Supplemental digital content is available in the text.
Key Words: metrics, measures, framework, feasibility, point-of-care
Abstract
Objective
Pilot (feasibility) studies form a vast majority of diagnostic studies with point-of-care technologies but often lack use of clear measures/metrics and a consistent framework for reporting and evaluation. To fill this gap, we systematically reviewed data to (a) catalog feasibility measures/metrics and (b) propose a framework.
Methods
For the period January 2000 to March 2014, 2 reviewers searched 4 databases (MEDLINE, EMBASE, CINAHL, Scopus), retrieved 1441 citations, and abstracted data from 81 studies. We observed 2 major categories of measures, that is, implementation centered and patient centered, and 4 subcategories of measures, that is, feasibility, acceptability, preference, and patient experience. We defined and delineated metrics and measures for a feasibility framework. We documented impact measures for a comparison.
Findings
We observed heterogeneity in reporting of metrics as well as misclassification and misuse of metrics within measures. Although we observed poorly defined measures and metrics for feasibility, preference, and patient experience, in contrast, acceptability measure was the best defined. For example, within feasibility, metrics such as consent, completion, new infection, linkage rates, and turnaround times were misclassified and reported. Similarly, patient experience was variously reported as test convenience, comfort, pain, and/or satisfaction. In contrast, within impact measures, all the metrics were well documented, thus serving as a good baseline comparator. With our framework, we classified, delineated, and defined quantitative measures and metrics for feasibility.
Conclusions
Our framework, with its defined measures/metrics, could reduce misclassification and improve the overall quality of reporting for monitoring and evaluation of rapid point-of-care technology strategies and their context-driven optimization.
Recently, in the context of implementation research with point-of-care technologies/rapid diagnostic tests (POCTs/RDTs) for human immunodeficiency virus (HIV), a discussion on clear reporting of measures and metrics beyond accuracy and impact has intensified. Against this backdrop, 2 broad categories of measures have been observed in the deployment of POCT-based strategies: (1) implementation research–centered outcomes (IROs), feasibility, and impact measures and (2) patient-centered outcomes (PCOs) (ie, preference, acceptability, patient experience measures).1–3 Although impact and accuracy measures remain clearly defined in literature, in contrast, a concurrent lack of clarity in documentation and reporting of measures/metrics for feasibility persists.4 Although feasibility studies form the bulk of diagnostic literature, their measures/metrics merit a scrutiny. Although new and well-defined measures/metrics such as test efficacy rate continue to be proposed, they are rarely deployed.4–8 Existing checklists have focused on reporting only on test accuracy (ie, Standards for Reporting of Diagnostic Accuracy),9 study quality (Grading of Recommendations, Assessment, Development and Evaluation),10 or reporting of biases (Quality Assessment of Diagnostic Accuracy Studies). We observed a persistent lack of clarity on feasibility measures/metrics and patient-reported outcomes (acceptability, preference, patient experience).11 Inconsistencies in definitions for measures/metrics also compound confusion, and the absence of a reporting framework often results in misuse and misclassification, consequently impacting study and metric reporting quality.12 Feasibility studies are often chosen for transition to scale, and a clear reporting framework for metrics is pertinent. Clarity in metrics will aid objectives, power, and sample size estimations. In addition to the wide variety of benchmarks used to document feasibility, inconsistencies in definitions and creative reporting, either related to the processes or effect of strategies, have led to improper use of definitions. Moreover, a lack of clarity on which metric to use in which context persists in the extant literature, either in relation to research and design of studies or in the implementation of programs.13 Taken together, these inconsistencies and the inbuilt heterogeneity therein impact the overall quality of research, its quantification, and, furthermore, policy recommendations that emerge from scientific evidence. This reveals a lack of basic understanding of the optimal usage for metrics, especially in studies that evaluate POCT-based diagnostics and linked treatment.
Proof-of-concept studies (pilot/feasibility) are particularly relevant in diagnostics. Pilots provide a holistic assessment of performance of a program/device/initiative before a controlled trial or quasirandomized impact assessment–based scale-up study can be planned or conducted. Pilots are very popular, in part because it is difficult to mount trials with time/resource constraints and unclear impacts on clinical decisions and patient wellness decisions. A vast majority of pilot studies explore feasibility and patient-centered outcomes. Patient-centered outcomes are also in evolution.
With the recent shift in research on diagnostics taking center stage in developing settings for improving the quality of care, and in parallel in developed settings with companion, molecular diagnostics for personalized medicine, and emergent threat of antimicrobial resistance, these measures/metrics needed to be revisited. In this context, we felt a need to synthesize evidence and harmonize the reporting of outcome measures/metrics. Furthermore, to respond to the need, we proposed a reporting framework to inform funding, policy decisions, and guideline development for POCT pilots. In an era where real-time diagnosis at the point of clinical care is rapidly becoming mainstream, the time to clarify such measures and metrics, beyond accuracy and impact, is long overdue. With this in mind, our objective was to call for standardized reporting of measures/metrics used in HIV POCTs/RDTs and propose a reporting framework.
METHODS
Our specific aims were the following:
To underline the heterogeneity in reporting, measuring, and defining measures and metrics related to feasibility and patient-reported/centered outcomes, and
To develop an improved framework of reporting and documentation with a goal to develop the overall quality of reporting for pilot studies (Table 1 refers to our framework).
TABLE 1.
