though thyroidectomy difficulty was similar, patients who did not receive pre-operative KI were more likely to experience transient complications compared with patients in the KI group (Table 2). Transient hypoparathyroidism was three times more likely to occur after thyroidectomy in patients not given KI (25.8% versus 7.1%, p = 0.049). Transient hoarseness persisting at least 2 weeks was also more common in the group not given KI (16.1%) compared with those in the KI group (0%) (p = 0.009). Of those who had intra-operative recurrent laryngeal nerve monitoring (n = 54), 19.2% (n = 5) of those in the no KI group had a signal loss compared with 3.6% (n = 1) of those in the KI group (p = 0.06). Only one definite nerve injury was identified in the no KI group (3.2%) and confirmed with laryngoscopy compared with none in the KI group (p = 0.25). No patients in either group experienced permanent hypoparathyroidism, permanent hoarseness, or a hematoma, and no patients required readmission. The patient with a nerve injury had complete resolution of vocal cord function which was also confirmed with laryngoscopy.
Table 2.
Operative Outcomes and Surgical Complications
| Variable | KI not planned N=31 | KI planned N=28 | P |
|---|---|---|---|
| Duration of Surgery, n (%), n=58 | 0.38 | ||
| < 1 hour | 1 (3.3) | 0 (0) | |
| 1–2 hours | 6 (20) | 10 (35.7) | |
| 2–3 hours | 19 (63.3) | 14 (50) | |
| > 3 hours | 4 (13.3) | 4 (14.3) | |
| PACU PTH, mean ± SD, pg/mL | 39 ± 30 | 43 ± 35 | 0.54 |
| 2 week Calcium, mean ± SD, mg/dL | 9.3 ± 0.5 | 9.1 ± 0.5 | 0.23 |
| 2 week PTH, mean ± SD, pg/mL | 41 ± 24 | 57 ± 33 | 0.06 |
| Thyroid Weight (g), mean ± SD | 30.0 ± 21.9 | 24.2 ± 10.0 | 0.33 |
| Transient hypoparathyroidism, n (%) | 8 (25.8) | 2 (7.1) | 0.049 |
| Transient hoarseness, n (%) | 5 (16.1) | 0 (0) | 0.009 |
| Nerve injury, n (%) | 1 (3.2) | 0 (0) | 0.25 |
| Emergency Department Visit, n (%) | 2 (6.5) | 0 (0) | 0.10 |
KI- Potassium Iodide, PACU- Post-Operative Anesthesia Care Unit, PTH- Parathyroid Hormone, SD- standard deviation.
Per Protocol Analysis
In general, per protocol analysis comparing patients who did not take KI to those who took KI as prescribed did not impact the difficulty or safety outcomes of thyroidectomy in these patients (Table 3). Of the 3 patients in the group prescribed KI who did not take it as planned, one patient had transient hypoparathyroidism, so the difference in the rate of transient hypoparathyroidism increased between patients who did not take KI (26.5%) compared with those who took KI as prescribed (4.0%) (p = 0.014), but this difference was already significant in the intention to treat analysis.
Table 3.
