Supplemental Digital Content is Available in the Text.
Inhibition of K2P potassium channels by pyrethroid insecticides contribute to activate primary sensory neurons to cause paraesthesias and painful sensations.
Keywords: Nociceptor, K2P channels, Paraesthesia, Background current, Membrane potential, Mechanical sensitivity, Insecticide
Abstract
Pyrethroid insecticides are widely used for pest control in agriculture or in human public health commonly as a topical treatment for scabies and head lice. Exposure to pyrethroids such as permethrin or tetramethrin (TM) causes sensory alterations such as transient pain, burning, stinging sensations, and paraesthesias. Despite the well-known effects of pyrethroids on sodium channels, actions on other channels that control sensory neuron excitability are less studied. Given the role of 2-pore domain potassium (K2P) channels in modulating sensory neuron excitability and firing, both in physiological and pathological conditions, we examined the effect of pyrethroids on K2P channels mainly expressed in sensory neurons. Through electrophysiological and calcium imaging experiments, we show that a high percentage of TM-responding neurons were nociceptors, which were also activated by TRPA1 and/or TRPV1 agonists. This pyrethroid also activated and enhanced the excitability of peripheral saphenous nerve fibers. Pyrethroids produced a significant inhibition of native TRESK, TRAAK, TREK-1, and TREK-2 currents. Similar effects were found in transfected HEK293 cells. At the behavioral level, intradermal TM injection in the mouse paw produced nocifensive responses and caused mechanical allodynia, demonstrating that the effects seen on nociceptors in culture lead to pain-associated behaviors in vivo. In TRESK knockout mice, pain-associated behaviors elicited by TM were enhanced, providing further evidence for a role of this channel in preventing excessive neuronal activation. Our results indicate that inhibition of K2P channels facilitates sensory neuron activation and increases their excitability. These effects contribute to the generation of paraesthesias and pain after pyrethroid exposure.
1. Introduction
Pyrethroid insecticides have been highly used since the 1970s for domestic pest control and in public health, commonly as topical treatments for scabies and head lice. Their use is still very important for control of mosquito populations and malaria spread despite the increasing incidence of resistance.23 They are more than 2000 times more toxic to insects than to humans because of insects' increased sodium channel sensitivity, lower body temperature, and smaller size.42 High doses of pyrethroids can cause paralysis, ataxia, convulsions, hypersensitivity, choreoathetosis, tremor, and in extreme cases, death by provoking neuronal hyperexcitability.6,37,47,48 More commonly, local effects are seen after skin contact or ingestion. Tactile, painful, and paraesthetic effects including tingling, itching, burning, and stinging sensations of the skin have been associated with repetitive activity in skin nerve terminals because of the known effects of pyrethroids on Na+ channels,41,43 where a slowing of the kinetics of channel opening and closing results in delayed and prolonged openings. These effects diminish action potential threshold and cause repetitive firing of sensory neurons, which is thought to underlie the reported abnormal sensations. At higher doses, the effects on sodium tail currents are large enough to prevent the generation of further action potentials, thus producing a conduction block.6,29,30 Inhibitory effects on chloride, calcium, and GABA-induced chloride currents have also been described.6,30,37
Direct effects of permethrin (PM), deltamethrin (DM), and tetramethrin (TM) have been partially reported in neurons but they were mainly attributed to modulation of Na+ channels, where they increased the duration of action potentials and produced spontaneous discharges.21,43 Similar effects of pyrethroids across disparate types of nociceptors with quite different complements of Nav channels (sensitive or resistant to tetrodotoxin) suggest that Nav channels are not solely responsible for nociceptor activation at body temperature.21,43
Two-pore domain potassium channels (K2P) carry most of the “leak” current and are expressed in different subpopulations of sensory neurons, including nociceptors. Their electrophysiological properties allow them to carry K+ currents over a wide range of membrane potentials and as such they are key determinants of neuronal excitability, contributing to the likeliness of depolarizing stimuli to achieve action potential threshold, as well as shaping the neuron firing response.18,35 TREK-1, TREK-2, TRAAK, and TRESK make a major contribution to leak currents in trigeminal and dorsal root ganglion (DRG) neurons,15,22,45,50 where they have been implicated in pain induced by mechanical, thermal and chemical stimuli, perception of warmth and cold, and neuropathic and inflammatory pain.1,3,28,31,36,45
Because of their important role in setting neuronal excitability, we studied whether K2P channels are implicated in the excitation of sensory neurons by pyrethroids. Here, we report that pyrethroids inhibit K2P channels, activate sensory neurons and peripheral nerve fibers, producing aversive behavioral responses in mice. Our results reveal a new target of pyrethroid action, suggesting that their K2P channel blocking effects contribute to the mechanisms underlying a persistent activation of sensory neurons that can lead to painful and paraesthetic sensations.
2. Methods
2.1. Animal behavior
2.1.1. Animals
All behavioral and experimental procedures were performed in accordance with the recommendations of the International Association for the Study of Pain (IASP) and were reviewed and approved by the Animal Care Committee of the University of Barcelona and by the Department of the Environment of the Generalitat de Catalunya, Catalonia, Spain (Ref. 8468, 8548, 6595). Male C57BL/6N mice between 8 and 15 weeks old were used in all experimental procedures (cellular cultures, electrophysiology, calcium imaging, and behavior), except for single nerve fiber recordings, where CD1 mice were used due to higher availability. Mice were housed at 22°C with free access to food and water in an alternating 12-hour light and dark cycle. Animals were housed individually for all behavioral assays. Behavioral measurements were taken in a quiet room, taking great care to minimize or avoid discomfort of the animals. TRESK (Kcnk18) knockout (KO) mice and wild-type (WT) littermates were obtained from the KOMP Repository (Mouse Biology Program, University of California, Davis, CA). The TRESK KO mouse was generated by replacing the complete Kcnk18 gene by a ZEN-UB1 cassette according to the VelociGene's KOMP Definitive Null Allele Design.
At 3 weeks of age, WT or KO newborn mice were weaned, separated, and identified by ear punching. Genomic DNA was isolated from tail snip samples with Maxwell Mouse Tail DNA Purification Kit (Promega, Madison, WI). Polymerase chain reaction (PCR) was performed with primers to detect the Kcnk18 gene: forward primer (TUF) 5- GAGGAGAACCCTGAGTTGAAGAAG -3 and reverse primer (TUR) 5- GCACCTCCGAGGCAGTAAC -3 or the inserted cassette in the KO mice: forward primer (REG-Neo-F) 5- GCAGCCTCTGTTCCACATACACTTCA and reverse primer (gene-specific) 5- AGACTTCTCCCAGGTAACAACTCTGC -3. The PCR mixture contained 1-μL DNA sample, 2.5-μL PCR buffer (10× concentration), 2-μL dNTP mixture (2.5 mM), 0.5-μL (20 μM) forward and reverse primers, 0.2-μL Taq DNA polymerase (5 U/μL), 1.7-μL MgCl2 (25 mM), 6.5-μL betaine (5 M), 0.325-μL dimethyl sulfoxide [DMSO], and 9.7-μL water. Polymerase chain reaction amplifications were performed with 31 cycles in a programmable thermal cycler (Eppendorf AG, Hamburg, Germany). The program used was 94°C for 5 minutes and cycles of 94°C for 15 seconds, 60°C for 30 seconds, 72°C for 40 seconds, and with a final extension at 72°C for 5 minutes. Polymerase chain reaction products were analyzed by electrophoresis on 1% agarose gels.
2.1.2. Evaluation of nocifensive behavior
Using a 30-g needle, 10 μL of a solution containing 100-μM TM or vehicle (control; DMSO 0.1% in phosphate buffered saline [PBS]) was administered intradermally in the plantar surface of the hind paw of 2 independent groups of mice for each genotype. Mice behavior was observed and the number of flinches and lickings of the paw were counted for a 15-minute period starting immediately after the injection. On the day previous to testing, animals were habituated to the testing room and to the handling procedure. The experimenter and the person analyzing data were blind to the mice genotype. After evaluation of nocifensive behavior, mechanical and thermal sensitivity of each mouse was assessed as detailed below.
2.1.3. Mouse mechanical sensitivity
To assess mechanical sensitivity, the withdrawal threshold to punctate mechanical stimuli of the hind paw was determined 30 minutes before and 15 minutes after TM injection in the hind paw (as previously described) by the application of calibrated von Frey filaments (North Coast Medical, Inc, Morgan Hill, CA). The von Frey filaments (size: 2.44, 2.83, 3.22, 3.61, 3.84, 4.08, 4.17, 4.31, and 4.56; equivalent to [in grams] 0.04, 0.07, 0.16, 0.4, 0.6, 1, 1.4, 2, and 4, respectively) were applied perpendicular to the plantar surface of the hind paw and gently pushed to the bending point for 5 seconds. The 50% withdrawal threshold was determined using the up-down method as previously described.11 A brisk hind paw lift in response to von Frey filament stimulation was regarded as a withdrawal response.
2.1.4. Mouse thermal sensitivity
The heat sensitivity of mice was assessed by measuring hind paw withdrawal latency from a radiant infrared source (Hargreaves' Method) using the Ugo Basile (Italy) Model 37370 Plantar test. Mice were acclimatized to the testing room for at least 30 minutes and each measurement was the mean of 3 trials. Withdrawal latency was measured 30 minutes before injecting TM in the hind paw and 15 minutes after injection.
2.1.5. Blinking test
To assess the mouse nocifensive response to noxious stimulation, 5 μL of vehicle in saline solution or the drug of interest were topically applied to the anterior ocular surface of an eye. Mice were lightly restrained (by grasping the scruff between the thumb and forefinger), so that the solution remained on the corneal surface and the number of blinks (complete lid closure) counted over a 2-minute period. After the observation time, the ocular surface was abundantly washed with PBS solution, and mice were returned to their home cages.