Framework for Reporting of Measures and Metrics
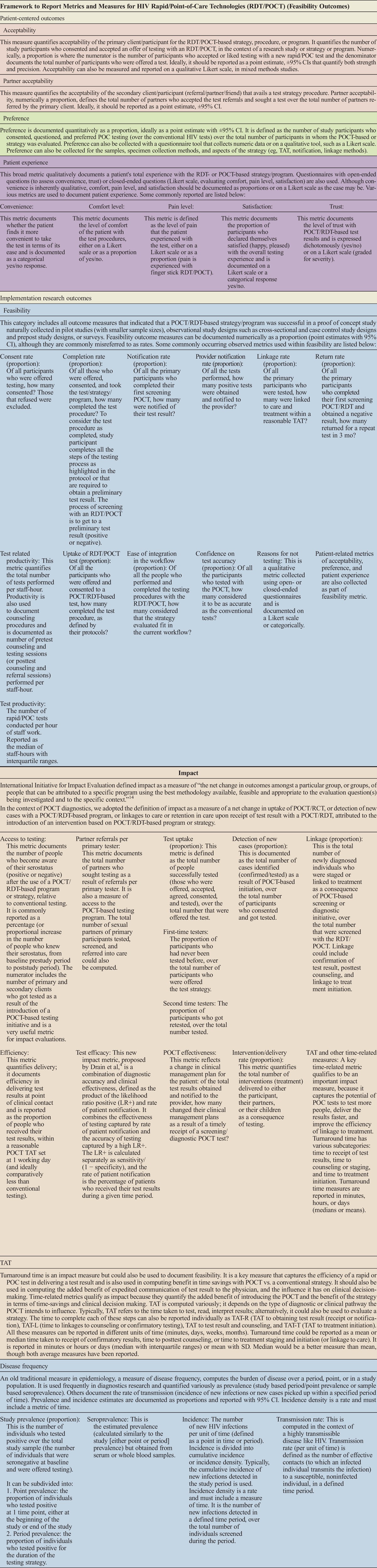
Recently, we classified outcomes for syphilis POCTs beyond accuracy. We organized outcomes into 2 broad categories: (a) IROs, feasibility, and prevalence and (b) PCOs, that is, acceptability, preference, patient experience, etc.6 Impact measures have been reported for a comparison. In this systematic review, we revisit the framework and reporting of metrics and measures for HIV POCTs/RDTs. We collated and synthesized all available evidence and aligned it as per a framework.
Search Methodology
We systematically searched published literature on rapid tests and POCTs for HIV from January 1, 2000 to March 6, 2014. We searched for data in 4 electronic databases: MEDLINE, EMBASE, CINAHL, and Scopus.
Our search string was the following: HIV [MeSH] OR Acquired Immunodeficiency Syndrome [MeSH], OR “HIV Antigens” [tiab], OR “HIV Antibodies” [tiab]) AND (“rapid test” [tiab] OR “point-of-care” [tiab] OR “test” [tiab]) AND (“acceptability” [tiab] OR “preference” [tiab] OR “cost” [tiab] OR “feasibility” [tiab] OR “concordance” [tiab], OR “prevalence” [tiab] OR “impact” [tiab] OR “field performance” [tiab]).
We followed the Cochrane methodology for systematic reviews. Our search strategy aimed to review all studies that documented any measure or metric related to implementation of HIV testing strategies using rapid and POCT tests. Two reviewers (T.C. and R.V.) independently screened and reviewed the full text of the articles and abstracted data. Criteria for study inclusion were determined by discussion among 2 primary reviewers, and, in cases of reviewer discordance, a third reviewer was consulted (N.P.P.). Figure 1 illustrates our study selection process.
FIGURE 1.
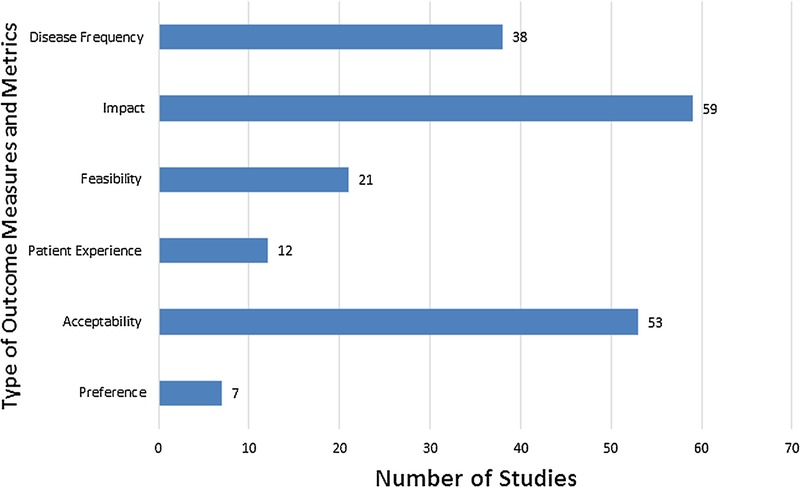
Distribution of included studies by measures. This figure can be viewed online in color at www.poctjournal.com.
Studies were considered eligible if they satisfied all of the following criteria:
Documented the use of HIV point-of-care or rapid tests;
Evaluated at least 1 implementation research– or patient-centered outcome;
Were conducted in humans or in human samples;
Were written in English, French, Spanish, or Portuguese.
Exclusion Criteria
Editorials, news reports, reviews, modeling studies, and studies that only evaluated laboratory tests/surveys on risk behavior were excluded. Data were abstracted from studies published in English (n = 78) and in French, Spanish, or Portuguese (n = 3) using a standardized data abstraction form and reporting framework created for this review. We collected metrics for each measure, evaluated them against our framework (refer to Table 1), proposed working definitions,6 and subclassified metrics. We included mixed methods studies15–27 but excluded those analyzing costs (economic outcomes) for a separate review.