Per Protocol Analysis of Thyroidectomy Difficulty and Operative Outcomes
| Variable | KI not taken N=34 | KI taken N=25 | P |
|---|---|---|---|
| Thyroidectomy Difficulty | |||
| Vascularity, mean ± SD | 3.3 ± 1.0 | 2.4 ± 1.2 | 0.009 |
| Friability, mean ± SD | 2.2 ± 1.2 | 1.7 ± 0.9 | 0.16 |
| Mobility/Fibrosis, mean ± SD | 2.3 ± 1.1 | 2.7 ± 0.9 | 0.19 |
| Gland Size, mean ± SD | 2.6 ± 0.8 | 2.4 ± 0.7 | 0.23 |
| Total TDS, mean ± SD | 10.5 ± 3.0 | 9.2 ± 3.0 | 0.12 |
| Difficult Thyroidectomy, mean ± SD | 15 ± 55.6 | 10 ± 43.5 | 0.39 |
| Operative Outcomes | |||
| Duration of Surgery, n (%), n=58 | 0.39 | ||
| < 1 hour | 1 (3.0) | 0 (0) | |
| 1–2 hours | 7 (21.2) | 9 (36.0) | |
| 2–3 hours | 21 (63.6) | 12 (48.0) | |
| > 3 hours | 4 (12.1) | 4 (16.0) | |
| PACU PTH, mean ± SD, pg/mL | 37.8 ± 29.3 | 43.9 ± 36.4 | 0.54 |
| 2 week Calcium, mean ± SD, mg/dL | 9.3 ± 0.5 | 9.1 ± 0.5 | 0.15 |
| 2 week PTH, mean ± SD, pg/mL | 41.5 ± (26.6 | 56.9 ± 31.4 | 0.55 |
| Thyroid Weight (g), mean ± SD | 29.7 ± 21.4 | 23.8 ± 9.1 | 0.35 |
| Surgical Complications | |||
| Transient hypoparathyroidism, n (%) | 9 (26.5) | 1 (4.0) | 0.014 |
| Transient hoarseness, n (%) | 5 (14.7) | 0 (0) | 0.016 |
| Nerve injury, n (%) | 1 (2.9) | 0 (0) | 0.29 |
| Emergency Department Visit, n (%) | 2 (5.9) | 0 (0) | 0.13 |
KI- Potassium Iodide, TDS- Thyroidectomy Difficulty Scale, PACU- Post-Operative Anesthesia Care Unit, PTH- Parathyroid Hormone, SD- standard deviation.
Discussion
Although the necessity of KI in the preparation of Graves’ patients for thyroidectomy is debated, its impact on the ease and safety of surgery is not well documented. Prior studies are small or have suboptimal control groups. Therefore, we sought to compare the effects of KI on operative difficulty and surgical complications exclusively in patients with Graves’ disease at a high-volume center. We found that overall operative difficulty was not statistically greater in patients without KI, but these patients experienced higher transient complications.
We used an intention to treat analysis in order to compare the practice of routinely prescribing KI to the practice of never prescribing KI. Although there were 3 patients that did not take KI as planned, our approach provided a realistic picture of outcomes in a practice where KI is prescribed for all patients with Graves’ disease. To address the conservative bias inherent to intention to treat analyses, we also reported data from a per protocol analysis where we grouped the aforementioned 3 patients with the group that did not take KI. Overall, outcomes were similar with both analyses indicating that bias did not skew our results drastically.
The thyroidectomy difficulty scale has a high inter-rater correlation and also correlates well with operative times and surgical complications.19 Our data show that overall difficulty scores were not significantly different between patients prepared for surgery with and without KI. Even though patients without KI had only a 1-point greater mean difficulty, that small increase took the mean beyond the threshold of what is considered a difficult thyroidectomy based on the point at which operative times tended to be above the surgeon’s average time.11 This small shift resulted in a 10% higher chance of a difficult thyroidectomy in patients without KI. While these differences were not statistically significant, they do suggest that KI may be beneficial in facilitating an easier thyroidectomy. In a small randomized trial, subjective difficulty of thyroidectomy was not significantly less with KI although only one-third of the planned participants were accrued, and the trial may have been underpowered to detect a difference.15
Despite not being able to identify a significant overall improvement in the ease of thyroidectomy with KI, thyroid vascularity was significantly less in patients given KI. This finding is in agreement with prior reports. Erbil and colleagues12 demonstrated a significant decrease in blood flow on Doppler ultrasonography, intraoperative blood loss, and micro-vessel density on pathology in patients treated with KI preoperatively.12 Similarly, Whalen and colleagues15 also showed that KI decreased intraoperative blood loss during thyroidectomy.15 The current study, grading thyroid vascularity on a 5-point Likert scale, was able to translate what has been quantitatively measured to what is subjectively appreciated at the time of surgery. Yet the subjective decrease in gland vascularity may be counterbalanced by an increase in fibrosis (albeit non-significant) for patients who receive KI, which may partially explain why a significant increase in gland vascularity for patients who did not get KI did not result in a greater overall thyroidectomy difficulty score.