2.2. Single nerve fiber recording
2.2.1. Animals
Adult outbred CD1 female mice (n = 18, mean body weight 32 ± 1.8 g) bred at the University Animal House were used (obtained from Harlan, Spain). European Union and State legislation for the regulation of animal experiments was followed. All experimental protocols were approved by the University of Alcala Committee on Animal Research and the Regional Government (project license: ES280050001165).
2.2.2. Surgical extraction and maintenance of the skin flap
Mice were killed by cervical dislocation, and the saphenous nerve together with the skin flap was excised and pinned down to a Sylgard based recording chamber, corium side up. The preparations were superfused with oxygenated synthetic interstitial fluid (SIF, composition [in mM]: 108 NaCl, 3.48 KCl, 0.7 MgSO4, 26 NaHCO3, 1.7 NaH2PO4, 1.53 CaCl2, 9.6 sodium gluconate, 5.55 glucose, and 7.6 sucrose) at a rate of 5 mL per minute. Recording temperature was monitored and maintained at 32°C ± 1°C by means of a Peltier device (Warner Instruments, Hamdem, CT). A second preparation from the same animal was stored in oxygenated SIF at 4°C and used if required, on the same day. There were no detectable differences in results between the first and second preparations studied.
2.2.3. Electrical recording and stimulation
The electrical activity from sensory fibers was recorded by means of suction microelectrodes obtained from glass pipettes (20 to 30 μm external tip diameter) filled with SIF as previously described.38 The microelectrode tip was carefully placed in contact with the proximal end of the nerve trunk under visual guidance by using a micromanipulator and negative pressure was applied to isolate thin nerve filaments. Controlled electrical pulses of supramaximal intensity for C-fibers (0.5 ms pulse width, maximum strength up to 10 mA) were delivered to the nerve trunk via a thin bipolar tungsten electrode (WPI, World Precision Instruments, Sarasota, FL) to identify the fibers present in the filament on the basis of their conduction velocity (CV). The experiments only proceeded when a single fiber was recorded or at least could be clearly differentiated from background discharges and other units on the basis of spike amplitude and shape. The presence of mechanosensitivity was explored by gently touching the skin-flap with a smooth-tipped glass rod (diameter = 0.5 mm); on location of the receptive field (RF), mechanical thresholds were established by means of gravity-driven von Frey hairs as described in detail.51,52
Electrical signals were recorded by means of a Dagan EX4-400 amplifier (Dagan, Minneapolis, MS), digitized at 20 KHz (Power 1401, CED) and stored for offline analysis. Fibers were classified according to their CV into A-units (CV >1 m/s) or C-units (CV <0.8 m/s) on the basis of pilot experiments using whole nerve recordings, which showed a CV of ∼0.8 m/s for the C-fiber volley. After electrical and mechanical characterization, the RF of the fiber under study was isolated by means of a hollow steel cylinder (internal diameter ∼6 mm) placed on top of the skin and medical grease (WPI, Germany) was added to improve isolation. To reproduce mechanical stimulation in both intensity and location, the von Frey probes were mounted on a micromanipulator and moved down until the probe tip (0.6 mm diameter) contacted perpendicularly the most sensitive spot of the RF. The following probe above threshold intensity was used during the experimental protocol, in which stimuli (∼5 seconds duration) were applied at ∼5-minute intervals.
Tetramethrin was dissolved in DMSO at 10 mM and stored at 4°C. Tetramethrin was diluted in SIF to final concentrations of 25, 50, 100, or 200 μM immediately before use. Tetramethrin (∼300 μL) was directly applied inside the cylinder and maintained for ∼10 minutes and the mechanical responsiveness was tested at least twice. A change in baseline firing was taken as an indication of excitatory response. For mechanoinsensitive fibers, electrical stimulation was applied to assess for the presence of the fiber. The magnitude of the mechanical response was quantified as the total number of spikes counted during the 5-second stimulation. Mean values during control and those obtained during exposures to each TM concentration were used for analysis and graphical representations. The maximum discharge frequency during the stimuli was also noted.
Waveforms were analyzed off-line with Spike 2 software (CED, Cambridge Electronic Design, Cambridge, United Kingdom). Autocorrelograms were performed for each of the recorded fibers to evaluate the success of the spike sorting. Statistical analyses were performed in Graphpad Prism 5 (GraphPad Software, San Diego, CA) on the raw data using Wilcoxon matched pairs test. The level of statistical significance was set at P < 0.05. Values are quoted as mean ± SEM.
2.3. Culture of dorsal root ganglion neurons
Mice were euthanized by decapitation under anesthesia (isoflurane) and thoracic, lumbar, and cervical DRG were removed for neuronal culture as previously described.9,45 Briefly, DRG were collected and maintained in cold (4°C-5°C) Ca2+- and Mg2+-free PBS solution (Sigma) supplemented with 10-mM glucose, 10-mM Hepes, 100-U.I./mL penicillin, and 100-μg/mL streptomycin until dissociation. Subsequently, ganglia were incubated with collagenase CLS I (1 mg/mL; Biochrome AG, Berlin, Germany) for 1 hour 45 minutes followed by 15-minute trypsin treatment (0.25%; Sigma) before mechanical dissociation with fire-polished Pasteur pipettes of decreasing diameter.
Neurons found in the pellet were suspended in 2-mL Dulbecco's Modified Eagle's medium [DMEM] + 10% fetal bovine serum (FBS; Sigma), centrifuged at 1000 rpm for 5 minutes and resuspended in the culture medium (DMEM + 10% FBS, 100-μg/mL penicillin/streptomycin, and 100-mg/mL L-glutamine). Cell suspensions were transferred to poly-L-lysine/laminin–coated 12-mm diameter glass coverslips and incubated at 37°C in humidified 5% CO2 for up to 1 day before being used for patch-clamp electrophysiological recordings or calcium imaging experiments. Nerve growth factor or other growth factors were not added.
2.4. Calcium imaging
Dorsal root ganglion neurons obtained as described above were loaded with 5-μM fura-2/AM (Calbiochem, San Diego, CA) for 45 to 60 minutes at 37°C in culture medium. Coverslips with fura-2 loaded cells were transferred into an open flow chamber (0.5 mL) mounted on the stage of an inverted Olympus IX70 microscope equipped with a TILL monochromator as a source of illumination. Pictures were taken with an attached cooled CCD camera (Orca II-ER, Hamamatsu Photonics, Shizuoka, Japan) and were digitized, stored, and analyzed on a PC computer using Aquacosmos software (Hamamatsu Photonics). After a stabilization period, image pairs were obtained alternately every 4 seconds at excitation wavelengths of 340 (λ1) and 380 nm (λ2; 10 nm bandwidth filters) to excite the Ca2+ bound and Ca2+ free forms of this ratiometric dye, respectively. The emission wavelength was 510 nm (120-nm bandwidth filter). Typically, 20 to 40 cells were present in a field and [Ca2+]i values were calculated and analyzed individually for each single cell from the 340- to 380-nm fluorescence ratios at each time point. Only neurons that produced a response >10% of the baseline value and that, at the end of the experiment, produced a Ca2+ response to KCl-induced depolarization (50 mM) were included in the analysis. Several experiments with cells from different primary cultures were used in all the groups assayed. The extracellular (bath) solution used was 140-mM NaCl, 4.3-mM KCl, 1.3-mM CaCl2, 1-mM MgCl2, 10-mM glucose, and 10-mM HEPES, at pH 7.4 with NaOH. In extracellular Ca2+-free experiments, the bath solution used was 145-mM NaCl, 5-mM KCl, 3-mM MgCl2, 10-mM glucose, 1-mM EGTA, and 20-mM HEPES, at pH 7.4 with NaOH (nominally Ca2+ free, estimated <10 nM).
2.5. Electrophysiological recording
2.5.1. Recordings in sensory neurons
Electrophysiological recordings were performed as previously described.8,9 Briefly, recordings were performed with a patch-clamp amplifier (Axopatch 200B; Molecular Devices, Union City, CA) and restricted to small and medium DRG neurons (<30-μm diameter; <45 pF), which largely correspond to nociceptive neurons.27 Patch electrodes were fabricated in a Flaming/Brown micropipette puller P-97 (Sutter instruments, Novato, CA). Electrodes had a resistance between 2 and 4 MΩ when filled with intracellular solution (in mM): 140 KCl, 2.1 CaCl2, 2.5 MgCl2, 5 EGTA, 10 HEPES, and 2 ATP at pH 7.3. Bath solution (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 5 glucose at pH 7.4. The osmolality of the isotonic solution was 310.6 ± 1.8 mOsm/Kg. In experiments using extracellular high K+, the solution used was (in mM) 15 NaCl, 135 KCl, 2 CaCl2, 2 MgCl2, and 10 HEPES at pH 7.4. Membrane currents were recorded in the whole-cell patch clamp configuration, filtered at 2 kHz, digitized at 10 kHz, and acquired with pClamp 10 software. Data were analyzed with Clampfit 10 (Molecular Devices) and Prism 5 (GraphPad Software, Inc, La Jolla, CA). Series resistance was always kept below 15 MΩ and compensated at 70% to 80%. All recordings were performed at room temperature (22°C-23°C), 18 to 24 hours after dissociation. To study the effects of pyrethroids on sensory neuron excitability, after achieving the whole-cell configuration in the patch clamp technique, the amplifier was switched to current-clamp bridge mode. Only neurons with a resting membrane voltage below −50 mV were considered for the study.