RESULTS
Please refer to Tables 1A–E of included studies (see Tables 1A–E, Supplemental Digital Content, http://links.lww.com/POC/A14).
A total of 81 studies met our inclusion criteria (refer to Fig. 1). These studies evaluated either IRO and/or PCO. Within IRO, 59 studies accounted for impact measures that were documented for a comparison. Of the remaining studies, 38 reported disease frequency measures and 21 documented feasibility measures. Among PCO, 53 studies reported on acceptability, 12 reported on patient experience, and 7 on preference measures (we included impact measures to serve as a reference for a comparison with feasibility measures).
Acceptability
In our framework (refer to Table 1), we defined acceptability as a proportion: the number of primary clients who consented and accepted to be tested with a POCT over the total number of participants in the study, strategy, or program.
Of the 53 studies reporting on acceptability measure, 81% (n = 43/53) documented it well and counted only acceptability of tests as a metric, but 15% of studies (n = 8/53)21,28–34 misclassified it; they counted refusal to test as acceptability. The other 2 studies35,36 combined within acceptability several processes like consent, testing, and study procedures.37 We classified flow of participants throughout a study and documented these metrics with greater clarity. Confusion on what defines acceptability prevailed in 15% studies. Furthermore, 4 studies incorrectly referred to acceptability as a rate (a misnomer; not a proportion).23,38–40 Other studies were creative in the use of acceptability, with use of metrics such as partner testing41 or the number of visits needed to test.42 Regarding precision, 81% (47/53) of studies documented these metrics as a proportion, but only 3 (6%) reported the precision with 95% confidence intervals (CIs).21,24,43
Preference
As per our framework (refer to Table 1), we defined preference as the proportion of study participants who preferred the POCT or rapid test strategy/program over the conventional HIV test/strategy/program. Only 1 study accurately described preference in line with our framework.40
Within preference, various metrics and comparators were reported by studies. Of 7 studies, 5 (71%) reported preference for type of testing strategy (ie, POCT only vs conventional). The remaining 2 studies reported on another metric, as in preference for the number of POCT tests performed,44 preference for test site,40 or preference for the type of specimen used, instead of preference for the POCT strategy itself. Other preference metrics were preference for the time to receive the POC test results or preference for the receipt of test results.45–47 Furthermore, 2 studies misclassified preference: either reporting it as uptake, which is an impact measure,48 or reporting preference as the “quality of test experience.”49 Five studies explored reasons to prefer POCT,15,40,46,50,51 either qualitatively, on a Likert scale,40,46 or quantitatively,15,50,51 with an odds ratio (with 95% CIs).46
Patient Experience
Patient experience is largely a qualitative outcome/measure but was also expressed quantitatively in many of the included studies. As per our framework, of 81 studies, 12 (15%) reported patient experience with various metrics including satisfaction, access, convenience, and level of comfort. The Likert scale was used in only 1 study to evaluate the overall satisfaction with POCT52; patient experience was also documented using preference for test sample in 4 studies. As for 2 other studies (3%), ease of test execution,41,53 patient's level of comfort,53 and access and convenience of POCT were reported.41
Feasibility
Our framework defines feasibility as a category encompassing outcome measures that indicate how successful a POCT/RDT-based strategy or program is, in a context in which the strategy/program/intervention was evaluated in a population group and in a small proof-of-concept study (refer to Table 1).
Following our definition, 21 (26%) of 81 studies reported on feasibility; however, the definitions of feasibility varied across studies. Two studies (2/21, 10%) concluded that the test or strategy was feasible without any data nor metrics to support this claim.39,54 Various metrics were used to report and define the feasibility outcome, including among others consent rate, completion rate,42 uptake,55 and offer rate (3/21, 14%).42,56,57 For example, in 1 study, an offer rate was defined as the proportion of those who were offered the test over the total eligible patients.56,57 In another study, offer rate was defined as the proportion of patient visits during which testing was offered42 or as “missed opportunities” in the third study.57
Heterogeneity in reporting persisted in definitions and documentation, impairing clarity. For example, completion rate (of test procedure) was reported in 4 studies (19% of the 21 observed)33,42,56,58 but defined in only 2.56,58 Whereas in 1 study it was reported as a percentage of women tested during labor,33 in another it was reported as test completion rate per patient visit. Numerators and denominators changed adding to heterogeneity.42 Likewise, the return rate was documented in 3 studies and reported inconsistently, either as (1) the proportion of individuals tested who returned for posttest counseling,59 (2) the proportion of individuals who successfully retested after having deferred testing,60 or (3) the proportion of individuals who received a repeat test.30 The linkage metric was also documented inconsistently depending on the type of posttest linkage initiated (eg, referral, care/treatment, counseling) and reported as the proportion of referrals to HIV care16 or the number (not proportion) of infected women who received treatment.61 Besides quantitative reporting, the qualitative documentation of measures was also impaired. Measures such as ease of testing (as in procedure),16,50,58 workflow integration19,29,52,62,63 (38%), the impressions of participants,16 perception of patients,52 perceptions of performance58 (2/81, 3%), and the ease of test execution41,53 were reported. These measures also need to be defined.