Even in the conservatively biased intention to treat analysis, KI was associated with significantly less transient hoarseness and hypoparathyroidism. Additionally, the only documented nerve injury occurred in a patient that did not receive KI. Conversely, Shinall and colleagues16 found that patients with Graves’ disease not prepared with KI had similar rates of transient nerve palsies and less transient hypoparathyroidism than patients with toxic multinodular goiters after thyroidectomy. They concluded that pre-operative KI was not necessary.16 In a separate study looking exclusively at patients with Graves’ disease, Shinall and colleagues17 found that noncompliance with American Thyroid Association recommendations for the preoperative preparation of patients with Graves’ disease (which includes KI administration) was not associated with increased complications but their study cohort only included 3 patients who received KI preparation17. Even though defined slightly differently in the studies by Shinall and colleagues, their observed rates of transient hypoparathyroidism (27–31%) were comparable to the group of patients not prepared with KI (26%) in the current study and substantially higher than the rate in the group that did receive KI (7%).16,17 These data suggest that the lack of a perceived benefit from KI administration in those studies is likely related to the limitations of the control groups rather than KI truly having no impact.
Although it stands to reason that increased frequency of transient complications would, on a larger scale, predict increased permanent complications, the current study did not record any permanent complications. Given the absence of permanent complications, even in the absence of KI administration, total thyroidectomy is a safe and effective treatment option for patients with Graves’ disease.
The current study does have limitations. Although a relatively large population of patients with Graves’ disease is represented, the size of our cohort prevented subgroup and multivariate analyses and places our results at risk for type II errors. Additionally, wide swings in proportions may have occurred with 1 or 2 events. Another limitation is the fact that surgeons were not blinded to the patients’ preoperative preparation. It is difficult to quantify bias that may have been incorporated into the thyroidectomy difficulty scale scores, although the general consensus in the group prior to this study was that KI administration probably did not matter and that its routine use was likely not necessary. In this regard, our findings are in contrast to what our surgeons expected based on their beliefs prior to the study, which actually strengthens the significance of our results. Lastly, this prospective study is not a randomized clinical trial. While a clinical trial could help decrease selection bias, patients in the current study either did or did not receive KI based on when they presented to clinic, not based on specific surgeon preferences or disease characteristics. Furthermore, other attempts at studying KI in a trial have been limited by the few participants enrolled which resulted in insufficient power to detect a difference in complications rates.12,15
In conclusion, preoperative KI administration noticeably decreases gland vascularity but did not affect the overall difficulty of thyroidectomy for patients with Graves’ disease. Total thyroidectomy is safe as a treatment for Graves’ disease given that permanent complications (hypoparathyroidism and hoarseness) were absent with or without KI. However, preparation with KI is associated with a lower rate of transient hoarseness and transient hypoparathyroidism, suggesting that including KI in preoperative protocols does confer a safety advantage.