2.5.2. Electrophysiology in transfected cells
HEK293T cells, cultured in DMEM supplemented with 10% FBS, 100-μg/mL penicillin/streptomycin, and 100-mg/mL L-glutamine at 37°C in humidified 5% CO2, were seeded on 12-mm diameter poly-L-lysine coated glass coverslips 24 hours before transfection. Cells were transiently transfected using X-tremeGENE 9 transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. Rat TRESK (kindly provided by S. Yost, University of California-San Francisco) and Mouse TRESK (kindly provided by P. Enyedi, Semmelweis University, Budapest, Hungary) and Human TRESK (kindly provided by Y. Sano, Astellas Pharma, Japan) were subcloned into the pIRES2-EGFP vector (Clontech) to form the rTRESK-pIRES2-GFP, mTRESK-pIRES2-GFP, and hTRESK-pIRES2-GFP constructs, respectively. The vectors pCD8-hTREK-2 and pCD8-mTRAAK (kindly provided by F. Lesage, Institut de Pharmacologie Moléculaire et Cellulaire-CNRS, Valbonne, France) were cotransfected with pEGFP-C3 vector to allow identification of transfected cells. hTREK-1-pIRES-EGFP was kindly provided by S. Bhattacharya (Bascom Palmer Eye Institute, University of Miami). Cells were used for electrophysiological recordings 24 to 48 hours after transfection. Patch clamp recordings were performed as described for sensory neurons using identical solutions.
2.6. Drugs
Tetramethrin, DM, and PM were obtained from Sigma-Aldrich (Madrid, Spain) and initially diluted in DMSO at 100 mM. For in vitro and in vivo experiments, the stock solution prepared in a glass container was subsequently diluted in the appropriate medium and used at the stated concentrations (0.2 to 100 μM). The final concentration of DMSO was 0.1% or below. Pyrethroids were added to the recording chamber with a pipette to avoid possible sticking of these highly lipophilic compounds to the plastic tubing. Menthol (100 μM), allyl isothiocyanate (AITC; 100 μM) and capsaicin (1 μM), tetraethylammonium (TEA; 1 mM), 4-aminopyridine (4-AP; 1 mM), CdCl2 (100 μM) and CsCl (1 mM), iberiotoxin (50 nM), arachidonic acid (10 μM), cloxyquin (100 μM), thapsigargin (1 μM), and A-803467 (1 μM) were also purchased from Sigma (Madrid, Spain). Tetrodotoxin (1 or 7 μM) was obtained from Alomone Labs (Jerusalem, Israel).
2.7. Data analysis
Data are presented as mean ± SEM. Statistical differences between different sets of data were assessed by performing paired or unpaired Student t tests or Wilcoxon matched pairs test, as indicated, using Prism 5 (GraphPad Software, Inc) data analysis software. The significance level was set at P < 0.05 in all statistical analyses.
3. Results
3.1. Tetramethrin-activated sensory neurons are mainly nociceptors
Sensory alterations produced by pyrethroids require the activation of sensory nerve endings in the skin or viscera. To study the response of sensory neurons to pyrethroid exposure, we used intracellular calcium imaging in DRG sensory neurons labeled with Fura-2 calcium dye that permits the analysis of larger cell populations. Tetramethrin (20 μM) application increased intracellular Ca2+ in 124 DRG neurons of 265 (46.8% response; Figs. 1A–C) with a mean Ca2+ increase of 1.60 ± 0.04-fold (ratio F340/F380). Interestingly, in most responding neurons (64.5%), the Ca2+ response followed an oscillatory pattern (Figs. 1A and B), whereas in 35.5% of the cells, TM produced a single Ca2+ peak. This oscillatory pattern was never observed when the same cells were stimulated with well-known agonists of different TRP channels present in cold (menthol, a TRPM8 agonist) or nociceptive sensory neurons (AITC and Capsaicin, agonists of TRPA1 and TRPV1, respectively). As shown in Figure 1C, TM-responding neurons had a significantly smaller diameter (18.1 ± 0.4 μm; range 8.3-34.8; Fig. 1C; n = 124) than nonresponding cells (19.9 ± 0.6 μm; range 8.3-44.0; P < 0.05). In addition, a high percentage of TM-responding cells were also positive for TRPA1 (62.9% also responded to AITC) or for TRPV1 (45.2% also responded to capsaicin), whereas TRPM8-positive TM responders formed a minor population (12.1% responded to menthol but not to AITC) (Fig. 1D). All together, these results (presence of TRPA1/V1 and small cell size) indicate that many of the cells stimulated by pyrethroids were nociceptors. Because the Ca2+ increase triggered by pyrethroids can be due to an entry of extracellular Ca2+, a release from intracellular stores or both, we performed experiments in the absence of extracellular Ca2+ (nominally Ca2+-free solution) to study the origin of the Ca2+ peak. In Ca2+-free conditions, none of 126 neurons studied produced any response after application of TM (20 μM; supplemental Fig. 1A, available at http://links.lww.com/PAIN/A485). Reintroduction of extracellular Ca2+ produced an increase in cytosolic Ca2+ in 70 of 126 neurons (55.6%), indicating that an entry of extracellular Ca2+ is mediating the effects of pyrethroids. Also, in the presence of thapsigargin (1 μM) to empty the intracellular calcium stores, the effect of TM was still present in a similar percentage of neurons (50.8%; supplemental Fig. 1B, C, available at http://links.lww.com/PAIN/A485).
Figure 1.
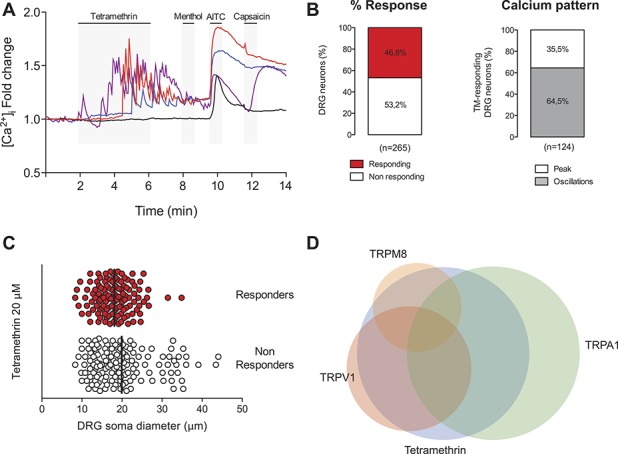
Tetramethrin (TM) excites a subset of sensory neurons with characteristics of nociceptors. (A) Wild-type mouse DRG sensory neurons were exposed to TM (20 μM), the TRPM8 agonist menthol (100 μM), the TRPA1 agonist allyl isothiocyanate (AITC, 100 μM), and the TRPV1 agonist capsaicin (1 μM), and intracellular calcium was measured by Fura-2 ratiometric imaging. A series of representative traces are shown. Tetramethrin induced a characteristic oscillatory calcium pattern in a large percentage of cells (red, purple, and blue traces). Calcium responses show that some cells are only activated by TM, whereas others respond also to agonists of TRPA1 or TRPV1. A non-TM responsive neuron that responds to AITC is shown in the black trace. (B) Left: quantification of the percentage of neurons responding to TM. Right: percentage of TM-responding cells showing an oscillatory calcium pattern or responding with a single calcium peak. (C) Soma diameter distribution of DRG neurons sensitive and insensitive to TM. Black bars show the mean soma value in each group. (D) Venn diagram showing the relative size and overlap between the population of TM-activated neurons and those activated by menthol, AITC, or capsaicin. DRG, dorsal root ganglion.
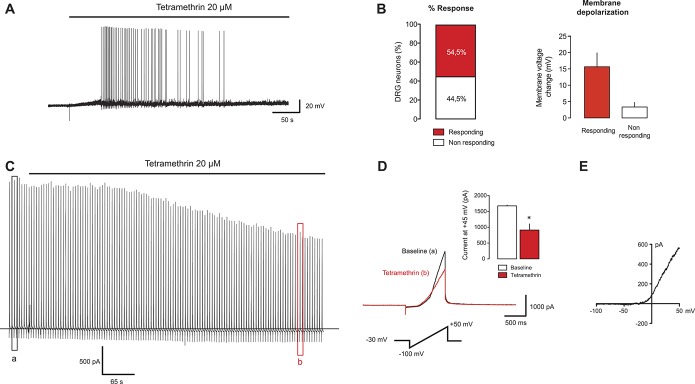
The effects of pyrethroids on sensory neuron excitability are usually attributed to effects on voltage-gated Na+ (Nav) channels (increased action potential duration and depolarizing after potentials), but less is known about their effects on other conductances that control resting membrane potential and neuronal excitability. For this matter, we tested whether small- and medium-sized DRG neurons in culture (which are mainly nociceptors) were depolarized and fired action potentials when challenged with TM, a type I pyrethroid. Twenty-two DRG neurons with a mean capacitance of 22.7 ± 2.6 pF and a resting membrane potential of −54.9 ± 1.1 mV were recorded using the current clamp mode of the patch-clamp technique. Twelve of these neurons (∼54.5% response) were depolarized by 15.7 ± 4.2 mV and fired action potentials (34.7 ± 12.7 spikes) in response to TM (20 μM, Figs. 2A and B), whereas 10 neurons showed a small depolarization (3.7 ± 1.4 mV) that did not reach action potential threshold (Fig. 2B). To examine the possible involvement of K+ currents active at the subthreshold range of membrane potentials, we recorded the effect of TM on membrane current in the presence of a general Na+, K+, and Ca2+ channel blocker cocktail (TTX, TEA, 4-AP, Cs+, and Cd2+) at a slightly depolarized holding potential (−30 mV). Under these conditions, TTX-sensitive Nav, Kv, KCa, and Cav channels should make a minimal contribution and noninactivating K+ currents will be more activated, allowing assessment of the contribution of other channels (including K2P channels) to the TM response. After TM application, a negative shift in the holding current was observed (ΔIhold = −29.9 ± 11.1 pA; n = 6), suggesting a possible inhibition of active resting conductances (Fig. 2C). Tetramethrin also significantly reduced whole-cell currents elicited by depolarizing ramps in the presence of the channel blocker cocktail (Figs. 2C–E). The reversal potential of the depolarization-activated current was −45.9 ± 5.1 mV, indicating that K+ is not the only contributor to this current and TTX-resistant persistent Na+ current and/or Cl− currents may make some contribution. In another set of experiments using the same experimental protocol, to exclude possible contributions of Nav channel to the effect produced by TM, we included a high concentration of TTX (7 μM) and the specific Nav1.8 blocker A-803467 (1 μM) to minimize sodium currents. In these conditions, similar inhibitory effects were found after TM application (−76.5%; n = 5; P < 0.001; not shown) on the total current elicited by a depolarizing ramp and measured at +45 mV (as in Fig. 2D).