Other feasibility metrics:
Turnaround Time
Turnaround time (TAT) measures capture the efficiency of the test in delivering a result and can be computed in several ways depending on the type of diagnostic or clinical pathway that the POCT aims to influence. Turnaround time typically refers to how long it takes to test, read, and interpret the results, but the time to complete each of these steps can also be reported separately. Alternatively, TAT may refer to how long it takes to complete a specific step of the clinical pathway, such as the time to receive a confirmatory test result, time to receive posttest counseling, time to treatment initiation, or time to staging (or linkage to care). Across studies, TAT was defined in terms of availability of test result and reported in 3 studies.16,49,64 In 1 study, TAT was documented qualitatively.16 Only 1 study proposed a clear definition for TAT. Three different metrics were related to TAT: (1) proportion of tests results available within 1 hour, (2) median test duration, and (3) time between sample collection.49
Productivity
Productivity appeared in 2 studies and was defined differently. In 1 study, it was reported as the total number of tests carried out per staff-hour,65 and the other defined productivity as the mean number of visits per patient (reported as mean ± SD).66
Trust
On this measure, study participants were asked whether they would choose a POCT in the future and whether they trusted their test results; the results were either reported as proportions or using Likert scores. Two studies documented patient confidence on the accuracy of POCT.50,52
Test Volume
Test volume refers to the volume of tests performed in a defined time period. For this measure, 1 study documented the change in the annual demand for HIV tests and the change in ordering tests.38 Other studies documented the change in the number of patients seeking rapid testing.28,30
Rapid Test Awareness
One study reported on the increase in awareness of rapid tests, before and after the introduction of the tests.28
Impact (as a Comparator of Measure/Metrics Within it)
Impact definitions have been clarified by the International Initiative for Impact Evaluation (3ie). Impact has been defined by 3ie as “the net change in outcomes amongst a particular group, or groups of people that can be attributed to a specific program using the best methodology available, feasible and appropriate to the evaluation question(s) being investigated and to the specific context.”14 This definition is very broad and encompasses a range of contexts, settings, programs, and interventions. We documented them as a comparator to demonstrate the contrast in reporting of feasibility metrics and measures.
Of 81 studies, 59 (73%) reported on a total of 163 impact metrics, with some studies often reporting 2 metrics. We classified these metrics into the following categories: uptake, detection of new cases, first time testers, receipt rate (proportion), linkage rate (proportion), intervention delivery rate (proportion), partner notification rate (proportion), referral rate (proportion), and TAT. Of these, detection of new cases was the most common metric (72/163, 44%), followed by first time testers (21/163, 13%), test result receipt rate (19/163, 12%), linkage rate (16/163, 10%), and test delivery rate (15/163, 9%). Uptake, TAT, partner notification, and referrals accounted for only 12% (20/163) of impact measures. Only 3 studies reported metrics perfectly in line with our framework.25,39,67
In terms of break up, metrics were separately reported as follows, and in some studies, these metrics were mixed up or creatively reported. (a) Increase in uptake: Uptake of testing was documented by 2 studies, that is, Anaya et al68 and Herbert et al,63 but reportedly misclassified as testing rate. Metsch et al69 reported on the likelihood (as adjusted risk ratio with CIs) of completing POCT strategy as uptake, whereas a third documented the proportion of participants tested as uptake.70 (b) Receipt of test results: 14 studies reported the receipt of test results as a proportion, 2 as a rate,25,28 and 3 documented the likelihood of receipt as an odds70 or risk ratio25,71 with 95% CIs. (c) New case detection: A total of 41 studies documented detection of new cases (proportion) often without CIs. (d) Rate of delivery of linked intervention: Rate was reported in 10 studies, documented in detail in 6,72 and reported variously as either a cumulative probability,21 sometimes accurately as a rate73 or as the number of cases where test results were not received in time with POCT;49 as the number of patients whose treatment changed because of a positive POCT result,38 or a decrease in unnecessary postexposure prophylaxis among health care workers with POCT.26 (e) First time testers: One of the best-defined metrics, reported in 18 (30%) of 59 studies as the proportion of those who were being tested for the first time often without CIs;72 one study reported it as a number alone,40 whereas another reported it as missed opportunities.74 (f) Linkage (proportion): Linkage was defined inconsistently, either as a proportion of patients who adhered to their first medical appointment or of those who completed follow-up.38,51,75 Only 1 study reported linkages with CIs,43 and another as a “high proportion of failure to return for confirmatory testing.”23 (g) Test efficiency: Test efficiency was documented by 2 studies as the proportion of actionable test results49 or those test results that were “resolved” at a screening visit.22 (h) Turnaround time: The TAT was defined inconsistently, either defined as the time taken to test,49,72,76 the total time to referral to an intervention,72 or the time between sample collection and test result.49 Turnaround time was reported as a median or a range.49,72 (i) Partner notification: Partner notification or referral rates (proportions) were documented in only 4 studies. Notification was reported either as the number19 or as the proportion72 of participants who disclosed their serostatus with their partners or as the proportion of patients who would recommend an HIV self-test to others.48 Partner referral was also documented qualitatively.41 (j) Mortality (testing rate): Ashby et al77 and van Rooyen et al55 documented the number of deaths as part of roll out of testing strategy. Of 45, only 7 studies (16%) reported 95% CIs.