Footnotes
Disclosure Information: Nothing to Disclose
To be Presented as a podium presentation at the annual meeting for the American Association of Endocrine Surgeons, April 2017 in Orland, FL
References
- 1.Streetman DD, Khanderia U. Diagnosis and treatment of Graves disease. The Annals of pharmacotherapy. 2003;37(7–8):1100–9. doi: 10.1345/aph.1C299. [DOI] [PubMed] [Google Scholar]
- 2.Burch HB, Cooper DS. Management of Graves Disease: A Review. Jama. 2015;314(23):2544–54. doi: 10.1001/jama.2015.16535. [DOI] [PubMed] [Google Scholar]
- 3.Elfenbein DM, Schneider DF, Havlena J, Chen H, Sippel RS. Clinical and socioeconomic factors influence treatment decisions in Graves’ disease. Annals of surgical oncology. 2015;22(4):1196–9. doi: 10.1245/s10434-014-4095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stalberg P, Svensson A, Hessman O, Akerstrom G, Hellman P. Surgical treatment of Graves’ disease: evidence-based approach. World journal of surgery. 2008;32(7):1269–77. doi: 10.1007/s00268-008-9497-9. [DOI] [PubMed] [Google Scholar]
- 5.Barakate MS, Agarwal G, Reeve TS, Barraclough B, Robinson B, Delbridge LW. Total thyroidectomy is now the preferred option for the surgical management of Graves’ disease. ANZ journal of surgery. 2002;72(5):321–4. doi: 10.1046/j.1445-2197.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- 6.Palit TK, Miller CC, 3rd, Miltenburg DM. The efficacy of thyroidectomy for Graves’ disease: A meta-analysis. The Journal of surgical research. 2000;90(2):161–5. doi: 10.1006/jsre.2000.5875. [DOI] [PubMed] [Google Scholar]
- 7.Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17(3):456–520. doi: 10.4158/ep.17.3.456. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZW, Masterson L, Fish B, Jani P, Chatterjee K. Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy. The Cochrane database of systematic reviews. 2015;(11):CD010576. doi: 10.1002/14651858.CD010576.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Bargren A, Schaefer S, Chen H, Sippel RS. Total thyroidectomy: a safe and effective treatment for Graves’ disease. The Journal of surgical research. 2011;168(1):1–4. doi: 10.1016/j.jss.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid: official journal of the American Thyroid Association. 2016;26(10):1343–421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 11.Mok VM, Oltmann SC, Chen H, Sippel RS, Schneider DF. Identifying predictors of a difficult thyroidectomy. The Journal of surgical research. 2014;190(1):157–63. doi: 10.1016/j.jss.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbil Y, Ozluk Y, Giris M, Salmaslioglu A, Issever H, Barbaros U, et al. Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves’ disease. The Journal of clinical endocrinology and metabolism. 2007;92(6):2182–9. doi: 10.1210/jc.2007-0229. [DOI] [PubMed] [Google Scholar]
- 13.Feek CM, Sawers JS, Irvine WJ, Beckett GJ, Ratcliffe WA, Toft AD. Combination of potassium iodide and propranolol in preparation of patients with Graves’ disease for thyroid surgery. The New England journal of medicine. 1980;302(16):883–5. doi: 10.1056/NEJM198004173021602. [DOI] [PubMed] [Google Scholar]
- 14.Jha CK, Bichoo RA, Yadav SK. Comment on article entitled “Randomized trial of a short course of preoperative potassium iodide in patients undergoing thyroidectomy for Graves’ disease”. American journal of surgery. 2016 doi: 10.1016/j.amjsurg.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Whalen G, Sullivan M, Maranda L, Quinlan R, Larkin A. Randomized trial of a short course of preoperative potassium iodide in patients undergoing thyroidectomy for Graves’ disease. American journal of surgery. 2016 doi: 10.1016/j.amjsurg.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Shinall MC, Jr, Broome JT, Baker A, Solorzano CC. Is potassium iodide solution necessary before total thyroidectomy for Graves disease? Annals of surgical oncology. 2013;20(9):2964–7. doi: 10.1245/s10434-013-3126-z. [DOI] [PubMed] [Google Scholar]
- 17.Shinall MC, Jr, Broome JT, Nookala R, Shinall JB, Kiernan C, Parks L, 3rd, et al. Total thyroidectomy for Graves’ disease: compliance with American Thyroid Association guidelines may not always be necessary. Surgery. 2013;154(5):1009–15. doi: 10.1016/j.surg.2013.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oltmann SC, Brekke AV, Schneider DF, Schaefer SC, Chen H, Sippel RS. Preventing postoperative hypocalcemia in patients with Graves disease: a prospective study. Annals of surgical oncology. 2015;22(3):952–8. doi: 10.1245/s10434-014-4077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider DF, Mazeh H, Oltmann SC, Chen H, Sippel RS. Novel thyroidectomy difficulty scale correlates with operative times. World journal of surgery. 2014;38(8):1984–9. doi: 10.1007/s00268-014-2489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]