Figure 2.
Tetramethrin (TM) activates sensory neurons. (A) Current-clamp recording on a DRG neuron activated by TM, a type I pyrethroid. Drug application depolarizes the cell sufficiently to trigger firing. (B) Quantification of the percentage of response (n = 22) and the amount of membrane depolarization induced by TM in neurons that fired action potentials (responding) or nonresponders. (C) Example of a membrane current recording in the presence of Na+, K+, and Ca2+ channel blockers (TTX, TEA, 4-AP, Cs+, and Cd2+) at a holding voltage of −30 mV. Depolarizing ramps from −100 to +50 mV were applied to monitor the effects of TM in the whole cell current. (D) Left: detail of depolarizing ramps before (a) and during (b) TM application as indicated on the recording shown in (C). Inset: quantification of the current inhibited by TM application (n = 6). Data are expressed as mean ± SEM. *P < 0.05; Student t test vs baseline. (E) Mean current subtracted (baseline current − current with TM) obtained from experiments as in (D). DRG, dorsal root ganglion.
3.2. Pyrethroids inhibit K2P channels
Of the different ion channels that are thought to participate in setting the membrane potential and regulating it in the subthreshold range, 4-AP-sensitive Kv, slowly-activating M channels (Kv7 and KCNQ), the Nav1.9, and “leak” K2P channels have been described as having the most significant influence.16 Within the K2P family, TREK-1, TREK-2, TRAAK, and TRESK are proposed to play a significant role in sensory neurons, especially those of small and medium size.15,22,45 To further analyze the effects of pyrethroids in the K2P channels expressed in native neurons, we searched for small/medium-sized neurons, where arachidonic acid (a known activator of TREK-1, TREK-2, and TRAAK19,26,35) or cloxyquin (a TRESK-activating compound24,49) increased the current measured at hyperpolarized voltages in high extracellular K+ solution. In these conditions, background potassium channels activated by each compound should carry the majority of the current measured at hyperpolarized potentials. Voltage ramps from −100 to +50 mV were first recorded in physiological solution and then the extracellular bath was replaced by high K+ solution (near symmetrical K+ concentration). Under these conditions, voltage ramps had a reversal potential near 0 mV, indicating that K+ was the major carrier of the current. As depicted in Figure 3A, addition of 10-μM arachidonic acid produced a 43% increase in the current measured at −95 mV in 5 of 9 neurons recorded (responding neurons: from −11.5 ± 3.6 to −16.1 ± 4.8 pA/pF; P < 0.05). When 20-μM TM was added (in the presence of arachidonic acid), a significant inhibition was observed (39 ± 6%; from −16.1 ± 4.8 to −10.7 ± 3.9 pA/pF; P < 0.05). In parallel experiments, 100-μM cloxyquin produced a significant current increase of 414% at −95 mV in 6 of 8 neurons recorded (from −12.2 ± 3.5 to −38.1 ± 6.6 pA/pF; P < 0.01: Fig. 3B). Again, addition of TM (in the presence of cloxyquin) significantly inhibited the current by 42% (from −38.1 ± 6.6 to −20.1 ± 4.6 pA/pF; P < 0.05). These results indicate that native currents mediated by background K+ channels were significantly affected by pyrethroid exposure.
Figure 3.
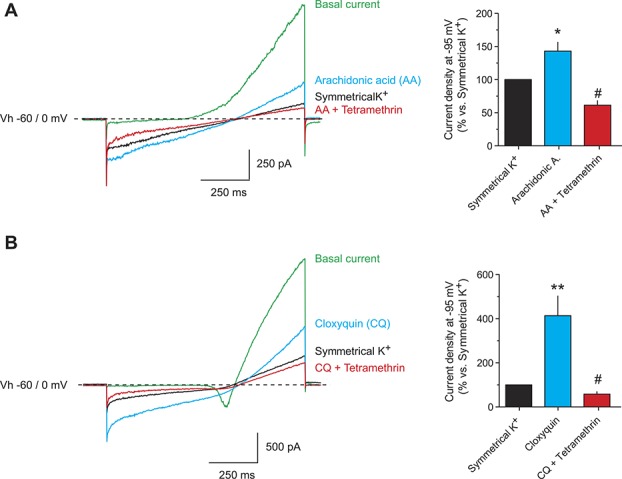
Pyrethroids inhibit native background currents in sensory neurons. (A and B) Representative recordings of whole-cell currents from small/medium-sized DRG sensory neurons. Depolarizing ramps from –100 to +50 mV were recorded from a holding voltage of –60 mV in physiological extracellular solution containing TEA (1 mM), 4-AP (1 mM), CdCl2 (100 μM), CsCl (1 mM), iberiotoxin (50 nM), and TTX (1 μM). Then, the extracellular solution was exchanged by a high K+ solution (135 mM) containing the same blockers. In near symmetrical K+ concentration, the current measured at hyperpolarized potentials should be mainly carried by background K+ channels. A known activator of TREK-1, TREK-2, and TRAAK (arachidonic acid, AA; 10 μM; A) or TRESK (cloxyquin, CQ; 100 μM; B) was used to specifically increase the current mediated by these channels. The effect of tetramethrin (TM, 20 μM) was then evaluated. Bar graphs show the quantification of the currents at −95 mV expressed as % of current recorded in symmetrical K+ conditions. Data are expressed as mean ± SEM of 5 to 6 neurons recorded in each condition. *P < 0.05, **P < 0.01 paired Student t test vs symmetrical K+. #P < 0.05; paired Student t test vs current activated by arachidonic acid or cloxyquin. DRG, dorsal root ganglion.
To corroborate the inhibitory effect found on native currents, and to further characterize the specific effects of pyrethroids on K2P channels, we studied them after expression in a heterologous system. Whole-cell currents from HEK293 cells transiently transfected with human TRESK (hTRESK) were recorded and responses to pyrethroids were measured. Treatment with 20-μM TM, DM, or PM had an inhibitory effect on hTRESK, decreasing its current by 33%, 30%, and 27%, respectively (Figs. 4A–C, G). Partial recovery of the current was seen after washout of the compounds. In a similar fashion, TM was also tested on rat and mouse TRESK orthologs, with similar inhibitory effects (Figs. 4G and H). The dose–response curve rendered a half-maximal inhibitory concentration (IC50) of 10.7 μM for human and 34.9 μM for mouse TRESK (Fig. 4H). To determine whether TM was acting directly on the channel or near it (through the plasma membrane), and to discard an action through intracellular messengers or interaction with soluble intracellular proteins, we performed recordings in outside-out patches from HEK293 cells transfected with mTRESK (supplemental Fig. 2, available at http://links.lww.com/PAIN/A485). In isolated membrane patches, an even greater inhibition of mTRESK current was observed after the application of TM 20 μM (75.6%; n = 7 independent patches) compared with whole-cell effects.
Figure 4.
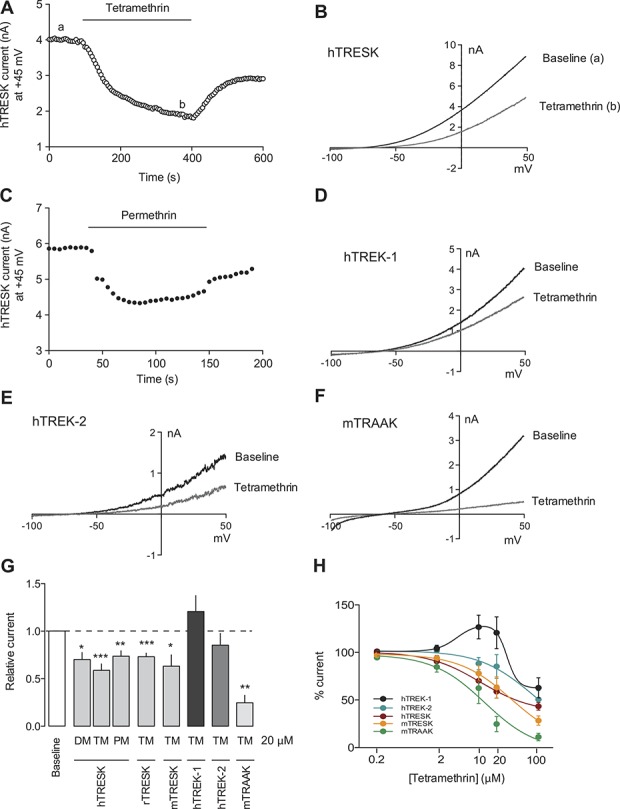
Pyrethroids inhibit K2P channels expressed in heterologous systems. (A) Whole-cell current in a HEK293 cell transfected with human TRESK. Depolarizing ramps from –100 to +50 mV every 5 seconds from a holding voltage of −60 mV were applied. Current measured at +45 mV in each ramp is plotted against time. Tetramethrin application (TM, 20 μM) produced a significant decrease in the current. Washout of the drug partially recovered current values. (B) Voltage ramps recorded in baseline (a) and during TM application (b) from the experiment shown in (A). (C) Permethrin (PM, 20 μM), a widely used drug as topical treatment for scabies and head lice, also inhibits hTRESK. TRESK current was recorded as in (A). (D–F) Effect of TM (100 μM) on human TREK-1 (D), human TREK-2 (20 μM, E), and mouse TRAAK (20 μM, F) expressed in HEK293 cells. (G) Quantification of inhibitory effects of pyrethroids in K2P channels. Currents were recorded as described for the experiment shown in (A). Tetramethrin, deltamethrin (DM), and PM were assayed at 20 μM. In each group, 6 to 10 cells were recorded per condition. Data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; and ***P < 0.001 Student t test vs baseline. (H) Dose–response relationships of hTRESK, mTRESK, hTREK-1, hTREK-2, and mTRAAK.