Measures of Disease Frequency (as a Comparator)
Precise definitions for measures of disease frequency are defined in many epidemiology textbooks. Prevalence was the most commonly reported measure, but only 10 (26%) of 38 studies reported it with 95% CIs;78 the remaining 28 studies were unclear, with 1 study reporting it as a relative risk.50 Period prevalence was defined accurately by 6 studies.79 A study confused the concepts of prevalence and incidence, reporting it as a new measure, “prevalence/rate of new incidence.”67 Incidence, on the other hand, was well defined.65 Transmission rate was not clearly reported.80
DISCUSSION
Using our proposed framework for feasibility, with clear standardized definitions (refer to Table 1), we attempted to reclassify and reevaluate metrics for feasibility, and patient-centered outcomes of preference, acceptability, and patient experience, that were reported with HIV point-of-care and rapid technologies. Across all studies, we observed heterogeneity and variability in reporting of various outcomes, inconsistent definitions, and documentation, with resultant misclassification of outcomes and measures.
Although feasibility, preference, and patient experience were the most frequently confused measures, acceptability was the best defined among them. Impact as a comparator was best defined. We attributed clarity in reporting impact to clear definitions outlined by the 3ie initiative.14
Another key finding was a lack of clarity on which metric to use, when, how, and in which context to use it; confusion prevailed, and careless numeric reporting of point estimates from feasibility studies without CIs was observed. Creative definitions and erroneous documentation generated confusion as to what was attempted, documented, and reported. Despite the reporting of a well-defined new impact measure called the test efficacy, the metric was not used at all by any study.4 This explains the disconnect in the application of clear metrics in diagnostics.
Oftentimes, qualitative research on patient experience with the POCT strategy provides a meaningful assessment of the utility of the strategy, compared with quantitative research with unclear metrics and measures.81,82 In this regard, a lack of clarity on the application of qualitative research metrics within mixed designs was also observed.
Incidentally, a time trend in reporting of outcomes beyond accuracy has been observed. Although the number of studies increased over time (refer to Fig. 2), the quality of reporting of measures/metrics remained unchanged. Although trends changed, test device evaluations were replaced by evaluations of test strategies/programs over time. Although our feasibility framework is aimed to improve clarity in reporting, the proposed measures/metrics will require a greater integration within observational and pilot trial designs. This framework could be adapted to other POCT initiatives targeted to other key sexually transmitted and blood borne infections (eg, hepatitis C virus, hepatitis B virus, syphilis, human papillomavirus, herpes simplex virus, chlamydia/gonorrhea) in the near future.
FIGURE 2.
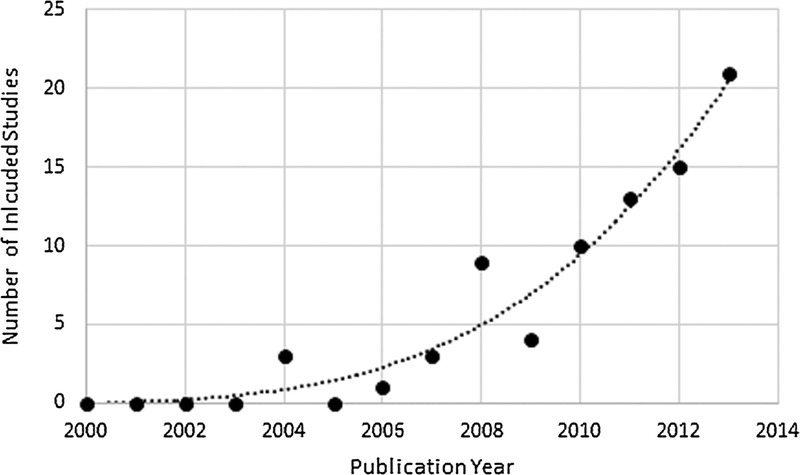
Number of included studies by publication year.
We do hope that new POCT devices will incorporate electronic documentation of measures/metrics with a digital data log in real time that automatically computes, plots, and displays key measures/metrics. This process will aid implementation and encourage donor agencies to monitor and document the impact of their interventions. This process will also reduce the extent of misclassification and further minimize errors in reporting of simple measures like proportions and TAT.
Strengths and Limitations of Review
A comprehensive search and use of a strong methodology were our strengths. Publication bias cannot be ruled out.
Implications for Research and Policy
This feasibility framework is aimed for pilot studies. It will be of interest to various stakeholders (ie, researchers, health care professionals, policy makers, laboratory professionals, funders, donors, front line health care professionals, and community-based organizations) that are involved in implementation, monitoring, and evaluation of POCT initiatives for HIV and related coinfections.
CONCLUSIONS
With this framework, we hope to improve the quality of collection, documentation, reporting, and classification of feasibility outcomes needed to evaluate HIV POCT/RDT-based programs and strategies. Clearly defined measures, and ideally, the use of standardized metrics, will facilitate a better comparison of different strategies, evaluations, and their context-driven optimization. Our findings will find resonance in the daily work needed for global implementation of HIV POCT/RDT policies, for both clinical/implementation research and global health practice.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This study was supported by Gates Foundation operating grant OPP1061487 and CIHR HIB-131558.
The authors also received FRQS Salary Award Junior 2 (2015–2018).
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.poctjournal.com).
REFERENCES
- 1.St-Louis P. Status of point-of-care testing: promise, realities, and possibilities. Clin Biochem. 2000;33(6):427–440. [DOI] [PubMed] [Google Scholar]
- 2.Pai NP, Sollis K, Peeling RW. Rapid hepatitis C tests: better than the gold standard? Expert Rev Mol Diagn. 2013;13(3):221–223. [DOI] [PubMed] [Google Scholar]
- 3.Banoo S, Bell D, Bossuyt P, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nature Rev Microbiol. 2008;6(11 Suppl.):S16–S26. [PubMed] [Google Scholar]
- 4.Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14(3):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontela PS, Pai NP, Schiller I, et al. Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: evaluation using QUADAS and STARD standards. PLoS One. 2009;4(11):e7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jafari Y, Johri M, Joseph L, et al. Poor reporting of outcomes beyond accuracy in point-of-care tests for syphilis: a call for a framework. AIDS Res Treat. 2014;2014:465932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White H. Theory-based impact evaluation: principles and practice. Journal of Development Effectiveness. 2009;1(3):271–284. [Google Scholar]
- 8.Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–W12. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 12.Hänscheid T, Rebelo M, Grobusch MP. Point-of-care tests: where is the point? Lancet Infect Dis. 2014;14(10):922. [DOI] [PubMed] [Google Scholar]
- 13.Pai NP, Balram B, Shivkumar S, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(5):373–380. [DOI] [PubMed] [Google Scholar]
- 14.International Initiative for Impact Evaluation (3ie). 3ie Principles for Impact Evaluation 2012-04-20, 2008. Available at: http://www.3ieimpact.org/media/filer_public/2012/04/20/principles-for-impact-evaluation.pdf. Accessed February 15, 2014.
- 15.Pai NP, Barick R, Tulsky JP, et al. Impact of round-the-clock, rapid oral fluid HIV testing of women in labor in rural India. PLoS Med. 2008;5(5):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker ML, Thompson LH, Pindera C, et al. Feasibility and success of HIV point-of-care testing in an emergency department in an urban Canadian setting. Can J Infect Dis Med Microbiol. 2013;24(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns F, Edwards SG, Woods J, et al. Acceptability, feasibility and costs of universal offer of rapid point of care testing for HIV in an acute admissions unit: results of the RAPID project. HIV Med. 2013;14(Suppl. 3):10–14. [DOI] [PubMed] [Google Scholar]
- 18.Carballo-Dieguez A, Frasca T, Balan I, et al. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS Behav. 2012;16(7):1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conners EE, Hagedorn HJ, Butler JN, et al. Evaluating the implementation of nurse-initiated HIV rapid testing in three Veterans Health Administration substance use disorder clinics. Int J STD AIDS. 2012;23(11):799–805. [DOI] [PubMed] [Google Scholar]
- 20.Mayhood MK, Afwamba IA, Odhiambo CO, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and determine HIV-1/2 rapid human immunodeficiency virus antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46(12):3946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo M, Varella I, Castro A, et al. HIV voluntary counseling and testing of couples during maternal labor and delivery: the TRIPAI couples study. Sex Transm Dis. 2013;40(9):704–709. [DOI] [PubMed] [Google Scholar]
- 22.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41(2):218–224. [DOI] [PubMed] [Google Scholar]
- 23.Mungrue K, Sahadool S, Evans R, et al. Assessing the HIV rapid test in the fight against the HIV/AIDS epidemic in Trinidad. HIV AIDS (Auckl). 2013;5:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble H, Wright G, Young E. HIV point of care testing in the emergency department. HIV Med. 2012;13:61. [Google Scholar]
- 25.Thomas R, Machouf N, Trottier B, et al. A new approach to encourage HIV testing in high-risk populations at the clinique l'actuel. Sex Transm Infect. 2011;87:A201. [Google Scholar]
- 26.Hoyos J, de la Fuente L, Fernandez S, et al. Street outreach rapid HIV testing in university settings: a priority strategy? Gac Sanit. 2012;26(2):131–137. [DOI] [PubMed] [Google Scholar]
- 27.Hoyos J, Fernandez-Balbuena S, de la Fuente L, et al. Never tested for HIV in Latin-American migrants and Spaniards: prevalence and perceived barriers. J Int AIDS Soc. 2013;16:18560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kania D, Fao P, Valéa D, et al. Low prevalence rate of indeterminate serological human immunodeficiency virus results among pregnant women from Burkina Faso, West Africa. J Clin Microbiol. 2010;48(4):1333–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin M, Mathema H, Stinson K, Jennings K. Acceptability, feasibility and impact of routine screening to detect undiagnosed HIV infection in 17 - 24-month-old children in the western sub-district of Cape Town. S Afr Med J. 2012;102(4):245–248. [PubMed] [Google Scholar]
- 30.Macgowan R, Margolis A, Richardson-Moore A, et al. Voluntary rapid human immunodeficiency virus (HIV) testing in jails. Sex Transm Dis. 2009;36(2 Suppl.):S9–S13. [DOI] [PubMed] [Google Scholar]
- 31.Manavi K, Williams G, Newton R. The uptake of HIV and syphilis testing in a nurse-delivered service during Gay Pride events. Int J STD AIDS. 2012;23(12):887–889. [DOI] [PubMed] [Google Scholar]
- 32.Martin EG, Salaru G, Paul SM, et al. Use of a rapid HIV testing algorithm to improve linkage to care. J Clin Virol. 2011;52(Suppl. 1):S11–S15. [DOI] [PubMed] [Google Scholar]