Similarly, human TREK-2 (hTREK-2) and mouse TRAAK (mTRAAK) currents were inhibited by TM application, with IC50 values of 75.6 and 12.6 μM, respectively (Figs. 4E–H). The effect of TM on TREK-1 was slightly different to its fellow family members and the dose–response relationship showed a biphasic curve: at the lower range of concentrations tested (2-20 μM), a stimulatory effect was observed while at a higher concentration (100 μM), the effect was an inhibition (Figs. 4D, G, and H). Nevertheless, the inhibitory effect on TREK-1 was much smaller than those observed on the other K2P channels tested. Altogether, the results obtained show that pyrethroids exert an inhibitory effect on the principal K2P channels expressed in small- and medium-diameter sensory neurons, suggesting that the effects of pyrethroids on these channels could contribute to depolarize and activate these neurons.
3.3. Tetramethrin actions on peripheral terminals of sensory nerve fibers
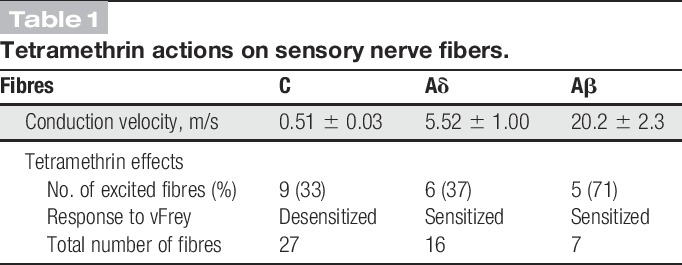
Using the saphenous nerve-skin preparation, we studied whether pyrethroids could alter sensory transduction in peripheral nerve terminals and, if so, which subsets of somatosensory nerve fibers were affected by exposure to TM. A total of 50 fibers recorded from 29 skin flaps were examined (Table 1). Among those, 27 were C-units (mean CV: 0.51 ± 0.03 m/s), 16 were Aδ (mean CV: 5.52 ± 1.0 m/s), and the remaining 7 were fast-conducting Aβ-fibers (mean CV: 20.2 ± 2.3 m/s) according to the classification provided by Zimmerman et al.52
Table 1.
Tetramethrin actions on sensory nerve fibers.
3.4. Tetramethrin effects on C- and Aδ-nociceptors
Our analysis revealed that TM had excitatory effects in a proportion of C- and Aδ-nociceptors; however, the type of effect differed between both groups. In addition, the mechanical responsiveness of both groups was differently altered in the presence of TM. At low concentrations (25-50 μM), TM had an excitatory effect in 6/20 C-mechanosensitive units (mean von Frey threshold 28.5 ± 7.6 mN), whereas vehicle used to dilute TM did not produce significant effects (not shown). Besides, 5 of 7 further C-mechanoinsensitive units were also excited. Within seconds after application started to fire at low frequencies (mean discharge 0.32 ± 0.13 Hz, range 0.04-1 Hz) for ∼15 minutes (an example of the activation of a C-mechanoinsensitive fiber is shown in Fig. 5; fiber 1). Higher concentrations (100-200 μM) did not further increased firing rates, but mechanical responses were significantly inhibited in 3 of the mechanosensitive units. In the remaining 14 C-mechanosensitive units unresponsive to TM, a similar inhibition of mechanical responsiveness was observed in 3 units (fiber 2, Fig. 5). In summary, at low concentrations (25-50 μM), TM evoked ongoing firing in about a third of C-nociceptors. At higher concentrations, TM had a clear desensitizing effect in the mechanical responsiveness of 50% of the mechanosensitive C-nociceptors (from 32 ± 7 to 7 ± 3 spikes, n = 7, P < 0.05 Wilcoxon matched pair test).
Figure 5.
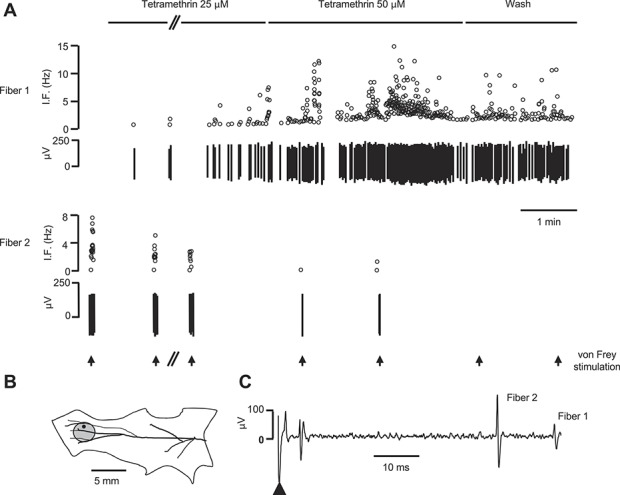
Tetramethrin (TM) activates cutaneous somatosensory C-fibers. Original recording of 2 single C-fibers recorded from the same nerve filament using the saphenous nerve-skin preparation. (A) The upper panel shows the firing of fiber 1 as instantaneous frequency (IF). This unit, which was mechanoinsensitive, exhibited an ongoing, dose-dependent, transient response to TM application. Washout started as soon as the response began to decline. The lower panel shows the mechanical responses of fiber 2 to a 16-mN vFrey filament which decreased and vanished during and after TM superfusion (>20 minutes after washout). Arrows under the traces mark application of vFrey filament for 5 seconds. The gap (//) represents a 5-minute interval. (B) Representation of the preparation with the mechanical receptive field of fiber 2 indicated by the gray circle. The black dot marks the site of electrical stimulation. (C) Electrically evoked responses of the fibers studied. According to latency and the recording distance (24 mm), the calculated conduction velocity of fibers 1 and 2 were 0.48 and 0.4 m/s, respectively. The arrowhead indicates the stimulation artifact.
A similar proportion of Aδ-nociceptors were excited by TM (6/16) at both low and medium-high concentrations in a concentration-dependent manner (25-100 μM; mean discharge 8.55 ± 7.5 Hz, range 0.26-46 Hz). Most of them (5/6) showed a transient or sustained activity in the form of bursts characterized by apparent intraburst frequencies above 200 Hz (see fiber 1 in Fig. 6 A and B for a representative example). The effect of TM on mechanical responses was evaluated in 13 of the 16 mechanosensitive units (mean von Frey threshold 9.27 ± 2.1 mN). In 10 of the units, the characteristic tonic discharge changed to a series of bursts of high frequency (up to 750-Hz intraburst frequency) as shown in Figure 6C. A desensitizing effect was only observed in one other Aδ-unit in which a single application of 200 μM inhibited evoked discharges from the exposed RF.
Figure 6.
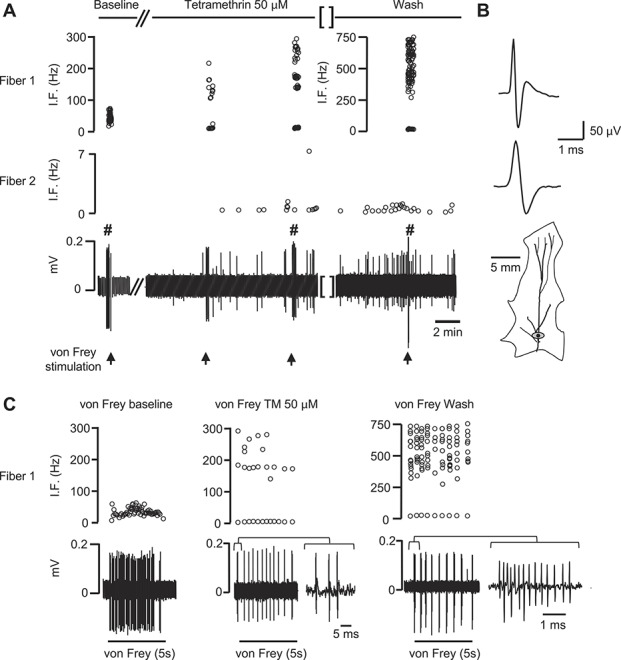
Pyrethroid exposure alters the firing pattern of cutaneous somatosensory fibers. Electrophysiological responses of 2 fibers recorded within the same filament before, during, and after application of TM. (A) Left panel: an Aδ-fiber (fiber 1) responded with an ongoing discharge when stimulated with a gravity-driven von Frey filament (11.4 mN). Fiber 2 was a C-type mechanoinsensitive fiber. Central panel: the basal activity of fiber 1 was unaffected but the responses to the 11.4 mN von Frey (arrow) consisted in a high-frequency burst, which built with time. Fiber 2 becomes activated after application of 50 μM TM. Right panel: the effects of TM on both fibers were irreversible and lasted >30 minutes after wash. (B) Averaged waveforms (>20 spikes) of fiber 1 and 2 in an expanded time base. The mechanical receptive field of fiber 1 (marked in gray) is shown below. The black dot indicates the site for electrical stimulation. Gap (//) represent a 5-minute interval and gap [ ] a 30-minute interval. (C) Expanded responses to von Frey application before (baseline), during application of 50 μM TM, and after washout in fiber 1 (marked with # in the original recording shown in (A)). The responses are presented as the original recording and as instantaneous frequency (IF). A burst in each condition has been further expanded to emphasize the presence of bursts with very short interspike intervals. TM, tetramethrin.