- 33.Mathe MK, Rigo J, Sontag D, et al. Prevalence of HIV infection among pregnant women. A study in rural Africa. Rev Epidemiol Sante Publique. 2008;56(6):407–413. [DOI] [PubMed] [Google Scholar]
- 34.Melvin AJ, Alarcon J, Velasquez C, et al. Rapid HIV type 1 testing of women presenting in late pregnancy with unknown HIV status in Lima, Peru. AIDS Res Hum Retroviruses. 2004;20(10):1046–1052. [DOI] [PubMed] [Google Scholar]
- 35.Jabbari H, Aghamollaie S, Esmaeeli Djavid G, et al. Frequency of HIV infection among sailors in south of Iran by rapid HIV test. AIDS Res Treat. 2011;2011:612475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menacho I, Sequeira E, Muns M, et al. Comparison of two HIV testing strategies in primary care centres: indicator-condition-guided testing vs. testing of those with non-indicator conditions. HIV Med. 2013;14(Suppl. 3):33–37. [DOI] [PubMed] [Google Scholar]
- 37.Mkwanazi NB, Patel D, Newell ML, et al. Rapid testing may not improve uptake of HIV testing and same day results in a rural South African community: a cohort study of 12,000 women. PLoS One. 2008;3(10):e3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullins TL, Braverman PK, Dorn LD, et al. Adolescent preferences for human immunodeficiency virus testing methods and impact of rapid tests on receipt of results. J Adolesc Health. 2010;46(2):162–168. [DOI] [PubMed] [Google Scholar]
- 39.Ndondoki C, Brou H, Timite-Konan M, et al. Universal HIV screening at postnatal points of care: which public health approach for early infant diagnosis in Cote d'Ivoire? PLoS One. 2013;8(8):e67996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson AK, Caldas A, Sebastian JL, et al. Community-based rapid oral human immunodeficiency virus testing for tuberculosis patients in Lima, Peru. Am J Trop Med Hyg. 2012;87(3):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gennotte AF, Semaille P, Ellis C, et al. Feasibility and acceptability of HIV screening through the use of rapid tests by general practitioners in a Brussels area with a substantial African community. HIV Med. 2013;14(Suppl. 3):57–60. [DOI] [PubMed] [Google Scholar]
- 42.Newbould C, Monrose C, Dodge J, et al. Don't forget the children — ongoing experience of a paediatric HIV unit using point-of-care tests in children born to HIV-positive parents — how far have we come? HIV Med. 2010;11:67–68. [Google Scholar]
- 43.Ouladlahsen A, Bensghir R, Karkouri M, et al. Benefit of the rapid test determine HIV1/2 in the clinical diagnosis of HIV infection in Ibn Rochd hospital of Casablanca, Morocco. Rev Epidemiol Sante Publique. 2012;60(4):333–338. [DOI] [PubMed] [Google Scholar]
- 44.Marsh KA, Reynolds GL, Rogala BE, et al. Who chooses a rapid test for HIV in Los Angeles County, California? Eval Health Prof. 2010;33(2):177–196. [DOI] [PubMed] [Google Scholar]
- 45.Jabbari H, Sharifi AH, SeyedAlinaghi S, et al. Assessing the prevalence of HIV among Afghan immigrants in Iran through rapid HIV testing in the field. Acta Med Iran. 2011;49(7):478–479. [PubMed] [Google Scholar]
- 46.Sattin RW, Wilde JA, Freeman AE, et al. Rapid HIV testing in a southeastern emergency department serving a semiurban-semirural adolescent and adult population. Ann Emerg Med. 2011;58(1 Suppl. 1):S60–S64. [DOI] [PubMed] [Google Scholar]
- 47.Scognamiglio P, Chiaradia G, Sciarrone MR, et al. Final results of an outreach program of HIV rapid testing among marginalized people living in Rome, Italy. Infection. 2011;39:S24. [Google Scholar]
- 48.Ramachandran R, Chandrasekaran V, Muniyandi M, et al. Prevalence and risk factors of HIV infection among clients attending ICTCs in six districts of Tamilnadu, South India. AIDS Res Treat. 2011;2011:650321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guenter D, Greer J, Barbara A, et al. Rapid point-of-care HIV testing in community-based anonymous testing program: a valuable alternative to conventional testing. AIDS Patient Care STDS. 2008;22(3):195–204. [DOI] [PubMed] [Google Scholar]
- 50.Robbins CL, Zapata L, Kissin DM, et al. Multicity HIV seroprevalence in street youth. Int J STD AIDS. 2010;21(7):489–496. [DOI] [PubMed] [Google Scholar]
- 51.Ruutel K, Ustina V, Parker RD. Piloting HIV rapid testing in community-based settings in Estonia. Scand J Public Health. 2012;40(7):629–633. [DOI] [PubMed] [Google Scholar]
- 52.Garrard N, Peck J, Ruf M, et al. Opt-out HIV testing pilot in termination of pregnancy services — 11-month service evaluation. HIV Med. 2010;11:69. [Google Scholar]
- 53.Tepper NK, Farr SL, Danner SP, et al. Rapid human immunodeficiency virus testing in obstetric outpatient settings: the MIRIAD study. Am J Obstet Gynecol. 2009;201(1):31e1–31e6. [DOI] [PubMed] [Google Scholar]
- 54.Theron GB, Shapiro DE, Van Dyke R, et al. Rapid intrapartum or postpartum HIV testing at a midwife obstetric unit and a district hospital in South Africa. Int J Gynaecol Obstet. 2011;113(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64(1):e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veloso VG, Bastos FI, Portela MC, et al. HIV rapid testing as a key strategy for prevention of mother-to-child transmission in Brazil. Rev Saude Publica. 2010;44(5):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viani RM, Araneta MR, Spector SA. Parallel rapid HIV testing in pregnant women at Tijuana General Hospital, Baja California, Mexico. AIDS Res Hum Retroviruses. 2013;29(3):429–434. [DOI] [PubMed] [Google Scholar]