3.5. Tetramethrin effects on Aβ-fibers
Tetramethrin excited 5/7 fibers at all concentrations assayed, within seconds of application the fibers started to fire at low frequency and in a concentration-dependent manner (mean discharge 5.4 ± 1.9 Hz, range 0.59-10.65 Hz). Furthermore, the characteristic tonic discharge evoked by mechanical stimulation changed to a series of bursts of very high frequency in the 4 fibers tested (up to 1000-Hz intraburst frequency).
3.6. Behavioral effects of pyrethroids
Because our data show that pyrethroids activate a subset of small- and medium-sized sensory neurons as well as nerve fibers that are thought to be involved in nociception, we examined the possible analgesic effects of TM by measuring mouse somatosensory behaviors. Injection of TM (10 μL, 100 μM) into the hind paw produced a significant increase in nocifensive behaviors (13.3 ± 2.4 flinches/lickings; n = 12; P < 0.001) compared with vehicle injection (0.3 ± 0.2; n = 10; Fig. 7A). Moreover, TM decreased the mechanical threshold for paw withdrawal measured using von Frey filaments 15 minutes after injection, compared with the value obtained 30 minutes before injection (Fig. 7B). Vehicle injection did not produce significant effects on mechanical threshold. By contrast, noxious thermal sensitivity was not significantly altered by TM exposure in the radiant heat paw withdrawal test (Fig. 7C). Commercial products for the treatment of head lice in humans or insecticides usually contain pyrethroid concentrations ranging between 0.25% and 1.5% (between 8 and 40 mM depending on the compound). To assess whether a relevant concentration used in commercial applications significantly affect nociceptor activity, we applied TM 1 mM to the corneal surface, which is similar to what occurs when a head lice shampoo gets to the eye. Blinking response, as a surrogate of nocifensive behavior, was largely increased on TM application compared with control vehicle (43.3 ± 9.5 vs 12.2 ± 1.6 blinks per 2 minutes; n = 5 each; P < 0.01). These results indicate that pyrethroid exposure produces painful responses and increases basal sensitivity to mechanical stimuli.
Figure 7.
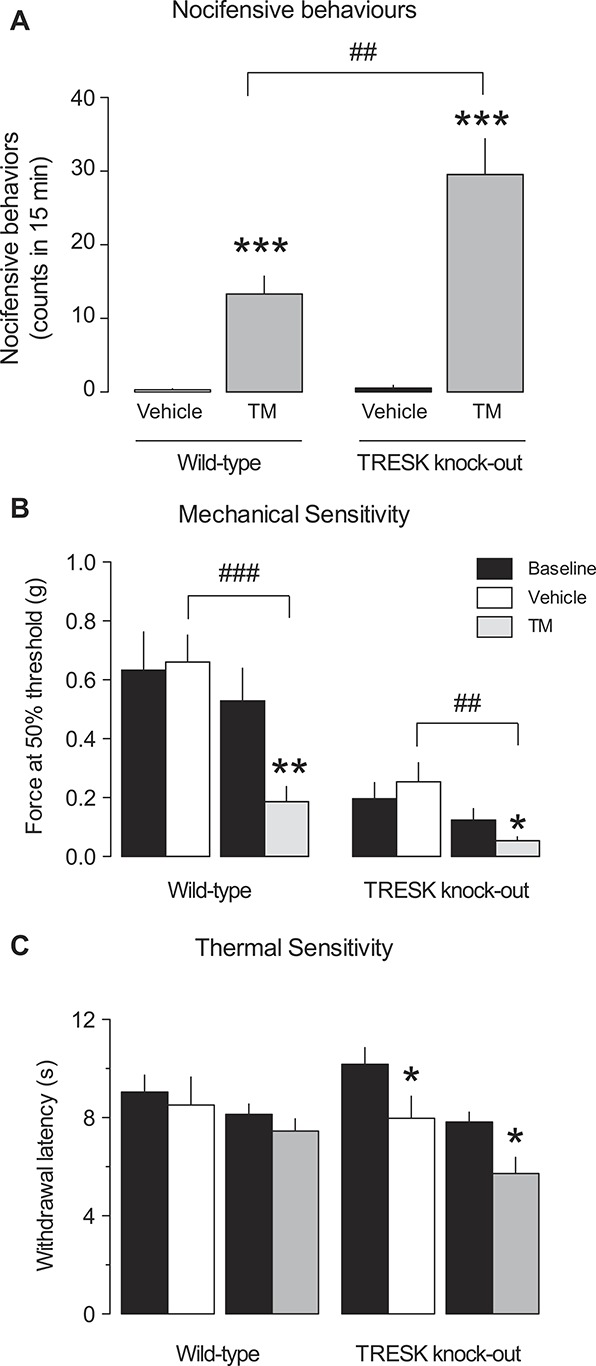
Pyrethroids produce pain-associated behavioral responses and decrease the threshold for mechanical stimuli. (A) Quantification of the number of flinching and licking behaviors after intradermal injection of tetramethrin (TM, 5 μL, 100 μM) or vehicle in the hind paw for wild-type and TRESK knockout mice. The mean number of counts over a period of 15 minutes is shown (n = 6-10 animals per group). ***P < 0.001 Student t test vs vehicle injection. ##P < 0.01 Student t test WT vs KO mice. (B) Mechanical sensitivity: von Frey response thresholds before (baseline) and 15 minutes after vehicle or TM injection (100 μM) for wild-type and TRESK knockout animals. *P < 0.05; **P < 0.01 Student t test vs baseline. ##P < 0.01; ###P < 0.001 Student t test vehicle vs TM. (C) Thermal sensitivity: latency of response to radiant heat stimuli applied to the hind paw of wild-type and TRESK knockout mice before (baseline) and after vehicle or TM injection (100 μM). *P < 0.05; Student t test vs baseline.
3.7. Lack of TRESK expression enhances pyrethroids effects
As mentioned earlier, K2P channels play a significant role in preventing sensory neuron depolarization. Therefore, inhibition of K2P channels by pyrethroids may contribute to increased neuronal excitability. Alternatively, although pyrethroids have inhibitory effects on K2P channels, the remaining K+ background current may still be acting as a “brake” to prevent excessive depolarization of sensory neurons. Our results (Figs. 3 and 4) demonstrated that one of the major inhibitory effects of pyrethroids is on TRESK current and, therefore, we examined whether a total lack of this channel further enhances or ameliorates pyrethroid effects. Using DRG neurons from TRESK KO animals, we examined the responses to TM in current-clamp and calcium imaging experiments similar to the analysis performed in WT animals. Twenty-four DRG neurons from TRESK KO mice were recorded in current-clamp mode with a mean capacitance of 20.5 ± 1.6 pF and a resting membrane potential of −58.0 ± 1.5 mV, which were not different from WT neurons. Twelve of these neurons were significantly depolarized (19.5 ± 4.4 mV) and fired action potentials in response to TM (50% response to 20 μM TM, data not shown). The remaining 12 neurons only showed a small depolarization (3.0 ± 2.6 mV) that was insufficient to trigger an action potential. When the effect of TM was assayed in calcium imaging experiments, 259 of 475 neurons elicited a significant intracellular calcium increase (54.5% response; Figs. 8A and B), which was similar to the value obtained in WT animals. Interestingly, the number of neurons showing oscillations in intracellular calcium levels was markedly decreased (30.2% compared with 64.5% in WT neurons; P < 0.001; Figs. 8A and B), and most neurons elicited a single and wider calcium peak in response to TM with a mean fold Ca2+ increase of 1.83 ± 0.04, which was significantly greater compared with WT neurons (P < 0.001). Similarly to WT animals, the neurons activated by TM in KOs were again smaller in size than nonactivated neurons (Fig. 8C). A significant percentage of neurons responding to TM were positive for TRPA1 (22.0%) and TRPV1 (66.7%; Fig. 8D), whereas a small proportion was positive for TRPM8 (4%; menthol positive, AITC negative). In summary, our electrophysiological and calcium imaging data showed similar overall effects of TM on primary sensory neurons from WT and KO animals. To further evaluate the contribution of TRESK, in WT animals, we pretreated neurons with cloxyquin (50 μM) to enhance TRESK currents. In these conditions, the percentage of neurons activated by TM application in Ca2+ imaging experiments was significantly decreased (supplemental Fig. 3, available at http://links.lww.com/PAIN/A485) as well as the number of neurons showing an oscillatory calcium pattern. These results suggest that TRESK function is important to prevent sensory neuron activation and that inhibition of the channel plays a significant role in the activation of these neurons by TM.
Figure 8.
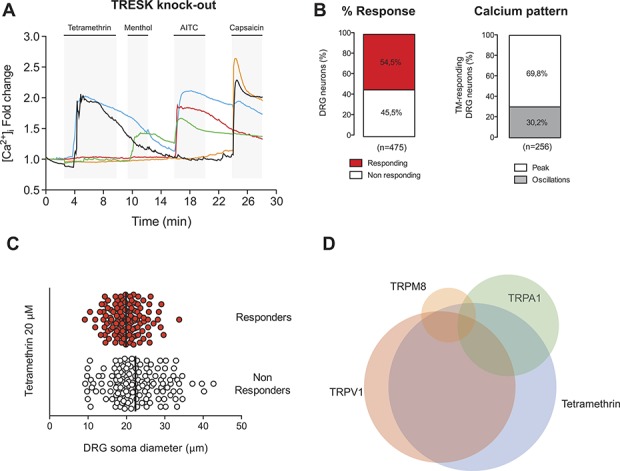
Tetramethrin (TM) effects on TRESK knockout sensory neurons. (A) Representative recordings of intracellular calcium in TRESK knockout mouse DRG sensory neurons. Tetramethrin (20 μM), menthol (100 μM), allyl isothiocyanate (AITC, 100 μM), and capsaicin (1 μM) were assayed. Tetramethrin activated a similar percentage of cells compared with wild-type neurons but a greater percentage of cells showed a single, larger calcium peak rather than an oscillatory pattern. (B) Left: quantification of the percentage of response to TM. Right: percentage of TM-responding cells showing an oscillatory calcium pattern or responding with a single calcium peak. (C) Soma diameter distribution of TRESK knockout DRG neurons displaying sensitivity and insensitivity to TM. Black bars show the mean soma value in each group. (D) Venn diagram showing the relative size and overlap between the population of TM-activated neurons and these activated by menthol, AITC, or capsaicin in the knockout mice. DRG, dorsal root ganglion.
We next tested whether deleting TRESK had any effects on TM responses at the behavioral level. Tetramethrin injection in the hind paw produced a significant increase in nocifensive behaviors compared with vehicle injection (TM: 29.5 ± 4.9; n = 10; vehicle: 0.5 ± 0.3 flinches/lickings; n = 8; P < 0.001; Fig. 7A). Comparison with WT responses revealed a significant increase in TM-induced nocifensive behaviors in KO animals (P < 0.01 vs TM in WT).
When mechanical sensitivity was assessed with von Frey filaments in KO animals under baseline conditions, the withdrawal threshold was found to be greatly reduced compared with WT animals (WT: 0.58 ± 0.08 vs KO: 0.15 ± 0.03 g; P < 0.001; n = 22 and 18 respectively), indicating that TRESK might play a role in the neuronal pathways detecting mechanical stimuli. The withdrawal threshold was further decreased after TM injection compared with vehicle in TRESK-deficient animals (P < 0.01; Fig. 7B). The mechanical threshold was also significantly smaller after TM application (P < 0.05) compared with the result obtained in WT animals. When thermal sensitivity was evaluated, no differences were found in baseline latencies between WT and KO animals. TM injection in the KO animals produced a small but significant decrease in latency compared with baseline levels, but this effect was also observed after vehicle injection, which could be related to an effect of the injection procedure specific to KO animals.
4. Discussion
Dermal exposure to pyrethroids causes paraesthesias (tingling, itching, burning, and stinging sensations) due to their ability to activate sensory neurons. Besides their well-known effects on Nav channels, we explored whether other conductances present in the action potential subthreshold region can also be affected, in particular, the K2P family of background channels. We report that pyrethroids inhibit K2P channels and activate a large percentage of DRG neurons, many of them positive for distinct nociceptive markers (TRPV1 and TRPA1). This correlates well with activation of Aδ- and C-fibers in response to TM as well as the nocifensive behaviors observed in mice.
Depolarization-induced neuronal firing observed after pyrethroid application is likely the result of either activation of a depolarizing conductance, inhibition of a K+ current, or both. Recordings of small sensory neurons in the presence of a cocktail of ion channel blockers that should minimize the contribution of Nav, Kv, KCa, and Cav channels, but that do not block K2Ps indicated that the TM-inhibited current had the kinetic characteristics of a K+ current. Nevertheless, any TTX-resistant Nav channels present could still be activated. In a different set of experiments, background currents were enhanced by arachidonic acid, a known activator of TREK-1/TREK-2/TRAAK, or by cloxyquin, that activates TRESK, allowing us to test the effects of TM in native cells. In both groups, TM produced a significant inhibition of the currents mediated by these channels. Similar inhibitory effects were corroborated in HEK293 cells expressing each type of channel. Taken together, our data indicate that pyrethroids inhibit K2P channels.
Acute and chronic effects of pyrethroids on Nav1.8 and Nav1.6 are well characterized,7,21,33 but these effects are unlikely to participate in a subthreshold depolarization because they are not active at rest. We and others found that responses to pyrethroids are still present in the presence of TTX (Fig. 2),21 thus the TTX-sensitive Nav1.7 does not seem to be necessary for pyrethroid effect. A possible effect on the TTX-resistant, noninactivating Nav1.9 channel (expressed in nonpeptidergic C-fibers) might be more relevant. Acute exposure to PM increased Nav1.9 current in muscle and vascular nociceptors, whereas chronic exposure produced an increased current without changing its voltage dependence.32 These acute and especially chronic effects have been implicated in the etiology of Gulf War Illness, a chronic pain syndrome linked to soldiers' exposure to pesticides and repellants, which contained significant amounts of PM.5 In view of our data, the participation of K2Ps should also be considered in the pathophysiological mechanism.
Currents mediated by Kv7 channels were initially upregulated and later downregulated on chronic exposure to pyrethroids.33,34 Initial upregulation was attributed to compensatory effects on nociceptor activation and the subsequent current decrease to persistent toxicity. Significant downregulation of delayed rectifier K+ currents has also been reported after chronic exposure, coincident with the presentation of pain behaviors in rats.34 These data suggest that the combined action of pyrethroids on different channels leads to neuronal activation and enhanced nociceptor excitability because of Na+ currents enhancement, decreased K+ currents, and possibly increased Ca2+ entry because of action potential broadening.
In heterologous systems and sensory neurons, pyrethroids significantly inhibited K2P currents, similar to hydroxy-α-sanshool, a compound extracted from Szechuan peppercorns, which inhibits TRESK, TASK-1, and TASK-3 and activates sensory neurons.4 Sanshool also inhibits Nav1.3, Nav1.7, and Nav1.8,44 and the subsequent blocking effect on action potential firing is thought to mediate its analgesic effects by silencing Aδ-nociceptors.44 The synthetic alkylamide isobutylalkenyl amide, a sanshool derivative, also inhibits TRESK, activates sensory neurons, and produces tingling, numbing, and burning sensations in humans and painful behaviors in rodents.2,40,45 Another alkylamide insecticide, BTG502, structurally related to sanshool, inhibits Nav channels by binding to a site that overlaps with the pyrethroid-binding site.17 These different compounds exhibit characteristic somatosensory effects via their actions on both Na+ and K+ channels expressed in sensory neurons and resulting sensations might depend on the particular neuronal subtypes affected. Effects on channels such as TRPs cannot be completely discarded, but these seem unlikely because we could find neurons not activated by TRPV1, TRPA1, or TRPM8 agonists that were still activated by TM (Fig. 1).
In peripheral nerve fibers, pyrethroids at low concentrations (20-50 μM), activated Aβ-, Aδ-, and C-fibers or potentiated their activation by other stimuli (eg, mechanical). Higher concentrations (100 to 200 μM) usually produced desensitization and inhibition of any previous mechanical response. Similar activating effects were reported for sanshool in Aβ-, Aδ-, and C-fibers which were associated with modulation of TRESK, TASK-1, or TASK-3 channels.25 Later, in fibers where sanshool did not evoke excitation, analgesic effects were linked to suppression of mechanical responses of somatosensory Aβ-mechanoreceptors and Aδ-mechanonociceptors, through inhibition of Na+ channels.44 Pyrethroids might induce similar effects depending on the concentration and the nerve fiber targeted, thus at low concentrations, activation or sensitization of C-fibers would be prominent, likely due to a combination of effects on Nav and K2P channels. This is well correlated with the increase observed in nocifensive behaviors when TM is injected into the mouse hind paw, which decreases mechanical withdrawal threshold. By contrast, high concentrations appear to desensitize C-fibers by sustained depolarization, possibly due to combined effects on Nav1.9 and K2P inhibition. This persistent depolarization will inactivate Nav channels and decrease mechanical responsiveness of nociceptive fibers. In addition, TM sensitized Aβ- and Aδ-fibers to mechanical stimulation, even in fibers not directly activated by the compound. Often, bursting activity in response to mechanical stimulation was observed, which has also been reported after sanshool application and attributed to K+ channel inhibition.25
TREK-1, TREK-2, and TRAAK are involved in the transduction of mechanical, noxious, and non-noxious thermal stimuli by sensory neurons.3,31,36 Despite TM inhibition of these channels, we did not find significant effects on thermal sensitivity in mice, although a more detailed characterization would be required. By contrast, as in nerve fibers, mechanical sensitivity was enhanced, providing strong evidence that the effects of pyrethroids are prominent in mechanoreceptive neurons. TRESK might be involved in the transduction of mechanical stimuli, not as a direct sensor but by regulating the excitability of sensory neurons involved in mechanodetection. TRESK is thought to prevent neuronal depolarization,18 and decreases in its expression after nerve injury or inflammation could contribute to neuronal hyperexcitability.15,28,45 In support of this role, we found an increased sensitivity to mechanical stimulation in TRESK KO animals, which is then further enhanced by TM exposure. In previous studies, knocking down TRESK using an siRNA increased mechanical sensitivity,45 which is consistent with a lack of this channel enhancing the peripheral and/or central excitability of the nociceptive pathway. Interestingly, TM still enhanced mechanical sensitivity in TRESK KO mice, suggesting that TRESK is not the only target of TM and that the absence of TRESK further potentiates the effects on other channels rather than preventing TM effects. We could hypothesize that the pharmacological inhibition of TRESK by TM in WT animals might contribute to the increased mechanical sensitivity because knocking down the channel produces a similar effect. A role for other channels, including K2Ps is also possible.