- 58.White DA, Scribner AN, Schulden JD, et al. Results of a rapid HIV screening and diagnostic testing program in an urban emergency department. Ann Emerg Med. 2009;54(1):56–64. [DOI] [PubMed] [Google Scholar]
- 59.Young PW, Mahomed M, Horth RZ, et al. Routine data from prevention of mother-to-child transmission (PMTCT) HIV testing not yet ready for HIV surveillance in Mozambique: a retrospective analysis of matched test results. BMC Infect Dis. 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choko AT, Desmond N, Webb EL, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8(10):e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castel AD, Magnus M, Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, D.C.: findings and lessons learned. Public Health Rep. 2012;127(4):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaydos CA, Solis M, Hsieh YH, et al. Use of tablet-based kiosks in the emergency department to guide patient HIV self-testing with a point-of-care oral fluid test. Int J STD AIDS. 2013;24(9):716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbert R, Ashraf AN, Yates TA, et al. Nurse-delivered universal point-of-care testing for HIV in an open-access returning traveller clinic. HIV Med. 2012;13(8):499–504. [DOI] [PubMed] [Google Scholar]
- 64.Nobrega I, Dantas P, Rocha P, et al. Syphilis and HIV-1 among parturient women in Salvador, Brazil: low prevalence of syphilis and high rate of loss to follow-up in HIV-infected women. Braz J Infect Dis. 2013;17(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell TV, Do AN, Setik E, et al. Sexual risk behaviors for HIV/AIDS in Chuuk State, Micronesia: the case for HIV prevention in vulnerable remote populations. PLoS One. 2007;2(12):e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parisi MR, Soldini L, Vidoni G, et al. Cross-sectional study of community serostatus to highlight undiagnosed HIV infections with oral fluid HIV-1/2 rapid test in non-conventional settings. New Microbiol. 2013;36(2): 121–132. [PubMed] [Google Scholar]
- 67.Seewald R, Bruce RD, Elam R, et al. Effectiveness and feasibility study of routine HIV rapid testing in an urban methadone maintenance treatment program. Am J Drug Alcohol Abuse. 2013;39(4):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anaya H, Feld J, Hoang T, et al. Implementing an HIV rapid testing intervention for homeless veterans in shelter settings within Los Angeles county. J Int Ass Physicians in AIDS Care. 2010;9(1):47. [Google Scholar]
- 69.Metsch LR, Feaster DJ, Gooden L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller S, Jones J, Erbelding E. Choice of Rapid HIV testing and entrance into care in Baltimore City sexually transmitted infections clinics. AIDS Patient Care STDS. 2011;25(4):237–243. [DOI] [PubMed] [Google Scholar]
- 71.Benzaken A, Pinto VM, Carvalho CH, et al. Increasing access to HIV and syphilis screening in remote areas using rapid tests. Sex Transm Infect. 2011;87:A2. [Google Scholar]
- 72.Ekouevi DK, Kariyiare BG, Coffie PA, et al. Feasibility and acceptability of rapid HIV screening in a labour ward in Togo. J Int AIDS Soc. 2012;15(2):17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ganesan A, Thatchinamoorthy G, Saramini S. Feasibility of testing antibodies to HIV from filter paper using HIV rapid test kits. J Int AIDS Soc. 2010;13. [Google Scholar]
- 74.Jerene D, Endale A, Lindtjorn B. Acceptability of HIV counselling and testing among tuberculosis patients in south Ethiopia. BMC Int Health Hum Rights. 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mikolasova G, Bonnach C, Monte CTW, et al. Relative low number of new HIV cases detected in rural district Bunda in Northwest Tanzania. Am J Trop Med Hyg. 2013;1:234. [Google Scholar]
- 76.Mwembo-Tambwe ANK, Kalenga MK, Donnen P, et al. HIV testing among women in delivery rooms in Lubumbashi, DR Congo: A catch-up strategy for prevention of mother-to-child transmission. [French] Depistage du VIH en salle de travail a Lubumbashi, Republique democratique du Congo. Une strategie de rattrapage dans le cadre de la prevention de la transmission de la mere a l'enfant. Rev Epidemiol Sante Publique. 2013;61(1):21–27. [DOI] [PubMed] [Google Scholar]
- 77.Ashby J, Braithewaite B, Walsh J, et al. HIV testing uptake and acceptability in an inner city polyclinic. AIDS Care. 2012;24(7):905–909. [DOI] [PubMed] [Google Scholar]
- 78.Qvist T, Cowan SA, Graugaard C, et al. High linkage to care in a community-based rapid HIV testing and counseling project among men who have sex with men in copenhagen. Sex Transm Dis. 2014;41(3):209–214. [DOI] [PubMed] [Google Scholar]
- 79.Anaya HD, Hoang T, Golden JF, et al. Improving HIV screening and receipt of results by nurse-initiated streamlined counseling and rapid testing. J Gen Intern Med. 2008;23(6):800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beckwith CG, Liu T, Bazerman LB, et al. HIV risk behavior before and after HIV counseling and testing in jail: a pilot study. J Acquir Immune Defic Syndr. 2010;53(4):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hernandez B, Mateo N, Walter J, et al. Targeted bedside emergency department HIV screening does not impact length of stay. Acad Emerg Med. 2013;1:S200. [Google Scholar]
- 82.Engel N, Davids M, Blankvoort N, et al. Making HIV testing work at the point of care in South Africa: a qualitative study of diagnostic practices. BMC Health Serv Res. 2017;17(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