According to recent reports, TRESK expression is restricted to some subtypes of sensory neurons,12,46 thus it is possible that despite the lack of this channel, remaining effects on other channels/neuronal subtypes are still prominent. Our analysis of the response of populations of primary sensory neurons did not reveal significant differences in the percentage of neurons activated by TM between WT and KO animals. However, calcium oscillations were seen in a large percentage of cells from WT animals while most cells in KO animals showed a single, longer calcium peak (Figs. 1A, B, 8A, and B). This larger calcium increase can produce a bigger neuronal activation or increased neurotransmitter release at the spinal synapse, thus producing greater pathway activation. In agreement, behavioral effects to TM injection were enhanced in KO mice. TRESK deletion might also increase the excitability of dorsal horn neurons because the channel was initially identified in the spinal cord, where it is highly expressed,39 and recent reports indicate that TRESK might play a role in the modulation of nociceptive information at this level.10,20 We did not explore further the change in the calcium pattern, but it is tempting to speculate that the TRESK activation by Ca2+/calcineurin13,14 might limit depolarization and Ca2+ influx, thus preventing excessive neuronal activation. In the absence of the channel, the loss of this Ca2+/calcineurin-activated K+ current will result in a more prolonged depolarization and a larger and longer Ca2+ influx. The results presented here provide the first demonstration that pyrethroids can inhibit K2P channels contributing to activation of pain pathways, where low concentrations will promote burning, itching, and stinging sensations, whereas desensitizing effects produced at higher concentrations can mediate tingling or numbing sensations.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supported by grants from Ministerio de Economia y Competitividad and Instituto de Salud Carlos III of Spain FIS PI11/01601 (X. Gasull), FIS PI14/00141 (X. Gasull), SAF2016-77585-R (C. Roza), RETICs Oftared RD12/0034/0003 (X. Gasull), and RD16/0008/0014 (X. Gasull) and Generalitat de Catalunya 2014SGR1165 (X. Gasull). J. P. Giblin was supported by a Ramón y Cajal Research Contract (Ministerio de Economia y Competitividad RYC-2011-08589). L. Bernal was supported by a FPU Scholarship (Ministerio de Educacion, Cultura y Deporte, Spain). A. Castellanos, A. Andres, G. Callejo, and X. Gasull performed electrophysiological recordings in neurons and cell lines and calcium imaging. A. Castellanos performed behavioral experiments. A. Castellanos, A. Andres, N. Comes, and J. P. Giblin performed cellular cultures, plasmid generation, and transfection. L. Bernal and C. Roza performed skin-nerve recordings. A. Castellanos, A. Andres, A. Gual, N. Comes, and X. Gasull participated in the design of the study and performed the statistical analysis. X. Gasull conceived the study, oversaw the research, and prepared the manuscript with help from all others. All authors read and approved the final manuscript.
Supplementary Material
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A485.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. J Neurosci 2014;34:1494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albin KC, Simons CT. Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS ONE 2010;5:e9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alloui A, Zimmermann K, Mamet J, Duprat F, Noël J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 2006;25:2368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci 2008;11:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, Graves JC, Hardie A, Knox ML, Meggs WJ, Nettleman MD, O'Callaghan JP, Smithson S, Steele L, White RF. Research advisory committee on Gulf War veterans' illnesses. Gulf War illness and the health of gulf war veterans. Washington, DC: Department of Veterans Affairs; 2008. http://www1.va.gov/rac-gwvi/. [Google Scholar]
- [6].Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol Rev 2005;24:93–106. [DOI] [PubMed] [Google Scholar]
- [7].Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, Burr S, Ray D. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology 2009;30:S17–31. [DOI] [PubMed] [Google Scholar]
- [8].Callejo G, Castellanos A, Castany M, Gual A, Luna C, Acosta MC, Gallar J, Giblin JP, Gasull X. Acid-sensing ion channels detect moderate acidifications to induce ocular pain. PAIN 2015;156:483–95. [DOI] [PubMed] [Google Scholar]
- [9].Callejo G, Giblin JP, Gasull X. Modulation of TRESK background K+ channel by membrane stretch. PLoS ONE 2013;8:e64471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao L, Loucif A, Saintot PP, Patel MK, Adams C, Kuan K, Fish R, Rigby M, Antonio B, Omoto K, Pryde D, Stevens EB. Investigating the role of TRESK in sensory afferent function using a selective opener. Program No. 534.02. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014. Online. [Google Scholar]
- [11].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [12].Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman G, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf C. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2014;3:e04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Czirják G, Enyedi P. Targeting of calcineurin to an NFAT-like docking site is required for the calcium-dependent activation of the background K+ channel, TRESK. J Biol Chem 2006;281:14677–82. [DOI] [PubMed] [Google Scholar]
- [14].Czirják G, Tóth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem 2004;279:18550–8. [DOI] [PubMed] [Google Scholar]
- [15].Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Döring F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol (Lond) 2007;585:867–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Du X, Hao H, Gigout S, Huang D, Yang Y, Li L, Wang C, Sundt D, Jaffe DB, Zhang H, Gamper N. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. PAIN 2014;155:2306–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du Y, Garden D, Khambay B, Zhorov BS, Dong K. Batrachotoxin, pyrethroids, and BTG 502 share overlapping binding sites on insect sodium channels. Mol Pharmacol 2011;80:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 2010;90:559–605. [DOI] [PubMed] [Google Scholar]
- [19].Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J 1998;17:3297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hwang HY, Zhang E, Park S, Chung W, Lee S, Kim DW, Ko Y, Lee W. Twik-related spinal cord K⁺ channel expression is increased in the spinal dorsal horn after spinal nerve ligation. Yonsei Med J 2015;56:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang N, Nutter TJ, Cooper BY. Molecular and cellular influences of permethrin on mammalian nociceptors at physiological temperatures. Neurotoxicology 2013;37:207–19. [DOI] [PubMed] [Google Scholar]
- [22].Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol 2006;291:C138–46. [DOI] [PubMed] [Google Scholar]
- [23].Kupferschmidt K. Pick your poison. Science 2016;354:171–3. [DOI] [PubMed] [Google Scholar]
- [24].Lengyel M, Dobolyi A, Czirják G, Enyedi P. Selective and state-dependent activation of TRESK (K2P 18.1) background potassium channel by cloxyquin. Br J Pharmacol 2017;174:2102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL. Physiological basis of tingling paresthesia evoked by hydroxy- -sanshool. J Neurosci 2010;30:4353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem 2000;275:28398. [DOI] [PubMed] [Google Scholar]
- [27].Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 2003;89:1588–602. [DOI] [PubMed] [Google Scholar]
- [28].Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K⁺ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci 2012;49:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Motomura H, Narahashi T. Interaction of tetramethrin and deltamethrin at the single sodium channel in rat hippocampal neurons. Neurotoxicology 2001;22:329–39. [DOI] [PubMed] [Google Scholar]
- [30].Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol 1996;79:1–14. [DOI] [PubMed] [Google Scholar]
- [31].Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J 2009;28:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nutter TJ, Cooper BY. Persistent modification of Nav1.9 following chronic exposure to insecticides and pyridostigmine bromide. Toxicol Appl Pharmacol 2014;277:298–309. [DOI] [PubMed] [Google Scholar]
- [33].Nutter TJ, Jiang N, Cooper BY. Persistent Na+ and K+ channel dysfunctions after chronic exposure to insecticides and pyridostigmine bromide. Neurotoxicology 2013;39:72–83. [DOI] [PubMed] [Google Scholar]
- [34].Nutter TJ, Johnson RD, Cooper BY. A delayed chronic pain like condition with decreased Kv channel activity in a rat model of Gulf War Illness pain syndrome. Neurotoxicology 2015;51:67–79. [DOI] [PubMed] [Google Scholar]
- [35].Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 1998;17:4283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pereira V, Busserolles J, Christin M, Devilliers M, Poupon L, Legha W, Alloui A, Aissouni Y, Bourinet E, Lesage F, Eschalier A, Lazdunski M, Noel J. Role of the TREK2 potassium channel in cold and warm thermosensation and in pain perception. PAIN 2014;155:2534–44. [DOI] [PubMed] [Google Scholar]
- [37].Ray DE, Forshaw PJ. Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J Toxicol Clin Toxicol 2000;38:95–101. [DOI] [PubMed] [Google Scholar]
- [38].Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. PAIN 2008;138:537–45. [DOI] [PubMed] [Google Scholar]
- [39].Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem 2003;278:27406. [DOI] [PubMed] [Google Scholar]
- [40].Sawyer CM, Carstens MI, Simons CT, Slack J, Mccluskey TS, Furrer S, Carstens E. Activation of lumbar spinal wide-dynamic range neurons by a sanshool derivative. J Neurophysiol 2009;101:1742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Song JH, Narahashi T. Differential effects of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant single sodium channels. Brain Res 1996;712:258–64. [DOI] [PubMed] [Google Scholar]
- [42].Song JH, Narahashi T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroid tetramethrin. J Pharmacol Exp Ther 1996;277:445–53. [PubMed] [Google Scholar]
- [43].Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the type II pyrethroid deltamethrin. J Pharmacol Exp Ther 1998;284:958–65. [PubMed] [Google Scholar]
- [44].Tsunozaki M, Lennertz RC, Vilceanu D, Katta S, Stucky CL, Bautista DM. A “toothache tree” alkylamide inhibits A mechanonociceptors to alleviate mechanical pain. J Physiol (Lond) 2013;591:3325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain 2011;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–53. [DOI] [PubMed] [Google Scholar]
- [47].Wakeling EN, Neal AP, Atchison WD. Pyrethroids and their effects on ion channels. In: Pesticides—advances in chemical and botanical pesticides. Rijeka, Croatia: InTech, 2012. p. 39–66. [Google Scholar]
- [48].Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol 2008;30:55–78. [DOI] [PubMed] [Google Scholar]
- [49].Wright PD, Weir G, Cartland J, Tickle D, Kettleborough C, Cader Z, Jerman J. Cloxyquin (5-Chloroquinolin-8-ol) is an activator of the two-pore domain potassium channel TRESK. Biochem Biophys Res Commun 2013;441:463–8. [PubMed] [Google Scholar]
- [50].Yamamoto Y, Hatakeyama T, Taniguchi K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neurosci Lett 2009;454:129–33. [DOI] [PubMed] [Google Scholar]
- [51].Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 1999;122:2245–57. [DOI] [PubMed] [Google Scholar]
- [52].Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc 2009;4:174–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.